Abstract
Objectives
Adults with congenital heart disease (ACHD) are a growing group with end-stage heart failure. We aim to describe the outcomes of ACHD patients undergoing assessment for orthotopic heart transplant (OHT).
Methods
Case notes of consecutive ACHD patients (>16 years) assessed for OHT between 2000 and 2016 at our centre were reviewed. Decision and outcome were reported as of 2017. Data were analysed in three groups: systemic left ventricle (LV), systemic right ventricle (RV) and single ventricle (SV).
Results
196 patients were assessed (31.8 years, 27% LV, 29% RV, 44% SV). 89 (45%) patients were listed for OHT and 67 (34%) were transplanted. 41 (21%) were unsuitable or too high risk and 36 (18%) were too well for listing. Conventional surgery was undertaken in 13 (7%) and ventricular assist device in 17 (9%) with 7 (4%) bridged to candidacy. Survival from assessment was 84.2% at 1 year and 69.7% at 5 years, with no difference between groups. Patients who were considered unsuitable for OHT (HR 11.199, p<0.001) and listed (HR 3.792, p=0.030) were more likely to die than those who were considered too well. Assessments increased over the study period.
Conclusions
The number of ACHD patients assessed for OHT is increasing. A third are transplanted with a small number receiving conventional surgery. Those who are unsuitable have a poor prognosis.
Keywords: congenital heart disease surgery, heart transplantation, congenital heart disease, complex congenital heart disease
Background
Most infants with complex congenital heart disease now survive to adulthood.1 Mortality in these adults is higher than the general population, with heart failure the leading cause of death.2–5 Strategies successful in acquired heart disease have uncertain survival benefit in the adult congenital heart disease (ACHD) heart failure population.6–8 Orthotopic heart transplantation (OHT) is effective for selected patients,9 10 but donor organs are scarce and competing demands from those with other conditions contribute to waiting list mortality.11–13 Despite better long-term outcomes from OHT, ACHD patients are at significant risk of peri-transplant mortality9 10 and the role of OHT continues to be debated.
The present study reports outcomes of consecutive ACHD patients referred to us for OHT assessment.
Methods
Study population
Consecutive ACHD patients (>16 years) assessed for OHT at our unit between 1 January 2000 and 1 January 2016 for OHT were included. Case records were retrospectively reviewed. Clinical decision and outcomes were reported from 1 January 2017.
Patients were categorised into three groups:
Left ventricle (LV): usual anatomic connections, two balanced ventricles with systemic LV
Right ventricle (RV): ventriculo-arterial (±atrioventricular) discordance, two balanced ventricles with systemic RV
Single ventricle (SV): unbalanced ventricles with a physiological SV circulation.
Assessment and decision process
Newcastle on Tyne Hospitals NHS Foundation Trust is a specialist adult and paediatric congenital heart disease surgical centre and a national cardiothoracic transplant centre. The unit carries out the majority of the UK’s OHTs and ventricular assist devices (VAD) for ACHD patients.
Following referral, patients were admitted at least once for inpatient assessment. Decision to list for transplantation was made at a multidisciplinary team (MDT) meeting and categorised as follows:
Unsuitable
Conventional surgery/intervention preferred
Too well and conventional surgery/intervention not indicated
Other (dual organ transplant, suitable but declined)
Suitable and listed for OHT.
Unsuitable patients were those with an absolute contraindication or who were considered too high risk for OHT. Patients considered too well were those without significant functional limitation (New York Heart Association (NYHA) 1 or 2) and where there was felt to be no clear survival advantage from transplantation. Urgent listing status was based on the National Health Service Blood and Transplant (NHS BT) urgent allocation scheme introduced in 1999.14 15 To assess change over time, results were compared over 4-year periods.
Statistics
Normality of data was assessed using the Kolgomorov–Smirnov Test. All continuous variables were not normally distributed (age, transpulmonary gradient (TPG), mean pulmonary artery pressure, pulmonary artery capillary wedge pressure, ventricular end diastolic pressure (EDP) and creatinine) and were expressed as median and interquartile range. Number of previous sternotomies was expressed as median and range. Groups were compared with the Kruskal–Wallis test and Dunn post-hoc test. Categorical data were expressed as absolute number and percentage and compared using the column proportion method. Survival from assessment was assessed using Kaplan–Meier plots and predictors were assessed using the Cox proportional hazards model. Survival from listing and transplant were compared across diagnostic groups with the log rank test. If patients underwent more than one assessment, the first date of assessment was considered. Significance was implied with a two-tailed p value <0.05 and adjusted with the Bonferroni correction. Data were analysed in SPSS v.24.
Ethics
The study was reviewed and non-requirement for approval by an NHS Research Ethics committee was confirmed by the chair of North East Tyne & Wear South Research Ethics committee.
