Abstract
Background
Although neutral oral contrast agents are widely in use, a consensus regarding a standardized protocol in abdominal staging CT does not exist.
Purpose
To test the null hypothesis that there is no quantitative or qualitative difference between water and mannitol for evaluation of the small bowel at abdominal staging CT.
Material and methods
180 patients prospectively underwent abdominal staging CT with oral administration of either 1 liter mannitol solution (n = 88) or water (n = 92). Intestinal distension was measured in 6 different segments of the small intestine. In addition, two radiologists separately evaluated diagnostic image quality with regards to luminal distension (three-point scale) in each segment and the possibility to rule out a possible underlying pathology. Quantitative and qualitative results were compared (Mann-Whitney test).
Results
Quantitatively, intestinal distension was comparable in all segments (p>0.05), except for the horizontal duodenum (p = 0.019). The mean luminal diameter over all intestinal segments was 19.0 mm (18.1–19.9 mm) for the water group and 18.4 mm (17.5–19.2 mm) for the mannitol group, respectively. Qualitatively, ratings were comparable for the first three segments, while distal segments were rated better using mannitol. Side effects were only observed using mannitol (n = 26; 29.5%).
Conclusions
Orally administered water and mannitol solution for evaluation of the small bowel at abdominal staging CT in clinical routine resulted in comparable results for the quantitative, but not for the qualitative analysis. Looking more differentiated at the overall performance, water has advantages in terms of patient comfort, side effects and costs, and can therefore be regarded as noninferior to mannitol in this specific patient group.
Introduction
Computed tomography (CT) staging examinations have become a cornerstone in diagnosis and follow-up of oncological disease. In case of abdominal imaging, the supplemental administration of a neutral or positive oral contrast agent for distension and assessment of the small intestine has been proven to reduce misinterpretations at CT, both false negative (i.e. tumors and polyps hidden by collapsed bowel loops) as well as false positive (simulation of wall thickening), and thus has been established in clinical routine [1–4].
Recently, the value of positive oral contrast agents has been questioned as the performance of neutral oral contrast agents was demonstrated to be comparable while less inconvenient, e.g. in oncologic staging, emergency settings or more specifically for the evaluation of inflammatory bowel disease [3–8]. Consequently, indications for positive contrast agents are nowadays mostly limited to particular conditions, e.g. to exclude anastomotic insufficiency after small bowel surgery.
Regarding specific protocols for oral administration of neutral contrast agents most experience is gathered from CT studies acquired with the purpose of distinct evaluation of the small bowel. These protocols often involve extensive patient preparation including a low fiber diet for up to three days. In addition, intensive bowel cleaning may be carried out the afternoon before the examination by oral administration of up to two liters of an isotonic non-absorbable electrolyte solution containing polyethylene glycol. For imaging, neutral contrast agents are then administered either orally (i.e. CT enterography) or through a naso-jejunal catheter (i.e. CT enteroclysis) in large amounts (ranging from 1500 to 2500 ml) [9]. A variety of different neutral oral contrast agents are being used including drinking water, oil emulsions or sugar solutions (e.g. mannitol, sorbitol, polyethylene glycol) [10–12], as these additives have been shown to decrease water reabsorption [13–16]. However, such extensive CT protocols are not only uncomfortable for patients, but also time consuming, cost intensive and not practicable in clinical routine with regards to the rising demand of CT examinations especially in an outpatient setting.
Although neutral oral contrast agents are widely in use, a consensus regarding a standardized protocol in abdominal staging CT does not exist. In this setting a dedicated evaluation of the small intestine is not in the focus of interest. To the best of our knowledge, there is no general agreement for the application of neutral contrast agents, including the specific type of contrast agent. The purpose of our study was therefore to test the null hypothesis that there is no quantitative or qualitative difference between non-sparkling mineral water and mannitol solution for evaluation of the small bowel at abdominal staging CT in clinical routine.
Materials and methods
Patients
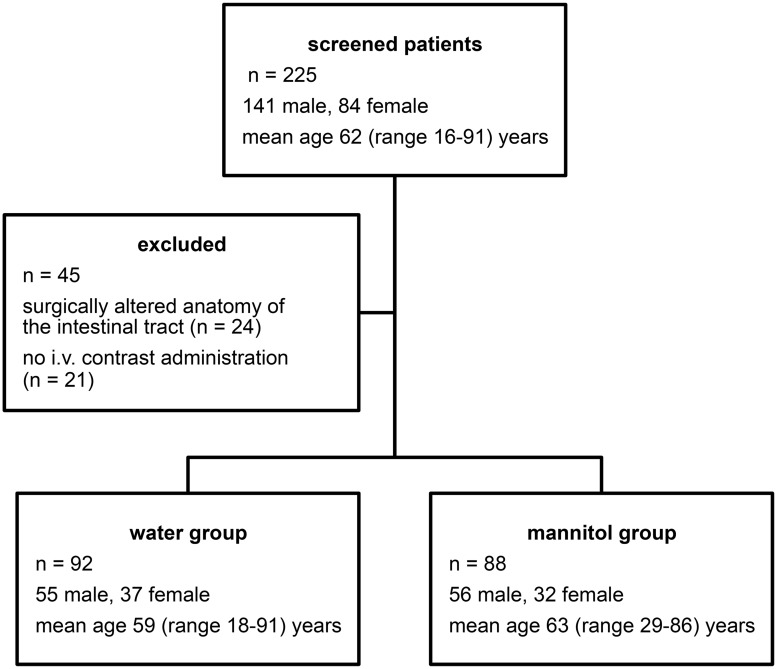
This prospective observational study was approved by the IRB of Hannover Medical School (Approval Number: 2252–2015). All patients gave written informed consent for study participation. Between March and May 2014, 225 patients referred for staging CT comprising either the abdomen only (including the pelvis) or thorax and abdomen were screened for possible study inclusion. Inclusion criteria were as follows: patient age ≥ 18 years, written informed consent for staging CT, oral administration of either water or mannitol solution. Exclusion criteria were as follows: patient age < 18 years, contraindications for intravenous iodinated contrast agent administration, surgically altered anatomy of the gastrointestinal tract (e.g. status post gastrostomy or ileostomy). 45 patients had to be excluded due to either surgically altered anatomy of the intestinal tract (n = 24) or lack of intravenous contrast administration (n = 21) (Fig 1). Consequently, the final study population consisted of 180 patients (69 female, 111 male; mean age 61 (range 18–91) years).
