Abstract
BACKGROUND
The value of early postoperative 18F-FET-PET in patients with glioblastoma (GBM) is unclear. Five-aminolevulinic acid (5-ALA) is used for fluorescence-guided resections in these patients and previous data suggest that fluorescence and 18F-FET-PET both demarcate larger tumor volumes than gadolinium enhanced magnet resonance imaging (MRI).
OBJECTIVE
To correlate fluorescence with enhancing volumes on postoperative MRI and 18F-FET-PET tumor volumes, and determine the value of postoperative 18F-FET-PET for predicting survival through observational study.
METHODS
GBM patients underwent fluorescence-guided resection after administration of 5-ALA followed by early postoperative MRI and 18F-FET-PET for determination of residual tissue volumes. All patients were treated with standard temozolomide radiochemotherapy and monitored for progression-free and overall survival (PFS, OS).
RESULTS
A total of 31 patients were included. For functional reasons, residual 5-ALA derived fluorescent tissue was left unresected in 18 patients with a median 18F-FET-PET volume of 17.82 cm3 (interquartile range 6.50-29.19). In patients without residual fluorescence, median 18F-FET-PET volume was 1.20 cm3 (interquartile range 0.87-5.50) and complete resection of gadolinium enhancing tumor was observed in 100% of patients. A 18F-FET-PET volume of above 4.3 cm3 was associated with worse OS (logrank P-value ≤ .05), also in patients with no residual contrast enhancing tumor on MRI. More patients in whom fluorescencing tissue had been removed completely had postoperative 18F-FET-PET tumor volumes below 4.3 cm3.
CONCLUSION
Postoperative 18F-FET-PET volumes predict OS and PFS. Resection of 5-ALA derived fluorescence beyond gadolinium enhancing tumor tissue leads to lower postoperative 18F-FET-PET tumor volumes and improved OS and PFS without additional deficits.
Keywords: Aminolevulinic acid, Fluorescence, Gadolinium, Glioblastoma, Tumor burden, (18F)fluoroethyltyrosine
ABBREVIATIONS
- CRET
complete resection of gadolinium enhancing tumor
- F-FET
F-Fluor-Ethyl-Tyrosine
- GBM
glioblastoma
- HR
hazard ratios
- MRI
magnet resonance imaging
- MWU
Mann–Whitney U
- OS
overall survival
- PET
positron emission tomography
- PFS
progression-free survival
- SUV
standardized uptake value
Glioblastoma (GBM), the most frequent malignant intrinsic brain tumor, carries a poor prognosis.1,2 Current treatment standard is resection and adjuvant radiochemotherapy. The target tissue to be resected is traditionally considered to be the gadolinium contrast enhancing (Gd+ ) tumor on T1 weighted magnet resonance imaging (MRI). Retrospective and prospective cohort studies have repeatedly demonstrated the resection of Gd+ tumor to be associated with improved prognosis.3-8 Removal of contrast enhancing tumor has been equated to “complete” resection.
As identification of tumor margins based on visual information or tactile tissue changes is difficult, techniques have evolved over time to overcome this issue.9 One technique is fluorescence-guided resection using 5-aminolevulinic acid (5-ALA). 5-ALA leads to accumulation of fluorescent protoporphyrin IX in non-necrotic marginal GBM tissue. Using an appropriate surgical microscope, the tumor can be visualized in real time during resection.10 A randomized controlled trial has shown the rates of complete resection of gadolinium enhancing tumor (CRET) to be increased, leading to approval with the European Medicines Agency as well as with the U.S. Food and Drug Administration.11 Despite its widespread use, little is known regarding the tissue volume that is actually visualized by fluorescence. Early on, our group suspected fluorescence to extend beyond the region of Gd+ on MRI12,13 as subsequently corroborated by others.13-15 Also, nonenhancing gliomas have been observed to accumulate visibly fluorescing tissue after administration of 5-ALA, the latter in about 20% of cases.16
For surgery of high-grade gliomas, it would appear crucial to determine the expected extent of fluorescent tissue beforehand. This is important when deciding on the type of intraoperative monitoring or mapping required for safe surgery when enhancing tumor is located in proximity to eloquent brain regions.14 Various MRI techniques have not yet been reliably able to predict the extent of fluorescence. Over the last years, positron emission tomography (PET) with radio labeled amid acids such as 18F-Fluor-Ethyl-Tyrosine (18F-FET-PET) has been established for glioma imaging. Its’ use is recommended throughout all stages of diagnostics and therapy.1718F-FET tracer uptake was found to correlate with the presence of intraoperative fluorescence after administration of 5-ALA.18 Similar to 5-ALA, 18F-FET-PET is capable of visualizing larger tumor volumes pre- and postoperatively than MRI with gadolinium contrast alone.19,20 FET-positive residual tumor volume, previously termed biological tumor volumes, correlates with worse survival in GBM patients undergoing resection and biopsy.21
Our study sought to investigate Gd+ and 18F-FET-PET tumor volumes after fluorescence-guided resection in GBM patients and to correlate residual volumes with survival data. To our knowledge, an analysis of the impact of residual FET-PET volume on survival in GBM has not yet been performed in patients with maximal resective surgery.
