Abstract
Objectives:
In this study, we sought to determine: 1) whether splenic activation after acute coronary syndrome (ACS) is linked to leukocyte pro-inflammatory remodeling, and 2) whether splenic activity independently predicts the risk of cardiovascular disease (CVD) events.
Background:
Preclinical data suggest the existence of a cardiosplenic axis, wherein activation of hematopoietic tissues (notably in the spleen) results in liberation of pro-inflammatory leukocytes and accelerated atherosclerotic inflammation. However, it is presently unknown whether: 1) a cardiosplenic axis exists in humans, and 2) whether splenic activation relates to CVD risk.
Methods:
18FDG-PET imaging was performed in 508 individuals across two studies. In the first study we performed FDG-PET imaging in 22 patients with recent ACS and 22 controls. FDG uptake was measured in spleen and arterial wall while pro-inflammatory gene expression of circulating leukocytes was assessed by qRT-PCR. In a second study, we examined the relationship between splenic tissue FDG uptake with subsequent CVD events during follow-up (median 4 years) in 464 patients who previously had undergone FDG-PET imaging.
Results:
Splenic activity increased after ACS and was significantly associated with multiple indices of inflammation: a) up-regulated gene expression of pro-inflammatory leukocytes, b) increased C-reactive protein, and c) increased arterial wall inflammation (FDG uptake). Moreover, in the second study, splenic activity (≥median) was associated with increased risk of CVD events (HR [95% CI] =3.3 [1.5, 7.3], p=0.003) which remained significant after adjustment for CVD risk factors (HR = 2.26 [1.01, 5.06], p=0.04) and for arterial FDG uptake (HR=2.68 [1.5–7.4], p=0.02).
Conclusions:
Our findings demonstrate increased splenic metabolic activity after ACS and its association with pro-inflammatory remodeling of circulating leukocytes. Moreover, we observe that metabolic activity of the spleen independently predicts risk of subsequent CVD events. Collectively, these findings provide evidence of a cardiosplenic axis in humans similar to that shown in preclinical studies.
Keywords: acute coronary syndrome, atherosclerosis, events, spleen, FDG, inflammation
Condensed Abstract
Preclinical data suggest the existence of a cardiosplenic axis, wherein activation of hematopoietic tissues (especially spleen) results in liberation of pro-inflammatory leukocytes and accelerated atherosclerosis. We sought to determine whether splenic activation after ACS is linked to leukocyte remodeling, and whether splenic activity predicts the risk of cardiovascular events. We performed 18FDG-PET imaging in individuals with recent ACS. In a second study, we examined the relationship between splenic FDG uptake and subsequent cardiovascular events. Our findings demonstrate increased splenic metabolic activity post-ACS and its association with pro-inflammatory leukocytes. Moreover, we observed that spleen activity independently predicts risk of subsequent events.
INTRODUCTION
Patients remain at an increased risk for recurrent cardiovascular disease (CVD) events in the weeks to months after an acute coronary syndrome (ACS).(1,2) However, the pathophysiologic basis for this increased risk remains unclear. Pre-clinical studies have shown that proliferation of monocyte progenitors and pro-inflammatory activation of monocytes within the hematopoietic tissues (i.e. bone marrow and spleen) may play an important role in accelerating atherosclerosis after myocardial infarction.(3,4) Preclinical studies demonstrated that following myocardial infarction in mice, monocyte progenitor cells departed bone marrow niches and resulted in amplified extramedullary monocytopoiesis. (3) Observation of activation of the inflammatory cell milieu and migration of pro-inflammatory monocytes from spleen to heart in animal models of heart failure,(5) have given rise to the concept of a cardiosplenic axis. Recently, the concept of a cardiosplenic axis has been extended to stable atherosclerosis in murine models as well;(6) however, it is presently unknown whether such an axis exists in humans.
Imaging with 18fluorodeoxyglucose-positron emission tomography (FDG-PET) provides a non-invasive measure of tissue glycolysis(7) and is used clinically for the evaluation of tumors (8) and infectious foci.(9) The biological basis for FDG accumulation within mononuclear inflammatory cells lies in the fact that macrophages have a high metabolic rate,(10) especially after pro-inflammatory activation,(11,12) and hence avidly accumulate FDG.(13) Additionally, cellular accumulation of FDG is increased in rapidly proliferating cells.(14) Since in animal models of myocardial infarction, splenic activation is marked by proliferation of monocyte progenitor cells and pro-inflammatory activation of monocytes,(3,4) we sought to evaluate splenic activation in humans using FDG PET/CT imaging. Further, we investigated whether splenic metabolic activity relates to pro-inflammatory gene expression of circulating leukocytes.
In the first study, we tested the hypothesis that the metabolic activity of hematopoietic tissues (i.e. bone marrow [BM] and spleen) occurs in humans with recent ACS. In the second study and in a separate population, we investigated whether metabolic activity of these hematopoietic tissues predicts the risk of subsequent CVD events. To do so, we evaluated BM and splenic metabolic activity, by FDG uptake, in a group of individuals with no known atherosclerotic disease who had undergone clinically indicated FDG-PET/CT scans and for whom clinical follow-up data were available. We then assessed whether the baseline hematopoietic tissue FDG signal: 1) correlated with arterial wall inflammation, and 2) independently predicted the subsequent development of incident CVD events.
