Abstract
Marantic endocarditis is a rare non-infectious endocarditis that mostly affects the aortic and mitral valves. It is often an autopsy finding that is most commonly seen in advanced malignancies thought to be due to a hypercoagulable state. When diagnosed antemortem, it typically presents with signs and symptoms of embolisation. We report a case of a 44-year-old Caucasian man with marantic endocarditis secondary to metastatic small cell lung cancer. Our patient presented with a short history of lower back pain with no signs/symptoms of embolisation, and a pansystolic murmur on initial physical examination. Serial blood cultures were negative and transthoracic echocardiography revealed vegetation on the posterior leaflet of the mitral valve. Further imaging in the form of MRI spine and CT thorax/abdomen/pelvis showed pulmonary lesions with liver and bony metastasis. Subsequent image-guided biopsy confirmed metastatic small cell lung cancer of T1N2M1c grade, which was treated with palliative chemotherapy and radiotherapy.
Keywords: valvar diseases, cancer - see oncology
Background
Marantic endocarditis (also known as non-bacterial thrombotic endocarditis, Libman-Sacks endocarditis or verrucous endocarditis), first described by Armand Trousseau in 1888, is a rare non-infectious endocarditis characterised by the deposition of sterile platelet thrombi on mostly aortic and mitral valves.1 It most commonly affects patients between the fourth and eighth decades of life with no sex predilection. Importantly, it is often found postmortem with rates in autopsy series ranging from 0.9%–1.6%1–7; however, when diagnosed antemortem, presentation is typical with signs and/or symptoms of systemic embolisation.1 8
Interestingly, 80% of marantic endocarditis is seen in advanced malignancies, particularly adenocarcinoma of the lung, pancreas, colon, prostate, ovary and the biliary system.1 3 9 10 It is less commonly seen in inflammatory conditions such as systemic lupus erythematosus, antiphospholipid syndrome, rheumatoid arthritis and sepsis.1 11–14
Case presentation
A 44-year-old Caucasian man presented to the emergency department with a 10-day history of lower back pain. He described the pain as 'electric shock-like' and non-radiating in nature. He denied any history of saddle anaesthesia, weight loss, fever or trauma; however, he reported a 2-day history of mild dysuria and haematuria.
His medical history included hypertension treated with ramipril 10 mg once daily, amlodipine 5 mg once daily and bisoprolol 2.5 mg once daily. He did not have any history of drug allergy.
He had never smoked, but had a history of passive exposure to cigarette smoking. He was employed as a customer service manager, with no significant family history of note.
On physical examination, observations revealed a respiratory rate of 16 breaths/min, oxygen saturation 96% on room air, blood pressure 140/103 mmHg, heart rate 115 beats/min and temperature 37.7°C. He had no peripheral stigmata of endocarditis; however, he had a pansystolic murmur heard louder in the apex, radiating to the axilla, with normal jugular venous pressure, no pitting oedema and clear chest on auscultation. In addition, there was tenderness in the lumbar (L3-L4) vertebrae with no paraspinal tenderness or focal neurological deficit.
Inpatient investigations included urinalysis, ECG, blood tests and serial blood cultures, chest and lumbar spine radiographs, transthoracic echocardiogram (TTE), MRI whole spine and CT scan of thorax, abdomen and pelvis.
He was started on intravenous gentamicin and vancomycin for infective endocarditis according to the local hospital protocol while some test results were awaited.
Investigations
Initial ECG showed sinus rhythm with normal PR interval. No abnormality was detected on urinalysis. Blood results were unremarkable except for raised C-reactive protein 83 mg/L (<5 mg/L), with normal white cell count 8.6×109/L (4.0–11.0) and abnormal alkaline phosphatase 262 U/L (30–130), and alanine transaminase 71 U/L (<50). Chest and lumbar spine radiographs were unremarkable ( figures 1 and 2 ). Three sets of blood culture were taken before the initiation of antimicrobial therapy but they had no microbial growth after 14 days.
Figure 1.
Chest radiograph on admission which was unremarkable.
Figure 2.
Lumbar spine radiograph demonstrating straightening of the normal lumbar lordosis and minor posterior disc space narrowing at L5/S1 with no obvious lesions or fractures.
TTE showed normal left ventricular size and hyperdynamic systolic function with an echogenic mobile structure attached to the posterior leaflet of the mitral valve (figures 3–5).
