SUMMARY
BACKGROUND:
People living with the human immunodeficiency virus (PLWH) may be particularly vulnerable to the consequences of non-tuberculous mycobacteria (NTM) given their defective T cell-mediated immunity and high rates of structural lung disease.
OBJECTIVE:
To determine the prevalence of NTM in PLWH hospitalized with pneumonia and to assess the potential predictors of NTM isolation.
METHODS:
Secondary data analysis of a prospective cohort study (2007–2011) of early bronchoscopy in PLWH presenting with suspected pneumonia was undertaken. Subjects with any species of NTM, henceforth described as ‘NTM of undetermined significance’ (NTM-US), isolated from sputum or bronchoalveolar lavage fluid (BALF), were included in the analysis. Potential predictors were chosen a priori.
RESULTS:
Among 196 HIV-infected subjects hospitalized with pneumonia, 96 had respiratory samples positive for NTM-US, with 91% of all NTM-US isolated from sputum compared with BALF. The overall prevalence of NTM-US was 49% (96/196). More NTM subjects were smokers (P = 0.08), with a history of chronic obstructive pulmonary disease (P = 0.08). Among those with pathogenic NTM, 39% (34/88) would have met American Thoracic Society microbiologic criteria for NTM pulmonary disease (17% of total cohort).
CONCLUSIONS:
Respiratory cultures, predominantly sputum samples, were positive for NTM-US in 45% of HIV-infected subjects admitted to hospital for pneumonia. Further research is needed to characterize the prevalence of NTM in PLWH and help establish specific diagnostic criteria in this population.
Keywords: HIV, immune deficiency, mycobacteria, NTM, pneumonia
RÉSUMÉ
CADRE:
Les personnes vivant avec le virus de l’immunodéficience humaine (PLWH) peuvent être particulièrement vulnérables aux conséquences des mycobactéries non-tuberculeuses (NTM) en raison de l’altération de leur immunité médiée par les lymphocytes T et les taux élevés de pathologie pulmonaire structurelle.
OBJECTIF:
Déterminer la prévalence des NTM chez les PLWH hospitalisés avec une pneumonie et évaluer les facteurs de prédiction potentiels d’isolation de NTM.
MÉTHODES:
Analyse des données secondaires d’une étude de cohorte prospective (2007–2011) de bronchoscopies précoces chez des PLWH se présentant avec une suspicion de pneumonie. Les sujets, hébergeant une quelconque espèce de NTM, ici décrits comme NTM de signification indéterminée (NTM-US), isolée à partir de crachats ou de lavage broncho-alveolaire (BAL), ont été inclus dans l’analyse. Les facteurs de prédiction potentiels ont été choisis a priori.
RÉSULTATS:
Parmi 196 sujets infectés par le VIH hospitalisés avec une pneumonie, 96 ont eu des échantillons respiratoires positifs pour NTM-US avec 91% de toutes les NTM-US isolées à partir des crachats par comparaison au BAL. La prévalence d’ensemble des NTM-US a été de 49% (96/196). Les patients NTM ont plus souvent été des fumeurs (P = 0,08) avec des antécédents de bronchopneumopathie obstructive chronique (P = 0,08). Parmi ceux ayant une NTM, 39% (34/88) auraient répondu aux critéres microbiologiques de l’American Thoracic Association de maladie pulmonaire NTM (17% de la cohorte).
CONCLUSIONS:
Les cultures respiratoires, surtout les échantillons de crachats, ont été positives aux NTM-US chez 45% des patients infectés par le VIH admis pour pneumonie. Davantage de recherche est requise pour caractériser la prévalence des NTM chez les PLWH et contribuer à établir des critères de diagnostic spécifiques dans cette population.
RESUMEN
MARCO DE REFERENCIA:
Las personas infectadas por el virus de la inmunodeficiencia humana (PLWH) son especialmente vulnerables a las consecuencias de la infección por micobacterias atípicas diferentes de Mycobacterium tuberculosis (NTM), dada su deficiente respuesta inmunitaria mediada por los linfocitos T y a la alta proporción de lesiones estructurales pulmonares.
OBJETIVO:
Determinar la prevalencia de NTM en los PLWH hospitalizados por neumonía y evaluar los posibles factores pronósticos de aislamiento de NTM.
MÉTODOS:
Se llevó a cabo un análisis secundario de los datos de un estudio de cohortes prospectivo (2007–2011) de la broncoscopia temprana en PLWH con presunción de neumonía. En el análisis se incluyeron pacientes con cualquier especie de NTM, denominadas con este fin NTM de significación clínica indeterminada (NTM-US), aisladas de muestras de esputo o lavado broncoalveolar (BAL). Los posibles factores pronósticos se escogieron a priori.
