Abstract
The use of anti-chlamydial antibiotics for trachoma control is based on the assumption that most people with clinically active disease have conjunctival infection with Chlamydia trachomatis. In high prevalence areas, this is generally true. As prevalence decreases, however, the positive predictive value of clinical signs for C. trachomatis infection also decrease. In this paper, the case for using laboratory assays to guide trachoma control strategies is presented, molecular methods for diagnosis (such as a ligase chain reaction and a polymerase chain reaction [PCR]) are compared with earlier techniques, and recent findings of ongoing studies using a quantitative PCR are reviewed. In addition, the contribution of genotyping to our understanding of the epidemiology and biology of C. trachomatis is considered.
Introduction
Trachoma is caused by ocular infection with the intracellular bacterium Chlamydia trachomatis,1 and antibiotics have played a key role in trachoma control efforts since the late 1930s.2 However, sulfonamides proved to be too toxic to be used in mass treatment campaigns,3 and tetracycline cannot be given systemically to children. The impact of topical tetracycline was limited, presumably (at least in part) because compliance was poor with the prolonged courses needed to cure infection.4 The demonstration that a single oral dose of azithromycin is as effective in the treatment of active trachoma as a six-week course of tetracycline ointment5–8 was a major advance. When given to whole communities, azithromycin produces a greater reduction in the prevalence of conjunctival C. trachomatis infection than does topical tetracycline.9 This has encouraged the World Health Organization (WHO) to set 2020 as the target date for elimination of trachoma as a public health problem.10
The surgery, antibiotics, facial cleanliness, and environmental improvement (SAFE) strategy for the elimination of blinding trachoma relies heavily on the use of mass treatment of endemic communities with antibiotics. The evidence base for the antibiotics component is stronger than that for the other aspects of SAFE.4,11 However, azithromycin is an expensive drug, and although it is being generously donated by its manufacturers to control programs in 10 countries, it is important that it is targeted to the communities, and the population groups within those communities, where it will have maximum impact.
Why is A Laboratory Test Needed for Trachoma Control?
The WHO recommends that whole communities should be treated with antibiotics when the prevalence of follicular active trachoma (TF) exceeds 20% in children 1–10 years old.12 The prevalence of active trachoma is determined by clinical examination of the everted upper eyelid of a sample of children, and TF is defined according to the WHO simplified system (Table 1).13 In communities with a prevalence of TF in children less than 20%, selective mass treatment is recommended, but there is no consensus as to who should be treated. Various strategies have been proposed, including the treatment of all children with active disease and their household contacts, the treatment of all children less than 10 years of age, and the treatment of all women and children. The relative impact of these different strategies has not been determined. The only comparison performed to date evaluated (in a low prevalence area) mass treatment of 1–10-year-old children against treatment of children with active disease plus members of their households; no significant differences in outcome were found.14 Furthermore, it is not clear what the time interval between treatments should be, nor how many rounds of treatment should be given.
Table 1. The World Health Organization simplified clinical grading system for trachoma13.
| TF: Trachomatous inflammation—follicular: the presence of five or more follicles at least 0.5 mm in diameter, in the central part of the upper tarsal conjunctiva |
| TI: Trachomatous inflammation—intense: pronounced inflammatory thickening of the upper tarsal conjunctiva obscuring more than half the normal deep tarsal vessels |
| TS: Trachomatous conjunctival scarring: the presence of easily visible scars in the tarsal conjunctiva |
| TT: Trachomatous trichiasis: at least one eyelash rubs on the eyeball, or evidence of recent removal of in-turned eyelashes |
| CO: Corneal opacity: easily visible corneal opacity over the pupil, so dense that at least part of the pupil margin is blurred when viewed through the opacity |
The purpose of antibiotic treatment is to cure ocular C. trachomatis infection. Community-based mass treatment is given in the hope that this will reduce the reservoir of infection in the community, leading to reduced or interrupted transmission. Unfortunately, the presence or absence of TF, defined according to WHO criteria, does not correlate perfectly with the presence of ocular C. trachomatis infection, for the following reasons: 1) C. trachomatis is not the only cause of follicular conjunctivitis; 2) there is a delay between the establishment of infection and the appearance of the characteristic signs of active trachoma in humans and in primate models;15–17 and 3) the subconjunctival follicles on which the clinical diagnosis is based can persist for weeks or months after the infection has resolved.15–19 Laboratory tests for the identification of individuals with ocular C. trachomatis infection can 1) help target antibiotic treatment to groups in whom it will have most impact; 2) be used to measure the impact of antibiotic treatment and of interventions to reduce transmission; 3) help to determine the optimal frequency of community-based treatment; and 4) be useful in identifying communities in which transmission has been interrupted, or reduced to such a level that control efforts are no longer required. Laboratory tests that allow the infecting serotype or strain to be identified can shed additional light on the way in which infection is transmitted, and on sources of reinfection after community-based treatment.
