Abstract
A 75-year-old man with stage IV chronic kidney disease due to type 2 diabetes mellitus, presented with increasing proteinuria and rapidly declining renal function despite excellent glycaemic control. Investigations organised to assess his suitability for renal transplantation included an abdominal CT scan, which revealed extensive intra-abdominal lymphadenopathy. A 17fluorodeoxyglucose (FDG)-positron emission tomography scan to further characterise the lymphadenopathy demonstrated activity in the lymph nodes, as well as both kidneys. Following a lymph node biopsy and flow cytometry he was diagnosed with a marginal zone lymphoma. A subsequent kidney biopsy confirmed lymphomatous infiltration of the kidney. Marginal zone lymphoma is an uncommon type of non-Hodgkin's lymphoma, and renal involvement is rare. This case highlights the importance of considering alternative diagnoses when there is deviation from the expected clinical trajectory and the importance of liaising with colleagues in other disciplines to enable an accurate diagnosis to be made.
Keywords: chronic renal failure, proteinurea, haematology (incl blood transfusion), pathology, diabetes
Background
This case report highlights a rare finding of lymphomatous renal infiltration with a low grade non-Hodgkin's lymphoma (NHL) and has several valuable clinical lessons.
First, it demonstrates the importance of clinicians considering alternative diagnoses in a chronic kidney disease (CKD) patient with deteriorating renal function out of keeping with the primary renal pathology.
Second, the case highlights the importance of a renal biopsy in patients with deteriorating renal function to identify the renal pathology.
Finally, the case emphasises the importance of liaising with laboratory staff to ensure that samples are suitably triaged to maximise diagnostic yield. Flow cytometry is not routinely performed on renal biopsies but was an important component of this patient’s diagnosis.
Case presentation
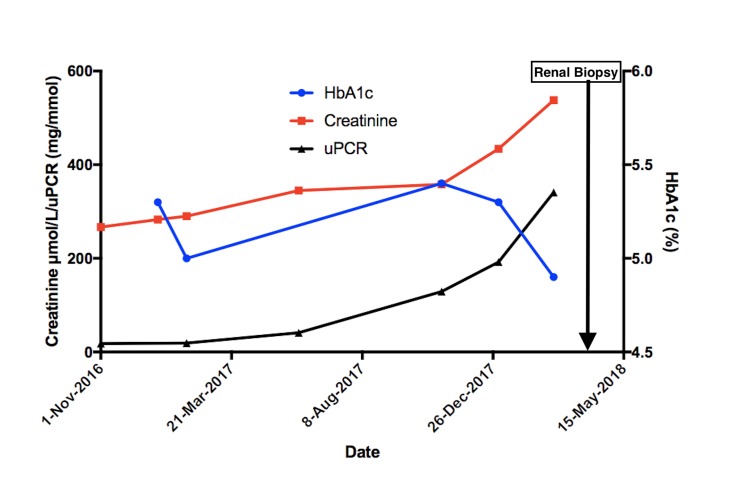
A 75-year-old man with stage IV CKD on a background of previously poorly controlled type 2 diabetes mellitus (T2DM) was reviewed in his routine renal outpatient appointment. His renal function had been slowly worsening for the past 2 years; however, on this occasion a marked increase in creatinine and proteinuria was noted despite excellent blood glucose control during the preceding 6 months, with an average glycated haemoglobin (HbA1c) of 5% (figure 1).
Figure 1.
Renal function and glycaemia control over time. HbA1c: glycated haemoglobin; uPCR: urine protein creatinine ratio.
Apart from the T2DM complicated by neuropathy and nephropathy, his past medical history also included hypertension.
He was compliant with all of his medications with no significant recent medication changes and his regular medications included gliclazide, moxonidine, telmisartan/amlodipine, oral iron sulfate and vadadustat (an oral prolyl hydroxylase inhibitor).
He was an ex-smoker having smoked during his teenage years and he consumed less than 3 units of alcohol/week.
There was no family history of renal disease or haematological malignancies.
Examination revealed moderate lymphadenopathy in the left cervical region. There was no other peripheral lymphadenopathy and no hepatosplenomegaly. The remainder of the examination was normal.
Plans for renal replacement therapy were made, including commencement of work-up to determine suitability for renal transplantation and to investigate the lymphadenopathy. A subsequent CT of his abdomen revealed extensive lymphadenopathy. Due to progressive fluid overload and worsening biochemical parameters, he was commenced on haemodialysis.