METHODS
Over-all Study Schema
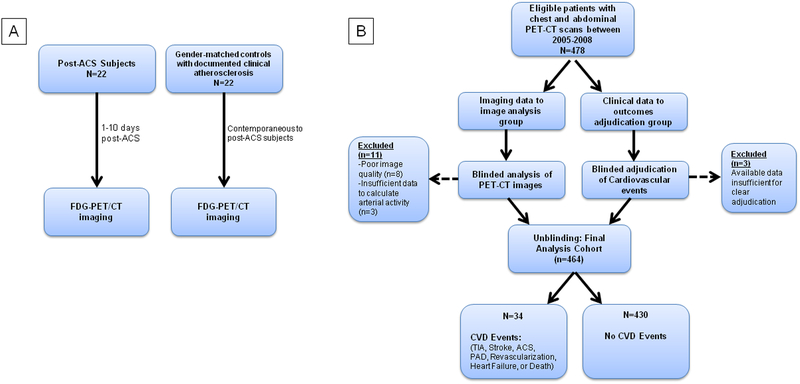
We performed two separate studies (Figure 1). The first study, “ACS Study”, was designed to test the hypotheses that metabolic activity of the hematopoietic tissues of bone marrow and spleen was more prominent after ACS, and associated with levels of serum pro-inflammatory biomarkers, pro-inflammatory gene expression of circulating leukocytes, and arterial wall inflammation. To test these hypotheses, 44 patients were prospectively recruited at Massachusetts General Hospital. FDG-PET/CT imaging was performed in all the subjects, and FDG uptake was assessed in the BM, spleen, arterial wall, and in control tissues. Additionally serum biomarker assays and Quantitative real time-polymerase chain reaction (qRT-PCR) of pro-inflammatory gene expression in circulating leukocytes was performed.
Figure 1: Schematic Diagrams of Studies.
In the first study (A), 22 post-ACS patients underwent FDG-PET/CT imaging within 10 days of their event. We compared bone marrow, splenic and arterial activity in these patients with 22 gender-matched control subjects. In the second study (B), we studied 464 subjects who had undergone FDG-PET/CT imaging for various clinical indications and assessed whether bone marrow and splenic activity was associated with future CVD events.
The second study, “Clinical Outcomes Study”, was conducted in a separate population and was designed to test the hypothesis that hematopoietic tissue (BM and spleen) metabolic activity was: 1) associated with arterial wall inflammation, and 2) independently predicts subsequent risk of incident CVD events. To test these hypotheses, we evaluated BM and splenic activity (using FDG PET/CT imaging) in 464 individuals consecutively identified from a database of patients who had undergone clinically indicated FDG-PET/CT scans. Thereafter, the development of incident CVD events was adjudicated by blinded Cardiologists, and the relationship between hematopoietic tissue activity and subsequent CVD risk was assessed.
Study Subjects
Study One: ACS Study
Forty-four subjects were prospectively recruited into this study. Twenty-two individuals with acute coronary syndrome (ACS group) and 22 individuals without recent ACS (control group) were recruited (Figure 1A).
The ACS group was identified according to pre-specified criteria. Eligible patients were adults (age 38–69 yrs) with recent ACS (defined as myocardial infarction or unstable angina documented by electrocardiogram [ECG], cardiac enzymes or angiogram), clinically stable and able to undergo an FDG-PET/CT scan within 10 days of the incident CVD event. The control group consisted of 22 individuals with documented clinical atherosclerotic disease who were gender-matched to the ACS group. The control group underwent contemporaneous FDG-PET/CT imaging and was selected among male or female subjects, 35–80 years of age, with documented clinical atherosclerotic disease (i.e. coronary artery disease, peripheral arterial disease, or carotid disease), but no history of ACS within the previous 6 months. Exclusion criteria for both groups included systemic chronic inflammatory conditions, type I diabetes or fasting plasma glucose > 175 mg/dL, presence of severe heart failure or severe LV dysfunction, or significant radiation exposure within the preceding 12 months. Additionally, individuals were excluded from participation if they had recent evidence of cardiogenic shock, sustained ventricular tachyarrhythmia, or hypoxemia (defined as O2 saturation <90% or requirement for >2 L/min supplemental oxygen to maintain O2 saturation >90%).
Study Two: Clinical Outcomes Study
Using a retrospective study design, 464 individuals who underwent prior PET/CT imaging at the Massachusetts General Hospital between 2005 and 2008 were included in this analysis (Figure 1B). We included all of the individuals with PET/CT image of chest and abdomen who met the following pre-defined inclusion criteria: 1) either absence of prior cancer diagnosis or remission from cancer at the time of PET imaging and throughout the follow-up period, 2) age ≥ 30 years, 3) no prior history of cardiovascular disease, 4) absence of acute or chronic inflammatory or autoimmune disease (based on documented medical history) or use of chronic anti-inflammatory therapy. Subjects were required to have at least 3 clinical visit notes (spanning ≥ 1 year) to ensure availability of sufficient clinical data and to determine clinical status at the time of PET imaging.
FDG-PET/CT imaging
FDG-PET/CT imaging was performed using previously reported, reproducible and validated approaches.(15) Briefly, 18F-FDG was administered intravenously (approximately 10 mCi for a 70 kg patient) after an overnight fast and imaging was performed 90 minutes after FDG injection using PET/CT.
