Abstract
This survey was to investigate the short-term effect of particulate matters (PMs) exposure on clinical and microbiological variables, especially septic emboli, in infective endocarditis (IE). The study analyzed 138 IE patients in Far Eastern Memorial Hospital from 2005 to 2015 and clinical variables were retrospectively requested. The data of air quality were recorded and collected by a network of 26 monitoring stations spreading in Northern part of Taiwan. We found that IE patients with septic emboli were found to be exposed to a significantly higher level of PM2.5 (32.01 ± 15.89 vs. 21.70 ± 13.05 μg/m3, P < .001) and PM10 (54.57 ± 24.43 vs 40.98 ± 24.81 μg/m3, P = .002) on lag 0 day when compared to those without. Furthermore, multivariate regression analysis revealed that that ambient exposure to PM2.5 (odds ratio: 3.87, 95% confidence interval: 1.31–8.31; P = .001) and PM10 (odds ratio: 4.58, 95% confidence interval: 2.03–10.32; P < .001) significantly increased risk of septic emboli in IE patients. To our knowledge, this is the first study demonstrating that short-term exposure to PMs was associated with septic emboli in IE.
Keywords: infective endocarditis, particulate matter 10, particulate matter 2.5, septic emboli
1. Introduction
Accumulating evidence has revealed that exposure to ambient particulate matter (PM2.5 and PM10) was associated with numerous cardiovascular (e.g., ischemic heart diseases, heart failure, cerebrovascular diseases) and pulmonary diseases (e.g., chronic obstructive pulmonary diseases, respiratory tract infection, lung cancer).[1,2] Recently, PM2.5 exposure was found to contribute to the occurrence and adverse outcomes of other non-cardiopulmonary diseases, including out-of-hospital cardiac arrest (OHCA),[3–5] Parkinson disease,[6] ischemic stroke,[7] liver-[8] and kidney-related diseases,[9,10] cancer,[11,12] and so on. However, the relationship between exposure to air pollutants and infective endocarditis (IE) remains to be explored.
The infection in the endocardium with vegetation or intracardiac abscess, namely IE, is relatively rare but could be life-threatening with high mortality rate of 9% to 30%.[13] IE could also be accompanied with a broad spectrum of systemic complications because of septic emboli, leading to localized thrombosis, metastatic infection and systemic immune reaction. It was reported that approximately 12% to 40% of IE patients experienced embolic events.[13] Besides, several risk factors were identified for IE, including cardiac (valvular heart disease,[14] replacement of prosthetic heart valve, previous IE history[15]) and non-cardiac (intravenous drug use,[16,17] dental infection,[18,19] hemodialysis[20,21] or peritoneal dialysis[22]). Nevertheless, the association of air pollutants with septic emboli, other risk factors in clinical and microbial species in IE patients remains unknown.
Therefore, we aimed to investigate the effect of PM2.5 and PM10 exposure on clinical and microbiological variables in IE patients. We also investigated whether septic emboli were associated with clinical and microbiological features as well as PM2.5 and PM10 exposure in IE patients.
2. Experimental section
2.1. Study design and patient population
From May 1st, 2005 to December 31st, 2015, patients visiting the outpatient department or emergency department in Far Eastern Memorial Hospital with international classification diseases, 9th edition (ICD-9) code of 421.0, 421.1, 421.9, 424.90, 424.91, or 424.99 were retrospectively reviewed. Those who had blood cultures collected on the visiting day and met the modified Duke criteria for definite and possible IE were enrolled. Clinical data including age, gender, predisposing clinical factors (prior history of IE, valvular heart disease or placement of prosthetic valve, intravenous drug use, hemodialysis or peritoneal dialysis, dental infections), microbiological results of blood culture, surgical intervention or antibiotic treatment and length of hospital stay were collected via reviewing the electronic medical record. The report of cardiac echography (by transthoracic or transesophageal echography) was acquired and the heart valve location of vegetation or lesion was recorded. Besides, the presence of septic emboli was considered with completion of imaging study and verified by the radiologist. The residential district in individuals was also collected. The research approval was acquired from the research ethics committee of FEMH (106130-E) and the investigation was conducted in accordance with the Declaration of Helsinki in 1964.
2.2. Data collection and analysis for PM2.5 and PM10
The report of air quality status in Taiwan from May 1st, 2005 to December 31st, 2015 was referred to the Taiwan Air Quality Monitoring Network manipulated by the Environmental Protection Administration. The data of air quality were recorded by a network of 26 monitoring stations spreading in Northern part of Taiwan (Taipei city, New Taipei city and Taoyuan city). The parameters of air quality, PM2.5 and PM10, recorded by the nearest monitoring station to patients’ residential district were collected. The 24-hour mean concentration of PM2.5 and PM10 on the visiting day (lag 0 day), as well as the lag 1 and lag 2 day of each patient were acquired for analysis of the short-term effect of air pollutant exposure on clinical and microbiological features in IE patients.
