ABSTRACT
Epidemiologic cohort studies enrolling a large percentage of vegetarians have been highly informative regarding the nutritional adequacy and possible health effects of vegetarian diets. The 2 largest such cohorts are the European Prospective Investigation into Cancer and Nutrition–Oxford (EPIC-Oxford) and the Adventist Health Study–2 (AHS-2). These cohorts are described and their findings discussed, including a discussion of where findings appear to diverge. Although such studies from North America and the United Kingdom have been important, the large majority of the world's vegetarians live in other regions, particularly in Asia. Findings from recent cohort studies of vegetarians in East and South Asia are reviewed, particularly the Tzu Chi Health Study and Indian Migration Study. Important considerations for the study of the health of vegetarians in Asia are discussed. Vegetarian diets vary substantially, as may associated health outcomes. Cohort studies remain an important tool to better characterize the health of vegetarian populations around the globe.
Keywords: epidemiologic studies, vegetarians, plant-based diet pattern, chronic disease risk, Adventist Health Study, Indian Migration Study, EPIC-Oxford, Tzu Chi Health Study
Introduction
People around the world have adhered to various plant-based, or vegetarian, diets since antiquity with a variety of motivations, including ethical and religious concerns. In the 19th and early 20th centuries, vegetarian diets were promoted by multiple prominent figures for their health benefits and vegetarian societies were organized. But only in the last 50 y have the health effects of vegetarian diets been studied with more scientific rigor. Whereas early studies often focused on examining vegetarians for possible nutrient deficiencies, the focus has expanded to evaluating possible health benefits of these long-standing real-world dietary patterns.
Much of the scientific investigation of the health effects of vegetarian diets has been accomplished through observational epidemiologic studies, particularly prospective cohort studies including vegetarians and nonvegetarians. In particular, 2 active centers of study have contributed a large part of our current knowledge on vegetarian diets and health: studies in the United Kingdom culminating in the current Oxford cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC) study (EPIC-Oxford) and studies of Seventh-day Adventists in the United States culminating in the current Adventist Health Study—2 (AHS-2). Although these have been important and informative studies, they may not be representative of many vegetarian populations around the world, particularly the large number of vegetarians in Asia. Fortunately, efforts are being made toward the scientific study of the health effects of vegetarian diets in both East Asia and South Asia.
At the recent Seventh International Congress on Vegetarian Nutrition, a symposium featured epidemiologic studies of vegetarians, including not only the well-established EPIC-Oxford and AHS-2 studies, but also epidemiologic studies of vegetarians in both the East Asian and South Asian contexts. This article reviews these cohort studies and their findings. In the context of these findings, we also discuss the consistency of the findings, potential reasons for apparent discrepancies, the varied nature of vegetarian diets, and the importance of epidemiologic studies of vegetarian diets in Asia. We begin with descriptions of the relevant cohorts and a summary of prominent and recent findings from each.
Vegetarian Cohort Studies and Findings
EPIC-Oxford
EPIC-Oxford is a cohort of ∼65,000 men and women aged ≥20 y who were recruited throughout the United Kingdom between 1993 and 2000. Recruitment was targeted to include a high proportion of non–meat-eaters, and the cohort includes 52% meat-eaters, 15% who eat fish but not meat, 29% lacto-ovo-vegetarians, and 4% vegans (1). Participants completed a food frequency and lifestyle questionnaire at recruitment, 20,000 provided blood samples, and 31,000 completed 7-d food diaries within a few months after recruitment. To follow their diet and health, participants have been asked to complete follow-up questionnaires at ∼5, 10, and 15 y after recruitment, and their health is also followed through record linkage to data held by the National Health Service in the United Kingdom to provide information on diagnoses of cancer, other hospital diagnoses, and causes of death. In some analyses the data from EPIC-Oxford have been combined with the earlier Oxford Vegetarian Study, a smaller (11,000 participants) but broadly similar cohort recruited from Oxford in the 1980s (2).
At recruitment, 66% of vegetarians had followed their diet for >5 y, and at 5-y follow-up 85% of vegetarians were still following a vegetarian diet (3). Compared with meat-eaters, vegetarians in EPIC-Oxford have relatively high intakes of carbohydrates, polyunsaturated fat, dietary fiber, folic acid, vitamin C, vitamin E, and magnesium, and relatively low intakes of protein, saturated fat, retinol, vitamin B-12, and zinc (1). Examination of biomarkers showed that, compared with meat-eaters, vegetarians have relatively low circulating concentrations of long-chain n–3 fatty acids, vitamin B-12, and vitamin D, with even lower concentrations in vegans (4–6). Metabolic profiles also differ between diet groups, for example vegans have relatively low plasma concentrations of lysine and methionine, but relatively high concentrations of glycine (7).
Assessment of diet ∼15 y after recruitment showed that overall compliance with population dietary goals was high in all the diet groups, but that the meat-eaters slightly exceeded the limit for saturated fat and did not reach the goal for dietary fiber, whereas the vegans may be at risk of dietary deficiency of vitamin B-12 and iodine (8). Examination of health behaviors showed that, compared with meat-eaters, vegetarian women were less likely to participate in screening for breast cancer or take hormonal therapy for menopause, and vegetarian men were less likely to report prostate-specific antigen testing for prostate cancer (9).
