LA was added to GA for BM procedures in patients with neuroblastoma to compare pain, opioid usage, and QoL.
Abstract
BACKGROUND:
Pediatric patients with cancer undergo repeated painful procedures, including bone marrow aspirations and biopsies (BMABs). Optimal management of procedure-related pain can reduce discomfort, anxiety, and distress.
METHODS:
Children with neuroblastoma were randomly assigned to 1 of 2 arms on a prospective, single-blind, crossover trial conducted at Memorial Sloan Kettering Cancer Center from October 2016 to January 2018 (www.clinicaltrials.gov, identifier NCT02924324). Participants underwent 2 sequential BMABs: one with general anesthesia (GA) alone, the other with GA plus local anesthesia (LA) (GA + LA). The objective was to assess procedure-related pain and its interference with quality of life (QoL) with GA versus GA + LA. Primary outcome was percentage of participants requiring postprocedural opioids. Secondary outcomes were total opioid and nonopioid analgesics, pain scores, time to first analgesic, QoL, and toxicity. Management of postprocedural pain was standardized.
RESULTS:
Of 56 participants randomly assigned (3–16.5 years old), 46 completed both procedures. There was no significant difference in percentage of participants requiring opioids with GA versus GA + LA (24% vs 20%, P = .5). Pain scores in the recovery room were significantly lower for GA + LA versus GA (median [IQR]: 0 [0–2] vs 2 [0–4], P = .002). There were no statistically significant differences in total opioid or nonopioid analgesic, 6- and 24-hour pain scores, median time to first analgesic, or pain interference. No adverse events occurred.
CONCLUSIONS:
LA was associated with significant improvement in pain scores in the immediate recovery period. LA did not reduce postprocedural opioid use, nor did it improve QoL for patients undergoing BMAB with GA.
What’s Known on This Subject:
Children with cancer undergo repeated painful procedures, including bone marrow aspirations and biopsies. No standardized clinical practice guidelines exist for procedure-related pain management. Nearly all centers provide general anesthesia, and many centers use local anesthesia for the procedure.
What This Study Adds:
With this trial, we provide new perspective on the burden of bone marrow procedures with general anesthesia. Many children reported minimal postprocedural pain or opioid usage. Local anesthesia was safely added with improvement in multidimensional pain outcomes for some patients.
Children with cancer undergo bone marrow aspirations and biopsies (BMABs) at regular intervals for diagnostic evaluation and disease surveillance. Patients have reported that BMABs are painful.1–3 Health care professionals and parents also acknowledge that these procedures are painful to children4 and a frequent cause of distress.5 Repeated exposure to painful procedures during childhood not only exacerbates anxiety and distress but may have long-term consequences.6,7 Strategies to reduce pain and anxiety are integral to the care of pediatric patients with cancer.8
Pediatric oncology departments have variable institutional practices to reduce bone marrow (BM) procedure–related pain and anxiety; however, there are no universally accepted practice guidelines. We surveyed 7 major pediatric oncology centers, and all reported routine use of general anesthesia (GA) combined with either systemic analgesia or local anesthesia (LA) for BM procedures. The routine practice at our institution, Memorial Sloan Kettering Cancer Center (MSK), is to provide GA with propofol and no LA. Propofol has amnestic properties but lacks analgesic properties.9 In contrast, LA limits the initiation of nociceptive pathways before traumatic insult occurs, allowing for its analgesic effect.
To better understand BMAB-related pain for pediatric patients at MSK, we conducted an institutional review board–exempt needs assessment of 26 patients with any oncologic diagnosis who underwent BMAB. We asked for patient-reported pain scores on the Wong-Baker FACES Pain Rating Scale10 (WBFPRS), analgesics consumed, and whether postprocedure activity varied from their baseline. Eight patients (31%) required opioids for analgesia during the procedure or within 24 hours. Many children reported persistent pain on the WBFPRS (46%, n = 12) and reduced activity 24 hours postprocedure (31%, n = 8). On the basis of these findings, we identified the need to improve the experience of our pediatric patients undergoing BMAB.
The aim of this trial was to investigate whether subcutaneous and periosteal infiltration of LA would reduce the percentage of patients with neuroblastoma requiring postprocedural opioids and improve short-term quality of life (QoL). We hypothesized that the addition of LA would alleviate procedure-related pain and improve QoL.
