Abstract
Patients with medullary thyroid cancer (MTC) are often diagnosed with spread tumour disease and the development of better systemic treatment options for these patients is important. Treatment with the radiolabelled somatostatin analogue 177Lu-octreotate is already a promising option but can be optimised. For example, combination treatment with another substance could increase the effect on tumour tissue. Gemcitabine is a nucleoside analogue that has been shown to sensitise tumour cells to radiation. The aim of this study was to investigate potentially additive or synergistic effects of combining radiation with gemcitabine for treatment of MTC. Nude mice transplanted with patient-derived MTC tumours (GOT2) were divided into groups and treated with radiation and/or gemcitabine. Radiation treatment was given as 177Lu-octreotate or external beam radiotherapy (EBRT). The volume of treated and untreated tumours was followed. The absorbed dose and amount of gemcitabine were chosen to give moderate tumour volume reduction when given as monotherapy to enable detection of increased effects from combination treatment. After follow-up, the mice were killed and tumours were immunohistochemically (IHC) analysed. Overall, the animals that received a combination of EBRT and gemcitabine showed the largest reduction in tumour volume. Monotherapy with EBRT or gemcitabine also resulted in a clear detrimental effect on tumour volume, while the animals that received 177Lu-octreotate monotherapy showed similar response as the untreated animals. The GOT2 tumour was confirmed in the IHC analyses by markers for MTC. The IHC analyses also revealed that the proliferative activity of tumour cells was similar in all tumours, but indicated that fibrotic tissue was more common after EBRT and/or gemcitabine treatment. The results indicate that an additive, or even synergistic, effect may be achieved by combining radiation with gemcitabine for treatment of MTC. Future studies should be performed to evaluate the full potential of combining 177Lu-octreotate with gemcitabine in patients.
Introduction
Medullary thyroid cancer (MTC) accounts for about 1–2% of all thyroid cancers [1]. It originates from the calcitonin-producing parafollicular C-cells of the thyroid and occurs either sporadically or as a hereditary form in the multiple endocrine neoplasia type 2 syndrome, often caused by mutations in the RET proto-oncogene [2–4]. Many patients with MTC present with metastatic disease at the time of diagnosis and curative surgery can only be performed in patients with limited or no tumour spread to local lymph nodes [5, 6]. Based on 1252 cases between 1973 and 2002 registered in the Surveillance, Epidemiology, and End Results (SEER) database, the 10-year survival for patients with MTC confined to the thyroid gland is about 95% compared with 40% for patients diagnosed with distant metastases [7]. Therefore, there is a need for better systemic therapy strategies for metastatic disease.
For distant metastases from the more common papillary and follicular thyroid cancer, systemic therapy with radioiodine (131I) is a well-established treatment technique with high response rates [8]. However, since MTC originates from the C-cells of the thyroid, it lacks the transmembrane protein NIS (sodium/iodine symporter) that is responsible for transporting iodide into the cell, and MTC can therefore not be treated with radioiodine. Instead, many MTCs express somatostatin (SST) receptors (SSTRs), which is a characteristic feature also for other types of neuroendocrine tumours (NETs) [9]. Therefore, high receptor-specific binding of radiolabelled SST analogues, e.g. 111In-octreotide or 177Lu-octreotate, can be achieved in MTC [10, 11]. Treatment with radiolabelled SST analogues is one type of peptide receptor radionuclide therapy (PRRT). In a clinical phase II trial, PRRT with 90Y-octreotide for patients with metastatic MTC was associated with long-term survival benefit [12]. However, few patients are cured with the current standardised treatment protocol. There is a clear need for optimisation and one option could be to combine PRRT with another drug [13].
Gemcitabine is a nucleoside analogue that has shown anti-tumour activity in many different cancer types, including MTC [14, 15]. After entering a cell, gemcitabine is activated by phosphorylation and the active metabolites gemcitabine diphosphate and triphosphate are generated. These metabolites are responsible for the cytotoxic effect by 1) incorporation in the DNA, which inhibits DNA polymerases, leading to G1/S cell cycle arrest or cell death, and by 2) interfering with the enzyme ribonucleotide reductase responsible for DNA synthesis and repair [16].
Chemotherapeutic drugs can enhance the cell-killing effect of radiation, a process called radiosensitisation [17]. In preclinical studies, gemcitabine has a well-documented radiosensitising effect on many different cancer types [18–22]. Furthermore, several clinical studies have evaluated the use of gemcitabine in combination with external beam radiotherapy (EBRT), most frequently for pancreatic cancer [23–27]. For treatment of metastatic MTC, it is of value to investigate the efficiency of the combination of gemcitabine and radiation, both as EBRT and as systemic radionuclide therapy using radiolabelled SST analogues, e.g. 177Lu-octreotate. To the authors’ knowledge, no such investigations have yet been made.
The aim of this study was to investigate the potentially additive or synergistic effects of combining ionising radiation (177Lu-octreotate and EBRT) with gemcitabine for treatment of MTC. The study was performed in MTC-bearing mice.
Material and methods
Radiopharmaceutical
177Lu-octreotate was purchased from IDB Holland (IDB Holland BV, Baarle-Nassau, the Netherlands). Radiolabelling was performed according to the manufacturer’s instructions. Instant thin layer chromatography (ITLC) was used to measure the amount of peptide bound 177Lu, which was determined to be over 99%. All syringes containing 177Lu-octreotate were measured in a well-type ionisation chamber (CRC-15R, Capintec, Ramsey, New Jersey, USA) before and after injection to determine the amount of injected radiopharmaceutical into each animal.
Animal model
GOT2 are MTC cells that have been successfully transplanted to nude mice [28]. Originally, MTC cells were collected from a patient with sporadic, RET-driven MTC in Gothenburg. By serially transplanting GOT2 tumours to new generations of mice, we have used this patient-derived xenograft model for studies of MTC for over 15 years. The collection of tumour tissue from the patient was performed during surgery in 2001. At that time, formal ethical approval by an ethics committee was optional according to Swedish law, but was in this case not regarded necessary. The patient provided oral informed consent for the collection according to standard procedure at the time. The consent was verified by two surgeons, one pathologist and one medical physicist. The principles expressed in the Declaration of Helsinki was followed.
In this study, small GOT2 tumour tissue samples (ca. 1x1x1 mm3) were transplanted subcutaneously in the neck of 4–5 weeks old female BALB/c nude mice (Charles River Laboratories, Sulzfeld, Germany) under anaesthesia by intraperitoneal (i.p.) injection of Ketaminol® vet. (Intervet AB, Stockholm, Sweden) and Domitor® vet. (Orion Pharma AB Animal Health, Sollentuna, Sweden). An i.p. injection of Antisedan® vet. (Orion Pharma AB Animal Health) was used as antidote to anaesthesia. After about 2 months, tumours appeared close to the transplanted location. Digital callipers were used to measure the tumour length, width, and height. Then, the tumour volume was calculated by assuming an ellipsoidal shape. The experiments were initiated when the tumours reached a volume of about 200–2000 mm3 (mean = 570 mm3, SD = 406 mm3). All mice were given water and autoclaved food ad libitum. The experiments were approved by the Ethical Committee on Animal Experiments in Gothenburg, Sweden (permit no. 107–15).