Compared to the meat-eaters, the vegetarians in EPIC-Oxford have a lower BMI (in kg/m2) and prevalence of obesity, less weight gain during follow-up, lower non–HDL cholesterol, lower systolic blood pressure, and a lower prevalence of hypertension; these differences were generally greater in vegans than in lacto-ovo-vegetarians (10–14).
The RR of ischemic heart disease in vegetarians compared with nonvegetarians was 0.68 (95% CI: 0.58, 0.81) (15). However, the risk of death from ischemic heart disease was not significantly different between vegetarians and nonvegetarians (RR: 0.99; 95% CI: 0.79, 1.23) (16). Results for incident stroke have not yet been published, but cerebrovascular disease mortality did not differ significantly between vegetarians and meat-eaters (RR: 1.11; 95% CI: 0.76, 1.62) (16).
For cancer at specific sites, the risk in vegetarians was significantly lower than that in meat-eaters for cancers of the stomach (RR: 0.37; 95% CI: 0.19, 0.69), bladder (RR: 0.62; 95% CI: 0.49, 0.84), and lymphatic and hematopoietic tissue (RR: 0.64; 95% CI: 0.49, 0.84). There were no significant differences between vegetarians and meat-eaters in the risks for other common cancer sites including cancers of the colorectum, breast, or prostate. The RR of being diagnosed with any type of cancer in vegetarians compared with meat-eaters was 0.88 (95% CI: 0.82, 0.95) (17). When the vegetarians were subdivided into lacto-ovo-vegetarians and vegans, RRs for all cancers combined compared with meat-eaters were 0.89 (95% CI: 0.83, 0.96) for lacto-ovo-vegetarians and 0.81 (95% CI: 0.66, 0.98) for vegans.
For conditions diagnosed in hospital other than cancer, vegetarians had relatively low risks of kidney stones, cataracts, and diverticular disease, but not gallstones (18–21).
In comparisons with the whole UK population, all-cause mortality was low in both vegetarians and nonvegetarians (standardized mortality ratios were 52% for both diet groups) (3), and in a formal comparison (including data from the Oxford Vegetarian Study) all-cause mortality did not differ between vegetarians and regular meat-eaters (death rate ratio: 1.02; 95% CI: 0.94, 1.10) (16).
AHS-2
AHS-2 is a North American cohort (22) consisting of 96,000 subjects who were enrolled between 2002 and 2007. All subjects are Seventh-day Adventists, aged between 30 and 111 y at enrollment, ∼65% are female, and 26% are Black (African-American or West Indian living in the United States or Canada).
An extensive questionnaire was obtained at study baseline including sections on medical history, an FFQ, physical activity questions, female questions, and social and demographic variables. Vital status was ascertained by annual matching with the National Death Index, and incident cancers were found by matching with 49 of the 50 state cancer registries and the Washington, DC registry. Follow-up reflected in analytic data sets averages ∼8 y, producing now >10,000 deaths and >5000 incident cancers. The FFQ has been validated by comparison with repeated 24-h dietary recalls (23, 24) and also with biomarkers of dietary intake (25).
Approximately 50% of AHS-2 subjects (n = 42,500) are nonvegetarian, eating meats on average ∼3 times/wk. The remainder (n = 53,500) are vegetarian (broadly defined), divided between lacto-ovo-, vegan-, pesco-, and semi-vegetarians. Adventist vegetarians have typically subscribed to their current dietary patterns for many years. Moreover, the same subjects, as they age, infrequently change their diets after age 55 y. When they do so, it is much more likely to be in the direction of eating fewer animal products (26).
The following is a summary of some of the most important recent results from AHS-2. All reported findings are from multivariate-adjusted analyses. When not otherwise specified, findings reported for vegetarians are for the combination of vegans, lacto-ovo-vegetarians, pesco-vegetarians, and semi-vegetarians compared with nonvegetarians.
Vegetarians have lower all-cause mortality (RR: 0.82; 95% CI: 0.72, 0.94), particularly in males, and specifically for cardiovascular, renal, and endocrine diseases, but not for cancer mortality. In females, trends are toward lower mortality in vegetarians but effect sizes are smaller and often nonsignificant (27).
Regarding cancer risk, vegetarians have a lower incidence of all cancers combined (RR: 0.92; 95% CI: 0.85, 0.99), and particularly of gastrointestinal cancers (RR: 0.76; 95% CI: 0.63, 0.90) (28). The incidence of 3 major cancers has been examined separately. Vegetarians have lower incidence of colorectal cancer (RR: 0.78; 95% CI: 0.64, 0.95), this being particularly evident in pesco-vegetarians (29). Higher dairy consumption is associated with a lower incidence of colorectal cancer; in the AHS-2 data the association is with higher dairy calcium for colon cancer, but with some other noncalcium component for rectal cancer. Milk is thus negatively associated with both cancers (30). Vegans (but not vegetarians more generally) have a significant 35% lower incidence of prostate cancer than nonvegetarians (31). Vegans (but not vegetarians more generally) may have a lower incidence of breast cancer than nonvegetarians with a separated breast cancer–free survival curve, but P = 0.09, suggesting the need for further evaluation with larger numbers (32).