Investigations
As shown in figure 1, there was a significant decline in renal function with the creatinine rising to 538 µmol/L from 385 µmol/L in a 4-month period. He developed heavy proteinuria of 3.4 g/day, which was not evident in a urine sample done 2 months prior (figure 1). His urinalysis also showed microscopic haematuria. His albumin was 23 g/L; however, his cholesterol profile was normal.
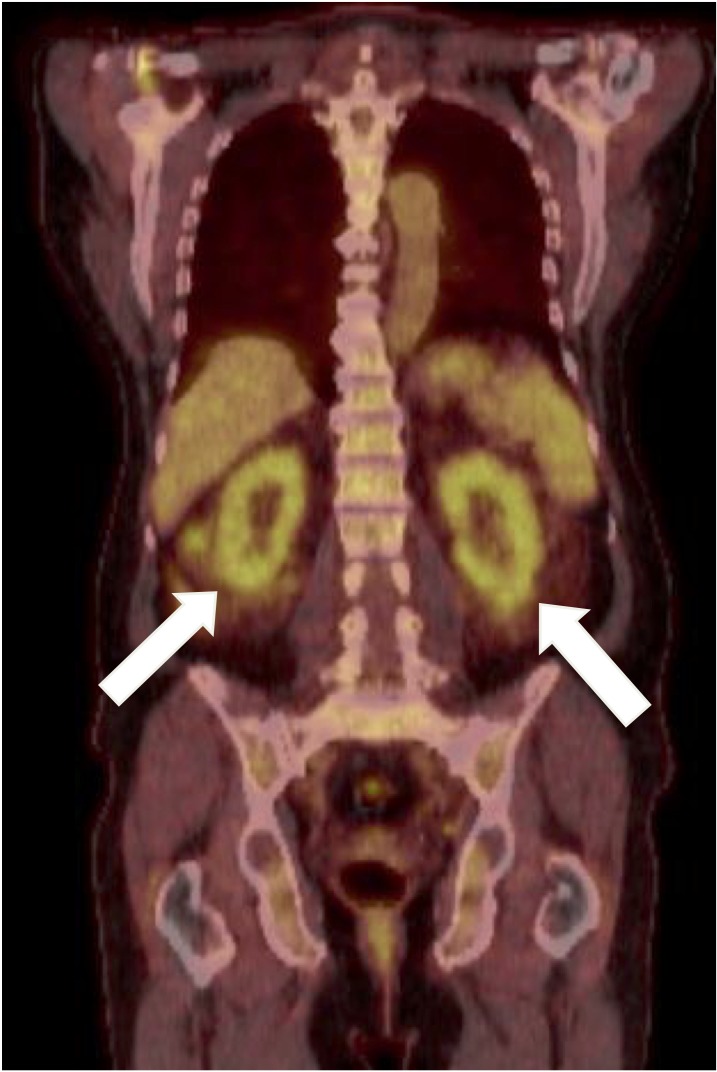
A CT scan of his abdomen reported multiple enlarged lymph nodes in the iliac, inguinal and para-aortic regions, with the largest lymph node measuring 9 mm in size. The kidney size was preserved, with a length of 105 mm on the left side and 112 mm on the right side. A subsequent 17fluorodeoxyglucose (FDG)-positron emission tomography (PET) scan demonstrated low to moderate activity in the cervical, axillary and abdominopelvic areas. Splenic activity was also increased suggesting infiltration and there was mild prominence of marrow activity. There was increased renal activity bilaterally and associated perinephric stranding (figure 2).
Figure 2.
17FDG positron emission tomography scan showing increased activity in the kidneys. FDG, fluorodeoxyglucose.
He proceeded to have a bone marrow biopsy and excisional cervical lymph node biopsy. Both showed evidence of a CD5 positive B-cell population with IgG kappa light chain restriction consistent with a low grade B-cell marginal zone lymphoma.
Following review at a multidisciplinary meeting, a renal biopsy was recommended as the FDG-avidity in the kidneys was considered atypical and the faster than expected decline in renal function raised the suspicion of renal infiltration by the lymphoma. The conclusion by the multidisciplinary meeting was that the presence and extent of renal involvement by the lymphoma would impact the treatment recommendations made, including the consideration to solely monitor this patient if there was no evidence of renal infiltration.