Fig 1. Patient cohort.
225 patients referred for abdominal staging CT were screened for possible study inclusion. Consequently, the final study population consisted of 180 patients.
CT imaging
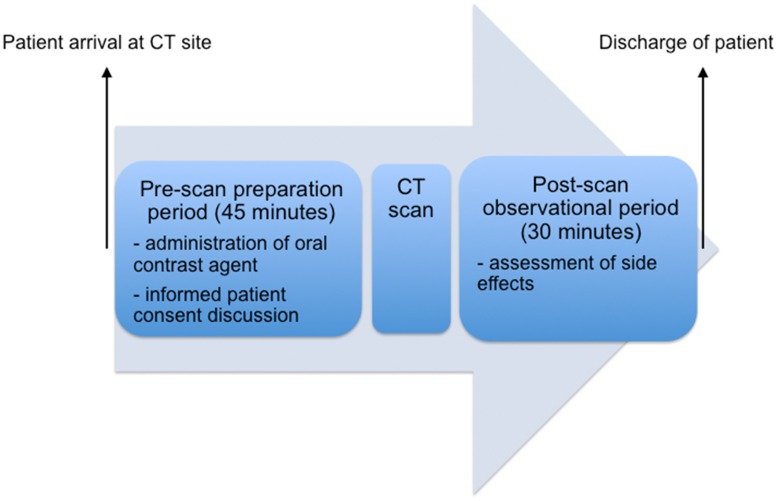
Approximately 45 minutes prior to image acquisition patients consecutively received either 1 liter non-sparkling water (group 1; n = 92) or 1 liter mannitol solution (group 2; n = 88) as a neutral oral contrast agent, both served at room temperature (Fig 2). Allocation of the specific type of oral contrast agent was performed prospectively in dependence on the time period imaging was carried out. Consecutive patients undergoing staging CT between March 15th and April 14th all received water, consecutive patients undergoing staging CT between April 15th and May 16th all received mannitol. Staging CT was performed in the supine position either on a 64-slice scanner (n = 132; Lightspeed VCT, GE HealthCare, Milwaukee, WI, USA) or a 16-slice scanner (n = 48; Lightspeed, GE HealthCare, Milwaukee, WI, USA). Images were acquired in the portal venous phase, after injection of 100 ml of a nonionic iodinated contrast agent (Xenetix® 350, Guerbet, France) followed by a 40 ml saline flush, injected at a flow rate of 4 ml per second. Image acquisition started 15 seconds after bolus detection in the spleen (threshold 250 Hounsfield Units). Scan parameters for image acquisition on the 64-slice scanner were as follows: tube current 120 kV automatic tube modulation, table feed 39.37 mm/ gantry rotation, 1.25 mm slice collimation, 1.0 mm reconstruction interval. Respective parameters for image acquisition on the 16-slice scanner were: tube current 120 kV, automatic tube modulation, table feed 27.5 mm/ gantry rotation, 1.25 mm slice collimation, 1.0 mm reconstruction interval. After completion of the CT scan patients remained in the department over a period of 30 minutes for observational purposes and assessment of potential side effects.
Fig 2. Timeline of the visit of a patient undergoing (thoraco-) abdominal staging CT.
After arrival at the CT site, patients received 1 liter of an oral neutral contrast agent and were instructed to drink the full amount within 45 minutes prior to image acquisition. After completion of the CT scan, patients remained in the department over a period of 30 minutes for observational purposes and assessment of potential side effects.