RESULTADOS:
De los 196 pacientes infectados por el VIH hospitalizados por neumonía, 96 tuvieron muestras respiratorias positivas para NTM-US y el 91% de todas estas micobacterias se aisló de muestras de esputo, en comparación con el BAL. La prevalencia global de NTM-US fue 49% (96/196). Más pacientes con NTM eran fumadores (P = 0,08) y tenían antecedente de enfermedad pulmonar obstructiva crónica (P=0,08). De los pacientes con NTM patógenas, el 39% (34/88) habría cumplido los criterios microbiológicos de enfermedad pulmonar por NTM de la American Thoracic Association (17% de toda la cohorte).
CONCLUSIÓN:
Los cultivos de muestras respiratorias, sobre todo de las muestras de esputo, fueron positivas para NTM-US en el 45% de las PLWH hospitalizadas por neumonía. Se precisan nuevas investigaciones que permitan caracterizar la prevalencia de NTM en las PLWH y contribuyan a definir criterios diagnósticos específicos en esta población.
WITH THE ADVENT of combined antiretroviral therapy (cART), the risk of disseminated Mycobacterium avium complex (MAC) has declined and the clinical importance of non-tuberculous mycobacteria (NTM) isolation in respiratory specimens has become less clear.1–5 Given their faulty T-cell-mediated immunity, human immunodeficiency virus (HIV) infected patients possess an immunologic vulnerability which may make them particularly susceptible to pulmonary infection with NTM. Alternatively, the emergence of chronic comorbidities in people living with HIV (PLWH), such as chronic obstructive pulmonary disease (COPD), may be permissive to NTM infection.6,7
Epidemiologic studies have shown that NTM prevalence is increasing, with pulmonary disease representing the most common clinical syndrome.8–10 As the prevalence of HIV infection increases due to longer life expectancies with cART, PLWH may represent a unique cohort susceptible to NTM-related chronic respiratory illness.
Current guidelines from the American Thoracic Society(ATS)/Infectious Diseases Society of America do not specifically address NTM in HIV-infected individuals, in whom diagnostic criteria and clinical outcomes may hold important consequences for differentiation between colonization, transient infection, subclinical or overt, active disease.11
In a retrospective analysis of HIV-related hospital admissions to a hospital in metropolitan Florida, USA, NTM was responsible for 11% of respiratory illnesses and was associated with an increased duration of hospitalization.12 In another study of HIV-infected patients hospitalized between 1989 and 2002, a single positive respiratory culture for Mycobacterium kansasii, positive sputum smear microscopy for NTM and lack of adequate mycobacterial treatment were risk factors for increased mortality.13
It is not known whether the predictors and associated conditions of NTM disease described in non-HIV-infected populations are valid in HIV-infected patients with NTM. One observational study which categorized NTM culture positivity in PLWH as infection vs. colonization concluded that NTM infection should be suspected in any patient with longstanding symptoms, anemia, low CD4 count and several positive sputum acid-fast bacilli (AFB) cultures.14
Taken together, the studies mentioned above highlight the need for improved understanding of the importance of NTM isolation in PLWH, including which species are pathogenic, because diagnosis and disease manifestations may differ from those in the immunocompetent population.
We wished to determine the prevalence of NTM in PLWH presenting to an acute care hospital with pneumonia. Associations between known risk factors for NTM disease in the general population were assessed in HIV-infected subjects. We hypothesized that NTM is highly prevalent in PLWH with active pneumonia, and that the risk factors identified in non-HIV-infected patients are associated with NTM in PLWH. This was the first study in which PLWH presenting with acute respiratory illness underwent systematic clinical, radiographic and microbiologic evaluation to enable determination of the prevalence and associated risk factors of NTM in this population.
METHODS
Study design
Data obtained from an HIV-associated Pneumonia Management Program (HMP) implemented at Louisiana State University, New Orleans (LSUHSC-NO), LA, USA, between 2007 and 2011 were used for this retrospective analysis. Under this clinical management program, early bronchoscopy was implemented to promote rapid microbiological diagnosis in HIV-infected subjects admitted with pneumonia. A presumptive diagnosis of pneumonia required 1) clinical suspicion of a lower respiratory tract infection; and 2) either a new or changing radiographic infiltrate or unexplained hypoxia in the absence of an infiltrate. Expectorated or induced sputum was also collected and sent for AFB, fungal and bacterial cultures.
Handling of sputum and bronchial washings
All respiratory tract sampling was performed for clinical indications. Bilateral, large-volume (⩾7180 ml) bronchoalveolar lavages (BALs) were done for diffuse infiltrates, and directed BALs were undertaken for focal opacities. AFB culture was performed using liquid and solid media. NTM were identified on liquid media using high-performance liquid chromatography combined with the Runyon classification or a molecular probe. Final NTM identification was based on solid media culture.