Combination therapy experiments
GOT2-bearing mice (n = 48) were divided into groups of 5–12 mice per group. The tumour size distribution within each group was kept as similar as possible. Three groups were used for combination studies of 177Lu-octreotate and gemcitabine, another three groups were used for combination studies of EBRT and gemcitabine, and one group was used as untreated control animals (Table 1). The 177Lu activity, absorbed dose, and amount of gemcitabine were chosen to give low to moderate tumour volume reduction as monotherapy to enable detection of any additive or synergistic effects in the combination therapy groups.
Table 1. GOT2-carrying nude mice were treated according to the presented schedules with 177Lu-octreotate (Lu), gemcitabine (Gem) and/or external beam radiotherapy (EBRT), or left untreated as control animals.
| Administered amount: Lu (MBq), Gem (mg/kg) or EBRT (Gy) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Group | T (d) | Day 0 | Day 3 | Day 7 | Day 10 | Day 13 | n | F (d) |
| Lu | 96 | 10 | 0 | 0 | 0 | 0 | 5 | 16 |
| Gem high | 96 | 125 | 125 | 125 | 60 | 60 | 5 | 16 |
| Lu + Gem high | 96 | 10+125 | 0+125 | 0+125 | 0+60 | 0+60 | 5 | 16 |
| EBRT | 62 | 5 | 0 | 0 | 0 | 0 | 5 | 44 |
| Gem low | 137 | 60 | 60 | 60 | 60 | 60 | 10 | 44 |
| EBRT + Gem low | 62 | 5+60 | 0+60 | 0+60 | 0+60 | 0+60 | 6 | 58 |
| Untreated control | 145 | - | - | - | - | - | 12 | 30 |
T, median time between transplantation and treatment start; n, number of animals in each group; F, follow-up time.
177Lu-octreotate (10 MBq, 0.1 ml) was administered by intravenous injection in the tail vein as a single treatment on day 0. Gemcitabine (Active Biochem LTD, Hong Kong, China) was obtained as a powder, which was dissolved in saline solution, and 60 or 125 mg/kg was administered by i.p. injection twice a week for a total of 2.5 weeks starting on day 0. Prior to each injection of gemcitabine, the mice were weighed and the injected volume (0.15–0.20 ml) was adjusted to administer the correct dose to each mouse. No symptoms of toxic side effects were seen for the animals receiving 177Lu-octreotate. However, the initial twice weekly gemcitabine dose of 125 mg/kg for the groups used for combination treatment studies of 177Lu-octreotate and gemcitabine resulted in weight loss for many animals. Therefore, the gemcitabine dose was lowered to 60 mg/kg from day 10 for these animals. Unfortunately, this change did not improve the condition of the animals and this treatment group was only followed for 16 days (Table 1). For the same reason, the animals used for combination treatment studies of EBRT and gemcitabine received 60 mg/kg from day 0.
For EBRT, the animals were anaesthetised (by i.p. injection of Ketaminol® vet. and Domitor® vet.), placed on their side on a tissue-equivalent polystyrene bed and individually irradiated using a Varian linear accelerator with 6 MV photon beam (nominal energy) at a gantry angle of 0 degrees (Varian Medical Systems, Palo Alto, California, USA). To obtain a relatively uniform dose distribution in the tumour and to minimise air gaps, tissue-equivalent material was fitted around the mouse and the tumour. The tumour was covered with 15 mm tissue-equivalent material and the centre of the tumour was positioned at isocenter at a depth of about 20 mm (depending on the size of the tumour). A 30x30 mm2 irradiation field was then used to deliver 5 Gy to the tumour. Using this setup, adjacent normal tissues were irradiated to some extent (although with lower exposure than in other positioning). However, the animals showed no apparent signs of side effects from the EBRT during the follow-up time of the animals.
Internal dosimetry
For 177Lu-octreotate exposure, estimations of mean absorbed dose, D, were made according to the Medical Internal Radiation Dose (MIRD) formalism [29]:
| (1) |
where à is the time-integrated activity, the product EiYi is the energy emitted per decay for the ith nuclear transition, ϕi is the absorbed fraction and M is the mass of the tissue of interest. Only the contribution from beta particles was considered. Therefore, ΣiEiYi was set to 147.9 keV and the absorbed fraction was set to 1 [30, 31]. The time-integrated activity from time of injection to infinity time was estimated based on previously published biodistribution data of 177Lu-octreotate in BALB/c nude mice carrying GOT2 tumours, by fitting a mono-exponential curve to the time-activity concentration data [32].
Post-treatment follow-up
After start of treatment, the animals were monitored and tumour volume was measured twice a week. For each mouse, the relative tumour volume was defined as the tumour volume at a given point-in-time divided by the tumour volume at day 0 (start of treatment). Each group was followed until most of the tumours in a group had regrown (relative tumour volume at least larger than 1, but usually ca. 3–4). In addition, a mouse that met one of the following criteria was killed: 1) the tumour reached a volume corresponding to more than 10% of the total body weight, 2) the body weight was reduced by more than 10%, or 3) the mice showed any signs of lower health status. The mice were killed by cardiac puncture under anaesthesia (Pentobarbitalnatrium vet., Apotek Produktion & Laboratorier AB, Huddinge, Sweden) and tumours were fixed in formalin for histological studies. The follow-up time for each group can be seen in Table 1.
Immunohistochemistry
The formalin-fixed tumours were embedded in paraffin and processed for histological examination by standard procedures. The tumours were sliced into 4-μm sections and stained with haematoxylin and eosin (H&E) and Masson’s trichrome (MT) for examination of morphology and fibrosis, respectively.
For immunohistochemical (IHC) analysis, tumour sections were placed on glass slides and treated with EnVision™ FLEX Target Retrieval Solution (high pH) using a PT-Link (Dako, Glostrup, Denmark). The IHC staining was performed using an Autostainer Link using EnVision™ FLEX according to the manufacturer’s instructions (Dako). In each run, positive and negative controls were included. To analyse cell proliferation, a primary antibody for Ki67 (AB9260, Merck Millipore, Burlington, Massachusetts, USA) was used. To verify MTC origin of the tumours, tumour tissue sections from the untreated control group were stained using antibodies for MTC markers chromogranin A (ab68271, Abcam, Cambridge, England), synaptophysin (ab16659, Abcam), and calcitonin (A0576, Dako). Imaging for figure presentation of MTC markers was performed using a microscope (20x magnification, Eclipse E1000, Nikon Instruments, Amsterdam, Netherlands) equipped with a camera (ProgRes C7, Jenoptik, Jena, Germany).