Methods
Trial Design
This was a prospective, single-blind, crossover, randomized clinical trial (www.clinicaltrials.gov, identifier NCT02924324). Patients who were previously scheduled for upcoming BMAB were recruited during routine outpatient visits and included on study for 2 BMABs. Eligible participants were randomly assigned and allocated by a computer-based algorithm to 1 of 2 arms representing a specific sequence of interventions. Arm AB included propofol (GA) for the first procedure and propofol plus ropivacaine (GA + LA) for the second. Arm BA included GA + LA for the first procedure followed by GA for the second. For this crossover study, we postulated that a participant’s response for the second procedure would not be affected by the intervention from the first. We could confidently assume this absence of a period effect given an estimated 2-month interval between procedures, which significantly exceeds the duration of action (DOA) of GA and GA + LA.
Written informed consent for this MSK Institutional Review Board–approved trial was obtained from parents of all participants; participants ≥7 years old provided assent.
Eligibility
Inclusion criteria included the following: patient age 3 to 18 years, neuroblastoma diagnosis, English-speaking, and ≥1 previous BMAB to avoid confounding pain from newly diagnosed disease. Exclusion criteria included the following: allergy to ropivacaine, other amides, or propofol; a chronic daily opioid requirement; low performance status (Lansky/Karnofsky score <60); unwillingness to forego peri-procedural opioids; and concurrent painful procedures.
Interventions
Ropivacaine is an amide LA similar to lidocaine; its lower lipid solubility provides a better safety profile with less cardiovascular and central nervous system toxicity.11 Its fast onset of action within 3 to 15 minutes after local infiltration12 and its DOA of 3 to 15 hours12 make ropivacaine a reasonable choice for a short procedure with a limited recovery period. GA was provided by an anesthesiologist in a procedure room. After induction of GA, LA was provided by 1 of 4 pediatric oncologists previously trained by a pediatric anesthesiologist (R.S.D.) on subcutaneous and periosteal infiltration of ropivacaine to ensure technique standardization. Ropivacaine 0.5% was prescribed by using weight-based dosing of 2 mg/kg (0.5 mg/kg per site at each of 4 sites: bilateral posterior and anterior iliac crests) with a maximum dose of 25 mg per site. All procedures consisted of bone marrow aspirations (BMAs) at 4 sites and bone marrow biopsies (BMBs) at either 2 posterior or all 4 sites (4 + 2 or 4 + 4).
After the procedure, participants were monitored during emergence by nurses in a recovery room (RR). Once fully awake, participants were asked to report their pain by severity by using a modified WBFPRS (0 = no pain to 10 = most pain, intermediate options 2, 4, 6, 8). Advantages of this validated subjective pain scale include its reliability over time, ease of administration, preference by children, and routine use at MSK.13,14 Pain was managed according to a postprocedure pain management algorithm (Supplemental Fig 4), based on the World Health Organization analgesic ladder. Interventions were based on pain severity with supportive measures of heat packs and nonopioid analgesics for mild pain and escalating to opioids for moderate to severe pain, defined as pain >4 on the WBFPRS. Parents followed a similar algorithm at home (Supplemental Fig 5).
Multidimensional outcome domains for pediatric pain clinical trials are defined in consensus guidelines.13,15 Our trial incorporated these domains by using a QoL metric adapted from the National Institutes of Health (NIH) Patient-Reported Outcomes Measurement Information System (PROMIS) Parent-Proxy Pain Interference Scale.16,17 The full item bank is validated for use by parents of 5 to 17 year olds.16,17 We extrapolated its use and selected 4 of 13 items to assess how procedural pain affects physical recovery (activity and sleep) and social and emotional functioning (Supplemental Fig 6). Scores of 4 (agree) and 5 (strongly agree) were used in comparative analysis to determine major interference of pain on these domains. This adapted metric was approved for use by the MSK Behavioral Research Methods Core.
Parents recorded pain scores and interventions at 6 and 24 hours postprocedure. If the participant reported a pain score of ≥2, parents were asked to complete the QoL metric at that time to assess pain interference. A study investigator collected data within 72 hours postprocedure.
Outcome Measures
Primary outcome was the percentage of participants who required postprocedural opioids with GA + LA versus GA. The aim was to determine if LA achieved opioid sparing. Opioid sparing has been identified as a practical and feasible surrogate primary end point in pediatric pain trials.18,19
Time to first opioid, total opioid consumption, and relative differences in pain scores are also commonly studied outcomes13,18,19 and were selected as secondary outcomes. Time to first nonopioid analgesic and total nonopioid analgesic were assessed. An additional secondary outcome was QoL, using the previously described metric.