Figure 3.
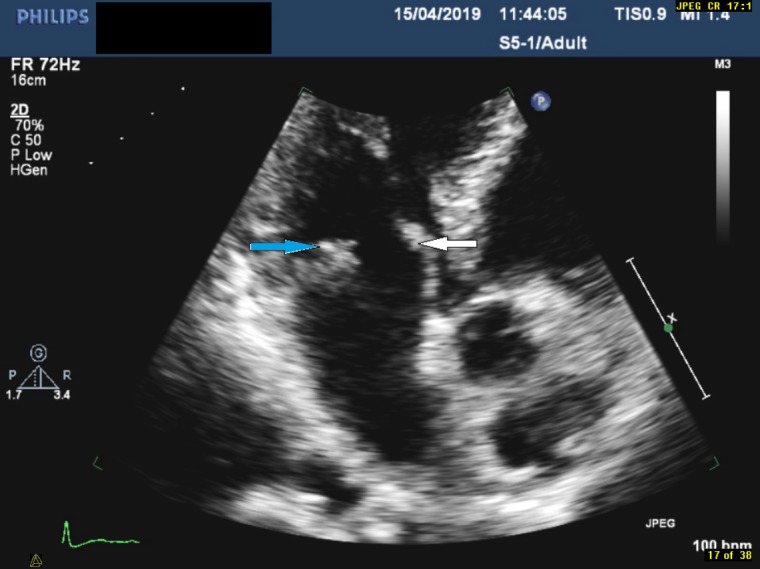
Focused view of the mitral valve in the apical three-chamber view demonstrating echogenic mass on the posterior leaflet (indicated by the blue arrow) and thickened anterior leaflet (indicated by the white arrow).
Figure 4.
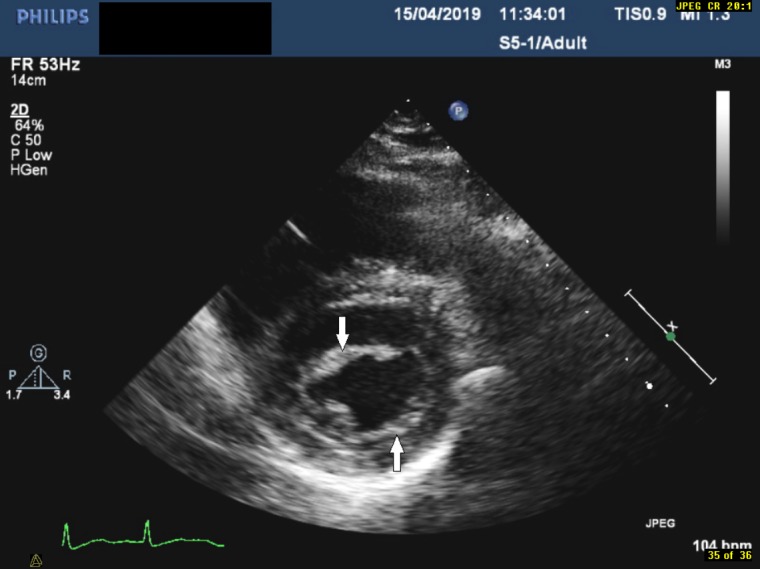
Parasternal short axis view demonstrating a thickened mitral valve (indicated by the arrows).
Figure 5.
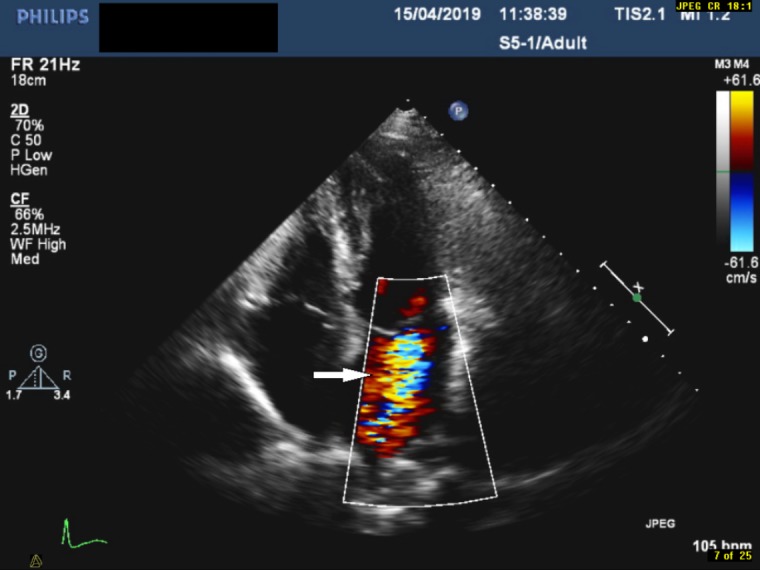
Apical four-chamber view with colour flow doppler demonstrating mitral regurgitation (indicated by the arrow).