Data collection
Study data were obtained using a defined variable set compiled by interviewing the subjects at the time of enrollment in the HMP. For variables not included in the original database, such as clinical diagnosis of COPD, diabetes mellitus (DM) or gastroesophageal reflux disease (GERD), a retrospective chart analysis was performed.
We included all positive NTM results in the overall prevalence estimate and characterized these patients as ‘NTM of undetermined significance’ (NTM-US). To investigate mycobacteria henceforth accepted as clinically relevant in the immunocompetent population, we further categorized the following NTM as ‘pathogenic’ (NTM-P): Mycobacterium avium complex (MAC), M. kansasii, M. fortuitum, M. chelonae and M. abscessus. We considered M. gordonae, M. terrae complex, M. scrofulaceum, M. mucogenicum and M. xenopi to be non-pathogenic. As the pathogenicity of M. gordonae in immunosuppressed patients is controversial, the data were also analyzed with M. gordonae categorized as a ‘pathogen’. To assess potential predictors for mycobacterial infection, the following variables were chosen a priori: CD4 count, viral load, cART use, smoking history, previous or current use of NTM prophylaxis, serum albumin, hematocrit and associated diagnosis of COPD, DM or GERD. Using these same predictors, we compared subjects in whom only slow-growing mycobacteria (SGM) (MAC and M. kansasii) were identified vs. those with only rapid-growing mycobacteria (RGM) (M. fortuitum, M. abscessus, M. chelonae). While the prevalence of NTM-US was determined by including subjects with at least one isolate of NTM, we also sought to ascertain the number of subjects who met ATS microbiologic criteria necessary for diagnosis of NTM-related pulmonary disease, and thereby identified those with at least two sputum samples or one BAL-positive for NTM-P. For all subjects with NTM-P, available chest computed tomography (CT) reports were reviewed for findings commonly associated with pulmonary mycobacterial disease. Finally, to determine the most frequently isolated non-mycobacterial co-organisms, we compared non-AFB microbiologic data between HIV-infected subjects with and without NTM-US.
Statistical analysis
Descriptive statistics were means for continuous variables, medians for non-parametric data and proportions for categorical variables. Bivariate analysis was performed using Student’s t-tests for means, Wilcoxon rank-sum testing for non-parametric data and χ2 testing with Fisher’s exact tests for samples with <5 observations. P < 0.05 was considered statistically significant. All statistical analyses were performed using STATA v 13.0 (STATA Corp, College Station, TX, USA).
Ethics statement
All subjects participated voluntarily, and we ensured the confidentiality and anonymity of research participants. The protocols for the parent study and this retrospective analysis were approved by the Institutional Review Board of LSUHSC-NO.
RESULTS
Cohort characteristics
Of 196 HIV-infected subjects hospitalized with pneumonia between 2007 and 2011, 165 underwent both sputum and collection of bronchoalveolar lavage fluid (BALF). Subjects were severely immunosuppressed (median CD4 64 cells/mm3), with 30% receiving cART upon hospital admission. The cohort was predominantly middle-aged (median age 44 years), male (71%), with a history of incarceration (71%) or homelessness (51%) who self-identified as African-American (87%).
Prevalence of non-tuberculous mycobacteria
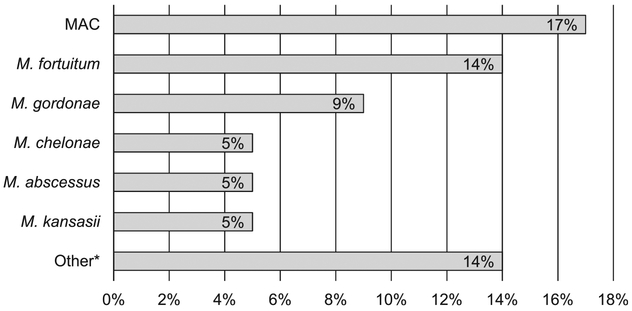
Ninety-six subjects (49%) had respiratory samples positive for NTM-US, with NTM-P strains identified in 73 (82 subjects if M. gordonae is included as a pathogen). Across all encounters, 187 isolates of NTM-US were identified, 138 (or 155 isolates [83%] with M. gordonae) of which were for NTM-P. Of the positive 187 isolates (91%), 170 were identified using sputum collection. MAC was the most frequent NTM identified in 34 subjects (17%), followed by M. fortuitum in 28 (14%) and M. gordonae in 17 (9%) (Figure 1). An additional 32 respiratory specimens in 27 subjects were positive for other NTM species, including M. terrae complex, M. xenopi, M. scrofulaceum, M. mucogenicum and undifferentiated Group IV RGM. Twenty-three subjects had more than one species of NTM co-identified, with M. gordonae or M. fortuitum being the most common.
