Abstract
A 64-year-old woman presented with bilateral visual loss with shimmering photopsias as the only clinical manifestation of an occult pancreatic ampullary adenocarcinoma causing duct dilatation. Abnormal electroretinograms led to suspicion of cancer-associated retinopathy (CAR), and CT of the abdomen showed an underlying pancreatic malignancy, detected with subclinical liver function tests following diagnosis of CAR. Biopsy showed a T2N0M0 ampullary adenocarcinoma. The patient was managed with Whipple’s procedure and adjuvant chemotherapy and has made a good recovery with no progression of her retinopathy. To our knowledge, this is one of the first descriptions of CAR in the context of pancreatic malignancy. It is atypical in its asymmetric presentation and favourable patient outcome. CAR is an important diagnosis to make, as ocular manifestations can be the only indication of an occult malignancy, resulting in a swifter diagnosis and potentially life-saving early intervention.
Keywords: ophthalmology, retina, pancreatic cancer
Background
Cancer-associated retinopathy (CAR) is the the most common of a very rare group of paraneoplastic visual syndromes, including melanoma-associated retinopathy (MAR), paraneoplastic optical neuropathy and bilateral diffuse uveal melanocytic proliferation. It usually affects individuals in middle age.1 Associated symptoms include photosensitivity, photopsia, central scotomas or nyctalopia, and normal or minimal changes on fundus examination. CAR is classically bilateral, occasionally asymmetric and leads to progressive blindness over the years.2
Ampullary tumours of the pancreas are relatively rare, accounting for 7% of periampullary lesions but 20% of obstructive lesions at the common bile duct. Most commonly, they present with obstructive jaundice, and in ~86% of patients, they are associated with elevated serum Ca 19-9.3 They do not classically present with ocular symptoms. However, in this patient, the ocular manifestations resulted in an earlier diagnosis that emphasises the importance of recognising CAR and the risk of an underlying malignancy early.
Case presentation
A 64-year-old woman presented with gradual blurring of her vision, worse in the left eye (LE), with visual snow (a symptom described as seeing white or black dots throughout part or whole of the visual fields), alongside shimmering photopsias and floaters over the previous 6 months.
Her ocular history was notable for a previous partial posterior vitreous detachment and a history of Meibomian gland disease. Systemically, aside from osteoarthritis and hypothyroidisim, she had no other complaints. Her father had glaucoma, but there was no other family history of disease, including no background of autoimmune diseases. She had never smoked and drank alcohol rarely.
Three months prior to presentation, she had undergone bilateral cataract surgery in her LE and then in her right eye (RE). However, as vision had not improved significantly, as expected, after this procedure, she was referred to ophthalmology for further investigation.
On attending the ophthalmology clinic, her best-corrected visual acuity was 6/30 in the RE and 6/38 in the LE, corrected by pinhole to 6/24 bilaterally, which had deteriorated from 6/12 and 6/6 months previously. She described a round shadow in the peripheral visual field that had existed prior to her cataract surgery, which was demonstrated by a paracentral scotoma on formal Humphrey visual field testing (figure 1).
Figure 1.
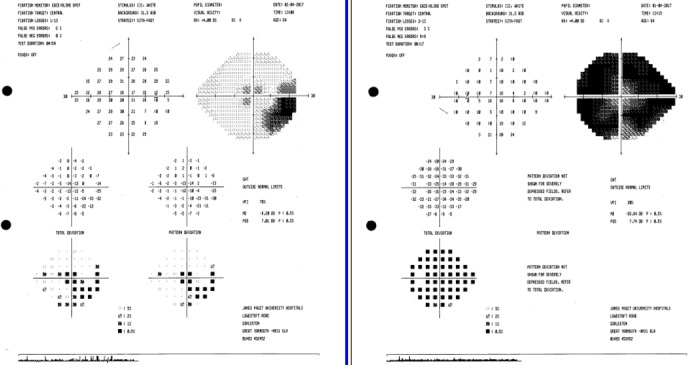
Paracentral scotoma in the right eye on visual field testing.
External examination showed orthophoric eyes with full range of ocular motion. The patient was unable to read any Ishihara colour plates and was only able to read the last plate with encouragement. There was no relative afferent pupillary defect. Cornea and anterior chambers were clear, and intraocular pressures were normal at 14 mm Hg in the RE and 13 mm Hg in the LE. Optical nerve appearance and function were normal, with no relative pupillary defect.
Dilated fundus examination showed flat retinas with no evidence of tears (illustrated in figure 2). Optical discs were healthy with cup:disc ratio within normal limits, with no vessel attenuation and no signs of retinal tears.
Figure 2.
