Supplemental Digital Content is available in the text
Keywords: cardiopulmonary resuscitation, heart arrest, medical device, observational study, prognosis
Abstract
This study aimed to investigate the prognostic difference between AUTOPULSE and LUCAS for out-of-hospital cardiac arrest (OHCA) adult patients.
A retrospective observational study was performed nationwide. Adult OHCA patients after receiving in-hospital mechanical chest compression from 2012 to 2016 were included. The primary outcomes were sustained return of spontaneous circulation (ROSC) of more than 20 minutes and survival to discharge.
Among 142,906 OHCA patients, 820 patients were finally included. In multivariate analysis, female (OR, 0.57; 95% CI, 0.33–0.99), witnessed arrest (OR, 2.10; 95% CI, 1.20–3.69), and arrest cause of non-cardiac origin (OR, 0.25; 95% CI, 0.10–0.62) were significantly associated with the increase in ROSC. LUCAS showed a lower survival than AUTOPULSE (OR, 0.23; 95% CI, 0.06–0.84), although it showed no significant association with ROSC. Percutaneous coronary intervention (OR, 6.30; 95% CI, 1.53–25.95) and target temperature management (TTM; OR, 7.30; 95% CI, 2.27–23.49) were the independent factors for survival. We categorized mechanical CPR recipients by witness to compare prognostic effectiveness of AUTOPULSE and LUCAS. In the witnessed subgroup, female (OR, 0.46; 95% CI, 0.24–0.89) was a prognostic factor for ROSC and shockable rhythm (OR, 5.04; 95% CI, 1.00–25.30), percutaneous coronary intervention (OR, 12.42; 95% CI, 2.04–75.53), and TTM (OR, 9.03; 95% CI, 1.86–43.78) for survival. In the unwitnessed subgroup, no prognostic factors were found for ROSC, and TTM (OR, 99.00; 95% CI, 8.9–1100.62) was found to be an independent factor for survival. LUCAS showed no significant increase in ROSC or survival in comparison with AUTOPULSE in both subgroups.
The in-hospital use of LUCAS may have a deleterious effect for survival compared with AUTOPULSE.
1. Introduction
The 2 recently used mechanical CPR devices are AUTOPULSE (AutoPulse Resuscitation System Model 100, ZOLL, CA) and LUCAS (LUCASTM2 Chest Compression System, JOLIFE AB Inc., Lund, Sweden). AUTOPULSE has a cardiac and thoracic pump mechanism.[1] A load-distributing band consists of a cover plate and 2 bands integrated with a compression pad with a Velcro fastener. Attached to a platform under the patient, the band is automatically adjusted to the patient and provides compressions to the patient's chest in the region of the heart.[2] By contrast, LUCAS has a cardiac pump mechanism.[1] A back plate is positioned underneath the patient as a support for the external chest compressions. An upper part with a suction cup is attached to the back plate through a claw lock on each side. This suction cup is positioned on the sternum and is capable of active decompression.[3] Unlike manual CPR that has a cardiac pump mechanism alone, the thoracic pump of AUTOPULSE and the active decompression of LUCAS are added in these mechanical CPR devices. Adding these unique mechanisms is deemed to enhance CPR quality compared with manual CPR. Therefore, mechanical CPR using AUTOPULSE and LUCAS could be associated with an increased rate of ROSC.[4]
However, several previous studies reported that the use of mechanical CPR devices was highly associated with post-mortem complications such as visceral injury or rib fracture during CPR. AUTOPULSE has a high incidence of pneumothorax and hematoma.[5] In addition, LUCAS has a high probability of sternum and rib fracture.[6,7]
In a recent study by Khan et al, the survival and post-mortem complications between AUTOPULSE and LUCAS were assessed indirectly using a Bayesian network meta-analysis.[8] The study reported no significant differences in survival at hospital discharge or 30 days between AUTOPULSE and LUCAS. In the comparison of post-mortem complications, AUTOPULSE showed a higher incidence of pneumothorax and hematoma and an equal incidence of rib or sternum fracture to LUCAS.[5–7]
Obtaining large samples or permission from arrest patients during CPR remains difficult when performing well-designed studies or randomized controlled trials for a direct comparison. Thus, we compared AUTOPULSE directly with LUCAS using nationwide observational studies. This study aimed to assess the efficacy of in-hospital use of AUTOPULSE and LUCAS in adult OHCA patients.
2. Methods
2.1. Study design and settings
This work is a population-based retrospective observational study using nationwide data from the Out of Hospital Cardiac Arrest Surveillance (OHCAS) conducted by the Korean Centers for Disease Control and Prevention (KCDC) from January 2012 to December 2016. OHCAS was performed in the 17 provinces of Republic of Korea. The local ethics committee approved this study in 2019 (Kangnam Sacred Heart Hospital Institutional Review Board; IRB No. 2019-01-016), and informed consent was not wavered. The KCDC approved the use of this data in this study.