2.3. Statistical analysis
Statistical analyses were performed using the SPSS (version 19.0; SPSS Inc., Chicago, IL) statistical software for data analysis. The descriptive data were expressed as the mean ± standard deviation or median with interquartile ranges (IQR). For categorical data, Pearson's chi-squared test or Fisher exact test was performed; and for continuous data, the student's t test was applied for statistical analyses. The optical cut-off values were determined by receiver operating characteristic (ROC) analysis with a high Youden index value. Besides, univariate analysis for clinical and microbiological features as well as parameters of air quality was performed. The above factors with the p value of 0.10 and less would be further adjusted in the multivariate binary logistic regression models. The data was presented with odds ratio (OR) with 95% confidence interval (CI). Statistical significance was considered if the P value was less than .05.
3. Results
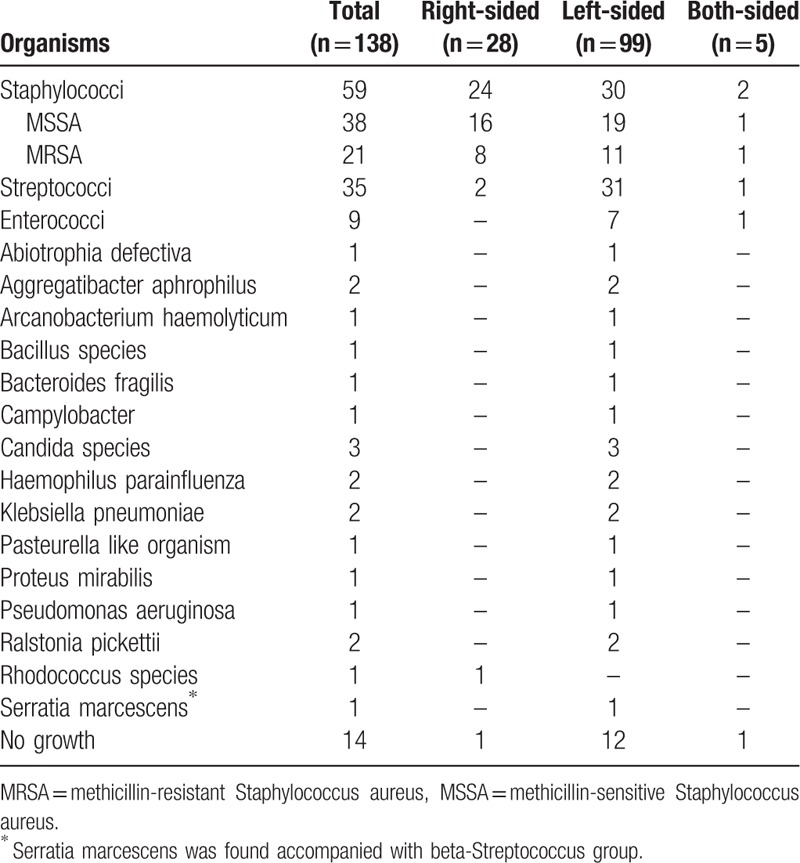
During the study interval, a total of 295 patients with ICD-9 code of 421.0, 421.1, 421.9, 424.90, 424.91, or 424.99 were retrospectively reviewed; and 138 who met the modified Duke criteria for definite and possible IE were enrolled. The demographic data of the study population were shown in Table 1. In microbiological distribution in these IE patients (Table 2), Staphylococci infection (n = 59, 42.8%) was the most common, followed by Streptococci (n = 35, 25.4%), Enterococci (n = 9, 6.5%) and Candida infections (n = 3, 2.2%). Of IE patients with Staphylococci infection, 21 were infected by methicillin-resistant Staphylococcus aureus (MRSA). Besides, other species of microorganism, including Abiotrophia defectiva, Aggregatibacter aphrophilus, Arcanobacterium haemolyticum, Bacillus species, Bacteroides fragilis, Campylobacter, Haemophilus parainfluenza, Klebsiella pneumoniae, Pasteurella like organism, Proteus mirabilis, Pseudomonas aeruginosa, Ralstonia pickettii, Rhodococcus species, and Serratia marcescens, were yielded in blood culture in IE. There was negative finding in blood culture of 14 IE patients.
Table 1.
The demographic data in patients with infectious endocarditis.