Fundus photography showing a normal fundus in the right (A) and left (B) eyes.
Investigations
Initial work-up included an optical coherence tomography (OCT) scan. This showed loss of the photoreceptor inner segment–outer segment junction, findings consistent with an autoimmune retinopathy (AIR) (figure 3). There was also a mild left epiretinal membrane, though this was not consistent with the severity of visual loss. Fundus autofluorescence was unremarkable (figure 4) apart from a single focus of perivascular hypofluorescence on the left. There was no hyperautofluorescence. MRI of the brain and the orbit did not show any abnormality.
Figure 3.

Optical coherence tomography findings showing the characteristic loss of photoreceptor inner segment–outer segment junctions as seen in autoimmune retinopathy in the right (A) and left (B) eyes.
Figure 4.
Fundus autofluorescence showing a single focus of perivascular hypofluorescence on the left with no evidence of any hyperautofluorescence.
Subsequently, electroretinography was undertaken in order to measure the electrical responses of various cell types in the retina. ISCEV (International Society for Clinical Electrophysiology of Vision) standard full-field flash electroretinogram (ERG) using gold electrodes showed archetypal findings with severely diminished a-waves and b-waves indicative of an unusual AIR (figure 5). Focal ERG was undetectable and pattern ERG showed minimal residual activity in each eye. Visual evoked potentials were absent or small, again consistent with retinopathy.
Figure 5.
Abnormal ERGs: (A) pattern ERG demonstrating lack of response to stimulus even at a large field stimulus, (B) scotopic ERG showing diminished B-wave, and (C) photopic responses showing bilaterally delayed and small B-waves. ERG, electroretinogram.
A full blood count showed elevated liver transaminases consistent with an obstructive picture, with bilirubin of around 20–30 mmol/L. The Ca 19–9 reading was 10 5 months after diagnosis.
CT of the chest abdomen and pelvis (figure 6) showed severe extrahepatic and intrahepatic biliary dilatation, with dilatation of the pancreas and gallbladder with an 11 mm enhancing nodule at the common bile duct. There was no significant local lymphadenopathy and no obvious gallstones. There was a large heterogenous lesion in the right lobe filling between the arterial and venous phases of the liver, consistent with a haemangioma. An exophytic nodule from the right lobe of the thyroid was also visible as a 17 mm heterogeneously enhancing nodule.
Figure 6.

CT of the abdomen demonstrating intrahepatic biliary duct dilatation (white arrows) and marked dilatation of the common bile duct (red arrow).
Endoscopic retrograde cholangiopancreatography biopsy demonstrated a 17 mm ampullary adenocarcinoma of stage T2N0 lesion in histopathology, as well as confirming pancreatic and common bile duct dilatation.
Differential diagnosis
Given that the electrophysiology appeared to suggest an unusual retinopathy, with severely diminished a-waves and b-waves, the likelihood of an AIR was the foremost differential. As AIR can be part of a paraneoplastic syndrome, which is a response to a primary elsewhere,4 an extensive investigation is deemed necessary in order to rule out a malignancy, and CAR was indeed diagnosed following CT and endoscopy findings.
Nevertheless, until that point, non-paraneoplastic autoimmune retinopathies (NPAIRs) could be deemed indistinguishable from CAR.5 Both are characterised by similarly presenting symptoms and bilateral subacute visual loss, which tends to be more rapid in NPAIR.
NPAIR is more prevalent in patients who have a history of autoimmune disease.
Other possibilities included MAR, which classically presents years after diagnosis6 in patients with cutaneous melanoma, however, and is more prevalent in men. It is rarer than CAR and presents with normal dark-adapted A-wave.
Differential diagnoses for such a condition might include white-dot syndrome spectrum disorders, such as acute zonal occult outer retinopathy. These can present similarly to autoimmune retinopathies in terms of symptomatology, visual field and ERG findings, and can be bilateral too. However, the absence of the distinctive findings clinically and on fundus autofluorescence renders this much less likely,7 as does the classically spontaneously remitting nature of this disease.
Retinal degenerative disorders (such as retinitis pigmentosa), uveitis syndrome and infectious retinopathies can also cause similar symptoms and electrophysiological findings. However, in all cases, the lack of any distinctive fundus findings, nor intraocular inflammation, renders these less entities less likely.
Outcome and follow-up
The patient was given Creon supplements and, after further blood tests and a multidisciplinary team discussion, underwent pancreatoduodenectomy (Whipple’s) procedure 7 months after first presenting, removing 70×45 mm distal stomach, 135×24 mm of the duodenum and 50×65×38 mm of the head of the pancreas.