Image analysis
While blinded to all clinical and temporal data, images were analyzed at a central core laboratory at Massachusetts General Hospital using Leonardo TrueD software (Siemens Forchheim, Germany). Arterial inflammation was measured within pre-defined sections of the three target vessels (right and left carotid, and aortic wall) using previously validated methods (see supplement for details).(15,16) Bone marrow FDG uptake was measured by placing an ROI over axial sections of individual vertebrae from T1 to L5. SUVmax for each vertebra was recorded and bone marrow activity was calculated as mean of SUVmax of all vertebrae in the imaging field. Similarly, splenic FDG uptake was assessed by placing ROIs in 3 orthogonal planes (axial, sagittal and coronal planes) (see supplemental figure 1). SUVmax was recorded in each plane, and splenic activity was calculated as mean of SUVmax values of the 3 planes. Intra-reader reproducibility for measurement of bone marrow FDG uptake was as intra-class correlation coefficient (ICC) = 0.99, (p<0.001) and percent variance = 2.2±2.1%. Reproducibility for splenic FDG uptake measurement was as ICC = 0.96 (p<0.001), and percent variance = 4.7±3.8%. For evaluations of the correlation of FDG uptake between tissues (with-in patients) we additionally employed uncorrected SUVs to avoid the contribution that a common background value would add to the relationship.
Gene expression assays
Inflammatory gene expression was assessed by Quantitative real time-polymerase chain reaction (qRT-PCR) of peripheral blood leukocyte pro-inflammatory mRNA. (See supplement for details of assessment of inflammatory gene expression)
Adjudication of CVD Events
Events were clinically adjudicated by 2 cardiologists who were blinded to all imaging data. Using clinically available records, incident CVD events included: ischemic stroke or transient ischemic attack (TIA), ACS, revascularization (coronary, carotid, or peripheral), unstable angina, heart failure, or CVD death. (See supplement for additional details)
Statistical Analysis
Descriptive data are presented as mean ± standard deviation (SD) for continuous parametric variables, median (interquartile range [IQR]) for continuous non-parametric data and frequency with proportions for nominal variables as appropriate. Independent samples t-test was used for cross-sectional comparison of normally distributed continuous variables (such as bone marrow, spleen and arterial FDG uptake) between ACS and control groups. Mann-Whitney U test was employed for the similar analyses of continuous variables without normal distribution (such as CRP). Fisher’s exact test was performed for comparison of dichotomous variables. Pearson correlation coefficient (R) was used to assess correlations between continuous variables once normal distribution was verified and Spearman ρ was reported as correlation coefficient for non-normally distributed variables. For comparison of means, 95% CI are provided. Kaplan-Meier estimates of CVD free events of patients stratified by median of FDG uptake in spleen and BM expressed as SUVmax were calculated. Cox Proportional Hazards Regression was used to calculate hazard ratios (HR) and 95% confidence intervals (CI). Framingham Risk Score (FRS) was not normally distributed among the study subjects and we converted it to an ordinal variable (low [<10], intermediate [10–20] and high risk [>20]) and used the ordinal variable for the analyses. Two-tailed probability values are reported and statistical significance is defined as P<0.05. All statistical analyses were performed using SPSS version 22 (IBM, Armonk, NY).
RESULTS
Study One: ACS Study
Baseline characteristics:
There were no significant differences between ACS vs. controls, for age or major CVD risk factors, except for current smoking (p=0.04). All the individuals in the control group had documented clinically diagnosed CAD. Baseline patient characteristics and demographics are summarized in Table 1. Additional information on clinical presentations of ACS patients is detailed in Supplemental Table 1.
Table 1.
Baseline Characteristics of ACS Study Subjects
| Acute Coronary Syndrome (n=22) |
Controls (n=22) |
p-value | |
|---|---|---|---|
| Age | 58±8.5 | 62±10 | 0.19 |
| Male gender | 16 (72%) | 16 (72%) | 0.63 |
| Current smoker | 9 (41%) | 3 (14%) | 0.04 |
| Diabetes Mellitus | 4 (18%) | 3 (14%) | 0.50 |
| Hypertension | 17 (77%) | 18 (82%) | 0.50 |
| BMI (kg/m2) | 31.5±5.6 | 29.2±3.4 | 0.16 |
| Baseline values | |||
| Total cholesterol, mg/dl | 173.2±38.2 | 159.8±21.9 | 0.21 |
| LDL cholesterol, mg/dl | 95.1±31.6 | 92.8±18.5 | 0.80 |
| HDL cholesterol, mg/dl | 42.8±12.7 | 53.1±16.2 | 0.03 |
| Triglycerides, mg/dl | 174.2±94.6 | 120.4±42.1 | 0.04 |
| FDG Uptake* | |||
| Bone Marrow | 2.9±0.5 | 2.4±0.6 | 0.01 |
| Spleen | 2.6±0.6 | 2.1±0.3 | 0.03 |
| Arterial wall | 2.7±1.2 | 2.2±0.6 | 0.04 |
| Control Tissues | |||
| Subcutaneous Adipose Tissue | 0.16±0.05 | 0.17±0.06 | 0.57 |
| Pectoralis Muscle | 0.41±0.07 | 0.43±0.12 | 0.53 |
Values are presented as mean ± standard deviation or n (%).
FDG uptake in bone marrow, spleen and control tissues is reported as SUV whereas arterial FDG uptake is reported as TBR.