Tumour sections stained for Ki67 and MT were digitalised using a digital slide scanner at 40x magnification (Leica SCN400 Slide Scanner, Leica Microsystems, Germany). Then, quantitative analysis of histological features were performed on these digitalised sections (resolution 0.25x0.25 μm2) using an in-house developed MATLAB tool (R2017a, MathWorks, Natick, Massachusetts, USA). In brief, tumour tissue was delineated by drawing a region of interest (ROI) around the tumour, excluding the tumour capsule. Thereafter, colour thresholds were applied to segment and remove regions of cracks (background) or folds caused by histological processing, and to segment positively stained tumour tissue. Necrotic tumour regions were easily discernible in the MT images (and verified on H&E sections), which facilitated automatic segmentation of tumour tissue into necrotic or non-necrotic tumour (denoted as viable). By aligning the digital Ki67 and MT sections by semi-automatic image registration (MATLAB control point selection tool), the segmentation mask for viable/necrotic tumour tissue defined from MT staining was applied also to the Ki67 sections. The colour thresholds and corresponding segmentation of tumour tissues were approved by a board-certified pathologist (O.N.). Ki67 was quantified only in viable tumour, whereas MT (blue regions representing collagen) was quantified for the entire tumour section (excluding background and artefacts).
Data calculations and statistical analyses
The relative tumour volume was calculated individually for each mouse. Then, mean relative tumour volumes for each group was calculated and used for the statistical analyses. Group differences were analysed for all measurements after study start when all groups were still followed (i.e., day 3–16 or day 3–30, Table 1). For each of these measurements, overall differences between all groups were analysed using one-way ANOVA in GraphPad Prism 7.04 (GraphPad Software, La Jolla, San Diego, California, USA). Furthermore, group-to-group differences were analysed using Student’s t-tests. All reported p-values were adjusted to account for multiple comparisons using Bonferroni-Holm correction [33]. An adjusted p-value of less than 0.05 was considered statistically significant for all tests.
The interaction effect between radiation and gemcitabine was analysed using the Bliss independence model [34]. Based on the mean relative tumour volumes, the predicted additive fractional response for a given combination, Fadd, was calculated using
| (2) |
where Frad and Fgem is the fractional response from monotherapy with radiation (177Lu-octreotate or EBRT) and with gemcitabine, respectively [35]. The fractional response was calculated using
| (3) |
where RTVmono and RTVcontrol is the mean relative volume in a monotherapy group and in the untreated control group, respectively. Lastly, Fadd was compared with the measured effect in the combination therapy group. The measured effect was assumed to be synergistic, additive or antagonistic if it was larger than, equal to or smaller than Fadd, respectively.
Results
Anti-tumour effects of 177Lu-octreotate and/or gemcitabine
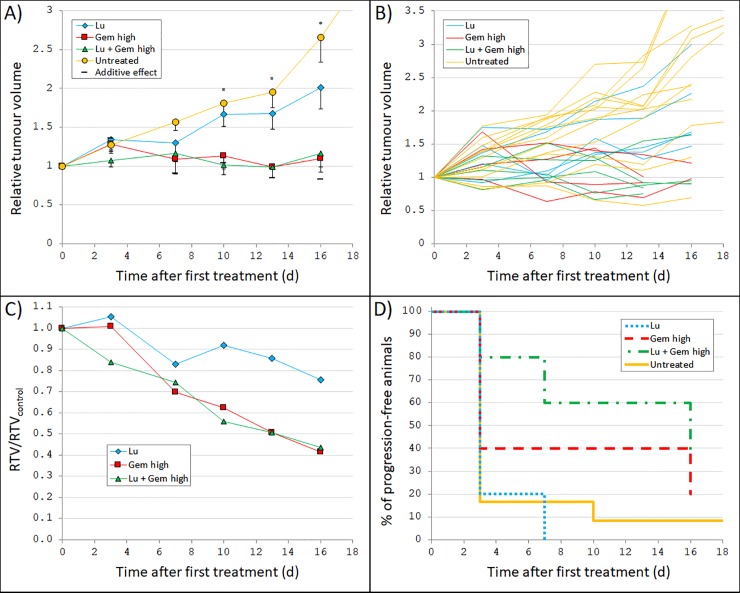
The administered activity of 10 MBq 177Lu-octreotate in this study resulted in a mean absorbed dose to tumour of 0.13 Gy. Tumour volume vs. time curves for nude mice carrying the patient-derived MTC model GOT2 treated with 10 MBq 177Lu-octreotate and/or 125 mg/kg gemcitabine (125 mg/kg initially but lowered to 60 mg/kg) are shown in Fig 1A–1C. ANOVA analyses of the relative tumour volumes revealed statistically significant overall differences between all four groups at day 10–16 (p = 0.428, 0.071, 0.009, 0.006, and 0.049 at day 3, 7, 10, 13, and 16, respectively). Absolute tumour volumes (mm3) are provided in S1 Fig.
Fig 1. Tumour growth after 177Lu-octreotate and/or gemcitabine treatment.
GOT2-carrying nude mice were treated with 10 MBq 177Lu-octreotate (Lu) and/or 125 mg/kg (initially, but lowered to 60 mg/kg) gemcitabine twice weekly (Gem high). The 177Lu activity and amount of gemcitabine were chosen to give low to moderate effect as single treatment to enable detection of any additive or synergistic effects. (A) Mean relative tumour volume (RTV) vs. time after first treatment. In addition to the measured values for each group, also the calculated relative tumour volume given a predicted additive response (Eq (2)) is shown. Error bars show SEM. A star indicate that the ANOVA analyses (performed at day 3–16) resulted in a statistically significant difference between the group means. (B) RTV vs. time after first treatment for each individual mouse. (C) Mean RTV for each treatment group divided by mean RTV for the untreated control group (RTV/RTVcontrol) vs. time after first treatment. (D) Percentage of animals in each group without tumour progression vs. time after first treatment. Not all animals reached tumour progression before the end of follow-up, and therefore, some lines end at values higher than 0%. The line for the untreated control animals continues past 16 days because the follow-up time was longer for these animals. Note differences in scale of the x-axis compared with Fig 2.
Given as monotherapy, gemcitabine resulted in a detrimental effect on tumour growth. At day 13, the relative tumour volume for the animals treated with gemcitabine was 51% of the relative volume for the untreated control animals (p = 0.045, Fig 1A). Furthermore, the animals that received only 177Lu-octreotate showed similar response as the untreated control animals (p = 0.249 at day 16). Consequently, the animals treated with a combination of both 177Lu-octretoate and gemcitabine showed no statistically significant difference in tumour volume compared with the animals treated with gemcitabine only (p = 0.430 at day 16).
In Fig 1D, Kaplan-Meier curves of progression-free survival (PFS) are shown. Tumour progression was defined as occurring when a tumour was larger than at start of treatment (after initial treatment response) or when an animal was killed. The longest time to progression (TTP) was seen for the combination therapy group, while the 177Lu-octreotate group showed similar PFS as the untreated control group, and gemcitabine monotherapy resulted in a prolonged TTP. For example, two weeks after start of treatment, about 90% of the control animals and all 177Lu-octreotate-treated animals had reached tumour progression, with corresponding values of 60% and 40% for the gemcitabine and combination therapy groups, respectively.