Results
Study population
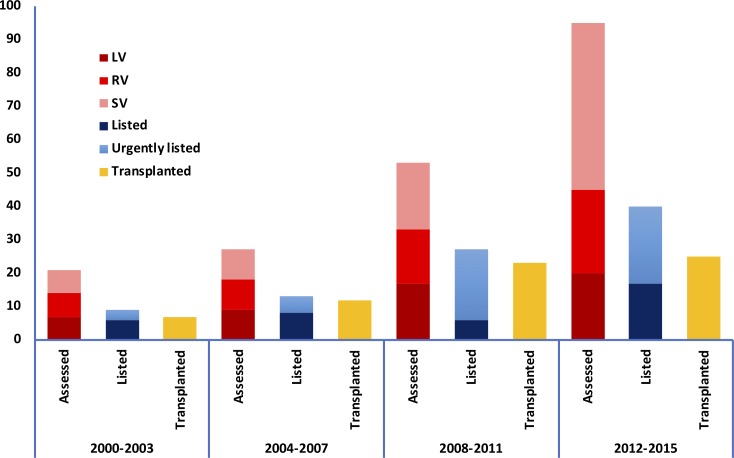
Between 2000 and 2016, 196 patients (67% male, 31.8 years (IQR 23.4–41.1)) were assessed for OHT (tables 1 and 2, figure 1).
Table 1.
Patient demographics and diagnoses at assessment for cardiac transplantation
| All | LV | RV | SV | P value | |
| n=196 | n=53 | n=57 | n=86 | ||
| Male | 131 (67%) | 38 (72%) | 42 (74%) | 51 (59%) | 0.137 |
| Age (years) | 31.8 (23.4–41.1) | 37.2 (23.3–46.3) | 34.2 (28.6–39.8) | 28.3 (21.5–35.0) | <0.001 |
| Diagnosis | |||||
| Right heart pathology (tetralogy of Fallot, pulmonary stenosis) |
18 | 18 | - | - | |
| Left heart pathology (aortic stenosis, Shone complex) | 11 | 11 | - | - | |
| Other (ventricular septal defect, venous drainage abnormalities) | 7 | 7 | - | - | |
| Ebstein anomaly | 13 | 13* | - | - | |
| Transposition of the great arteries | 38 | ||||
| Rastelli repair | 3 | - | - | ||
| Takedown Mustard/arterial switch | 1 | - | - | ||
| Mustard/Senning | - | 34 | - | ||
| CCTGA | 23 | - | - | ||
| Unoperated | 10 | ||||
| Operated | 13 | ||||
| Single ventricle | 86 | ||||
| Balanced (unoperated) | - | - | 4 | ||
| Pulmonary artery band | - | - | 3 | ||
| Shunt (various) | - | - | 11 | ||
| Glenn | - | - | 10† | ||
| Fontan | - | - | |||
| Atriopulmonary | 20 | ||||
| Lateral tunnel | 8 | ||||
| TCPC | 17‡ | ||||
| Kawashima | 8 | ||||
| Unknown | 5 |
*Six with Glenn.
†Three with forward flow, one with concomitant systemic–pulmonary shunt.
‡Five conversions.
CCTGA, congenitally corrected transposition of the great arteries; LV, left ventricle group; RV, right ventricle group; SV, single ventricle group; TCPC, total cavo-pulmonary connection
Table 2.
Comorbidities at assessment for heart transplantation
| All | LV | RV | SV | P values | |
| n=196 | n=53 | n=57 | n=86 | ||
| Previous sternotomies | 1 (0–5) | 2 (1–5) | 1 (0–4) | 2 (0–5) | <0.001 |
| Transpulmonary gradient (mm Hg) | 6 (4–11) | 6 (4–10) | 12 (6–17) | 5 (4–7) | <0.001 |
| PCWP/ventricular EDP (mm Hg) | 13 (10–24) | 18 (10–26) | 23 (12–30) | 12 (9–14) | <0.001 |
| MPAP (mm Hg) | 20 (15–35) | 24 (15–38) | 37 (20–45) | 17 (13–21) | <0.001 |
| Creatinine (µmol/L) | 98 (82–120) | 93 (79–129) | 100 (88–119) | 94 (79–118) | 0.179 |
| Renal replacement therapy | 2 | 1 | 0 | 1 | |
| Diabetes mellitus | 3 | 0 | 3 | 0 | |
| Thyroid dysfunction | 18 | 6 | 3 | 9 | |
| Neurological (prior stroke/cerebral abscess/epilepsy/head injury) | 23 | 6 | 7 | 10 | |
| Chromosomal abnormality | 1 | 1 | 0 | 0 | |
| Liver disease (viral hepatitis) | 19 | 2 | 2 | 15 | |
| Protein losing enteropathy | 7 | 0 | 0 | 7 |
EDP, end diastolic pressure; LV, left ventricle group; MPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; RV, right ventricle group; SV, single ventricle group.