Sample Size
On the basis of our needs assessment, we hypothesized that 30% of participants would require opioid analgesics with GA alone. A sample size of 45 participants (providing outcomes from 90 procedures) allowed 80% power to detect an absolute difference of 20% in proportions between GA and GA + LA (with a type I error rate of 0.05). Target enrollment of 50 participants allowed for an anticipated drop-out rate of 10%. We assumed a conservative correlation of 0.4 between the 2 outcomes (measured from 2 procedures) from the same participant. This power calculation was generated by using the PROC POWER Procedure in SAS 9.4 (SAS Institute, Inc, Cary, NC), specifying paired proportions and correlation for McNemar’s exact conditional testing. This sample size allowed for an interim assessment for futility at the halfway point (23 of 45 participants with both procedures completed). Interim analysis results were reviewed by the MSK Data Safety Monitoring Board, and the trial was permitted to proceed.
Blinding
Study participants, their parents, RR nurses, and study investigators involved in data collection were blinded to the randomization and allocation. At the time of each procedure, the attending oncologist administering LA, procedure room anesthesiologist, and pharmacy staff were unblinded.
Statistical Methods
Analyses were conducted at the procedure level while addressing the correlation between outcomes from 2 procedures contributed by the same participant arising from this crossover trial. The primary outcome of proportion requiring postprocedural opioids was evaluated with a generalized estimating equations (GEE) model for binary outcomes with logit link function. Fixed effects in the model included intervention (GA or GA + LA) and procedure order (first or second). Outcomes were clustered by participant with an exchangeable correlation structure for intraparticipant correlation. The GEE models appropriately account for correlation among outcomes from the same participants and allow for the inclusion of participants who only contributed 1 of 2 planned procedures. The secondary outcome of need for nonopioid analgesics within 24 hours was analyzed similarly. Continuous outcomes such as pain scores were analyzed similarly with GEE for continuous outcomes (gaussian link function). Because of nonconvergence, total opioid consumption within 24 hours among those who required opioids was compared between interventions by Wilcoxon rank-sum test. Total consumption among those who required nonopioid analgesics was analyzed similarly. Individual items on the NIH-PROMIS metric were defined as major interference if the item score was a 4 or 5. The proportions with major interference were compared between interventions by using Fisher’s exact test. These analyses were considered exploratory because they are of secondary interest, and we do not have previous data to estimate statistical power.
Results
Participant Flow
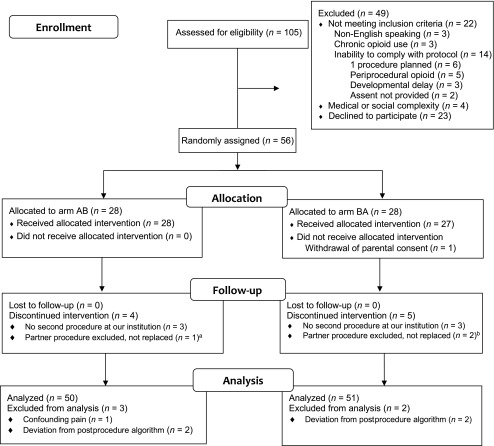
Between October 2016 and January 2018, 105 patients were assessed for eligibility (Fig 1). Fifty-six participants were randomly assigned (accrual rate of 53.3%) and allocated to Arm AB (n = 28) or BA (n = 28, one withdrew consent before the first procedure). Forty-six participants completed both interventions with 2 evaluable procedures (92 total); 9 participants had data from one evaluable procedure for analysis. Final analysis using an intent-to-treat approach for primary outcome was performed on 101 evaluable procedures: 50 individual procedures (GA) and 51 individual procedures (GA + LA). The trial was stopped once accrual goals were met. Follow-up was completed in January 2018. In Table 1, we show participant demographics and clinical characteristics at study enrollment.
FIGURE 1.
CONSORT diagram of participant flow. a Disease progression and painful procedures prior to BMAB. b Deviation from postprocedure algorithm, replacement procedure not scheduled before study closure (n = 1); patient death before replacement procedure (n = 1). CONSORT, consolidated standards of reporting trials.
TABLE 1.