Abbreviations: BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; SUV, standardized uptake value; TBR, target-to-background ratio
Hematopoietic tissue metabolic activity is up-regulated after ACS:
We observed a significantly higher splenic FDG uptake in individuals with recent ACS (SUV: 2.6±0.6 vs. 2.1±0.3; p=0.03). Similarly, bone marrow FDG uptake was substantially higher in ACS compared to controls (SUV: 2.9±0.5 vs. 2.4±0.6; p=0.01). In contrast, FDG uptake in control tissues (subcutaneous adipose tissue [SAT] and pectoralis muscles) was not increased in ACS compared to control subjects (SAT SUV: 0.16±0.05 vs. 0.17±0.06; p=0.57; pectoralis muscle SUV: 0.41±0.07vs. 0.43±0.12; p=0.53) (Table 1).
Hematopoietic tissue metabolic activity correlates with CRP and Pro-inflammatory gene expression in leukocytes:
Serum CRP concentrations were significantly higher in the ACS group vs. control subjects (median [IQR]: 7.70 [1.45, 21.2] vs. 2.47 [1.00, 4.13], p=0.04). In addition, CRP correlated with BM FDG uptake (ρ=0.62, p=0.002) and splenic FDG uptake (ρ=0.44, p=0.04). There was a strong correlation between CRP and BM FDG uptake (ρ=0.66, p=0.02) in post-ACS patients whereas no significant correlation was observed in controls (ρ=0.48, p=0.13). Further, we observed a significant correlation between hematopoietic tissue FDG uptake and the expression of several genes associated with pro-inflammatory activation within circulating leukocytes. (Table 2)
Table 2.
Relationship between Hematopoietic Tissue Activity and Inflammatory Biomarkers
| Bone marrow FDG Uptake | Spleen FDG Uptake | |||
|---|---|---|---|---|
| correlation coefficient | p value | correlation coefficient | p value | |
| Serum Biomarkers | ||||
| CRP | 0.62 | 0.002 | 0.44 | 0.04 |
| TNF | 0.19 | 0.46 | 0.39 | 0.44 |
| IL1-beta | 0.43 | 0.09 | −0.37 | 0.47 |
| Gene Expression in Leukocytes | ||||
| CD36 | 0.05 | 0.85 | 0.51 | 0.03 |
| MSR-1 | 0.53 | 0.02 | 0.48 | 0.04 |
| S100A9 | 0.15 | 0.54 | 0.59 | 0.01 |
| TLR-2 | 0.19 | 0.45 | 0.45 | 0.06 |
Bone marrow and spleen FDG uptakes are analyzed as standardized uptake value (SUV). Correlation coefficients represent Spearman ρ. Correlations are assessed in the entire population (n=44) including both post-ACS and control subjects. In analyses that were limited to the sub-groups, we did not observe significant correlations except for between S100A9 expression and splenic activity in the control group (ρ=0.8, p=0.02).
CRP, C-reactive protein; TNF, tumor necrosis factor; MSR-1, Macrophage Scavenger Receptor 1; TLR-2, toll-like receptor 2
Relationships with arterial inflammation:
As expected, the mean arterial FDG uptake (TBR) was greater in patients with ACS compared to controls (2.7±1.2 vs. 2.2±0.6; p=0.04) (Table1). Moreover, BM and splenic metabolic activity (SUV) significantly correlated with arterial inflammation (TBR) (r= 0.34, p=0.03 and r=0.46, p=0.01; BM and spleen, respectively). Similarly, the uncorrected arterial FDG signal (SUV) also correlated with BM and splenic SUV (r= 0.67, p<0.001 and r=0.37, p=0.04; BM and spleen, respectively). In contrast, FDG uptake in the control tissues did not correlate with FDG uptake in the artery wall (r=0.11, p=0.39 and r=0.20, p=0.10; for the artery wall vs. SAT and pectoralis muscle, respectively).
Additionally, we observed a significant correlation between arterial inflammation (TBR) and CRP (ρ=0.45, p=0.04). Arterial inflammation (TBR) was associated with mRNA levels of CD16 and toll-like receptor 4 (TLR-4) in the peripheral leukocytes such that tertiles of mean arterial TBR correlated with mRNA levels of CD16 (ρ=0.47, p=0.04) and TLR-4 (ρ=0.49, p=0.03).
Study Two: Clinical Outcomes Study
Baseline characteristics:
In the second study, we evaluated 464 participants with follow-up for 6.5 years (median 4 years). Thirty-four individuals developed CVD events (2 per 100 person-years at risk) during this period. Baseline characteristics and demographics are presented in Table 3.
Table 3.