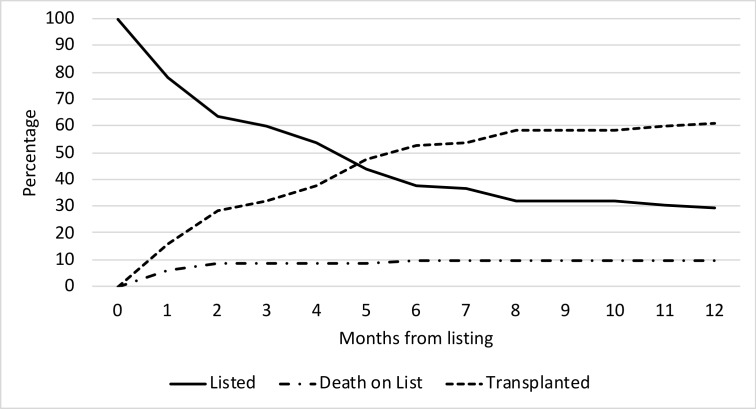
Figure 1.
Trends in assessments and transplants over time. Increased number of assessments when comparing each 4 year period with growth in both the total number of single ventricle patients assessed and the proportion of single ventricle patients assessed. The total number of transplants has remained fixed when comparing the two most recent periods. LV, left ventricle group; RV, right ventricle group; SV, single ventricle group.
Outcome following assessment for OHT
The outcome for each group of patients is described below and summarised in tables 3 and 4 and figure 1.
Table 3.
Outcomes following assessment for heart transplantation
| All patients | LV | RV | SV | P values | |
| n=196 | n=53 | n=57 | n=86 | ||
| Too high risk | 41 (21%) | 13 (25%) | 13 (23%) | 15 (17%) | NS |
| Too well | 36 (18%) | 13 (25%) | 10 (18%) | 13 (15%) | NS |
| MCS | 17 (9%) | 3 (6%)* | 13 (23%)* | 1 (1%)* | <0.05 |
| Conventional surgery or intervention | 13 (7%) | 7 (13%) | 1 (2%) | 5 (6%) | NS |
| Listed | 89 (45%) | 20 (38%) | 27 (47%) | 42 (49%) | NS |
| Urgently listed | 52 (27%) | 9 (17%) | 15 (26%) | 28 (33%) | NS |
| Assessment to list (months) | 3.5 (IQR 0.5–10.6, range 0–92.7) |
1.3 (IQR 0.1–6.9, range 0–38.3) |
2.3 (IQR 0.2–7.2, range 0–92.7) |
4.7 (IQR 1.9–11.6, range 0–47.28) |
0.058 |
| Transplanted | 67 (34%) | 16 (30%) | 18 (32%) | 33 (38%) | NS |
NS, not significant on column proportion analysis at p<0.05 level.
*Significant difference between indicated columns on column proportion analysis at p<0.05 level.
Urgently listed patients shown as a proportion of the total number within the group.
LV, left ventricle group; MCS, mechanical circulatory support; RV, right ventricle group; SV, single ventricle group.
Table 4.
Factors associated with survival in patients following assessment for orthotopic heart transplantation in Cox model
| Variables | HR | 95% CI | P value | ||
| Lower | Upper | ||||
| Age | 1.003 | 0.980 | 1.026 | 0.809 | |
| Male | 0.961 | 0.552 | 1.672 | 0.887 | |
| Ventricular morphology | |||||
| Left ventricle | Reference category | ||||
| Right ventricle | 0.643 | 0.307 | 1.347 | 0.242 | |
| Single ventricle | 1.045 | 0.567 | 1.929 | 0.887 | |
| Listing decision | |||||
| Too good | Reference category | ||||
| Listed | 3.792 | 1.138 | 12.638 | 0.030 | |
| Unsuitable | 11.199 | 3.296 | 38.048 | <0.001 | |
Unsuitable for OHT
Forty-one of 196 (21%) were considered too high risk or inappropriate for OHT. Primary reasons were:
Elevated TPG: Fourteen patients had a TPG too high for OHT. Five were LV patients (TPG 14–40 mm Hg), and three had aortic valve replacements as children with chronically elevated EDP due to longstanding patient–prothesis mismatch (two underwent conventional surgery). One was an SV patient who had previously undergone pulmonary artery banding. Eight were RV patients (TPG 16–56 mm Hg); four received VAD intended to reduce TPG (three died at 5 days, 4 months and 7 months, and one remains on support at 17 months). Six further RV patients had an initial TPG too high for OHT which was successfully reduced after VAD insertion allowing listing.16
Anatomical unsuitability: Three SV cases: diffusely hypoplastic pulmonary arteries (1), multiple areas of peripheral pulmonary artery stenosis (2).
High panel reactive antibodies: Two cases, one underwent conventional surgery.
Co-morbidity: Twelve patients (LV 4, SV 8). Six had multiple comorbidities (including concerns about future self-care, cerebral abscesses, sepsis and bronchiolitis obliterans). Five SV patients had had advanced Fontan associated liver disease (FALD) with four unsuitable for combined heart–liver transplant (CHLT). One SV patient had hepatocellular carcinoma, was actively treated and remains under assessment for CHLT.
Too unwell: Seven patients (LV 2, RV 4, SV 1). Two (1 LV, 1 RV) were pre-VAD era, one RV patient was anatomically unsuitable for VAD (multiple muscular ventricular septal defects), one RV patient was stabilised on VAD but subsequently died from a complication of a non-cardiac procedure, and one RV patient was too unwell for active management. One SV patient underwent Fontan revision and an Ebstein patient underwent tricuspid valve replacement with extracorporeal membrane oxygenation (ECMO) decannulation.