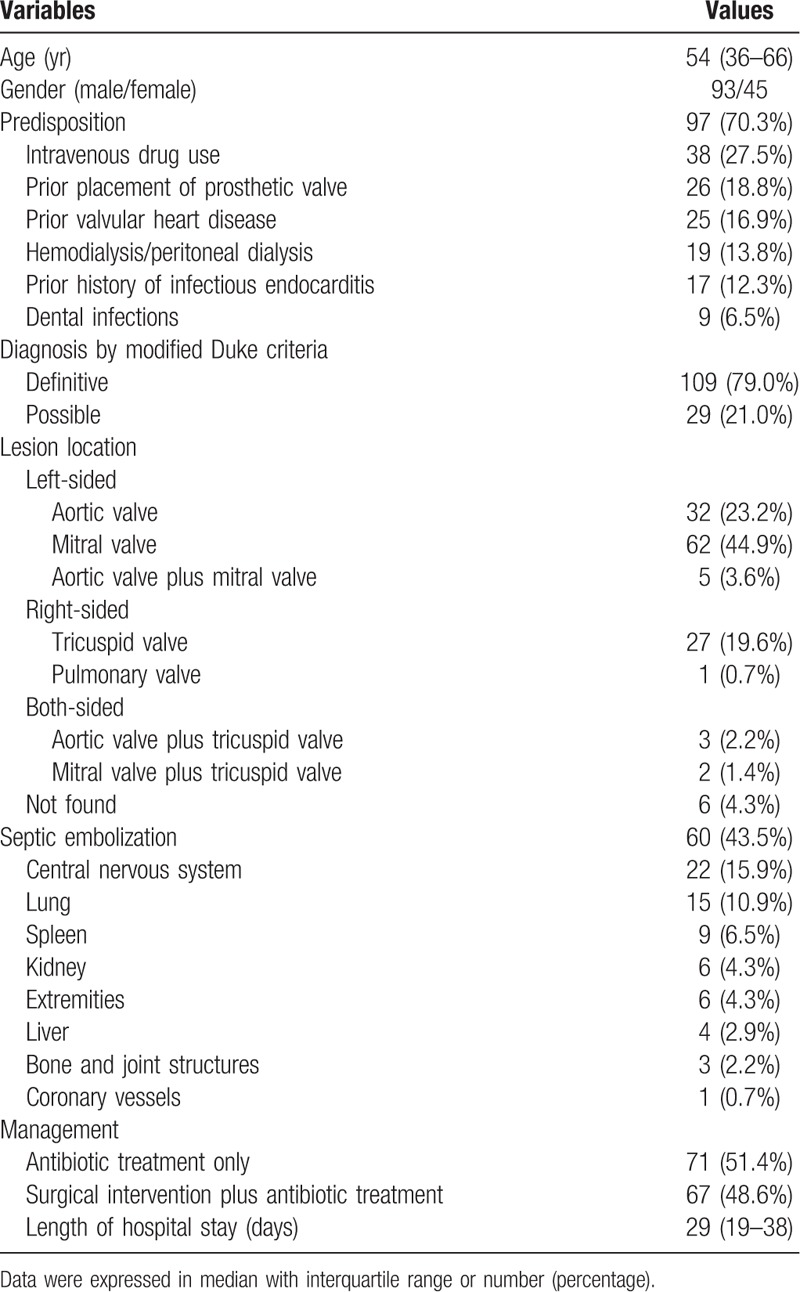
Table 2.
The microbiological distribution in patients with infectious endocarditis.