METHODS
Study Design and Patients
From April 8, 2015, 31 adult patients with Gd+ GBM and subsequent fluorescence-guided resection were included in this exploratory prospective observational single-center study. Surgical aim was safe complete resection of enhancing tumor or cytoreductive surgery in tumors extending into crucial cortex or white matter tracts. Early postoperative MRI was obtained within 48 h after surgery. Early postoperative 18F-FET-PET was performed at the earliest time point possible (median 6 d, interquartile range 4-7 d, average 6.25 d; SD 3.78) but within 15 d after surgery as previously recommended.19 All patients were treated with concomitant radiochemotherapy with temozolomide followed by sequential chemotherapy with temozolomide alone.1 Patients were followed up every 3 mo with serial MRI and clinical examination. Progression was assessed in accordance with the criteria defined by the Response Assessment in Neuro-Oncology Working Group.22 The last visit for survival analysis was on August 2, 2018. Median follow-up was 2.5 yr (1.7-3.3 CI). No patient was lost to follow-up. Full ethical approval was obtained at the regional ethical review committee. This study is reported according to the STROBE guidelines for reporting observational studies.23
Fluorescence-Guided Resection
Inraoperatively, neuronavigation, ultrasound, and 5-ALA derived fluorescence were used. 5-ALA (Gliolan®, Medac, Wedel, Germany) was administered at 20 mg per kilogram b.w. orally 4 h prior to induction of anesthesia. Amicroscope with appropriate filters (Pentero with BLUE400 filter option, Zeiss Meditech, Oberkochen, Germany) was used for fluorescence visualization. Surgical goal was the complete resection of visible fluorescence while respecting eloquent brain. Residual fluorescence at the end of surgery was documented in the surgical report. Functional MRI and fiber tracking studies were obtained as necessary, as well as methods of intraoperative mapping and monitoring including awake craniotomies for language mapping.24 Three primary neuro-oncological surgeons including the senior author were involved in patient treatment. All resections were finalized by the senior author. Preoperatively, patients received 4 mg dexamethasone 3 times a day for duration of 3 d, which was tapered postoperatively.
MRI Protocol
Early postoperative MRI was obtained within 48 h after surgery including contrast enhanced sequences. Images were segmented for volumetric analysis using an established semiautomatic technique (Medical Imaging Toolkit, German Cancer Research Center, Division of Medical Image Computing, Heidelberg, Germany, open source, www.mitk.org) by a neuroradiologist (P.S.) blinded to survival data and extent of resection. Residual enhancing tumor volumes of less than .175 cm3 were considered to represent “complete” resections in accordance with previous publications.11,14
18F-FET-PET Protocol
Patients were studied after overnight fasting and after informed consent. 18F-FET PET-CT was performed as previously described in 12 patients with minor modifications.25 Static images were acquired 20 to 40 min after intravenous injection of 3 MBq 18F-FET/kg body weight using a hybrid PET–computed tomography device (mCT, Siemens, Erlangen, Germany). Images were processed and reconstructed with software as supplied by the manufacturer, including homogeneous attenuation correction of the head based on contours extracted from the emission data. In 19 patients, 40 min dynamic FET-PET acquisition was started immediately after injection of the tracer using list mode and a MR-PET device (Siemens, Erlangen, Germany). These images were reconstructed as four 10 min frames the third and fourth frame comparable with the static images. PET images were coregistered with early postoperative MRI using Syngo.via software (Siemens, Erlangen, Germany). Volumes of interest were placed on the tumor to detect the maximal standardized uptake value (SUV). A standardized circular reference region encompassing grey and white matter was drawn on axial slices at the level above the side ventricles in the unaffected hemisphere or in the anterior of posterior half of the brain in case of bilateral tumors. Uptake ratios were calculated as tumor maximum SUV/reference mean SUV. To assess for tumor volume, a spherical volume of interest was defined covering all visible tumor lesions and carefully avoiding structures with physiological uptake like venous sinuses and the scalp or the base of the scull. Within this volume only voxels exceeding the threshold of 1.8 x mean activity of the reference ROI were used to calculate the 18F-FET-PET positive tumor volume.21 Analyses were performed by a nuclear medicine specialist (M.W.) blinded to survival data and extent of resection according to other modalities.
Due to logistical reasons, mainly short-term availability that would not interfere with the timing of urgent surgery in GBM, the number of patients with preoperative 18F-FET-PET was too low to allow meaningful analysis.
Histopathological and Molecular Analysis
Tissue diagnoses were obtained locally in accordance with the 2016 World Health Organization classification of tumors of the central nervous system.2
Survival Data Analysis and Statistics
Overall survival (OS) was defined as time from day of surgery until death from any cause. Patients alive were censored at time of last contact. Progression-free survival (PFS) was defined as time from day of surgery until diagnosis of progressive disease according to MRI criteria or death from any cause. Patients without such event were censored at last contact. Standard descriptive analyses were performed. Categorical variables are shown as absolute and relative frequencies and continuous variables are presented as mean ± standard deviation or median and interquartile range (IQR, 25% quartile-75% quartile). Fisher's exact test was calculated to compare categorical variables and two-sided non-parametric Mann–Whitney U (MWU) test were performed for continuous variables. Wilcoxon signed rank test was used to compare related samples. OS and PFS were analyzed using time-to-event methods: Kaplan–Meier estimates, log-rank tests, and univariate Cox regressions.26,27 Results are reported as hazard ratios (HR) and corresponding 95% confidence interval (95% CI). Exploratory cut-off point determination of residual 18F-FET-PET volumes was performed to predict OS and PFS based on the maximally selected standardized log rank statistics using the R package maxstat.28 Exact P-values (pmax) for these maximally selected Gauss statistics were calculated.29 Additionally, time-dependent ROC curves from censored event data using Kaplan–Meier method were estimated using the R package survivalROC28 for the continuous variable residual 18F-FET-PET.30 Area under the curve (AUC), sensitivity and specificity were calculated to predict 1-yr OS or PFS. As sensitivity analysis, another definition of PFS was analysed, where patients alive without progression were censored at day of their last MRI without progression. Statistical analyses were performed using R 3.5.1,28 SAS software version 9.4 for Windows (SAS Institute, Cary, North Carolina) and IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, New York). Inferential statistics such as P-values and confidence intervals were intended to be exploratory and not confirmatory. P-values represent only an unadjusted metric measure of evidence against the respective null hypothesis and were used only to generate new hypotheses. Therefore, neither global nor local significance levels were determined, and no adjustment for multiplicity was applied. P-values ≤ .05 were considered statistically noticeable.