Image analysis
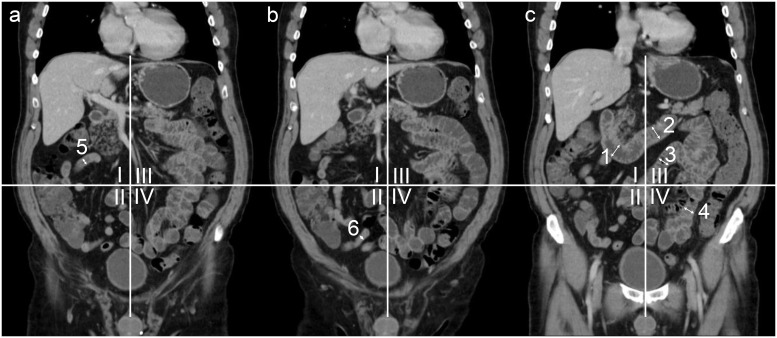
Image analysis was performed using a thin client PACS viewer (Visage 7, Pro Medicus, Richmond, Australia). Readouts were performed using sagittal, coronal and transverse reformations in 3 mm average intensity projections. In order to assess the effect of the administered oral contrast agents on the small intestine, the following segments (n = 6) were defined: descending duodenum, horizontal duodenum, proximal jejunum, distal jejunum, proximal ileum and distal ileum. The anatomy of the small intestine was primarily assessed in coronal view. While the duodenal segments can be easily identified, ileal and jejunal loops may be more difficult to distinguish. In order to identify these segments reliably, the abdomen was divided into four quadrants with the umbilicus as the center point, as has been previously suggested by Minordi et al [11]. Small intestine in the left upper quadrant of the abdomen was defined as proximal jejunum, small bowel loops in the left lower quadrant as distal jejunum, loops in the right upper quadrant as proximal ileum and loops in the right lower quadrant as distal ileum [9,11], respectively (Fig 3).
Fig 3. Coronal reformatted CT (three consecutive slices (a-c)) for demonstration of small bowel segment definition.
The abdomen is divided into 4 quadrants with the umbilicus as the center point. Representative measurements of the descending (1) and horizontal duodenum (2). Jejunal and ileal segments were defined using the 4-quadrant model: I = upper right quadrant, including the proximal ileum (5); II = lower right quadrant, including the distal ileum (6); III = upper left quadrant, including the proximal jejunum (3); IV = lower left quadrant, including the distal jejunum (4).
Quantitative analysis
For quantitative analysis, the luminal distension of each bowel segment at one representative location was measured by two radiologists in consensus (one radiology resident with two years and one board certified radiologist with eleven years of experience in abdominal imaging, respectively). Since the intestinal wall could not always be distinguished from the lumen, the total diameter of the bowel was measured. Both radiologists were blinded to the administered oral contrast agent.
Qualitative analysis
Qualitative image analysis was performed by two different readers (radiology residents with three and five years of experience in abdominal imaging, respectively), who were also blinded to the type of oral contrast agent administered. Both readers independently assessed the diagnostic image quality in terms of the possibility to rule out a potential underlying pathology (two-point scale; yes or no). Further, the degree of luminal distension was evaluated on a three-point scale (0 = unsatisfactory distension, 1 = good distension, 2 = optimal distension).
Radiation dose
The radiation dose was calculated for each CT exam using the computed tomography dose index (CTDI). Thoraco-abdominal examinations were scanned in one continuous scan, therefore the radiation dose of the thorax could not be excluded. Furthermore, a normalized CTDI was calculated dividing the CTDI by the body mass index (BMI).
Statistical analysis
Statistical analysis was performed using JMP Pro 11 software (SAS Institute; U.S.A.) and GraphPad Prism 6 (GraphPad Software, Inc.; U.S.A.). Data are presented as mean with standard deviation. To test for potential differences in age and sex distribution between patients in the water and mannitol group the Kolmogorov-Smirnov test (after testing for a Gaussian and equal distribution using the Shapirow-Wilk normality test) was performed.
Quantitative and qualitative results as well as radiation doses were compared between the two study groups applying a Mann-Whitney test. In addition, a subgroup analysis was performed depending on scanner type. Further, interrater agreement was assessed using the intraclass correlation coefficient (ICC), applying the two-way mixed model [17]. ICC was interpreted as follows: a value less than 0.20 indicated poor agreement, a value of 0.21–0.40 fair agreement, a value of 0.41–0.60 moderate agreement, a value of 0.61–0.80 substantial agreement, and a value of 0.81–1.00 almost perfect agreement. For all measurements, a p-value less than 0.05 was considered indicative of a significant difference.
Results
Patients
In the group receiving water as an oral contrast agent, a thoraco-abdominal CT was acquired in 84 patients; in 8 patients only images of the abdomen were acquired. Respective numbers in the group receiving mannitol solution as an oral contrast agent were 86 and 2 patients. Patient characteristics as well as indications for staging CT in terms of underlying malignancy are presented in detail in Table 1. There were no significant differences between both study groups with regards to sex and age distribution (p>0.05).
Table 1. Characteristics of patients receiving either water or mannitol solution as neutral oral contrast agent.
| water group | mannitol group | |
|---|---|---|
| No. of patients (total n = 180) | 92 | 88 |
| Sex | ||
| Number of male patients | 55 | 56 |
| Number of female patients | 37 | 32 |
| Age (y) | 59 (18–91) | 63 (29–86) |
| Body mass index (kg/m2) | 26.5 ± 5.4 | 25.7 ± 5.9 |
| Primary tumor | ||
| lip, oral cavity and pharynx | 0 | 4 |
| digestive organs | 28 | 23 |
| respiratory and intrathoracic organs | 22 | 27 |
| bone and articular cartilage | 1 | 0 |
| melanoma and other malignant neoplasms of skin | 9 | 10 |
| mesothelial and soft tissue | 5 | 5 |
| breast | 3 | 3 |
| female genital organs | 3 | 1 |
| male genital organs | 4 | 5 |
| urinary tract | 7 | 2 |
| thyroid and other endocrine glands | 3 | 1 |
| ill-defined, secondary and unspecified sites | 0 | 2 |
| lymphoid, hematopoietic and related tissue | 7 | 5 |
Underlying malignancies were classified according to the World Health Organization (WHO)[18]. Age: mean with range in parenthesis. Body mass index (BMI): mean with SD.
