Abstract
Nontuberculous mycobacteria (NTM) are important pathogens in humans, and hospital-based studies have shown an increased incidence of NTM infection. However, little is known about the treatment pattern of NTM infection with respect to the number of cases per population in South Korea. This study evaluated the trends in the incidence of NTM infection, respiratory comorbidities, and treatment patterns in South Korea.
National claims data from the Health Insurance Review and Assessment service database for the years 2009 to 2015 were reviewed, and codes related to NTM infection, respiratory comorbidities occurring from one year before NTM infection and associated treatments were identified.
In total, 52,551 patients were included in the study and the average annual incidence per 100,000 person-years was 12.8. The annual incidence was found to have increased from 6.6 to 26.6 per 100,000 persons. Accompanied comorbidities were tuberculosis (33.7%), followed by bronchial asthma (33.2%), chronic obstructive pulmonary disease (25.6%), and lung cancer (5.8%). A total of 76.6% of patients did not receive any combination treatment within one year after the diagnosis of NTM infection. Macrolide-based treatment was administered to 18.8% of patients.
A dramatic increase in the incidence of NTM infection was noted in the population of South Korea. Approximately three-fourth of the patients with NTM infection were clinically observed without treatment for at least 1 year after the identification of NTM infection and most patients who treated NTM infection received macrolide-based combination therapy.
Keywords: incidence, nontuberculous mycobacteria, South Korea, treatment pattern
1. Introduction
Nontuberculous mycobacteria (NTM) are opportunistic pathogens prevalent in soil and water systems. Infection with some NTM species can result in diseases of the lungs, skin and soft tissue, and lymphatic system. Pulmonary diseases are among the most common diseases caused by NTM infection,[1] with an increasing incidence and prevalence of NTM-induced pulmonary disease (NTMPD) being recognized in Western and Asian countries.[2]
An increasing annual incidence of NTMPD was noted in Germany during 2009 to 2014, increasing from 2.3 to 3.3 cases per 100,000 population.[3] The annual site-specific prevalence in the US between 2004 and 2006 increased from 1.4 to 6.6 cases per 100,000 population, and it appears that NTM exceeded the incidence of TB as the major cause of mycobacterial lung disease.[4] A population-based study conducted in Japan reported 14.7 cases per 100,000 person-years of NTMPD in 2014, an incidence rate 2.6 times higher than that calculated for 2007.[5]
Although such an increase was evaluated in South Korea,[6–11] analyses of the treatment pattern of NTM infection in addition to the incidence of NTM infection using a nationwide population-based cohort studies are lacking. Furthermore, few studies have been conducted into the identification of comorbidities prior to NTM diagnosis[12,13] or into the evidence-based management of NTM in a population-based cohort.[14] In this study, we analyzed the incidence, comorbidities, and treatment patterns of NTM infection in South Korea using claims data from the Health Insurance Review and Assessment service (HIRA) between 2009 and 2015.
2. Materials and methods
2.1. Data sources
The HIRA database, a nationwide claims database, covers the claims of the entire South Korean population. HIRA database includes patient demographics as well as a record of diagnoses (as determined by the International Classification of Diseases, Tenth Revision [ICD-10]), interventions, and prescriptions. The HIRA service provided data for this study only after anonymization, in line with the Personal Information Protection Act. This retrospective cohort study follows the principles of the declaration of Helsinki. The Institutional Review Board of the HIRA approved this retrospective cohort study (Approval no. 2018–053–001). The requirement for informed patient consent was waived by the board.
2.2. Study design
The data used in this analysis were obtained from all claims registered between January 2009 and December 2015. The incidence of NTM infections was estimated by A31 codes, other than A31.1 which codes for cutaneous mycobacterial infection. New cases of NTM infection were identified when an A31 code was first entered into the HIRA database from 2009 to 2015. If patients had previously attended a clinic for NTM during 2007 to 2008, we excluded them from the incidence estimates. Patients who had more than two visits within 180 days after A31 coding were enrolled for analyses. Population data were extracted from records using the ICD-10 diagnosis code (A31) and stratified according to age and sex. The annual incidence rate of NTM infection was calculated by dividing the number of newly diagnosed NTM infection cases by the total Korean population in each year. Data on the Korean population were acquired from the Statistics Korea database (http://kostat.go.kr) for the period 2009 to 2015.