Baseline Characteristics of the Clinical Outcomes Study
| Full Cohort (n=464) | Individuals Without Subsequent CVD (n=430) | Individuals With Subsequent CVD (n=34) | p-value | |
|---|---|---|---|---|
| Age | 55 [44–46] | 54 [44–65] | 66 [60–78] | <0.001 |
| Male gender | 201 (43) | 186 (43) | 15 (44) | 0.53 |
| Current smoker | 46 (10) | 36 (8.5) | 10 (29) | 0.001 |
| Hypertension | 158 (34) | 139 (32) | 19 (56) | 0.006 |
| Diabetes Mellitus | 41 (9) | 34 (8) | 7 (20) | 0.02 |
| Statin use | 89 (19) | 75 (17) | 14 (41) | 0.002 |
| BMI (kg/m2) | 26 [23–31] | 26 [23–31] | 27 [24–32] | 0.33 |
| Dyslipidemia | 127 (27) | 112 (26) | 15 (44) | 0.02 |
| Baseline values | ||||
| Total cholesterol, mg/dl | 192±45 | 193±45 | 184±41 | 0.32 |
| LDL cholesterol, mg/dl | 111±38 | 112±32 | 107±34 | 0.49 |
| HDL cholesterol, mg/dl | 56±18 | 57±19 | 50±14 | 0.04 |
| Triglycerides, mg/dl | 124±72 | 121±72 | 140±71 | 0.18 |
| FRS* | ||||
| <10 | 186 (82) | 168 (85) | 18 (60) | 0.08 |
| 10–20 | 36 (16) | 24 (12) | 12 (40) | <0.001 |
| >20 | 5 (2) | 5 (2.5) | 0 (0) | 0.68 |
| FDG Uptake† | ||||
| Bone Marrow | 2.2 [1.9–2.6] | 2.2 [1.9–2.6] | 2.4 [2.1–2.8] | 0.03 |
| Spleen | 2.1 [1.8–2.4] | 2.1 [1.8–2.3] | 2.2 [2.1–2.5] | 0.01 |
| Arterial wall | 1.9 [1.8–2.2] | 1.9 [1.8–2.2] | 2.2 [1.9–2.3] | <0.001 |
| Subcutaneous Adipose Tissue (SAT) | 0.17 [0.14–0.2] | 0.16 [0.14–0.2] | 0.19 [0.13–0.22] | 0.28 |
Values are presented as mean ± standard deviation, median [IQR] or n (%).
A subset of 227 subjects provided all the required data to calculate FRS.
FDG uptake in bone marrow, spleen and SAT is reported as SUV whereas arterial FDG uptake is reported as TBR.
Abbreviations: BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein
CVD Events:
The events were characterized as following: 8 acute coronary syndromes (6 acute myocardial infarction and 2 unstable angina), 8 coronary revascularizations, 7 strokes, 2 transient ischemic attacks, 2 carotid endarterectomy, 4 new-onset angina (1 with and 3 without obstructive disease documented on coronary catheterization), 2 new diagnoses of peripheral artery disease (PAD), 2 peripheral revascularizations secondary to PAD, and 1 subsequent deaths due to acute myocardial infarction. The distribution of events between males and females was not statistically significant (15 males and 19 females, p=0.54).
Hematopoietic tissue activity correlates with arterial inflammation:
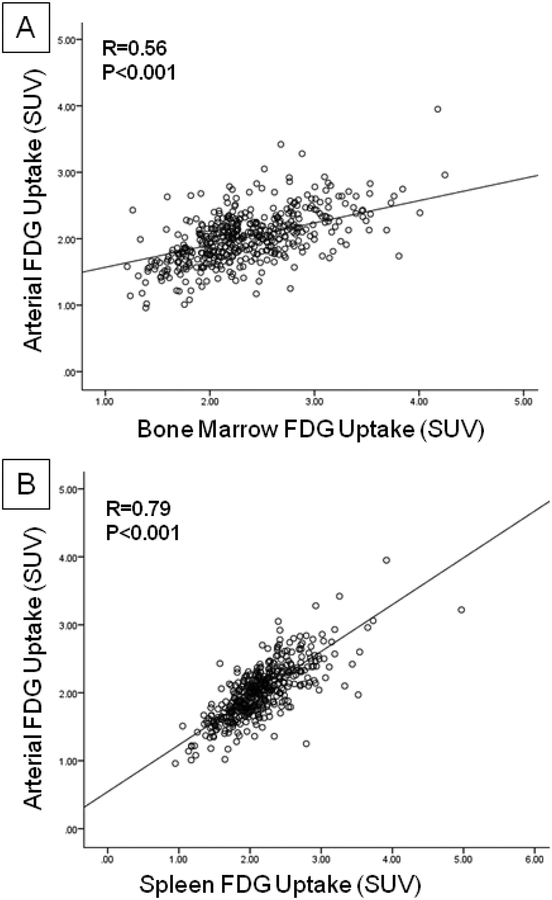
BM metabolic activity (FDG uptake) significantly correlated with arterial SUV (r=0.56, p<0.001; Figure 3A) as well as background corrected arterial TBR (r=0.22, p<0.001). Similarly, splenic activity significantly correlated with arterial SUV (r=0.79, p<0.001; Figure 3B) and arterial TBR (r=0.23, p<0.001). Additionally, we observed a strong positive correlation between BM and splenic activation (r=0.71, p<0.001). In contrast, there was no significant correlation between SAT metabolic activity and arterial inflammation (r=−0.074, p=0.11).
Figure 3: Correlation Between FDG Uptake in Bone Marrow, Spleen and Arterial Wall.
In the clinical outcomes study, a strong correlation between baseline arterial FDG uptake with (A) bone marrow and (B) spleen was observed.
Bone marrow and splenic activation are associated with subsequent risk of CVD events:
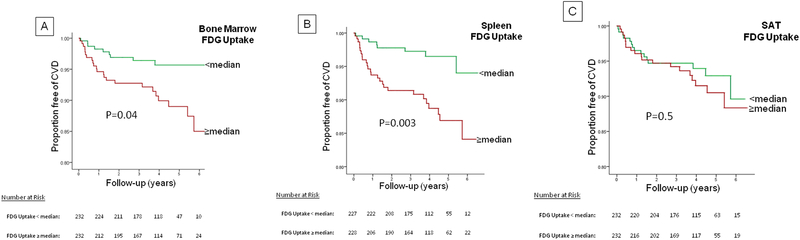
Patients with higher BM activity (≥median) had an increased risk for subsequent CVD events (HR [95% CI] =2.15 [1.05, 4.41], p=0.04; Figure 4A). However, in a Cox-regression model, the association was no longer significant after adjusting for FRS (p=0.12), statin use (p=0.06) or for arterial FDG uptake (p=0.14).