Other reasons: In three cases listing was declined due to active smoking, excessive body mass index or medication compliance issues.
In addition to these 41 patients, seven who were initially unsuitable were bridged to transplant candidacy and listing and two underwent dual organ transplant (see below).
Conventional surgery
Conventional surgery or intervention (without VAD) was offered to 18/196 patients (9%); in 11 as the preferred option, while seven were unsuitable for transplant. Two patients were subsequently listed for OHT: one underwent successful OHT 13 months following aortic valve replacement; the other died on the waiting list 12 months after Fontan conversion and desensitisation. At study closure, three patients were awaiting Fontan revision, one declined surgery and another died while considering it (see online supplementary table).
heartjnl-2019-314711supp001.docx (14.7KB, docx)
Too well
Thirty-six of 196 (18%) patients (LV 13, RV 10, SV 13) were considered too well for OHT and conventional surgery or intervention was not indicated. Two later died: one with repaired tetralogy of Fallot and right heart failure 12 years after declining reassessment; and the other 18 months after assessment with worsening Fontan failure. An additional 14 patients were initially considered too well for transplant and subsequently listed (median 23.2 months from first assessment, IQR 14.5–94.0). No patient moved from the too well group to the unsuitable group.
Other
One Fontan patient with significant FALD underwent CHLT.17 One patient with Shone complex (TPG did not fall with VAD) underwent heart–lung transplant. Four patients had not completed the assessment process at study closure. Fourteen patients were regarded suitable for OHT but declined listing (four were temporarily listed).
Listed for OHT
Eighty-nine of 196 patients (45%) were listed for OHT, 52 urgently. There were no differences in rates of listing or transplantation between diagnostic groups. Seven of 89 (9%) had been bridged to candidacy with VAD: six RV patients whose pulmonary vascular resistance fell and one following conventional surgery. One underwent conventional surgery as the initial treatment of choice but was listed after deteriorating.
Ventricular assist devices
VAD (with or without concomitant surgery) was used in 17 patients (Heartware (Medtronic, Framingham, MA, USA: RV 11, LV 2), Berlin Heart EXCOR (Berlin Heart GmbH, Berlin, Germany: RV 1, LV 1, SV 1), SynCardia Total Artificial Heart (SynCardia Systems Inc, Tucson, AZ, USA: RV 1)). Outcomes of some of the RV cohort have previously been reported.16 Ten of 17 (59%) patients were subsequently listed, five were transplanted, two died on the waiting list and three remained on the list at study close. Of the seven who did not become eligible for OHT, four died, one did not achieve a fall in TPG after 18 months and underwent heart–lung transplant, and two remained on support at study close.
Survival following assessment
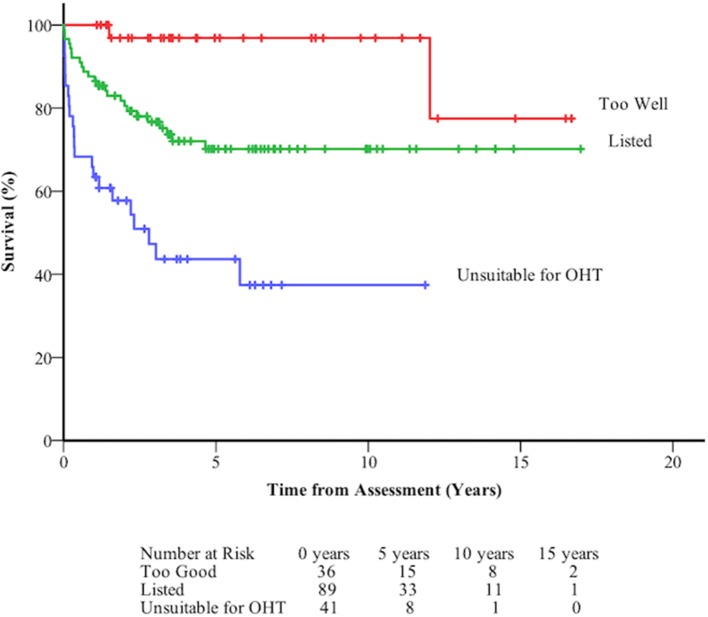
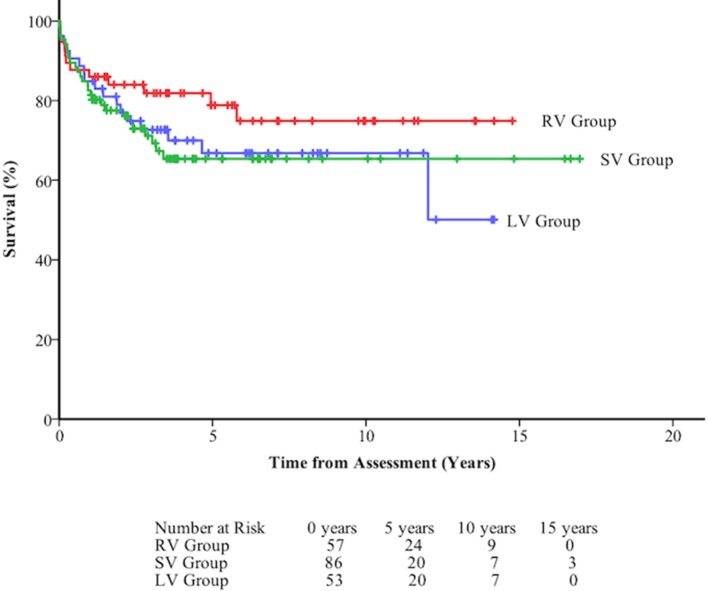
Overall survival was 84.2±2.6% at 1 year, 69.7±3.6% at 5 years, and 68.5±3.8% at 10 years. Survival from assessment was not significantly influenced by diagnostic group (figure 2, table 4). Survival following assessment varied according to decision. Those deemed unsuitable for OHT were 11 times more likely to die than those who were considered too well for OHT (figure 3, table 4).