2.2. Population
A total of 142,905 out-of-hospital cardiac arrest (OHCA) patients between January 2012 and December 2016 were obtained. This study included all adult patients (older than 18 years of age) who survived to hospitalization and received in-hospital cardiopulmonary resuscitation by mechanical CPR devices after prehospital manual CPR by emergency medical technicians (EMT), AUTOPULSE, or LUCAS for more than 20 minutes in the emergency room. The exclusion criteria were as follows: trauma related, dead on arrival (DOA), pre-hospital ROSC, do not resuscitate (DNR), transfer out from emergency room, age of less than 18 years, and patients who received manual CPR. Pre-hospital ROSC was excluded in this study because our aim was to evaluate the effect of in-hospital use of mechanical CPR device on outcome of OHCA patients. Trauma patients were excluded from this study because traumatic OHCA has a different pathophysiology and usually require various interventions in contrast to OHCA of medical causes.[9] Furthermore, mechanical CPR could be contraindicated in traumatic OHCA, especially caused by thoracic trauma injuries.[10] These exclusions were meant to reduce heterogeneity in the population while maintaining generalizability to most patients with sudden cardiac arrest. Other well-designed studies also excluded trauma patients for these reasons.[9,10]
2.3. Variables
Information on individual factors (age, sex), initial monitored rhythm (shockable vs non-shockable), etiologic factors (cardiac vs non-cardiac), witnessed cardiac arrest, bystander CPR, target temperature management (TTM), percutaneous coronary intervention (PCI), extracorporeal membrane oxygenation (ECMO), pacemaker, and mechanical CPR devices (AUTOPULSE vs LUCAS) were collected. Shockable rhythm was defined as ventricular fibrillation and pulseless ventricular tachycardia. Arrest cause of cardiac origin was defined as failure of cardiac function, such as cardiac tamponade, ischemic heart diseases, arrhythmias, or cardiac cause suspected for unanticipated arrest patients. TTM included both external and intravascular cooling devices. PCI included ballooning and primary stenting. Pacemaker refers to the insertion of a temporary or permanent pacemaker. The appliance of external cardiac pacemaker was not included.
2.4. Outcome measures
The primary outcome measures were sustained ROSC for more than 20 minutes in the hospital and survival to hospital discharge.
2.5. Statistical analysis
Data analyses were performed using the R version 3.3.2 (http://www.web-r.org) software. Descriptive statistics were used to describe the baseline characteristics of the study participants and to present the categorical variables as frequencies and percentages. Non-normally distributed data are presented as medians with interquartile ranges. In the univariate analysis, the Mann–Whitney U test was used for comparing the continuous variables and the chi-square or Fisher exact test for the categorical variables. A propensity–score matching (PSM) analysis was performed to adjust the possible confounding factors. To identify the predictors of the outcomes, the effect of statistically significant covariates after PSM was evaluated by adjusted odds ratios from the multivariate logistic regression of stepwise backward elimination. A P value of less than .05 was considered statistically significant.
3. Result
3.1. Patient characteristics
Among the 142,905 OHCA patients, the following were excluded: trauma patients (n = 35,932), DOA patients (n = 43,751), pre-hospital ROSC (n = 5807), DNR patients (n = 3846), patients younger than 18 years (n = 1076), and patients transferred out from the emergency room (n = 30). Of the remaining 52,463 OHCA patients, the additional exclusion of manual CPR (n = 19,045) and missing data (n = 32,598) was conducted. The remaining enrolled adult OHCA patients who received in-hospital mechanical CPR by AUTOPULSE (n = 671) or LUCAS (n = 149) accounted for 820 (Fig. 1). This whole group was then divided into two subgroups: the group of witnessed patients [AUTOPULSE (n = 422) and LUCAS (n = 95)] and the group of unwitnessed patients [AUTOPULSE (n = 249) and LUCAS (n = 54)]. These 3 groups underwent 1:1 PSM and a multivariable logistic regression analysis to identify the independent predictors of good outcomes.
Figure 1.