Quantitative assessment of intestinal distension
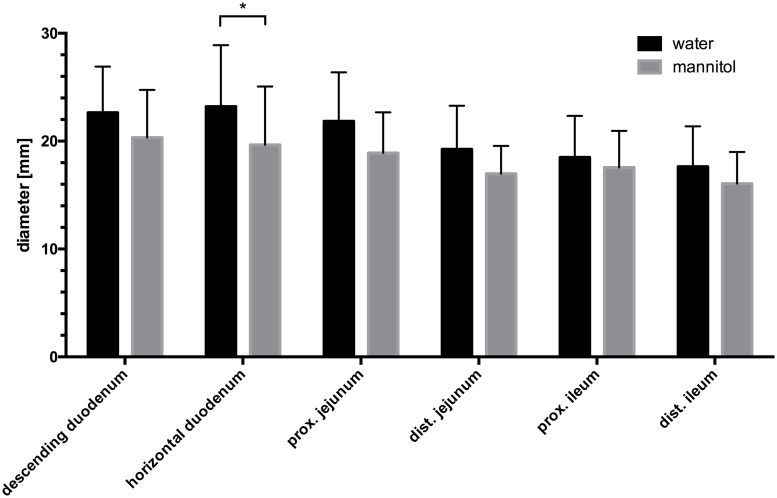
Quantitatively, there was no statistically significant difference between patients receiving water and mannitol solution with regards to intestinal distension in almost all segments of the small intestine (p>0.05). Only in the horizontal duodenum intestinal distension was slightly better after water administration, with a mean diameter of 22.0 mm as compared to a mean diameter of 20.2 mm in the mannitol group (p = 0.019). In both groups, a decrease of the mean intestinal diameter was observed from oral to aboral (Fig 4). At subgroup analysis depending on scanner type, only distension in the horizontal duodenum was slightly better in examinations acquired on the 64-row CT using water as compared to mannitol (mean diameter of 22.6 mm vs. 20.6 mm; n = 71 and n = 61, respectively; p = 0.034). No significant difference between water and mannitol was observed in scans acquired at the 16-row CT (n = 21 and n = 27, respectively).
Fig 4. Mean diameter of each intestinal segment.
Whiskers: upper confidence limit. Black columns: water group; grey columns: mannitol group. *: p< 0.05. Note the decrease of the diameter of the intestinal segments from proximal to distal.
Qualitative assessment of intestinal distension
Comparing the percentage of segments evaluated as diagnostic in terms of the possibility to rule out a potential underlying pathology, there was no statistically significant difference in the descending duodenum, horizontal duodenum or the proximal jejunum (both readers p>0.05; Table 2). Regarding the distal jejunum, both readers evaluated less segments as diagnostic in the water group as compared to the mannitol group (reader 1: 35% vs. 65%, p<0.0001; reader 2: 23% vs. 50%, p<0.001, respectively). Regarding the proximal and distal ileum, statistically significant more segments were rated as diagnostic after mannitol administration by reader 1 (water group vs. mannitol group: 43% vs. 67%, p = 0.002 and 56 vs. 75%; p = 0.009), whereas reader 2 found no significant difference between the two groups (64% vs. 77%, p = 0.054; and 48% vs. 52%, p = 0.553). Table 3 includes a subgroup analysis with further division of the water and mannitol group depending on the CT scanner used. A substantial bias introduced by the different CT could however not be found.
Table 2. Percentage of intestinal segments rated as diagnostic on a two-point scale in terms of the possibility to rule out a potential underlying pathology.
| Segment | water group (n = 92) | mannitol group (n = 88) | p-value | |||
|---|---|---|---|---|---|---|
| R1 [%] | R2 [%] | R1 [%] | R2 [%] | R1 | R2 | |
| descending duodenum | 34 | 51 | 32 | 44 | 0.790 | 0.366 |
| horizontal duodenum | 18 | 33 | 18 | 30 | 0.961 | 0.659 |
| proximal jejunum | 8 | 18 | 8 | 20 | 0.934 | 0.740 |
| distal jejunum | 35 | 23 | 65 | 50 | <0.0001 | <0.001 |
| proximal ileum | 43 | 64 | 67 | 77 | 0.002 | 0.054 |
| distal ileum | 56 | 48 | 75 | 52 | 0.009 | 0.553 |
| Mean percentage over all segments | 32 | 40 | 44 | 46 | ||
R1 = reader 1; R2 = reader 2; p-values: Mann-Whitney-test.