Figure 1.
Frequency of distribution of isolation of NTM species (% of total cohort). MAC = M. avium complex; NTM = non-tuberculous mycobacteria.
We found that 17% (34/196) of subjects in the total cohort, and 39% (34/88) of those with NTM-P identified met ATS microbiologic criteria for NTM pulmonary disease. Among those who met the ATS criteria, 88% (30/34) were based on ⩾2 positive sputum samples. Of 88 subjects with NTM-P, 48 (55%) had chest CT, 60% (29/48) of whom had imaging findings consistent with NTM-related pulmonary disease. Such findings included the following terminology: ‘pulmonary nodules’, ‘tree-in-bud changes’, ‘bronchiectasis’, ‘cavitation/necrosis’, ‘nodular focus of airspace disease’, ‘nodular infiltrates’ and ‘calcified granulomas’. Bronchiectasis was specifically mentioned as a finding in 15% (7/48) of chest CT reports.
Predictive factors for non-tuberculous mycobacteria
No difference was observed with regard to age, sex, race, CD4 count, viral load, MAC prophylaxis, hematocrit, cART use or other clinical parameters evaluated in subjects with and without NTM-US isolated. However, more NTM-positive subjects had a history of COPD (P = 0.08) or of being ever smokers (P = 0.08) (Table 1).
Table 1.
Patient-based predictors of NTM-positive respiratory specimens in HIV-infected subjects
| Positive for NTM (n = 96) % | Negative for NTM (n = 100) % | P value | |
|---|---|---|---|
| Age, years, mean ± SD | 45 ± 9 | 44 ± 9 | 0.31 |
| Male sex | 70 | 71 | 0.85 |
| Black race | 85 | 88 | 0.59 |
| CD4 count, cells/mm3, median [IQR] | 65 [19–129] | 48 [9–135] | 0.41 |
| Viral load,* copies/ml, median [IQR] | 97 719 [5886–242 553] | 65 475 [1303–265 608] | 0.56 |
| cART use | 27 | 33 | 0.37 |
| On MAC prophylaxis | 7 | 9 | 0.66 |
| Ever smoker | 83 | 73 | 0.08 |
| Albumin, g/dl, mean ± SD | 2.96 ± 0.56 | 2.92 ± 0.59 | 0.59 |
| Esophageal reflux | 21 | 15 | 0.26 |
| COPD | 40 | 28 | 0.08 |
| Diabetes mellitus | 15 | 11 | 0.48 |
| Hematocrit, %, mean ± SD | 34 ± 6 | 34 ± 6 | 0.95 |
Not collected upon hospitalization.
NTM = non-tuberculous mycobacteria; HIV = human immunodeficiency virus; SD = standard deviation; IQR = interquartile range; cART = combined antiretroviral therapy; MAC = M. avium complex; COPD = chronic obstructive pulmonary disease.
Relative to subjects with concomitant growth of MAC plus another NTM-P strain, those with only MAC were older (47 vs. 42 years, P = 0.04). There were no other significant differences in CD4 count, viral load, cART use, MAC prophylaxis, hematocrit, history of smoking, GERD, COPD or DM (Table 2). When comparing subjects with SGM vs. RGM, those with SGM had a significantly lower CD4 count. However, among other predetermined predictive factors, irrespective of M. gordonae inclusion, there were no significant differences (Table 3; Appendix Table A*).
Table 2.
Patient-based predictors of MAC vs. MAC + other NTM respiratory specimens in HIV-infected subjects
| MAC-positive (n = 18) % | MAC + other NTM (n = 16) % | P value | |
|---|---|---|---|
| Age, years, mean ± SD | 47 ± 8 | 42 ± 7 | 0.04 |
| Male sex, | 83 | 69 | 0.32 |
| CD4 count, cells/mm3, median [IQR] | 32 [18–83] | 25 [11–106] | 0.82 |
| cART use | 33 | 31 | 0.90 |
| On MAC prophylaxis | 11 | 25 | 0.39 |
| Ever smoker | 83 | 56 | 0.13 |
| Esophageal reflux | 17 | 6 | 0.60 |
| COPD | 39 | 31 | 0.64 |
| Diabetes mellitus | 6 | 19 | 0.32 |
| Hematocrit, %, mean ± SD | 35 ± 5 | 32 ± 6 | 0.12 |
MAC = M. avium complex; NTM = non-tuberculous mycobacteria; HIV = human immunodeficiency virus; SD = standard deviation; IQR = interquartile range; cART = combined antiretroviral therapy; COPD = chronic obstructive pulmonary disease.
Table 3.