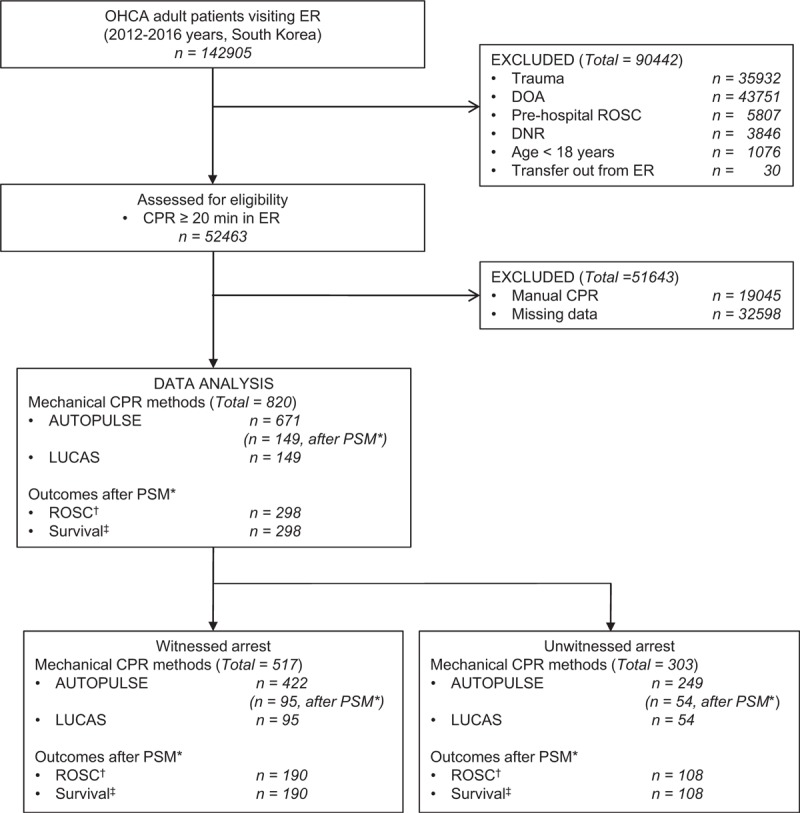
Flow diagram of the study population. CPR, cardiopulmonary resuscitation, DNR, do not resuscitate, DOA, dead on arrival, ER, emergency room; OHCA, out-of-hospital cardiac arrest, PSM, propensity score matching, ROSC, return of spontaneous circulation. ∗ 1:1 propensity score matching to select the participants in both the witnessed and unwitnessed groups. †ROSC was defined as sustained circulation more than 20 minutes. ‡Survival was defined as survival at hospital discharge.
3.2. All OHCA patients: AUTOPULSE vs LUCAS
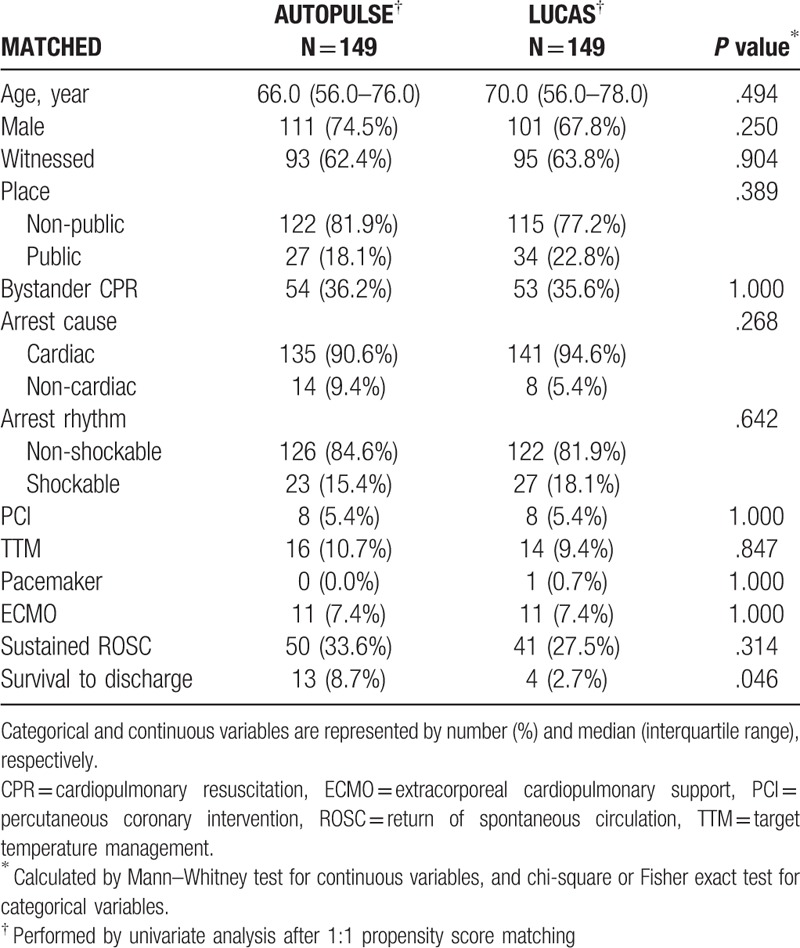
Unmatched univariate analysis revealed that PCI and ECMO were significant confounders (P = .009; P = .015, respectively; Supplementary Table 1). After adjusting these confounders by performing PSM, all of the confounders were fully adjusted. LUCAS showed a lower survival than AUTOPULSE (P = .046) and an equal ROSC to AUTOPULSE (P = .314) (Table 1).
Table 1.
Matched univariate analysis for mechanical cardiopulmonary resuscitation of all arrest patients.
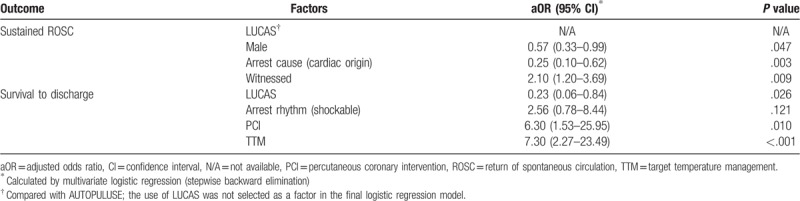
After a multivariate logistic regression analysis by stepwise backward elimination, female (odds ratio (OR), 0.57; 95% confidential interval (CI), 0.33–0.99), witnessed arrest (OR, 2.10; 95% CI, 1.20–3.69), and arrest cause of non-cardiac origin (OR, 0.25; 95% CI, 0.10–0.62) were found to be significantly associated with increased ROSC. LUCAS showed a lower survival than AUTOPULSE (OR, 0.23; 95% CI, 0.06–0.84) and showed no significant association with ROSC. PCI (OR, 6.30; 95% CI, 1.53–25.95) and TTM (OR, 7.30; 95% CI, 2.27–23.49) were independent factors for survival to discharge (Table 2).
