Abstract
We present two clinical cases of lymphadenopathy after vaccination with the human papillomavirus (HPV) 9-valent vaccine: an asymptomatic 11-year-old boy with inferior cervical and supraclavicular lymphadenopathy, and a 13-year-old girl who presented with lymphadenopathy. In both cases, medical history was unremarkable and there was no recent infection, or other clinical findings. Both adolescents had received the HPV 9-valent vaccine in the previous week. In the first case, blood tests, ultrasonography and biopsy were performed, while in the second, a watchful waiting strategy was adopted. In both cases, the lymphadenopathy resolved spontaneously. The boy received the second dose of the vaccine 6 months later and lymphadenopathy reappeared. The Naranjo scale was applied, classifying the events as definite (in the case of the boy) and probable (girl) adverse drug reactions. The vaccine is safe, but recognising this minor adverse event is important to prevent unnecessary investigation and reduce patient and parental anxiety.
Keywords: human papilloma virus, unwanted effects/adverse reactions, preventative paediatrics, vaccination/immunisation, immunological products and vaccines
Background
Due to the risk of cervical cancer caused by human papillomavirus (HPV), the European Medicines Agency (EMA) approved the bivalent (HPV16/18) and the quadrivalent (HPV6/11/16/18) HPV vaccines in 2006 and 2007, respectively. In 2015, the EMA approved the 9-valent HPV vaccine (HPV6/11/16/18/31/33/45/52/58), which is currently indicated for active immunisation of individuals from the age of 9 years against HPV-associated diseases.1 HPV vaccination has proved to be effective and safe, without major adverse reactions, and has been adopted into national immunisation programmes of most European Union countries.2
While most industrialised countries have introduced routine female HPV vaccination into their national immunisation programmes, routine vaccination of male children/adolescents and men is currently only implemented in few countries (including Australia, Canada, the USA and Austria). Vaccination of males may further reduce the incidence of cervical cancer and precancerous lesions via herd protection and reduce the incidence of anal, penile, head and neck cancers.3 4
The WHO, Food and Drug Administration, Advisory Committee on Immunization Practices, National Advisory Committee on Immunization, Australian Technical Advisory Group on Immunisation, National Health Service (NHS), Portugal Directorate-General of Health (DGS) and other regulatory agencies continue to recommend HPV vaccination because it is effective, cost-effective and safe.5–11
The most common adverse reactions observed with the 9-valent HPV vaccine are injection-site adverse reactions (pain, swelling and erythema) and headache. Other commonly reported adverse reactions are dizziness, nausea, fever, fatigue and pruritus or bruising at the injection site. In the postmarketing experience section of the summary of product characteristics, there are some very rare events reported voluntarily as injection-site cellulitis, immune thrombocytopenic purpura, anaphylactic reactions, bronchospasm, urticaria, acute disseminated encephalomyelitis, Guillain-Barré syndrome, syncope, vomiting, arthralgia, myalgia, asthenia, chills and malaise.1
We reviewed the current literature and found a single case report of lymphadenopathy after HPV vaccination, described in a 26-year-old woman. We did not find any reports of post-HPV 9-valent vaccination lymphadenopathy in children.12
Case presentation
Case 1
An asymptomatic healthy 11-year-old boy presented to a routine paediatric appointment 4 days after he had received his first dose of the HPV 9-valent vaccine in the right deltoid muscle. There was no history of fever, weakness, fatigue, night sweats, recent diseases or travels, nor close contact with cats or other animals. Apart from pectus excavatum, he had no relevant personal or family medical history. Physical examination revealed a non-tender right-sided inferior cervical and supraclavicular lymph node, rubbery and mobile, without erythema or other inflammatory signs. The largest diameter was approximately 1 cm. There was no other palpable lymphadenopathy elsewhere, and no splenomegaly. There were no signs of inflammation in the right deltoid region. The rest of his physical examination was unremarkable. Ten days after the first appointment, he was reassessed, and the abnormal lymph node had increased in size on physical examination.