RESULTS
A total of 31 patients (18 females, 13 males) were analyzed. Median age was 66.8 yr (interquartile range 51.2-71.5 yr). CRET was achieved in 18 (58%) patients. Complete resection of all fluorescing tissue was achieved in 13 (41.9%) patients, all of which with CRET. One patient (3%) suffered permanent hemiparesis from surgery (see Table 1 for patient characteristics).
TABLE 1.
Patient Characteristics
| Characteristic | Overall | 18F-FET-PET Volume ≤ 4.3 cm3 | 18F-FET-PET Volume > 4.3 cm3 | P value |
|---|---|---|---|---|
| Number of cases | 31 | 11 | 20 | – |
| Age in years | 66.8 (51.2 - 71.5) | 55.1 (41.74 - 68.67) | 67.2 (57.99 - 74.33) | .064† |
| Female/Male (n,%) | 18/13 (58.1%/41.9%) | 7/4 (63.6%/36.4%) | 11/9 (55%/45%) | .718# |
| MGMT Methylation (n,%) | 18 (58.1%) | 8 (72.7%) | 10 (55.6%) | .276# |
| IDH Mutation (n,%) | 2 (6.5%) | 2 (18.2%) | 0 (0%) | .118# |
| Residual Fluorescence (n,%) | 18 (58%) | 2 (18.2%) | 16 (80%) | .002# |
| No Residual Fluorescence (n,%) | 13 (41.9%) | 9 (81.8%) | 4 (20%) | |
| Early Postoperative Gd+ Volume in cm3 | 0 (0 - 1.1) | 0 (0 - 0.02) | 0.51 (0.04 - 1.99) | .005† |
| CRET (%) | 18 (58%) | 11 (100%) | 7 (35%) | < .001# |
| Early Postoperative 18F-FET-PET Volume in cm3 | 7.3 (2.2 - 25.3) | 1.07 (0.22 - 2.91) | 13.98 (7.46 - 28.55) | – |
| Surgically acquired permanent motor or language deficit (n,%) | 1 (3%) | 0 (0%) | 1 (5%) | > .99# |
Data are presented median (25% quantile-75% quantile) or frequencies (percentages). Statistically noticeable differences between 18F-FET-PET Volume ≤ 4.3 cm3 and > 4.3 cm3 are marked in bold (P ≤ .05). CRET complete resection of enhancing tumor; Gd+ gadolinium contrast enhancing tumor; IDH isocitrate dehydrogenase; IQR interquartile range; MGMT O6-methylguanine-DNA methyltransferase; †Mann–Whitney U test, #Fisher's exact.
Early Postoperative Tumor Volumes
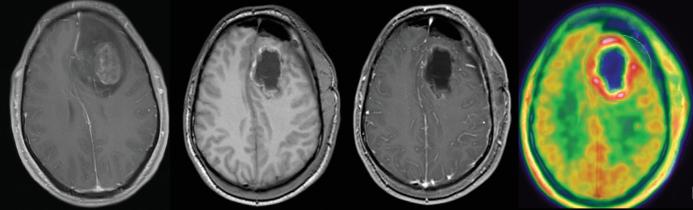
Median residual Gd+ tumor volume was 0 cm3 (IQR 0-1.1 cm3) and residual 18F-FET-PET+ volume 7.3 cm3 (IQR 2.2-25.3 cm3; P < .0001, Wilcoxon signed rank test). 18F-FET-PET positive volumes exceeded Gd+ volumes in all patients. Figure 1 depicts a case of complete resection of enhancing right temporal GBM, whereas 18F-FET-PET showed a residual tumor volume of more than 4.3 cm3.
FIGURE 1.
Complete resection (center left without and center right with contrast) of a gadolinium contrast enhancing left frontal glioblastoma (far left image). Early postoperative 18F-FET-PET (far right image) showed residual tumor volume of more than 4.3 cm3.
Stratifying for residual fluorescence, fluorescence-guided resection lead to 100% CRET when no residual fluorescence was noted at the end of surgery. The median Gd+ volume was 0 cm3 (IQR 0-0 cm3). In the group with residual fluorescence, CRET was achieved in 5/18 (28%) patients. In this group, median Gd+ volume was 0.55 cm3 (IQR 0.15-2.34 cm3; P < .001, MWU test). All patients with residual fluorescence showed 18F-FET tracer uptake. Median 18F-FET-PET volume in patients with residual fluorescence was 17.82 cm3 (IQR 6.25-33.14 cm3) and larger than the median volume of 1.2 cm3 (IQR 0.55-6.38 cm3) in patients without residual fluorescence (P < .001, MWU test).