Subsequently, antimicrobial therapy was escalated to vancomycin, gentamicin, rifampicin and doxycycline to cover for blood culture-negative endocarditis. Additional blood tests for culture-negative micro-organisms (Coxiella burnetii, Bartonella sp, Legionella spp, Mycoplasma and Brucella) were also performed at this stage and came back negative.
Following the severe nature of the back pain, and tenderness in the L3-L4 vertebrae, we requested an MRI whole spine. This showed widespread areas of low T1 and high T2 signal change throughout the spinal column, involving anterior and posterior elements suspicious of widespread bone metastases (figures 6 and 7). Consequently, a CT scan of thorax, abdomen and pelvis showed multiple cavitating and subpleural lesions in hemithoraces, widespread liver lesions, lytic lesions in the spine with para-aortic adenopathy (figures 8–10). Further blood tests for HIV and tumour markers such as alpha-fetoprotein, beta human chorionic gonadotrophins, prostate specific antigen, carcinoembryonic antigen were normal, except for CA199 elevated at 1919 KU/L (0–35).
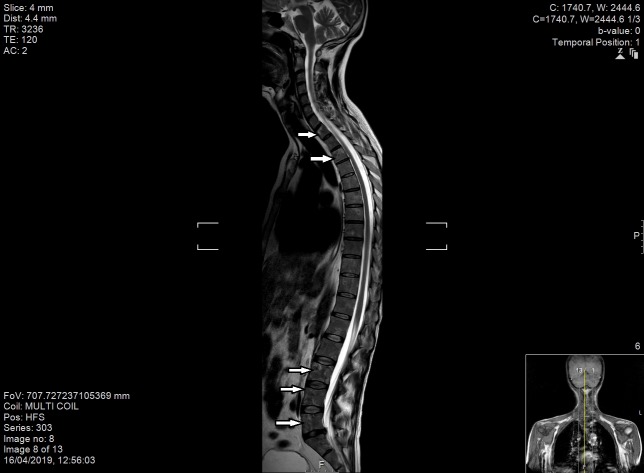
Figure 6.
MRI spine demonstrating widespread areas of low T1 and high T2 signal change throughout the spinal column, involving anterior and posterior elements (indicated by the arrows).
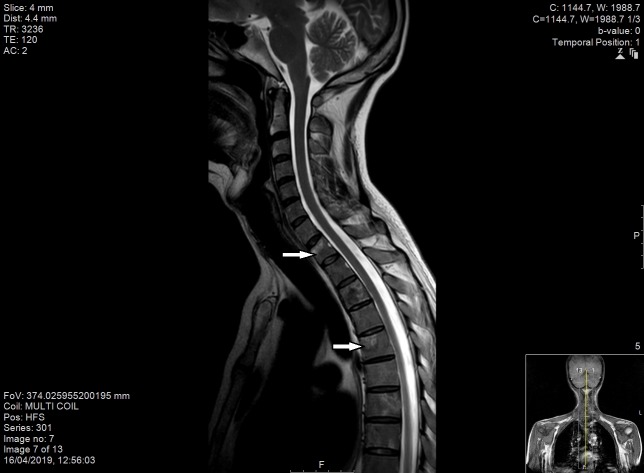
Figure 7.
MRI spine demonstrating widespread areas of low T1 and high T2 signal change throughout the spinal column, involving anterior and posterior elements (indicated by the arrow).
