Abstract
Nasopharyngeal carcinoma (NPC), an uncommon malignancy in Western Countries and Radiotherapy, remains an effective treatment. Its side effects are classified as either immediate or late; postradiation necrosis is as an important late side effect with a strong impact on the prognosis in patients with NPC. We report the case of 65-year-old Caucasian man presenting with a deep necrotic ulcer of the nasopharynx and osteoradionecrosis of the skull base that appeared 3 months after radiotherapy for nasopharyngeal carcinoma. Conservative treatment was applied with surgical management of the ulcer. Clinical and radiological outcomes are presented. Radiotherapy remains a good treatment option with varying degrees of side effects, in particular, postradiation necrosis and ulcer. Multiple options of treatment have been described. However, the surgical management could be indicated in cases of deep ulcer with life-threatening prognosis.
Keywords: imaging and management, nasopharyngeal carcinoma, necrosis, osteoradionecrosis, radiotherapy, ulcer
Background
Nasopharyngeal carcinoma (NPC) is rare in the Western Countries and more common in Southeast Asia.1 Radiotherapy remains a treatment option for NPC. However, side effects induced by radiation could be acute or late. Late side effects develop after several months or years and could include both the bones and soft tissues of the neck and nasopharynx.2
Radiation-induced necrosis of the nasopharynx, as its name suggests, is necrosis of the tissues of the nasopharynx, such as the mucosa, muscles, parapharyngeal tissues and skull base.3 Due to the anatomy of the nasopharynx and the approximately of the internal carotid artery (ICA), This necrosis could induced an erosion of the ICA with the risk of rupture and becomes life threatening.
Traditionally, postradiation nasopharyngeal ulcers (PRNUs) are divided into mucosal ulcers induced by radiation and tumour necrosis-induced ulcers.4
The management of radiation-induced necrosis and ulcers of the nasopharynx depend on the severity of the UPRNN and varies from conservative medical treatment to surgical debridement.
We report a case of radiation-induced deep ulcer of the nasopharynx associated with skull base osteoradionecrosis and clivus bone exposure. Surgical management by using multiple grafts followed by a customised medical treatment was applied. Clinical, endoscopic and radiological outcomes are presented below.
Case presentation
A 65-year-old Caucasian man presented in January 2018, following the appearance since 3 months of a unilateral left-sided nasal obstruction associated with permeant pulsatile tinnitus of the left ear. His medical background showed an adenocarcinoma of the prostate staged pT2 cN0 M0 treated surgically in 2016. The fibroendoscopic examination of the nose showed an exophytic lesion in the nasopharynx on the left side. The examination of the left ear showed a retraction of the tympanic membrane with a signs of a retrotympanic effusion. The rest of the clinical examination was normal.
Multiple biopsies of the left-sided nasopharyngeal mass were done, and the histology confirmed the diagnosis of an undifferentiated Epstein-Barr virus (EBV) positive carcinoma of the nasopharynx. An initial radiological evaluation with CT and positron emission tomography (PET)-CT were performed that revealed a hypermetabolic lesion in the nasopharynx on the left side (standardised uptake volume (SUV) max at 17.5) with suspicious hypermetabolic cervical and retropharyngeal lymph nodes on both sides (SUV max at 14.2 and 12.3), without evidence of a distant metastasis (figure 1A). The disease was staged as cT2 cN2 cM0, and the patient was treated by radiotherapy with a total dose of 69.96 Gy on the tumour side and 52.8 Gy on the lymph nodes bilaterally with concomitant chemotherapy (weekly carboplatin 2 area under the curve, 90 mg intravenously with Taxol 50 mg/m²).
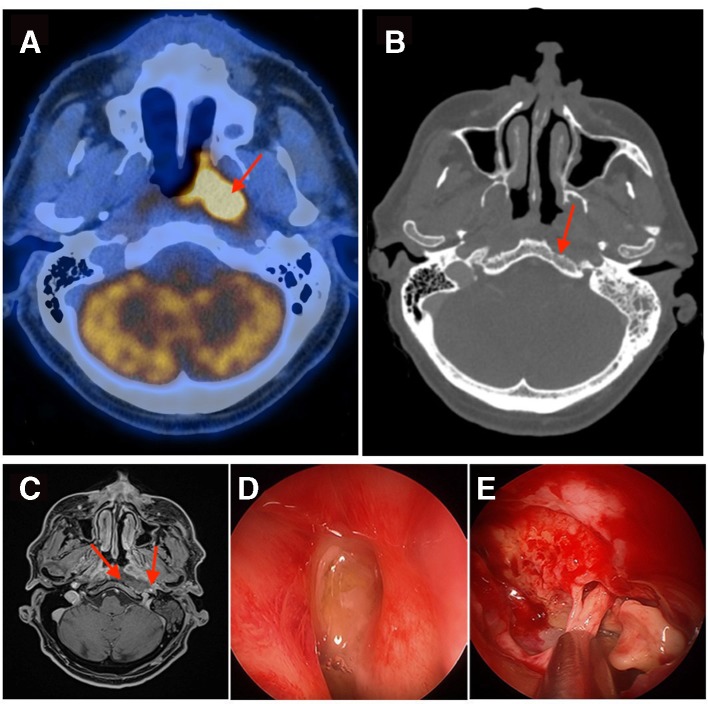
Figure 1.