Which Laboratory Test is Most Useful?
In the 1960s, large epidemiologic studies of trachoma were conducted in The Gambia, Saudi Arabia, Taiwan, and Ethiopia in preparation for vaccine trials. Infected individuals were identified by staining conjunctival smears for chlamydial inclusions or by culture in fertile hen eggs. The staining method lacked sensitivity, and culture was laborious, expensive, and required a cold chain for the transport of specimens. Subsequent studies in Iran used the presence of antibodies to C. trachomatis in tears detected by the micro-immunofluorescence test of Wang and Grayston20 as a proxy for infection.21 This method could also be used to identify the infecting serotype, and helped to define the importance of transmission within the household unit.
In the 1980s, antigen detection methods (direct fluorescence or enzyme immunoassay) were used to identify C. trachomatis-infected individuals in Tanzania and The Gambia.22,23 The sensitivities of these tests were similar to culture, but they were simpler to perform, and did not require that specimens be kept cold. It was suggested that the presence of chlamydial antigen in samples that were negative by culture implied that persistent, non-replicating C. trachomatis may be present in some individuals, and contribute to the pathogenesis of blinding trachoma,24 but it is possible that this phenomenon merely reflected the difficulty inherent in culturing C. trachomatis from samples collected in the field.
Molecular Tools
Molecular methods, in particular nucleic acid amplification tests (NAATs), for the detection, quantification, and genotyping of ocular C. trachomatis infection have furthered our understanding of the epidemiology of trachoma in the past decade. New insights provided by NAATs can be used to refine our strategies for the control of blinding trachoma, as described below.
NAATs to diagnose infection
NAATs are significantly more sensitive than other methods of diagnosing C. trachomatis infection.25 The application of these tests in the diagnosis of ocular C. trachomatis infection in trachoma-endemic communities has led to some surprising findings. For example, in Nepal, very few infected individuals were identified in communities with clear clinical evidence of blinding trachoma, whereas in Tanzania, The Gambia, and Egypt, a high prevalence of infection was found in individuals living in endemic communities, even for subsets without clinical evidence of active disease. In-house polymerase chain reaction (PCR) assays were first used to diagnose ocular infection in trachoma-endemic communities in Tanzania and The Gambia.16,26
In Tanzania, a PCR assay targeting the outer membrane protein A (ompA) gene detected C. trachomatis in a higher proportion of subjects with active trachoma than did the direct fluorescent antibody (DFA) assay (Syva Microtrak™; Syva, Palo Alto, CA). Fifty-four percent of those with only follicular trachoma (TF) were positive by the PCR (compared with 28% by the DFA), as were 95% of those with intense trachoma (TI) (compared with 60% by the DFA). However, a surprisingly high proportion of subjects without clinical evidence of active trachoma according to WHO criteria were positive by the PCR (24% compared with 1% by the DFA).26
In The Gambia, a PCR assay targeting the cryptic chlamydial plasmid detected C. trachomatis-specific DNA in 144 (72%) of 200 individuals with active trachoma and in 85 (7.5%) of 1,132 without active trachoma, according to the WHO criteria. Conjunctival follicles were present in 31 (36%) of 85 of these individuals, and corneal vascularization (pannus), typical of trachoma, was present in 11 (13%). The PCR-positive subjects without active trachoma by WHO criteria at baseline were significantly more likely to have developed active trachoma according to these criteria one and six months later than were PCR-negative subjects.16
Several NAATs are commercially available for the diagnosis of C. trachomatis infection. These were developed for the diagnosis of genital infection, and only two, the Amplicor™ PCR assay developed by Roche Diagnostic Systems (Pleasanton, CA), and the LCx™ ligase chain reaction (LCR) developed by Abbott Laboratories (Abbott Park, IL), have been used to diagnose ocular infection in trachoma.
In the Surkhet District of Nepal, 726 children 1–10 years old were examined in six villages.19 Forty-six (6%) were found to have clinical evidence of trachoma, according to the simplified WHO criteria.13 Swabs were taken from these 46 children and from 44 controls without clinical trachoma. They were stored at 4°C, transported to San Francisco, California, and tested by the LCR. None of the 90 swabs were positive. Twelve samples from clinically active children were retested, after dilution, to rule out the presence of inhibitors, and retested again with a separate LCR that targeted a different C. trachomatis gene. All were consistently negative.19
In the Kailali District of Nepal, eye swabs were taken (by the same group of investigators) from 24 children with clinically active trachoma in four villages in which the prevalence of active disease was < 10% in children 1–10 years old. C. trachomatis was detected by the LCR in only 2 (8%) of 24. In contrast, in another village in Kailali in which the prevalence of active trachoma was 39% in children 1–10 years old, C. trachomatis was detected by the LCR in 15 (63%) of the 24 children with active disease from whom swabs were taken.18
As part of the same study, in Dongfang District of Hainan Province in China, eye swabs were taken from 25 individuals with active trachoma. The overall prevalence of active disease in children 1–7 years old was 2% in the district, although there was a relatively high prevalence of trachomatous scarring and of trichiasis among adults (15% and 3%, respectively), suggesting that trachoma had been a more serious problem in the recent past. C. trachomatis was detected by the LCR in 2 (8%) of 25 subjects.18
These results suggest that in communities where the prevalence of active trachoma (determined clinically) is < 10%, the prevalence of ocular C. trachomatis infection is extremely low. It seems unlikely that sustained transmission of infection occurs in such communities, suggesting that antibiotic treatment is unnecessary there for the control of blinding trachoma.
