ABSTRACT
Evidence suggests that eating nuts may reduce the risk of cardiovascular disease (CVD). We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) evaluating almond consumption and risk factors for CVD. MEDLINE, Cochrane Central, Commonwealth Agricultural Bureau, and previous systematic reviews were searched from 1990 through June 2017 for RCTs of ≥3 wk duration that evaluated almond compared with no almond consumption in adults who were either healthy or at risk for CVD. The most appropriate stratum was selected with an almond dose closer to 42.5 g, with a control most closely matched for macronutrient composition, energy intake, and similar intervention duration. The outcomes included risk factors for CVD. Random-effects model meta-analyses and subgroup meta-analyses were performed. Fifteen eligible trials analyzed a total of 534 subjects. Almond intervention significantly decreased total cholesterol (summary net change: −10.69 mg/dL; 95% CI: −16.75, −4.63 mg/dL), LDL cholesterol (summary net change: −5.83 mg/dL; 95% CI: −9.91, −1.75 mg/dL); body weight (summary net change: −1.39 kg; 95% CI: −2.49, −0.30 kg), HDL cholesterol (summary net change: −1.26 mg/dL; 95% CI: −2.47, −0.05 mg/dL), and apolipoprotein B (apoB) (summary net change: −6.67 mg/dL; 95% CI: −12.63, −0.72 mg/dL). Triglycerides, systolic blood pressure, apolipoprotein A1, high-sensitivity C-reactive protein, and lipoprotein (a) showed no difference between almond and control in the main and subgroup analyses. Fasting blood glucose, diastolic blood pressure, and body mass index significantly decreased with almond consumption of >42.5 g compared with ≤42.5 g. Almond consumption may reduce the risk of CVD by improving blood lipids and by decreasing body weight and apoB. Substantial heterogeneity in eligible studies regarding almond interventions and dosages precludes firmer conclusions.
Keywords: systematic review, meta-analysis, blood lipids, risk factors, metabolic syndrome, hypertension, hyperglycemia, hypercholesterolemia, obesity
Introduction
Almonds contain a variety of nutrients and phytochemicals that have individually been related to cardiovascular benefits (1). Tree nuts, including almonds, are good sources of “healthy” mono- and polyunsaturated fats that have been shown to lower blood cholesterol concentrations and contain a variety of other potentially cardioprotective components, such as dietary fiber, vitamin E, selenium, magnesium, copper, potassium, β-sitosterol, and ω-3 (n–3) fatty acids (1). The qualified health claim for tree nuts and heart health by the US FDA, passed in 2003, states, “Scientific evidence suggests but does not prove that eating 1.5 ounces (42.5 g) per day of most nuts, as part of a diet low in saturated fat and cholesterol, may reduce the risk of heart disease” (2). Four large epidemiologic cohort studies, namely the Nurses’ Health Study (3), the Physicians’ Health Study (4), the Adventist Health Study (5), and the Iowa Women's Health Study (6), have shown a clear inverse dose response between nut consumption and coronary heart disease (CHD) risk (7). In combined analyses of these cohorts, there was an average of 37% reduction in risk of CHD death or an average 8.3% risk reduction of CHD death for each serving (42.5 g) of nuts per week (8).
A serving of 42.5 g of almonds, ∼35 almonds or a 1.5 handful, is packed with 246 calories and is also very nutrient dense, containing 9 g of protein, 21 g of fat (13 g from monounsaturated, 5 g from polyunsaturated, 2 g from saturated), 9 g of carbohydrates and 5 g of fiber (9). In the 2017–2018 crop year, American almond consumption per capita per year was reported to have increased from its 1980 value of 0.42 pounds (190.5 g) to 2.36 pounds (1070 g) (10). Although almond consumption has increased dramatically, this per-capita consumption would only yield a current daily intake of as little as 2.93 g. This daily intake meets only ∼7% of the FDA-qualified health claim of 42.5 g, which translates into per-person almond consumption for only 25 d/y. Therefore, American almond consumption per capita is well below the FDA-recommended daily serving size of tree nuts.
Modifiable cardiovascular risk factors, including hyperlipidemia, inflammation, blood pressure, blood glucose and insulin concentrations, metabolic syndrome, and body weight/fat/composition have the potential to be favorably altered by almond consumption (11). Even though a previous systematic review examined the effect of almond consumption on blood lipid concentrations, there has not been an in-depth, critical, and comparative analysis of the contemporary literature reporting on all biomarkers of cardiometabolic risk (12). Given the evidence that individual nutrients found in almonds are related to a decreased risk of cardiovascular disease (CVD), the purpose of this systematic review is to critically appraise and summarize the data related to almond consumption and biomarkers of CVD risk.
Methods
Overview
This systematic review of the literature compared the effects of almond consumption to no almond consumption interventions on risk factors of CVD. The review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidance on conduct and reporting of systematic reviews (13). To evaluate the effect of almond consumption, we applied the analytic framework (Supplemental Figure 1), addressing the relevant clinical outcomes and biomarkers of CVD risk.
Data sources and study eligibility
We searched MEDLINE, the Cochrane Central Register of Controlled Trials, and Commonwealth Agricultural Bureau (CAB) Abstracts from 1990 through June 2017 for risk factor outcomes of CVD, including blood pressure, fasting blood glucose, and body weight. For lipid outcomes, the same databases were searched between 2015 and June 2017. Eligible studies for the outcomes of lipids published between 1990 and 2014 were obtained from a previous systematic review (12). The electronic search strategy combined terms for almond and risk factors for development of CVD (Supplemental Table 1). The search was limited to randomized controlled trial (RCT) design, the highest level of evidence, which was also the design of interest in the previously published systematic review. We supplemented with studies identified in the bibliography of recovered articles.
Study selection criteria
Six researchers screened citations in duplicate. Studies accepted based on abstract screening were retrieved. Full-text articles were screened in duplicate for eligibility. Disagreements in screening and study selection were resolved by consensus in group meetings.
We included English-language studies of almond interventions among adults (≥18 y of age) who were either healthy or had risk factors for CVD (e.g., dyslipidemic, diabetic, or hypertensive) at baseline. The comparators of interest were no almond consumption or dietary substitutions containing no almond. The outcomes of interest were total cholesterol (TC), LDL cholesterol, HDL cholesterol, LDL cholesterol/HDL cholesterol, TC/HDL cholesterol, triglycerides (TGs), blood pressure [systolic blood pressure (SBP), diastolic blood pressure (DBP)], body weight, BMI, fasting blood glucose (FBG), glycated hemoglobin A1c (HbA1c), high-sensitivity C-reactive protein (hsCRP), apoA-I, apoB, and lipoprotein (a) [Lp(a)].
The minimum study duration for all outcomes was 3 wk and for blood glucose was 1 wk; no sample size restriction was applied.
Study exclusion criteria
We excluded studies conducted in animals, children, pregnant women, and any trials that involved a weight-loss or lifestyle modification program. We excluded studies that did not assess the effect of almond intervention on relevant outcomes of interest, used statins in both intervention and control, subjects with CVD at baseline, missing dietary information, or study strata (multiple intervention or control arms within a trial) that were not matched for energy intake during the intervention (Supplemental Table 2).
Data extraction and quality assessment
Data from each study were extracted by 1 of 2 investigators (ML-B, LK) and confirmed by ≥1 other (JW). The extracted data included: study design; baseline participant characteristics; follow-up period; sample size; details of the almond intervention and control (e.g., inclusion, exclusion criteria and funding source) (Supplemental Table 3); population characteristics (Supplemental Table 4); all reported outcomes of interest, duration, dose, diet comparisons, methods of ensuring compliance, and reported adverse reactions (Supplemental Table 5); and dietary information (Supplemental Table 6).
We applied the Cochrane risk-of-bias tool for RCTs (14) and nutrition-specific risk-of-bias items identified from a critical appraisal of systematic reviews in nutrition (15). We assessed the methodologic quality of each study outcome based on predefined criteria, in accordance with the Agency for Healthcare Research and Quality recommendations for systematic reviews (16). Risk of bias for individual domains was assessed in duplicate and discrepancies were resolved by consensus in group meetings with senior investigators (Supplemental Table 7).