Table 3. Percentage of intestinal segments rated as diagnostic on a two-point scale in terms of the possibility to rule out a potential underlying pathology by CT-Scanner.
| Segment | water group (n = 92) | mannitol group (n = 88) | p-value | |||
|---|---|---|---|---|---|---|
| R1 [%] | R2 [%] | R1 [%] | R2 [%] | R1 | R2 | |
| 64-row CT | n = 71 | n = 61 | ||||
| descending duodenum | 35 | 51 | 36 | 44 | 0.921 | 0.463 |
| horizontal duodenum | 18 | 32 | 25 | 30 | 0.382 | 0.724 |
| proximal jejunum | 8 | 21 | 10 | 15 | 0.787 | 0.348 |
| distal jejunum | 34 | 24 | 61 | 43 | 0.002 | 0.023 |
| proximal ileum | 46 | 65 | 66 | 70 | 0.029 | 0.489 |
| distal ileum | 58 | 52 | 74 | 48 | 0.055 | 0.604 |
| Mean percentage over all segments | 33 | 41 | 45 | 42 | ||
| 16-row CT | n = 21 | n = 27 | ||||
| descending duodenum | 29 | 52 | 22 | 44 | 0.628 | 0.597 |
| horizontal duodenum | 19 | 33 | 4 | 30 | 0.091 | 0.796 |
| proximal jejunum | 5 | 10 | 4 | 33 | 0.881 | 0.056 |
| distal jejunum | 38 | 19 | 74 | 67 | 0.014 | 0.001 |
| proximal ileum | 33 | 62 | 70 | 93 | 0.012 | 0.011 |
| distal ileum | 52 | 33 | 78 | 63 | 0.069 | 0.045 |
| Mean percentage over all segments | 29 | 35 | 42 | 55 | ||
R1 = reader 1; R2 = reader 2; p-values: Mann-Whitney-test.
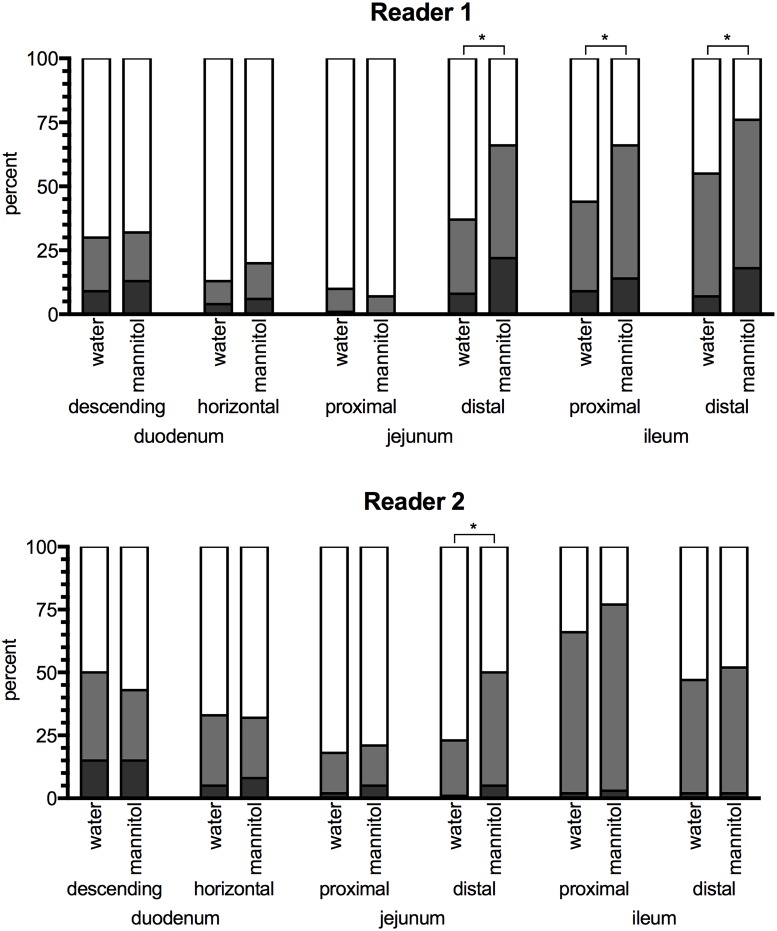
Evaluating the degree of luminal distension as assessed on a three-point scale, no statistically significant differences between water and mannitol solution were observed in the three proximal diagnostically satisfactory intestinal segments (p>0.05 for both readers and all segments). However, both readers rated intestinal distension of the distal jejunum statistically significant better after administration of mannitol solution (both readers p<0.0001). In the two ileal segments, significantly better distension was observed only for reader 1 (p = 0.004 and 0.0001), whereas reader 2 rated both segments comparable (p = 0.098 and 0.471) (Fig 5). Overall, less than half of all intestinal segments were rated as diagnostic satisfactory (reader 1: water group 32%, mannitol group 44%; reader 2: water group 40%, mannitol group 46%).
Fig 5. Rating of intestinal distension.
Luminal distension was evaluated on a three-point-scale. Each bar, representing one segment, is divided according to the percentage of given ratings (white = unsatisfactory distension, grey = good distension, dark grey = optimal distension). *: p< 0.05.
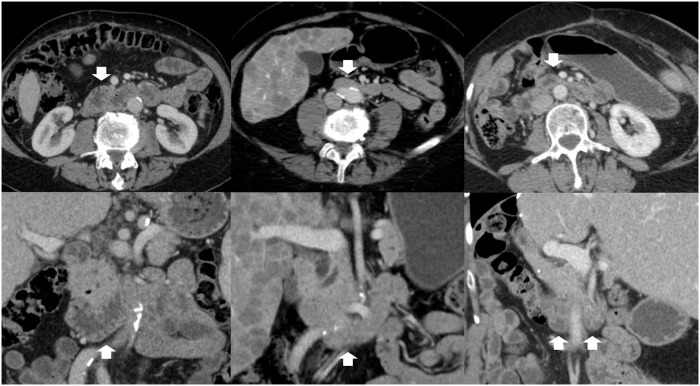
Interrater agreement for assessment of intestinal distension in individual segments after water and mannitol administration was moderate, with a mean ICC of 0.52 and 0.49, respectively. Interrater agreement for individual intestinal segments dependent on the type of oral contrast agent is presented in detail in Table 4. Exemplary images of agreement and disagreement of both readers is presented in Fig 6.