The LCR was used by Schachter and others to detect ocular C. trachomatis infection in endemic communities in The Gambia, Tanzania, and Egypt.9 Trachoma was highly endemic in all communities, with the prevalence of active disease, according to the WHO simplified criteria,13 ranging from 30% in The Gambia to 64% in Tanzania, among children 1–10 years old. Among individuals with mild active trachoma (TF), C. trachomatis was detected in 41% in Tanzania, 52% in The Gambia, and 61% in Egypt. The corresponding prevalences of C. trachomatis infection in children with severe active disease (TI) were 55%, 70%, and 81%, respectively. Surprisingly, the prevalence of C. trachomatis infection was also high among subjects without clinically active disease in The Gambia (462 of 1,470, 31%) and Egypt (466 of 1,661, 28%). Of concern for trachoma control programs, the prevalence of infection was high among adults, who are not normally considered a significant reservoir of infection, in both countries (> 20% in Egypt, and > 30% in The Gambia among subjects > 10 years old). In Tanzania, the prevalence of infection among those without active disease was considerably lower in this study (83 of 1,809, 4.6%)9 than in the previous Tanzanian study of Bobo and others,26in which it was 24%. Less than 10% of those > 10 years old were infected in the later study.
What significance do these findings have for control programs? Initially, they suggest that adults are as important a reservoir of infection as children. Moreover, the prevalence of infection was similar in men and women at all three sites, suggesting that targeting women and children alone may not be an effective control strategy. Conversely, the fact that most subjects without active disease show negative results for C. trachomatis in less sensitive (non-NAAT) tests suggest that they harbor low numbers of organisms. Does this represent productive infection with replicating organisms that could be transmitted to others, or merely contamination with dead organisms or chlamydial DNA?
Quantitative PCR
This question could be addressed in various ways. The number of organisms or the amount of C. trachomatis DNA in ocular samples from those with and without clinical signs could be measured using real-time quantitative DNA amplification methods; markers of replication and viability could be measured, e.g., isolation in tissue culture or by mRNA expression; longitudinal studies could document the presence and amount of C. trachomatis shedding and corresponding clinical signs; and household contacts of these subjects could be followed up to determine which subjects are most likely to infect their household contacts.
The first report of a semi-quantitative PCR method to determine the amount of chlamydial DNA in eye swabs in a trachoma endemic population described a longitudinal study conducted in Kongwa District, Tanzania.27 Fifty-three children from 33 families were examined every week for three months. Children with active disease had significantly higher chlamydial loads than those with no signs. Those with persistent infection had higher chlamydial loads than those with sporadic infections, and were more likely to have intense inflammatory trachoma (TI).13
A quantitative PCR (Q-PCR) directed at the ompA gene has now been developed using the LightCycler (Roche Molecular Systems, Branchburg, NJ).28,29 The target is a 123-basepair sequence of ompA constant domain 3, which is known to be highly conserved among C. trachomatis strains. OmpA is found in a single copy on the chlamydial chromosome. The LightCycler uses a true kinetic (real-time) quantitative PCR format. Reactions take place in closed glass capillaries. The high surface-to-volume ratio of capillaries minimizes the cost of reagents and allows rapid heat transfer and therefore rapid thermal cycling.30 Because capillaries are optically clear, analysis of product generation can occur through detection and quantification of a fluorescent reporter (SYBR Green I; Molecular Probes, Eugene, OR) after each extension step.30,31 Unfortunately, a small sample volume also has a disadvantage: it increases the effect of sampling variability, particularly for low-copy number positive samples. This means that the sensitivity of the test is lower than commercial qualitative PCRs, despite the fact that the LightCycler assay is optimized to reproducibly amplify one copy of the target sequence if present in a capillary. To compensate for this disadvantage, swabs can first be screened using a qualitative PCR or LCR, such as the Amplicor PCR, which is known to be highly sensitive.25 Positive specimens are then quantified in the LightCycler. In addition, quantifying two separate aliquots of each positive specimen should reduce random error due to sampling variation.