Pre-existing respiratory comorbidities were also retrieved from the claims database to evaluate the prevalence of accompanying diseases in NTM infection. Data on tuberculosis (A15-A19), chronic obstructive pulmonary disease (COPD, J42-J44), bronchial asthma (J45-J46), bronchiectasis (J47), interstitial lung disease (J84), and lung cancer (C34) were extracted from the HIRA database for patients who had experienced previous pulmonary problems and visited clinics more than once because of these comorbidities within one year before the identification of NTM infection. To analyze the treatment pattern of NTM infection, we searched pharmacy records for patients with A31 codes who were treated within one year after NTM infection according to American Thoracic Society (ATS) and Infectious Disease Society of America (IDSA) guidelines[15] and insurance coverage in South Korea. Inventory lists of the prescriptions were used to evaluate the treatment compounds: ethambutol, rifampin, isoniazid, clarithromycin, azithromycin, cefoxitin, imipenem, streptomycin, or amikacin.
2.3. Statistical analysis
Descriptive statistical analyses were performed on all patients. Continuous and categorical variables are presented as means ± standard deviation and numbers (%). The incidence rate and corresponding 95% confidence intervals (CIs) were calculated using the Poisson distribution. Comparisons between categorical variables were made using the Chi-square test. A two-tailed P value < .05 was considered to indicate statistical significance. All statistical analyses were performed using the R program (ver. 3.4.0; R Development Core Team, Vienna, Austria).
3. Results
3.1. Patient characteristics
In total, 96,040 patients with NTM infection between January 2009 and December 2015 were identified. The total number of patients with NTM infection according to the inclusion criteria during the study period was 52,551 (Fig. 1), and the number of NTM infection increased from 3,293 in 2009 to 13,543 in 2015. There were 22,494 male and 30,057 female patients. The mean age of patients with NTM infection was 53.0 ± 19.9 years.
Figure 1.
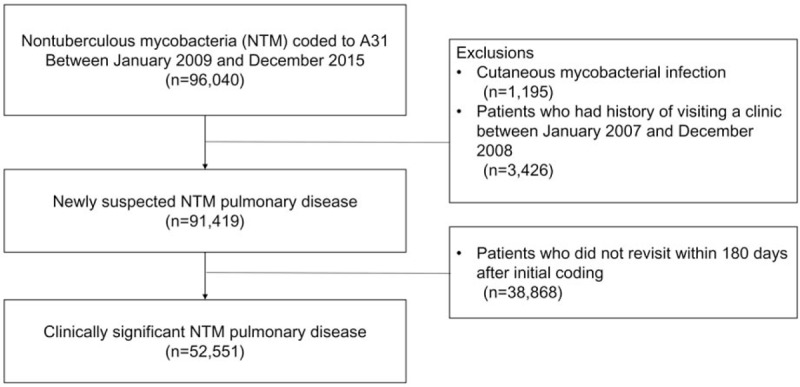
Flow chart of patient selection for this study.
3.2. Incidence rate
The average incidence rate of NTM infection was 12.8 cases per 100,000 person-years. The annual incidence (per 100,000 persons) of patients with NTM infection increased every year, from 6.6 in 2009 to 26.6 in 2015 (P < .001, Table 1) An upward trend in the incidence of NTM infection was found during the study period in both sexes (P < .001, Fig. 2). The annual incidence according to age group also showed similar trends of incidence in each group (P < .001, Fig. 3).
Table 1.
The incidence of nontuberculous mycobacterial pulmonary disease.
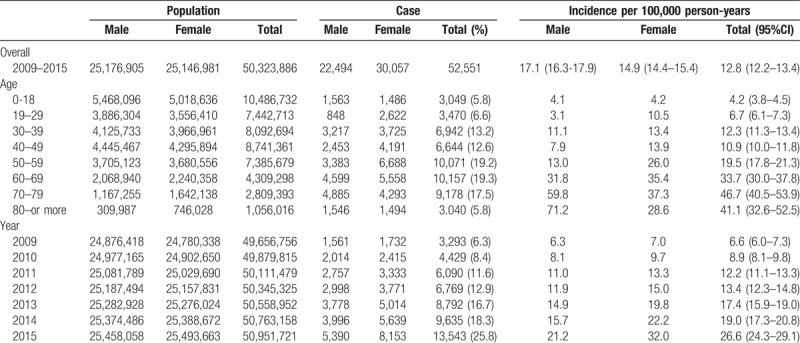
Figure 2.