Cardiovascular mortality is strongly negatively associated with a factor weighing heavily on nut and seed proteins, and strongly positively associated with a factor weighing heavily on meat proteins (33). This is after adjusting for different categories of fatty acid, raising the notion of proteins themselves as possible risk factors. Further, risk of coronary artery disease (34), as well as traditional coronary risk factors, such as blood cholesterol, blood pressure, risk of diabetes, and C-reactive protein, are all much lower in vegetarians (35–37). Much of this is also true in Black subjects (38). BMI is also much lower in vegetarians (36, 38) and further, having breakfast and eating most calories before late afternoon is associated with less increase in weight before age 60 y and a faster loss of weight after age 60 y (39). Over decades this translates to a mean difference of 5–6 kg.
In summary, AHS-2 findings with regard to vegetarianism do show considerable internal consistency by helping explain the well-known lower rates of cancer, cardiovascular disease, and all-cause mortality among Adventists (40); findings for these major endpoints are also consistent with the effects of these diets on many risk factors. Findings identifying certain vegetarian-related foods (e.g. red meat, nuts and seeds, dairy) that associate with disease clearly deserve further investigation.
The Tzu Chi Health Study
The Tzu Chi Health Study (TCHS) recruited 6002 participants in Taiwan (including 4625 certified Tzu Chi volunteers and 1377 non–Tzu Chi volunteers) from 2005 to 2007. Tzu Chi volunteers are Buddhist volunteers who are devoted to community service, local and international disaster relief, recycling, and various other volunteer projects initiated by the Buddhist Tzu Chi Foundation. Before becoming certified as Tzu Chi volunteers, these individuals went through ≥2 y of training and were required to quit smoking and alcohol-drinking. In addition, volunteers are encouraged to consume vegetarian diets for reasons of compassion (toward animals) and environmental conservation.
At baseline, all participants received a detailed health examination and were interviewed by trained research assistants on demographic information, medical history, lifestyle (smoking, alcohol drinking, physical activities), and diet. Diet was assessed through an FFQ with good reliability and validity among cohort participants (41). Approximately one-third of the cohort participants were vegetarians at baseline. Participants were followed up in 2 ways: 1) every 3 y, participants were invited back for a follow-up health examination; 2) baseline data were linked to the National Health Insurance Database and the National Death Registry at the Health and Welfare Data Center of Taiwan.
Besides avoidance of meat, fish, and seafood, vegetarians in this cohort also consumed more soy products, vegetables, and whole grains, but similar amount of fruits and dairy, compared with nonvegetarians (42). At baseline, vegetarians had a lower prevalence of diabetes than nonvegetarians (independent of BMI) in all men (OR: 0.49; 95% CI: 0.28, 0.89), premenopausal women (OR: 0.26; 95% CI: 0.06, 1.21), and postmenopausal women (OR: 0.25; 95% CI: 0.15, 0.42) (42) and a 21% lower prevalence of nonalcoholic fatty liver (OR: 0.79; 95% CI: 0.68, 0.91) mainly due to lower BMI (43), whereas a vegetarian diet was not associated with prevalence of ultrasound-detected gallstones (44). In the longitudinal follow-up, where diabetes incidence was identified by abnormal fasting glucose, hemoglobin A1C, and a disease questionnaire, a BMI-independent lower risk of diabetes was observed in those with a consistent vegetarian diet (HR: 0.65; 95% CI: 0.46, 0.92) and in those converting from nonvegetarian to vegetarian (HR: 0.47; 95% CI: 0.30, 0.71) (45).
Indian Migration Study and other studies of Indian vegetarians
The Indian Migration Study (IMS) is a sib-pair study of 7067 adults aged ≥20 y located in 4 regions—representing northern (Lucknow), central (Nagpur), south-central (Hyderabad), and southern (Bangalore) India. Factory workers who migrated from rural to urban areas (mean ± SD duration of migration: 20 ± 5.4 y) and their spouses, along with a 25% random sample of urban nonmigrants and their spouses, were invited to participate in the study from 2005 to 2007. Eligible migrant and nonmigrant participants identified a sibling matched by gender and similar in age in a rural or urban area, representing a total of 18 states across India. Of 7594 eligible adults, 7102 (94%) completed a clinical examination, anthropometric measurements, fasting blood sample collection, and an interviewer-administered questionnaire, which included information on sociodemographic factors, lifestyle factors, and medical history (46). Diet was assessed using a validated interviewer-administered semiquantitative FFQ on 184 commonly consumed food items across 4 major regions and 18 states (47).
The prevalence of vegetarians (defined as no meat or fish or eggs or poultry) in IMS (32.8%) in 2005–2007 (48) was slightly higher than the national prevalence based on the National Family Health Survey-3 (NFHS-3) in 2005–2006 (29.0%) (49), a nationally representative cross-sectional repeated survey historically focused on maternal and child health issues, representing 29 states, >100,000 households, and nearly 200,000 men and women aged 15–49 y. Table 1 describes the IMS and NFHS-3.
TABLE 1.