Figure 2.
Death-free survival at long term follow-up of 196 patients assessed for orthotopic heart transplant (OHT) following assessment according to the decision made by the multidisciplinary team.
Figure 3.
Death-free survival at long term follow-up of 196 patients assessed for orthotopic heart transplant following assessment by diagnostic group. LV, left ventricle; RV, right ventricle; SV, single ventricle.
Outcome and survival following listing
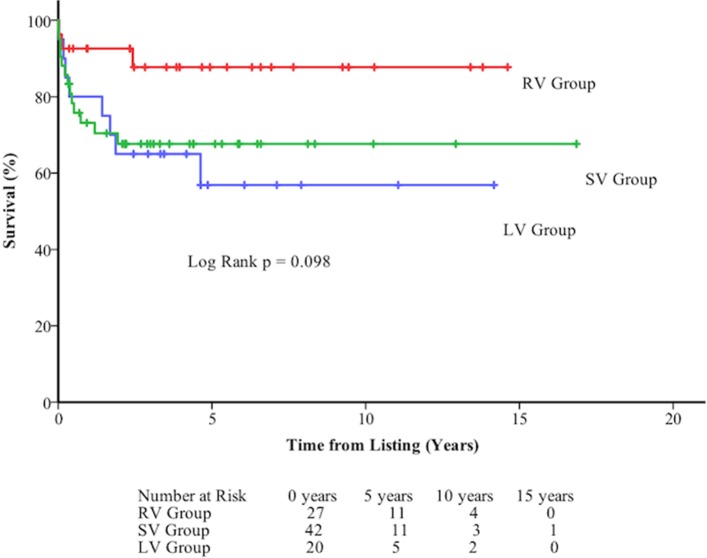
Nine of 89 (10%) (2 LV, 2 RV, 5 SV) patients listed for transplant died on the waiting list (five on the urgent list). Median time from listing to death was 24 days (range 2–885 days) while median time from listing to transplant was 104 days (range 5–1284) and from urgent listing to transplant was 33 days (range 1–507). Sixty-seven of 89 (75.2%) were transplanted. Thirteen were still waiting at study close. Overall survival following listing was 80.6±4.2% at 1 year, 70.6±5.2% at 5 years, and 70.6±5.2% at 10 years. There was no significant difference in survival between diagnostic groups (figure 4, figure 5).
Figure 4.
Death-free survival at long term follow-up of 89 patients listed for orthotopic heart transplant following listing by diagnostic group. LV, left ventricle; RV, right ventricle; SV, single ventricle.
Figure 5.
Competing interest curve following listing for transplant. All patients start on the curve as listed with the timing of possible outcomes transplant or death on the waiting list shown by the other curves. No patients were removed from the list for clinical deterioration.
Survival following transplant
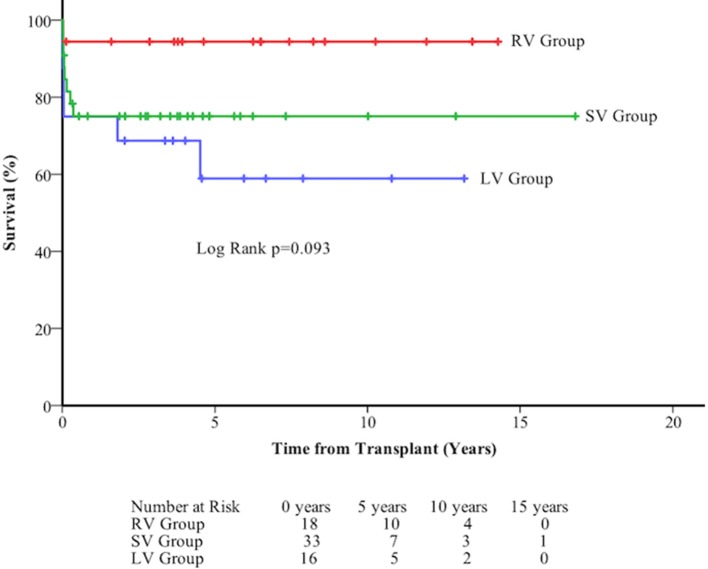
Overall survival following transplant was 85.0±4.4% at 30 days, 80.4±4.9% at 1 year, 75.9±5.6% at 5 years, and 75.9±5.6% at 10 years. There was no difference between diagnostic groups (figure 6).