Anti-tumour effects of EBRT and/or gemcitabine
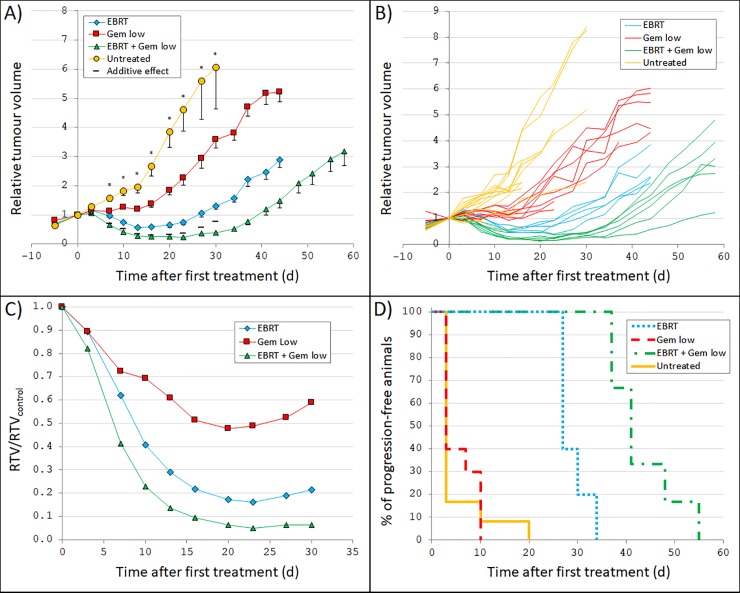
Tumour volume vs. time curves for the animals treated with 5 Gy EBRT and/or 60 mg/kg gemcitabine can be seen in Fig 2A–2C. ANOVA analyses revealed statistically significant overall differences between all four groups at all follow-up measurements, except for at 3 days after treatment (p = 0.156 at day 3, and p<0.001 at day 7–30). Absolute tumour volumes (mm3) are provided in S1 Fig.
Fig 2. Tumour growth after external beam radiotherapy and/or gemcitabine treatment.
GOT2-carrying nude mice were treated with 5 Gy external beam radiotherapy (EBRT) and/or 60 mg/kg gemcitabine twice weekly (Gem low). The absorbed dose and amount of gemcitabine were chosen to give low to moderate effect as single treatment to enable detection of any additive or synergistic effects. (A) Mean relative tumour volume (RTV) vs. time after first treatment. In addition to the measured values for each group, also the calculated relative tumour volume given a predicted additive response (Eq (2)) is shown. Error bars show SEM. A star indicate that the ANOVA analyses (performed at day 3–30) resulted in a statistically significant difference between the group means. (B) RTV vs. time after first treatment for each individual mouse. (C) Mean RTV for each group divided by mean RTV in the untreated control group (RTV/RTVcontrol) vs. time after first treatment. (D) Percentage of animals in each group without tumour progression vs. time after first treatment. Note differences in scale of the x-axis compared with Fig 1.
Given as monotherapy, both gemcitabine and EBRT resulted in a clear detrimental effect on tumour growth. Compared with the tumour volume in the untreated control group, the largest effect of the gemcitabine treatment was seen after 20 days when the relative volume was 48% of the relative volume in the untreated group (p<0.001). For the animals treated with EBRT, a clear initial tumour volume reduction was seen. Seven days after treatment, the mean relative tumour volume was 0.97 and reached a minimum after 13 days when the relative volume was 0.57, corresponding to 29% of the relative volume in the untreated group (p<0.001). Thereafter, the tumours started to regrow with a growth rate similar to what was seen in the gemcitabine group and in the untreated group (Fig 2A).
Overall, the animals that received a combination of EBRT and gemcitabine showed the largest reduction in tumour volume over time of all groups (Fig 1–Fig 2). One week after start of treatment, the relative volume was 0.65 and reached a minimum of 0.23 at 23 days after treatment start, corresponding to only 5% of the relative volume in the untreated control group (p<0.001). Furthermore, the analyses of interaction effects showed that the treatment effect for these animals was larger than the calculated predicted additive response for several measurements after start of treatment (Fig 2A).
The PFS curves showed a substantially prolonged TTP for the animals treated with EBRT monotherapy, with a median TTP of 27 days (Fig 2D). However, the TTP for the gemcitabine-treated animals was similar to that of the untreated control group, with a median TTP of 3 days for both groups. Overall, the animals that received a combination of EBRT and gemcitabine showed the longest TTP of all groups, with a median TTP of 41 days (Fig 2D). Altogether, the results show that gemcitabine clearly enhances the anti-tumour effect of radiation therapy on GOT2.
Immunohistochemical analyses
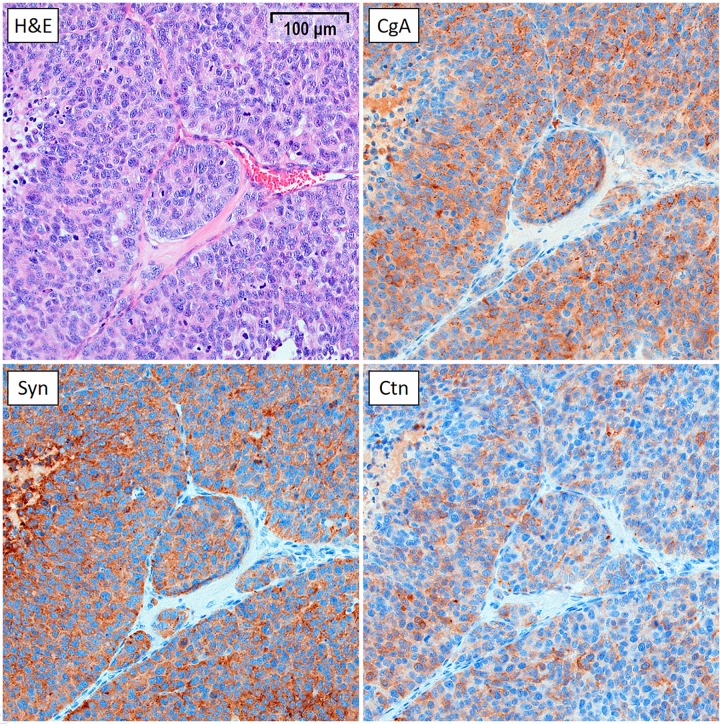
The stained tumours in the untreated control group showed a high and specific expression of MTC markers chromogranin A, synaptophysin, and calcitonin, and were morphologically consistent with MTC (Fig 3).
Fig 3. IHC staining of a representative GOT2 tumour (20x magnification).
The tumour was harvested from an animal in the untreated control group 30 days after start of treatment. The growth curve for this tumour was similar to the mean growth curve of the control group. The tumour was stained with haematoxylin and eosin (H&E), as well as for MTC markers chromogranin A (CgA), synaptophysin (Syn), and calcitonin (Ctn).