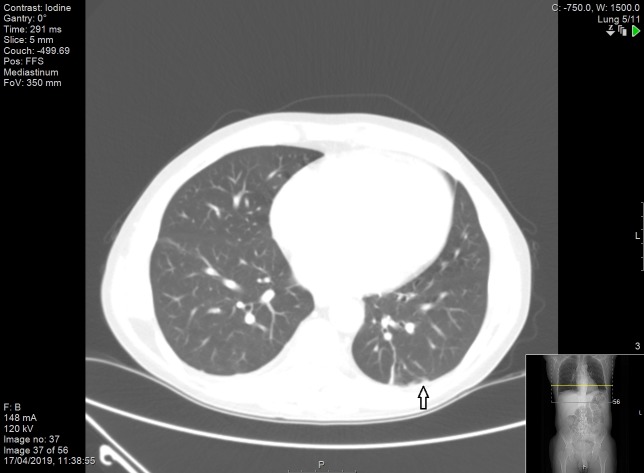
Figure 8.
CT scan of the thorax demonstrating multiple subpleural solid pulmonary lesions (indicated by the arrow).
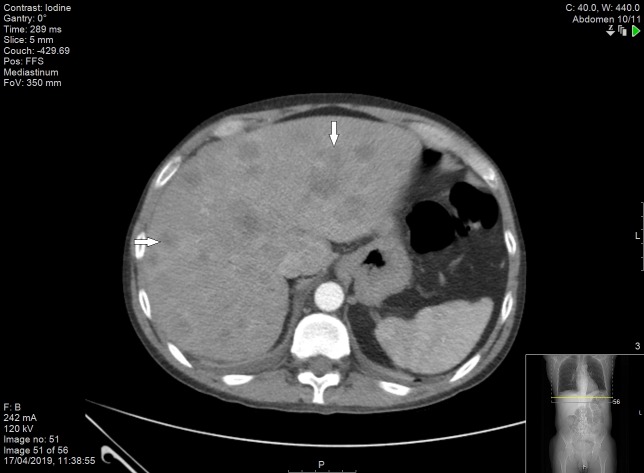
Figure 9.
CT scan of abdomen and pelvis demonstrating enlarged liver with multiple lesions (indicated by the arrows).
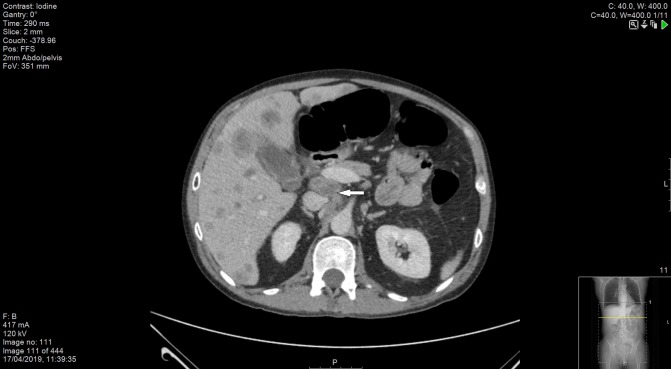
Figure 10.
CT scan of abdomen and pelvis demonstrating significant para-aortic adenopathy (indicated by the arrow).
He underwent an ultrasound-guided biopsy of the liver lesions with subsequent immunohistochemistry confirming chemosensitive metastatic small cell lung cancer of T1N2M1c grade. Antimicrobial therapy was subsequently stopped as the echogenic vegetation on the posterior leaflet of the mitral valve was thought to be marantic endocarditis. Anticoagulation was considered for secondary prevention of thromboembolic phenomenon but the patient was deemed unsuitable due to the advanced stage of lung cancer, and the patient himself was not keen on anticoagulation.
Differential diagnosis
Infective endocarditis.
Atrial myxoma.
Fibroelastoma.
Lambl’s excrescences.
Treatment
Carboplatin etoposide.
Radiotherapy L2-L4 8 Gy single fraction.
Outcome and follow-up
Pain control with analgesia and lower back radiotherapy.
Discharged home and subsequently started treatment with carboplatin etoposide.
Palliative care input.
Discussion
It is often very difficult to distinguish marantic endocarditis from infective endocarditis, primarily due to lack of specific laboratory tests for marantic endocarditis. Typically, patients with marantic endocarditis are asymptomatic until embolisation (cerebrovascular or systemic) occurs. Cerebrovascular embolisation can present with signs and symptoms of stroke or transient ischaemic attack while systemic embolisation can manifest as peripheral arterial embolism or mesenteric ischaemia.1 In our case, the patient presented with lower back pain, and a pansystolic murmur on heart auscultation. This is of particular importance as cardiac murmurs are infrequently noted in marantic endocarditis and occur in <50% of the cases.1 It is plausible that the pansystolic murmur heard from heart auscultation is due to mitral regurgitation as shown by the echocardiogram (figure 5). However, our patient had no clinical signs/symptoms of heart failure. The mitral regurgitation revealed by echocardiography is consistent with the case report by Mantovani et al, 15 although their patient had symptoms of heart failure.