She subsequently underwent four cycles of adjuvant chemotherapy comprising capecitabine and gemcitabine as per the ESPAC-4 trial. Since completion of surgery, the patient has generally been well but has had stomach concerns, which have been managed with domperidone 20 mg.
Her AIR has not progressed in the 30 months since her pancreatoduodenectomy, with stable 6 monthly visual fields and electrophysiology testing. Subjectively, there has been no deterioration in her symptoms, and she has benefited from supportive therapy (magnification to overcome the reduction in her central vision). Given the abeyance in the condition’s progression, she is now undergoing annual review.
Discussion
CAR was first described by Sawyer et al8 as a paraneoplastic syndrome culminating in an AIR. It is most commonly associated with small-cell lung carcinoma,9 colonic tumours10 and gynaecological tumours11 12 and in 50% of patients presenting with symptoms prior to the diagnosis of malignancy.
Patients often describe symptoms of glare, scotomas, nyctalopia and decreased colour perception. Fundus examination can often be normal, although CAR has been associated with retinal atrophy, vessel attenuation or late-stage optical nerve pallor. One other report exists of a postulated CAR relating to a previously diagnosed pancreatic malignancy13; however, there is a degree of uncertainty about the diagnosis, which may have been subacute glaucoma, and the patient did not attend follow-up appointments.
ERG is important to the diagnosis of CAR, with abnormal retinograms in both light and dark adaptations. It is also relevant in distinguishing CAR from melanoma-associated retinopathy, which has a more significant drop in b-wave amplitude with a normal dark-adapted a-wave.14 Other potential findings include fundus autofluorescence displaying a hyperautofluorescent ring in the parafoveal region and OCT thinning of the inner retinal layers.14
CAR may be associated with retinal antigens. This is thought to be a B-cell drive response to antigens expressed by tumours and the retina,15 of which the first shown was recoverin,16 but later, other proteins, including alpha-enolase17 and transducin, were later shown to be relevant. The associated prevalence of each antibody was reported in one case series as alpha enolase (30%), transducin (17%), carbonic anhydrase (14%) and recoverin (10%), which totals 65% of cohort.18 However, it is important to bear in mind that the same antibodies have been found in the healthy population, which includes patients with uveitic syndrome, and no standards for antibody detection exist.19 Given that there is a great degree of uncertainty surrounding the use of these antiretinal antibodies, from their measurement and interpretation to identifying which ones are pathogenic, their diagnostic use is therefore considered limited.5 Based on these studies, no antiretinal antibodies were performed for our patient, as it was judged that the results of these would not change the management.
For most patients with CAR, the visual prognosis is poor with eventual blindness. Similarly, survival is poor. Cancer removal alone does not appear to resolve CAR20; however, in the case of our patient, the progression of the condition was halted. We can only postulate that swift surgical management of the malignancy contributed towards this outcome; however, it is atypical, given the characteristic pattern of CAR, which usually leads to progression of retinopathy and mortality.
Various treatment regimes have been postulated, including combinations of high-dose systemic steroids and immunomodulators, such as rituximab, alemtuzemab, intravenous immunoglobulin, infliximab, azothiaprine and cyclosporine with anecdotal effect.21 A triple-therapy regime combining prednisolone, ciclosporin and azathioprine appears to be associated with modest improvement in visual acuity and fields.22 However, care must be taken with balance of immunomodulation in the context of a systemic malignancy, and given the rarity of this condition, there are not enough valid studies to recommend any specific immunomodulatory regime.
Learning points.
Cancer-associated retinopathy (CAR) needs to be considered in instances of unexplained or subacute visual loss or field changes.
CAR often presents with near-normal funduscopy.
Electrodiagnostic tests are critical to evaluating retinal function for early diagnosis of CAR.
The presence of serum antiretinal antibodies is not diagnostic for CAR and must be correlated with clinical findings.
Ocular manifestations may be the only indication of a carcinoma, and swift diagnosis can be potentially life-saving.