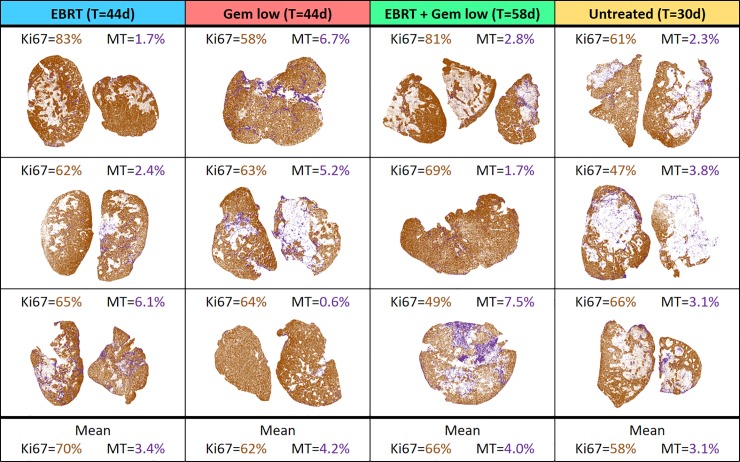
The percentage of Ki67-positive tissue (in viable tumour areas) was similar in all groups with an overall median of 64% (Fig 4). The percentage of MT-positive tissue (in whole tumour) was also similar in all groups, with an overall median of 3.0%. However, several tumour sections from mice that received radiation and/or gemcitabine treatment showed higher percentage of MT-positive staining (higher than 6%) compared with the untreated control where all values were similar to the overall median.
Fig 4. Overview of the results of the quantitative IHC analyses.
Analyses were performed for the animals treated with 5 Gy external beam radiotherapy (EBRT) and/or 60 mg/kg gemcitabine (Gem low) as well as for the untreated control animals. Quantification was made for digitalised histological sections stained with Ki67 (shown in brown) and Masson’s trichrome (MT, shown in purple). For each tumour, the percentage of the area positively stained for Ki67 (in viable tumour only) and MT (in the whole tumour area) was determined. Included is also the follow-up time, T, for each group.
Discussion
In this study, we have investigated the therapeutic effect of combining ionising radiation with gemcitabine for treatment of medullary thyroid cancer (MTC). Using a patient-derived MTC animal model (GOT2), we have provided results that indicate that an additive, or possibly even synergistic, combinatorial effect could be achieved. Furthermore, given as monotherapy, both gemcitabine and irradiation appeared to have a clear detrimental effect on tumour volume. It should be noted that suboptimal monotherapy doses and treatment protocols were used in order to enable detection of an increased effect from the combination treatment. Higher doses or a more optimal treatment protocol, e.g. repeated or differently scheduled treatment, would probably have resulted in a much higher effect on tumour volume in each of the monotherapy groups.
For the combination of irradiation and gemcitabine to be of value for patients with metastatic disease, systemic radionuclide therapy is needed (e.g. 177Lu-octreotate) instead of EBRT. Even though the 177Lu-octreotate monotherapy group showed similar response as the untreated control group in this study, previous data indicate that 177Lu-octreotate treatment is an option for patients with metastatic MTC with high SSTR expression [10, 11]. The biodistribution of 177Lu-octreotate in GOT2 nude mice has previously been studied [32]. The tumour uptake was comparable to what has been seen for radiolabelled SST analogues in patients with MTC, with a ratio of activity concentration in tumour and blood (T/B) of about 50 [10, 11]. However, there is a large individual variation between patients, and some patients have also shown much higher T/B values of up to 350, and these patients should be the most suitable for 177Lu-octreotate treatment. Furthermore, it is possible that the administered amount of 10 MBq 177Lu-octreotate used in this study was too low (absorbed dose to the GOT2 tumours was only 0.13 Gy). In a similar study, where 177Lu-octreotate was evaluated for treatment of small-intestine NETs in a xenograft animal model (GOT1), a clear effect on tumour volume was seen after administration of 15 MBq 177Lu-octreotate (resulting in 2.7 Gy to the tumour) [36, 37]. Since EBRT gave such high radiobiological effect, we believe that the poor response for the animals that received 177Lu-octreotate monotherapy could have been significantly improved if a higher absorbed dose would have been achieved. Because of the relatively low SSTR-expression in the GOT2 tumours, it would have been difficult to increase the absorbed dose by simply increasing the administered activity, due to tumour SSTR saturation. Unfortunately, there is, to our knowledge, no other MTC animal model available with higher SSTR-expression to better reflect the high uptake seen in some MTC patients; GOT2 is, as far as we know, the model with the highest uptake [32]. Therefore, we used EBRT in this study to be able to deliver a higher absorbed dose to the tumours and show that MTC is sensitive to irradiation.
Combination therapy offers many potential benefits for patients and can still be warranted even if there is no synergistic, but instead an additive or even antagonistic, effect between the drugs combined [38]. Firstly, if two drugs with non-overlapping toxic effects are used, the total administered amount can be increased while still keeping side effects below acceptable limits for the patient. Thus, the non-specific toxicity produced by a high dose of a single drug can be reduced. Another reason for using combination therapy is that a synergistic interaction effect on tumour tissue between the two drugs can occur if chosen wisely, increasing the tumour-killing potential. Furthermore, if a tumour develops resistance against the first drug, which is a common phenomenon in cancer therapy, the second drug can be an important next treatment step for the patient. It is also possible that, if administered at the same time, one of the drugs inhibits the development of resistance to the other drug. Lastly, both within-patient tumour heterogeneity and patient-to-patient variability are two additional arguments to use combination therapy. Within-patient tumour heterogeneity includes both differences within a given tumour and differences between two tumours, e.g. a primary tumour and its metastases, and in both situations, using more than one drug may be crucial to be able to target different populations of tumour cells and kill all cancer cells in the tumours if some cancer cells are not affected by only one drug. Furthermore, given a patient-to-patient variability, the use of drug combinations give each individual patient a better chance that at least one drug will be effective [39].
The results in this study show that an additive, or possibly even synergistic, effect could be achieved when combining irradiation and gemcitabine for treatment of MTC. A synergistic interaction effect could be explained by the mechanism of action of gemcitabine. By acting as a nucleoside analogue of deoxycytidine, gemcitabine can inhibit DNA synthesis and cause cell death [16]. Through this incorporation into the DNA, gemcitabine can also inhibit DNA repair of genomic damage caused by the radiation, leading to a theoretical interaction effect between irradiation and gemcitabine. Additionally, gemcitabine interferes with the enzyme ribonucleotide reductase, which is involved in DNA synthesis and repair. Lastly, there are also data suggesting that SST receptor (SSTR) expression can be upregulated by gemcitabine, further increasing the potential of radiolabelled SST analogue therapy for patients with metastatic MTC [40, 41]. This could be very useful if gemcitabine is administered before each injection of 177Lu-octreotate and would be interesting for future investigations.