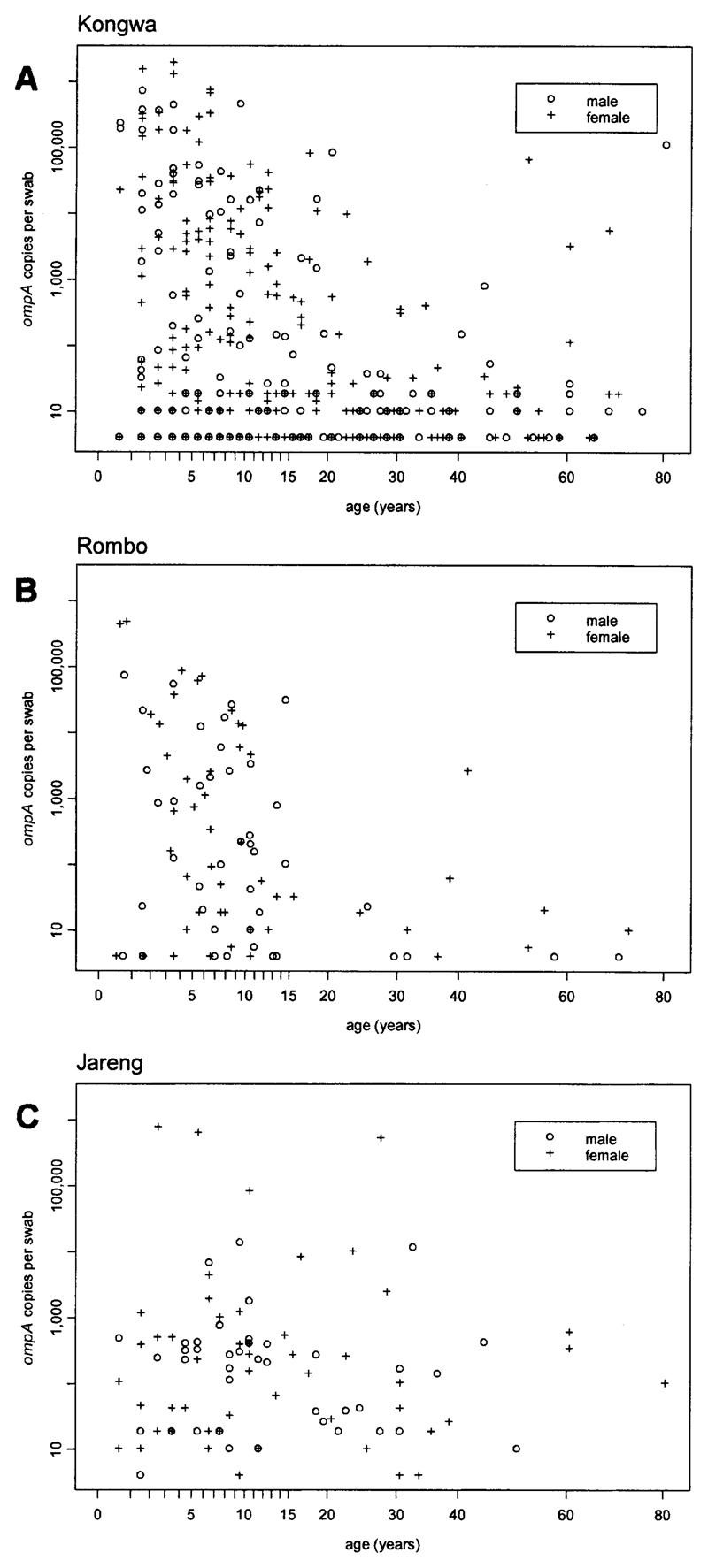
Quantitative PCR has recently been used to determine the distribution of ocular C. trachomatis infection in three African trachoma-endemic communities chosen to be representative of populations with differing levels of disease endemicity.29 Two of these communities were in Tanzania (Rombo and Kongwa Districts), and one in The Gambia (Jareng District). Samples were taken from each consenting individual at baseline using a dry sterile Dacron swab applied in a highly standardized fashion to the everted upper tarsal conjunctiva. A plot of the number of copies of ompA determined per swab versus the age of the individual is shown for each site in Figure 1. Chlamydial loads are shown on the vertical axis against a log scale; uninfected individuals (load = 0) are therefore not represented on the plot. There was a strong tendency for higher loads to be found in younger children in Kongwa and Rombo, the two communities with higher prevalences of disease. High loads were more diffusely distributed by age in Jareng, which had the lowest prevalence of disease. One possible explanation for this association is that the propensity to develop protective immunity to ocular C. trachomatis infection is dependent on the level of endemicity. Ninety percent of the total community ocular load of C. trachomatis was found in children 0–6.5 years old in Kongwa, in children 0–6.6 years old in Rombo, and in individuals 0–27.5 years old in Jareng.29
Figure 1.