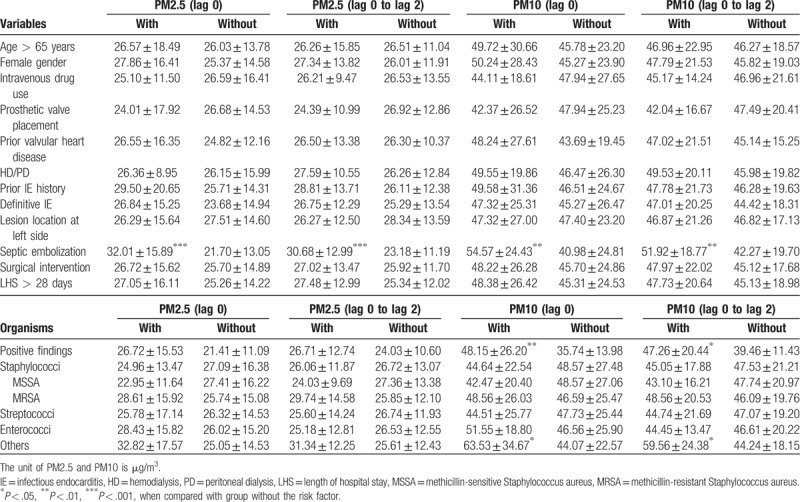
As shown in Table 3, the association of PM2.5 and PM10 exposure with the clinical and microbiological features in IE patients was further investigated. The patients were divided into 2 groups with and without the specified factors; and the exposed levels of PM2.5 and PM10 on lag 0 day, and mean PM2.5 and PM10 from lag 0 to lag 2 days were compared in the two groups. The results revealed that the exposed concentrations of PM2.5 on lag 0 day (32.01 ± 15.89 vs 21.70 ± 13.05 μg/m3, P < .001), mean PM2.5 from lag 0 to lag 2 days (30.68 ± 12.99 vs 23.18 ± 11.19 μg/m3, P < .001), PM10 on lag 0 day (54.57 ± 24.43 vs 40.98 ± 24.81 μg/m3, P = .002) and mean PM10 from lag 0 to lag 2 days (51.92 ± 18.77 vs 42.27 ± 19.70 μg/m3, P = .004) were significantly higher in the group with septic emboli when compared to the group without. Besides, the exposed concentrations of PM10 on lag 0 day (54.57 ± 24.43 vs 40.98 ± 24.81 μg/m3, P = .010) and mean PM10 from lag 0 to lag 2 days (51.92 ± 18.77 vs 42.27 ± 19.70 μg/m3, P = .039) were significantly higher in the group with positive findings in blood culture in comparison with the group without. It was also revealed that the exposed concentrations of PM10 on lag 0 day (54.57 ± 24.43 vs 40.98 ± 24.81 μg/m3, P = .024) and mean PM10 from lag 0 to lag 2 days (51.92 ± 18.77 vs 42.27 ± 19.70 μg/m3, P = .013) were significantly higher in the group with infection by other microorganisms except Staphylococci, Streptococci, and Enterococci when compared to the group without. There was no statistical difference between groups with and without other specified factors.
Table 3.
The clinical and microbiological features of patients with infectious endocarditis exposed to PM2.5 and PM10 concentrations.
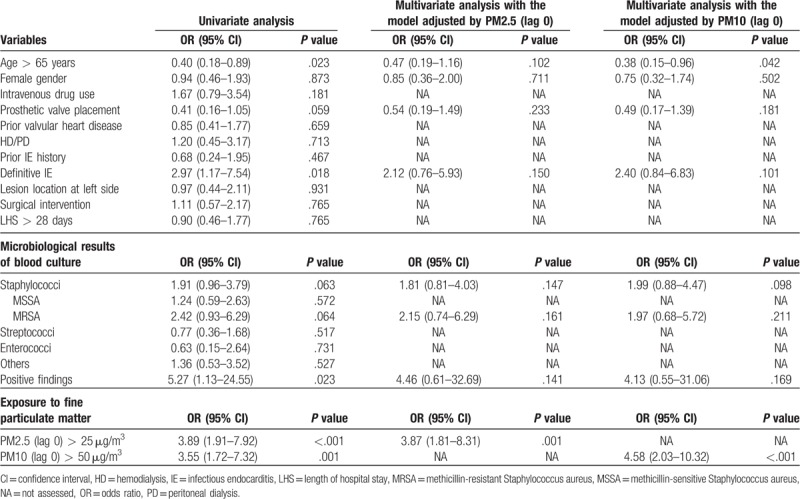
Since we found that exposed concentration of air pollutants was significantly higher in IE patients with septic emboli than those without, we further performed univariate analysis to find out if there were any confounding factors. As shown in Table 4, definite IE diagnosed by the modified Duke criteria (OR: 2.97, 95% CI: 1.17–7.54; P = .018), positive findings in blood culture (OR: 5.27, 95% CI: 1.13–24.55; P = .023), PM2.5 of 25 μg/m3 and more on lag 0 day (OR: 3.89, 95% CI: 1.91–7.92; P < .001) and PM10 of 50 μg/m3 and more on lag 0 day (OR: 3.55, 95% CI: 1.72–7.32; P = .001) were significantly associated with septic emboli in IE patients. Besides, Staphylococci (OR: 1.91, 95% CI: 0.96–3.79; P = .063) and MRSA infections (OR: 2.42, 95% CI: 0.93–6.29; P = .064) were seemingly associated with septic emboli in IE patients. In contrast, age of 65 years and more (OR: 0.40, 95% CI: 0.18–0.89; P = .023) was significantly reversely associated with septic emboli in IE patients; and prior placement of prosthetic valve (OR: 0.41, 95% CI: 0.16–1.05; P = .059) was seemingly reversely associated with septic emboli in IE patients. The multivariate binary logistic regression analyses were then performed by2 separate models with adjustment of PM2.5 and PM10 accompanied with adjustment of the above variables. In the model adjusted by PM2.5 and the above variables, PM2.5 of 25 μg/m3 and more on lag 0 day (OR: 3.87, 95% CI: 1.31–8.31; P = .001) was significantly associated with septic emboli in IE patients. We also performed the model adjusted by PM10 and the above variables, which showed that PM10 of 50 μg/m3 and more on lag 0 day (OR: 4.58, 95% CI: 2.03–10.32; P < .001) was significantly associated with septic emboli; and age of 65 years and more (OR: 0.38, 95% CI: 0.15–0.96; P = .042) was significantly reversely associated with septic emboli in IE patients.