Table 2.
Matched multivariate analysis for all arrest patients (n = 298) in the comparison of AUTOPULSE and LUCAS.
3.3. Witnessed OHCA patients: AUTOPULSE VS LUCAS
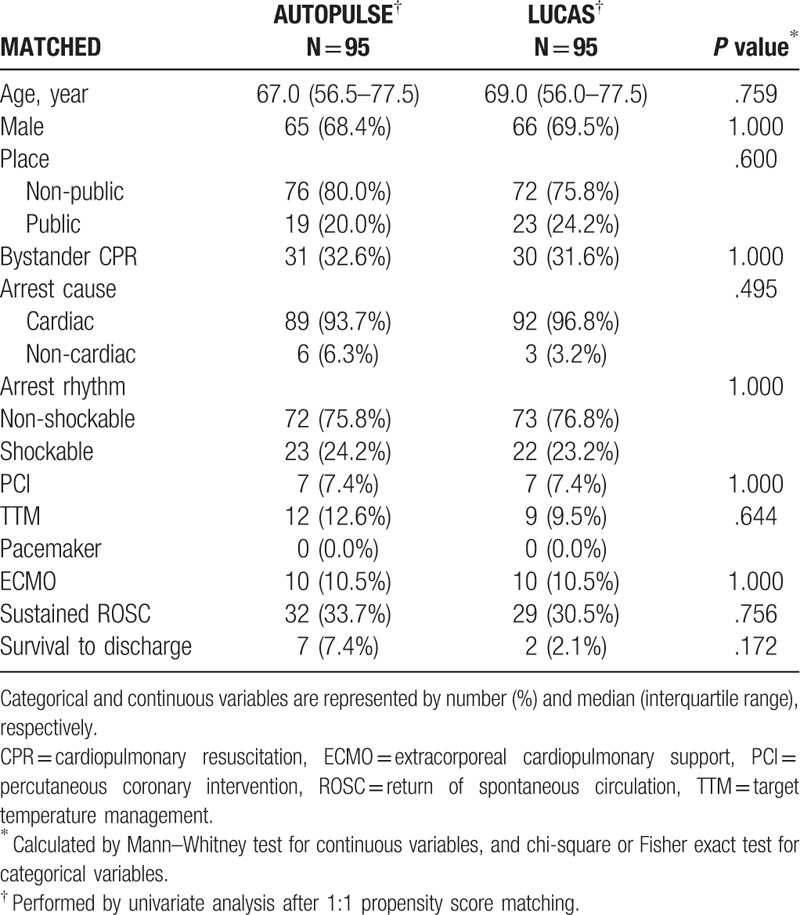
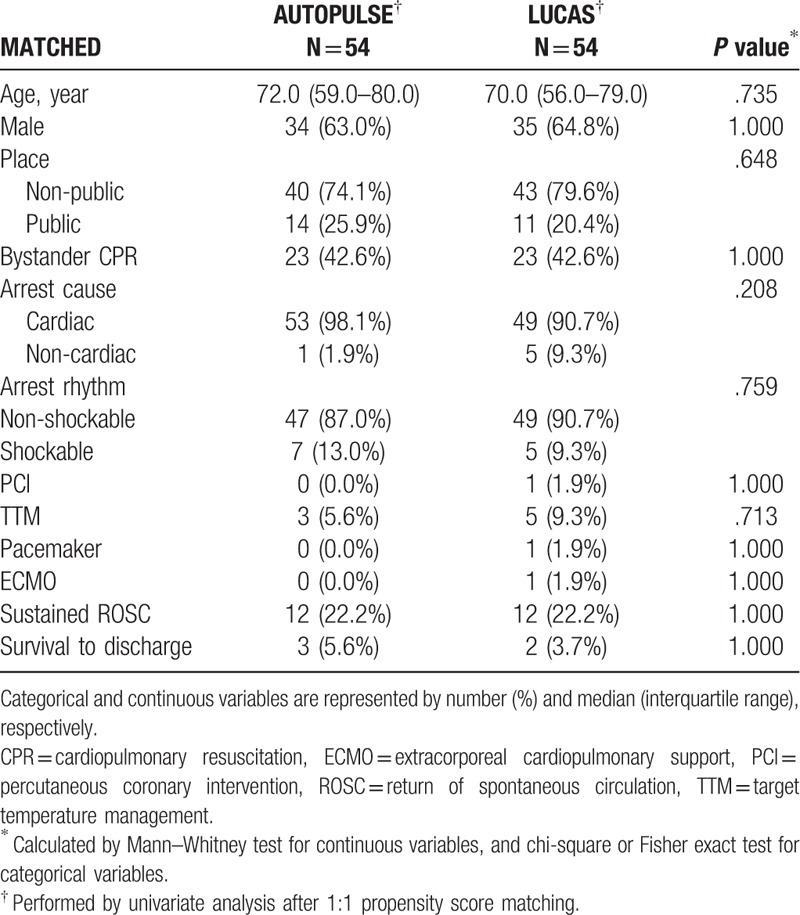
Unmatched univariate analysis revealed that PCI and ECMO were significant confounders (P = .032; P = .014, respectively; Supplementary Table 2). After adjusting these confounders by performing PSM, all of the confounders were fully adjusted. AUTOPULSE showed an equal ROSC and survival to LUCAS confounders (P = .756; P = .172, respectively; Table 3).