Data synthesis
The main analysis included all studies that had an almond intervention, compared with no almond, and assessed similar outcomes. Multiple intervention or control arms within a trial are referred to as strata hereinafter. We extracted the mean baseline values and measures of variance (SD or SE) in both intervention and control. We estimated net differences for each continuous outcome through the use of the following equations:
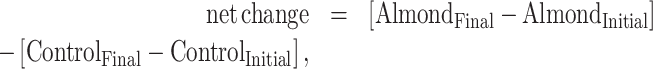 |
in cases of randomized parallel controlled trials;
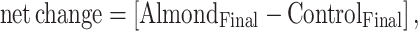 |
in cases of randomized crossover trials.
We calculated the SE of net change from the formula:
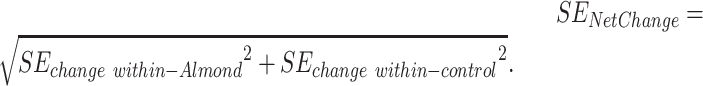 |
In addition, for blood lipids (TC, LDL cholesterol, HDL cholesterol, and TG), we estimated the percentage net changes in comparison with baseline values. We collected data for lipid ratios (TC/HDL cholesterol and LDL cholesterol/HDL cholesterol) when reported, but we did not estimate ratios from individual results. For outcomes that did not have sufficient quantitative data (e.g., HbA1c), results were synthesized qualitatively.
Main analysis
We performed both fixed-effects and random-effects model meta-analyses when ≥3 studies reported the same outcome. We presented results of random-effects meta-analysis as this model can estimate the mean of a distribution of true effects (16). We tested between-study heterogeneity with the Q statistic (significant when P < 0.10) and quantified its extent with I2 (17). I2 > 50% was deemed as having significant heterogeneity. When trials reported multiple strata, but shared either a common intervention or a common control group, we examined all available groups in separate meta-analyses. When trials examined different doses of almond or different intervention durations, in the main analysis, we included the strata closest to an almond dose of 42.5 g, and with similar intervention duration across studies.
Subgroup and sensitivity analyses
When appropriate, we performed subgroup meta-analyses by healthy compared with at risk of CVD at baseline; almond dose (≤42.5 g or >42.5 g); almond intervention duration (≤6 wk or >6 wk); and almonds consumed alone compared with almonds mixed with foods. The specific cutoffs for the subgroup analyses were established for the following reasons: healthy compared with at risk of CVD at baseline was determined to evaluate if baseline health status had an effect on outcomes; ≤42.5 g or >42.5 g was based on FDA-recommended intake for nut consumption; ≤6 wk compared with >6 wk duration of intervention was decided as the duration to stabilize an intervention effect on lipid outcomes [generally, it takes at least 3–4 wk to observe an effect on lipids (18)]. Additional subgroups were explored to assess their effects on the outcomes including consumption of almonds alone compared with almonds mixed with foods, and plasma lipids compared with serum lipids. Subgroup analyses required ≥3 strata in a subgroup and were not performed when there were insufficient numbers of studies. To explore potential heterogeneity, sensitivity analysis was conducted to exclude 1 trial with medicated participants [Supplemental Table 8 (19)]. Sensitivity analyses were also conducted by including [Supplemental Table 9 (20–23)] and by excluding other strata that were not relevant (22, 24–26). We also assessed the impact of each study on the combined effect by leaving out 1 study at a time (data not shown). Random-effects meta-regression was performed to assess the impact of study-level variables (mean age, percentage male, and almond dose) on the outcome of interest. All analyses were performed with R statistical software (meta).
Results
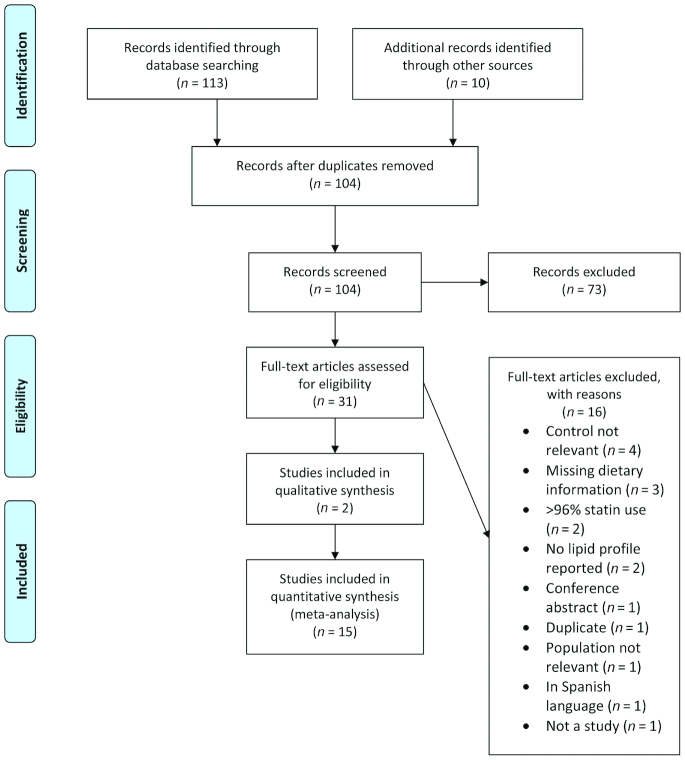
The literature search retrieved 104 unique citations identified through searches, 31 of which were assessed for eligibility as full-text articles. In total, 15 RCTs were eligible (Figure 1); Table 1 describes the study and participant characteristics at baseline (19–33).
FIGURE 1.
Study flow diagram depicting the review process following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
TABLE 1.