Figure 2 compares 18F-FET-PET and Gd+ tumor volumes according to residual or no residual intraoperative fluorescence. The distributions of Gd+ and 18F-FET-PET tumor volumes are additionally illustrated as a scatter plot in Figure 3. All data points were located above the bisectrix, illustrating 18F-FET-PET tumor always to exceed Gd+ volumes.
FIGURE 2.
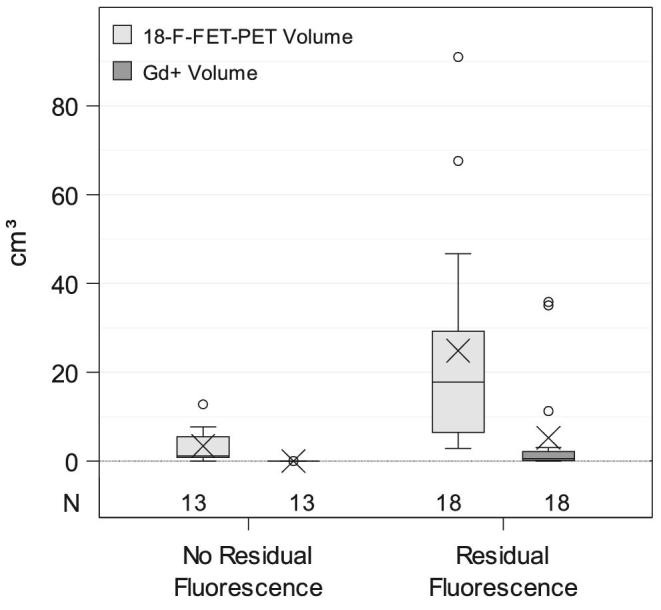
Boxplots showing the distribution of 18F-FET-PET and Gd+ tumor volumes according to residual or no residual intraoperative fluorescence noted at the end of the operation. An X denotes the mean.
FIGURE 3.
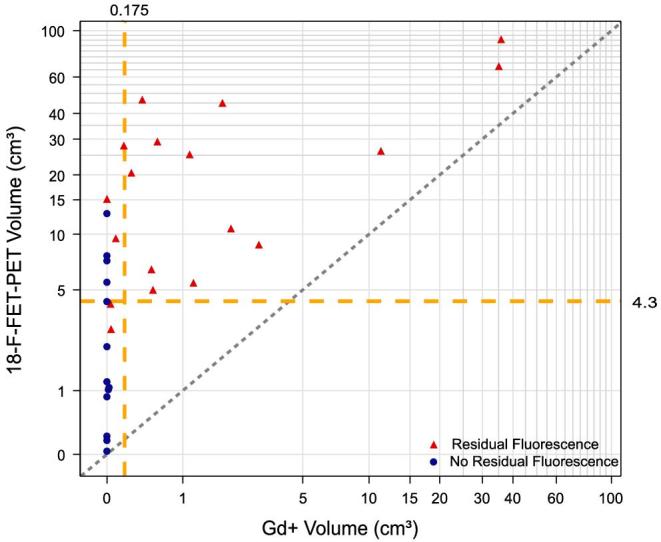
Scatter plot of Gd+ and 18F-FET-PET volumes of each patient. Dotted bisectrix refers to perfect matching absolute values of Gd+ and 18F-FET-PET volumes. Intersected lines at x and y axes refer to cut-off values of 0.175 cm3 for Gd+ (indicating a “complete” resection by MRI standards) and 4.3 cm3 for 18F-FET respectively, the cut-off found for survival with this modality. For the group with no residual fluorescence median Gd+ volume was 0 cm3 (IQR 0-0) and median 18F-FET-PET volume was 1.2 cm3 (IQR 0.05-6.08). In the group with residual fluorescence Gd+ volume was 0.55 cm3 (IQR 0.15-2.34) and 18F-FET-PET volume was 17.82 cm3 (IQR 6.25-33.14). The graph indicates “complete” resections based on visible fluorescence to extend well into the residual 18F-FET-PET volume.
We also stratified tumors by their localization according to the system proposed by Sawaya et al. into grade I to III tumors to determine whether location influenced residual tumor volumes.31 We found no significant influence regarding the distribution of grades I to III on residual 18F-FET-PET volumes.