The IHC analyses showed high expression of all MTC markers and a morphology consistent with that of MTC, verifying the MTC origin of the GOT2 tumours. The percentage of viable tumour area positively stained for Ki67 (indicating proliferative activity) was similar in all groups. This can be explained by the fact that the tumours were harvested for histological analysis at the end of follow-up (day 30–58), when all tumours had started to regrow. The amount of MT staining was also similar on a group level. However, some tumours in the treatment groups had a higher percentage of area positively stained for MT compared with untreated tumours. High amounts of positive MT staining indicates high levels of fibrosis, which in turn could be a sign of scarring due to treatment-related effects.
The experience of using gemcitabine as a treatment for patients with MTC is limited. In a study where gemcitabine (1000 mg/m2) was used to treat two patients with MTC, no apparent effect was seen [42]. However, for other cancer types, gemcitabine has been widely used for many years. In 1996, the U.S. Food and Drug Administration (FDA) approved gemcitabine for treatment of pancreas cancer and non-small cell lung cancer. Since then, it has also been approved for treatment of bladder, ovarian and breast cancer in combination with other drugs. The recommended dose of gemcitabine is 1000 mg/m2 on days 1 and 8 of each 21 day cycle [43]. At this dose level, gemcitabine is associated with side effects and among the most common (≥20% of patients) are nausea, vomiting, anemia, hepatic transaminitis, neutropenia, increased alkaline phosphatase, proteinuria, fever, hematuria, rash, thrombocytopenia, dyspnea, and peripheral edema. At lower doses, gemcitabine is less toxic and when used as a radiosensitiser rather than single-treatment agent, it is possible that much lower doses than 1000 mg/m2 can be used, minimizing the side effects while still increasing the therapeutic effect on tumour tissue. In a study on locally advanced head and neck cancer, gemcitabine appeared to be a strong radiosensitiser at doses much lower than those required for cytotoxic effects [24]. The amount of gemcitabine used in the present study was based on previous studies of gemcitabine in animal models, but also chosen to give low to moderate effect as monotherapy to enable the detection of any increased effect when the treatment was combined with irradiation. The dose of 60 mg/kg used for the mice in this study would correspond to about 180 mg/m2 for humans [44], which is considerably lower than the recommended 1000 mg/m2. Therefore, we believe that patients with MTC could benefit from the suggested combination therapy, while still being able to limit the dose of gemcitabine to keep side effects at an acceptable level.
The clinical experience of PRRT (e.g. 177Lu-octreotate or 90Y-octreotide) for MTC is relatively limited but larger than that of gemcitabine for MTC [12, 45–49]. Treatment outcomes differ between studies, but generally, response rates were around 40–70% with an estimated prolonged median survival of about 1–2 years together with very few side effects. It should be mentioned that these studies evaluating PRRT for MTC included relatively few patients. For other more common types of NET, PRRT has been widely used for over a decade and in studies including a much larger number of patients, PRRT has proven to be an efficient and safe treatment associated with few side effects [50–52]. However, few patients are cured with the current standard treatment protocol. An increased amount of administered activity should result in better treatment results but probably also a higher incidence of side effects. In recent years, 177Lu-octreotate has become more commonly used than 90Y-octreotide and in 2017 and 2018, respectively, the European Medicines Agency (EMA) and the FDA, approved the use of 177Lu-octreotate for the treatment of gastroenteropancreatic NETs.
Conclusions
The results from this study showed additive, or even synergistic, therapeutic effects in MTC-bearing nude mice after radiation and gemcitabine treatment. The results indicate that it can be possible to achieve similar combinatorial effects also in patients with MTC. Future studies should be performed to evaluate the full potential of using 177Lu-octreotate for treatment of highly SSTR-expressing MTCs, both as monotherapy and in combination with gemcitabine.
Supporting information
GOT2-carrying nude mice were treated with (A) 10 MBq 177Lu-octreotate (Lu) and/or 125 mg/kg gemcitabine twice weekly (Gem high), and (B) 5 Gy external beam radiotherapy (EBRT) and/or 60 mg/kg gemcitabine twice weekly (Gem low). Error bars show SEM.
(TIF)
Acknowledgments
The authors thank Gülay Altiparmak and Ulric Pedersen for their expert technical assistance with the IHC staining and image processing.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This study was supported by grants from the Swedish Research Council (grant no. 21073), the Swedish Cancer Society (grant no. 3427), BioCARE – a National Strategic Research Program at the University of Gothenburg, the Swedish state under the agreement between the Swedish government and the county councils – the ALF-agreement (ALFGBG-725031), and the King Gustav V Jubilee Clinic Cancer Research Foundation to EFA, as well as grants from the Sahlgrenska University Hospital Research Funds, the Assar Gabrielsson Cancer Research Foundation, the Adlerbertska Research Foundation, the Herbert & Karin Jacobsson Foundation, the Royal Society of Arts and Sciences in Gothenburg (KVVS), and the Wilhelm and Martina Lundgren Research Foundation to VS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wells Jr SA, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma: the American Thyroid Association Guidelines Task Force on medullary thyroid carcinoma. Thyroid. 2015;25(6):567–610. 10.1089/thy.2014.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulligan LM, Kwok JB, Healey CS, Elsdon MJ, Eng C, Gardner E, et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature. 1993;363(6428):458 10.1038/363458a0 [DOI] [PubMed] [Google Scholar]
- 3.Hofstra RM, Landsvater RM, Ceccherini I, Stulp RP, Stelwagen T, Luo Y, et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature. 1994;367(6461):375 10.1038/367375a0 [DOI] [PubMed] [Google Scholar]