Study design and participant characteristics of trials included in meta-analyses1
| Author, year (ref) | Country | Study design (duration) | n enrolled (n analyzed) | Male % | Mean age, y | Mean BMI, kg/m2 | Ethnicity | Co-conditions | Almond, g/d | Diet comparisons |
|---|---|---|---|---|---|---|---|---|---|---|
| Abazarfard et al., 2014 (27) | Iran | RCTP (12 wk) | 108 (100) | 0 | i: 42; c: 43 | i: 29.9; c: 29.4 | NR | Generally healthy overweight or obese 100% | 50 | Low-calorie diet with almonds vs. low-calorie diet without almonds |
| Berryman et al., 2015 (28) | USA | RCTC (6 wk) | 61 (48) | 45.8 | 50 | 26.2 | White 94%, African American, 2%, Asian 4% | Generally healthy 100% | 43 | Cholesterol-lowering diet with almonds vs. cholesterol-lowering diet with banana muffin and butter |
| Damasceno et al., 2011 (24) | Spain | RCTC (4 wk) | 26 (18) | 50 | 56 | 25.7 | NR | Moderate hypercholesterolemia 100% | 50–75 (22% of total energy) | Mediterranean diet counseling with almonds vs. Mediterranean diet counseling with virgin olive oil |
| Hollis and Mattes, 2007 (29) | USA | RCTC (10 wk) | 24 (20) | 0 | 24 | 25.9 | NR | Generally healthy 100% | 60 | Customary diet with almonds vs. customary diet without almonds |
| Jenkins et al., 2002 (25) | Canada | RCTC (4 wk) | 43 (27) | 56 | 64 | 25.7 | NR | Hyperlipidemic 100%2 | 37 | Self-selected low-fat therapeutic diet with almond muffin vs. self-selected low fat therapeutic diet with muffin |
| Kurlandsky and Stote, 2006 (26) (strata: dark chocolate + almond vs. dark chocolate) | USA | RCTP (6 wk) | Total: 52 (47) strata: ∼24–25 (23) | 0 | i: 46; c: 37 | i: 27.2; c: 23.9 | NR | Healthy and normocholesterolemic 100%3 | 60 | Self-selected low-fat diet with dark chocolate and almonds vs. self-selected low-fat diet with dark chocolate |
| Li et al., 2011 (30) | Taiwan | RCTC (4 wk) | 20 (20) | 45 | 58 | 26 | Chinese 100% | Type 2 diabetic, not medicated 100% | 56 | NCEP step 2 diet with almonds vs. NCEP step 2 diet without almonds |
| Lovejoy et al., 2002 (20) | USA | RCTC (4 wk) | 34 (30) | 43 | 544 | 33.0 | NR | Type 2 diabetic, not medicated 100% | 57–113 (10% of total energy) | High-fat diet with almonds vs. high-fat diet without almonds |
| Sabate et al., 2003 (21) | USA | RCTC (4 wk) | 27 (25) | 56 | 41 | NR | White 40%, Hispanic 28%, Asian 20%, African American 12% | Generally healthy or mildly hypercholesterolemic 100% | 34 g/d per 2000 kcal (10% isoenergetic replacement | NCEP step 1 diet with almonds vs. NCEP step 1 diet without almonds |
| Spiller et al., 1998 (31) (strata: almonds vs. cheddar cheese, butter and rye) | USA | RCTP (4 wk) | Total: 51 (45); strata: 33 (30) | 27 | 53 | NR | NR | Hypercholesterolemic 100% | 100 | Whole and unrefined foods background diet with almonds vs. whole and unrefined foods background diet with cheddar crackers |
| Sweazea et al., 2004 (19) | USA | RCTP (12 wk) | 24 (21) | 43 | i: 58; c: 55 | i: 37.2; c: 33.5 | NR | Type 2 diabetic, medicated 100% | 43 | Customary diet with almonds vs. customary diet without almonds |
| Tamizifar et al., 2005 (32) | Iran | RCTC (4 wk) | 35 (30) | 57 | 56 | 24.1 | NR | Hyperlipidemic 100% | 25 | NCEP step 1 diet with almonds vs. NCEP step 1 diet without almonds |
| Tan and Mattes, 2013 (22) (strata: almonds as afternoon snack vs. no nuts or seeds) | USA | RCTP (4 wk) | Total: 150 (137); strata: 60 (55) | 29 | i: 29 | i: 28.2; c: 27.0 | NR | Generally healthy but at risk of type 2 diabetes 100% | 43 | Customary diet with almonds as afternoon snack vs. customary diet without nuts or seeds |
| Wien et al., 2010 (33) | USA | RCTP (16 wk) | 65 (65) | 26 | i: 53; c: 54 | i: 30; c: 29 | i: White 38%, Hispanic 12%, African American 44%, Asian 6%, c: White 40% Hispanic 15%, African American 27%, Asian 18% | Prediabetic 100% | 60 | ADA diet and energy intake deficits of 250–500 kcal with almonds vs. ADA diet and energy intake deficits of 250–500 kcal without tree nuts or peanuts |
| Zaveri and Drummond, 2009 (23) (strata: almond snack vs. no snacks) | Scotland | RCTP (12 wk) | Total: 45 (36); strata: 31(23) | 100 | i: 40; c: 44; t: 40 | i: 30.0; c: 30.0 | NR | Generally healthy 100% | 56 | Customary diet with almonds vs. customary diet without snacks |
ADA, American Diabetes Association; c, control; i, intervention; NCEP, National Cholesterol Education Program; NR, not reported; RCTC, randomized controlled trial, crossover; RCTP, randomized controlled trial, parallel; ref, reference; t, total, both control and intervention.
Three men and 5 women were taking the following medications: a hypolipidemic agent (statin; n = 2); blocking agents (n = 3); ACE inhibitors (n = 3); angiotensin II ATI receptor blockers (n = 1); thiazide diuretics (n = 2), levothyroxine (n = 2); and hormone replacement therapy (n = 2). Fourteen subjects were following therapeutic NCEP Step 2 diets.
Subjects were allowed to be taking oral contraceptives and hormone replacement therapy.
Included 4 dropout participants.
Qualitative review
HbA1c
Two trials analyzed a total of 51 participants with type 2 diabetes and reported the effect of almond intake on HbA1c measurements [Table 2 (19, 20)]. HbA1c was reported in 2 trials: a 12-wk parallel trial with 21 subjects with type 2 diabetes taking medication (19), and a 4-wk crossover trial with 30 subjects with type 2 diabetes not taking medication (20). Neither study found a difference in HbA1c between groups.
TABLE 2.
Study design and participant characteristics of trials in qualitative review for HbA1c measurements1
| Author, year (ref) | Country | Study design, (duration) | n enrolled (n analyzed) | Male, % | Mean age, y | Mean BMI, kg/m2 | Ethnicity | Co-conditions | Almond, g/d | Diet comparisons |
|---|---|---|---|---|---|---|---|---|---|---|
| Lovejoy et al., 2002 (20) | USA | RCTC (4 wk) | 34 (30) | 43 | 542 | 33.0 | NR | Type 2 diabetic, not medicated 100% | 57–113 (10% of total energy) | High-fat diet with almonds vs. high-fat diet without almonds |
| Sweazea et al., 2014 (19) | USA | RCTP (12 wk) | 24 (21) | 43 | i: 58; c: 55 | i: 37.2; c: 33.5 | NR | Type 2 diabetic, medicated 100% | 43 | Customary diet with almonds vs. customary diet without almonds |
c, control; HbA1c, glycated hemoglobin; i, intervention; NR, not reported; RCTC, randomized controlled trial, crossover; RCTP, randomized controlled trial, parallel; ref, reference.
Includes 4 drop-out participants.
Description of trials included in meta-analysis
Fifteen RCTs analyzed a total of 534 subjects (19–33). Seven studies were parallel, randomized trials (19, 22, 23, 26, 27, 31, 33) and the rest were crossover designs (20, 21, 24, 25, 28–30, 32). Three studies enrolled 100% female subjects (26, 27, 29) and 1 study enrolled 100% male subjects (23). Eight studies enrolled generally healthy participants (21–23, 26–29, 33) and the remaining 7 studies enrolled participants who were at a higher risk of CVD (19, 20, 24, 25, 30–32). The study durations included in the meta-analysis ranged between 4 and 16 wk; in crossover studies, the washout period ranged between 5 d and 3 wk. Only 1 crossover study did not include a washout period (21). The dose of almond intake ranged between 25 and 100 g/d. Two studies reported the dose of almond intake as a percentage of total energy and reported a range for almond dose (20, 24). The majority of the studies compared almond intake with no almond intake, except for 6 studies in which the control involved a substitution that varied considerably across studies [Table 1 (20, 24–26, 28, 31)]. Nine studies examined almonds alone; 3 studies examined almonds incorporated into snack foods such as muffins, cookies, or trail mix (20, 25, 26). Six studies used raw almonds (24, 25, 27–29, 31), 2 studies used roasted almonds (22, 30), 1 study used either raw or roasted (33), and 5 studies did not specify between raw and roasted (19–21, 23, 26). Two studies incorporated the almonds either into meals or provided them on their own (21, 30), and 1 study used almond powder, but did not mention how it was consumed (32). Nine of the studies were conducted in the United States, 2 in Iran, and 1 each in Canada, Spain, Taiwan, and Scotland (Table 1). All trials reported the effect of almond intake on TC and LDL cholesterol, except for 2 studies that focused only on changes in body weight or BMI (23, 29). Overall, reporting of adequate randomization was high, except for 5 studies that did not report a method of randomization (20, 23, 25, 26, 30). Reporting of participant and outcome assessor blinding, allocation concealment, and intention to treat analysis was unclear in most studies (Supplemental Table 7).