Baseline Participant Demographics and Clinical Characteristics
| Arm AB | Arm BA | Total | |
|---|---|---|---|
| (n = 28) | (n = 27) | ||
| Sex, n (%) | |||
| Male | 15 (54) | 19 (70) | |
| Female | 13 (46) | 8 (30) | |
| Race, n | 42 | ||
| White | 4 | ||
| African American | 4 | ||
| Asian American | 1 | ||
| American Indian or Alaskan Native | |||
| Not reported | 3 | ||
| Unknown | |||
| Ethnicity, n | 2 | ||
| Non-Hispanic | 50 | ||
| Hispanic or Latino | 1 | ||
| Unknown | 5 | ||
| Age at enrollment in y, median (IQR) | 7.3 (5.5–9.9) | 5.8 (4.1–8.8) | |
| Lansky score at enrollment, n (%) | |||
| 80 | 1 (3.6) | 0 (0) | |
| 90 | 3 (11) | 4 (15) | |
| 100 | 24 (86) | 23 (85) | |
| No. BMA per procedure, n (%) | |||
| 4 | 28 (100) | 27 (100) | |
| No. BMB per procedure, n (%) | |||
| 2 | 21 (75) | 23 (85) | |
| 4 | 7 (25) | 4 (15) | |
| No. procedures completed, n | |||
| 1 procedure | 4 | 5 | |
| 2 procedures | 24 | 22 | |
| No. evaluable procedures, n | 52 | 49 |
Primary Outcome Analysis
In total, only 22 of 101 procedures (22%) required postprocedural opioids within 24 hours (Table 2). There was no statistically significant difference in the proportion of participants who required postprocedural opioids with GA versus GA + LA (24% vs 20%; odds ratio: 0.79; 95% confidence interval: 0.37–1.69; P = .5).
TABLE 2.
Characterization of Postprocedure Analgesic Consumption
| Total No. Procedures (n = 101) | Pa | ||
|---|---|---|---|
| GA, n = 50 | GA + LA, n = 51 | ||
| Primary outcome, n (%) | |||
| Require postprocedural opioid | 12 (24) | 10 (20) | .5 |
| Secondary outcomes: opioid analgesia | n = 12 | n = 10 | |
| Total opioid in mg mEqIV/kg/d, median (IQR) | 0.2 (0.2–0.5) | 0.2 (0.1–0.3) | .6 |
| Time to first opioid in min, median (IQR) | 62.0 (36–423) | 223.0 (30–360) | .5 |
| Secondary outcomes: nonopioid analgesia | |||
| Require postprocedural nonopioid analgesic, n (%) | 17 (34) | 15 (29) | .5 |
| Time to first nonopioid analgesic, median (IQR) | 248.0 (48–525) | 360.0 (160–410) | .3 |
P values extracted from GEEs with participant-level clusters and intervention (GA versus GA + LA) and procedure number fixed effects.
Secondary Outcome Analysis
Opioid Analgesia
In the 22 procedures in which postprocedure opioids were used, no difference was noted in total opioids used in milligrams of morphine equivalents (mEqIVs) per kilograms per day between procedures with GA (median [IQR]: 0.2 mg mEqIV per kilogram per day [0.2–0.5]) versus GA + LA (0.2 mg mEqIV per kilogram per day [0.1–0.3]), P = .6 (Table 2). The median time to first opioid was shorter in procedures with GA (62 minutes [35.5–422.5]) than with GA + LA (223 minutes [30.0–360]), but this difference was not statistically significant, P = .5 (Table 2).
Nonopioid Analgesia
A slightly higher proportion of participants required nonopioid analgesia after procedures with GA (17 out of 50 = 34%) than with GA + LA (15 out of 51 = 29%); this difference was not statistically significant, P = .5. The median time to first nonopioid analgesia was shorter for procedures with GA (248 minutes [48–525]) than with GA + LA (360 minutes [130–410]); this difference was not statistically significant, P = .3 (Table 2).
Pain Scores on WBFPRS
Pain scores in the RR were significantly higher when participants had GA versus GA + LA (median [IQR]: 2 [0–4] vs 0 [0–2], P = .002). No significant differences were noted in pain scores between interventions at 6 and 24 hours postprocedure: (2 [0–4] vs 2 [0–4] and 0 [0–2] vs 0 [0–2], respectively) (Fig 2).
FIGURE 2.
Postprocedure pain scores with or without LA. Each diamond (♦) represents an individual pain score (GA [n = 50], GA + LA [n = 51]). Red lines represent median pain scores. Distribution of RR pain scores was significantly lower for GA + LA than GA (median [IQR]: 0 [0–2] vs 2 [0–4], P = .002). No difference was observed 6 or 24 hours postprocedure. a Indicates a statistically significant difference between pain score distributions. A, RR. B, 6 hours postprocedure. C, 24 hours postprocedure.