Individual ocular chlamydial loads versus age in three trachoma-endemic communities, measured as the quantitative polymerase chain reaction–determined number of copies of the outer membrane protein A (ompA) gene per standard conjunctival swab. Only data for individuals with positive swabs are displayed. Prevalences of active disease in study subjects were A, 312 (36%) of 871 in Kongwa, central Tanzania; B, 174 (18%) of 956 in Rombo, northern Tanzania; and C, 103 (8%) of 1,319 in Jareng, The Gambia.
There were no significant differences in either the prevalence or load of infection between males and females at any of the three sites. This was consistent with the findings for prevalence of infection by the LCR in the study of Schachter and others,9 but is somewhat at odds with current treatment protocols in low prevalence areas, which often recommend antibiotics for adult women but not adult men.
The relationship between infection and disease is also of interest. Loads were highest in those with severe inflammatory trachoma (TI), but a surprisingly high proportion of individuals with trachomatous conjunctival scarring (TS), without evidence of active disease, had low-copy number infections.29 The latter finding is important because there is some evidence that women with TS are more likely to develop trichiasis if they test positive for ocular C. trachomatis.32 However, given the low copy numbers, it seems unlikely that these older individuals with TS alone are an important reservoir of infection.
In contrast to those with high copy numbers, C. trachomatis was isolated in tissue culture from only a small proportion of those with low ompA loads. This might mean that few of these individuals are infected with live, replicating organisms, or that there is a stage in the natural history of ocular chlamydial infection during which non-culture test results are positive but viable organism cannot be isolated.24 Follow-up studies are in progress to determine whether this group of individuals represents a source of infection for their household contacts.
Following baseline swabbing, mass distribution of single-dose azithromycin was undertaken in all three communities, and at 2, 6, 12, and 18 months, swabs were obtained from all consenting residents for determination of ocular chlamydial load. At the time this review was prepared, complete post-treatment Q-PCR data were available from only one site. At that site (Kahe Mpya, Rombo District in northern Tanzania), nearly 94% of 978 resident individuals took azithromycin at baseline, and an additional 4% were given two tubes of 1% tetracycline eye ointment because according to Tanzanian guidelines they were ineligible to receive azithromycin. An adjusted geometric mean of the total load of ocular C. trachomatis in the community was reduced to 14% of its baseline level two months after mass treatment, 8% at six months, and 5% at 12 months.33 In summary, this study used Q-PCR to provide evidence of the dramatic and sustained effect of single-dose azithromycin on the prevalence and intensity of ocular chlamydial infection.
The Q-PCR provides useful information on which to base antibiotic treatment strategies by identifying the groups with the highest infectious load: these groups need to be targeted for treatment. The optimal treatment strategy may differ in communities with different levels of endemicity.
Molecular strain typing (genotyping)
Small studies conducted in Taiwan and Iran in the 1970s and 1980s, which used the micro-immunofluorescence test to type ocular isolates or tear antibody, showed that a single serovar of C. trachomatis usually accounts for all infections in any given household, confirming the importance of transmission within the family.34,35 Molecular genotyping, usually performed by sequencing the ompA gene after its amplification by a PCR, has enabled us to conduct much larger studies. These investigations have generally confirmed that a single household contains a single genotype, that more than one genotype is present in most if not all trachoma-endemic communities, and that single nucleotide polymorphisms are commonly found in ocular strains.
The ompA gene codes for the chlamydial major outer membrane protein (MOMP) that contains the serovar-specific epitopes of C. trachomatis. It is a strong candidate for a chlamydial vaccine since MOMP-specific monocolonal antibodies neutralize infection in vitro.36 There is evidence from non-human primate models that resistance to ocular C. trachomatis is serovar specific.37 It was therefore interesting to see whether it is possible for a single ompA genotype to persist in a trachoma endemic community, or whether persistence in the community requires the appearance of mutations in ompA to escape immunologic pressure. A longitudinal study in two Gambian villages identified four variant strains of C. trachomatis serovar A and four of serovar B. Each variant differed from the prototype strain by a single nucleotide. Two genovar A and two genovar B variants comprised 87% of the strains. Although there was some flux in the prevalence of individual strains over time, their overall distribution remained remarkably stable over a 22-month period. There was no evidence of major antigenic shift resulting from recombination at ompA locus.38,39 This is in one sense encouraging, since it seems that C. trachomatis is not prone to rapid mutation in the ompA gene that might compromise vaccine efficacy, but conversely it implies that a given strain of C. trachomatis can persist for long periods in individuals and communities in spite of the presence of an immune response to that genotype.