- 4.Marsh DJ, Learoyd DL, Andrew SD, Krishnan L, Pojer R, Richardson AL, et al. Somatic mutations in the RET proto‐oncogene in sporadic medullary thyroid carcinoma. Clinical Endocrinology. 1996;44(3):249–57. 10.1046/j.1365-2265.1996.681503.x [DOI] [PubMed] [Google Scholar]
- 5.Pelizzo M, Boschin I, Bernante P, Toniato A, Piotto A, Pagetta C, et al. Natural history, diagnosis, treatment and outcome of medullary thyroid cancer: 37 years experience on 157 patients. European Journal of Surgical Oncology (EJSO). 2007;33(4):493–7. 10.1016/j.ejso.2006.10.021 [DOI] [PubMed] [Google Scholar]
- 6.Machens M, Andreas, Hinze M, Raoul, Thomusch M, Oliver, Dralle M, Henning. Pattern of nodal metastasis for primary and reoperative thyroid cancer. World Journal of Surgery. 2002;26(1):22–8. 10.1007/s00268-001-0176-3 [DOI] [PubMed] [Google Scholar]
- 7.Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer. 2006;107(9):2134–42. 10.1002/cncr.22244 [DOI] [PubMed] [Google Scholar]
- 8.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli J, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. The Journal of Clinical Endocrinology & Metabolism. 2006;91(8):2892–9. [DOI] [PubMed] [Google Scholar]
- 9.Mato E, Matías-Guiu X, Chico A, Webb SM, Cabezas R, Berná L, et al. Somatostatin and Somatostatin Receptor Subtype Gene Expression in Medullary Thyroid Carcinoma 1. The Journal of Clinical Endocrinology & Metabolism. 1998;83(7):2417–20. [DOI] [PubMed] [Google Scholar]
- 10.Forssell-Aronsson E, Nilsson O, Benjegard SA, Kölby L, Bernhardt P, Mölne J, et al. (111)In-DTPA-D-Phe(1)-octreotide binding and somatostatin receptor subtypes in thyroid tumors. Journal of Nuclear Medicine. 2000;41(4):636 [PubMed] [Google Scholar]
- 11.Forssell-Aronsson E, Bernhardt P, Nilsson O, Tisell L-E, Wängberg B, Ahlman H. Biodistribution data from 100 patients iv injected with 111In-DTPA-D-Phe1-octreotide. Acta Oncologica. 2004;43(5):436–42. 10.1080/02841860410030670 [DOI] [PubMed] [Google Scholar]
- 12.Iten F, Müller B, Schindler C, Rochlitz C, Oertli D, Mäcke HR, et al. Response to [90Yttrium-DOTA]-TOC treatment is associated with long-term survival benefit in metastasized medullary thyroid cancer: a phase II clinical trial. Clinical Cancer Research. 2007;13(22):6696–702. [DOI] [PubMed] [Google Scholar]
- 13.Forssell-Aronsson E, Spetz J, Ahlman H. Radionuclide therapy via SSTR: future aspects from experimental animal studies. Neuroendocrinology. 2013;97(1):86–98. 10.1159/000336086 [DOI] [PubMed] [Google Scholar]
- 14.Hung WW, Wang CS, Tsai KB, Ou‐Yang F, Shin SJ, Hsiao PJ. Medullary thyroid carcinoma with poor differentiation and atypical radiographic pattern of metastasis. Pathology International. 2009;59(9):660–3. 10.1111/j.1440-1827.2009.02423.x [DOI] [PubMed] [Google Scholar]
- 15.Dadan J, Wołczyński S, Sawicki B, Chyczewski L, Azzadin A, Dziecioł J, et al. Preliminary evaluation of influence of gemcitabine (Gemzar) on proliferation and neuroendocrine activity of human TT cell line: immunocytochemical investigations. Folia Histochemica et Cytobiologica. 2001;39(2):187–8. [PubMed] [Google Scholar]
- 16.Pauwels B, Korst AE, Lardon F, Vermorken JB. Combined modality therapy of gemcitabine and radiation. The Oncologist. 2005;10(1):34–51. 10.1634/theoncologist.10-1-34 [DOI] [PubMed] [Google Scholar]
- 17.Wardman P. Chemical radiosensitizers for use in radiotherapy. Clinical Oncology. 2007;19(6):397–417. 10.1016/j.clon.2007.03.010 [DOI] [PubMed] [Google Scholar]
- 18.Shewach DS, Hahn TM, Chang E, Hertel LW, Lawrence TS. Metabolism of 2′, 2′-difluoro-2′-deoxycytidine and radiation sensitization of human colon carcinoma cells. Cancer Research. 1994;54(12):3218–23. [PubMed] [Google Scholar]
- 19.Lawrence TS, Chang EY, Hahn TM, Hertel LW, Shewach DS. Radiosensitization of pancreatic cancer cells by 2′, 2′-difluoro-2′-deoxycytidine. International Journal of Radiation Oncology • Biology • Physics. 1996;34(4):867–72. [DOI] [PubMed] [Google Scholar]
- 20.Mason KA, Milas L, Hunter NR, Elshaikh M, Buchmiller L, Kishi K, et al. Maximizing therapeutic gain with gemcitabine and fractionated radiation. International Journal of Radiation Oncology • Biology • Physics. 1999;44(5):1125–35. [DOI] [PubMed] [Google Scholar]
- 21.Milas L, Fujii T, Hunter N, Elshaikh M, Mason K, Plunkett W, et al. Enhancement of tumor radioresponse in vivo by gemcitabine. Cancer Research. 1999;59(1):107–14. [PubMed] [Google Scholar]
- 22.Pauwels B, Korst AE, Pattyn GG, Lambrechts HA, Van Bockstaele DR, Vermeulen K, et al. Cell cycle effect of gemcitabine and its role in the radiosensitizing mechanism in vitro. International Journal of Radiation Oncology • Biology • Physics. 2003;57(4):1075–83. [DOI] [PubMed] [Google Scholar]
- 23.Blackstock AW, Bernard SA, Richards F, Eagle KS, Case LD, Poole ME, et al. Phase I trial of twice-weekly gemcitabine and concurrent radiation in patients with advanced pancreatic cancer. Journal of Clinical Oncology. 1999;17(7):2208–. 10.1200/JCO.1999.17.7.2208 [DOI] [PubMed] [Google Scholar]
- 24.Eisbruch A, Shewach DS, Bradford CR, Littles JF, Teknos TN, Chepeha DB, et al. Radiation concurrent with gemcitabine for locally advanced head and neck cancer: a phase I trial and intracellular drug incorporation study. Journal of Clinical Oncology. 2001;19(3):792–9. 10.1200/JCO.2001.19.3.792 [DOI] [PubMed] [Google Scholar]
- 25.McGinn CJ, Zalupski MM, Shureiqi I, Robertson JM, Eckhauser FE, Smith DC, et al. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. Journal of Clinical Oncology. 2001;19(22):4202–8. 10.1200/JCO.2001.19.22.4202 [DOI] [PubMed] [Google Scholar]
- 26.Dueñas-González A, Zarbá JJ, Patel F, Alcedo JC, Beslija S, Casanova L, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. Journal of Clinical Oncology. 2011;29(13):1678–85. 10.1200/JCO.2009.25.9663 [DOI] [PubMed] [Google Scholar]
- 27.Loehrer PJ, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. Journal of Clinical Oncology. 2011;29(31):4105–12. 10.1200/JCO.2011.34.8904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johanson V, Ahlman H, Bernhardt P, Jansson S, Kölby L, Persson F, et al. A transplantable human medullary thyroid carcinoma as a model for RET tyrosine kinase-driven tumorigenesis. Endocrine-Related Cancer. 2007;14(2):433–44. 10.1677/ERC-06-0033 [DOI] [PubMed] [Google Scholar]