QoL
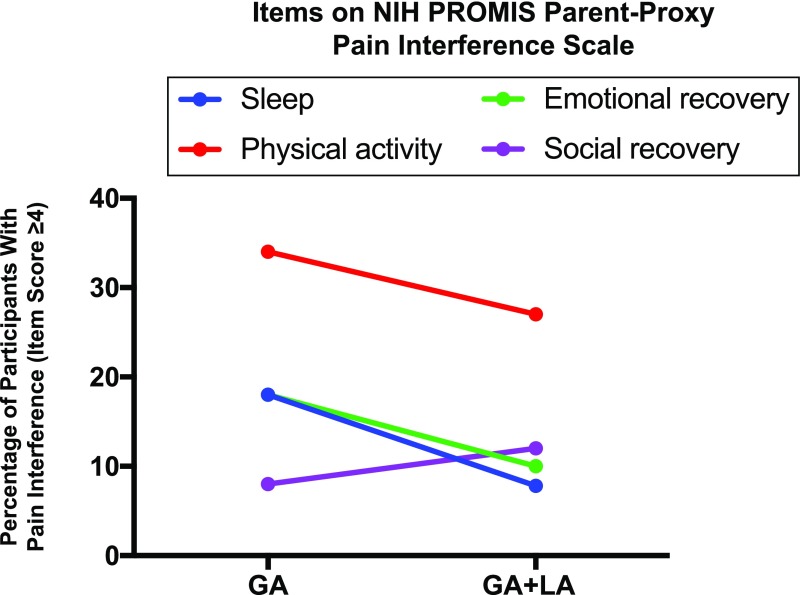
Comparative analysis of major interference did not demonstrate a statistically significant difference in pain interference with sleep, physical activity, or social or emotional recovery in procedures with GA + LA versus GA (Fig 3). Physical activity was most affected by pain regardless of intervention (GA = 34% and GA + LA = 27%, P = .5). For other QoL domains, major interference was reported in 7.8% to 18% of participants across both interventions.
FIGURE 3.
Pain interference on QoL within 24 hours of BMAB. Pain interference with sleep, physical activity (“run”), social recovery (“have fun”), and emotional recovery (“get along with others”) on a Likert scale (1 = strongly disagree [that pain interfered], 2 = disagree, 3 = neither agree nor disagree, 4 = agree, 5 = strongly agree). Major interference is a score of 4 or 5.
Unplanned Subset Analysis
We observed that older participants were more affected by BMAB. To better investigate differences in pain and opioid use by age, we performed an unplanned subset analysis of the 55 participants using 2 age groups: <8 years old (n = 31, 56%) and ≥8 years old (n = 24, 44%). Within each age group, participants were evenly distributed between arms AB and BA (younger: AB [n = 16], BA [n = 15]; older: AB [n = 12], BA [n = 12]). Although no statistically significant difference was noted between interventions for the primary outcome, a greater percentage of older participants required opioids across both interventions (GA ≥8: 38%; GA + LA ≥8: 32%) when compared with younger participants (GA <8: 14%; GA + LA <8: 10%).
Adverse Events
No adverse events occurred. Two participants reported numbness without pain or motor deficit in the anterolateral thigh after a BMAB with GA + LA. This on-target effect was attributed to a likely peripheral lateral femoral cutaneous nerve block during an anterior iliac crest BMAB. Numbness resolved within expected DOA of ropivacaine.
Discussion
Pediatric patients with cancer undergo frequent painful procedures that increase anxiety and distress and may negatively affect future responses to pain and behavior toward subsequent interventions.5,6,20 No universally accepted guidelines exist for management of BMAB-related pain. Several groups have studied various pharmacologic and nonpharmacologic interventions,21–26 and many centers routinely use LA with GA for BMAB, but no study has specifically investigated the efficacy of GA + LA. In this crossover trial, we assessed whether GA + LA could achieve opioid sparing and improve short-term QoL in children with neuroblastoma who endure repeated BMAB. We were unable to demonstrate significant opioid sparing or improvement in short-term QoL, but pain scores in the recovery period were significantly lower in the GA + LA versus GA group, demonstrating less pain immediately postprocedure.
There were no differences in pain scores at 6 and 24 hours, suggesting that the analgesic effect of ropivacaine only provided short-term benefit. When given by subcutaneous injection, ropivacaine has rapid absorption27 and may provide as little as 3 hours of analgesia. Although not statistically significant, the time to first analgesics (opioid and nonopioid) was longer, and the total use of nonopioid analgesics was lower in the GA + LA versus GA groups, supporting an analgesic effect of LA. Further studies in which longer-acting local anesthetics for BMABs are used might achieve prolonged analgesia and lower total opioid and nonopioid analgesic consumption.