Case 2
A 13-year-old girl presented to paediatric emergency department complaining of a swelling in the neck 5 days after receiving the first dose of the HPV 9-valent vaccine. The patient’s personal medical background and family medical history were both unremarkable. She was otherwise asymptomatic and, apart from a palpable non-tender left-sided inferior cervical lymph node with a diameter of approximately 1.5 cm (figure 1), the physical examination was normal.
Figure 1.
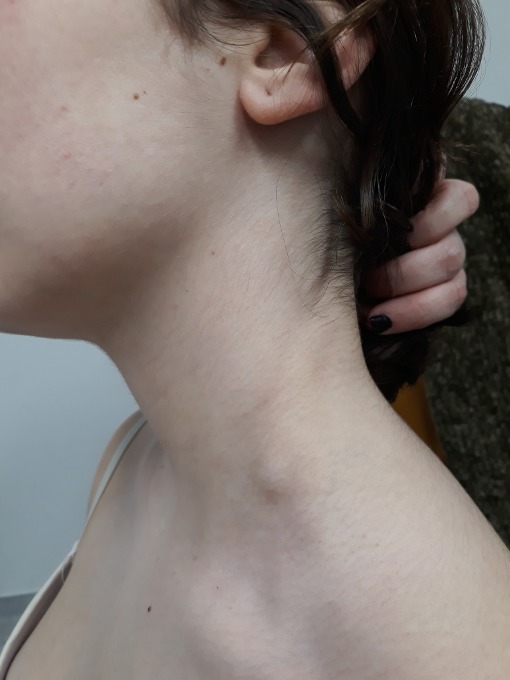
Palpable non-tender left-sided inferior cervical lymph node with a diameter of approximately 1.5 cm.
Investigations
Case 1
After the first appointment, the 11-year-old boy underwent blood tests that were unremarkable (haemoglobin 137 g/L, leucocytes 7.7×109/L—4.10×109/L neutrophils and 3.04×109/L lymphocytes, platelets 284×109/L, erythrocyte sedimentation rate 3 mm/hour, c reactive protein 0.09 mg/dL, lactate dehydrogenase 249 U/L, aspartate transaminase 23 U/L and alanine transaminase 22 U/L). A peripheral blood smear was normal. An ultrasound was performed that revealed four nodular structures, the largest with a long axis of 15 mm (the remaining structures measured 9, 8 and 7.2 mm), all well-delineated and with increased Doppler signal, findings suggestive of recent developing adenopathies (figures 2 and 3). When the patient was reassessed 10 days after the first appointment, blood tests were repeated, and the results were unremarkable. Epstein-Barr virus, Toxoplasma gondii, cytomegalovirus and Bartonella henselae serologies were all negative. Thirteen days after the first ultrasound, the patient repeated sonographic evaluation showing the same four ganglionic structures with the following dimensions: 14×6.8, 9.3×4.4, 8.6×4.6 and 5.7×2.7 mm (figure 4). All lymph nodes were rounded, strongly hypoechogenic, with little Doppler signal, not suggestive of reactive changes. Chest X-ray was normal. The patient underwent a biopsy of the largest two lymph nodes, which showed exuberant follicular lymphoid hyperplasia and hyperplasia of the parafollicular areas without changes of the mantle layer (figure 5). The biopsy showed non-specific reactive hyperplasia with no granulomas and no features of lymphoma.
Figure 2.
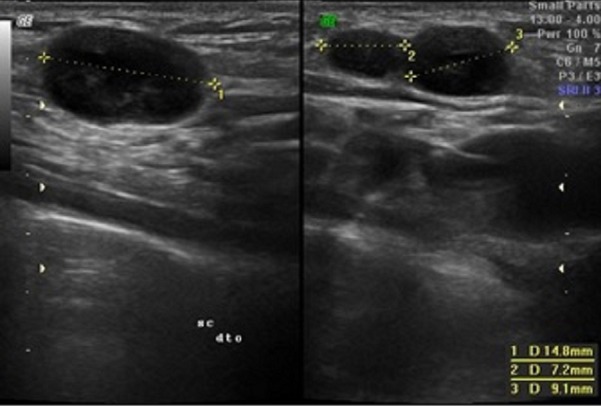
The first ultrasound of the 13-year-old boy showing three nodular structures, the largest with a bigger axis of 14.8 mm, both well-delineated.