Table 4. Intraclass coefficient for assessment of interrater agreement.
| Segment | water group (n = 92) | mannitol group (n = 88) |
|---|---|---|
| interrater agreement | interrater agreement | |
| descending duodenum | 0.563 (0.332–0.713) | 0.548 (0.314–0.703) |
| horizontal duodenum | 0.680 (0.479–0.799) | 0.652 (0.469–0.771) |
| proximal jejunum | 0.623 (0.432–0.750) | 0.059 (-0.379–0.367) |
| distal jejunum | 0.348 (0.035–0.562) | 0.516 (0.242–0.688) |
| proximal ileum | 0.402 (0.106–0.601) | 0.638 (0.447–0.763) |
| distal ileum | 0.475 (0.211–0.651) | 0.531 (0.179–0.720) |
Upper and lower confidence limits in parenthesis.
Fig 6. Interrater agreement horizontal duodenum.
Exemplary slices of three patients (columns) in transverse (first row) and coronal plane (second row). First column: both readers rated the horizontal duodenum as diagnostic and degree of luminal distension as good; second column: both readers rated the horizontal duodenum as not diagnostic and degree of luminal distension as unsatisfactory. Third column; R1 rated the horizontal duodenum as diagnostic and degree of luminal distension as good while R2 did not.
Side effects
Side effects attributed to the oral contrast agent were observed only after mannitol administration, summing up to 29.5% (n = 26). Specifically, patients complained about diarrhea (n = 22; 25%), abdominal pain (n = 9; 10%) and nausea (n = 5; 6%, in one case with vomiting (1%). There were no adverse events attributed to the oral administration of water or to the intravenous contrast injection.
Radiation dose
A comparison of the CTDI is shown in Table 5. Mean CTDI values were slightly higher in the water group compared to the mannitol group on both scanners without reaching statistical significance (p = 0.682). A subanalysis of each CT scanner alone showed statistically significant higher CTDI values for the water group compared to the mannitol group (p = 0.029) on the 16-row CT. However, after normalizing the CTDI values by a division with the patient’s body mass index in order to reduce the influence of patient’s weight and size, no significant difference could be found (p = 0.469).
Table 5. Radiation dose for both study groups based on the CT scanner used for imaging.
| 16-row CT | 64-row CT | 16- and 64-row CT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| water group (n = 21) | mannitol group (n = 27) | p | water group (n = 71) | mannitol group (n = 61) | p | water group (n = 92) | mannitol group (n = 88) | p | |
| BMI [kg/m2] | 27.1 (24.4–29.7) | 24.2 (21.7–26.7) | 0.112 | 26.4 (25.1–27.6) | 26.4 (24.9–27.8) | 0.900 | 26.5 (24.4–27.6) | 25.7 (24.4–27.0) | 0.330 |
| CTDI | |||||||||
| absolute [mGy] | 14.7 (13.6–15.9) | 12.9 (11.6–14.1) | 0.048 | 11.4 (10.4–12.4) | 11.5 (10.0–12.9) | 0.553 | 12.2 (11.3–13.0) | 11.9 (10.8–13.0) | 0.494 |
| normalized [mGy*m2*kg-1] |
0.54 (0.52–0.57) | 0.63 (0.38–0.90) | 0.344 | 0.43 (0.40–0.45) | 0.42 (0.38–0.46) | 0.478 | 0.46 (0.43–0.48) | 0.49 (0.41–0.57) | 0.942 |
Mean Body mass index (BMI) of the subgroups. Computed tomography dose index (CTDI) as absolute values and normalized (CTDI divided by BMI). Data is presented as mean with upper and lower confidence intervals in parenthesis.
Discussion
In this prospective study we evaluated water and mannitol solution as neutral oral contrast agents for assessment of the small bowel in patients undergoing (thoraco-) abdominal staging CT. Even though individual distal segments were rated better at qualitative assessment using mannitol solution, this trend could not be verified quantitatively. Moreover, mannitol administration was associated with side effects in 29.5% of examinations, while patients receiving water reported no side effects. Radiation dose as assessed by CTDI was slightly lower after mannitol solution without reaching statistical significance.
Similar to our results, in a study comparing different administration techniques for CT enterography and enteroclysis, Paparo et al observed a declining intestinal distension from proximal to distal small bowel (jejunum to terminal ileum) using up to 2 liters of a neutral contrast agent administered orally or via a naso-jejunal tube. The reported intestinal distension of the ileal segments is in the range of our measurements, even though patients in our study received only 1 liter of an oral contrast agent. Interestingly, in their study a lower distension of the jejunum (mean 13 mm; range 10–25 mm) was observed as compared with the ileum (mean 17 mm; range 10–21 mm) [9]. Applying the contrast agent via a naso-jejunal tube (CT enteroclysis) improved distension of the jejunum (mean 27 mm; range 17–32 mm) only. Even though in our present study the distension of the jejunum was not as low as reported by Paparo, qualitative ratings were worse compared to the other intestinal segments. Hence, as Paparo pointed out, if the focus of the examination is a possible pathology in the jejunum, e.g. in patients with celiac disease, this might selectively affect this diagnostic accuracy. In such cases other techniques for intestinal distension (e.g. CT enteroclysis) should be considered. However, for patients undergoing (thoraco-) abdominal staging CT, the distension of jejunal loops achieved in the context presented in this study was acceptable.