Table 4.
The association of clinical and microbiological features and fine particulate matter with septic emboli in patients with infectious endocarditis.
Besides, considering that intravenous drug use (27.5%) may be more closely related to PM2.5 and PM10, we then excluded 38 patients with intravenous drug use for subgroup analysis. It was found that the exposed concentration of PMs remains significantly higher in the group with septic emboli when compared with those without. We also further performed univariate analysis and the results showed that definite IE diagnosed by the modified Duke criteria (OR: 3.50, 95% CI: 1.19–10.31, P = .018), PM2.5 of 25 μg/m3 and more on lag 0 day (OR: 2.92, 95% CI: 1.27–6.70, P = .010) and PM10 of 50 μg/m3 and more on lag 0 day (OR: 3.09, 95% CI: 1.34–7.15, P = .007) were significantly associated with septic emboli in IE patients. Multivariate binary logistic regression analyses were further performed with confounding factors of age, gender and definite IE. In the model adjusted by PM2.5 and the above variables, PM2.5 of 25 μg/m3 and more on lag 0 day (OR: 2.75, 95% CI: 1.15–6.55; P = .023) was significantly associated with septic emboli in IE patients. Additionally, in the model adjusted by PM10 and the above variables, PM10 of 50 μg/m3 and more on lag 0 day (OR: 3.20, 95% CI: 1.32–7.77; P = .010) was significantly associated with septic emboli in IE patients. Compared with the original results including IE patients with IV drug abuse and the non-including group, it was shown that exposure to PMs remained an association with septic emboli in IE. The above results suggested that exposure to PM2.5 and PM 10 could be a critical factor for contribution to septic emboli in IE patients.
4. Discussion
Our main findings indicated that in IE patients with septic emboli and positive findings in blood culture, especially in which the species except Staphylococci, Streptococci and Enterococci were yielded, were found to be exposed to a significantly higher level of PMs in short-term exposure when compared to those without. We also found that ambient exposure to PM2.5 and PM10 could contribute to septic emboli in IE patients. To the best of our knowledge, this investigation is the first study to explore the association of short-term exposure to PMs with septic emboli in IE.
Septic emboli could increase the risk of critical sequelae and even mortality in IE, if not properly managed.[23] In the previous report, it was shown that about 12% to 40% of patients with IE experienced embolic events.[13] The frequency of location where septic emboli were found could vary in different surveys. In the literature review, central nervous system (48–65%) was the most common place to be affected by septic emboli in IE, followed by extremities (30%), spleen (19–30%), lung (14–25%), kidney (6–14%), bone and joint structures (11%), liver (3–11%), coronary vessels (6%) and so on.[23–25] Besides, previous studies revealed that patients with left-sided IE and lesion on the mitral valve had a higher risk of septic emboli when compared to those with right-sided IE and lesion on the aortic valve, respectively.[26,27] In our study, neither left-sided (crude OR: 0.88, 95% CI: 0.38–2.03, P = .764) nor mitral valve-involved (crude OR: 0.87, 95% CI: 0.37–2.06, P = .752) IE has increased likelihood of septic emboli. Besides, it was reported that those who had IE due to Staphylococcus aureus, Streptococcus bovis or fungal infections or with vegetations sized over 10 mm could have a higher risk of experiencing embolic events.[28,29] In our study, IE Patients with MRSA infection were prone to have septic emboli when compared to those without, and there was no significant difference after adjustment with confounding variables in multivariate logistic regression. Notably, we found that the age of 65 years and more was significantly reversely associated with septic emboli in IE, which was somewhat different from the literature reviews.[30,31] This could be explained that the age of patients developing septic emboli in IE seemed to be younger (mean: 37.2 years; standard deviation: 12.4 years) in our study population when compared with that reported (mean: 38.1–46.3 years; standard deviation: 14.7–19.5 years) in the previous study.[30] And whether ambient exposure to air pollutants contributed to septic embolization in IE had not been explored yet in the previous studies.