Figure 3.
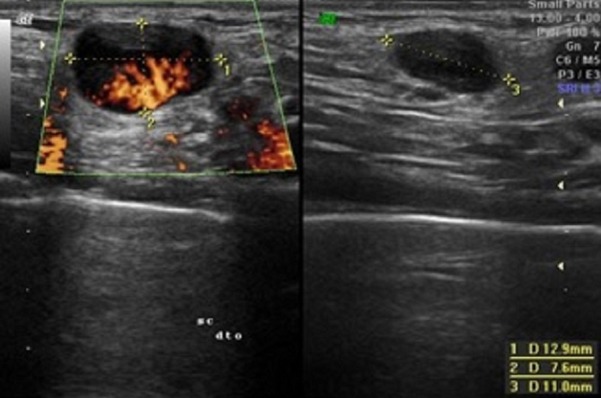
Increased Doppler signal, in the largest nodular structure, suggesting a recently developing adenopathy.
Figure 4.
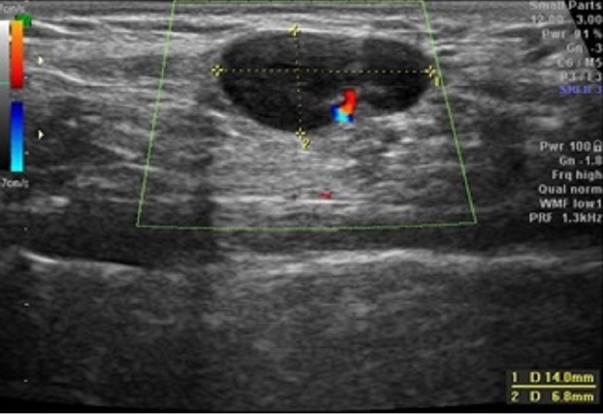
The second ultrasound of the 13-year-old boy 13 days after the first. The largest adenopathy is represented with similar dimensions but with a weaker Doppler signal.
Figure 5.
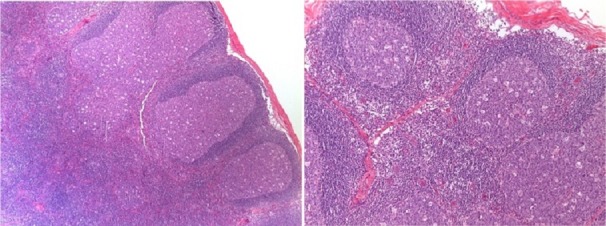
Exuberant follicular lymphoid hyperplasia in the lymph node cortical region, and no changes in the mantle layer. H&E staining, magnification ×4 and ×10.
Case 2
A watchful waiting strategy was adopted, and no investigations were performed.
Differential diagnosis
In a paediatric population, lymphadenopathy is a very common finding and usually provoked by a reaction to viral antigens; therefore, the most common cause is viral infection; however, a postvaccination aetiology should be considered. Lymphadenopathy can also be caused by bacteria (acute bacterial lymphadenitis), fungi and parasites. Less frequently, there are other non-infectious causes, such as malignancies, metastasis and some specific diseases.13 An enlarged lymph node in the supraclavicular location is a rarer and more worrisome finding suggesting either primary lymphatic malignancy, infection in the mediastinum or a metastatic malignancy from the abdomen.14
In both cases, patients had no history of a viral prodrome, and the lymph nodes were not tender to palpation, which helps to exclude lymphadenopathy secondary to viral infection. In the absence of fever, tenderness on palpation and overlying erythema acute bacterial lymphadenitis are less likely. There was no history or findings that would point towards a malignant diagnosis, such as night sweats, fatigue, easy bleeding or bruising, weight loss, or firm and indurated masses on physical examination.
Treatment
Four weeks after the first presentation, the 11-year-old boy underwent an excisional biopsy.
There was no therapeutic intervention in the second case.
Outcome and follow-up
One month after the procedure, the 11-year-old boy had no palpable lymphadenopathy in the cervical and supraclavicular regions. Six months after the first vaccination, the patient received the second dose of the HPV vaccine in the left deltoid region and subsequently developed lymphadenopathy in the same region with the same clinical findings. He was reassessed 3 months after the second dose and there were no palpable lymphadenopathy. We calculated the Naranjo score and the result was 10, suggestive of a ‘definite’ adverse drug reaction (ADR).