Subjective ratings of intestinal distension and diagnostic quality were carried out in this study, reflecting how confident the two readers were in ruling out a possible underlying pathology. Regarding the two duodenal segments, our readers did not find significant differences between the water and mannitol group. The interrater agreement of the ratings was moderate to strong. Lowest percentages in terms of diagnostic quality were found for the proximal jejunum, also reflected by a high percentage of segments rated as unsatisfied distended and only poor to moderate agreement. A low distension might explain these conflicting results. The same may be true for the distal segments, in that a decreasing intestinal distension impedes bowel assessment.
This study focused on neutral oral contrast agents. A comparison with positive oral contrast agents was not carried out. In a recent study, Kammerer et al stated that for the majority of clinical indications neutral enteric contrast in abdominal CT imaging offers the most advantageous combination of bowel delineation, pathology detection and diagnostic reliability [19]. Other studies stated that water provides a sufficient enteric contrast for imaging specific gastrointestinal pathologies, e.g. chronic intestinal inflammation [2,7,20–23]. The use of positive oral contrast agents may be limited to distinct clinical questions such as pathologies with a lower density, e.g. fistulas or anastomotic insufficiency [19].
As known from the use of positive oral contrast agents, patients more often accept drinking the full amount of necessary neutral oral contrast agents without any additives [19]. In contrast to the mannitol group no patient in the water group reported any side effects (no diarrhea, nausea, vomiting or abdominal pain). Moreover, drinking water is advantageous in terms of hydration after i.v. contrast application [24]. Furthermore, from an economical point of view, using water without mannitol as an oral contrast agent results in slightly lower costs (difference around € 4.00 per patient).
Our study had several limitations. The same time schedule was applied for administration of the negative oral contrast agents 45 minutes prior to CT examination, although water may have a faster transit time compared to mannitol. Thus the clinical workflow established in our institute has not to be changed, which might introduce unnecessary errors. Evaluation of different time schemes was not carried out. For quantitative measurements the total diameter as the intestine was regarded as the luminal diameter, as the intestinal wall was not always distinguishable from the lumen. Thus intestinal wall thickening (caused by e.g. contractions or wall edema) may have distorted the measurements. We tried to compensate for this using with the additional qualitative assessment. Qualitative assessment was carried out by two relatively inexperienced radiology residents, which in part might be a possible explanation for discrepancies regarding the evaluation of intestinal distension. The chosen three-point scale for assessment of the distension might have lowered reproducibility as well. Our readers were asked how confident they were in terms of ruling out a possible underlying pathology without any comparison to a reference standard (e.g. pathologic diagnosis).
In conclusion, we accept the null hypothesis that there is no difference between orally administered water and mannitol solution for evaluation of the small bowel at abdominal staging CT in clinical routine only for the quantitative, but not for the qualitative analysis. Furthermore, looking more differentiated at the overall performance, water has advantages in terms of patient comfort, side effects and costs, and can therefore be regarded as noninferior to mannitol in this specific patient group.
Acknowledgments
The authors thank Dr. Babak Alikhani for support regarding data analysis.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Diederichs G, Franiel T, Asbach P, Romano V, Hamm B, Rogalla P. Intravenöse Kontrastmittel oral appliziert: Eine wohlschmeckende Alternative zum herkömmlichen oralen Kontrastmittel in der Computertomografie. Fortschr Röntgenstr. 2007;179: 1061–1067. [DOI] [PubMed] [Google Scholar]
- 2.Megibow AJ, Babb JS, Hecht EM, Cho JJ, Houston C, Boruch MM, et al. Evaluation of Bowel Distention and Bowel Wall Appearance by Using Neutral Oral Contrast Agent for Multi–Detector Row CT 1. Radiology. 2006;238: 87–95. 10.1148/radiol.2381041985 [DOI] [PubMed] [Google Scholar]
- 3.Harieaswar S, Rajesh A, Griffin Y, Tyagi R, Morgan B. Routine Use of Positive Oral Contrast Material Is Not Required for Oncology Patients Undergoing Follow-up Multidetector CT 1. Radiology. 2009;250: 246–253. 10.1148/radiol.2493080353 [DOI] [PubMed] [Google Scholar]
- 4.Paulsen SR, Huprich JE, Fletcher JG, Booya F, Young BM, Fidler JL, et al. CT Enterography as a Diagnostic Tool in Evaluating Small Bowel Disorders: Review of Clinical Experience with over 700 Cases1. RadioGraphics. 2006;26: 641–657. 10.1148/rg.263055162 [DOI] [PubMed] [Google Scholar]