Meta-analysis results
Blood lipids
TC
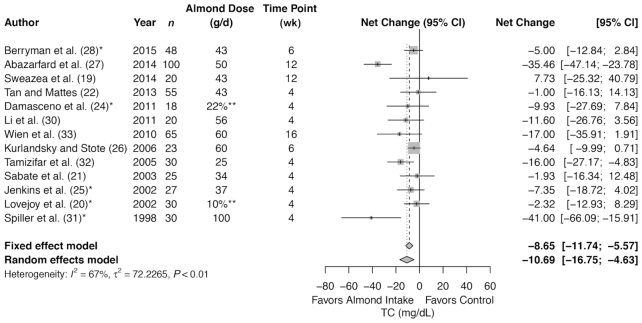
Meta-analysis of 13 trials (491 subjects in total) showed a significant decrease in TC with almond intervention compared with control (summary net change: −10.69 mg/dL; 95% CI: −16.75, −4.63 mg/dL) (Figure 2), but with a significant heterogeneity (I2 = 67%, P < 0.01). The summary estimates remained significant for the following subgroups: at increased risk for CVD at baseline and healthy at baseline; >42.5 g and ≤42.5 g; ≤6 wk (Table 3); almonds consumed either alone or mixed with foods; and for TC analyzed in plasma or serum (Supplemental Table 10). There was no difference in summary estimates between almond compared with control for >6 wk of almond intervention.
FIGURE 2.
Forest plots of almond intake on blood lipids: TC. *Control involves a substitution. **Dose as a percentage of energy intake, not in grams per day. TC, total cholesterol.
TABLE 3.
Meta-analysis reporting the effect of almond compared with control on blood lipids and other biomarkers of CVD risk1
| Subgroup analysis of baseline health status | Subgroup analysis of dosage | Subgroup analysis of duration | ||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | n studies (n subjects) | Main analysis, net change (95% CI) | Healthy | At risk of CVD | ≤42.5 g | >42.5 g | ≤6 wk | >6 wk |
| TC, mg/dL | 13 (491) | −10.69 (−16.75, −4.63)2 | −10.55 (−20.39, −0.70)2 | −10.72 (−18.26, −3.18)2 | −6.48 (−11.35, −−1.61)2 | −15.83 (−26.67, −5.00) 2 | −7.18 (−11.46, −2.90)2 | −19.14 (−40.90, 2.62) |
| I2 = 67% | I2 = 81% | I2 = 42% | I2 = 0% | I2 = 80% | I2 = 29% | I2 = 73% | ||
| LDL-C, mg/dL | 13 (491) | −5.83 (−9.91, −1.75)2 | −1.32 (−4.04, 1.40) | −10.46 (−17.82, −3.09)2 | −6.67 (−13.31, −0.02)2 | −5.03 (−10.11, 0.06) | −7.09 (−11.75, −2.44)2 | 0.44 (−3.33, 4.22) |
| I2 = 61% | I2 = 9% | I2 = 61% | I2 = 50% | I2 = 62% | I2 = 57% | I2 = 2% | ||
| HDL-C, mg/dL | 12 (461) | −1.26 (−2.47, −0.05)2 | −0.74 (−1.97, 0.50) | −1.88 (−4.26, 0.50) | −0.73 (−3.42, 1.96) | −1.17 (−2.44, 0.10) | −1.66 (−3.18, −0.14) 2 | −0.32 (−1.96, 1.32) |
| I2 = 16% | I2 = 0% | I2 = 25% | I2 = 52% | I2 = 0% | I2 = 19% | I2 = 0% | ||
| TGs, mg/dL | 12 (461) | −11.63 (−23.47, 0.21) | −15.76 (−33.93, 2.41) | −6.32 (−−21.26, 8.62) | −7.84 (−19.21, 3.52) | −16.06 (−38.87, 6.75) | −5.18 (−12.62, 2.26) | −29.07 (−77.86, 19.72) |
| I2 = 71% | I2 = 84% | I2 = 28% | I2 = 21% | I2 = 84% | I2 = 24% | I2 = 82% | ||
| Body weight, kg | 11 (432) | −1.39 (−2.49, −0.30)2 | −1.55 (−2.70,−0.40)2 | 0.05 (−3.44, 3.53) | −0.17 (−2.35, 2.01) | −1.80 (−3.07, −0.54)2 | 0.10 (−2.12, 1.92) | −1.93 (−3.23, −0.63)2 |
| I2 = 0% | I2 = 0% | I2 = 0% | I2 = 0% | I2 = 0% | I2 = 0% | I2 = 0% | ||
| FBG, mg/dL | 9 (391) | −1.92 (−4.55, 0.71) | −0.93 (−4.06, 2.21) | −6.08 (−10.77, −1.40)2 | −−0.04 (−1.85, 1.78) | −4.11 (−7.43, −0.80)2 | −0.97 (−3.00, 1.06) | −2.18 (−9.38, 5.01) |
| I2 = 62% | I2 = 78% | I2 = 0% | I2 = 0% | I2 = 34% | I2 = 16% | I2 = 65% | ||
| SBP, mm Hg | 7 (306) | 1.27 (−2.63, 5.18) | 1.61 (−5.69, 8.91) | 0.13 (−3.46, 3.73) | −0.04 (−−3.27, 3.19) | 2.07 (−6.31, 10.44) | 0.03 (−3.13, 3.19) | 2.02 (−7.23, 11.27) |
| I2 = 51% | I2 = 78% | I2 = 0% | I2 = 0% | I2 = 75% | I2 = 0% | I2 = 75% | ||
| DBP, mm Hg | 7 (305) | −1.51 (−3.96, 0.94) | −1.79 (−6.46, 2.88) | −0.82 (−3.38, 1.74) | 0.88 (−1.92, 3.67) | −3.15 (−5.77, −0.54)2 | 0.13 (−2.11, 2.37) | −4.24 (−6.68, −1.81)2 |
| I2 = 49% | I2 = 76% | I2 = 0% | I2 = 0% | I2 = 35% | I2 = 0% | I2 = 0% | ||
| TC/HDL-C | 5 (235) | −0.30 (−0.62, 0.02) | −0.47 (−0.95, 0.02) | −0.17 (−0.57, 0.23) | −0.28 (−0.49, −0.07)2 | −0.30 (−1.12, 0.52) | N/A | N/A |
| I2 = 77% | I2 = 77% | I2 = 66% | I2 = 0% | I2 = 93% | ||||
| LDL-C/HDL-C | 6 (168) | −0.11 (−0.23, 0.00) | −0.17 (−0.34, 0.00) | −0.07 (−0.22, 0.08) | −0.16 (−0.32, −0.01) 2 | −0.06 (−0.22, 0.11) | N/A | N/A |
| I2 = 0% | I2 = 0% | I2 = 0% | I2 = 0% | I2 = 0% | ||||
| BMI, kg/m2 | 6 (284) | −0.33 (−1.08, 0.43) | −0.27 (−1.37, 0.82) | 0.11 (−1.34, 1.56) | 0.22 (−2.18, 2.62) | −0.25 (−1.25, 0.74) | 0.13 (−1.15, 1.40) | −0.33 (−1.51, 0.86) |
| I2 = 21% | I2 = 44% | I2 = 0% | I2 = 0% | I2 = 49% | I2 = 0% | I2 = 40% | ||
| hsCRP, mg/L | 4 (114) | −0.74 (−1.49, 0.01) | N/A | N/A | N/A | N/A | N/A | N/A |
| I2 = 50% | ||||||||
| apoA-I, mg/dL | 5 (138) | 1.37 (−3.04, 5.78) | N/A | N/A | 1.72 (−3.17, 6.62) | −0.14 (−10.31, 10.03) | N/A | N/A |
| I2 = 0% | I2 = 0% | I2 = 0% | ||||||
| apoB, mg/dL | 5 (138) | −6.67 (−12.63, −0.72)2 | N/A | N/A | −4.54 (−8.23,−0.84)2 | −11.79 (−27.05, 3.47) | N/A | N/A |
| I2 = 50% | I2 = 0% | I2 = 69% | ||||||
| Lp(a), mg/dL | 4 (118) | 0.91 (−0.60, 2.42) | N/A | N/A | N/A | N/A | N/A | N/A |
| I2 = 0% | ||||||||
Meta-analyses were conducted with the use of the random-effects model. Main analysis: all studies from Table 1, includes both no-almond controls and isocaloric snack substitutions. I2 is an indicator of between-comparison heterogeneity. I2 > 50% was deemed as having significant heterogeneity. CVD, cardiovascular disease; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL-C, HDL cholesterol; hsCRP, high-sensitivity C-reactive protein; LDL-C, LDL cholesterol; Lp(a), lipoprotein (a); N/A, not applicable; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
Significant decrease in the outcome was observed.