Given the technical difficulties with the renal biopsy there were limited samples available for analysis. However, with knowledge of the clinical context, the pathologist had requested priority for a core to be triaged for flow cytometry. As a result, no samples were available for immunofluorescence or electron microscopy.
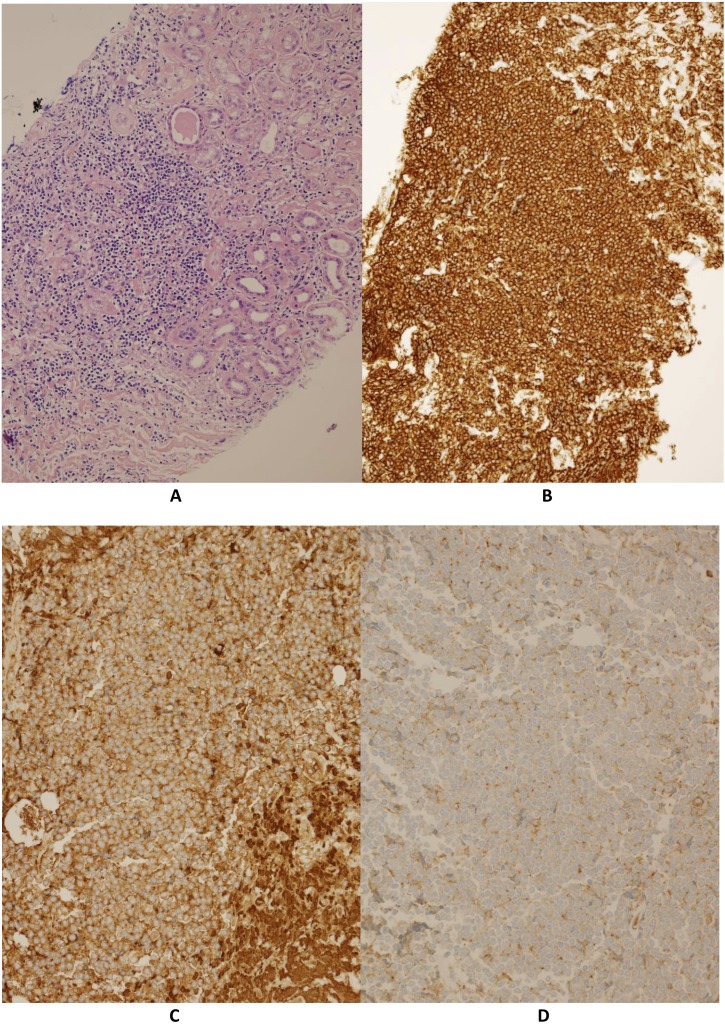
The renal biopsy revealed widespread lymphocytic infiltration throughout both the renal parenchyma and perinephric fat, with extensive tubular atrophy and significant interstitial fibrosis causing glomeruli ischaemia. The glomeruli did not show any evidence of mesangial hypercellularity, endocapillary proliferation or agyrophilic basement membrane spikes, although focal basement membrane splitting was evident. The sample flow cytometry confirmed a kappa light chain restricted population of CD20 positive B-cells expressing CD5. The findings were concordant with the lymph node biopsy and consistent with marginal zone lymphoma invading the kidney (figure 3).
Figure 3.
(A) Lymphocytic infiltration of the kidney, (B) infiltrating cells are CD20 positive, (C and D) kappa light chain restriction of the cells.
Differential diagnosis
Differential diagnoses for a patient with increasing proteinuria (eventually within nephrotic range) include focal segmental glomerulosclerosis, minimal change disease, membranous glomerulopathy, membranoproliferative glomerulonephritis and worsening diabetic nephropathy. However, these renal pathologies, with the exception of diabetic nephropathy, do not usually present with impaired renal function unless they are in the chronic phase or associated with marked volume disturbances. While there was microscopic haematuria, given the lack of serological markers of autoimmune disease and the clinical presentation, other differentials, such as anti-neutrophil cytoplasmic antibodies (ANCA)-associated vasculitis, IgA nephropathy, anti-glomerular basement membrane (GBM) disease and lupus nephritis were considered unlikely.
Apart from diabetic nephropathy (an unlikely cause in this case given this patient's excellent glycaemic control as demonstrated by his HbA1c), most of these pathologies have both primary and secondary causes. Important secondary causes to consider include infection, malignancy, medication (again an unlikely differential in this case) and connective tissue disorders.