Survival Analyses
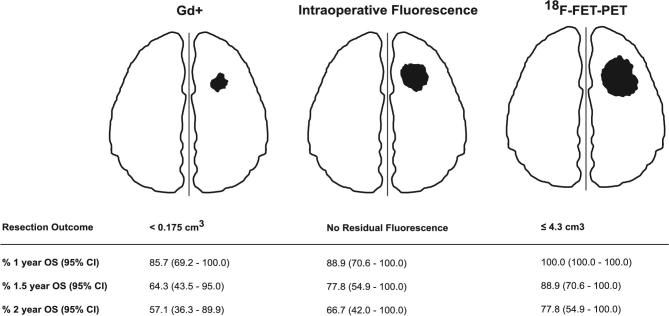
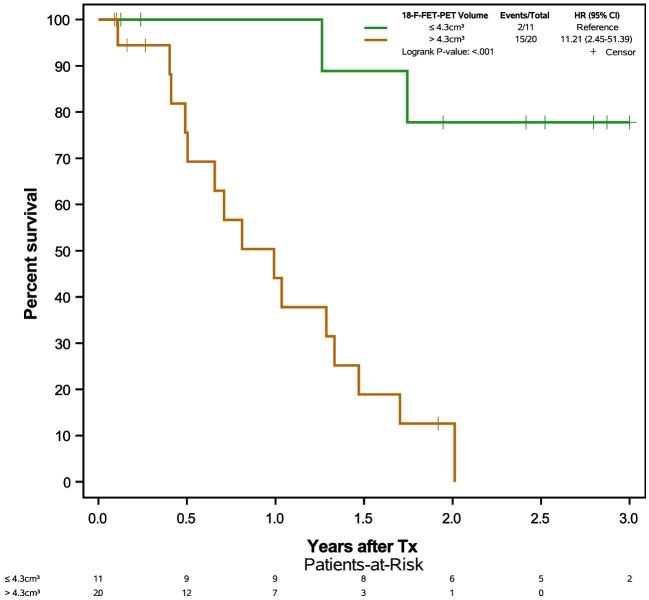
A synopsis of different resection target volumes and their impact on OS is displayed in Figure 4. Stratifying for residual Gd+ volumes, a residual Gd+ volume < 0.175 cm3 (CRET) prolongs OS (Figure 5) as expected. The same was observed for PFS (logrank P-value .008; HR 3.37, 95% CI 1.31-8.70), see Supplemental Digital Content. Exploratory cut-off point determination of residual 18F-FET-PET volumes based on maximally selected standardized logrank statistics found 4.3 cm3 to be the cut-off point for OS and PFS with adjusted exact P-values pmax of .002 for OS and .09 for PFS. Using 18F-FET-PET volume as continuous variable, time dependent AUC, sensitivity, and specificity were calculated to predict 1-yr OS or PFS. AUC, sensitivity, and specificity were 0.75, 100%, and 55.8% for 1-yr OS and 0.70, 86.6%, and 63.2% for 1-yr PFS, respectively. This analysis again indicated 4.3 cm3 to be the relevant cut off. To examine the robustness of this result, a sensitivity analysis was performed using an alternate definition of PFS, where patients alive without progression were censored at day of last MRI without progression. Cut-off point determination led to the same results (data not shown). Extent of tumor resection leading to an early postoperative 18F-FET-PET volume of ≤4.3 cm3 noticeably prolonged OS (Figure 6).
FIGURE 4.
Schematic synopsis of different resection target volumes and their impact on overall survival.
FIGURE 5.
Kaplan–Meier plots of overall survival times stratified by residual gadolinium contrast enhancing (Gd+ ) volumes on early postoperative MRI.
FIGURE 6.
Kaplan–Meier plots of overall survival times stratified by 18F-FET-PET tumor volumes in early postoperative 18F-FET-PET.
In univariable Cox regressions, MGMT status, age, and 18F-FET-PET volume were predictive for OS (Table 2). Volume of gadolinium contrast enhancing tumor, 18F-FET-PET volume, and residual fluorescence were collinear, as expected from Gd+ and 18F-FET-PET volume distributions. However, a meaningful multivariable analysis of these variables could not be performed, given of the small number of patients in this study.
TABLE 2.
Univariable Cox Regression Analysis of Prognostic Factors of Overall Survival
| Univariable Cox regression | ||
|---|---|---|
| Independent variables | HR (95% CI) | P value |
| Age | 1.02 (1.02-1.118) | .005 |
| Sex | 0.60 (0.23-1.604) | .310 |
| Gd+ volume | 1.09 (1.02-1.163) | .012 |
| 18F-FET-PET volume | 1.03 (1.01-1.059) | .006 |
| Residual fluorescence | 5.77 (1.59-20.96) | .008 |
| MGMT methylation | 0.47 (0.18-1.24) | .125 |
Results are reported as hazard ratios, 95% confidence intervals and P-values from Wald-tests. Gd+ gadolinium contrast enhancing tumor; MGMT O6-methylguanine-DNA methyltransferase.
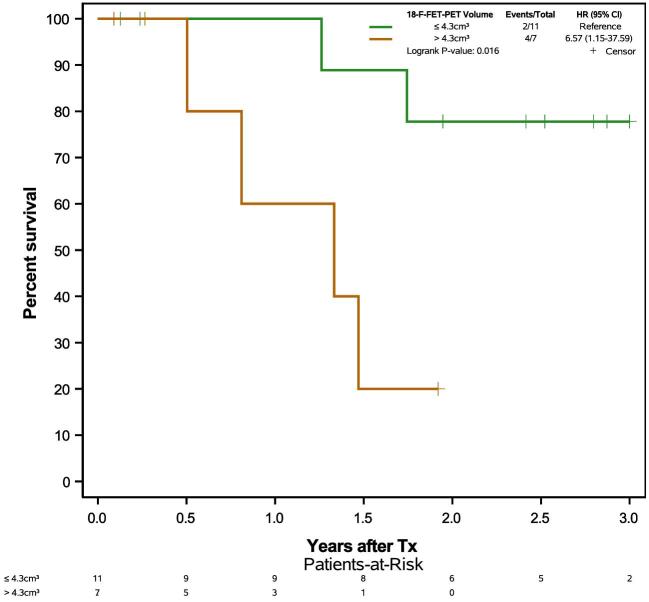
We additionally analyzed only patients with CRET to determine whether resecting 18F-FET-PET positive, but gadolinium negative marginal tumor influenced outcome. In this subgroup of 18 patients with CRET, residual 18F-FET-PET volume ≤4.3 cm3 was similarly associated with prolonged OS (Figure 7). Complete resection of fluorescent tissue resulted in a larger proportion of patients with postoperative 18F-FET-PET tumor burdens of <4.3 cm3 compared to incomplete resections (P = .002, Fisher).