(A) Pretreatment PET-CT image shows the hypermetabolic mass on the left side of the nasopharynx. (B) Axial CT image (bony window) shows the osteoradionecrosis of the skull base with the erosion of the clivus bone on the left side. (C) Axial MRI T1 image after injection of gadolinium shows the presence of wide cavity, loss of the soft tissues and exposure of the left-sided ICA to the nasopharynx air. (D) Intraoperative endoscopic view shows an important purulent discharge at the level of the nasopharynx on the left side. (E) Intraoperative endoscopic view shows a deep ulcer and necrosis on the posterior wall of the nasopharynx with erosion of the clivus bone on the left side. ICA, internal carotid artery; PET, positron emission tomography.
Radiological evaluation by PET-CT 3 months after the end of the treatment showed a complete response at the level of the cervical lymph nodes on both sides but persistence of mild hypermetabolic activity in the nasopharynx; on the left side that was considered as an inflammatory reaction induced by radiation therapy.
The patient presented 4 months following treatment completion, sever left-sided otalgia with hemicranial pain. The fibroendoscopic examination of the nose showed a large ulcer with mucosal necrosis and purulent discharge on the posterior wall of the nasopharynx. Radiological evaluation by CT (figure 1B) showed erosions of the clivus bone that were compatible with osteitis associated with diffuse inflammation in the retropharyngeal space and the skull base. Additionally, there was a large abscess causing a compression of the internal carotid artery on the left side.
An intravenous antibiotic (piperacillin (Tazobac)—4.5 g three times per day) was applied, and the patient underwent a surgical debridement with drainage of the abscess by an intranasal endoscopic approach (figure 1D and E). The bacterial biological analysis of the discharge taken from the fluid of the abscess showed S taphylococcus aureus and anaerobic germs. Biopsies of the ulcer and the surrounding tissues confirmed eradication of tumour. The antibiotic was changed to intravenous coamoxicillin (1 g two times per day) and followed for an additional 6 weeks associated with daily nasal wash with saline solution. The follow-up at 3 weeks postsurgical debridement showed a marked decrease in the inflammatory signs and biological parameters.
Radiological evaluation with an MRI (figure 1C) 2 months after the conservative treatment was performed and showed the persistence of a deep ulcer on the posterior wall of the nasopharynx, exposure of the clivus bone, the deep extension of the necrosis of the soft tissues and the threat of rupture of the internal carotid artery, a surgical closure of the defect of the nasopharynx was performed.
The surgical procedure was done by a transnasal endoscopic approach; the edges of the ulcer have been cleaned, fat grafts have been harvested from the periumbilical region and a fascia lata graft was taken from the region of the lateral thigh on the left side. A first layer of fascia lata was applied on the clivus bone and the deep part of the ulcer, the cavity of the defect was filled by the fat grafts and a second layer of fascia lata was applied on the surface. This was then fixed to the surrounding mucosa using the adhesive tissues (figure 2A–D).