Infiltrative conditions of the kidneys, such as lymphomatous involvement are often associated with renal enlargement, which was not a prominent feature in our patient.1–4 Although diabetes can also cause renal enlargement, his diabetic control had been good and there was no evidence of diabetic glomerular changes on the biopsy. Any enlargement in renal size was therefore likely offset by the reduction in kidney volume that is commonly seen in CKD.
Due to the limited renal biopsy samples, and lack of immunofluorescence and electron microscopy samples it is difficult to ascertain exactly what glomerulopathy was present to explain the increased proteinuria. We suspect, given the focal basement membrane splitting on light microscopy that higher resolution imaging would confirm widespread basement membrane pathology with associated podocyte effacement.
Treatment
After discussions with the patient, he was commenced on a rituximab, cyclophosphamide, vincristine and prednisolone chemotherapy treatment regime for his marginal zone lymphoma with renal infiltration.
In this patient there were two reasons that this chemotherapy regime was offered and commenced, rather than the decision to solely monitor him. One being the significant infiltration by the lymphoma of both kidneys into the surrounding perinephric tissue, seen on imaging and supported by the renal biopsy. This was considered likely to become symptomatic in the near future without treatment. The other was the possibility of some recovery of renal function, although he was advised this was unlikely given he had already commenced dialysis.
Outcome and follow-up
Despite having completed six cycles of chemotherapy with repeat PET scans demonstrating a good treatment response, his serial creatinine and urea levels have remained static, and he has minimal urine output. He has therefore remained on haemodialysis.
The light chain restriction seen on serum electrophoresis has also resolved since the commencement of chemotherapy. Given this patient has remained dialysis dependent no further assessments of proteinuria have been performed.
With his recently treated lymphoma, he is currently ineligible for a renal transplant. He continues to have regular follow-up with both the renal and haematology services.
Discussion
Marginal zone lymphoma is an uncommon type of low grade NHL, with the incidence rate of 1.8 per 100 000 people in the USA in 2016.5
Several case series from autopsies performed in patients who had lymphoma show that the presence of malignant lymphocytes in the kidneys may occur more commonly than clinically suspected.6–10 One small case series demonstrated that while 14% of patients had been diagnosed with renal infiltration by their lymphoma, autopsies showed that there were actually 34% of patients with renal involvement.10 This under diagnosis is likely due to most patients lacking clear clinical and/or radiological findings of renal lymphocytic invasion.8 9 However, clinically relevant renal involvement in low grade B-cell lymphoproliferative malignancies does remain rare.1–4 7 11–24
There are only eight biopsy proven cases of renal involvement by marginal zone lymphoma in the published literature.1 4 20–22 24 Of these, three presented with proteinuria and an associated acute kidney injury, two presented with nephrotic syndrome alone, two presented with acute kidney injury alone and in one case the diagnosis was made following a nephrectomy, which was done in a patient with proteinuria and worsening renal failure who was found to have a renal mass on imaging.1 4 20–22 24
This case highlights the importance of considering alternate and additional diagnoses in CKD patients whose disease is progressing at a rate out of keeping with their expected trajectory. This point is reiterated in a retrospective study published in 2013 involving 611 renal biopsies on diabetic patients.25 This showed that only 227 (37%) of these patients showed pure diabetic changes on renal biopsy, with 384 (63%) of these patients displaying evidence of additional non-diabetic diseases.25
An interesting factor in this case is the patient’s use of vadadustat. Vadadustat is an oral prolyl hydroxylase (PHD) inhibitor currently in a phase III clinical trial for the management of CKD-associated anaemia.26–29 Phase II studies have shown vadadustat to be an effective treatment for CKD related anaemia.26–29 There is a theoretical concern regarding the increased risk of tumour progression due to vascular endothelial growth factor promotion through PHD inhibition.26–29 However, there is not yet sufficient long-term data to support this hypothesis, and therefore it is unclear whether this medication would have had any effect on this patient’s lymphoma progression.
Also noteworthy is that while light microscopy showed features consistent with lymphocytic infiltration of the kidneys, flow cytometry played an important role in providing confirmatory evidence of renal involvement by the marginal zone lymphoma.
Flow cytometry is not a routine investigation for renal biopsies and requires samples to be collected into culture media such as Roswell Park Memorial Institute media (rather than buffer and fixative, which is the standard renal biopsy collection method). The tissue sample was only requested to be taken for flow cytometry by the pathologist after clinical details for this specific case was provided by the clinician. This highlights the importance of communication of the clinical context with the pathologist analysing and interpreting the biopsy specimens to ensure appropriate triage of tissue to maximise diagnostic yield.