Patient-based predictors of SGM vs. RGM respiratory specimens in HIV-infected subjects
| NTM-positive SGM* (n = 28) % | NTM-positive RGM† (n = 27) % | P value | |
|---|---|---|---|
| Age, years, mean ± SD | 45 ± 9 | 44 ± 8 | 0.52 |
| CD4 count, cells/mm3, median [IQR] | 27 [11–84] | 92 [16–152] | 0.04 |
| Viral load,‡ copies/ml, median [IQR] | 112 311 [2 823–251 488] | 123 104 [20 594–211 022] | 0.89 |
| cART use | 21 | 26 | 0.69 |
| On MAC prophylaxis | 11 | 4 | 0.61 |
| Ever smoker | 82 | 89 | 0.70 |
| COPD | 32 | 44 | 0.35 |
| Esophageal reflux | 14 | 7 | 0.67 |
| Diabetes mellitus | 7 | 15 | 0.36 |
| Hematocrit, %, mean ± SD | 34 ± 6 | 34 ± 7 | 0.94 |
MAC, M. kansasii.
M. fortuitum, M. abscessus, M. chelonae.
Not collected upon hospitalization.
SGM = slow-growing mycobacteria; RGM = rapid-growing mycobacteria; HIV human immunodeficiency virus; NTM = non-tuberculous mycobacteria; SD = standard deviation; IQR = interquartile range; cART = combined antiretroviral therapy; MAC = M. avium complex; COPD = chronic obstructive pulmonary disease.
Organisms concomitantly isolated with non-tuberculous mycobacteria
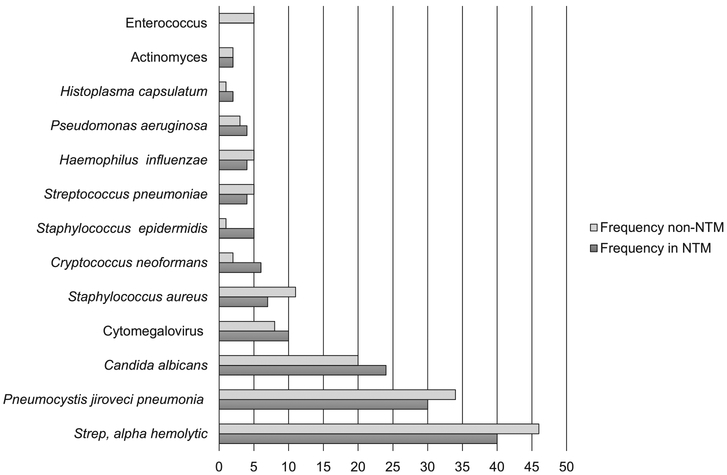
The most commonly identified co-occurring nonmycobacterial organisms are listed in Figure 2. Among all non-mycobacterial co-isolates, those most commonly identified were alpha hemolytic streptococcus, Pneumocystis jirovecci and Candida albicans.
Figure 2.
Number of co-organisms isolated in patients with and without NTM. * Enterococcus demonstrated a trend towards significance (P = 0.06). There were no other statistically significant differences (P < 0.05) between the number of co-organisms isolated between patients with and without NTM. NTM = non-tuberculous mycobacteria.
DISCUSSION
Considerable evidence suggests that the worldwide NTM prevalence is increasing.15–20 The importance of routine isolation of NTM in HIV-infected individuals remains incompletely understood. Similarly, it is not known if persistent, qualitative T-cell defects in HIV-infected patients, irrespective of CD4+ count, makes them inherently more vulnerable to NTM-related pulmonary disease.
We observed a prevalence of nearly 50% of NTM isolated from respiratory samples of HIV-infected pneumonia subjects. The increased prevalence of NTM in our cohort may be directly representative of the generally disproportionate burden of NTM in the Southeast region of the United States. One study identifying geographic clusters of NTM in immunocompetent hosts found the relative risk of pulmonary NTM to be 6.5 when residing in Plaquemines Parish, LA, USA, an area adjacent to our catchment region.21,22 Environmental exposures associated with geographic location, in combination with our cohort’s high prevalence of homelessness, may have contributed to the high prevalence of NTM. Furthermore, use of dual culture systems may have increased assay sensitivity. Liquid media in conjunction with molecular analysis leads to the identification of more NTM, particularly RGM, than if only solid media are used.11,23 An increased sensitivity of liquid media for NTM in HIV-infected patients is likely because a greater prevalence of NTM was observed in Thailand and Viet Nam than in Cambodia, where only solid media were implemented.9
We observed a higher prevalence of M. fortuitum and M. gordonae than has been described previously. Our findings deviated from studies strictly implicating MAC and M. kansasii as common and clinically important NTM pathogens in PLWH.1,13,24 These observations suggest the importance of diagnostic techniques in establishing the prevalence of previously undetected NTM species. Furthermore, a proportion of the subjects in our study demonstrated CT findings characteristic of pulmonary mycobacterial disease, thereby underlining the need for continued investigation into the clinical relevance of these organisms in PLWH.
