There is ample evidence that inflammatory processes and signaling play a critical role in the progression of most cancers (1), especially as potent initiators at sites of chronic injury (2). In these chronic inflammation–associated cancers (CIACs), tissue injury is caused by sustained or recurrent infection, irritation, or trauma. This distinct class of malignancies, which includes acid reflux–associated esophageal adenocarcinoma (EAC), smoking-associated lung squamous cell carcinoma, Helicobacter pylori infection–associated gastric adenocarcinoma, and inflammatory bowel disease–associated colon adenocarcinoma, is responsible for 20 to 25% of all cancer deaths worldwide, killing more than 2 million people annually. Compared with other tumor types, CIACs tend to have a poorer prognosis, attributable to a high propensity for metastasis and drug resistance. The underlying mechanisms by which chronic inflammation promotes the development of such lethal cancers are still poorly understood.
EAC is a typical example of a CIAC. The clinical course of this disease progresses through stages characterized by increased alterations of the tissue landscape: metaplasia, dysplasia, and carcinoma. These stages are shared by other CIACs and may suggest a common biological mechanism linking chronic inflammation to carcinogenesis (3) (see the figure). In 10 to 15% of patients with repeated acid reflux, the persistent injury and associated chronic inflammation leads to the activation of an adaptive metaplastic response, defined as the replacement of one tissue type by a different tissue type not normally present at that location. Metaplasia in the injured esophagus, called Barrett esophagus, is characterized by the replacement of the native squamous esophageal epithelium, most often with columnar intestinal and gastric epithelium. Barrett esophagus is not inherently premalignant, as the resultant tissue is well suited to protecting the esophagus from acid exposure owing to the presence of intestinal goblet cells, which naturally elaborate acid-neutralizing mucus. However, metaplasia is associated with a higher frequency of dysplasia, which in turn occasionally develops into malignancy, suggesting that this process is error prone.
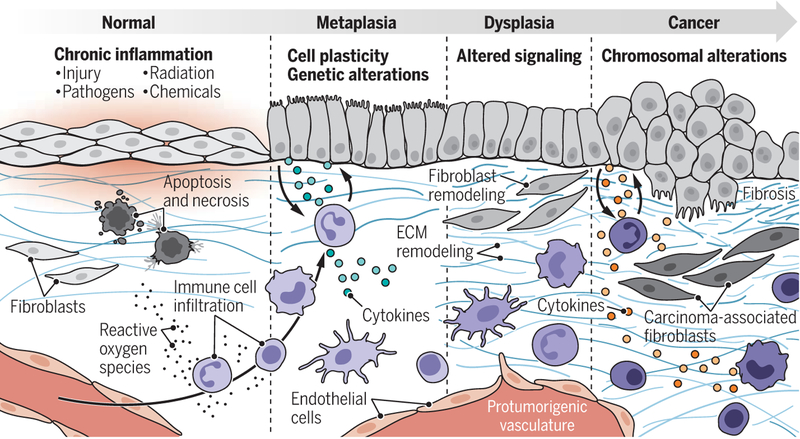
Chronic inflammation–associated cancer pathogenesis.
The pathogenic evolution of chronic inflammation–associated cancers (CIACs) involves metaplasia, dysplasia, and then cancer. During this process, multiple changes take place within the tissue microenvironment, including changes in stromal cells (immune cells, endothelial cells, and fibroblasts) and the extracellular matrix (ECM).
The progression of Barrett esophagus to EAC is rare (1 in 1000 Barrett esophagus cases). However, epidemiological, physiological, molecular, and histological lines of evidence strongly support the origin of most EACs from the cellular field of Barrett esophagus. Patients diagnosed with Barrett esophagus have a 40-fold increased lifetime risk of developing EAC and a 0.1 to 0.3% risk of progression to EAC per year. Critically, the 5-year survival rate for patients with EAC is 15%. Unlike symptomatic patients, many patients with acid reflux and associated Barrett esophagus are asymptomatic and are therefore not followed clinically through endoscopic surveillance to detect EAC. Indeed, 85% of patients with EAC first present in the clinic with late-stage, metastatic, unresectable, and highly drug-resistant cancers with limited treatment options. Identifying the rare subset of Barrett esophagus patients who will progress to EAC is an unmet clinical challenge, as is the search for effective therapeutics to treat patients at various stages of EAC progression. To improve the diagnosis and treatment of these diseases, the following question must be answered: How does chronic injury and the ensuing chronic inflammation lead to tumorigenesis?