Our patient presented with atypical signs and symptoms of infective endocarditis, and the pre-test probability for infective endocarditis was low as per the modified Duke criteria.16 Importantly, multiple negative blood cultures, as well as negative tests for specific micro-organisms responsible for blood culture-negative endocarditis played a pivotal role in ruling out infective endocarditis in our patient. Blood culture-negative endocarditis is an important diagnosis to be excluded in our patient because it accounts for 2.5% – 70% of all cases of endocarditis depending on the country.17 Factors such as early use of antimicrobial therapy prior to blood culture and fastidious zoonotic micro-organisms may contribute to high incidence of blood-culture negative endocarditis, although geographical variation exists. However, in our patient, three blood cultures were taken prior to initiating antimicrobial therapy. Importantly, tests for the fastidious micro-organisms were negative.
Echocardiography is vital in the detection of valvular vegetations; however, it is equally difficult to differentiate sterile thrombus from infective endocarditis. Echocardiographic features of marantic endocarditis vegetation may include small and sessile (typically ≤10 mm) with irregular borders, broad-based, easily friable (leading to embolisation), heterogeneous echo density and a characteristic absence of independent motion of the vegetations on the cardiac valves (two-thirds involving the basal and mid-portions of the mitral valve, up to a quarter involving the aortic valve and less commonly both valves).1 8 Although, transoesophageal echocardiogram (TOE) is more sensitive than TTE, two-dimensional TTE is the first choice with TOE reserved for cases where TTE is unrevealing, with suitable patients.1 Our echocardiographic findings are consistent with the findings of previously published cases.8 18
The aetiology of marantic endocarditis remains somewhat unclear. It is strongly associated with advanced malignancies and autoimmune conditions.1 3 9 10 However, endothelial injury in the setting of a hypercoagulable state is thought to play a pivotal role in its pathogenesis. It is believed that activation of the coagulation system by malignancies, disseminated intravascular coagulation, and conditions such as antiphospholipid syndrome and systemic lupus erythematosus trigger the release of proinflammatory cytokines such as tumour necrosis factor and interleukin-1. These proinflammatory cytokines in turn cause endothelial damage, which triggers platelet interwoven with fibrin strands, immune complexes and mononuclear cells deposition on the cardiac valves.1 8 This can lead to systemic embolisation, physical disabilities and death if untreated.
Treatment for marantic endocarditis is not well established in literature. Systemic anticoagulation to prevent thromboembolic phenomena can be considered unless there is contraindication such as central nervous system haemorrhage, in addition to therapy directed at treating the underlying cause.1 8 Lifelong therapeutic dose of subcutaneous low molecular weight heparin or intravenous unfractionated heparin is the anticoagulant treatment of choice. This is because vitamin K antagonist such as warfarin, or other agents that inhibit direct thrombin and factor Xa such as dabigatran, rivaroxaban, edoxaban and apixaban have been shown to be less-effective at reducing the rate of recurrent embolisation.1 8 Our patient was not started on any anticoagulant due to the stage of the cancer and patient's wishes.
Finally, incidental finding of the changes in the T1 and T2-weighted signals on the MRI whole spine prompted the search for an underlying malignancy, which led to the diagnosis of marantic endocarditis, having excluded blood culture-negative endocarditis. We note from previously published cases8 16 that malignancies often trigger the search for marantic endocarditis and/or vice versa. We believe that some of the barriers to antemortem diagnosis of marantic endocarditis would be removed if clinicians have a high index of suspicion for the disease in patients with known malignancies or autoimmune diseases, and have blood culture-negative valvular vegetations.
Learning points.
Blood culture-negative endocarditis should be excluded in patients with marantic endocarditis.
Malignancies and inflammatory conditions such as systemic lupus erythematosus antiphospholipid syndrome should be actively sought when marantic endocarditis is suspected.
A traumatic lower back pain in young patients should not be dismissed as another sacroiliitis.