Description of reviewed studies of Indian vegetarians
| Study, study design | Population | Location | Dietary data |
|---|---|---|---|
| The Indian Migration Study, 2006— Sib-pair study in industrial populations of 4 Indian cities |
Participants from 4 geographical regions and 18 states across India: urban migrants, their spouses, and their rural-dwelling siblings; urban nonmigrants, their spouses, and their urban-dwelling siblings | Lucknow, Nagpur, Hyderabad, and Bangalore (n = 7067, mean age 40.8 y) | Validated interviewer-administered, 184-item semiquantitative FFQ |
| National Family Health Survey, 2005–2006— Survey on the lines of the Demographic and Health Surveys |
Representative nationwide sample of participants across 29 states | Nationwide sample across 29 states: n = 124,385 women aged 15–49 y and 74,369 men aged 15–54 y residing in 109,041 households with 99% of the country's representative population | Diet and health information at individual level gathered by face-to-face interviews conducted in the respondents’ homes |
In both the IMS and NFHS-3, vegetarians had a higher standard of living and were less likely to smoke and drink alcohol. In the IMS, lacto-vegetarians (32.8%) did not differ from nonvegetarians with respect to age and use of smokeless tobacco. In the IMS, vegetarians were less likely to be physically active, whereas in NFHS-3, there was no clear pattern in frequency of TV watching between the groups (49).
In the IMS, vegetarians consumed greater amounts of legumes, vegetables, roots and tubers, dairy, and sugar, whereas nonvegetarians had a greater intake of cereals, fruits, spices, salt, fats, and oils. In multivariate analyses adjusting for sociodemographic variables, total energy, and sib-pair, vegetarians consumed more carbohydrates, vitamin C, and folate and lower amounts of fat, protein, vitamin B-12, and zinc (48). However, RDA comparisons indicated that a greater proportion of vegetarians consumed adequate amounts of protein and micronutrients (iron, calcium, vitamin C, and folate) and also consumed less total energy than nonvegetarians in different regions and locations. Overall, Indian vegetarian diets were found to be adequate to sustain nutritional demands according to RDAs, with less fat. Lower vitamin B-12 (β: −1.4 μg/d; 95% CI: −1.2, −1.5 μg/d; P < 0.0001) bioavailability remains a concern and requires exploration of acceptable dietary sources for vegetarians (48).
A principal components analysis in IMS revealed 3 main patterns, with an “animal-food” pattern (red meat, poultry, fish/seafood, eggs) associated with higher levels of obesity and central obesity (50). In multivariate analyses, a vegetarian diet was inversely associated with cardiovascular disease risk factors; vegetarians had lower concentrations of total cholesterol (β: −0.1 mmol/L; 95% CI: −0.03, −0.2 mmol/L; P = 0.006), TGs (β: −0.05 mmol/L; 95% CI: −0.007, −0.1 mmol/L; P = 0.02), LDL cholesterol (β: −0.06 mmol/L; 95% CI: −0.005, −0.1 mmol/L; P = 0.03), and fasting blood glucose (β: −0.07 mmol/L; 95% CI: −0.2, 0.01 mmol/L; P = 0.09), and lower systolic blood pressure (β: −0.9 mm Hg; 95% CI: −1.9, 0.08 mm Hg; P = 0.07) and diastolic blood pressure (β: −0.7 mm Hg; 95% CI: −1.2, −0.07 mm Hg; P = 0.02) when compared with nonvegetarians (51), although the prevalence of diabetes and hypertension was not significantly different. When evaluating dietary patterns, a high intake of the “animal food” pattern was also positively associated with concentrations of total cholesterol, LDL cholesterol, HDL cholesterol, and fasting blood glucose, and higher systolic blood pressure and diastolic blood pressure (52). And the NFHS-3 data suggested a positive association between prevalent diabetes and a nonvegetarian diet (49).
Discussion
Comparison of the cohorts
Table 2 provides a summary comparison of the cohorts. EPIC-Oxford had earlier enrollment and thus has had longer follow-up than AHS-2, with the Tzu Chi and IMS studies overlapping with the end of AHS-2 enrollment. AHS-2 is the largest study, and along with EPIC-Oxford are an order of magnitude larger than the 2 Asian cohorts. Women predominate in all but the IMS. The percentage of vegetarians is similar across the studies, depending on the definition used, but the proportions of different vegetarian diets (e.g., vegan compared with lacto or lacto-ovo) vary. Smoking is much lower in the AHS-2 and Tzu Chi populations, owing to religious proscription in these communities. Alcohol use is also low in these populations and relatively low in the IMS, but much more common in EPIC-Oxford.
TABLE 2.
Comparison of 4 epidemiologic cohort studies of vegetarians1
| Adventist Health Study–2 | EPIC-Oxford Study | Tzu Chi Health Study | Indian Migration Study | |
|---|---|---|---|---|
| Recruitment period | 2001–2007 | 1993–1999 | 2005–2007 | 2005–2007 |
| Country | United States and Canada | United Kingdom | Taiwan | India |
| Participants, n | 96,469 | 65,000 | 6002 | 6555 |
| Female, % | 65 | 78 | 63 | 42 |
| Vegetarians, % | 36 | 33 | 30 | 33 |
| Smokers, % | ||||
| Vegetarian | ∼0.1 | ∼10 | 0.03 | 7.5 |
| Nonvegetarian | ∼2 | ∼12 | 4.47 | 11.8 |
| Alcohol consumers, % | ||||
| Vegetarian | ∼2 | ∼79 | 0.25 | 5.7 |
| Nonvegetarian | ∼10 | ∼85 | 6.33 | 21.3 |
1EPIC-Oxford, European Prospective Investigation into Cancer and Nutrition–Oxford.