Inflammation is not fundamentally a pathogenic process. The inflammatory response is an essential component of the host immune reaction to microbial infection and tissue injury. Inflammation is usually self-limiting; however, the inflammatory response is poorly adapted to resolving persistent injurious stimuli. When injury persists, cycles of tissue destruction perpetuate the inflammatory response and establish a chronic inflammatory state associated with an unresolved DNA damage response, secretion of proinflammatory cytokines, and reactive oxygen species (ROS). Consequently, the prevailing paradigm for conceptualizing the relationship between chronic inflammation and cancer initiation focuses on an increase in the epithelial mutation rate and genomic instability in the perturbed environment, as well as the activation of oncogenic signaling pathways by inflammatory mediators. However, as with Barrett esophagus, an intriguing feature of many CIACs that is not often observed in non-CIACs is the presence of a metaplastic reaction at the site of injury. Increased cellular plasticity and the reexpression of pathways associated with embryogenesis and fetal development (4, 5) have been observed in the context of the metaplastic reaction and other adaptive responses to injury. Could such distinct alterations play a role in the development of aggressive tumor phenotypes associated with CIACs?
Cellular plasticity can be enhanced and potentially stabilized by nonmutational events in the chronic inflammatory microenvironment. For instance, the cytokine milieu generated by chronic inflammation promotes epigenetic changes within epithelial cells that are often associated with cell-fate plasticity. Chronic inflammation was shown to drive the epigenetic silencing of the gene encoding the tumor suppressor p16 in a chronic inflammation–associated lung cancer mouse model (6). Previous work has demonstrated that repression of p16 expression not only relaxes cell cycle checkpoint response, which is important for maintaining genomic stability, but also increases the expression of chromatin remodeling proteins and, consequently, activates epigenetic plasticity (4). Loss of p16 activity also reduces control on centrosome number (which is important for accurate cell division) and activates a transcriptional stress response supporting multiple malignant traits (7). Thus, cells in which p16 activity is silenced through DNA hypermethylation acquire a multitude of phenotypes that poise the cells for cell fate plasticity as well as malignant potential. Epigenetic silencing of p16 expression is common at the metaplastic stage in a substantial subset of CIACs, including injury-associated squamous cell carcinoma of the lung, H. pylori infection–associated gastric cancer, chronic pancreatitis–associated pancreatic cancer, and acid reflux–associated EAC.
It is postulated that, in addition to promoting cellular plasticity, chronic inflammation also disrupts homeostatic epithelial-mesenchymal interactions and alters contextual cues that direct cellular communities to adopt new compositional states, which can be either protective (metaplasia) or potentially pathogenic (malignancy) (8, 9). Developmental biology has taught us that tissue organization and cell identity are coordinated by epithelial-mesenchymal interactions, which include soluble factors, cell-cell interactions, cell shape, cell–extracellular matrix (ECM) interactions, as well as mechanical and physical forces (8, 9).
Recent work demonstrates that epithelial-mesenchymal interactions continue to dictate tissue structure and function even in adult organisms. For example, implantation of neural stem cells or human embryonic stem cells into mouse mammary fat pads reprograms the cells into milk-producing mammary epithelium (10). Additionally, in both the developmental and adult contexts, much of the instructive information for cellular identity is contained within the ECM, a network of proteins that constitutes the non-cellular component of the stroma. This is evidenced by experiments demonstrating that decellularized ECM can recapitulate many of the instructive features of mesenchyme in guiding cell fate and state (9). For example, decellularized ECM from rat mammary glands in either virgin or lactating states is sufficient to reprogram mammary epithelial cells to recapitulate the physiological state of the tissue from which the ECM was derived (11). These studies inform us that the stroma, and the ECM in particular, is dominant and dynamic in dictating development and cellular differentiation. The composition and structure of the ECM are also therefore likely critical to cellular decisions between a metaplastic or a malignant path.