LDL cholesterol
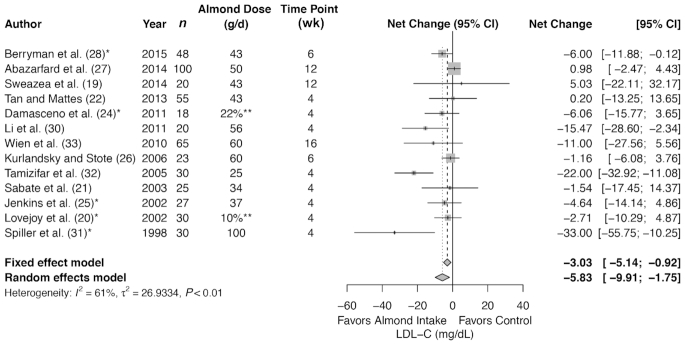
Meta-analysis of 13 trials (491 subjects in total) showed a significant decrease in LDL cholesterol with almond intervention compared with control (summary net change: −5.83 mg/dL; 95% CI: −9.91, −1.75 mg/dL) (Figure 3), but with a significant heterogeneity (I2 = 61%, P < 0.01). The summary estimate of studies remained significant for subjects who were at increased risk for CVD at baseline; ≤42.5 g dose; ≤6 wk (Table 3); and for LDL cholesterol analyzed in serum (Supplemental Table 10). There was no difference in summary estimates between almond compared with control for the following subgroups: healthy at baseline; >42.5 g almond intervention; >6 wk; method of almond consumption; and for LDL cholesterol analyzed in plasma.
FIGURE 3.
Forest plots of almond intake on blood lipids: LDL cholesterol. *Control involves a substitution.**Dose as a percentage of energy intake, not in grams per day. LDL-C, LDL cholesterol.
HDL cholesterol
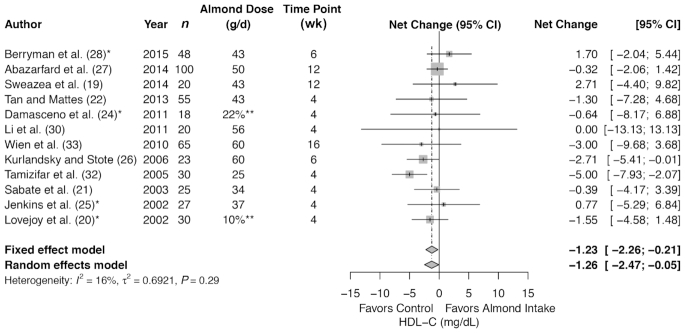
Meta-analysis of 12 trials (461 subjects in total) showed a significant decrease in HDL cholesterol with almond compared with control (summary net change −1.26 mg/dL; 95% CI: −2.47, −0.05 mg/dL) (Figure 4), but without heterogeneity (I2 = 16%, P = 0.29). The summary estimates remained significant for the ≤6 wk of almond intervention. There was no difference in summary estimates between almond compared with control for the following subgroups: healthy at baseline or at risk for CVD at baseline; ≤42.5 g or >42.5 g almond intervention; >6 wk; almonds consumed either alone or mixed with food; and for HDL cholesterol analyzed in plasma or serum.
FIGURE 4.
Forest plots of almond intake on blood lipids: HDL cholesterol. *Control involves a substitution. **Dose as a percentage of energy intake, not in grams per day. HDL-C, HDL cholesterol.
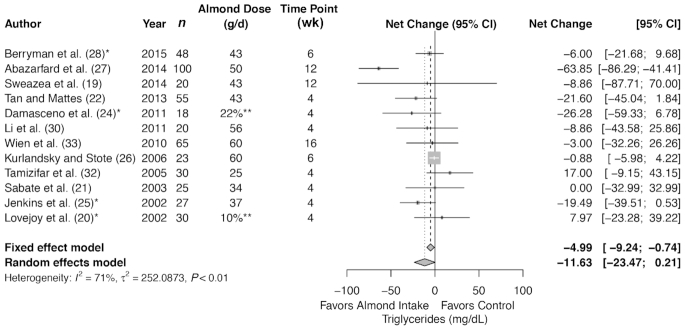
TGs
Meta-analysis of 12 trials (461 subjects in total) showed no difference in TGs with almond intervention compared with control (summary net change: −11.63 mg/dL; 95% CI: −23.47, 0.21 mg/dL) (Figure 5), but with a significant heterogeneity (I2 = 71%, P < 0.01). There was no difference in summary estimates between almond compared with control in any of the subgroups (Table 3), except when almonds were consumed alone (Supplemental Table 10).
FIGURE 5.
Forest plots of almond intake on blood lipids: triglycerides. *Control involves a substitution. **Dose as a percentage of energy intake, not in grams per day.
TC/HDL cholesterol
Meta-analysis of 5 trials (235 subjects in total) showed no difference in TC/HDL cholesterol with almond compared with control (summary net change: −0.30; 95% CI: −0.62, 0.02 mg/dL) (Supplemental Figure 2), and with significant heterogeneity (I2 = 77%, P < 0.01). The main summary estimate of studies was significant for the subgroup of ≤42.5 g dose (summary net change: −0.28; 95% CI: −0.49, −0.07) (Table 3) and for TC/HDL cholesterol analyzed in plasma (Supplemental Table 10). There was no difference in any of the other subgroups.
LDL cholesterol/HDL cholesterol
Meta-analysis of 6 trials (168 subjects in total) showed no difference in LDL cholesterol/HDL cholesterol with almond compared with control (summary net change −0.11; 95% CI: −0.23; 0.00) (Supplemental Figure 3), and without heterogeneity (I2 = 0%, P = 0.75). The summary estimate of studies was significant for the subgroup of ≤42.5 g dose (summary net change: −0.16; 95% CI: −0.32, −0.01) (Table 3) and when almonds were consumed alone (summary net change: −0.18; 95% CI: −0.34, −0.02) (Supplemental Table 10). There was no difference in any of the other subgroups.
Other biomarkers of CVD risk
Meta-analysis of 11 trials (432 subjects in total) showed significant decrease in body weight in almond, as compared with control (summary net change: −1.39 kg; 95% CI: −2.49, −0.30 kg) (Supplemental Figure 4) and without heterogeneity (I2 = 0%, P = 0.87). The summary estimate of studies remained significant for healthy at baseline; >42.5 g dose; and >6 wk (Table 3). Meta-analysis of 5 trials (138 subjects in total) showed a significant decrease in apoB with almond compared with control (summary net change −6.67 mg/dL; 95% CI: −12.63, −0.72 mg/dL) (Supplemental Figure 5), but with heterogeneity (I2 = 50%, P = 0.09). The summary estimate remained significant for the subgroup of ≤42.5 g dose and when almonds were consumed alone (Supplemental Table 10).
There was no difference between almond and control for FBG in the main analysis (Supplemental Figure 6). The summary estimate of studies for FBG was significant only for the subgroup at risk for CVD at baseline (summary net change: −6.08 mg/dL; 95% CI: −10.77 −1.40 mg/dL) without heterogeneity (I2 = 0%, P = 0.94); and >42.5 g dose (summary net change: −4.11 mg/dL; 95% CI: −7.43, −0.80 mg/dL), with mild heterogeneity (I2 = 34%, P = 0.19).