Table 3.
Matched univariate analysis for mechanical cardiopulmonary resuscitation of witnessed arrest patients.
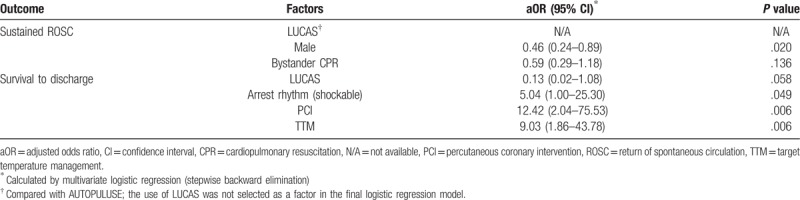
After a multivariate logistic regression analysis by stepwise backward elimination, female (OR, 0.46; 95% CI, 0.24–0.89) was found to be associated with ROSC. Shockable rhythm (OR, 5.04; 95% CI, 1.00–25.30), PCI (OR, 12.42; 95% CI, 2.04–75.53), TTM (OR, 9.03; 95% CI, 1.86–43.78) were independently associated with survival. LUCAS showed no difference with AUTOPULSE in both ROSC and survival to discharge (Table 4).
Table 4.
Matched multivariate analysis for witnessed arrest patients (n = 190) in the comparison of AUTOPULSE and LUCAS.
3.4. Unwitnessed OHCA patients: AUTOPULSE vs LUCAS
Unmatched univariate analysis revealed that bystander CPR was a significant confounder (P = .008; Supplementary Table 3). After adjusting this confounder by performing PSM, all of the confounders were fully adjusted. AUTOPULSE showed an equal ROSC and survival to the LUCAS confounders (P = 1.000; P = 1.000, respectively; Table 5).
Table 5.
Matched univariate analysis for mechanical cardiopulmonary resuscitation of unwitnessed arrest patients.
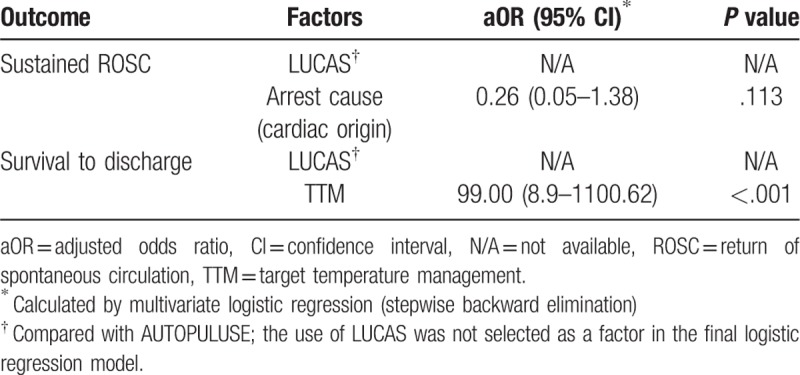
After a multivariate logistic regression analysis by stepwise backward elimination, no single factor was found to have a significant influence on ROSC. TTM (OR, 99.00; 95% CI, 8.9–1100.62) was independently associated with survival. LUCAS showed no difference with AUTOPULSE in both ROSC and survival to discharge (Table 6).
Table 6.
Matched multivariate analysis for unwitnessed arrest patients (n = 108) in the comparison of AUTOPULSE and LUCAS.
4. Discussion
Mechanical CPR devices are introduced as probable alternatives to manual CPR. Theoretical advantages of mechanical CPR make them attractive. Relieving rescuer fatigue, consistent and reliable chest compression are the main advantages.[11] However, studies are still yet conflicting, with some proving disadvantages and lack of clinical benefit of mechanical CPR. Some drawbacks of mechanical CPR are pneumothorax, rib fracture, and visceral injuries.[5–7] Despite its inability to replace manual CPR,[12] studies are continuously progressing and several types of mechanical CPR devices have been proposed.[13,14]
However, there have been no direct comparative randomized control trials or nationwide studies so far between LUCAS and AUTOPULSE. Only a recent systematic review by Khan et al. indirectly compared LUCAS with AUTOPULSE using a Bayesian network meta-analysis.[8] In the study of Khan et al, only patients from Europe, the United States, and Canada were included, and patients from Asia were excluded. To secure wide representation and to reach a robust conclusion on the efficacy of mechanical CPR devices, nationwide studies in Asia, such as our study, should be conducted.[5,15–20]
Our study is the first to directly compare LUCAS with AUTOPULSE using nationwide data. This study also demonstrated that LUCAS showed a lower survival of all arrest patients in South Korea than AUTOPULSE, although it showed no significant association with ROSC in CPR. Therefore, we could assume that the in-hospital use of LUCAS could have a deleterious effect on survival compared with AUTOPULSE.