PM2.5 and PM10, of which aerodynamic diameter less than 2.5 and 10 mm, respectively, were mainly composed of sulfate, nitrate, ammonium, organic and elemental carbons, trace metals, geological materials, and so on.[32,33] The significant impact of PM2.5 on health has been reported, affecting the occurrence and outcomes in a spectrum of diseases. Either long-term or short-term PM exposure was focused and investigated in the previous studies. In the surveys concerning short-term PM exposure, it was observed that ambient exposure to high PM2.5 concentrations for lag 0 through lag 1 day increased the risk of hospital admission and subsequent management because of OHCA, ischemic stroke, cardiovascular and respiratory diseases.[1,3–5,7] Besides, it was suggested that the level of PM2.5 at lag 2 day affected the outcomes of respiratory infection to the greatest extent.[1] In the present study, we also investigated the short-term effect of PM2.5 and PM10 on events of septic emboli in IE. Our results revealed that exposure to both high PM2.5 and PM10 levels for lag 0 through lag 2 day was associated with septic embolization in IE patients. However, the detailed mechanism of PM exposure facilitating septic embolization in IE remains to be explored. Researchers found that airborne microbes such as bacteria, fungi and viruses were identified in the composition of PM2.5 and PM 10 via DNA detection and sequencing analysis.[34] Besides, another study revealed that PM2.5 exposure contributed to pneumococcal adhesion to human epithelial cells, thereby increasing the risk of pneumococcal pneumonia.[35] Nevertheless, the current evidence is insufficient to explain why IE patients exposed to high PM2.5 and PM10 levels have increased risk of developing septic emboli. One of the possible explanations is that PM-induced chronic inflammation could increase susceptibility to septic embolization in IE. The association of exposure to air pollutants with septic embolization in IE should be interpreted very carefully.
The impact of PM2.5 on health and the involved mechanism have been investigated and proposed. Prior studies indicated that PM2.5 triggered inflammatory response, oxidative stress and mitochondrial dysfunction in animal and cell line models.[36–38] Furthermore, it was demonstrated that acute PM exposure increased plasma expression of proinflammatory cytokines including interleukin (IL)-6 and tumor necrosis factor (TNF)-alpha, and activated polymorphonuclear leukocytes systemically.[39] Another study revealed that PM2.5-induced inflammatory response was suppressed by endotoxin neutralizer and knock-out of toll-like receptor (TLR)-4, while oxidative stress was not. Additionally, antioxidant treatment suppressed PM2.5-induced oxidative stress rather than the inflammatory response.[40] Although the above results suggested that environmental PM2.5 elicited systemic inflammation and oxidative stress by separate pathways, the link between PM2.5-induced inflammation with affected immunity and septic embolization remains to be disclosed; and further investigation remains warranted.
There are several major limitations in the present study. First, this is a retrospective study design in a single medical center and thus the case number is limited. Besides, the measured PM levels could have been inadequate indices of actual exposure in individuals due to the unavailability of indoor air quality, leading to overestimated or underestimated correlation between environmental PMs and septic embolization in IE.
5. Conclusions
In the present study, we demonstrated that IE patients with septic emboli and positive findings in blood culture were exposed to a significantly higher level of PMs in short-term exposure when compared to those without. Our results also suggested that the exposure to PM2.5 and PM10 contributed to septic emboli in IE.
Author contributions
Conceptualization: Fu-Chien Hsieh, Chih-Chun Chang.
Data curation: Chun-Yen Huang, Sheng-Feng Lin.
Formal analysis: Sheng-Feng Lin, Chih-Chun Chang.
Funding acquisition: Fu-Chien Hsieh.
Investigation: Fu-Chien Hsieh, Chun-Yen Huang, Sheng-Feng Lin, Jen-Tang Sun, Tzung-Hai Yen, Chih-Chun Chang.
Methodology: Fu-Chien Hsieh, Chih-Chun Chang.
Project administration: Fu-Chien Hsieh.
Resources: Chun-Yen Huang, Jen-Tang Sun.
Supervision: Fu-Chien Hsieh.
Writing – original draft: Chun-Yen Huang, Chih-Chun Chang.
Writing – review & editing: Fu-Chien Hsieh, Sheng-Feng Lin, Tzung-Hai Yen.
Footnotes
How to cite this article: Hsieh FC, Huang CY, Lin SF, Sun JT, Yen TH, Chang CC. Short-term exposure to particulate matters is associated with septic emboli in infective endocarditis. Medicine. 2019;98:45(e17899).
Abbreviations: CI = confidence interval, ICD = international classification diseases, IE = infective endocarditis, IL = interleukin, IQR = interquartile ranges, MRSA = methicillin-resistant Staphylococcus aureus, OHCA = out-of-hospital cardiac arrest, OR = odds ratio, PM = particulate matter, ROC = receiver operating characteristic, TLR = toll-like receptor, TNF = tumor necrosis factor.