There was no difference between almond and control for DBP in the main analysis (Supplemental Figure 7). The summary estimate of studies for DBP was significant for >42.5 g dose (summary net change: −3.15 mm Hg; 95% CI: −5.77, −0.54 mm Hg) with mild heterogeneity (I2 = 35%, P = 0.21) and >6 wk (summary net change: −4.24 mm Hg; 95% CI: −6.68, −1.81 mm Hg) without heterogeneity (I2 = 0%, P = 0.51).
Systolic blood pressure, BMI, hsCRP, apoA-I, and Lp(a) showed no difference in summary estimates with almond intervention compared with control in either main analysis or subgroup analyses (Table 3, Supplemental Figures 8–12).
Subgroup analyses for healthy at baseline or at risk for CVD at baseline and for >6 wk compared with ≤6 wk could not be conducted due to an insufficient number of studies for hsCRP, apoA-I, apoB, and Lp(a). Subgroup analyses for almond consumed alone or mixed with foods could not be conducted for SBP, DBP, body weight, and BMI (Supplemental Table 10).
Percent net change for blood lipids
Meta-analysis of 13 trials (491 subjects in total) showed a significant decrease in TC and LDL cholesterol with almond intervention compared with control [(summary % net change: −5.10; 95% CI: −8.67, −1.53), and with significant heterogeneity (I2 = 66%, P < 0.01) and (summary % net change: −4.11; 95% CI: −7.48, −0.74), and without heterogeneity (I2 = 42%, P = 0.05), respectively]. Meta-analysis of 12 trials (461 subjects in total) showed no difference in HDL cholesterol with almond intervention compared with control (summary % net change: −1.64; 95% CI: −4.42 1.14), and without heterogeneity (I2 = 0%, P = 0.75). Meta-analysis of 11 trials (299 subjects in total) showed a significant decrease in TGs with almond intervention compared with control (summary % net change: −10.15; 95% CI: −19.71, −0.60), and with significant heterogeneity (I2 = 66%, P < 0.01). Percent net changes for TC and LDL cholesterol concurred with summary net changes, but did not for HDL cholesterol and TGs. More detailed information is provided in Table 4.
TABLE 4.
Blood lipid percent net change analysis reporting the effect of almond intervention1
| Outcome | n studies (n subjects) | % net change (95% CI) |
|---|---|---|
| TC, mg/dL | 13 (491) | −5.10 (−8.67,−1.53)2 |
| I2 = 66.2% | ||
| LDL-C, mg/dL | 13 (491) | −4.11 (−7.48, −0.74)2 |
| I2 = 42.4% | ||
| HDL-C, mg/dL | 12 (461) | −1.64 (−4.42, 1.14) |
| I2 = 0.0% | ||
| TGs,3 mg/dL | 11 (299) | −10.15 (−19.71, −0.60)2 |
| I2 = 65.7% |
Meta-analyses were conducted with the use of the random-effects model. I2 is an indicator of between-comparison heterogeneity. I2 > 50% was deemed as having significant heterogeneity. HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; TC, total cholesterol; TG, triglyceride.
Significant decrease in the outcome was observed.
We had insufficient data to calculate percentage net change for Lovejoy et al., 2002 (20), and therefore it was excluded from this analysis.
Sensitivity analyses
Excluding a trial with medicated subjects
Excluding 1 study that included subjects who were taking medications (19), the summary estimate of studies with nonmedicated subjects concurred with the main results (Supplemental Table 8).
Excluding 1 study at a time
When the impact of each study on the combined effect was assessed by omitting 1 study at a time, the magnitude of the combined effect and its significance were not affected for TC and LDL cholesterol. We determined that omitting Tamizifar et al. (32) and Kurlandsky and Stote (26) individually (−0.71 mg/dL; 95% CI: −1.80, 0.39 mg/dL and −1.01 mg/dL; 95% CI: −2.33, 0.30, respectively), resulted in the smallest decrease in HDL cholesterol that was no longer significant (data not shown); in total, omitting 1 study at a time for each of the following 8 studies resulted in no difference between groups for the outcome of HDL cholesterol (20–22, 24, 26, 30, 32, 33). Excluding Tamizifar et al. (32) and Lovejoy et al. (20), individually, resulted in a decrease in TG that was significant (−14.28 mg/dL; 95% CI: −26.71, −1.86 mg/dL and −13.17 mg/dL; 95% CI: −25.74, −0.60 mg/dL, respectively). The exclusion of Lovejoy et al. (20) resulted in significant reductions in LDL cholesterol/HDL cholesterol (−0.17; 95% CI: −0.31, −0.03) and TC/HDL cholesterol (−0.42; 95% CI: −0.69, −0.15). Omitting Tan and Mattes (22) yielded a significant decrease in DBP (−2.45 mm Hg; 95% CI: −4.60, −0.30 mm Hg). Leaving out Berryman et al. (28) and Jenkins et al. (25), individually, resulted in a larger decrease in apoB that was no longer significant (−8.31 mg/dL; 95% CI: −18.65, 2.03 mg/dL and −8.34 mg/dL; 95% CI: −17.60, 0.92 mg/dL, respectively). Omitting Zaveri and Drummond (23) resulted in a significant decrease in BMI (−0.72 kg/m2; 95% CI: −1.29, −0.16 kg/m2). Systolic blood pressure, FBG, apoA-I, hsCRP, and Lp(a) remained unchanged when omitting 1 study a time (data not shown).
Replacing strata of same studies
Replacing strata of same studies resulted in changes in the combined effects of HDL cholesterol, TGs, and TC/HDL cholesterol (Supplemental Table 9), but not in other outcomes. Replacing Sabate et al. (21) half-dose stratum (34 g of almonds) for the full-dose stratum (68 g of almonds) resulted in a nonsignificant decrease in HDL cholesterol (−1.16 mg/dL; 95% CI: −2.43, 0.12 mg/dL) and a significant decrease in TGs (−11.93 mg/dL; 95% CI: −23.76, −0.10 mg/dL). Replacing the Lovejoy et al. (20) high-fat control stratum for the low-fat control stratum resulted in a significant decrease in TC/HDL cholesterol (−0.31; 95% CI: −0.59, −0.04). Replacing the Tan et al. (22) afternoon snack for the morning snack and replacing the Zaveri and Drummond (23) 12-wk duration for the 6-wk duration did not result in any significant changes in the combined effect of any outcome.
Conclusions
Our review of RCTs indicates that TC, LDL cholesterol, HDL cholesterol, body weight, and apoB were significantly reduced with almond compared with no almond control or substitution. The largest reductions with almond intervention compared with control were found for TC, apoB, and LDL cholesterol. Although almond intervention with >42.5 g compared with control significantly decreased TC, body weight, DBP and FBG, almond consumption of ≤42.5 g compared with control significantly decreased LDL cholesterol/HDL cholesterol, TC/HDL cholesterol, and apoB. In addition, body weight and DBP significantly decreased when the intervention duration was >6 wk. There was no significant difference in other outcomes, including TGs, SBP, hsCRP, apoA-I, and Lp(a). The significant reductions in TC, LDL cholesterol, HDL cholesterol, and body weight with almond intervention remained consistent in sensitivity analyses, and TC and LDL cholesterol reductions remained consistent across the subgroups tested. However, when almonds were mixed with foods or when lipids were analyzed in plasma, not only did the reductions in TC remain consistent, but it had the largest reductions for the comparison of plasma and serum analyses (−22.63 mg/dL compared with −4.93 mg/dL).
Two potential mechanisms could explain the effect of almond consumption on blood lipids and other biomarkers of CVD risk. Almonds contain β-sitosterol, a phytosterol that is very structurally similar to the body's cholesterol, and therefore possibly in competition for absorption in the digestive system (1), resulting in lower concentrations of circulating LDL cholesterol. The lower circulating LDL cholesterol could potentially reduce the chances for an initiation of a cascade of events accompanying fatty streak and foam cell formation in the subendothelial space of arteries, and thereby reduce atherosclerotic CVD risk (34). The second mechanism is via the beneficial effects of weight reduction on hypertension and dyslipidemia (35). In our analyses, we found that body weight was significantly reduced with almond consumption compared with no almond or substitution.