A provocative finding in our study was that the overwhelming majority of NTM identified were isolated from sputum rather than from BALF. One possible explanation is that increased environmental exposure in the New Orleans area facilitated NTM upper airway colonization, of which sputum sampling may be more reflective.25 Similar findings have been observed in studies assessing the shared upper and lower airway microbiota in patients with asthma, COPD or bronchiectasis.26–31 An alternative explanation could be that expectorated sputum reflects sampling of multiple bronchial segments, thus contributing to a higher yield of NTM than BALF taken from a single bronchial segment. In one study comparing the characteristics of patients diagnosed with pulmonary MAC using sputum vs. BALF, the authors found significantly more patients diagnosed using sputum to have left upper division abnormalities and a higher number of affected lobes on chest imaging.32 Furthermore, the large aliquots used in BAL may have a dilutional effect, whereby sensitivity for NTM identification is reduced. The clinical relevance of NTM isolation from sputum compared with BALF in PLWH requires further study.
ATS guidelines recommend combined clinical and microbiologic criteria to establish a diagnosis of NTM pulmonary disease. In our study, 35% (34/96) of subjects in whom NTM was identified would have met the microbiologic criteria for NTM disease.11 The majority of these subjects met the criteria based on sputum results; however, there were insufficient data to determine whether the NTM were the primary pathogens responsible for the patient’s presenting illness, co-pathogens or colonizers. In a study of non-HIV-infected patients, even a single sputum isolation of NTM in the presence of young age (⩽65 years) and bronchiectasis indicated a higher risk of subsequent future culture positivity and NTM lung disease.33 The 49% prevalence of at least a single isolation of NTM in our young adult cohort suggests the need to follow HIV-infected patients closely (perhaps with sputum surveillance) for the development of mycobacterial-related lung disease. Whether single sputum isolation of NTM leads to clinically significant disease in PLWH remains to be elucidated in future prospective studies.
Our study suggests an association of COPD and history of smoking in subjects with isolation of NTM on culture, although this association was not statistically significant. Similar risk factors have also been reported in non-HIV-infected patients, implying that smoking-related impaired mucociliary clearance, structural lung disease, or some combination thereof, is mechanistically contributory.34 More specifically, precocious emphysema, which has been well described in PLWH, may predispose patients to NTM infection and explain the prevalence of NTM observed in our young cohort.6,7 Our study was limited because pulmonary function results were not available to quantify obstruction severity. Hence, we had to rely on chart documentation, which could have led to misclassification of our COPD diagnosis. Furthermore, as a standardized protocol for chest imaging was not employed, lung anatomy was not uniformly measured.
We did not find any difference in viral load when comparing patients with and without NTM, nor in those with SGM vs. RGM isolation. Viral load data available for analysis in our study were not collected at the time of acute illness, so such data may not be reflective of NTM-related disease in our cohort. This remains an important point for further analysis as the contribution of mycobacterial illness to viral replication has been well described.35,36 Also of interest is the significantly lower CD4 count found in subjects with SGM than those with respiratory isolation of RGM. While it is known that a CD4 count,<50 cells/mm3 is a risk factor for disseminated MAC, this finding may also highlight a potential predisposition for SGM-related pulmonary disease that requires further investigation.20,24,37
Our study had four important limitations. First, as our study was retrospective, so the potential contribution of confounding factors and missing data may have limited our findings. Second, given the pragmatic nature of the parent study, a strict protocol was not in place for timing, number and adequacy of respiratory mycobacterial samples. For those in whom sputum samples were collected, the number of samples was highly variable and therefore may have contributed to sampling bias. Third, our study was not specifically designed to assess NTM infection. The specific contribution of NTM to clinical and radiographic findings is therefore unclear. Finally, as our cohort of HIV-infected subjects was profoundly immunocompromised and had low cART compliance, it is potentially not reflective of the overall HIV population.
CONCLUSION
Respiratory cultures were commonly positive for NTM in HIV-infected subjects admitted to hospital for pneumonia, more than one third of which would have met ATS criteria for NTM disease due to potentially pathogenic strains. Further investigation should explore the short and long-term sequelae of routinely identified NTM in HIV-infected patients, and evaluate the utility of early NTM diagnosis and treatment strategies.
Acknowledgements
This research was made possible by grants supported by the National Institutes of Health, Bethesda, MD, USA, and Louisiana Clinical & Translational Science Center, Baton Rouge, LA, USA (UH2 AA026226, P60 AA009803, R24AA19661, PO1 HL076100, U54 GM104940).
APPENDIX
Table A.