All of the studies have provided nutrient profiles of vegetarian diets compared with nonvegetarians, have assessed cardiometabolic risk factors, and have reported associations with prevalent conditions such as obesity, diabetes, and hypertension. Given their larger size and longer follow-up, only AHS-2 and EPIC-Oxford have reported prospective results for mortality and cancer incidence at this time. We next discuss the consistency of the findings of these 2 large cohort studies of vegetarians, and then discuss further important distinctions relating to the Asian cohorts.
Consistency of results from AHS-2 and EPIC-Oxford
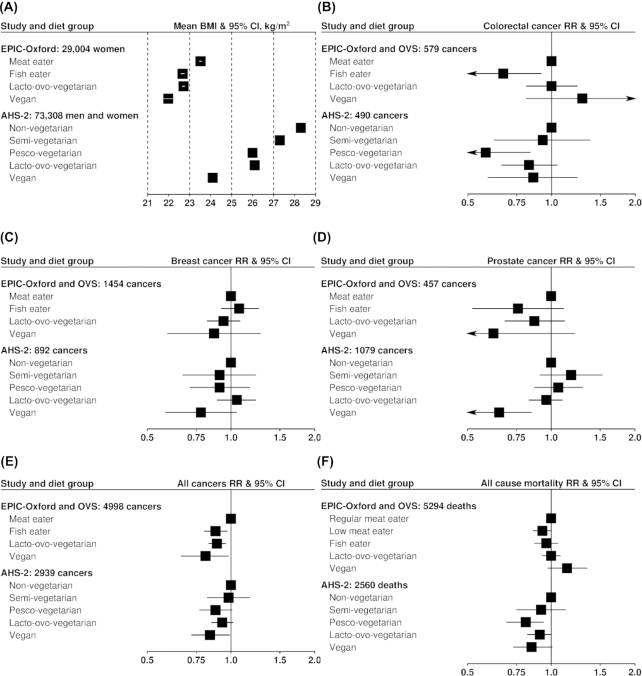
Many similarities but also some differences appear to be present when comparing the 2 main published contemporary sources of data regarding vegetarianism, risk of cancer, and overall mortality, namely AHS-2 (United States) and EPIC-Oxford (United Kingdom). In general, AHS-2 shows significant advantages in incidence of major cancers (colorectal, prostate, breast) and overall mortality for the vegetarians, whereas EPIC-Oxford does not find clearly supportive evidence. However, it seems that in some cases these differences are readily compatible with chance. Figure 1 provides a visual comparison of EPIC-Oxford and AHS-2 results for BMI, several cancer outcomes, and all-cause mortality.
FIGURE 1.
Comparisons of selected findings from Oxford cohorts and AHS-2. (A) BMI: EPIC-Oxford from (10); AHS-2 from (27). Note: no 95% CIs available for AHS-2. (B) Colorectal cancer: EPIC-Oxford and OVS from (17); AHS-2 from (29). (C) Breast cancer: EPIC-Oxford and OVS from (17); AHS-2 from (32). (D) Prostate cancer: EPIC-Oxford and OVS from (17); AHS-2 from (31). (E) All cancers: EPIC-Oxford and OVS from (17); AHS-2 from (28). (F) All-cause mortality: EPIC-Oxford and OVS from (16); AHS-2 from (27). AHS-2, Adventist Health Study—2; EPIC-Oxford, European Prospective Investigation into Cancer and Nutrition—Oxford; OVS, Oxford Vegetarian Study.
There is a similar BMI association, declining from nonvegetarians to vegans, with other groups intermediate; however, the decline is steeper in AHS-2, where BMI is higher overall.
For colorectal cancer, the findings appear compatible. AHS-2 has reported lower colorectal cancer for vegetarians than for nonvegetarians (29), whereas EPIC-Oxford has not. However, in AHS-2, the vegetarian group included pesco- and semi-vegetarians, and pesco-vegetarians partially drove the association. Both AHS-2 and EPIC-Oxford show significantly lower incident colorectal cancer for pesco-vegetarians (i.e., fish-eaters) than for nonvegetarians (i.e., non–meat-eaters).
For both breast cancer and prostate cancer incidence, both studies suggest possibly lower risk for vegans; but only in AHS-2 do these findings achieve or approach significance, likely because of the larger number of vegans in that study.
For all incident cancers combined, fish-eaters, lacto-ovo-vegetarians, and vegans appear to have lower overall rates than nonvegetarians, with the lowest point-estimates of risk being for vegans in both studies.
The results for all-cause mortality appear less compatible, with associations in EPIC-Oxford more null, but tending towards lower mortality for pesco-vegetarians, lacto-ovo-vegetarians, and vegans in AHS-2. In AHS-2, where these findings tend to be significant, the effect sizes for mortality are modest. However, relatively small differences in total mortality do translate to 1–2 y of extra life (53) that is probably of relatively good quality (54). Cancer mortality does not appear to differ by diet group in EPIC-Oxford (16) or in AHS-2 (27).