Figure 6.
Death-free survival at long term follow-up of 67 patients following orthotopic heart transplant by diagnostic group. LV, left ventricle; RV, right ventricle; SV, single ventricle.
Discussion
The growing ACHD population with heart failure has resulted in rising numbers being referred for OHT assessment. Further increases are expected, particularly in the single ventricle population who already account for more than half the assessments but <5% of congenital heart disease.1 18–20 Given the heterogeneity of the population, different therapeutic approaches to address the failing circulation must be considered. We have defined three sub-groups by morphological characteristics (LV, RV, SV) who, in our experience, can also be grouped by their management strategies.
In the LV group, sub-pulmonary right ventricular function is often the dominant clinical problem limiting conventional medical strategies and use of VADs.6 7 21 The RV group, mainly composed of those with atrial switch procedures, present challenges from baffle obstruction, persistent shunts and higher TPGs—presumably due to chronically elevated EDP and, perhaps, a latent effect of the left to right shunt in early childhood. Conventional medical therapy is ineffective in RV patients, but they do comparatively well in terms of survival at all stages following assessment.6–8 This is partly due to the successful reduction in TPG with VAD and bridge to OHT and candidacy.16 VAD should be incorporated into national heart failure strategies for this group, with patients and their physicians educated accordingly. The SV group has significant comorbidity with limited options for medical therapy or VAD.6 7 22 23 Optimal timing for listing and transplanting these patients is therefore key to improving outcomes. Despite comorbidity, survival from all time points was comparable with other diagnoses, reflecting expertise gained over recent years in assessing and supporting this complex group.
Twenty per cent of ACHD patients referred for transplant were unsuitable and prognosis was poor. Elevation in the TPG and co-morbidity were key factors. Although calculation of pulmonary flow and resistance can be performed in ACHD patients, flow is often difficult to calculate reliably. We therefore tend to use TPG in the majority when deciding on the degree of pulmonary vascular disease and its impact on transplant eligibility. Both can be used in the context of transplant assessment.24 Of those with high TPG, three had left ventricular outflow obstruction due to patient–prosthesis miss-match following aortic valve replacement as children, leading to irreversible pulmonary vascular disease. Close, expert follow-up of ACHD patients is essential to ensure early surgical intervention where appropriate and avoid such complications. RV patients were most frequently found to be unsuitable due to high TPG. Some of those turned down were early in our series predating our use of VAD, which we have subsequently shown to be effective in reducing TPG and perhaps should even be considered for destination treatment in selected cases.16 Extra cardiac comorbidity is particularly pertinent to the Fontan population, with FALD (and associated renal dysfunction) being the primary reason for turning these patients down for isolated OHT. The point at which liver disease precludes OHT and the role of CHLT remain ongoing areas for investigation.23 Our Fontan liver surveillance programme has evolved over the study period. At present, those with cirrhosis but normal synthetic function, normal hepatic venous anatomy, good liver volume and no significant portal hypertension or hepatocellular carcinoma are considered for heart-only transplantation.
Death on the transplant waiting list is a well-recognised problem.11–13 Earlier listing and transplantation before major comorbidities develop, particularly in those unsuitable for VAD, may address this. The disadvantages ACHD patients face on the waiting list are well described and changes in UK listing criteria and proposed changes in the USA may address this.11–13 15 25 Our experience with Fontan patients deteriorating on the list and arriving for transplant in a worse clinical condition has led us to keep many patients waiting in hospital for daily review and proactive management (eg, milrinone infusion) to ensure they are optimised when an organ becomes available. The limitation of any strategy with transplant at its centre will always be donor supply and in our cohort, despite increasing numbers being assessed and listed and a small increase in OHTs, the proportion of those transplanted fell. This is reflected in the UK OHT numbers which have remained fixed since 2013 despite a rise in those patients listed and the growth in the failing UK ACHD population.26 27 Two patients in our cohort received dual organ transplantation. Limited donor availability is even more pertinent to these patients and this is unlikely to be a realistic solution for very many ACHD patients.28
Although prognostic biomarkers are being sought for this heterogeneous population, robust clinically useful markers to guide decision making, particularly as patients approach transplant, are still to be established.29 OHT is an emotive subject, and the ability to conduct clinical trials to determine its effectiveness compared with other strategies is fraught with significant ethical and methodological barriers. Mortality as the sole and preferred marker of outcome in ACHD transplantation should also be questioned. Survival does not consistently equate to benefit and patient reported outcome measures should also be considered highly relevant in this complex situation.