Although community-based antibiotic treatment can dramatically reduce the prevalence of ocular chlamydial infection for 18 months or more if a high enough coverage is achieved,33 it is often followed by rapid re-emergence of infection when coverage is low or moderate. In designing the optimal regimen for antibiotic treatment, it is important to know if re-emergent infection represents treatment failure, reinfection from untreated members of the community, or reinfection from sources outside the community. In theory, strain typing may enable us to distinguish between these possibilities; but in practice, since most households contain only one strain, it has been difficult to distinguish treatment failure from reinfection at the household level.38,40,41
In addition to furthering our understanding of the epidemiology of infection and impact of treatment, molecular approaches are of fundamental importance to basic science research, which may ultimately lead to the development of the long-awaited vaccine against trachoma. For example, C. trachomatis strains isolated from eyes in trachoma-endemic communities are almost invariably of serovar A, B, Ba, or C, whereas genital isolates are almost always of serovars D-K. The biologic differences underlying this distinct tropism were unknown until recently, when it was shown that a wide range of ocular strains all contained a mutation in the tryptophan synthase (trpBA) gene that rendered it non-functional; whereas all genital strains contained a functioning trpBA gene.42 This observation is likely to be of fundamental importance to our understanding of host-pathogen relationships.
Financial support
Anthony W. Solomon is supported at the London School of Hygiene and Tropical Medicine by the International Trachoma Initiative (grant #01-034). This work was supported by the International Trachoma Initiative, the Wellcome Trust/Burroughs Wellcome Fund, and the Medical Research Council, United Kingdom.
References
- 1.Collier LH, Duke-Elder S, Jones BR. Experimental trachoma produced by cultured virus. Br J Ophthalmol. 1958;42:705–720. doi: 10.1136/bjo.42.12.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freyche MJ. Antibiotics and sulfonamides in the treatment of trachoma. Bull World Health Organ. 1950;2:523–544. [PMC free article] [PubMed] [Google Scholar]
- 3.Schachter J. Rifampin in chlamydial infections. Rev Infect Dis. 1983;5(suppl 3):S562–S564. doi: 10.1093/clinids/5.supplement_3.s562. [DOI] [PubMed] [Google Scholar]
- 4.Kuper H, Solomon AW, Buchan J, Zondervan M, Foster A, Mabey D. A critical review of the SAFE strategy for the prevention of blinding trachoma. Lancet Infect Dis. 2003;3:372–381. doi: 10.1016/s1473-3099(03)00659-5. [DOI] [PubMed] [Google Scholar]
- 5.Bailey RL, Arullendran P, Whittle HC, Mabey DC. Randomised controlled trial of single-dose azithromycin in treatment of trachoma. Lancet. 1993;342:453–456. doi: 10.1016/0140-6736(93)91591-9. [DOI] [PubMed] [Google Scholar]
- 6.Tabbara KF, Abu-el-Asrar A, al-Omar O, Choudhury AH, al-Faisal Z. Single-dose azithromycin in the treatment of trachoma. A randomized, controlled study. Ophthalmology. 1996;103:842–846. doi: 10.1016/s0161-6420(96)30605-2. [DOI] [PubMed] [Google Scholar]
- 7.Dawson CR, Schachter J, Sallam S, Sheta A, Rubinstein RA, Washton H. A comparison of oral azithromycin with topical oxytetracycline/polymyxin for the treatment of trachoma in children. Clin Infect Dis. 1997;24:363–368. doi: 10.1093/clinids/24.3.363. [DOI] [PubMed] [Google Scholar]
- 8.Bowman RJ, Sillah A, Van Dehn C, Goode VM, Muquit M, Johnson GJ, Milligan P, Rowley J, Faal H, Bailey RL. Operational comparison of single-dose azithromycin and topical tetracycline for trachoma. Invest Ophthalmol Vis Sci. 2000;41:4074–4079. [PubMed] [Google Scholar]
- 9.Schachter J, West SK, Mabey D, Dawson CR, Bobo L, Bailey R, Vitale S, Quinn TC, Sheta A, Sallam S, Mkocha H, et al. Azithromycin in control of trachoma. Lancet. 1999;354:630–635. doi: 10.1016/S0140-6736(98)12387-5. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Global Elimination of Blinding Trachoma. Geneva: World Health Organization; 1998. 51st World Health Assembly, Resolution 51.11. Text at http://who.int/pbd/trachoma/wha51-e.htm: World Health Organization. [Google Scholar]