This deleterious result of LUCAS may be related to patients’ age and chest configuration, as these factors may be associated with the cardiac pump only mechanism of LUCAS during CPR.[1,21] In old-age patients with increased anterior–posterior chest diameters, the so-called “barrel chests,” the cardiac pump mechanism alone may be insufficient.[1] By contrast, the thoracic pump mechanism of AUTOPULSE can increase the intra-thoracic pressure in barrel chests, generating forward blood flow from the heart.[22] We assumed that the high proportion of elderly patients in this study (69.7% of over 60 years of age) was associated with a high proportion of barrel chests, which could benefit from AUTOPULSE. This outcome may be attributed to the better prognosis of AUTOPULSE.
We also found several prognostic factors related to good prognosis. Non-cardiac origin arrest cause, female patients, and witnessed arrest were associated with increased ROSC.(Table 2) As cardiac origin included presumed cardiac cause not fully diagnosed, it was inappropriate as a standard for subgroup analysis. In some previous studies, witnessed arrest was reported to be a good prognostic factor in OHCA patients.[23–25] Thus, we performed a subgroup analysis for the witnessed and unwitnessed subgroups. In witnessed subgroup, female was found to have significant influence on ROSC. Shockable rhythm, PCI, and TTM were associated with survival. In unwitnessed subgroup, TTM was independently associated with survival. LUCAS showed no difference with AUTOPULSE in both subgroups.
Significant prognostic factors from this study differed from other former studies.[23–29] In one study of 1528 witnessed arrest patients,[25] the researchers found that gender, age, public location, and shockable rhythm were significantly associated with survival to hospital discharge In our study, however, shockable rhythm, PCI, and TTM were significantly associated with survival. We suspected that this difference was due to the former study lacking in-hospital procedures data such as PCI and TTM, which have been known to be important prognostic factors.[25–27,29] Other reasons for different risk factors are such as PSM not performed in former studies and discrepancy in the EMS systems including ACLS.[23–29]
No-flow time and low-flow time were also important factors in the outcome of cardiac arrest patients.[11,30] No-flow time is defined as the time during which no chest compression occurs.[31] Low-flow time is defined as time with active CPR by a bystander or a medical provider.[32] However, flow time of OHCA was not provided from the raw data of the Out-of-Hospital Cardiac Arrest Surveillance (OHCAS). Nevertheless, we categorized mechanical CPR recipients by witness (witnessed vs un-witnessed) to consider indirectly the effect of no-flow time because witnessed OHCA patients were more likely to have shorter no-flow time than unwitnessed OHCA patients. But in this study, we could not reflect the effect of low flow time on outcome of OHCA patients by categorizing by witness because the raw data did not provide ROSC time of OHCA patients. The outcome of OHCA treated with mechanical CPR in this study could be changed if no flow time or low flow time were to be identified.
4.1. Limitations
This study has some limitations.
First, the issues of selection bias remained in the comparison between LUCAS and AUTOPULSE. This comparison was not prospectively randomized because of the nature of retrospective observational study. In addition, the differences in medical systems among various medical facilities could have affected the outcomes. If this study was performed in other countries or races besides South Korea, the results would not be similar to those of this study. Even if the appropriate statistical method, such as score matching, was applied to control the confounders, the original bias could not be removed. Thus, the results of this comparative study should be cautiously interpreted considering the occurrence of bias.
Second, the important confounders were not fully adjusted. In the raw data from the OHCAS, the underlying medical condition of patients or the severity score (e.g., Acute Physiology and Chronic Health Evaluation) was not included. Patient's underlying disease, hemodynamic status, laboratory findings and mental status were not provided. Previous well-designed studies also did not provide information on these confounders.[8] Nevertheless, we consider the difference in these confounders between mechanical CPR devices to affect the outcomes of this study.
Third, only the short-term outcome was measured in this study. To compare LUCAS with AUTOPULSE in a more precise manner, a long-term functional outcome will be necessarily in future studies.
Fourth, the effect of pre-hospital manual CPR could not be measured quantitatively for LUCAS or AUTOPULSE. The differences in CPR duration, expertise, or number of EMT between both groups could cause an imbalanced manual CPR effect on outcome, which could have occurred in this study.
5. Conclusion
The in-hospital use of LUCAS may have a deleterious effect on survival compared with AUTOPULSE. Nevertheless, these results should be cautiously interpreted considering the possible bias. Further nationwide studies are needed to measure long-term outcome and should also include the in-hospital data of patients.
Author contributions
Conceptualization: Hyun Tae Kim, Jae Guk Kim, Yong Soo Jang, Gu Hyun Kang, Wonhee Kim, Hyun Young Choi, Gwang Soo Jun.