FIGURE 7.
Kaplan–Meier plot of overall survival in all patients with complete resection of gadolinium contrast enhancing tumor on early postoperative MRI, stratified by residual 18F-FET-PET volume in early postoperative 18F-FET-PET.
All patients were treated by surgery and concomitant radiochemotherapy with temozolomide followed by adjuvant temozolomide. We did not note significant differences in secondary therapies after tumor recurrence in patients with initial CRET regarding re-operation, second line chemotherapeutic strategies or re-irradiation strategies. Approximately one third of these patients received secondary therapies and the numbers were too low to allow meaningful statistical corroboration.
In univariable Cox regression, it was found that per 1 cm3 residual 18F-FET-PET volume the risk of death increased by a factor of 2. Univariate Cox-Regression found a hazard ratio of 1.04 (95% CI 1.01-1.06) for worse OS with every 1 cm3 residual 18F-FET-PET volume. For PFS the hazard ratio was found to be 1.02 (0.988%-1.054 95% CI).
DISCUSSION
Currently, MRI is the imaging modality of choice for GBM, and gadolinium enhancing tumor is the resection target volume. Even so, it is well known that infiltrating tumor cells by far exceed Gd+ tumor volumes, leading to inevitable recurrence at the margin of the resection.32 5-ALA derived fluorescence has been shown to mark tumor tissue volume exceeding Gd+ tumor volume.3,12,33,34 Studies correlating completeness of tumor resection with outcome have largely examined the percentage of tumor removal. Even though this concept has been popularized, it does not represent the biological reality of the tumor burden.22 Furthermore, residual tumor volumes on MRI appear to predict survival in a continuous but not “all or nothing” relationship.35 In accordance with previous studies, we found that postoperative 18F-FET-PET tumor volumes exceed Gd+ volumes and that fluorescence-guided resection using 5-ALA can be relied upon for identifying tissue that is a part of the Gd+ volume, but also part of the 18F-FET volume, exceeding the Gd+ volume. Several histopathological sampling studies have demonstrated that tumor tissue exceeds 5-ALA derived fluorescence.34,36 Removing all fluorescing tissue reduced the residual density of tumor cells to about 10%.37
We demonstrate that by resecting beyond the Gd+ volume, the residual 18F-FET-PET volume can be further reduced, which affects survival. To our knowledge, this is the first demonstration that supramarginal resections as quantified by early postoperative 18F-FET-PET serve to improve survival in patients with GBM.
Similarly, previous work found postoperative 18F-FET-PET volumes to influence survival independent of age or MGMT promotor methylation, although the cut-off was more than twice as high as in our patients.21 However, almost half of patients had only received biopsies. Therefore, while this study indicates a principle value of 18F-FET-PET for predicting prognosis, it does not assess whether resections outside the gadolinium enhancing portions of GBM confers an additional survival advantage, provided this is done safely. With our observations we demonstrate this to be the case.
The knowledge, that residual postoperative 18F-FET-PET volumes after surgery in patients with GBM will affect PFS and OS might affect the overall management of patients. We show that the use of 5-ALA-induced porphyrins can help reduce the residual 18F-FET-PET tumor volume, underlining the importance of using fluorescence for malignant glioma surgery. Apart from mere surgical aspects, our observations demonstrate 18F-FET-PET to be useful for estimating residual tumor burden and prognosis on a more biological level. This might translate into decisions regarding adjuvant therapies, such as extended course of temozolomide. Gadolinium enhancement indicates blood-brain barrier breakdown or increased vascular volume in GBM, rather than tumor cell infiltration. Accordingly, 18F-FET-PET has been proposed as an additional tool in resection planning and for defining additional tissue volumes to be incorporated in radiotherapy planning.38,39
From a surgical perspective, we observed that complete resection of fluorescence allows additional tumor removal beyond Gd+. This volume, visualized by 18F-FET-PET may be used as a new definition of resection target volume. The concept of “supramarginal” resections, ie, beyond the contrast enhancing part of the tumor, has previously been put forward,40-42 using FLAIR, DTI abnormalities or functional limits to define the borders of resection, provided such extensions of resection can be achieved safely. Deficits from surgery in GBM patients have been associated with decreased survival.43 The judicious use of intraoperative monitoring in conjunction with intraoperative fluorescence has already proved for achieving CRET.14 Future studies are needed to investigate safety and feasibility of extended 18F-FET-PET based resections.
Limitations
One limitation of this study is the small number of patients. Since the resulting effect measures are to some extent uncertain, leading to large confidence intervals, the reported results should be interpreted carefully. The determination of the cut-off value was explorative and due to the distribution of the few observed 18F-FET-PET volumes more precise cut-off estimation was not possible. Due to the sample size, it was also not possible to perform multivariable analyses including relevant covariates and confounders. Consequently, a prospective study is required to confirm results and to validate the cut-off value. Further, the mode of acquiring 18F-FET-PET needs to be further investigated, eg, regarding timing or the definition of the optimal tumor to brain ratio when calculating 18F-FET-PET volumes. Authors have used different ratios of 1.6-2.0 resulting in variations in tumor volumes.44
Importantly, PET volumetry depends on the definition of thresholds. We conducted our study at a tumor to brain ratio of 1.8, in accordance with the largest previous study21 and a consensus publication of nuclear medicine and neuro-oncological societies.45
Finally, our assessment of residual fluorescence was subjective, which is inherent to this method. Residual fluorescence hidden under blood or behind overhanging brain margins can never be ruled out completely. 5-ALA fluorescence-guided resection is an optical but not spectroscopic method. However, all resections in this study were finalized by the senior author having profound experience with the method. To further overcome these limitations we are in the active planning stage for a multicentric approach to prospectively analyzing the relationship between CRET, 5-ALA derived fluorescence and 18F-FET-PET and their usefulness for driving resection, looking at safety and outcomes.