From an unplanned subset analysis, we noted that one-third of participants aged 8 and older required postprocedure opioids in both intervention groups compared with 10% in the younger cohort, indicating that older patients may endure more suffering from BMAB. There are several plausible explanations for this finding. BMAB may be a greater physical insult in older patients because of more subcutaneous tissue and denser cortical bone. Additionally, older children are more likely to have anticipatory procedure-related anxiety and can better express other multidimensional components of pain, particularly with greater exposure to painful procedures over time. Despite this study’s use of validated metrics, there are inherent developmental limitations in accurately capturing self-reports of pain in younger children, and parents may underestimate pain.28 Future studies of BMAB in varying age groups of pediatric patients with cancer may help us better understand age-related differences in pain and opioid use observed in this study.
Interestingly, we observed that the burden of BMAB in this trial was lower than expected from our needs assessment. Fewer participants required opioid analgesia, median pain scores were mild to moderate across all time points in both interventions, and pain interference with QoL was infrequent, other than in the domain of physical recovery. On the basis of the above discussion, the young age of most of our patients may have skewed our data toward lower reported procedural pain and impact on QoL. However, given these findings, nonpharmacologic interventions should be considered to reduce BMAB pain. Although there are few well-designed studies of these interventions in pediatric patients with cancer, some techniques have shown positive effects on BMAB-related pain, including hypnosis, relaxation, deep breathing, and procedure practice.24,26,29–31 The incorporation of nonpharmacologic interventions into current pharmacologic approaches should be explored as a means to mitigate BMAB-related pain and distress and potentially reduce opioid use.
Anecdotally, several parents noted that their child had a better experience with an on-study BMAB than with previous procedures and requested LA for future (off-study) procedures. Because parents were blinded to the intervention, it is not possible to ascertain whether this perceived improvement was related to LA. However, this reduction in parental distress could positively affect a child’s future experience with BMAB.32
This trial was a single-blind crossover study in which participants served as their own control between procedures and were blinded to the order of intervention. These elements limited intraparticipant variability in pain tolerance, procedure-related distress, and reporting of pain and QoL outcomes. Another strength of this study was the use of the postprocedure pain management algorithm to standardize interventions according to pain severity.
A limitation of this trial was that it was conducted at a single institution. Our neuroblastoma surveillance practice requires 4 BMA sites and 2 to 4 BMB sites. Anterior BMAB is not part of routine surveillance elsewhere; therefore, our results might not be generalizable to all pediatric patients with cancer undergoing BMAB. Additionally, study eligibility criteria excluded those with chronic pain and those unwilling to forego periprocedural opioids. This selection bias may contribute to the discrepancy with our needs assessment because those patients who are more likely to have lower pain tolerance and use opioids to manage pain were excluded. Another selection bias occurred when parents who did not perceive their child to experience pain after previous BMABs declined to participate.
Conclusions
Developing strategies to minimize pain related to treatments and procedures will reduce anxiety and distress experienced by children with cancer and is imperative to improving their QoL. In this trial, it was shown that LA may safely benefit some children, especially older patients, undergoing BMAB. Although opioids may be necessary for some patients for procedure-related pain, further studies of nonpharmacologic interventions or longer-acting local anesthetics may help reduce overall opioid consumption after painful procedures. A better understanding of age-related differences in the burden of BM procedures and potential interventions is also necessary to optimize this experience for children and adolescents.
Acknowledgments
We have many people to thank for their time and contribution to successful trial completion. Most importantly, we thank the patients with neuroblastoma and their families who considered or participated in our trial. Neuroblastoma attending physician Dr Brian Kushner provided feedback on trial design and results, participated in trial implementation, and revised the manuscript, and Dr Nai-Kong Cheung provided feedback on trial design and results. We are grateful for our clinical research team: Jennifer Bonura, Jessica Sollitto, Nikolette Zamarra, Allison Zambardino, and Dr Karima Yataghene. Dr Thomas Atkinson, head of the MSK Behavioral Research Methods Core, provided input on our QoL metric. Finally, we relied daily on procedure and RR session assistants and nurses, procedure room anesthesiologists, pharmacy staff, and the neuroblastoma nurse practitioners and administrative staff.