In the previous systematic review and meta-analysis of 18 RCTs (total 837 subjects) on blood lipids and almond consumption, Musa-Veloso et al. (12) found that TC, LDL cholesterol, TC/HDL cholesterol, and TGs were significantly reduced, whereas HDL cholesterol and LDL cholesterol/HDL cholesterol were not affected by almonds. Our analyses for the outcomes of TC and LDL cholesterol with almond consumption compared with no almond control were similar to those of Musa-Veloso et al. (12), but our analyses found larger reductions for both of the outcomes. Our results did not concur with the prior review for the outcomes of HDL cholesterol and TGs. We found that HDL cholesterol was significantly decreased, and TGs showed no difference. The inclusion of Tamizifar et al. (32) and Kurlandsky and Stote (26) in our analysis may have resulted in significant decreases in HDL cholesterol, which disappeared when these studies were omitted individually or together. In both studies, HDL cholesterol increased substantially in the control, much higher than in the almond intervention. The control diet in Tamizifar et al. (32) had higher dietary fiber and MUFA contents compared with almond diet, possibly resulting in substantial increases in HDL cholesterol in the control. Meanwhile, the best-matched comparison in Kurlandsky and Stote (26) was the almond and chocolate compared with chocolate control, which had grossly underpowered sample sizes of ∼11–12 subjects per group; it is unclear why this study showed higher reductions in HDL cholesterol in the control.
Musa-Veloso et al. (12) also found that the significant decrease in TGs was dependent on the inclusion of a single trial—Abazarfard et al. (27)—and therefore, it is plausible that almond consumption may not have had a significant effect on TGs. Our results concurred with those of Musa-Veloso et al (12) for the subgroup analysis of the following outcomes: TC, LDL cholesterol, TC/HDL cholesterol, LDL cholesterol/HDL cholesterol. Our subgroup analysis of subjects who were healthy compared with at risk of CVD was similar to the subgroup analysis by Musa-Veloso et al. (12) of optimal compared with not optimal baseline lipid concentrations. Our subgroup analysis found a significant decrease in TC and LDL cholesterol in both groups of subjects who were at risk of CVD or who had suboptimal baseline lipid concentrations. Additionally, in our analyses, even those who were healthy at baseline had a significant decrease in TC. Both reviews acknowledged the high degree of clinical and statistical heterogeneity across studies.
Our methodology differed from the previous systematic review and meta-analysis, which is one of the major strengths of our analyses. The previous review included information from all strata from each study in their analyses. In contrast, we selected the most appropriate stratum that had an almond dose closer to 42.5 g, a control that was most closely matched for macronutrient composition and energy intake, and similar intervention duration. The remaining strata were either tested in sensitivity analyses or were completely excluded, if they were ineligible. Another major difference and strength is that we had stricter inclusion criteria in terms of energy intake and eligible population; this resulted in exclusion of 5 studies that were included in the previous review [Supplemental Table 2 (36–40)]. We integrated all available evidence on cardiometabolic risk in our review by quantifying additional biomarkers such as body weight, BMI, SBP, DBP, FBG, apoA-I, apoB, hsCRP, and Lp(a).
Most of the limitations largely arise from the different study designs and methodologies of the RCTs included in this review. We tried to mitigate this limitation by selecting and including relevant and comparable strata across studies. Nevertheless, there was considerable heterogeneity in terms of almond consumption—for example, some studies used almond consumption as a percentage of energy intake, whereas others used a fixed amount of almond regardless of energy intake. Studies failed to recognize potential interactions of almonds mixed with food components and some studies did not report whether almonds were mixed in foods or were consumed alone. There were differences in lipid measurement; some studies reported plasma lipids whereas others reported serum lipids. Although most studies used a food log, diet diary, or dietary intake report as a measure of compliance, their frequency of assessment was highly variable, ranging from daily, to 3–4 d, or to a certain number of diet diaries (19, 20, 22, 23, 26, 28–33). Adding to the heterogeneity, studies also used other methods of compliance such as 24-h recalls (25, 27, 31), diet recalls (24, 25), fasting α-tocopherol (22, 29, 33), food weigh-in (21, 28), fatty acid composition of TGs (21), and even breath samples (22). Nevertheless, the different types of control strata among eligible studies was the largest limitation in terms of heterogeneity and this needs to be properly addressed in future almond trials.
Although RCTs are deemed as the highest level of evidence and provide causal inferences, designing diet-related nutrition research can be challenging with regard to control selection, patient blinding, health motivation, compliance with the intervention, attrition, etc. Additional RCTs are warranted with a more homogeneous selection of controls that match the macronutrient composition and energy intake of the almond intervention, but differ only in terms of the bioactive components of almonds that may have a beneficial effect on the biomarkers of CVD risk. Furthermore, it is possible that the results may be affected based on how almonds are consumed, either alone or mixed with foods that may influence their bioabsorption and bioavailability. Thus, future RCTs should examine these potential interactions between almond and added food components. It is plausible that the ability to detect long-term effect of almond consumption on many of the outcomes in our analyses may have been underpowered because only 3 studies were >6 wk in duration (19, 27, 33). No major adverse effects attributable to intervention or control were reported in RCTs. More long-term RCTs with almonds are needed to examine whether there are sustained beneficial effects.
In conclusion, our findings further add to the literature that the consumption of almonds compared with controls decreases TC and LDL cholesterol, but also significantly decreases other CVD risk factors such as body weight. In addition, this review suggests that the benefits of almond consumption may extend to healthy subjects as well as those at risk of CVD. Lastly, responses to certain outcomes may be dose specific. Substantial heterogeneity in the eligible studies regarding almond intervention, comparators, and doses preclude firmer conclusions.
Supplementary Material
Acknowledgments
We thank Muriel Powers, MFA, Project Coordinator at Tufts CTSI for proofreading the manuscript. The authors’ responsibilities were as follows—GR and EJJ: conceived and designed the experiments; ML-B, LK, and EEA: performed the analyses; ML-B, JW, and EEA: synthesized the data; ML-B, JW, GR, and EJJ: wrote the paper; GR and EJJ: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
Part of the results were presented as a poster at the ASN 2018 meeting.
Supported by the Almond Board of California; USDA 8050-51000-095-01S. The funder did not have a role in the study selection, quality assessment, data synthesis, or manuscript preparation.
Author disclosures: GR was a consultant for Porter Novelli; EJJ received funds from the Almond Board of California for a clinical trial at the time of the study. MAL-B, JW, EEA, and LK, no conflicts of interest.