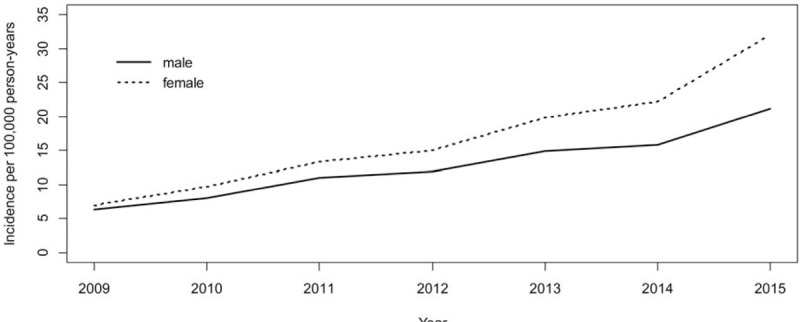
The annual incidence of nontuberculous mycobacterial pulmonary disease by sex and year, 2009 to 2015.
Figure 3.
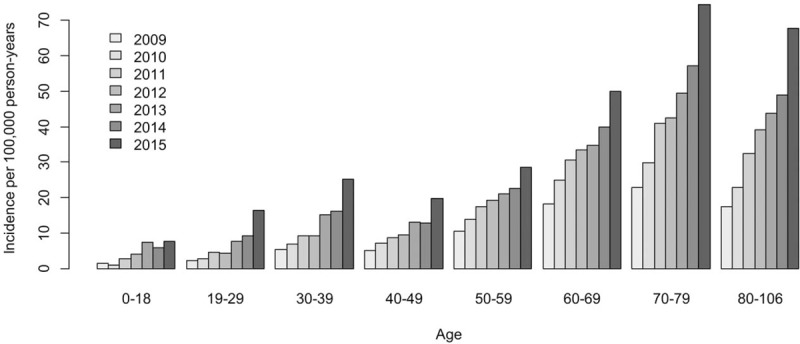
The annual incidence of nontuberculous mycobacterial pulmonary disease by age and year, 2009 to 2015.
3.3. Respiratory comorbidity
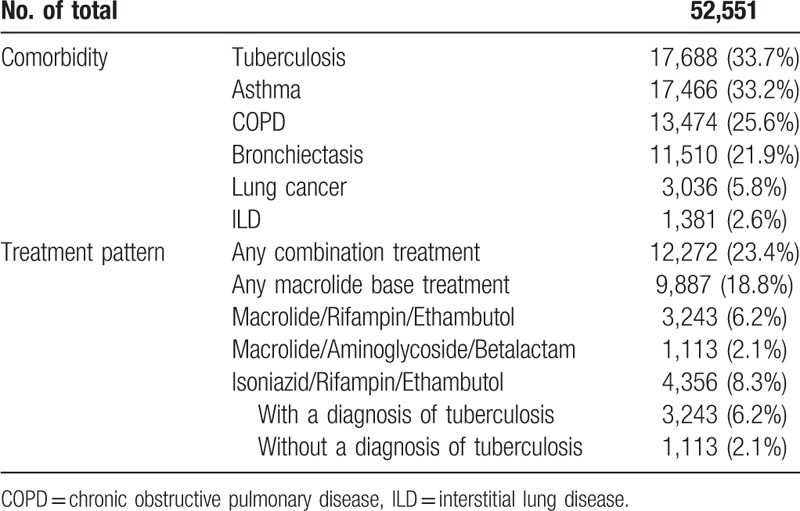
The most frequent concomitant diagnosis throughout the study period was tuberculosis (33.7%), followed by bronchial asthma (33.2%), COPD (25.6%), bronchiectasis (21.9%), lung cancer (5.8%), and interstitial lung disease (2.6%) (Table 2).