The diagnosis of NHL with renal involvement is significant and has the potential to have an impact on the decision made for the most appropriate NHL treatment and the ongoing management of his CKD, including suitability for transplantation.
Learning points.
Consider alternate renal pathology in a patient with progressive deterioration of their glomerular filtration rate out of keeping with their primary renal pathology.
The importance of renal biopsy in identifying the cause of deteriorating renal function, which in this case was the rare finding of lymphomatous renal infiltration by a low grade non-Hodgkin's lymphoma.
Discussion of clinical cases, especially abnormal presentations, with the pathologist to facilitate appropriate triage of the biopsy to maximise diagnostic yield.
Taking into consideration a patient’s indications for intervention and how this will change patient outcomes.
Footnotes
Correction notice: This article has been corrected since it was first published online. This particluar patient was on trial drug "Vadadustat" rather than "Daprodustat".
Contributors: Patient is under the clinical care of AC and GC. Literature review and first draft written by AS. Significant edits and contributions by AC, GC and DW. Pathology images provided by DW. All authors have been involved in this patient's investigation and clinical care.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Yaomura T, Hayashi H, Kanoh T, et al. [A case of non-Hodgkin's lymphoma presenting with acute renal failure diagnosed by renal biopsy]. Nihon Jinzo Gakkai Shi 1999;41:505–10. [PubMed] [Google Scholar]
- 2.Kasmani R, Marina VP, Abidi S, et al. Minimal change disease associated with malt lymphoma. Int Urol Nephrol 2012;44:1911–3. 10.1007/s11255-011-9992-z [DOI] [PubMed] [Google Scholar]
- 3.Peces R, Vega-Cabrera C, Peces C, et al. [MALT B cell lymphoma with kidney damage and monoclonal gammopathy: a case study and literature review]. Nefrologia 2010;30:681–6. 10.3265/Nefrologia.pre2010.Jun.10428 [DOI] [PubMed] [Google Scholar]
- 4.Lubas A, Mróz A, Smoszna J, et al. Membranoproliferative glomerulonephritis, mantle cell lymphoma infiltration, and acute kidney injury. Int Urol Nephrol 2013;45:1489–94. 10.1007/s11255-012-0210-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan E, Hin C, Ooi M, et al. Non-Hodgkin's lymphoma. England: BMJ. Available: https://bestpractice-bmj-com.qelibresources.health.wa.gov.au/topics/en-gb/312
- 6.Li S-J, Chen H-P, Chen Y-H, et al. Renal involvement in non-Hodgkin lymphoma: proven by renal biopsy. PLoS One 2014;9:e95190 10.1371/journal.pone.0095190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.On W, Udberg M. Acute kidney injury as first presentation of lymphoma: the role of renal biopsy. Case Rep Child Meml Hosp Chic 2013;2013 10.1136/bcr-2013-202196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richards MA, Mootoosamy I, Reznek RH, et al. Renal involvement in patients with non-Hodgkin's lymphoma: clinical and pathological features in 23 cases. Hematol Oncol 1990;8:105–10. 10.1002/hon.2900080206 [DOI] [PubMed] [Google Scholar]
- 9.Richmond J, Sherman RS, Diamond HD, et al. Renal lesions associated with malignant lymphomas. Am J Med 1962;32:184–207. 10.1016/0002-9343(62)90289-9 [DOI] [PubMed] [Google Scholar]
- 10.Cohen LJ, Rennke HG, Laubach JP, et al. The spectrum of kidney involvement in lymphoma: a case report and review of the literature. Am J Kidney Dis 2010;56:1191–6. 10.1053/j.ajkd.2010.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Salam S, Shaaban A, Alketbi M, et al. Acute kidney injury secondary to renal large B-cell lymphoma: role of early renal biopsy. Int Urol Nephrol 2011;43:237–40. 10.1007/s11255-010-9728-5 [DOI] [PubMed] [Google Scholar]
- 12.Dauchy F-A, Etienne G, Deminière C, et al. [Lymphoma with initial renal involvement: four cases]. Rev Med Interne 2006;27:909–15. 10.1016/j.revmed.2006.07.015 [DOI] [PubMed] [Google Scholar]