Patient-based predictors of SGM (including M. gordonae) vs. RGM respiratory specimens in HIV-infected subjects
| NTM-positive SGM* (n = 37) % | NTM-positive RGM† (n = 27) % | P value | |
|---|---|---|---|
| Age, years, mean ± SD | 45 ± 9 | 44 ± 8 | 0.62 |
| CD4 count, cells/mm3, median [IQR] | 34 [13–95] | 107 [40–162] | 0.01 |
| Viral load,‡, copies/ml median [IQR] | 97 622 [2 823–288 346] | 97 816 [20 594–190 712] | 0.71 |
| cART use | 24 | 26 | 0.88 |
| On MAC prophylaxis | 8 | 4 | 0.63 |
| Ever smoker | 84 | 89 | 0.72 |
| COPD | 38 | 44 | 0.60 |
| Esophageal reflux | 24 | 7 | 0.10 |
| Diabetes mellitus | 11 | 15 | 0.71 |
| Hematocrit, % mean ± SD | 34 ± 6 | 34 ± 7 | 0.96 |
MAC, M. kansasii.
M. fortuitum, M. abscessus, M. chelonae.
Not collected upon hospitalization.
SGM = slow-growing mycobacteria; RGM = rapid-growing mycobacteria; HIV = human immunodeficiency virus; NTM = non-tuberculous mycobacteria; SD = standard deviation; IQR = interquartile range; cART = combined antiretroviral therapy; MAC = M. avium complex; COPD = chronic obstructive pulmonary disease.
Footnotes
Conflicts of interest: none declared.
The appendix is available in the online version of this article, at http://www.ingentaconnect.com/content/iuatld/ijtld/2019/00000023/00000004/art000
References
- 1.Havlik JA Jr, Horsburgh CR Jr, Metchock B, Williams PP, Fann SA, Thompson SE 3rd. Disseminated Mycobacterium avium complex infection: clinical identification and epidemiologic trends. J Infect Dis 1992; 165: 577–580. [DOI] [PubMed] [Google Scholar]
- 2.Horsburgh CR Jr. Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med 1991; 324: 1332–1338. [DOI] [PubMed] [Google Scholar]
- 3.Statistics Center, HIV/AIDS, Centers for Disease Control and Prevention. HIV in the United States: statistics overview. Atlanta, GA, USA: CDC, 2018. [Google Scholar]
- 4.Centers for Disease Control and Prevention, US Government. Today’s HIV/AIDS epidemic factsheet. Atlanta, GA, USA: CDC, 2016. [Google Scholar]
- 5.Horsburgh CR Jr., Epidemiology of disease caused by non-tuberculous mycobacteria. Semin Respir Infect 1996; 11: 244–251. [PubMed] [Google Scholar]
- 6.Leader JK, Crothers K, Huang L, et al. Risk factors associated with quantitative evidence of lung emphysema and fibrosis in an HIV-infected cohort. J Acquir Immune Defic Syndr 2016; 71: 420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz PT, King MA, Pacht ER, et al. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med 2000; 132: 369–372. [DOI] [PubMed] [Google Scholar]
- 8.Winthrop KL, Mcnelley E, Kendall B, et al. Pulmonary non-tuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med 2010; 182: 977–982. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy KD, Cain KP, Winthrop KL, et al. Non-tuberculous mycobacterial disease in patients with HIV in Southeast Asia. Am J Resp Crit Care Med 2012; 185: 981–988. [DOI] [PubMed] [Google Scholar]
- 10.Mirsaeidi M, Farshidpour M, Allen MB, Ebrahimi G, Falkinham JO. Highlight on advances in non-tuberculous mycobacterial disease in North America. BioMed Res Int 2014: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of non-tuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175: 367–416. [DOI] [PubMed] [Google Scholar]
- 12.Miguez-Burbano MJ, Flores M, Ashkin D, Rodriguez A, Granada AM, Quintero N, Pitchenik A. Non-tuberculous mycobacteria disease as a cause of hospitalization in HIV-infected patients. Int J Infect Dis 2006;10: 47–55. [DOI] [PubMed] [Google Scholar]
- 13.Marras TK, Morris A, Gonzalez LC, Daley CL. Mortality prediction in pulmonary Mycobacterium kansasii infection and human immunodeficiency virus. Am J Resp Crit Care Med 2004; 170: 793–798. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez-Uria G, Falco V, Martin-Casabona N, et al. Non-tuberculous mycobacteria in the sputum of HIV-infected patients: infection or colonization. Int J STD AIDS 2009; 20: 193–195. [DOI] [PubMed] [Google Scholar]
- 15.Adjemian J, Olivier KN, Seitz AE, et al. Prevalence of non-tuberculous mycobacterial lung disease in US Medicare beneficiaries. Am J Respir Crit Care Med 2012; 185: 881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strollo SE, Adjemian J, Adjemian MK, Prevots DR. The burden of pulmonary non-tuberculous mycobacterial disease in the United States. Ann Am Thorac Soc 2015; 12: 1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowman S, Burns K, Benson S, et al. The antimicrobial susceptibility of non-tuberculous mycobacteria. J Infect 2016; 72: 324–331. [DOI] [PubMed] [Google Scholar]