Table 2.
Patient comorbidity and treatment pattern.
3.4. Treatment pattern
Only 12,272 patients (23.4%) were treated with combination medication for one year following NTM infection. Among the patients, 9,887 (18.8%) were treated with macrolide-based combination regimens, 9,277 (17.7%) with macrolide/rifampin/ethambutol, and 820 (1.6%) were treated with regimens that included a macrolide, aminoglycoside, and beta-lactam. A total of 4356 (8.3%) patients were treated with isoniazid/rifampin/ethambutol. Among them, 3,243 (6.2%) patients had a concomitant code for tuberculosis and 1113 (2.1%) patients were coded NTM infection without tuberculosis (Table 2).
4. Discussion
Increased rates of NTM infection have frequently been reported worldwide. In a single-center study, the number of patients with NTM lung disease increased from 82 to 133, while the number of TB patients decreased from 436 to 276 during 2002 to 2008.[16] In contrast to the stationary incidence of TB, NTM lung disease might be on the increase in South Korea. Another hospital-based study revealed that the annual incidence of NTM lung disease had increased from 1.82 in 2006 to 4.38 in 2010 per 100,000 population in patients and outpatients.[17] The present population-based cohort study was able to confirm these trends of NTM incidence, showing an increase in the NTM infection rate throughout the study period in line with reports from hospital-based studies. In the present study, and using data from the HIRA-registered population, the incidence of NTM infection increased steeply in 2015 compared to the incidence in 2009. Between 2009 and 2015 in South Korea, the annual incidence rate increased from 6.6 to 26.6 per 100,000 population. On the basis of previously reported evidence, the increasing prevalence of NTM pulmonary infection may now be explained. The increase in the rate of NTM infection may be caused by an actual increased incidence of the disease or an increase in detection due to a multifactorial combination of improved microbiological treatment for NTM and an increased awareness of NTM among clinicians.[4,18] Aging and associated comorbidity may also have contributed to increasing the rate of NTM infection as the elderly population increases in South Korea. According to improved mycobacterial diagnostics and easy access to chest computed tomography scans, it is now possible to suspect NTM pulmonary infection and diagnose it more accurately among patients.
NTM infection is more prevalent in patients aged over 65 years, and the rate of infection in this age group is expected to have increased two-fold by 2030[18]; this is considered a potential public health issue. The results of the present study suggest again that NTM infection is more prevalent in patients aged more than 60 years compared to those aged less than 60 years. The incidence rate showed increasing trends in terms of age as well as a greater prominence in males aged more than 60 years. These trends for old age and a greater increase in incidence in males may be related to comorbidities including COPD or malignancy. NTM patients had a significantly higher incidence of COPD than the non-NTM group (21.75 vs 6.11 per 1,000 person-years).[19] Among patients with cancer and concomitant NTM infection, lung cancer was the most common comorbidity, followed by hematologic malignancy and gastrointestinal tract cancer.[20]
Most patients infected with NTM in the present cohort had concomitant comorbidities. Bronchial asthma was the most common non-infectious lung disease, followed by airway COPD, bronchiectasis, lung cancer, and interstitial lung disease. Patients with bronchial asthma usually use inhaled steroids to control their symptoms, and this may be related to the risk for NTM lung infection.[21] Interestingly, 33.7% of patients had concomitant infections of pulmonary tuberculosis and NTM. It is possible to explain the high prevalence of tuberculosis by the increased chance of detecting NTM during sputum examinations, as well as the chance of NTM infection because of tuberculosis-related structural damage. As tuberculosis is the major comorbidity and the major potential etiological factor, increased efforts for tuberculosis control should be undertaken in South Korea.