- 5.Guimarães LS, Fidler JL, Fletcher JG, Bruining DH, Huprich JE, Siddiki H, et al. Assessment of appropriateness of indications for CT enterography in younger patients. Inflamm Bowel Dis. 2010;16: 226–232. 10.1002/ibd.21025 [DOI] [PubMed] [Google Scholar]
- 6.Hara AK, Alam S, Heigh RI, Gurudu SR, Hentz JG, Leighton JA. Using CT enterography to monitor Crohn’s disease activity: a preliminary study. AJR. 2008;190: 1512–1516. 10.2214/AJR.07.2877 [DOI] [PubMed] [Google Scholar]
- 7.Reittner P, Goritschnig T, Petritsch W, Doerfler O, Preidler KW, Hinterleitner T, et al. Multiplanar spiral CT enterography in patients with Crohn’s disease using a negative oral contrast material: initial results of a noninvasive imaging approach. Eur Radiol. 2002;12: 2253–2257. 10.1007/s00330-002-1361-y [DOI] [PubMed] [Google Scholar]
- 8.Shankar KR, Lloyd DA, Kitteringham L, Carty HM. Oral contrast with computed tomography in the evaluation of blunt abdominal trauma in children. Br J Surg. 1999;86: 1073–1077. 10.1046/j.1365-2168.1999.01192.x [DOI] [PubMed] [Google Scholar]
- 9.Paparo F, Garlaschi A, Biscaldi E, Bacigalupo L, Cevasco L, Rollandi GA. Computed tomography of the bowel: A prospective comparison study between four techniques. Eur J Radiol. 2013;82: e1–e10. 10.1016/j.ejrad.2012.08.021 [DOI] [PubMed] [Google Scholar]
- 10.Wold PB, Fletcher JG, Johnson CD, Sandborn WJ. Assessment of small bowel Crohn disease: noninvasive peroral CT enterography compared with other imaging methods and endoscopy—feasibility study. Radiology. 2003;229: 275–281. 10.1148/radiol.2291020877 [DOI] [PubMed] [Google Scholar]
- 11.Minordi LM, Vecchioli A, Mirk P, Bonomo L. CT enterography with polyethylene glycol solution vs CT enteroclysis in small bowel disease. BJR. 2011;84: 112–119. 10.1259/bjr/71649888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickhardt PJ, Choi JR, Hwang I, Butler JA, Puckett ML, Hildebrandt HA, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349: 2191–2200. 10.1056/NEJMoa031618 [DOI] [PubMed] [Google Scholar]
- 13.Schunk K, Kern A, Heussel C-P, Kalden P, Orth T, Wanitschke R, et al. [Hydro-MRT with fast sequences in Crohn’s disease: a comparison with fractionated gastrointestinal passage]. Fortschr Röntgenstr. 1999;170: 338–346. 10.1055/s-2007-1011051 [DOI] [PubMed] [Google Scholar]
- 14.Patak MA, Froehlich JM, Weymarn von C, Ritz MA, Zollikofer CL, Wentz K. Non-invasive distension of the small bowel for magnetic-resonance imaging. Lancet. 2001;358: 987–988. 10.1016/s0140-6736(01)06127-x [DOI] [PubMed] [Google Scholar]
- 15.Laghi A, Paolantonio P, Iafrate F, Altomari F, Miglio C, Passariello R. Oral contrast agents for magnetic resonance imaging of the bowel. Top Magn Reson Imaging. 2002;13: 389–396. [DOI] [PubMed] [Google Scholar]
- 16.Lauenstein TC, Schneemann H, Vogt FM, Herborn CU, Ruhm SG, Debatin JF. Optimization of oral contrast agents for MR imaging of the small bowel. Radiology. 2003;228: 279–283. 10.1148/radiol.2281020161 [DOI] [PubMed] [Google Scholar]
- 17.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychological Methods. American Psychological Association; 1996;1: 30–46. 10.1037/1082-989X.1.1.30 [DOI] [Google Scholar]
- 18.International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10)-WHO Version. Geneva: WHO, 2016. http://apps.who.int/classifications/icd10/browse/2016/en#/II
- 19.Kammerer S, Höink AJ, Wessling J, Heinzow H, Koch R, Schuelke C, et al. Abdominal and pelvic CT: is positive enteric contrast still necessary? Results of a retrospective observational study. Eur Radiol. 2015;25: 669–678. 10.1007/s00330-014-3446-9 [DOI] [PubMed] [Google Scholar]
- 20.Wittenberg J, Harisinghani MG, Jhaveri K, Varghese J, Mueller PR. Algorithmic approach to CT diagnosis of the abnormal bowel wall. RadioGraphics. 2002;22: 1093–109. 10.1148/radiographics.22.5.g02se201093 [DOI] [PubMed] [Google Scholar]
- 21.Hebert JJ, Taylor AJ, Winter TC. Comparison of colonic transit between polyethylene glycol and water as oral contrast vehicles in the CT evaluation of acute appendicitis. AJR 2006;187: 1188–1191. 10.2214/AJR.05.1073 [DOI] [PubMed] [Google Scholar]
- 22.Hebert JJ, Taylor AJ, Winter TC, Reichelderfer M, Weichert JP. Low-attenuation oral GI contrast agents in abdominal-pelvic computed tomography. Abdom Imaging. 2006;31: 48–53. 10.1007/s00261-005-0350-4 [DOI] [PubMed] [Google Scholar]
- 23.Angelelli G, Macarini L, Fratello A. Use of water as an oral contrast agent for CT study of the stomach. AJR. 1987;149: 1084–1084. 10.2214/ajr.149.5.1084 [DOI] [PubMed] [Google Scholar]
- 24.Kong D-G, Hou Y-F, Ma L-L, Yao D-K, Wang L-X. Comparison of oral and intravenous hydration strategies for the prevention of contrast-induced nephropathy in patients undergoing coronary angiography or angioplasty: a randomized clinical trial. Acta Cardiol. 2012;67: 565–569. 10.2143/AC.67.5.2174131 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.