Data curation: Hyun Tae Kim, Jae Guk Kim, Yong Soo Jang, Gu Hyun Kang, Wonhee Kim, Hyun Young Choi, Gwang Soo Jun.
Formal analysis: Hyun Tae Kim, Jae Guk Kim, Yong Soo Jang, Wonhee Kim, Gwang Soo Jun.
Funding acquisition: Jae Guk Kim.
Investigation: Hyun Tae Kim, Jae Guk Kim, Yong Soo Jang, Gu Hyun Kang, Hyun Young Choi, Gwang Soo Jun.
Methodology: Hyun Tae Kim, Jae Guk Kim, Yong Soo Jang, Gu Hyun Kang, Wonhee Kim, Gwang Soo Jun.
Project administration: Hyun Tae Kim, Yong Soo Jang.
Resources: Hyun Tae Kim, Jae Guk Kim, Yong Soo Jang.
Software: Hyun Tae Kim, Jae Guk Kim, Yong Soo Jang.
Supervision: Yong Soo Jang.
Validation: Hyun Tae Kim, Jae Guk Kim, Yong Soo Jang, Gu Hyun Kang.
Visualization: Hyun Tae Kim, Jae Guk Kim, Yong Soo Jang.
Writing – original draft: Hyun Tae Kim, Jae Guk Kim, Yong Soo Jang.
Writing – review & editing: Hyun Tae Kim, Jae Guk Kim, Yong Soo Jang, Gu Hyun Kang, Wonhee Kim, Hyun Young Choi.
Yong Soo Jang orcid: 0000-0001-5964-1580.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CPR = cardiopulmonary resuscitation, DNR = do not resuscitate, DOA = dead on arrival, ECMO = extracorporeal membrane oxygenation, EMS = emergency medical service, EMT = emergency medical technicians, KCDC = Korean Centres for Disease Control and Prevention, OHCA = out-of-hospital cardiac arrest, OHCAS = Out-of-Hospital Cardiac Arrest Surveillance, OR = odds ratio, PCI = percutaneous coronary intervention, PSM = propensity–score matching, ROSC = return of spontaneous circulation, TTM = targeted temperature management.
How to cite this article: Kim HT, Kim JG, Jang YS, Kang GH, Kim W, Choi HY, Jun GS. Comparison of in-hospital use of mechanical chest compression devices for out-of-hospital cardiac arrest patients. Medicine. 2019;98:45(e17881).
The authors have no funding and conflicts of interests to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Ewy GA. The mechanism of blood flow during chest compressions for cardiac arrest is probably influenced by the patient's chest configuration. Acute Med Surg 2018;5:236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].AutoPulse Resuscitation System - Zoll Medical. Available at: https://www.zoll.com/medical-products/resuscitation-system/autopulse/ems [access date July 1, 2019]. [Google Scholar]
- [3].LUCAS Chest Compression System - Physio Control. Available at: https://www.lucas-cpr.com/ [access date July 1, 2019]. [Google Scholar]
- [4].Seewald S, Obermaier M, Lefering R, et al. Application of mechanical cardiopulmonary resuscitation devices and their value in out-of-hospital cardiac arrest: a retrospective analysis of the German Resuscitation Registry. PloS One 2019;14:e0208113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Koster RW, Beenen LF, van der Boom EB, et al. Safety of mechanical chest compression devices AutoPulse and LUCAS in cardiac arrest: a randomized clinical trial for non-inferiority. Eur Heart J 2017;38:3006–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Oberladstaetter D, Braun P, Freund MC, et al. Autopsy is more sensitive than computed tomography in detection of LUCAS-CPR related non-dislocated chest fractures. Resuscitation 2012;83:e89–90. [DOI] [PubMed] [Google Scholar]
- [7].Smekal D, Lindgren E, Sandler H, et al. CPR-related injuries after manual or mechanical chest compressions with the LUCAS device: a multicentre study of victims after unsuccessful resuscitation. Resuscitation 2014;85:1708–12. [DOI] [PubMed] [Google Scholar]
- [8].Khan SU, Lone AN, Talluri S, et al. Efficacy and safety of mechanical versus manual compression in cardiac arrest - a Bayesian network meta-analysis. Resuscitation 2018;130:182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Couper K, Quinn T, Lall R, et al. Mechanical versus manual chest compressions in the treatment of in-hospital cardiac arrest patients in a non-shockable rhythm: a randomised controlled feasibility trial (COMPRESS-RCT). Scand J Trauma Resusc Emerg Med 2018;26:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang PL, Brooks SC. Mechanical versus manual chest compressions for cardiac arrest. Cochrane Database Syst Rev 2018;8:Cd007260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ong ME, Mackey KE, Zhang ZC, et al. Mechanical CPR devices compared to manual CPR during out-of-hospital cardiac arrest and ambulance transport: a systematic review. Scand J Trauma Resusc Emerg Med 2012;20:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li H, Wang D, Yu Y, et al. Mechanical versus manual chest compressions for cardiac arrest: a systematic review and meta-analysis. Scand J Trauma Resusc Emerg Med 2016;24:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jennings PA, Harriss L, Bernard S, et al. An automated CPR device compared with standard chest compressions for out-of-hospital resuscitation. BMC Emerg Med 2012;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Westfall M, Krantz S, Mullin C, et al. Mechanical versus manual chest compressions in out-of-hospital cardiac arrest: a meta-analysis. Crit Care Med 2013;41:1782–9. [DOI] [PubMed] [Google Scholar]