- 18.Ringshausen FC, Apel RM, Bange FC, et al. Burden and trends of hospitalisations associated with pulmonary non-tuberculous mycobacterial infections in Germany, 2005–2011. BMC Infect Dis 2013; 13: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olivier KN, Weber DJ, Wallace RJ Jr, et al. Non-tuberculous mycobacteria. I. Multicenter prevalence study in cystic fibrosis. Am J Resp Crit Care Med 2003; 167: 828–834. [DOI] [PubMed] [Google Scholar]
- 20.Henkle E, Winthrop K. Non-tuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med 2015; 36: 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adjemian J, Olivier KN, Seitz AE, Falkinham JO III, Holland SM, Prevots DR. Spatial clusters of non-tuberculous mycobacterial lung disease in the United States. Am J Respir Crit Care Med 2012; 186: 553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honda JR, Bernhard JN, Chan ED. Natural disasters and non-tuberculous mycobacteria. Chest 2014; 147: 304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Ingen J. Diagnosis of non-tuberculous mycobacterial infections. Semin Respir Crit Care Med 2013; 34: 103–109. [DOI] [PubMed] [Google Scholar]
- 24.Levy-Frebault V, Pangon B, Bure A, et al. Mycobacterium simiae and Mycobacterium avium-M. intracellulare mixed infection in acquired immune deficiency syndrome. J Clin Microbiol 1987; 25: 154–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith GS, Ghio AJ, Stout JE, et al. Epidemiology of non-tuberculous mycobacteria isolations among central North Carolina residents, 2006–2010. J Infect 2016; 72: 678–686. [DOI] [PubMed] [Google Scholar]
- 26.Hens G, Hellings PW. The nose: gatekeeper and trigger of bronchial disease. Rhinology 2006; 44: 179–187. [PubMed] [Google Scholar]
- 27.Hellings PW, Hens G. Rhinosinusitis and the lower airways. Immunol Allergy Clin North Am 2009; 29: 733–740. [DOI] [PubMed] [Google Scholar]
- 28.Shirahata Y. Correlation between upper airway tract and lower airway tract in the breakdown of sinobronchiectasis. Nippon Jibiinkoka Gakkai Kaiho 1990; 93: 1991–1998. [DOI] [PubMed] [Google Scholar]
- 29.Ramakrishnan VR, Ferril GR, Suh JD, Woodson T, Green TJ, Kingdom TT. Upper and lower airways associations in patients with chronic rhinosinusitis and bronchiectasis. Int Forum Allergy Rhinol 2013; 3: 921–927. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Xu Y, Jin J, Li R, Liu X, Sun Y. Chronic rhinosinusitis is associated with higher prevalence and severity of bronchiectasis in patients with COPD. Int J Chron Obstruct Pulmon Dis 2017; 12: 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philpott CM, McKiernan DC. Bronchiectasis and sino-nasal disease: a review. J Laryngol Otol 2008; 122: 11–15. [DOI] [PubMed] [Google Scholar]
- 32.Maekawa K, Naka M, Shuto S, et al. The characteristics of patients with pulmonary Mycobacterium avium-intracellulare complex disease diagnosed by bronchial lavage culture compared to those diagnosed by sputum culture. J Infect Chemother 2017; 23: 604–608. [DOI] [PubMed] [Google Scholar]
- 33.Lee MR, Yang CY, Shu CC, et al. Factors associated with subsequent non-tuberculous mycobacterial lung disease in patients with a single sputum isolate on initial examination. Clin Microbiol Infect 2015; 21: 1–7. [DOI] [PubMed] [Google Scholar]
- 34.Chan ED, Iseman MD. Underlying host risk factors for non-tuberculous mycobacterial lung disease. Semin Respir Crit Care Med 2013; 34: 110–123. [DOI] [PubMed] [Google Scholar]
- 35.Whalen C, Horsburgh C, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med 1995; 151: 129–135. [DOI] [PubMed] [Google Scholar]
- 36.Modjarrad K, Vermund S. Effect of treating co-infections on HIV-1 viral load: a systematic review. Lancet Infect Dis 2010; 10: 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayskaya MU, Otten TF, Ariel BM, Fedotova EP, Hunter RL, Nasyrov RA. Morphological manifestations of the atypical mycobacteriosis caused by non-tuberculous mycobacteria in the HIV-infected patients. Ann Clin Lab Sci 2014; 44: 131–133. [PubMed] [Google Scholar]