Limitations
This is a single centre experience reporting an MDT decision; other units will have different working practices. The level of risk that transplant centres are able to tolerate is multifactorial and influenced by experience with ACHD patients, overall transplant outcomes and the referral population. Patients have been grouped according to perceived clinical commonalities based on underlying diagnosis. They remain a heterogeneous group with various mechanisms of heart failure and indications for transplant. Analysing data by decision at a specific time point is complicated and limited as decisions and candidacy change along the patient pathway. Regional variation in patient populations and referral practices also influence the data presented. In particular a unit’s decision to carry out high risk conventional surgery in place of transplant assessment referral will vary widely. Our experience with high risk conventional surgery with mechanical support used as back up, an alternative treatment or bridge to candidacy, has evolved during the study period and is always considered by the MDT. The medium and long term outcomes of these alternate strategies are not yet established.
Conclusions
The ACHD population referred for OHT assessment will grow dramatically over the next few decades. OHT is a component of a multifaceted advanced heart failure strategy and should be provided by an MDT with expertise in all adjunctive areas to identify and manage the anatomical and physiological challenges. Recognition of the different sub-groups of ACHD heart failure patients should lead the medical community to develop condition-specific management strategies that include appropriate education and support for patients and carers.
Key questions.
What is already known on this subject?
Adults with moderate and complex congenital heart disease (ACHD) have higher mortality than the general population, with most deaths due to cardiovascular causes, particularly heart failure.
Cardiac transplant for ACHD has a relatively high early mortality; late outcome (beyond 8 years) is better than cardiac transplant for all other causes.
What this study adds
The number of ACHD patients referred for cardiac transplant assessment in the UK has quadrupled over 16 years. One third undergo transplant.
Survival from assessment is 85% at 1 year and 70% at 5 years. Underlying congenital diagnosis has no influence on subsequent mortality or chance of transplantation. Those considered unsuitable or too high risk have worse outcomes (survival 63% at 1 year, 44% at 5 years) than those who are listed (survival 88% at 1 year, 70% at 5 years) or those considered too well (survival 100% at 1 year, 97% at 5 years).
How this might impact on clinical practice
ACHD heart failure patients should be referred early for transplant assessment to ensure all alternative options are considered. Rigorous follow-up of patients considered too well is required to ensure that transplant candidacy is not lost due to the development of comorbidity.
Although effective, cardiac transplant is only available to a few ACHD patients and counselling of patients at all stages should reflect this.
Footnotes
Contributors: DSC, KJ, GP, AH, GP, AD, LC collected the data for the manuscript. DSC, KJ, LC analysed the data and prepared the manuscript. All authors edited the manuscript and approved the final version.
Funding: The authors would like to thank the Children’s Heart Unit Fund (CHUF, www.chuf.org.uk) for funding the colour printing and open access availability of this paper.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Khairy P, Ionescu-Ittu R, Mackie AS, et al. Changing mortality in congenital heart disease. J Am Coll Cardiol 2010;56:1149–57. 10.1016/j.jacc.2010.03.085 [DOI] [PubMed] [Google Scholar]
- 2. Verheugt CL, Uiterwaal CS, van der Velde ET, et al. Mortality in adult congenital heart disease. Eur Heart J 2010;31:1220–9. 10.1093/eurheartj/ehq032 [DOI] [PubMed] [Google Scholar]
- 3. Diller GP, Kempny A, Alonso-Gonzalez R, et al. Survival prospects and circumstances of death in contemporary adult congenital heart disease patients under follow-up at a large tertiary centre. Circulation 2015;132:2118–25. 10.1161/CIRCULATIONAHA.115.017202 [DOI] [PubMed] [Google Scholar]
- 4. Greutmann M, Tobler D, Kovacs AH, et al. Increasing mortality burden among adults with complex congenital heart disease. Congenit Heart Dis 2015;10:117–27. 10.1111/chd.12201 [DOI] [PubMed] [Google Scholar]
- 5. Opotowsky AR, Siddiqi OK, Webb GD. Trends in hospitalizations for adults with congenital heart disease in the U.S. J Am Coll Cardiol 2009;54:460–7. 10.1016/j.jacc.2009.04.037 [DOI] [PubMed] [Google Scholar]
- 6. Stout KK, Broberg CS, Book WM, et al. Chronic heart failure in congenital heart disease: a scientific statement from the American Heart Association. Circulation 2016;133:770–801. 10.1161/CIR.0000000000000352 [DOI] [PubMed] [Google Scholar]
- 7. Budts W, Roos-Hesselink J, Rädle-Hurst T, et al. Treatment of heart failure in adult congenital heart disease: a position paper of the working group of grown-up congenital heart disease and the heart failure association of the European Society of Cardiology. Eur Heart J 2016;37:1419–27. 10.1093/eurheartj/ehv741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roche SL, Redington AN. The failing right ventricle in congenital heart disease. Can J Cardiol 2013;29:768–78. 10.1016/j.cjca.2013.04.018 [DOI] [PubMed] [Google Scholar]
- 9. Irving C, Parry G, O’Sullivan J, et al. Cardiac transplantation in adults with congenital heart disease. Heart 2010;96:1217–22. 10.1136/hrt.2009.184713 [DOI] [PubMed] [Google Scholar]
- 10. Lund LH, Khush KK, Cherikh WS, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-fourth adult heart transplantation report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant 2017;36:1037–46. 10.1016/j.healun.2017.07.019 [DOI] [PubMed] [Google Scholar]
- 11. Alshawabkeh LI, Hu N, Carter KD, et al. Wait-list outcomes for adults with congenital heart disease listed for heart transplantation in the U.S. J Am Coll Cardiol 2016;68:908–17. 10.1016/j.jacc.2016.05.082 [DOI] [PubMed] [Google Scholar]
- 12. Everitt MD, Donaldson AE, Stehlik J, et al. Would access to device therapies improve transplant outcomes for adults with congenital heart disease? Analysis of the United Network for Organ Sharing (UNOS). J Heart Lung Transplant 2011;30:395–401. 10.1016/j.healun.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 13. Davies RR, Russo MJ, Yang J, et al. Listing and transplanting adults with congenital heart disease. Circulation 2011;123:759–67. 10.1161/CIRCULATIONAHA.110.960260 [DOI] [PubMed] [Google Scholar]
- 14. Banner NR, Bonser RS, Clark AL, et al. UK guidelines for referral and assessment of adults for heart transplantation. Heart 2011;97:1520–7. 10.1136/heartjnl-2011-300048 [DOI] [PubMed] [Google Scholar]
- 15. Zalewska K. Heart transplantation: selection criteria and recipient registration. http://odt.nhs.uk/pdf/heart_selection_policy.pdf (Accessed 1 May 2018).