Figure 4: Proportion Free of CVD Events Stratified by Bone Marrow, Spleen and SAT Metabolic Activity.
Individuals with higher (≥median), (A) bone marrow activity (FDG uptake), and (B) splenic activity at baseline had an increased risk for CVD events. Baseline SAT activity does not predict CVD events in individuals stratified into higher vs. lower FDG values. (C)
P-value from Kaplan-Meier survival curve was derived from log-rank test.
In contrast, splenic FDG uptake (≥median) was associated with an increased risk for CVD events (HR [95% CI] =3.3 [1.5, 7.3], p=0.003, Figure 4B), which remained significant after adjusting for CVD risk factors of age, male gender, smoking (HR=2.25 [1.01–5.04], p=0.04); and also after adjusting for hypertension, diabetes mellitus and dyslipidemia (HR=2.76 [1.24, 6.18], p=0.01). In two additional models, the association between splenic FDG uptake and CVD events remained significant after adjusting for FRS (HR=2.9 [1.2–6.9], p=0.01), and after adjusting for statin use (HR=2.8 [1.3–6.4], p=0.01).
The association between higher splenic activity and risk for CVD events remained significant after adjusting for history of prior cancer diagnosis, chemotherapy and radiotherapy (HR=3.37 [1.5–7.4], p=0.003), after adjusting for “time from chemotherapy to imaging” (HR=2.65 [1.03, 6.78], p=0.04), after excluding subjects with history of lymphoma and hematological malignancies (HR=3.4 [1.4–8.4], p=0.008), and after excluding subjects with prior history of any type of cancer (HR=5.1 [1.1–23.8]. p=0.04). In addition, we observed similar findings after adjusting for arterial TBR (HR=2.68 [1.5–7.4], p=0.02). In contrast, metabolic activity in the control tissue (SAT) was not associated with risk of CV events (HR [95% CI] =1.26 [0.64, 2.48], p=0.50, Figure 4C).
DISCUSSION
We observe that the BM and splenic tissue metabolic activity is substantially increased after ACS, and correlates with CRP and expression of pro-inflammatory markers of circulating leukocytes. Finally, we observe in a separate study group, that splenic tissue activity is independently associated with an increased risk of CVD events in individuals without known atherosclerosis. Thus these data suggest that in humans, BM and splenic activity are associated with an increase in pro-inflammatory mediators which may play a role in increased atherosclerotic plaque inflammation and an elevated risk of subsequent CVD events.
Inflammation in atherosclerosis
Inflammation plays a central role in the pathogenesis of atherosclerosis and its associated clinical complications.(17) The response to acute ischemic injury is believed to trigger a molecular and cellular systemic inflammatory response, whereby the recruitment of pro-inflammatory leukocytes results in deleterious effects on the blood vessel wall.(18) Numerous mediators contribute to this process, including chemokines, cytokines, proteases, and adhesion molecules, whereby their interactions contribute to atherosclerotic plaque instability.(19) Although statins appear to reduce vascular risk by favorably modifying this biology,(20) a substantial residual risk remains, thus there exists a strong impetus to further our knowledge of the underlying pathobiology of atherosclerosis.(21)
Metabolic activity of spleen and pro-inflammatory remodeling of leukocytes
Our data suggests that after ACS, increased metabolic activity of hematopoietic tissue (especially spleen), as assessed by FDG-PET, may represent an early step in a process resulting in pro-inflammatory remodeling of leukocytes (especially monocytes). This concept is supported by our observation that CRP, a marker of systemic inflammation, is strongly correlated to BM and splenic activity. These findings are consistent with those of Kim et al.(22) Moreover, in the current study, we extend the observations of Kim et al by providing novel data on the gene expression of pro-inflammatory leukocytes and also by evaluating the association between splenic activity and subsequent CVD events. We show that the gene expression of circulating pro-inflammatory monocytes (i.e. MSR-1,(23,24) S100A9(25), CD36(24), IL-1β and TLR-4(26)) is significantly correlated with BM and splenic activity. It is notable that pro-inflammatory gene activation within circulating leukocytes was more closely associated with metabolic activity of the spleen than it was for the bone marrow. Several reasons may account for this observation. Firstly, while the bone marrow is an important and steady source of circulating leukocytes, the spleen acts as a large reservoir and may contribute substantially to the pool of circulating leukocyets after myocardial infarction (27). Secondly, the spleen has been shown to be an important locus of myeloid cell production in animal models of atherosclerosis and myocardial infarction (3). Hence, the activation state of the spleen (more than the bone marrow) may closely relate to the pro-inflammatory state of circulating leukocytes, especially after injury or stress. Further studies are warranted to explore the precise underlying mechanisms.