Glossary
- BM
bone marrow
- BMA
bone marrow aspiration
- BMAB
bone marrow aspiration and biopsy
- BMB
bone marrow biopsy
- DOA
duration of action
- GA
general anesthesia
- GEE
generalized estimating equation
- LA
local anesthesia
- mEqIV
morphine equivalent
- MSK
Memorial Sloan-Kettering Cancer Center
- NIH
National Institutes of Health
- PROMIS
Patient-Reported Outcomes Measure Information System
- QoL
quality of life
- RR
recovery room
- WBFPRS
Wong-Baker FACES Pain Rating Scale
Footnotes
Dr Zarnegar-Lumley designed and executed the study, enrolled patients, collected and analyzed participant data, and wrote and revised the manuscript; Drs Lange, Mathias, Nakajima-Hatano, Offer, Ogu, and Ortiz designed and executed the study, enrolled patients, collected and analyzed participant data, and revised the manuscript; Dr Tan developed the statistical design, analysis, and wrote and revised the statistics section of the manuscript; Dr Kellick contributed to study design and execution and reviewed and revised the manuscript; Drs Basu, Modak, and Roberts contributed to study design and execution and data analysis and reviewed and revised the manuscript; Dr Dingeman designed the study, interpreted participant data, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
This trial has been registered at www.clinicaltrials.gov (identifier NCT02924324).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICTS OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
FUNDING: Funded in part through the National Institutes of Health (NIH) and National Cancer Institute (Cancer Center Support grant P30 CA008748). Dr Ortiz is supported by the NIH/National Cancer Institute (award K12CA184746). Funded by the National Institutes of Health (NIH).
Data Sharing Statement: Deidentified individual participant data will not be made available.
References
- 1.Vanhelleputte P, Nijs K, Delforge M, Evers G, Vanderschueren S. Pain during bone marrow aspiration: prevalence and prevention. J Pain Symptom Manage. 2003;26(3):860–866 [DOI] [PubMed] [Google Scholar]
- 2.Lidén Y, Landgren O, Arnér S, Sjölund KF, Johansson E. Procedure-related pain among adult patients with hematologic malignancies. Acta Anaesthesiol Scand. 2009;53(3):354–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tutelman PR, Chambers CT, Stinson JN, et al. Pain in children with cancer: prevalence, characteristics, and parent management. Clin J Pain. 2018;34(3):198–206 [DOI] [PubMed] [Google Scholar]
- 4.Po’ C, Benini F, Sainati L, et al. The opinion of clinical staff regarding painfulness of procedures in pediatric hematology-oncology: an Italian survey. Ital J Pediatr. 2011;37(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hedström M, Haglund K, Skolin I, von Essen L. Distressing events for children and adolescents with cancer: child, parent, and nurse perceptions. J Pediatr Oncol Nurs. 2003;20(3):120–132 [DOI] [PubMed] [Google Scholar]
- 6.Chen E, Zeltzer LK, Craske MG, Katz ER. Children’s memories for painful cancer treatment procedures: implications for distress. Child Dev. 2000;71(4):933–947 [DOI] [PubMed] [Google Scholar]
- 7.Hjortholm N, Jaddini E, Hałaburda K, Snarski E. Strategies of pain reduction during the bone marrow biopsy. Ann Hematol. 2013;92(2):145–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeltzer LK, Altman A, Cohen D, LeBaron S, Munuksela EL, Schechter NL. American Academy of Pediatrics report of the subcommittee on the management of pain associated with procedures in children with cancer. Pediatrics. 1990;86(5 pt 2):826–831 [PubMed] [Google Scholar]
- 9.Propofol. Lexicomp Online, Pediatric and Neonatal Lexi-Drugs Online. Available at: https://online.lexi.com/lco/action/login. Accessed June 14, 2019
- 10.Wong-Baker FACES Foundation Wong-Baker FACES Pain Rating Scale. Available at: www.WongBakerFACES.org. Accessed December 21, 2015
- 11.De Negri P, Ivani G, Tirri T, Del Piano AC. New local anesthetics for pediatric anesthesia. Curr Opin Anaesthesiol. 2005;18(3):289–292 [DOI] [PubMed] [Google Scholar]
- 12.Ropivacaine. Lexicomp Online, Pediatric and Neonatal Lexi-Drugs Online. Available at: https://online.lexi.com/lco/action/login. Accessed June 14, 2019
- 13.Stinson JN, Kavanagh T, Yamada J, Gill N, Stevens B. Systematic review of the psychometric properties, interpretability and feasibility of self-report pain intensity measures for use in clinical trials in children and adolescents. Pain. 2006;125(1–2):143–157 [DOI] [PubMed] [Google Scholar]