Supplemental Figures 1–12 and Supplemental Tables 1–10 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: CHD, coronary heart disease; CVD, cardiovascular disease; DBP, diastolic blood pressure; FBG, fasting blood glucose; HbA1c, glycated hemoglobin A1c; hsCRP, high-sensitivity C-reactive protein; Lp(a), lipoprotein (a); RCT, randomized controlled trial; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
References
- 1. Alasalvar C, Bolling BW. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br J Nutr. 2015;113:(Suppl 2):S68–78. [DOI] [PubMed] [Google Scholar]
- 2. US FDA. Qualified health claims, letter of enforcement discretion—nuts and coronary heart disease (docket no 02P-0505). Rockville (MD); 2003. pp. 1–4. [Google Scholar]
- 3. Hu FB, Stampfer MJ, Manson JE, Rimm EB, Colditz GA, Rosner BA, Speizer FE, Hennekens CH, Willett WC. Frequent nut consumption and risk of coronary heart disease in women: prospective cohort study. BMJ. 1998;317(7169):1341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Albert CM, Gaziano JM, Willett WC, Manson JE. Nut consumption and decreased risk of sudden cardiac death in the Physicians' Health Study. Arch Intern Med. 2002;162(12):1382–7. [DOI] [PubMed] [Google Scholar]
- 5. Fraser GE, Sabate J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study. Arch Intern Med. 1992;152(7):1416–24. [PubMed] [Google Scholar]
- 6. Ellsworth JL, Kushi LH, Folsom AR. Frequent nut intake and risk of death from coronary heart disease and all causes in postmenopausal women: the Iowa Women's Health Study. Nutr Metab Cardiovasc Dis. 2001;11(6):372–7. [PubMed] [Google Scholar]
- 7. Ang Y, Sabaté J. Nuts and health outcomes: new epidemiologic evidence. Am J Clin Nutr. 2009;89(5):S1643–8. [DOI] [PubMed] [Google Scholar]
- 8. Kelly JH Jr., Sabate J. Nuts and coronary heart disease: an epidemiological perspective. Br J Nutr. 2006;96:(Suppl 2):S61–7. [DOI] [PubMed] [Google Scholar]
- 9. USDA. Full report (all nutrients): 12061, nuts, almonds. USDA; 2018. [Google Scholar]
- 10. Almond Board of California. Annual Report: Almond Almanac. Modesto (CA); 2018. [Google Scholar]
- 11. Kamil A, Chen CY. Health benefits of almonds beyond cholesterol reduction. J Agric Food Chem. 2012;60(27):6694–702. [DOI] [PubMed] [Google Scholar]
- 12. Musa-Veloso K, Paulionis L, Poon T, Lee HY. The effects of almond consumption on fasting blood lipid levels: a systematic review and meta-analysis of randomised controlled trials. J Nutr Sci. 2016;5:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA et al.. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lichtenstein AH, Yetley EA, Lau J. Application of systematic review methodology to the field of nutrition. J Nutr. 2008;138(12):2297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- 18. Kris-Etherton PM, Dietschy J. Design criteria for studies examining individual fatty acid effects on cardiovascular disease risk factors: human and animal studies. Am J Clin Nutr. 1997;65(5 Suppl):S1590–6. [DOI] [PubMed] [Google Scholar]
- 19. Sweazea KL, Johnston CS, Ricklefs KD, Petersen KN. Almond supplementation in the absence of dietary advice significantly reduced C-reactive protein in subjects with type 2 diabetes. J Funct Foods. 2014;10:252–9. [Google Scholar]
- 20. Lovejoy JC, Most MM, Lefevre M, Greenway FL, Rood JC. Effect of diets enriched in almonds on insulin action and serum lipids in adults with normal glucose tolerance or type 2 diabetes. Am J Clin Nutr. 2002;76(5):1000–6. [DOI] [PubMed] [Google Scholar]
- 21. Sabate J, Haddad E, Tanzman JS, Jambazian P, Rajaram S. Serum lipid response to the graduated enrichment of a Step I diet with almonds: a randomized feeding trial. Am J Clin Nutr. 2003;77(6):1379–84. [DOI] [PubMed] [Google Scholar]
- 22. Tan SY, Mattes RD. Appetitive, dietary and health effects of almonds consumed with meals or as snacks: a randomized, controlled trial. Eur J Clin Nutr. 2013;67(11):1205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zaveri S, Drummond S. The effect of including a conventional snack (cereal bar) and a nonconventional snack (almonds) on hunger, eating frequency, dietary intake and body weight. J Hum Nutr Diet. 2009;22(5):461–8. [DOI] [PubMed] [Google Scholar]
- 24. Damasceno NR, Perez-Heras A, Serra M, Cofan M, Sala-Vila A, Salas-Salvado J, Ros E. Crossover study of diets enriched with virgin olive oil, walnuts or almonds. Effects on lipids and other cardiovascular risk markers. Nutr Metab Cardiovasc Dis. 2011;21:(Suppl 1):S14–20. [DOI] [PubMed] [Google Scholar]
- 25. Jenkins DJ, Kendall CW, Marchie A, Parker TL, Connelly PW, Qian W, Haight JS, Faulkner D, Vidgen E, Lapsley KG et al.. Dose response of almonds on coronary heart disease risk factors: blood lipids, oxidized low-density lipoproteins, lipoprotein(a), homocysteine, and pulmonary nitric oxide: a randomized, controlled, crossover trial. Circulation. 2002;106(11):1327–32. [DOI] [PubMed] [Google Scholar]
- 26. Kurlandsky DR, Stote KS. Cardioprotective effects of chocolate and almond consumption in healthy women. Nutr Res. 2006;26:509–16. [Google Scholar]
- 27. Abazarfard Z, Salehi M, Keshavarzi S. The effect of almonds on anthropometric measurements and lipid profile in overweight and obese females in a weight reduction program: a randomized controlled clinical trial. J Res Med Sci. 2014;19(5):457–64. [PMC free article] [PubMed] [Google Scholar]
- 28. Berryman CE, West SG, Fleming JA, Bordi PL, Kris-Etherton PM. Effects of daily almond consumption on cardiometabolic risk and abdominal adiposity in healthy adults with elevated LDL-cholesterol: a randomized controlled trial. J Am Heart Assoc. 2015;4(1):e000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hollis J, Mattes R. Effect of chronic consumption of almonds on body weight in healthy humans. Br J Nutr. 2007;98(3):651–6. [DOI] [PubMed] [Google Scholar]
- 30. Li SC, Liu YH, Liu JF, Chang WH, Chen CM, Chen CY. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism. 2011;60(4):474–9. [DOI] [PubMed] [Google Scholar]
- 31. Spiller GA, Jenkins DA, Bosello O, Gates JE, Cragen LN, Bruce B. Nuts and plasma lipids: an almond-based diet lowers LDL-C while preserving HDL-C. J Am Coll Nutr. 1998;17(3):285–90. [DOI] [PubMed] [Google Scholar]
- 32. Tamizifar B, Rismankarzadeh M, Vosoughi AA, Rafieeyan M, Tamizifar B, Aminzade A. A low-dose almond-based diet decreases LDL-C while preserving HDL-C. Arch Iran Med. 2005;8:45–51. [Google Scholar]
- 33. Wien M, Bleich D, Raghuwanshi M, Gould-Forgerite S, Gomes J, Monahan-Couch L, Oda K. Almond consumption and cardiovascular risk factors in adults with prediabetes. J Am Coll Nutr. 2010;29(3):189–97. [DOI] [PubMed] [Google Scholar]
- 34. Linton MF, Yancey PG, Davies SS, Jerome WGJ, Linton EF, Vickers KC. The role of lipids and lipoproteins in atherosclerosis. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM et al., editors. Endotext: South Dartmouth (MA); 2000. [Google Scholar]
- 35. Eckel RH. Obesity and heart disease: a statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation. 1997;96(9):3248–50. [DOI] [PubMed] [Google Scholar]
- 36. Cohen AE, Johnston CS. Almond ingestion at mealtime reduces postprandial glycemia and chronic ingestion reduces hemoglobin A(1c) in individuals with well-controlled type 2 diabetes mellitus. Metabolism. 2011;60(9):1312–7. [DOI] [PubMed] [Google Scholar]
- 37. Foster GD, Shantz KL, Vander Veur SS, Oliver TL, Lent MR, Virus A, Szapary PO, Rader DJ, Zemel BS, Gilden-Tsai A. A randomized trial of the effects of an almond-enriched, hypocaloric diet in the treatment of obesity. Am J Clin Nutr. 2012;96(2):249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jia X, Li N, Zhang W, Zhang X, Lapsley K, Huang G, Blumberg J, Ma G, Chen J. A pilot study on the effects of almond consumption on DNA damage and oxidative stress in smokers. Nutr Cancer. 2006;54(2):179–83. [DOI] [PubMed] [Google Scholar]
- 39. Ruisinger JF, Gibson CA, Backes JM, Smith BK, Sullivan DK, Moriarty PM, Kris-Etherton P. Statins and almonds to lower lipoproteins (the STALL Study). J Clin Lipidol. 2015;9(1):58–64. [DOI] [PubMed] [Google Scholar]
- 40. Wien MA, Sabate JM, Ikle DN, Cole SE, Kandeel FR. Almonds vs complex carbohydrates in a weight reduction program. Int J Obes Relat Metab Disord. 2003;27(11):1365–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.