The properties of the ECM are determined by complex interactions between fibroblasts and other mesenchymal cells, as well as immune and epithelial cells. As in cancer, the ECM is extensively remodeled during chronic inflammation, which includes the increased deposition of the ECM components collagen, periostin, and fibronectin; liberation of bio-active ECM fragments; and decreased deposition of decorin, matrilin, and nidogens 1 and 2 (9). Notably, the increased expression of ECM proteins and their posttranslational cross-linking increase tissue stiffness and alter mechanotransduction in inflamed tissue (12). Thus, epithelial cells activated to a plastic state and bereft of appropriate contextual cues may be at the highest risk of following a malignant cell state trajectory.
The idea that signaling from the stroma, as well as the instructive information contained within the ECM, may underlie tissue pathologies, including the development of malignancy, is not new (8, 9). This concept is supported by many experimental studies. For example, the ectopic expression of matrix metalloproteinase 3 (MMP3) results in ECM reorganization and is sufficient to induce malignant transformation of the epithelium, suggesting that epithelial cells lacking normative mesenchymal cues are more likely to adopt a malignant fate (13). Moreover, allowing carcinoma-associated fibroblasts to exchange signals with nontumorigenic epithelial cells redirects the epithelial cells to malignancy (14).
Previous studies have shown that normalizing cues from an embryonic milieu or a healthy stromal environment can force aggressive cancer cells to lose tumorigenic properties and exhibit benign and differentiated characteristics. For example, aggressive teratocarcinoma cells introduced into the environment of a blastocyst (a stage of embryonic development) contribute to functional tissues in newborn mice (9). Likewise, malignant breast cancer cells placed on embryonic mesenchymes, or decellularized ECM proteins from these mesenchymes, lose malignant properties and acquire more-differentiated phenotypes (15). Therefore, just as there are mesenchymal signals that can induce and maintain malignant epithelial states, there are also mesenchymal signals that can induce and maintain epithelial differentiation states.
Understanding the factors influencing the fidelity of the metaplastic response is critical to identifying the mechanisms underlying the genesis of CIACs and addressing their clinical consequences. Identifying common contextual elements (such as ECM proteins) favoring the maintenance of an adaptive protective metaplastic state that can no longer progress to malignancy may support the development of different approaches for early diagnosis, risk stratification, prevention, and therapeutic intervention to decrease the clinical burden of CIACs. This potential new class of therapy for epithelial cancers would restore homeostatic tissue interactions that normally prevent cancers from forming or advancing but that are often lost in the context of chronic inflammation. The severity and duration of chronic inflammation largely dictates the risk of future tumor formation, providing a considerable window for monitoring and intervention. A strategy combining current therapies that target cell intrinsic oncogenic pathways with newly developed therapies aimed at restoring tissue homeostasis could be a viable complement to overcome failures. These failures may result from focusing on therapeutic killing of mutant cancer cells without integrating the restoration of microenvironmental interactions that naturally participate in repressing malignant phenotypes.
ACKNOWLEDGMENTS
T.D.T. and P.G. thank J. Caruso and D. Pan for extensive discussions, the Cancer Research UK (CRUK) Grand Challenge STORMing Cancer team for critical remarks, and S. Huang for help with the figure. T.D.T. acknowledges support from National Cancer Institute grant R35 CA197694 and funding of the STORMing Cancer team by a CRUK Grand Challenge grant.
REFERENCES AND NOTES
- 1.Taniguchi K, Karin M, Nat. Rev. Immunol 18, 309 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A et al. , Nature 454, 436 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Barbera M, Fitzgerald RC, Surg. Oncol. Clin. N. Am 18, 393 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Li H et al. , Nature 460, 1136 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willet SG et al. , EMBO J. 37, e98311 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco D et al. , Neoplasia 9, 840 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berman H et al. , Cold Spring Harb. Symp. Quant. Biol 70, 317 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Ingber DE, Differentiation 70, 547 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Nelson CM, Bissell MJ, Annu. Rev. Cell Dev. Biol 22, 287 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth BW et al. , Proc. Natl. Acad. Sci. U.S.A. 105, 14891 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schedin P et al. , Mol. Carcinog 41, 207 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Northcott JM et al. , Front. Cell Dev. Biol 6, 17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sternlicht MD et al. , Cell 98, 137 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olumi AF et al. , Cancer Res. 59, 5002 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bischof AG et al. , Integr. Biol 5, 1045 (2013). [DOI] [PubMed] [Google Scholar]