- 16. Peng E, O’Sullivan JJ, Griselli M, et al. Durable ventricular assist device support for failing systemic morphologic right ventricle: early results. Ann Thorac Surg 2014;98:2122–9. 10.1016/j.athoracsur.2014.06.054 [DOI] [PubMed] [Google Scholar]
- 17. Duong P, Coats L, O’Sullivan J, et al. Combined heart-liver transplantation for failing Fontan circulation in a late survivor with single-ventricle physiology. ESC Heart Fail 2017;4:675–8. 10.1002/ehf2.12202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coats L, O’Connor S, Wren C, et al. The single-ventricle patient population: a current and future concern a population-based study in the North of England. Heart 2014;100:1348–53. 10.1136/heartjnl-2013-305336 [DOI] [PubMed] [Google Scholar]
- 19. Schilling C, Dalziel K, Nunn R, et al. The Fontan epidemic: population projections from the Australia and New Zealand Fontan Registry. Int J Cardiol 2016;219:14–19. 10.1016/j.ijcard.2016.05.035 [DOI] [PubMed] [Google Scholar]
- 20. Marelli AJ, Ionescu-Ittu R, Mackie AS, et al. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation 2014;130:749–56. 10.1161/CIRCULATIONAHA.113.008396 [DOI] [PubMed] [Google Scholar]
- 21. MacGowan GA, Schueler S. Right heart failure after left ventricular assist device implantation: early and late. Curr Opin Cardiol 2012;27:296–300. 10.1097/HCO.0b013e3283511e60 [DOI] [PubMed] [Google Scholar]
- 22. Murtuza B, Hermuzi A, Crossland DS, et al. Impact of mode of failure and end-organ dysfunction on the survival of adult Fontan patients undergoing cardiac transplantation. Eur J Cardiothorac Surg 2017;51:135–41. 10.1093/ejcts/ezw243 [DOI] [PubMed] [Google Scholar]
- 23. Greenway SC, Crossland DS, Hudson M, et al. Fontan-associated liver disease: implications for heart transplantation. J Heart Lung Transplant 2016;35:26–33. 10.1016/j.healun.2015.10.015 [DOI] [PubMed] [Google Scholar]
- 24. Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant 2016;35:1–23. 10.1016/j.healun.2015.10.023 [DOI] [PubMed] [Google Scholar]
- 25. Organ procurement and transplantation network. Modify adult heart allocation 2016 2nd round. https://optn.transplant.hrsa.gov/governance/public-comment/modify-adult-heart-allocation-2016-2nd-round/ (Accessed 1 May 2018).
- 26. NHS Blood and Transplant. Annual report on cardiothoracic organ transplantation. Report for 2016/2017. 2018. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/5418/cardiothoracic-annual-report-2016-17.pdf.
- 27. Dimopoulos K, Muthiah K, Alonso-Gonzalez R, et al. Heart or heart-lung transplantation for patients with congenital heart disease in England. Heart 2019;105:596–602. 10.1136/heartjnl-2018-313984 [DOI] [PubMed] [Google Scholar]
- 28. Bradley EA, Pinyoluksana KO, Moore-Clingenpeel M, et al. Isolated heart transplant and combined heart-liver transplant in adult congenital heart disease patients: insights from the united network of organ sharing. Int J Cardiol 2017;228:790–5. 10.1016/j.ijcard.2016.11.121 [DOI] [PubMed] [Google Scholar]
- 29. Van De Bruaene A, Hickey EJ, Kovacs AH, et al. Phenotype, management and predictors of outcome in a large cohort of adult congenital heart disease patients with heart failure. Int J Cardiol 2018;252:80–7. 10.1016/j.ijcard.2017.10.086 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2019-314711supp001.docx (14.7KB, docx)