F-CH and C-YH contributed equally to this work.
This study was supported by grants of Far Eastern Memorial Hospital (FEMH-2017-C-042 and FEMH-2019-C-053), Taiwan.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Dominici F, Peng RD, Bell ML, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006;295:1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Raaschou-Nielsen O, Andersen ZJ, Beelen R, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol 2013;14:813–22. [DOI] [PubMed] [Google Scholar]
- [3].Straney L, Finn J, Dennekamp M, et al. Evaluating the impact of air pollution on the incidence of out-of-hospital cardiac arrest in the Perth Metropolitan Region: 2000–2010. J Epidemiol Community Health 2014;68:6–12. [DOI] [PubMed] [Google Scholar]
- [4].Kang SH, Heo J, Oh IY, et al. Ambient air pollution and out-of-hospital cardiac arrest. Int J Cardiol 2016;203:1086–92. [DOI] [PubMed] [Google Scholar]
- [5].Zhao R, Chen S, Wang W, et al. The impact of short-term exposure to air pollutants on the onset of out-of-hospital cardiac arrest: a systematic review and meta-analysis. Int J Cardiol 2017;226:110–7. [DOI] [PubMed] [Google Scholar]
- [6].Shin S, Burnett RT, Kwong JC, et al. Effects of ambient air pollution on incident Parkinson's disease in Ontario, 2001 to 2013: a population-based cohort study. Int J Epidemiol 2018;47:2038–48. [DOI] [PubMed] [Google Scholar]
- [7].Matsuo R, Michikawa T, Ueda K, et al. Short-term exposure to fine particulate matter and risk of ischemic stroke. Stroke 2016;47:3032–4. [DOI] [PubMed] [Google Scholar]
- [8].Pan WC, Wu CD, Chen MJ, et al. Fine particle pollution, alanine transaminase, and liver cancer: a Taiwanese prospective cohort study (REVEAL-HBV). J Natl Cancer Inst 2015;108:djv341. [DOI] [PubMed] [Google Scholar]
- [9].Mehta AJ, Zanobetti A, Bind MA, et al. Long-term exposure to ambient fine particulate matter and renal function in older men: the veterans administration normative aging study. Environ Health Perspect 2016;124:1353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liu MH, Chan MJ, Hsu CW, et al. Association of uremic pruritus in hemodialysis patients with the number of days of high mean 24-hour particulate matter with a diameter of <2.5 μm. Ther Clin Risk Manag 2017;13:255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chang CC, Chung YH, Liou CB, et al. Influence of residential environment and lifestyle on multiple primary malignancies in Taiwan. Asian Pac J Cancer Prev 2015;16:3533–8. [DOI] [PubMed] [Google Scholar]
- [12].Kim HB, Shim JY, Park B, et al. Long-term exposure to air pollutants and cancer mortality: a meta-analysis of cohort studies. Int J Environ Res Public Health 2018;15:2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Colen TW, Gunn M, Cook E, et al. Radiologic manifestations of extra-cardiac complications of infective endocarditis. Eur Radiol 2008;18:2433–45. [DOI] [PubMed] [Google Scholar]
- [14].McKinsey DS, Ratts TE, Bisno AL. Underlying cardiac lesions in adults with infective endocarditis. The changing spectrum. Am J Med 1987;82:681. [DOI] [PubMed] [Google Scholar]
- [15].Carrel T, Schaffner A, Vogt P, et al. Endocarditis in intravenous drug addicts and HIV infected patients: possibilities and limitations of surgical treatment. J Heart Valve Dis 1993;2:140. [PubMed] [Google Scholar]
- [16].Mathew J, Addai T, Anand A, et al. Clinical features, site of involvement, bacteriologic findings, and outcome of infective endocarditis in intravenous drug users. Arch Intern Med 1995;155:1641–8. [PubMed] [Google Scholar]
- [17].Ortiz-Bautista C, López J, García-Granja PE, et al. Current profile of infective endocarditis in intravenous drug users: the prognostic relevance of the valves involved. Int J Cardiol 2015;187:472. [DOI] [PubMed] [Google Scholar]
- [18].Duval X, Millot S, Chirouze C, et al. Oral streptococcal endocarditis, oral hygiene habits, and recent dental procedures: a case-control study. Clin Infect Dis 2017;64:1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen SJ, Liu CJ, Chao TF, et al. Dental scaling and risk reduction in infective endocarditis: a nationwide population-based case-control study. Can J Cardiol 2013;29:429–33. [DOI] [PubMed] [Google Scholar]