Cardiosplenic axis
Both the BM and spleen may be involved in a highly coordinated and dynamic biologic interaction with the vascular wall resulting in accelerated atherogenesis after ACS in humans. In preclinical studies, the role of BM and spleen activity following acute MI is established. Prior preclinical studies support our findings in ACS patients, demonstrating a paradigm in which bone marrow and spleen-derived inflammatory cells provoke vascular inflammation. Additionally, preclinical studies have demonstrated that activation of inflammatory mononuclear cells in spleen plays a central role in progression of heart failure in response to cardiac-derived alarmins, which further emphasizes the presence of interplay between cardiovascular system and spleen -- cardiosplenic axis.(5) A recent autopsy study of patients following myocardial infarction show that pro-inflammatory monocytes are depleted from the BM and spleen, but are increased in atherosclerotic plaques while the spleen experiences a pronounced reduction of monocytes, suggesting a prominent role of the spleen in trafficking monocytes to atherosclerotic plaques.(28) The results of our clinical outcome study suggest that this interconnection between the spleen and arterial wall may be present in a stable setting as well.
In this study, we confirmed in humans, the paradigm-shifting preclinical observation (3) that BM and splenic activity occurs after ACS and is associated with increased arterial wall inflammation. It has been previously shown that apolipoprotein-E-deficient mice lacking bone marrow-derived progenitor cells or spleen(3) results in a reduction in atherosclerosis. In addition, this study for the first time raises the possibility that residual risk in stable patients might also be related to the spleen-atherosclerotic plaque axis. As such, the results of our study may encourage the development of novel therapeutic agents aimed at modulating the activity of progenitor cells to attenuate the systemic inflammatory milieu and its adverse clinical sequelae. The study also highlights that splenic metabolic activity may represent a novel target of future therapies designed to minimize atherosclerosis progression.
Limitations
This study is not without limitations. Due to ethical concerns, bone marrow and splenic biopsies were not obtained from patients limiting histologic correlation of the FDG signal. Flow cytometry analysis of peripheral blood leukocytes was not performed thus limiting evaluation of monocyte subsets. It is also worth noting that a large proportion of patients with ACS (41% of total study population) presented with unstable angina and many did not have increased levels of cardiac biomarkers. Hence, it is possible that in a population with a greater degree of myocardial injury, BM and splenic activation may be greater than what was observed in the ACS population. In addition, the design of this study precludes assessment of splenic activity prior to ACS. Given the retrospective nature of the clinical outcomes study and the fact that the study population was drawn from a clinical database consisting mostly of cancer survivors, the generalizability of the findings may be constrained. Other confounding factors might include history and type of cancer, as well as of chemo- or radio-therapy. Although we have tried to minimize the impact of these potential confounders, through statistical adjustments, we believe our findings warrant confirmation in prospective studies. Lastly, while we observed compelling inter-relationships between BM and splenic activation, leukocyte pro-inflammatory gene expression, and arterial inflammation, we acknowledge that these relationships do not necessarily indicate causality.
Conclusion
Our findings demonstrate that splenic metabolic activity is increased after ACS and correlates with pro-inflammatory remodeling of circulating leukocytes. Furthermore, we show for the first time that splenic activity correlates with arterial inflammation and is an independent predictor of the risk of incident CVD events. These observations suggest that the cardiosplenic axis may be clinically relevant in both acute and stable atherosclerotic vascular disease and provide impetus for further study of the mechanisms underlying the link between the hematopoietic tissues (BM and spleen) and progression of atherosclerotic cardiovascular diseases.
Supplementary Material
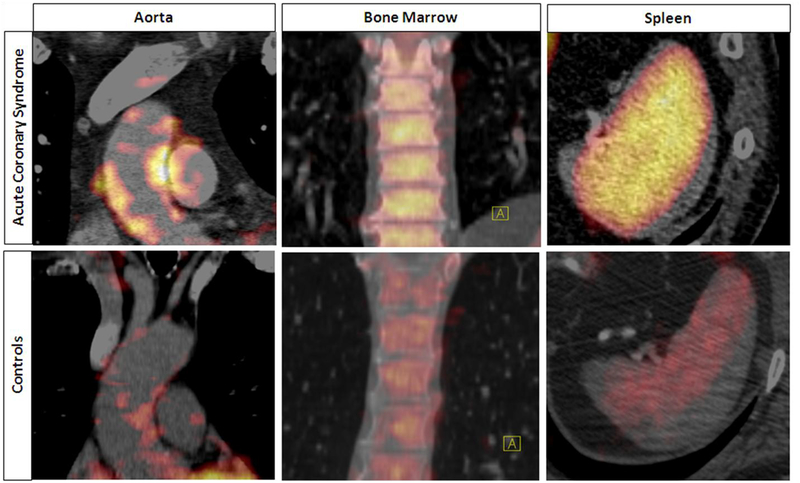
Figure 2: Increased Bone Marrow, Spleen and Arterial FDG Uptake After ACS.
Coronal views of aorta (Left), bone marrow (Middle) and axial view of spleen (Right). FDG uptake is significantly increased in the arterial wall (aorta), bone marrow and spleen in patients with ACS vs. controls.
Acknowledgments
Funding for the first study (ACS Study) was provided by Genentech, Inc, and BioInvent International AB. No funding from any source was provided for the second study (Clinical Outcomes Study).