CONCLUSION
Postoperative 18F-FET-PET volumes correlate with OS and PFS. Resection of 5-ALA derived fluorescence of GBM tissue leads to lower postoperative 18F-FET-PET tumor volumes and a higher number of patients below a calculated survival cut off of 4.3 cm3. 18F-FET-PET may potentially serve to better define the target volume, which should be resected in patients with GBM.
Disclosures
Dr Stummer reports consultant and lecture activities activity for medac (Wedel, Germany), Carl Zeiss Meditech (Oberkochen, Germany), and NxDc (Lexington, Kentucky). The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Supplemental Digital Content. Figures. Kaplan–Meier plots.
REFERENCES
- 1. Stupp R, Hegi ME, Mason WP et al.. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466. [DOI] [PubMed] [Google Scholar]
- 2. Louis DN, Perry A, Reifenberger G et al.. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803-820. [DOI] [PubMed] [Google Scholar]
- 3. Stummer W, Reulen HJ, Meinel T et al.. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62(3):564-576; discussion 564-576. [DOI] [PubMed] [Google Scholar]
- 4. Stummer W, Meinel T, Ewelt C et al.. Prospective cohort study of radiotherapy with concomitant and adjuvant temozolomide chemotherapy for glioblastoma patients with no or minimal residual enhancing tumor load after surgery. J Neurooncol. 2012;108(1):89-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stummer W, Tonn JC, Mehdorn HM et al.. Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. JNS. 2011;114(3):613-623. [DOI] [PubMed] [Google Scholar]
- 6. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. JNS. 2011;115(1):3-8. [DOI] [PubMed] [Google Scholar]
- 7. Kreth FW, Thon N, Simon M et al.. Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann Oncol. 2013;24(12):3117-3123. [DOI] [PubMed] [Google Scholar]
- 8. McGirt MJ, Chaichana KL, Gathinji M et al.. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. JNS. 2009;110(1):156-162. [DOI] [PubMed] [Google Scholar]
- 9. Albert FK, Forsting M, Sartor K, Adams HP, Kunze S. Early postoperative magnetic resonance imaging after resection of malignant glioma: Objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery. 1994;34(1):45-60; discussion 60-41. [DOI] [PubMed] [Google Scholar]
- 10. Stummer W, Stepp H, Moller G, Ehrhardt A, Leonhard M, Reulen HJ. Technical principles for protoporphyrin-IX-fluorescence guided microsurgical resection of malignant glioma tissue. Acta Neurochir (Wien). 1998;140(10):995-1000. [DOI] [PubMed] [Google Scholar]
- 11. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392-401. [DOI] [PubMed] [Google Scholar]
- 12. Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme utilizing 5-ALA-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg. 2000;93(6):1003-1013. [DOI] [PubMed] [Google Scholar]
- 13. Schucht P, Knittel S, Slotboom J et al.. 5-ALA complete resections go beyond MR contrast enhancement: Shift corrected volumetric analysis of the extent of resection in surgery for glioblastoma. Acta Neurochir. 2014;156(2):305-312; discussion 312. [DOI] [PubMed] [Google Scholar]
- 14. Schucht P, Beck J, Abu-Isa J et al.. Gross total resection rates in contemporary glioblastoma surgery: Results of an institutional protocol combining 5-aminolevulinic acid intraoperative fluorescence imaging and brain mapping. Neurosurgery. 2012;71(5):927-936; discussion 935-926. [DOI] [PubMed] [Google Scholar]
- 15. Aldave G, Tejada S, Pay E et al.. Prognostic value of residual fluorescent tissue in glioblastoma patients after gross total resection in 5-aminolevulinic Acid-guided surgery. Neurosurgery. 2013;72(6):915-921; discussion 920-911. [DOI] [PubMed] [Google Scholar]
- 16. Jaber M, Ewelt C, Wolfer J et al.. Is visible aminolevulinic acid-induced fluorescence an independent biomarker for prognosis in histologically confirmed (World Health Organization 2016) low-grade gliomas? Neurosurgery. 2019;84(6):1214-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Albert NL, Weller M, Suchorska B et al.. Response assessment in neuro-oncology working group and european association for neuro-oncology recommendations for the clinical use of PET imaging in gliomas. NEUONC. 2016;18(9):1199-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stockhammer F, Misch M, Horn P, Koch A, Fonyuy N, Plotkin M. Association of F18-fluoro-ethyl-tyrosin uptake and 5-aminolevulinic acid-induced fluorescence in gliomas. Acta Neurochir. 2009;151(11):1377-1383. [DOI] [PubMed] [Google Scholar]
- 19. Buchmann N, Klasner B, Gempt J et al. (18)F-Fluoroethyl-l-thyrosine positron emission tomography to delineate tumor residuals after glioblastoma resection: A comparison with standard postoperative magnetic resonance imaging. World Neurosurg. 2016;89:420-426. [DOI] [PubMed] [Google Scholar]
- 20. Pauleit D, Floeth F, Hamacher K et al.. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128(3):678-687. [DOI] [PubMed] [Google Scholar]