- 13.Domazetovski I, Jovanovic R, Kostadinova-Kunovska S, et al. Acute renal failure in a patient with diffuse large B-cell lymphoma: case report. Prilozi 2012;33:231–8. [PubMed] [Google Scholar]
- 14.Erdoğmuş Şiyar, Aktürk S, Kendi Çelebi Z, et al. Diffuse large B-cell lymphoma presenting with bilateral renal masses and hematuria: a case report. Turk J Haematol 2016;33:159–62. 10.4274/tjh.2015.0238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merta M, Jelínková E, Zabka J, et al. [Renal infiltration in lymphoma--diagnosis in renal biopsy (case report)]. Sb Lek 2002;103:405–9. [PubMed] [Google Scholar]
- 16.Monfared A, Orangpoor RO, Fakheri TF, et al. Acute renal failure and bilateral kidney infiltration as the first presentation of non-Hodgkin lymphoma. Iran J Kidney Dis 2009;3:50–3. [PubMed] [Google Scholar]
- 17.Sellin L, Friedl C, Klein G, et al. Acute renal failure due to a malignant lymphoma infiltration uncovered by renal biopsy. Nephrol Dial Transplant 2004;19:2657–60. 10.1093/ndt/gfh201 [DOI] [PubMed] [Google Scholar]
- 18.Takei T, Uchiyama M. Diffuse large B-cell lymphoma with involvement of the kidney. BMJ Case Rep 2012;2012 10.1136/bcr-2012-006281. [Epub ahead of print: 03 Jul 2012]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Törnroth T, Heiro M, Marcussen N, et al. Lymphomas diagnosed by percutaneous kidney biopsy. Am J Kidney Dis 2003;42:960–71. 10.1016/j.ajkd.2003.08.004 [DOI] [PubMed] [Google Scholar]
- 20.Chelioti E, Efthimiou E, Sotiraki M, et al. Splenic marginal zone lymphoma and concurrent membranoproliferative glomerulonephritis with IgMKappa deposits in a HCV-Seropositive patient. Nephrourol Mon 2014;6:e18391 10.5812/numonthly.18391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akiyama K, Itabashi M, Shiohira S, et al. [Successful rituximab therapy for MALT lymphoma complicating cryoglobulinemic glomerulonephritis]. Nihon Jinzo Gakkai Shi 2011;53:713–8. [PubMed] [Google Scholar]
- 22.Hoshino T, Iriuchijima H, Ogawa Y, et al. [Splenic marginal zone B-cell lymphoma with bilateral renal invasion after splenectomy]. Rinsho Ketsueki 2008;49:35–9. [PubMed] [Google Scholar]
- 23.Stokes MB, Wood B, Alpers CE. Membranoproliferative glomerulonephritis associated with low-grade B cell lymphoma presenting in the kidney. Clin Nephrol 2002;57:303–9. 10.5414/CNP57303 [DOI] [PubMed] [Google Scholar]
- 24.Mak SK, Wong PN, Lo KY, et al. Successful treatment of IgA nephropathy in association with low-grade B-cell lymphoma of the mucosa-associated lymphoid tissue type. Am J Kidney Dis 1998;31:713–8. 10.1053/ajkd.1998.v31.pm9531192 [DOI] [PubMed] [Google Scholar]
- 25.Sharma SG, Bomback AS, Radhakrishnan J, et al. The modern spectrum of renal biopsy findings in patients with diabetes. Clin J Am Soc Nephrol 2013;8:1718–24. 10.2215/CJN.02510213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akizawa T, Tsubakihara Y, Nangaku M, et al. Effects of Daprodustat, a novel hypoxia-inducible factor prolyl hydroxylase inhibitor on anemia management in Japanese hemodialysis subjects. Am J Nephrol 2017;45:127–35. 10.1159/000454818 [DOI] [PubMed] [Google Scholar]
- 27.Becker KA, Jones JJ. An emerging treatment alternative for anemia in chronic kidney disease patients: a review of Daprodustat. Adv Ther 2018;35:5–11. 10.1007/s12325-017-0655-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta N, Wish JB. Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors: A Potential New Treatment for Anemia in Patients With CKD. Am J Kidney Dis 2017;69:815–26. 10.1053/j.ajkd.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 29.Pergola PE, Spinowitz BS, Hartman CS, et al. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int 2016;90:1115–22. 10.1016/j.kint.2016.07.019 [DOI] [PubMed] [Google Scholar]