ABBREVIATIONS
- ACS
acute coronary syndrome
- BM
bone marrow
- CRP
C-reactive protein
- CVD
cardiovascular disease
- FDG
18-fluorodeoxyglucose
- FRS
Framingham Risk Score
- PET
positron emission tomography
- SAT
subcutaneous adipose tissue
- SUV
standardized uptake value
- TBR
target-to-background ratio
REFERENCES
- 1.Rosamond WD, Chambless LE, Folsom AR, et al. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987 to 1994. N Engl J Med 1998;339:861–7. [DOI] [PubMed] [Google Scholar]
- 2.Thune JJ, Signorovitch JE, Kober L, et al. Predictors and prognostic impact of recurrent myocardial infarction in patients with left ventricular dysfunction, heart failure, or both following a first myocardial infarction. Eur J Heart Fail 2011;13:148–53. [DOI] [PubMed] [Google Scholar]
- 3.Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature 2012;487:325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009;325:612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the Mononuclear Phagocyte Network Underlies Chronic Inflammation and Disease Progression in Heart Failure: Critical Importance of the Cardiosplenic Axis. Circulation Research 2014;114:266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins CS, Hilgendorf I, Weber GF, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med;19:1166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudd JH, Narula J, Strauss HW, et al. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: ready for prime time? J Am Coll Cardiol 2010;55:2527–35. [DOI] [PubMed] [Google Scholar]
- 8.Lardinois D, Weder W, Hany TF, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med 2003;348:2500–7. [DOI] [PubMed] [Google Scholar]
- 9.Jamar F, Buscombe J, Chiti A, et al. EANM/SNMMI Guideline for 18F-FDG Use in Inflammation and Infection. Journal of Nuclear Medicine 2013;54:647–658. [DOI] [PubMed] [Google Scholar]
- 10.Garedew A, Henderson SO, Moncada S. Activated macrophages utilize glycolytic ATP to maintain mitochondrial membrane potential and prevent apoptotic cell death. Cell Death Differ 2010;17:1540–50. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Prados J-C, Traves PG, Cuenca J, et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol 2010;185:605–614. [DOI] [PubMed] [Google Scholar]
- 12.Satomi T, Ogawa M, Mori I, et al. Comparison of Contrast Agents for Atherosclerosis Imaging Using Cultured Macrophages: FDG Versus Ultrasmall Superparamagnetic Iron Oxide. J Nucl Med 2013;54:999–1004. [DOI] [PubMed] [Google Scholar]
- 13.Kubota R, Kubota K, Yamada S, Tada M, Ido T, Tamahashi N. Microautoradiographic Study for the Differentiation of Intratumoral Macrophages, Granulation Tissues and Cancer Cells by the Dynamics of Fluorine-18-Fluorodeoxyglucose Uptake. J Nucl Med 1994;35:104–112. [PubMed] [Google Scholar]
- 14.Higashi K, Ueda Y, Yagishita M, et al. FDG PET Measurement of the Proliferative Potential of Non-Small Cell Lung Cancer. Journal of Nuclear Medicine 2000;41:85–92. [PubMed] [Google Scholar]
- 15.Tawakol A, Fayad ZA, Mogg R, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol 2013;62:909–17. [DOI] [PubMed] [Google Scholar]
- 16.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA 2012;308:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Libby P. Inflammation in atherosclerosis. Nature 2002;420:868–74. [DOI] [PubMed] [Google Scholar]
- 18.Kempe S, Kestler H, Lasar A, Wirth T. NF-kappaB controls the global pro-inflammatory response in endothelial cells: evidence for the regulation of a pro-atherogenic program. Nucleic Acids Res 2005;33:5308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the unstable plaque. Prog Cardiovasc Dis 2002;44:349–56. [DOI] [PubMed] [Google Scholar]
- 20.Murphy SA, Cannon CP, Wiviott SD, McCabe CH, Braunwald E. Reduction in recurrent cardiovascular events with intensive lipid-lowering statin therapy compared with moderate lipid-lowering statin therapy after acute coronary syndromes from the PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22) trial. J Am Coll Cardiol 2009;54:2358–62. [DOI] [PubMed] [Google Scholar]
- 21.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011;473:317–25. [DOI] [PubMed] [Google Scholar]
- 22.Kim EJ, Kim S, Seo HS, Kang DO. The Metabolic Activity of the Spleen and Bone Marrow in Patients with Acute Myocardial Infarction Evaluated by 18F-FDG PET Imaging. Circ Cardiovasc Imaging 2014. [DOI] [PubMed] [Google Scholar]
- 23.Croce K, Gao H, Wang Y, et al. Myeloid-related protein-8/14 is critical for the biological response to vascular injury. Circulation 2009;120:427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piechota M, Banaszewska A, Dudziak J, Slomczynski M, Plewa R. Highly upregulated expression of CD36 and MSR1 in circulating monocytes of patients with acute coronary syndromes. Protein J 2012;31:511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Averill MM, Barnhart S, Becker L, et al. S100A9 differentially modifies phenotypic states of neutrophils, macrophages, and dendritic cells: implications for atherosclerosis and adipose tissue inflammation. Circulation 2011;123:1216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blich M, Golan A, Arvatz G, et al. Macrophage activation by heparanase is mediated by TLR-2 and TLR-4 and associates with plaque progression. Arterioscler Thromb Vasc Biol 2013;33:e56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pittet MJ, Nahrendorf M, Swirski FK. The journey from stem cell to macrophage. Ann N Y Acad Sci 2014;1319:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Laan AM, Ter Horst EN, Delewi R, et al. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur Heart J 2013;35:376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.