- 21. Suchorska B, Jansen NL, Linn J et al.. Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology. 2015;84(7):710-719. [DOI] [PubMed] [Google Scholar]
- 22. Vogelbaum MA, Jost S, Aghi MK et al.. Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) Working Group. Neurosurgery. 2012;70(1):234-244; discussion 243-234. [DOI] [PubMed] [Google Scholar]
- 23. von Elm E, Altman DG, Egger M et al.. The Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet North Am Ed. 2007;370(9596):1453-1457. [DOI] [PubMed] [Google Scholar]
- 24. Suero Molina E, Schipmann S, Mueller I et al.. Conscious sedation with dexmedetomidine compared with asleep-awake-asleep craniotomies in glioma surgery: An analysis of 180 patients. J Neurosurg. 2018;129(5):1-8. [DOI] [PubMed] [Google Scholar]
- 25. Jaber M, Wolfer J, Ewelt C et al.. The value of 5-aminolevulinic acid in low-grade gliomas and high-grade gliomas lacking glioblastoma imaging features: An analysis based on Fluorescence, Magnetic Resonance Imaging, 18F-Fluoroethyl Tyrosine Positron Emission Tomography, and Tumor Molecular Factors. Neurosurgery. 2016;78(3):401-411; discussion 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaplan EL, Paul M. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53(282):457-481. [Google Scholar]
- 27. Cox D. Regression models and life tables. J Royal Statist Soc B. 1972;34:187-220. [Google Scholar]
- 28. A language and environment for statistical computing [computer program]. Vienna, Austria, 2018. [Google Scholar]
- 29. Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics. Comput Stat Data Anal. 2003;43:121-137. [Google Scholar]
- 30. Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56(2):337-344. [DOI] [PubMed] [Google Scholar]
- 31. Sawaya R, Hammoud M, Schoppa D et al.. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42(5):1044-1055; discussion 1055-1046. [DOI] [PubMed] [Google Scholar]
- 32. Watanabe M, Tanaka R, Takeda N. Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology. 1992;34(6):463-469. [DOI] [PubMed] [Google Scholar]
- 33. Idoate MA, Diez Valle R, Echeveste J, Tejada S. Pathological characterization of the glioblastoma border as shown during surgery using 5-aminolevulinic acid-induced fluorescence. Neuropathology. 2011;31(6):575-582. [DOI] [PubMed] [Google Scholar]
- 34. Roberts DW, Valdes PA, Harris BT et al.. Coregistered fluorescence-enhanced tumor resection of malignant glioma: Relationships between delta-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. Clinical article. JNS. 2011;114(3):595-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Skardelly M. The role of resection in primary glioblastoma – revisited. Deutsche Gesellschaft für Neurochirurgie. 69. Jahrestagung der Deutschen Gesellschaft für Neurochirurgie (DGNC), Joint Meeting mit der Mexikanischen und Kolumbianischen Gesellschaft für Neurochirurgie. Münster, 03.-06.06.2018; 2018; Münster, Germany. [Google Scholar]
- 36. Hauser SB, Kockro RA, Actor B, Sarnthein J, Bernays RL. Combining 5-aminolevulinic acid fluorescence and intraoperative magnetic resonance imaging in glioblastoma surgery: A histology-based evaluation. Neurosurgery. 2016;78(4):475-483. [DOI] [PubMed] [Google Scholar]
- 37. Stummer W, Tonn JC, Goetz C et al.. 5-Aminolevulinic acid-derived tumor fluorescence: The diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery. 2014;74(3):310-320; discussion 319-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oehlke O, Mix M, Graf E et al.. Amino-acid PET versus MRI guided re-irradiation in patients with recurrent glioblastoma multiforme (GLIAA) - protocol of a randomized phase II trial (NOA 10/ARO 2013-1). BMC Cancer. 2016;16(1):769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Floeth FW, Sabel M, Ewelt C et al.. Comparison of 18F-FET PET and 5-ALA fluorescence in cerebral gliomas. Eur J Nucl Med Mol Imaging. 2011;38(4):731-741. [DOI] [PubMed] [Google Scholar]
- 40. Li YM, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? JNS. 2016;124(4):977-988. [DOI] [PubMed] [Google Scholar]
- 41. Yan JL, van der Hoorn A, Larkin TJ, Boonzaier NR, Matys T, Price SJ. Extent of resection of peritumoral diffusion tensor imaging-detected abnormality as a predictor of survival in adult glioblastoma patients. JNS. 2017;126(1):234-241. [DOI] [PubMed] [Google Scholar]
- 42. Duffau H. Is supratotal resection of glioblastoma in noneloquent areas possible? World Neurosurg. 2014;82(1-2):e101-e103. [DOI] [PubMed] [Google Scholar]
- 43. McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65(3):463-470; discussion 469-470. [DOI] [PubMed] [Google Scholar]
- 44. Piroth MD, Holy R, Pinkawa M et al.. Prognostic impact of postoperative, pre-irradiation 18F-fluoroethyl-l-tyrosine uptake in glioblastoma patients treated with radiochemotherapy. Radiother Oncol. 2011;99(2):218-224. [DOI] [PubMed] [Google Scholar]
- 45. Law I, Albert NL, Arbizu J et al.. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [(18)F]FDG: Version 1.0. Eur J Nucl Med Mol Imaging. 2019;46(3):540-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.