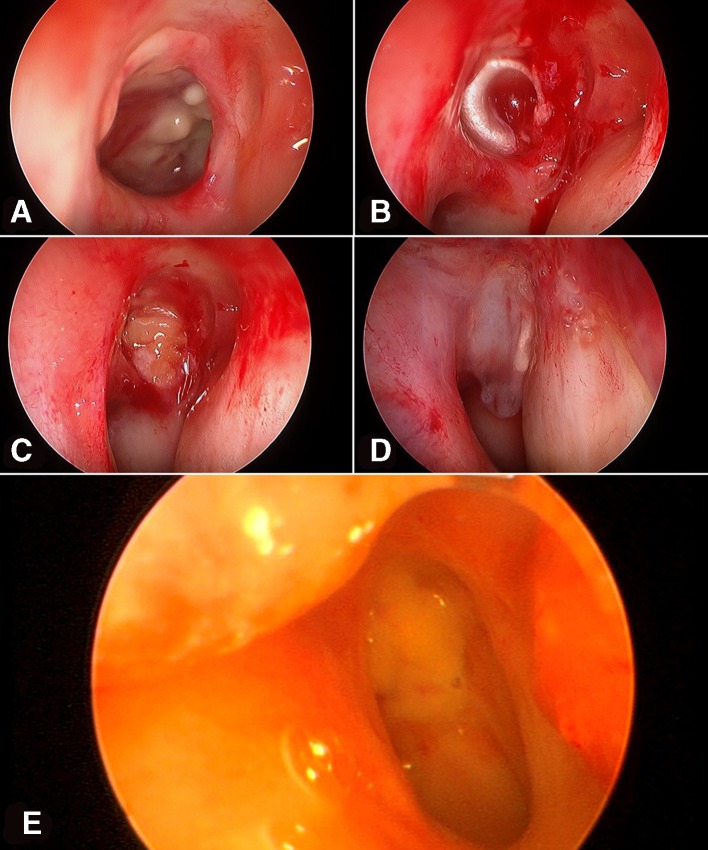
Figure 2.
The second surgical procedure. (A) Intraoperative endoscopic view shows the regression of the inflammatory signs with the persistence of a deep ulcer of the nasopharynx on the left side. (B) Endoscopic view shows the application of the first layer of fascia lata in the deep part of the cavity of the ulcer. (C) Endoscopic view shows the application of the fat grafts to filled the cavity of the ulcer. (D) Endoscopic view shows the application of the second layer of fascia lata on the surface of the mucosa of the posterior wall of the nasopharynx. (E) Endoscopic view 4 months after the surgery shows a good healing and integration of the grafts with a compete closure of the defect of the nasopharynx on the left side.
The postoperative care was simple; it included regular nose cleansing and use of intravenous antibiotics. The patient was discharged 2 weeks after the surgery; the antibiotic was changed to oral clindamycin (dose of 600 mg three times per day) and followed for additional 3 months.
The follow-up at 4 months after the surgery showed absence of any complications, good healing with complete integration of the grafts, complete closure of the defect of the nasopharynx and total regression of the inflammatory signs (figure 2E).
Outcome and follow-up
Radiological evaluation by PET-CT 3 months after the radiochimiotherapy showed a complete response at the level of the cervical lymph nodes on both side, persistence of mild hypermetabolic activity in the nasopharynx on the left side.
Follow- up at 4 months showed the development of radiation-induced deep ulcer and necrosis of the nasopharynx with osteoradionecrosis and abscess of the skull base without histological evidence of tumour recurrence. The management was included surgical debridement associated with medical treatment and surgical procedure to cover and protect the internal carotid artery.
Follow-up at 4 months showed a total regression of the inflammatory signs and parameters, absence of complications and complete closure of the defect of the nasopharynx.
Discussion
The radiotherapy is the mainstay of treatment for NPC. However, radical radiation can both significantly improve survival and cause consequential late effects. Of these late effects, postradiation nasopharyngeal necrosis results in poor quality of life and poor prognosis. If osteoradionecrosis appears, the bone and internal carotid artery lose soft tissue coverage and are exposed to the air cavity.
Radiation-induced necrosis of the nasopharynx is as a process with three stages4: the early stage, occurs at the level of the mucosa, including local denaturalisation and discontinuation; the second stage includes both the mucosa and the muscle and could extend to the tendon of the nasopharynx with more important defects in the paraphyrngeal space. The osteoradionecrosis of the skull base is seen in the third stage of necrosis and usually causes refractory headache.
Haemorrhage, a fatal event, is closely associated with radiation-induced nasopharyngeal necrosis. Weakening of the wall of postradiated vessels may result in dissection or rupture and produce a pseudoaneurysm. Without immediate treatment, rupture can be fatal because of the anatomy of the nasopharynx.
Ming et a l 5 reported a series of 67 nasopharyngeal carcinoma patients with postradiation necrosis treated at Sun Yat-sen University cancer Center in China. In this series, 18 patients (26,9%) died of acute haemorrhage within 2 years of being diagnosed with nasopharyngeal necrosis. In the clinical series reported by Fengqin et al 6 of 53 ulcers of postradiation nasopharyngeal necrosis in patients with nasopharyngeal carcinoma, 5 patients succumbed to mortality due to a massive bleeding. In both these two series witch are presented the largest number of cases of radiation-induced deep nasopharyngeal ulcer and necrosis, the management was varied from conservative treatment to surgical debridement. According to our knowledge, the use of a different grafts to cover the cavity of the ulcer and to protect the ICA has not been reported yet in the literature.