- 11.Emerson PM, Cairncross S, Bailey RL, Mabey DC. Review of the evidence base for the ‘F’ and ‘E’ components of the SAFE strategy for trachoma control. Trop Med Int Health. 2000;5:515–527. doi: 10.1046/j.1365-3156.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Primary Health Care Level Management of Trachoma. Geneva: World Health Organization; 1993. (WHO/PBL/93.33) [Google Scholar]
- 13.Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987;65:477–483. [PMC free article] [PubMed] [Google Scholar]
- 14.Holm SO, Jha HC, Bhatta RC, Chaudhary JS, Thapa BB, Davis D, Pokhrel RP, Yinghui M, Zegans M, Schachter J, Frick KD, et al. Comparison of two azithromycin distribution strategies for controlling trachoma in Nepal. Bull World Health Organ. 2001;79:194–200. [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor HR, Siler JA, Mkocha HA, Munoz B, West S. The natural history of endemic trachoma: a longitudinal study. Am J Trop Med Hyg. 1992;46:552–559. doi: 10.4269/ajtmh.1992.46.552. [DOI] [PubMed] [Google Scholar]
- 16.Bailey RL, Hampton TJ, Hayes LJ, Ward ME, Whittle HC, Mabey DC. Polymerase chain reaction for the detection of ocular chlamydial infection in trachoma-endemic communities. J Infect Dis. 1994;170:709–712. doi: 10.1093/infdis/170.3.709. [DOI] [PubMed] [Google Scholar]
- 17.Taylor HR, Johnson SL, Prendergast RA, Schachter J, Dawson CR, Silverstein AM. An animal model of trachoma II. The importance of repeated reinfection. Invest Ophthalmol Vis Sci. 1982;23:507–515. [PubMed] [Google Scholar]
- 18.Thein J, Zhao P, Liu H, Xu J, Jha H, Miao Y, Pizzarello L, Tapert L, Schachter J, Mabon M, Osaki-Holm S, et al. Does clinical diagnosis indicate ocular chlamydial infection in areas with a low prevalence of trachoma? Ophthalmic Epidemiol. 2002;9:263–269. doi: 10.1076/opep.9.4.263.1508. [DOI] [PubMed] [Google Scholar]
- 19.Baral K, Osaki S, Shreshta B, Panta CR, Boulter A, Pang F, Cevallos V, Schachter J, Lietman T. Reliability of clinical diagnosis in identifying infectious trachoma in a low-prevalence area of Nepal. Bull World Health Organ. 1999;77:461–466. [PMC free article] [PubMed] [Google Scholar]
- 20.Wang SP, Grayston JT. Immunologic relationship between genital TRIC, lymphogranuloma venereum, and related organisms in a new microtiter indirect immunofluorescence test. Am J Ophthalmol. 1970;70:367–374. doi: 10.1016/0002-9394(70)90096-6. [DOI] [PubMed] [Google Scholar]
- 21.Treharne JD. The community epidemiology of trachoma. Rev Infect Dis. 1985;7:760–764. doi: 10.1093/clinids/7.6.760. [DOI] [PubMed] [Google Scholar]
- 22.West SK, Rapoza P, Munoz B, Katala S, Taylor HR. Epidemiology of ocular chlamydial infection in a trachoma-hyperendemic area. J Infect Dis. 1991;163:752–756. doi: 10.1093/infdis/163.4.752. [DOI] [PubMed] [Google Scholar]
- 23.Mabey DC, Robertson JN, Ward ME. Detection of Chlamydia trachomatis by enzyme immunoassay in patients with trachoma. Lancet. 1987;2:1491–1492. doi: 10.1016/s0140-6736(87)92623-7. [DOI] [PubMed] [Google Scholar]
- 24.Schachter J, Moncada J, Dawson CR, Sheppard J, Courtright P, Said ME, Zaki S, Hafez SF, Lorincz A. Nonculture methods for diagnosing chlamydial infection in patients with trachoma: a clue to the pathogenesis of the disease? J Infect Dis. 1988;158:1347–1352. doi: 10.1093/infdis/158.6.1347. [DOI] [PubMed] [Google Scholar]
- 25.Schachter J, Moncada J. Nucleic acid amplification tests to diagnose Chlamydia trachomatis genital infection—the glass is more than half full. In: Schachter J, Christiansen G, Clarke IN, Hammerschlag MR, Kaltenboeck B, Kuo CC, Rank RG, Ridgway GL, Saikku P, Stamm WE, Stephens RS, et al., editors. Chlamydial Infections: Proceedings of the Tenth International Symposium on Human Chlamydial Infections; Antalya, Turkey: International Chlamydia Symposium; 2002. pp. 379–388. [Google Scholar]
- 26.Bobo L, Munoz B, Viscidi R, Quinn T, Mkocha H, West S. Diagnosis of Chlamydia trachomatis eye infection in Tanzania by polymerase chain reaction/enzyme immunoassay. Lancet. 1991;338:847–850. doi: 10.1016/0140-6736(91)91502-l. [DOI] [PubMed] [Google Scholar]