Footnotes
Contributors: WIU, IK and SG were involved in the care of the patient. WIU gathered the materials and wrote the manuscript. IK and SG proofread and edited the manuscript. RS provided the descriptions of the echographic images.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Bauer KA. Nonbacteria thrombotic endocarditis. Uptodate, 2019. [Google Scholar]
- 2. Deppisch LM, Fayemi AO. Non-bacBacterial thrombotic endocarditis: clinicopathologic correlations. Am Heart J 1976;92:723–9. 10.1016/s0002-8703(76)80008-7 [DOI] [PubMed] [Google Scholar]
- 3. Rosen P, Armstrong D. Nonbacterial thrombotic endocarditis in patients with malignant neoplastic diseases. Am J Med 1973;54:23–9. 10.1016/0002-9343(73)90079-X [DOI] [PubMed] [Google Scholar]
- 4. González Quintela A, Candela MJ, Vidal C, et al. Non-Bacterial thrombotic endocarditis in cancer patients. Acta Cardiol 1991;46:1–9. [PubMed] [Google Scholar]
- 5. Steiner I. [Nonbacterial thrombotic endocarditis--a study of 171 case reports]. Cesk Patol 1993;29:58–60. [PubMed] [Google Scholar]
- 6. Llenas-García J, Guerra-Vales JM, Montes-Moreno S, et al. Nonbacterial thrombotic endocarditis: clinicopathologic study of a necropsy series. Rev Esp Cardiol 2007;60:493–500. 10.1016/S1885-5857(07)60190-X [DOI] [PubMed] [Google Scholar]
- 7. Eiken PW, Edwards WD, Tazelaar HD, et al. Surgical pathology of nonbacterial thrombotic endocarditis in 30 patients, 1985–2000. Mayo Clin Proc 2001;76:1204–12. 10.4065/76.12.1204 [DOI] [PubMed] [Google Scholar]
- 8. Shibata N, Matsumoto K, Kitamura S, et al. Nonbacterial thrombotic endocarditis concomitant with repeated systemic embolization that received palliative care based on the antemortem diagnosis. Intern Med 2018;57:3559–63. 10.2169/internalmedicine.1381-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. el-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist 2007;12:518–23. 10.1634/theoncologist.12-5-518 [DOI] [PubMed] [Google Scholar]
- 10. Borowski A, Ghodsizad A, Cohnen M, et al. Recurrent embolism in the course of marantic endocarditis. Ann Thorac Surg 2005;79:2145–7. 10.1016/j.athoracsur.2003.12.024 [DOI] [PubMed] [Google Scholar]
- 11. Ferrans VJ, Rodríguez ER. Cardiovascular lesions in collagen-vascular diseases. Heart Vessels 1985;1:256–61. 10.1007/BF02072405 [DOI] [PubMed] [Google Scholar]
- 12. Wada H, Sase T, Yamaguchi M. Hypercoagulant states in malignant lymphoma. Exp Oncol 2005;27:179–85. [PubMed] [Google Scholar]
- 13. Hughson MD, McCarty GA, Sholer CM, et al. Thrombotic cerebral arteriopathy in patients with the antiphospholipid syndrome. Mod Pathol 1993;6:644–53. [PubMed] [Google Scholar]
- 14. Sharma S, Mayberry JC, Deloughery TG, et al. Fatal cerebroembolism from nonbacterial thrombotic endocarditis in a trauma patient: case report and review. Mil Med 2000;165:83–5. 10.1093/milmed/165.1.83 [DOI] [PubMed] [Google Scholar]
- 15. Mantovani F, Navazio A, Barbieri A, et al. A first described case of cancer-associated non-bacteria thrombotic endocarditis in the era of direct oral anticoagulants. Thromb Res 2017;149:45–7. 10.1016/j.thromres.2016.11.016 [DOI] [PubMed] [Google Scholar]
- 16. Sexton D, Chu VH. Clinical manifestations and evaluation of adults with suspected native valve endocarditis. Uptodate, 2019. [Google Scholar]
- 17. Fournier P-E, Gouriet F, Casalta J-P, et al. Blood culture-negative endocarditis: improving the diagnostic yield using new diagnostic tools. Medicine 2017;96:e8392 10.1097/MD.0000000000008392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Starobinska E, Robinson EA, Brucks E, et al. Marantic endocarditis: incidental infarcts leading to diagnosis of pancreatic cancer. BMJ Case Rep 2018;3:bcr-2018-224529 10.1136/bcr-2018-224529 [DOI] [PMC free article] [PubMed] [Google Scholar]