Several factors may help to explain the results which are divergent. First, the definition of a vegetarian is sufficiently nonspecific to accommodate a wide variety of diets under the same label. Both the US and UK studies have tried to minimize this by dividing vegetarians into subtypes as mentioned already. Despite this, it appears that British and US Adventist vegetarians, even within the same vegetarian category, do not eat the same foods or in the same proportions, aside from the absence or near absence of animal products. AHS-2 vegetarians, in addition to reduced intakes of meat, dairy, and eggs, also eat lower amounts of snack foods, sweets, refined grains, solid fats, and nonwater beverages than nonvegetarians, and eat higher amounts of fruits, vegetables, nuts and seeds, legumes and plant proteins, and whole grains (55). Vegetarians in EPIC-Oxford also report higher intakes of fruit and vegetables than nonvegetarians (3); relative intakes of other foods such as sweets have not been published from EPIC-Oxford, but in a similar population in UK-Biobank, vegetarians consumed more fruits, vegetables, legumes, nuts, and wholemeal cereals than regular meat-eaters, but similar amounts of refined cereals, desserts, sweets, and chocolate (56). This suggests that the diet of vegetarians in the UK may have a somewhat less healthy profile than that of vegetarians in AHS-2, at least in relation to consumption of refined cereals and sweets. This possible difference needs to be examined more carefully, but it might relate to different motivations for the dietary choices. One can speculate that Adventists’ health and religious motivations may lead to a particularly strong commitment to their diets and will often motivate choices of healthful foods. British vegetarians, possibly motivated more by ethical or environmental concerns, and also often highly committed vegetarians, might be somewhat less nutritionally informed or less inclined to choose healthful foods, beyond the avoidance of meat. AHS-2 subjects on average (26, 57) have subscribed to their current dietary pattern for many years, often lifelong, whereas the stability of vegetarian diets may be somewhat lower in EPIC-Oxford (3).
Differences in the mortality findings between EPIC-Oxford and AHS-2 therefore could be due to differences in dietary choices between the vegetarians in these studies, beyond meat avoidance. If so, it would suggest that mortality advantages of vegetarian diets may depend on a substitution of whole plant foods (rather than refined foods) for meat. This points to the limitations of defining diets as vegetarian only based on meat avoidance and the need for more emphasis on healthy plant-based diets, such as others have described (58, 59).
Confounding is another possible explanation for the divergent mortality results. In particular, the larger BMI gradient across dietary patterns in AHS-2 than in EPIC-Oxford suggests a possibility for greater confounding by adiposity. However, BMI adjustment made little difference to the mortality results in AHS-2 (27). That said, BMI is an imperfect measure of adiposity, including visceral adiposity, so residual confounding by adiposity is possible. Still, to the extent that the adiposity difference between diets is caused by the dietary patterns, which is very plausible, adjustment for adiposity would be adjusting for a causal intermediate and only isolating adiposity-independent mechanisms.
Another factor affecting both studies, but perhaps AHS-2 more so, is that the nonvegetarian comparison group is less different in their dietary choices than may be suggested by the label. The AHS-2 nonvegetarians have lower meat intakes than the general population. This may limit the ability to detect significant health associations or to fully test the potential effects of the vegetarian diets compared with more typical nonvegetarian diets in the population.
Considerations regarding studies of vegetarians in Asia
Vegetarian diets in Asia
Vegetarianism in Asia has traditionally been affiliated with religions including but not limited to Buddhism, Hinduism, Jainism, and I-Kuan Tao. These religions encourage avoidance of meat out of the concept of Ahimsa, or “nonviolence,” and vegetarianism is considered an act of compassion and believed to be beneficial for spiritual cultivation. Besides meat, many Buddhist vegetarians also avoid allium vegetables, such as garlic and onions. More recently, vegetarian movements in Asia, as in the rest of the world, may be motivated by health and environmental concerns. In East and Southeast Asia, vegetarians tend to replace meat and seafood with soy products, such as tofu, yuba, edamame, nato, soy milk, tempeh, miso, and meat analogues made of soy or gluten (seitan). Foods fortified with vitamin B-12, vitamin D, EPA, and DHA are less common than in North America or Europe. Consumption of dairy products among East Asians is generally much lower than in North Americans, and TCHS participants consume only about one-third as much dairy as those in AHS-2 (42, 55). Vegetarians had lower intake of saturated fat than nonvegetarians in EPIC-Oxford, AHS-2, and TCHS, but not in IMS (1, 42, 48, 60). Dietary habits of Asians may also be influenced by traditional medicine, such as Traditional Chinese Medicine or Ayurveda. For example, different herbal ingredients from Traditional Chinese Medicine may be added to cuisine according to the season, illness, or one's personal needs.
Vegetarian diets in India
In India a substantial proportion of the population are vegetarians, varying between 10% and 62% according to the region (61), in contrast to small proportions in the West (<5%) (62, 63). Vegetarianism in India is driven by faith, culture, or community, is generally lifelong, and is associated with unique spices, seasonings, and cooking patterns. With a higher prevalence of vegetarianism and lower or different propensity for confounding by behaviors such as physical activity or tobacco use, India offers an opportunity for a more robust evaluation of vegetarian diets and disease outcomes.