Footnotes
Twitter: @DrStrangetwit
Contributors: YY wrote the first draft of the report. NG and BJLB were the main care providers for the patient. NG prepared the report and wrote the final draft for submission. BJLB reviewed the report and approved the article prior to submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Braithwaite T, Vugler A, Tufail A. Autoimmune retinopathy. Ophthalmologica 2012;228:131–42. 10.1159/000338240 [DOI] [PubMed] [Google Scholar]
- 2.Weleber RG, Watzke RC, Shults WT, et al. Clinical and electrophysiologic characterization of paraneoplastic and autoimmune retinopathies associated with Antienolase antibodies. Am J Ophthalmol 2005;139:780–94. 10.1016/j.ajo.2004.12.104 [DOI] [PubMed] [Google Scholar]
- 3.Panzeri F, et al. Management of ampullary neoplasms: a tailored approach between endoscopy and surgery. World J Gastroenterol 2015;21:7970–87. 10.3748/wjg.v21.i26.7970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heckenlively JR, Ferreyra HA. Autoimmune retinopathy: a review and summary. Semin Immunopathol 2008;30:127–34. 10.1007/s00281-008-0114-7 [DOI] [PubMed] [Google Scholar]
- 5.Grewal DS, Fishman GA, Jampol LM, et al. Autoimmune retinopathy and antiretinal antibodies. Retina 2014;34:1023–41. 10.1097/01.iae.0000450880.26367.4e [DOI] [PubMed] [Google Scholar]
- 6.Chan JW. Paraneoplastic retinopathies and optic neuropathies. Surv Ophthalmol 2003;48:12–38. 10.1016/S0039-6257(02)00416-2 [DOI] [PubMed] [Google Scholar]
- 7.Fujiwara T, Imamura Y, Giovinazzo VJ, et al. Fundus autofluorescence and optical coherence tomographic findings in acute zonal occult outer retinopathy. Retina 2010;30:1206–16. 10.1097/IAE.0b013e3181e097f0 [DOI] [PubMed] [Google Scholar]
- 8.Sawyer RA, Selhorst JB, Zimmerman LE, et al. Blindness caused by photoreceptor degeneration as a remote effect of cancer. Am J Ophthalmol 1976;81:606–13. 10.1016/0002-9394(76)90125-2 [DOI] [PubMed] [Google Scholar]
- 9.Morita M, Fukuhara T, Takahashi H, et al. Small cell lung cancer and progressive retinopathy. Case Reports 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogra S, Sharp D, Danesh-Meyer H. Autoimmune retinopathy associated with carcinoid tumour of the small bowel. J Clin Neurosci 2014;21:358–60. 10.1016/j.jocn.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 11.Siggel R, Schick T, Grajewski RS, et al. Karzinomassoziierte Retinopathie (CAR) bei neuroendokrinem Uteruskarzinom. Ophthalmologe 2019;116:456–8. 10.1007/s00347-018-0751-8 [DOI] [PubMed] [Google Scholar]
- 12.Robertson DM. Non-cancerous ophthalmic clues to non-ocular cancer. Surv Ophthalmol 2002;47:397–430. 10.1016/S0039-6257(02)00332-6 [DOI] [PubMed] [Google Scholar]
- 13.Cristescu TR. Diagnosis difficulties in a patient with progressive loss of vision - a case report. Rom J Ophthalmol 2017;61:60–4. 10.22336/rjo.2017.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canamary AM, Takahashi WY, Sallum JMF, et al. Autoimmune retinopathy: a review. Int J Retin Vitr 2018;4 10.1186/s40942-017-0104-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohguro H, Nakazawa M. Pathological roles of recoverin in cancer-associated retinopathy. Photoreceptors and Calcium 2002;514:109–24. [DOI] [PubMed] [Google Scholar]
- 16.Adamus G, Guy J, Schmied JL, et al. Role of anti-recoverin autoantibodies in cancer-associated retinopathy. Investig Ophthalmol Vis Sci 1993;34:2626–33. [PubMed] [Google Scholar]
- 17.Dot C, Guigay J, Adamus G. Anti-α-enolase antibodies in cancer-associated retinopathy with small cell carcinoma of the lung. Am J Ophthalmol 2005;139:746–7. 10.1016/j.ajo.2004.10.044 [DOI] [PubMed] [Google Scholar]
- 18.Adamus G. Autoantibody targets and their cancer relationship in the pathogenicity of paraneoplastic retinopathy. Autoimmun Rev 2009;8:410–4. 10.1016/j.autrev.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faez S, Loewenstein J, Sobrin L. Concordance of antiretinal antibody testing results between laboratories in autoimmune retinopathy. JAMA Ophthalmol 2013;131:113–5. 10.1001/jamaophthalmol.2013.574 [DOI] [PubMed] [Google Scholar]
- 20.Goldstein SM, et al. Cancer-Associated retinopathy. Arch Ophthal 1999;117:1641–5. 10.1001/archopht.117.12.1641 [DOI] [PubMed] [Google Scholar]
- 21.Grange L, Dalal M, Nussenblatt RB, et al. Autoimmune retinopathy. Am J Ophthalmol 2014;157:266–72. 10.1016/j.ajo.2013.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreyra HA, et al. Management of autoimmune retinopathies with immunosuppression. Arch Ophthal 2009;127:390–7. 10.1001/archophthalmol.2009.24 [DOI] [PubMed] [Google Scholar]