- [15].Axelsson C, Nestin J, Svensson L, et al. Clinical consequences of the introduction of mechanical chest compression in the EMS system for treatment of out-of-hospital cardiac arrest-a pilot study. Resuscitation 2006;71:47–55. [DOI] [PubMed] [Google Scholar]
- [16].Hallstrom A, Rea TD, Sayre MR, et al. Manual chest compression vs use of an automated chest compression device during resuscitation following out-of-hospital cardiac arrest: a randomized trial. JAMA 2006;295:2620–8. [DOI] [PubMed] [Google Scholar]
- [17].Perkins GD, Lall R, Quinn T, et al. Mechanical versus manual chest compression for out-of-hospital cardiac arrest (PARAMEDIC): a pragmatic, cluster randomised controlled trial. Lancet (London, England) 2015;385:947–55. [DOI] [PubMed] [Google Scholar]
- [18].Rubertsson S, Lindgren E, Smekal D, et al. Mechanical chest compressions and simultaneous defibrillation vs conventional cardiopulmonary resuscitation in out-of-hospital cardiac arrest: the LINC randomized trial. JAMA 2014;311:53–61. [DOI] [PubMed] [Google Scholar]
- [19].Smekal D, Johansson J, Huzevka T, et al. No difference in autopsy detected injuries in cardiac arrest patients treated with manual chest compressions compared with mechanical compressions with the LUCAS device--a pilot study. Resuscitation 2009;80:1104–7. [DOI] [PubMed] [Google Scholar]
- [20].Wik L, Olsen JA, Persse D, et al. Manual vs. integrated automatic load-distributing band CPR with equal survival after out of hospital cardiac arrest. The randomized CIRC trial. Resuscitation 2014;85:741–8. [DOI] [PubMed] [Google Scholar]
- [21].Halperin HR, Tsitlik JE, Gelfand M, et al. A preliminary study of cardiopulmonary resuscitation by circumferential compression of the chest with use of a pneumatic vest. N Engl J Med 1993;329:762–8. [DOI] [PubMed] [Google Scholar]
- [22].Halperin HR, Paradis N, Ornato JP, et al. Cardiopulmonary resuscitation with a novel chest compression device in a porcine model of cardiac arrest: improved hemodynamics and mechanisms. J Am Coll Cardiol 2004;44:2214–20. [DOI] [PubMed] [Google Scholar]
- [23].Boyce LW, Vliet Vlieland TP, Bosch J, et al. High survival rate of 43% in out-of-hospital cardiac arrest patients in an optimised chain of survival. Neth Heart J 2015;23:20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Reynolds JC, Grunau BE, Rittenberger JC, et al. Association between duration of resuscitation and favorable outcome after out-of-hospital cardiac arrest: implications for prolonging or terminating resuscitation. Circulation 2016;134:2084–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yamaguchi Y, Woodin JA, Gibo K, et al. Improvements in out-of-hospital cardiac arrest survival from 1998 to 2013. Prehosp Emerg Care 2017;21:616–27. [DOI] [PubMed] [Google Scholar]
- [26].Bendz B, Eritsland J, Nakstad AR, et al. Long-term prognosis after out-of-hospital cardiac arrest and primary percutaneous coronary intervention. Resuscitation 2004;63:49–53. [DOI] [PubMed] [Google Scholar]
- [27].Callaway CW, Donnino MW, Fink EL, et al. Part 8: post-cardiac arrest care: 2015 American Heart Association Guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132(18 Suppl 2):S465–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cen Y, Zhang S, Shu Y, et al. Investigation of out-of-hospital cardiac arrest in Zhengzhou City and the risk factors of prognosis of cardiopulmonary resuscitation: case analysis for 2016–2018. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019;31:439–43. [DOI] [PubMed] [Google Scholar]
- [29].Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346:549–56. [DOI] [PubMed] [Google Scholar]
- [30].Tranberg T, Lassen JF, Kaltoft AK, et al. Quality of cardiopulmonary resuscitation in out-of-hospital cardiac arrest before and after introduction of a mechanical chest compression device, LUCAS-2; a prospective, observational study. Scand J Trauma Resusc Emerg Med 2015;23:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wiese CH, Bartels U, Schultens A, et al. Influence of airway management strategy on “no-flow-time” during an “advanced life support course” for intensive care nurses - a single rescuer resuscitation manikin study. BMC Emerg Med 2008;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dankiewicz J, Friberg H, Belohlavek J, et al. Time to start of cardiopulmonary resuscitation and the effect of target temperature management at 33 degrees C and 36 degrees C. Resuscitation 2016;99:44–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