India is undergoing an epidemiologic and nutritional transition (64), similar to that affecting many developing countries and regions (65), including China (66), Latin America and the Caribbean (67), and North Africa and the Middle East (68). Related to this nutritional transition, the prevalence of vegetarian diets is decreasing in India [∼10% in the past 10 y based on nationally representative surveys 3 and 4 (61, 69)]. There is stark heterogeneity across the country in diet composition, preparation, and spices and seasonings (e.g., only 29% of variance was explained from 3 IMS dietary patterns). The influence of these epidemiologic and nutritional transitions on the health effects of vegetarian diets is unclear, particularly with mixed health associations for health outcomes such as obesity. There are limited nutritional epidemiologic data with respect to variations in India's vegetarian diets (e.g., in certain regions such as Goa and certain vegetarian groups such as Jains and Buddhists) and a lack of evidence regarding possible undesirable influences of vegetarian diets (e.g., vitamin B-12 deficiency).
Animal food consumption among nonvegetarians in India is low and vegetarianism in India is a blend of healthy and unhealthy dietary practices. In the IMS, a principal components analysis–derived “animal-food” pattern yielded positive associations with central and overall obesity, although bivariate analyses comparing lacto-ovo-vegetarians and nonvegetarians did not yield significant differences in BMI in the same study population. Indian vegetarian dietary patterns may not be consistently healthier. For example, “cereals-savoury foods” in the IMS showed positive loadings for nuts and whole grains but also had positive loadings for refined grains and negative loadings for vegetables; the “fruit-veg-sweets-snacks” pattern showed positive loadings for fruits and vegetables as well as for snacks and sugar. In NFHS-3, lacto-ovo- (21.0) and lacto-vegetarians (21.2)—the latter representing the largest type of vegetarian pattern and one-fourth of India's population—had higher BMI levels than nonvegetarians (20.7).
Vegetarian patterns adopted in some other countries such as the United States, however, reveal healthier outcomes (70). For example, among American adults in the NHANES, vegetarians had significantly lower levels of overweight or obesity and central adiposity than nonvegetarians (70), whereas the same did not hold true in India. South Asian vegetarians (India and Pakistan) were more likely to consume more dairy, fried foods, and desserts (70). The difference between vegetarians and nonvegetarians in the United States also yielded significantly lower cardiovascular disease risk scores, whereas this was not true among the same comparison groups in South Asia (70). Vegetarians had nonsignificant positive associations with diabetes in India whereas vegetarians had nonsignificant inverse associations in the United States (70).
As with studies in the West, vegetarian dietary habits in India are potentially confounded to some degree by socioeconomic status and risk factors such as smoking and alcohol, although reverse patterns are observed for physical activity. An important distinction in Indian analyses is the comparison group, as nonvegetarians tend to have low amounts of meat consumption compared with the West and still eat high amounts of fruits and vegetables. In addition to the mixed evidence on healthy eating among vegetarians, and the confounding by demographic and risk factor variables, one must also consider the lower potential harm of substitution effects in Indian diets given the relatively low amount of meat consumption in nonvegetarians.
Meat intake and health outcomes in Asians compared with Westerners
Highly relevant to a discussion of vegetarian diets in Asian populations are findings on the associations of meat and animal protein with total mortality, cardiovascular diseases, and diabetes, which appear to differ between Asian studies and Western studies. In North American and European cohorts, red meat and processed meat have generally been associated with higher mortality (71–73). In American nurses and health professionals with ≥1 unhealthy lifestyle habit or risk factor, plant protein was beneficially, whereas animal protein was harmfully, associated with all-cause and cardiovascular mortality (74). On the other hand, in a pooled analysis of 8 cohorts across Asia (Asia Cohort Consortium), total meat was not associated with all-cause, cancer, or cardiovascular disease mortality, whereas red meat was associated with lower cardiovascular mortality (in men) and cancer mortality (in women) (75).
Meat and animal protein, particularly red meat and processed meat, have been associated with increased risk of diabetes in most cohort studies (76). However, in the Shanghai Women's Health Study, total meat was associated with a lower risk of diabetes, and surprisingly, red meat was even inversely associated with diabetes risk among those with BMI <25 (77). Nevertheless, positive associations between meat-rich patterns and diabetes have also been reported in some Asian ethnic populations, including the Singapore Chinese Health Study (78) and Japanese Americans of the Multiethnic Cohort (79). The effect of fish on diabetes risk also appears to be modified by geographical location, where a harmful association has been found in Americans, a null association in Europeans, and a protective association in Asians (80).