- 14.Tomlinson D, von Baeyer CL, Stinson JN, Sung L. A systematic review of faces scales for the self-report of pain intensity in children. Pediatrics. 2010;126(5). Available at: www.pediatrics.org/cgi/content/full/126/5/e1168 [DOI] [PubMed] [Google Scholar]
- 15.McGrath PJ, Walco GA, Turk DC, et al. ; PedIMMPACT . Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain. 2008;9(9):771–783 [DOI] [PubMed] [Google Scholar]
- 16.Varni JW, Thissen D, Stucky BD, et al. PROMIS® Parent Proxy Report Scales: an item response theory analysis of the parent proxy report item banks. Qual Life Res. 2012;21(7):1223–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varni JW, Thissen D, Stucky BD, et al. PROMIS® Parent Proxy Report Scales for children ages 5-7 years: an item response theory analysis of differential item functioning across age groups. Qual Life Res. 2014;23(1):349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kossowsky J, Donado C, Berde CB. Immediate rescue designs in pediatric analgesic trials: a systematic review and meta-analysis. Anesthesiology. 2015;122(1):150–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berde CB, Walco GA, Krane EJ, et al. Pediatric analgesic clinical trial designs, measures, and extrapolation: report of an FDA scientific workshop. Pediatrics. 2012;129(2):354–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Baeyer CL, Marche TA, Rocha EM, Salmon K. Children’s memory for pain: overview and implications for practice. J Pain. 2004;5(5):241–249 [DOI] [PubMed] [Google Scholar]
- 21.Holdsworth MT, Raisch DW, Winter SS, et al. Pain and distress from bone marrow aspirations and lumbar punctures. Ann Pharmacother. 2003;37(1):17–22 [DOI] [PubMed] [Google Scholar]
- 22.Abdolkarimi B, Zareifar S, Golestani Eraghi M, Saleh F. Comparison effect of intravenous ketamine with pethidine for analgesia and sedation during bone marrow procedures in oncologic children: a randomized, double-blinded, crossover trial. Int J Hematol Oncol Stem Cell Res. 2016;10(4):206–211 [PMC free article] [PubMed] [Google Scholar]
- 23.Iannalfi A, Bernini G, Caprilli S, Lippi A, Tucci F, Messeri A. Painful procedures in children with cancer: comparison of moderate sedation and general anesthesia for lumbar puncture and bone marrow aspiration. Pediatr Blood Cancer. 2005;45(7):933–938 [DOI] [PubMed] [Google Scholar]
- 24.Zeltzer L, LeBaron S. Hypnosis and nonhypnotic techniques for reduction of pain and anxiety during painful procedures in children and adolescents with cancer. J Pediatr. 1982;101(6):1032–1035 [DOI] [PubMed] [Google Scholar]
- 25.Kazak AE, Penati B, Brophy P, Himelstein B. Pharmacologic and psychologic interventions for procedural pain. Pediatrics. 1998;102(1 pt 1):59–66 [DOI] [PubMed] [Google Scholar]
- 26.Shokrani O, Saghaei M, Ashrafi F, Sadeghi A. Electrical stimulation of acupuncture points for analgesia during bone marrow aspiration and biopsy: a randomized double-blind placebo-controlled trial. Adv Biomed Res. 2014;3:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christie LEPJ, Weinberg GL. Local anesthetic systemic toxicity. Br J Anaesth Education. 2015;15(3):136–142 [Google Scholar]
- 28.Matziou V, Vlachioti E, Megapanou E, et al. Perceptions of children and their parents about the pain experienced during their hospitalization and its impact on parents’ quality of life. Jpn J Clin Oncol. 2016;46(9):862–870 [DOI] [PubMed] [Google Scholar]
- 29.Jay S, Elliott CH, Fitzgibbons I, Woody P, Siegel S. A comparative study of cognitive behavior therapy versus general anesthesia for painful medical procedures in children. Pain. 1995;62(1):3–9 [DOI] [PubMed] [Google Scholar]
- 30.Liossi C, Hatira P. Clinical hypnosis versus cognitive behavioral training for pain management with pediatric cancer patients undergoing bone marrow aspirations. Int J Clin Exp Hypn. 1999;47(2):104–116 [DOI] [PubMed] [Google Scholar]
- 31.Wall VJ, Womack W. Hypnotic versus active cognitive strategies for alleviation of procedural distress in pediatric oncology patients. Am J Clin Hypn. 1989;31(3):181–191 [DOI] [PubMed] [Google Scholar]
- 32.Link CJ, Fortier MA. The relationship between parent trait anxiety and parent-reported pain, solicitous behaviors, and quality of life impairment in children with cancer. J Pediatr Hematol Oncol. 2016;38(1):58–62 [DOI] [PubMed] [Google Scholar]