Although ATS/IDSA treatment guidelines were published in 2007,[15] consensus management of NTM infections were largely based on a small series of clinical trials and expert opinions with significant variability regarding the treatment of NTMPD, and adherence to these guidelines in clinical practice is poor.[1,22,23] The decision of how to treat NTMPD remains under clinical consideration because clinicians need to evaluate the risks and benefits of treatment using information about NTM species, image findings on chest computed tomography, and the patient's symptoms and conditions. Studies in the United States have reported that around half of patients with NTMPD receive treatment; 55% of these patients have Mycobacterium avium complex (MAC) and 44% have Mycobacterium abscessus.[23] In our study, 9,887 patients (18.8%) were treated with macrolide-based combination regimens within 360 days after notification of NTM infection. Approximately 76.6% of NTM infections were clinically observed without treatment for at least 1 year. However, given that not all NTM infections require treatment initiation at the time of diagnosis, non–pathogenic NTM species may be, in part, responsible for the increased incidence of NTM infection and the reduced treatment rate. Using the established diagnostic criteria, it is possible that a subset of cases were classified as NTM colonization instead of pathogenic infection. In a previous report, 188 (12%) of 1,548 NTM isolates were identified as Mycobacterium gordonae.[24] However, we were unable to identify NTM to the species level due to the inherent limitations of claims data; therefore, further studies on both the incidence of NTM species in South Korea and specific clinical treatment guidelines are needed.
The present study had several limitations. First, the incidence of NTM infection was determined only on the basis of ICD-10 codes, and may not reflect the true incidence of NTM pulmonary disease according to the definition used. Although the majority of NTM infections are pulmonary,[1,25] the results of an analysis of NTM infection involving only those cases with an ICD-10 code of A31 would not reflect the actual incidence of NTM pulmonary disease in South Korea. Second, the insurance claims data provided no detailed information related to the mycobacterial species isolated, history of TB, the radiological pattern of NTM disease, or the geographical region.
5. Conclusion
This nationwide population-based cohort study showed that the incidence of NTM infection in South Korea from 2009 to 2015 increased with increasing age. The most frequent combined comorbidity was tuberculosis, followed by bronchial asthma. Approximately three-fourth of the patients with NTM infection were clinically observed without treatment for at least 1 year after the identification of NTM infection, and most patients who treated NTM infection received macrolide-based combination therapy. Understanding the incidence of NTM infection and its treatment pattern is a major priority for optimizing infection control and resolving public health issues. The dramatic increase in incidence rates for NTM infection warrants clinical concerns considering the chronic nature and poor outcome and tolerability of treatments for NTM infection.
Author contributions
Conceptualization: Hye Ok Kim, Kyungjong Lee.
Data curation: Sang Moo Lee, Gi Hyeon Seo.
Investigation: Hye Ok Kim, Kyungjong Lee, Sangmi Ha, Gi Hyeon Seo.
Methodology: Hee Kyoung Choi, Sang Moo Lee, Gi Hyeon Seo.
Supervision: Hee Kyoung Choi, Gi Hyeon Seo.
Validation: Sangmi Ha, Sang Moo Lee, Gi Hyeon Seo.
Writing – original draft: Hye Ok Kim, Kyungjong Lee.
Gi Hyeon Seo orcid: 0000-0001-7414-0258.
Footnotes
Abbreviations: ATS = American Thoracic Society, COPD = chronic obstructive pulmonary disease, HIRA = Health Insurance Review and Assessment service, ICD-10 = International Classification of Diseases, Tenth Revision, IDSA = Infectious Disease Society of America, MAC = mycobacterium avium complex, NTM = nontuberculous mycobacteria, NTMPD = NTM-induced pulmonary disease.
How to cite this article: Kim HO, Lee K, Choi HK, Ha S, Lee SM, Seo GH. Incidence, comorbidities, and treatment patterns of nontuberculous mycobacterial infection in South Korea. Medicine. 2019;98:45(e17869).