- 27.Bobo LD, Novak N, Munoz B, Hsieh YH, Quinn TC, West S. Severe disease in children with trachoma is associated with persistent Chlamydia trachomatis infection. J Infect Dis. 1997;176:1524–1530. doi: 10.1086/514151. [DOI] [PubMed] [Google Scholar]
- 28.Mabey D, Holland M, Solomon A, Alexander N, Massae P, Aguirre A, Li S, Bailey R, West S, Foster A. The epidemiology of ocular Chlamydia trachomatis infection in a trachoma endemic community determined by quantitative PCR. In: Schachter J, Christiansen G, Clarke IN, Hammerschlag MR, Kaltenboeck B, Kuo CC, Rank RG, Ridgway GL, Saikku P, Stamm WE, Stephens RS, et al., editors. Chlamydial Infections: Proceedings of the Tenth International Symposium on Human Chlamydial Infections; Antalya, Turkey: International Chlamydia Symposium; 2002. pp. 495–498. [Google Scholar]
- 29.Solomon AW, Holland MJ, Burton MJ, West SK, Alexander ND, Aguirre A, Massae PA, Mkocha H, Munoz B, Johnson GJ, Peeling RW, et al. Strategies for control of trachoma: observational study with quantitative PCR. Lancet. 2003;362:198–204. doi: 10.1016/S0140-6736(03)13909-8. [DOI] [PubMed] [Google Scholar]
- 30.Wittwer CT, Ririe KM, Andrew RV, David DA, Gundry RA, Balis UJ. The LightCycler: a microvolume multisample fluorimeter with rapid temperature control. Biotechniques. 1997;22:176–181. doi: 10.2144/97221pf02. [DOI] [PubMed] [Google Scholar]
- 31.Raeymaekers L. Basic principles of quantitative PCR. Mol Biotechnol. 2000;15:115–122. doi: 10.1385/MB:15:2:115. [DOI] [PubMed] [Google Scholar]
- 32.Munoz B, Bobo L, Mkocha H, Lynch M, Hsieh YH, West S. Incidence of trichiasis in a cohort of women with and without scarring. Int J Epidemiol. 1999;28:1167–1171. doi: 10.1093/ije/28.6.1167. [DOI] [PubMed] [Google Scholar]
- 33.Solomon AW, Alexander ND, Holland MJ, Aguirre A, Massae PA, Natividad-Sancho A, Safari S, Peeling RW, Bailey RL, West SK, Foster A, et al. Longitudinal study of the effect of azithromycin on ocular Chlamydia trachomatis load in a trachoma-endemic community of Tanzania. Trans R Soc Trop Med Hyg. 2003 (in press) [Google Scholar]
- 34.Treharne JD. The microbial epidemiology of trachoma. Int Ophthalmol. 1988;12:25–29. doi: 10.1007/BF00133777. [DOI] [PubMed] [Google Scholar]
- 35.Grayston JT, Wang SP, Yeh LJ, Kuo CC. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985;7:717–725. doi: 10.1093/clinids/7.6.717. [DOI] [PubMed] [Google Scholar]
- 36.Zhang YX, Stewart S, Joseph T, Taylor HR, Caldwell HD. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987;138:575–581. [PubMed] [Google Scholar]
- 37.Wang SP, Grayston JT, Alexander ER. Trachoma vaccine studies in monkeys. Am J Ophthalmol. 1967;63(suppl):1615–1630. doi: 10.1016/0002-9394(67)94155-4. [DOI] [PubMed] [Google Scholar]
- 38.Hayes LJ, Pecharatana S, Bailey RL, Hampton TJ, Pickett MA, Mabey DC, Watt PJ, Ward ME. Extent and kinetics of genetic change in the ompA gene of Chlamydia trachomatis in two villages with endemic trachoma. J Infect Dis. 1995;172:268–272. doi: 10.1093/infdis/172.1.268. [DOI] [PubMed] [Google Scholar]
- 39.Hayes LJ, Bailey RL, Mabey DC, Clarke IN, Pickett MA, Watt PJ, Ward ME. Genotyping of Chlamydia trachomatis from a trachoma-endemic village in the Gambia by a nested polymerase chain reaction: identification of strain variants. J Infect Dis. 1992;166:1173–1177. doi: 10.1093/infdis/166.5.1173. [DOI] [PubMed] [Google Scholar]
- 40.Smith A, Munoz B, Hsieh YH, Bobo L, Mkocha H, West S. OmpA genotypic evidence for persistent ocular Chlamydia trachomatis infection in Tanzanian village women. Ophthalmic Epidemiol. 2001;8:127–135. doi: 10.1076/opep.8.2.127.4164. [DOI] [PubMed] [Google Scholar]
- 41.Bailey RL, Hayes L, Pickett M, Whittle HC, Ward ME, Mabey DC. Molecular epidemiology of trachoma in a Gambian village. Br J Ophthalmol. 1994;78:813–817. doi: 10.1136/bjo.78.11.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caldwell HD, Wood H, Crane D, Bailey R, Jones RB, Mabey D, Maclean I, Mohammed Z, Peeling R, Roshick C, Schachter J, et al. Polymorphisms in Chlamydia trachomatis tryptophan synthase genes differentiate between genital and ocular isolates. J Clin Invest. 2003;111:1757–1769. doi: 10.1172/JCI17993. [DOI] [PMC free article] [PubMed] [Google Scholar]