- [20].Robinson DL, Fowler VG, Sexton DJ, et al. Bacterial endocarditis in hemodialysis patients. Am J Kidney Dis 1997;30:521–4. [DOI] [PubMed] [Google Scholar]
- [21].Baroudi S, Qazi RA, Lentine KL, et al. Infective endocarditis in haemodialysis patients: 16-year experience at one institution. NDT Plus 2008;1:253–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tsai MJ, Yang WC, Chen TW, et al. Infective endocarditis giving rise to peritonitis in a patient on peritoneal dialysis. Perit Dial Int 2013;33:462–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Millaire A, Leroy O, Gaday V, et al. Incidence and prognosis of embolic events and metastatic infections in infective endocarditis. Eur Heart J 1997;18:677–84. [DOI] [PubMed] [Google Scholar]
- [24].Huang PH, Kang HC, Hsieh FC. Ralstonia pickettii-infected endocarditis complicated with possible septic emboli in kidney: a rare case report. J Urol Ren Dis 2018;2: JURD-180. [Google Scholar]
- [25].Luaces Méndez M, Vilacosta I, Sarriá C, et al. Hepatosplenic and renal embolisms in infective endocarditis. Rev Esp Cardiol 2004;57:1188–96. [PubMed] [Google Scholar]
- [26].Bayer AS, Bolger AF, Taubert KA, et al. Diagnosis and management of infective endocarditis and its complications. Circulation 1998;98:2936–48. [DOI] [PubMed] [Google Scholar]
- [27].Rohmann S, Erbel R, Görge G, et al. Clinical relevance of vegetation localization by transoesophageal echocardiography in infective endocarditis. Eur Heart J 1992;13:446–52. [DOI] [PubMed] [Google Scholar]
- [28].Ellis ME, Al-Abdely H, Sandridge A, et al. Fungal endocarditis: evidence in the world literature, 1965–1995. Clin Infect Dis 2001;32:50–62. [DOI] [PubMed] [Google Scholar]
- [29].Thuny F, Di Salvo G, Belliard O, et al. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation 2005;112:69–75. [DOI] [PubMed] [Google Scholar]
- [30].Aalaei-Andabili SH, Martin T, Hess P, et al. Management of Septic emboli in patients with infectious endocarditis. J Card Surg 2017;32:274–80. [DOI] [PubMed] [Google Scholar]
- [31].Hubert S, Thuny F, Resseguier N, et al. Prediction of symptomatic embolism in infective endocarditis: construction and validation of a risk calculator in a multicenter cohort. J Am Coll Cardiol 2013;62:1384–92. [DOI] [PubMed] [Google Scholar]
- [32].Ye B, Ji X, Yang H, et al. Concentration and chemical composition of PM2.5 in Shanghai for a 1-year period. Atmos Environ 2003;37:499–510. [Google Scholar]
- [33].Cheng Y, Lee S, Gu Z, et al. PM2.5 and PM10-2.5 chemical composition and source apportionment near a Hong Kong roadway. Particuology 2015;18:96–104. [Google Scholar]
- [34].Cao C, Jiang W, Wang B, et al. Inhalable microorganisms in Beijing's PM2.5 and PM10 pollutants during a severe smog event. Environ Sci Technol 2014;48:1499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mushtaq N, Ezzati M, Hall L, et al. Adhesion of Streptococcus pneumoniae to human airway epithelial cells exposed to urban particulate matter. J Allergy Clin Immunol 2011;127:1236–42. [DOI] [PubMed] [Google Scholar]
- [36].Guo Z, Hong Z, Dong W, et al. PM2.5-induced oxidative stress and mitochondrial damage in the nasal mucosa of rats. Int J Environ Res Public Health 2017;14:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jin X, Su R, Li R, et al. Crucial role of pro-inflammatory cytokines from respiratory tract upon PM2.5 exposure in causing the BMSCs differentiation in cells and animals. Oncotarget 2017;9:1745–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chen T, Zhang J, Zeng H, et al. The impact of inflammation and cytokine expression of PM2.5 in AML. Oncol Lett 2018;16:2732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Marchini T, Magnani ND, Paz ML, et al. Time course of systemic oxidative stress and inflammatory response induced by an acute exposure to Residual Oil Fly Ash. Toxicol Appl Pharmacol 2014;274:274–82. [DOI] [PubMed] [Google Scholar]
- [40].Bekki K, Ito T, Yoshida Y, et al. PM2.5 collected in China causes inflammatory and oxidative stress responses in macrophages through the multiple pathways. Environ Toxicol Pharmacol 2016;45:362–9. [DOI] [PubMed] [Google Scholar]