Two months after the first appointment, the 13-year-old girl was asymptomatic with no palpable lymphadenopathy on examination. She has not yet received a second dose of the HPV vaccine. The Naranjo score was 7, a ‘probable’ ADR.
Discussion
Confronted with these two cases of acute lymphadenopathy, we considered the most common aetiologies as possible causes. Once again, the most useful diagnostic tool was the history and physical examination. If the clinical diagnosis is questionable, imaging may be required. Ultrasonography can be used to further delineate the nature of the lymph node. Patients who continue to have persistent symptoms for more than 4–6 weeks despite appropriate therapies may require an excisional biopsy.13
In both cases, there was no history of a viral prodrome or active disease and no signs of local infection. In the first case, we decided to perform blood analysis, including serologies, ultrasounds and biopsies. This strategy was adopted due to the persistence of the supraclavicular lymphadenopathy 4 weeks after the initial presentation, despite the absence of other medical history, presenting complaints or examination findings suggestive of a malignancy aetiology. In the case of the 13-year-old girl, who had no medical history, current disease or worrying findings on clinical examination, we were able to adopt a more conservative approach. Our attitude in the second case was also probably influenced after a literature review found the previous case reported in this article.
After ruling out the most common and potentially serious aetiologies, we hypothesised that this could be a postvaccine reaction. As stated previously, the summary of product characteristics for the vaccine did not include lymphadenopathy as one of the possible adverse reactions.1 The safety of HPV vaccination has been evaluated carefully in several countries that have implemented it in national immunisation programmes. In Denmark and Sweden, there was no evidence of increased risk of autoimmune events, neurological events or venous thromboembolism.15 In Canada, confirmed adverse events following immunisation (AEFI) were reported at a rate of 1.9/10 000 doses. Primarily, the AEFI were allergic reaction (25%), rash (22%) and injection-site reaction (20%), while 26% of reports had a non-specific event. In the UK, the NHS classifies HPV vaccine side effects as very common (redness, swelling or pain at the site of the injection, and headaches), common (bruising or itching at the site of the injection, a high temperature or feeling hot and shivery, and nausea and pain in the arms, hands, fingers, legs, feet or toes), rare (an itchy red rash), very rare (difficulty breathing and restriction of the airways) and others (bruising or bleeding more easily, chills, weakness, tiredness or general feeling unwell, pain or tenderness in the joints or muscles, vomiting and seizures).16
Despite not having found any report of post-HPV vaccination lymphadenopathy in children, we decided to apply the Naranjo Adverse Drug Reaction Probability Scale, which is a simple method developed to assess the causality of ADRs in a variety of clinical situations and proved to offer a sensitive way to monitor ADRs and to be able to be applicable to postmarketing drug surveillance. It classifies the ADRs as definite, possible, probable or doubtful.17
By applying this score to the 11-year-old boy case, we reach a total score of 10, corresponding to the classification of a definite ADR which means the reaction followed a reasonable temporal sequence after a drug or in which a toxic drug level had been established in body fluids or tissues, followed a recognised response to the suspected drug, and was confirmed by improvement after stopping the drug and reappearance on re-exposure.
When applying the same scale to the 13-year-old girl, we obtained a lower score (7), representing a probable ADR. The patient had not yet received the second dose of the vaccine, so we could not confirm reappearance after the re-administration of the drug.
Despite the AEFI’s noticed in these two case reports, only previously described once, we do not think our findings should prevent the routine HPV vaccination. In both cases, the adverse events were non-serious conditions whose only impact was the over the investigation of the first case, which we hope to help prevent by making paediatricians aware that lymphadenopathy may follow HPV vaccination.
Learning points.
Paediatricians should be aware that lymphadenopathy may occur after human papillomavirus (HPV) vaccination to prevent unnecessary patient concern and procedures, such as blood analyses, ultrasound or lymph node biopsies.
The HPV vaccine has proved to be effective and safe and the knowledge of this minor adverse event should not prevent paediatricians from recommending this vaccination to the adolescent population.