- 29.Bolch WE, Eckerman KF, Sgouros G, Thomas SR. MIRD pamphlet no. 21: a generalized schema for radiopharmaceutical dosimetry—standardization of nomenclature. Journal of Nuclear Medicine. 2009;50(3):477–84. 10.2967/jnumed.108.056036 [DOI] [PubMed] [Google Scholar]
- 30.Miller WH, Hartmann-Siantar C, Fisher D, Descalle M-A, Daly T, Lehmann J, et al. Evaluation of beta-absorbed fractions in a mouse model for 90Y, 188Re, 166Ho, 149Pm, 64Cu, and 177Lu radionuclides. Cancer Biotherapy & Radiopharmaceuticals. 2005;20(4):436–49. [DOI] [PubMed] [Google Scholar]
- 31.Eckerman K, Endo A. ICRP Publication 107. Nuclear decay data for dosimetric calculations. Annals of the ICRP. 2008;38(3):7–96. 10.1016/j.icrp.2008.10.004 [DOI] [PubMed] [Google Scholar]
- 32.Dalmo J, Rudqvist N, Spetz J, Laverman P, Nilsson O, Ahlman H, et al. Biodistribution of 177Lu-octreotate and 111In-minigastrin in female nude mice transplanted with human medullary thyroid carcinoma GOT2. Oncology Reports. 2012;27(1):174–81. 10.3892/or.2011.1494 [DOI] [PubMed] [Google Scholar]
- 33.Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979:65–70. [Google Scholar]
- 34.Bliss C. The toxicity of poisons applied jointly. Annals of Applied Biology. 1939;26(3):585–615. [Google Scholar]
- 35.Yeh PJ, Hegreness MJ, Aiden AP, Kishony R. Drug interactions and the evolution of antibiotic resistance. Nature Reviews Microbiology. 2009;7(6):460–6. 10.1038/nrmicro2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dalmo J, Spetz J, Montelius M, Langen B, Arvidsson Y, Johansson H, et al. Priming increases the anti-tumor effect and therapeutic window of 177Lu-octreotate in nude mice bearing human small intestine neuroendocrine tumor GOT1. EJNMMI Research. 2017;7(1):6 10.1186/s13550-016-0247-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montelius M, Spetz J, Jalnefjord O, Berger E, Nilsson O, Ljungberg M, et al. Identification of potential MR-derived biomarkers for tumor tissue response to 177Lu-octreotate therapy in an animal model of small intestine neuroendocrine tumor. Translational Oncology. 2018;11(2):193–204. 10.1016/j.tranon.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greco WR, Faessel H, Levasseur L. The search for cytotoxic synergy between anticancer agents: a case of Dorothy and the ruby slippers? Journal of the National Cancer Institute. 1996;88(11):699–700. 10.1093/jnci/88.11.699 [DOI] [PubMed] [Google Scholar]
- 39.Palmer AC, Sorger PK. Combination cancer therapy can confer benefit via patient-to-patient variability without drug additivity or synergy. Cell. 2017;171(7):1678–91. 10.1016/j.cell.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fueger BJ, Hamilton G, Raderer M, Pangerl T, Traub T, Angelberger P, et al. Effects of chemotherapeutic agents on expression of somatostatin receptors in pancreatic tumor cells. Journal of Nuclear Medicine. 2001;42(12):1856–62. [PubMed] [Google Scholar]
- 41.Nayak TK, Atcher RW, Prossnitz ER, Norenberg JP. Enhancement of somatostatin-receptor-targeted 177 Lu-[DOTA 0-Tyr 3]-octreotide therapy by gemcitabine pretreatment-mediated receptor uptake, up-regulation and cell cycle modulation. Nuclear Medicine and Biology. 2008;35(6):673–8. 10.1016/j.nucmedbio.2008.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matuszczyk A, Petersenn S, Voigt W, Kegel T, Dralle H, Schmoll H, et al. Chemotherapy with paclitaxel and gemcitabine in progressive medullary and thyroid carcinoma of the follicular epithelium. Hormone and Metabolic Research. 2010;42(01):61–4. [DOI] [PubMed] [Google Scholar]
- 43.Full Prescribing Information for Gemcitabine Hydrochloride National Cancer Institute2018 [13 Mar 2018]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020509s077lbl.pdf.
- 44.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. Journal of Basic and Clinical Pharmacy. 2016;7(2):27 10.4103/0976-0105.177703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waldherr C, Schumacher T, Pless M, Crazzolara A, Maecke H, Nitzsche E, et al. Radiopeptide transmitted internal irradiation of non-iodophil thyroid cancer and conventionally untreatable medullary thyroid cancer using [90Y]-DOTA-D-Phe1-Tyr3-octreotide: a pilot study. Nuclear Medicine Communications. 2001;22(6):673–8. 10.1097/00006231-200106000-00011 [DOI] [PubMed] [Google Scholar]
- 46.Bodei L, Handkiewicz-Junak D, Grana C, Mazzetta C, Rocca P, Bartolomei M, et al. Receptor radionuclide therapy with 90Y-DOTATOC in patients with medullary thyroid carcinomas. Cancer Biotherapy and Radiopharmaceuticals. 2004;19(1):65–71. 10.1089/108497804773391694 [DOI] [PubMed] [Google Scholar]
- 47.Makis W, McCann K, McEwan AJ. Medullary thyroid carcinoma (MTC) treated with 177Lu-DOTATATE PRRT: a report of two cases. Clinical Nuclear Medicine. 2015;40(5):408–12. 10.1097/RLU.0000000000000706 [DOI] [PubMed] [Google Scholar]
- 48.Vaisman F, de Castro PHR, Lopes FPPL, Kendler DB, Pessoa CH, Bulzico DA, et al. Is there a role for peptide receptor radionuclide therapy in medullary thyroid cancer? Clinical Nuclear Medicine. 2015;40(2):123–7. 10.1097/RLU.0000000000000628 [DOI] [PubMed] [Google Scholar]
- 49.Salavati A, Puranik A, Kulkarni HR, Budiawan H, Baum RP. Peptide receptor radionuclide therapy (PRRT) of medullary and nonmedullary thyroid cancer using radiolabeled somatostatin analogues. Seminars in Nuclear Medicine. 2016;46(3):215–24. 10.1053/j.semnuclmed.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 50.Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. European Journal of Nuclear Medicine and Molecular Imaging. 2015;42(1):5–19. 10.1007/s00259-014-2893-5 [DOI] [PubMed] [Google Scholar]
- 51.Brabander T, Van der Zwan WA, Teunissen JJ, Kam BL, Feelders RA, de Herder WW, et al. Long-term efficacy, survival and safety of [177Lu-DOTA0, Tyr3] octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clinical Cancer Research. 2017;23(16):4617–24. 10.1158/1078-0432.CCR-16-2743 [DOI] [PubMed] [Google Scholar]
- 52.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. New England Journal of Medicine. 2017;376(2):125–35. 10.1056/NEJMoa1607427 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GOT2-carrying nude mice were treated with (A) 10 MBq 177Lu-octreotate (Lu) and/or 125 mg/kg gemcitabine twice weekly (Gem high), and (B) 5 Gy external beam radiotherapy (EBRT) and/or 60 mg/kg gemcitabine twice weekly (Gem low). Error bars show SEM.
(TIF)
Data Availability Statement
All relevant data are within the manuscript.