HOK and KL contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Kang YA, Koh WJ. Antibiotic treatment for nontuberculous mycobacterial lung disease. Expert Rev Respir Med 2016;10:557–68. [DOI] [PubMed] [Google Scholar]
- [2].Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis 2014;6:210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ringshausen FC, Wagner D, de Roux A, et al. Prevalence of nontuberculous mycobacterial pulmonary disease, Germany, 2009-2014. Emerg Infect Dis 2016;22:1102–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med 2010;182:970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Namkoong H, Kurashima A, Morimoto K, et al. Epidemiology of Pulmonary Nontuberculous Mycobacterial Disease, Japan(1). Emerg Infect Dis 2016;22:1116–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ko RE, Moon SM, Ahn S, et al. Changing epidemiology of nontuberculous mycobacterial lung diseases in a tertiary referral hospital in Korea between 2001 and 2015. J Korean Med Sci 2018;33:e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Koh WJ, Chang B, Jeong BH, et al. Increasing recovery of nontuberculous mycobacteria from respiratory specimens over a 10-year period in a tertiary referral hospital in South Korea. Tuberc Respir Dis (Seoul) 2013;75:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kwon YS, Koh WJ. Diagnosis of pulmonary tuberculosis and nontuberculous mycobacterial lung disease in Korea. Tuberc Respir Dis (Seoul) 2014;77:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim JK, Rheem I. Identification and distribution of nontuberculous mycobacteria from 2005 to 2011 in cheonan, Korea. Tuberc Respir Dis (Seoul) 2013;74:215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yoon HJ, Choi HY, Ki M. Nontuberculosis mycobacterial infections at a specialized tuberculosis treatment center in the Republic of Korea. BMC Infect Dis 2017;17:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee H, Myung W, Koh WJ, et al. Epidemiology of nontuberculous mycobacterial infection, South Korea, 2007-2016. Emerg Infect Dis 2019;25:569–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Andrejak C, Thomsen VO, Johansen IS, et al. Nontuberculous pulmonary mycobacteriosis in Denmark: incidence and prognostic factors. Am J Respir Crit Care Med 2010;181:514–21. [DOI] [PubMed] [Google Scholar]
- [13].Al-Houqani M, Jamieson F, Mehta M, et al. Aging, COPD, and other risk factors do not explain the increased prevalence of pulmonary mycobacterium avium complex in ontario. Chest 2012;141:190–7. [DOI] [PubMed] [Google Scholar]
- [14].Axson EL, Bloom CI, Quint JK. Nontuberculous mycobacterial disease managed within UK primary care, 2006-2016. Eur J Clin Microbiol Infect Dis 2018;37:1795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367–416. [DOI] [PubMed] [Google Scholar]
- [16].Park YS, Lee CH, Lee SM, et al. Rapid increase of non-tuberculous mycobacterial lung diseases at a tertiary referral hospital in South Korea. Int J Tuberc Lung Dis 2010;14:1069–71. [PubMed] [Google Scholar]
- [17].Lee SK, Lee EJ, Kim SK, et al. Changing epidemiology of nontuberculous mycobacterial lung disease in South Korea. Scand J Infect Dis 2012;44:733–8. [DOI] [PubMed] [Google Scholar]
- [18].Adjemian J, Olivier KN, Seitz AE, et al. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med 2012;185:881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yeh JJ, Wang YC, Sung FC, et al. Nontuberculosis mycobacterium disease is a risk factor for chronic obstructive pulmonary disease: a nationwide cohort study. Lung 2014;192:403–11. [DOI] [PubMed] [Google Scholar]
- [20].Lai CC, Tan CK, Cheng A, et al. Nontuberculous mycobacterial infections in cancer patients in a medical center in Taiwan, 2005-2008. Diagn Microbiol Infect Dis 2012;72:161–5. [DOI] [PubMed] [Google Scholar]
- [21].Brode SK, Campitelli MA, Kwong JC, et al. The risk of mycobacterial infections associated with inhaled corticosteroid use. Eur Respir J 2017;50. [DOI] [PubMed] [Google Scholar]
- [22].van Ingen J, Wagner D, Gallagher J, et al. Poor adherence to management guidelines in nontuberculous mycobacterial pulmonary diseases. Eur Respir J 2017;49. [DOI] [PubMed] [Google Scholar]
- [23].Adjemian J, Prevots DR, Gallagher J, et al. Lack of adherence to evidence-based treatment guidelines for nontuberculous mycobacterial lung disease. Ann Am Thorac Soc 2014;11:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Koh WJ, Kwon OJ, Jeon K, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest 2006;129:341–8. [DOI] [PubMed] [Google Scholar]
- [25].Adzic-Vukicevic T, Barac A, Blanka-Protic A, et al. Clinical features of infection caused by non-tuberculous mycobacteria: 7 years’ experience. Infection 2018;46:357–63. [DOI] [PubMed] [Google Scholar]