The HPV vaccine is recent, so postmarketing surveillance must be maintained to detect any possible adverse events following HPV immunisation.
Acknowledgments
We would like to acknowledge and thank Dr Merlin McMillan for revising the article’s grammar and idiom.
Footnotes
Contributors: MPP’s contribution was planning, conducting, reporting, and analysing and interpreting data. PF contributed by planning, reporting and interpreting data. ASN was responsible for acquisition of data, conception and design. All the authors met the four conditions recommended by ICMJE. MPP, PF and ASN equally contributed to the current work, by acquisition, analysis and interpretation of data, drafting the work and revising it critically for important intellectual content. All the authors approved the final version published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. EMA Summary of product characteristics. Available: https://www.ema.europa.eu/en/documents/product-information/gardasil-9-epar-product-information_en.pdf [Accessed May 2019].
- 2. Bruni L, Albero G, Serrano B, et al. ICO/IARC information centre on HPV and cancer (HPV information centre). human papillomavirus and related diseases in the world summary report 2019;22. [Google Scholar]
- 3. Masterson L, O'Mahony J, Lechner M. Expanding the benefits of HPV vaccination to boys and men. The Lancet 2016;388 10.1016/S0140-6736(16)32525-9 [DOI] [PubMed] [Google Scholar]
- 4. McKie R. Give HPV vaccine to boys to protect against cancers, experts say. The guardian, 2016. Available: https://www.theguardian.com/science/2016/jul/09/vaccine-boys-cancer-men-hpv [Accessed Jun 2019].
- 5. WHO Human papillomavirus vaccines: who position paper, may, 2017. Available: https://apps.who.int/iris/bitstream/handle/10665/255353/WER9219.pdf?sequence=1 [Accessed June 2019].
- 6. Centers for Disease Control and Prevention (CDC) Fda licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on immunization practices (ACIP). MMWR Morb Mortal Wkly Rep 2010;59:630–2. [PubMed] [Google Scholar]
- 7. Petrosky E, Bocchini JA, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the Advisory Committee on immunization practices. MMWR Morb Mortal Wkly Rep 2015;64:300–4. [PMC free article] [PubMed] [Google Scholar]
- 8. NACI Available: http://www.phac-aspc.gc.ca/publicat/ccdrrmtc/12vol38/acs-dcc-1/index-eng.php [Accessed Apr 2019].
- 9. ATAGI Available: http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/national-immunisationprogram-schedule [Accessed April 2019].
- 10. NHS Vaccinations. Available: http://www.nhs.uk/conditions/vaccinations/pages/vaccination-schedule-age-checklist.aspx [Accessed Apr 2019].
- 11. DGS Programa Nacional de Vacinação. Available: https://www.dgs.pt/paginas-de-sistema/saude-de-a-a-z/programa-nacional-de-vacinacao/normas-e-orientacoes.aspx [Accessed May 2019].
- 12. Studdiford J, Lamb K, Horvath K, et al. Development of unilateral cervical and supraclavicular lymphadenopathy after human papilloma virus vaccination. Pharmacotherapy 2008;28:1194–7. 10.1592/phco.28.9.1194 [DOI] [PubMed] [Google Scholar]
- 13. Weinstock MS, Patel NA, Smith LP, et al. Pediatric cervical lymphadenopathy. Pediatrics in Review 2018;39:433–43. 10.1542/pir.2017-0249 [DOI] [PubMed] [Google Scholar]
- 14. Kliegman R. Nelson textbook of pediatrics. 20th edn Philadelphia, PA: Elsevier, 2016: 2413. [Google Scholar]
- 15. Arnheim-Dahlström L, Pasternak B, Svanström H, et al. Autoimmune, neurological, and venous thromboembolic adverse events after immunisation of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: cohort study. BMJ 2013;347:f5906 10.1136/bmj.f5906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. NHS Hpv vaccine side effects, 2017. Available: https://www.nhs.uk/conditions/vaccinations/hpv-vaccine-cervarix-gardasil-side-effects/ [Accessed Apr 2019].
- 17. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–45. 10.1038/clpt.1981.154 [DOI] [PubMed] [Google Scholar]