The exact mechanism of PRNU is unknown; the infection was reported to play an important role in the process of nasopharyngeal necrosis.7 Infection of a local nasopharyngeal region may increase the energy demand by local tissues as well as oxygen and other metabolic demands, which leads to collagen destruction and cell death. Hypoxia and radiation factors such as dosage and dose rate also play an important role.8 Other factors such as the neoadjuvant chemotherapy, anaemia and malnutrition, T stage and the invasion of muscle have also an important role in the development of PRNU.
The treatment of PRNU is diverse and varies from conservative medical treatment to surgical endoscopic debridement. The choice of the treatment depends on the stage and the severity of the PRNU.
Our patient presented a deep PRNU classified as stage 3. The possibility of local tumour recurrence was ruled out. He underwent a conservative treatment for a period of 2 months which included intravenous and oral antibiotics associated with daily nasal rinçages and endoscopic debridement of the nasopharyngeal ulcer. The evolution of the local infection was favourable with a significant decrease in the inflammatory parameters. But due to the persistence of a wide cavity on the posterior wall of the nasopharynx and loss of the soft tissues with a high risk of rupture of the ICA and massive bleeding, we completed the conservative treatment with surgical closing of the cavity of the ulcer by using different grafts to protect the ICA and reduce the risk of rupture. According to our knowledge, a similar procedure in the management of PRNU has never been reported in the literature.
Learning points.
Nasopharyngeal carcinoma is a common malignancy in Southeast Asia but a very rare entity in Western Countries.
Radiotherapy remains a good treatment option but may cause both acute and late side effects.
Radiation-induced nasopharyngeal necrosis and ulcer could be life threatening.
Management should be immediate and adapted depending on the stage of the ulcer and the extension of the necrosis.
Coverage and protection of the internal carotid artery is very important to avoid fatal bleeding and death.
Footnotes
Contributors: BH was responsible for the reporting. MM was coauthor and responsible for the acquisition of data. RK was coauthor, responsible for the acquisition of data and design. SB was coauthor and also responsible for reporting.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Yu MC, Yuan J-M. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol 2002;12:421–9. [DOI] [PubMed] [Google Scholar]
- 2. Lee AW, Law SC, Ng SH, et al. Retrospective analysis of nasopharyngeal carcinoma treated during 1976-1985: late complications following megavoltage irradiation. Br J Radiol 1992;65:918–28. 10.1259/0007-1285-65-778-918 [DOI] [PubMed] [Google Scholar]
- 3. Hua Y-J, Chen M-Y, Hong M-H, et al. [Short-term efficacy of endoscopy-guided debridement on radiation-related nasopharyngeal necrosis in 20 nasopharyngeal carcinoma patients after radiotherapy]. Ai Zheng 2008;27:729–33. [PubMed] [Google Scholar]
- 4. Hua Y-J, Chen M-Y, Qian C-N, et al. Postradiation nasopharyngeal necrosis in the patients with nasopharyngeal carcinoma. Head Neck 2009;31:807–12. 10.1002/hed.21036 [DOI] [PubMed] [Google Scholar]
- 5. Ming YC, Hai QM, Rui S, et al. Clinical findings and imaging features of 67 nasopharyngeal carcinoma patients with postradiation nasopharyngeal necrosis. [DOI] [PMC free article] [PubMed]
- 6. Fengqin Y, Zhimin YE, FangzhengW LW, et al. Clinical and imaging characteristics of 53 ulcers of post-radiation nasopharyngeal necrosis in patients with nasopharyngeal carcinoma. [DOI] [PMC free article] [PubMed]
- 7. Hao SP, Chen HC, Wei FC, et al. Systematic management of osteoradionecrosis in the head and neck. Laryngoscope 1999;109:1324–7. 10.1097/00005537-199908000-00027 [DOI] [PubMed] [Google Scholar]
- 8. Huang X-M, Zheng Y-Q, Zhang X-M, et al. Diagnosis and management of skull base osteoradionecrosis after radiotherapy for nasopharyngeal carcinoma. Laryngoscope 2006;116:1626–31. 10.1097/01.mlg.0000230435.71328.b9 [DOI] [PubMed] [Google Scholar]