Several reasons may help to explain the inconsistencies. 1) Human diets typically contain a wide range of foods, and independent effects of individual foods may be difficult to single out. In Western populations, red meat intake tends to be correlated with intakes of refined carbohydrates, sugar-sweetened beverages, and high-fat dairy, together categorized as the Western dietary pattern (81, 82). However, in Asian populations, those consuming more meat or fish may also be consuming more healthful plant-based foods: in the Shanghai Women's Health Study, the dietary pattern cluster with the lowest total meat and red meat intake also had the lowest intakes of vegetables and fruits and highest intake of staple foods (likely refined carbohydrates), and participants in this cluster had the lowest socioeconomic status (83). In the Japanese Public Health Center-Based Prospective Study, higher fish intake was correlated with higher intakes of vegetables, fruit, soy, potatoes, seaweed, and mushrooms (84). 2) Meat and red meat consumption in some Asian countries may be an indicator for unmeasured socioeconomic factors, which may simultaneously suggest food security and better access to medical and preventive care. 3) Birth cohort effects may potentially play a role. Several Asian cohorts comprise populations that have lived through periods of conflict and famine in early life, which may lead to epigenetic alteration of metabolic risk (85). 4) The amount of red meat, particularly beef, consumed by Asians is typically much lower than that consumed by Americans, so potential risks may be more difficult to detect (75). 5) In developing economies, competing risk from infectious diseases may precede the development of chronic degenerative diseases. 6) Most chronic degenerative diseases are complex and multifactorial, and the diet–disease relation may be modified by genetics or other lifestyle factors. Previous studies have shown that the association between Western or meat-rich dietary patterns and diabetes is modified by genetics in Americans (86), and by smoking status in Singaporean Chinese (78). 7) Etiology for some diseases, such as diabetes, may potentially vary across ethnicities. For example, East Asian diabetics tend to be characterized by lower BMI (compared to the BMI of diabetics in Western countries) and low capacity to secrete insulin (87, 88). Japanese individuals with normal glucose tolerance were found to have a similar insulinogenic index as Caucasian diabetes patients (88). A recent study also confirmed that β-cell dysfunction contributes to a higher population attributable risk (than insulin resistance) for type 2 diabetes in a Korean population-based cohort (89).
Despite some inconsistencies in the relation of meat and fish to health outcomes, most prospective studies of Asians do support beneficial associations of plant-based foods such as soy, legumes, vegetables, and fruits, or of dietary patterns consisting largely of these foods (78, 90–92).
Importance of epidemiologic studies of vegetarians in Asia
The addition of Asian cohorts of vegetarians has great public health significance, because Asia has a long history of vegetarian tradition and culture, and is a continent where both the population and chronic degenerative disease incidences are on the rise. Vegetarian diets and many popular vegetarian foods, such as tofu, miso, and tempeh, have been consumed for hundreds, if not thousands of years in some Asian traditions, and there may be potential opportunities to study multigenerational effects of vegetarian diets and associated foods. These foods are typically consumed in greater amounts by Asians than by Westerners, and the inclusion of Asians provides a wider range of dietary variation to enable studies of dietary components and health outcomes. This may be particularly useful for diseases that are more prevalent among Asians such as diabetes and stroke (particularly hemorrhagic stroke). In addition, many Asian countries are undergoing economic and nutritional transitions; epidemiologic data from Asia may thus offer the opportunity to dynamically study vegetarian diets and their health associations during this period of nutritional transition. The inclusion of Asian cohorts also allows an opportunity to test the generalizability of previous findings for vegetarian diets in Western populations.
Conclusions
Epidemiologic cohorts with a large percentage of vegetarian subjects have contributed greatly to our understanding of both the nutritional adequacy and the health outcomes associated with these dietary patterns. The evidence from these studies has supported vegetarian diets as healthy dietary patterns associated with a reduction in several common disease risk factors and reduced risk of some chronic diseases of public health importance.
Much of this evidence comes from EPIC-Oxford and AHS-2, and their predecessor studies. These large prospective studies continue to contribute to our understanding of the health effects of vegetarian diets, as the results reviewed here indicate. Some findings from these studies have seemed to conflict; however, as discussed here, many of the findings for cancer outcomes appear consistent or at least compatible. Findings for all-cause mortality continue to differ, which may highlight the limitations of simple vegetarian categories in describing a healthy diet that might affect longevity.
Vegetarian diets, consistent with their simple definitions, that is, the absence of selected or all animal foods, allow great variations in the choices of other foods and their modes of preparation. It will be a valuable contribution to further refine our understanding of the range of “healthy” vegetarian diets. One cannot assume that simply avoiding animal foods will necessarily produce such a healthy diet.
There is a discrepancy between where vegetarian diets have mostly been studied and where they are most practiced. It is vitally important to better understand the health effects of vegetarian dietary patterns in both South and East Asia, and also in other parts of the world. The studies reviewed here begin to address this discrepancy and highlight important differences in the food consumption patterns and nutritional profiles of vegetarians in different regions, which may in turn lead to differences in associations with health outcomes.
Collectively these cohorts highlight the great diversity of vegetarian dietary patterns around the world. Studying these long-practiced real-world dietary patterns in different populations remains a high priority for nutritional science, chronic disease epidemiology, and public health.
Acknowledgments
We thank Dorairaj Prabhakaran (Public Health Foundation of India and Centre for Chronic Disease Control, Gurgaon, India); KM Venkat Narayan and Mohammed K Ali (Emory Global Diabetes Research Center, Rollins School of Public Health, Emory University), Nikhil Tandon (Department of Endocrinology and Metabolism, All India Institute of Medical Sciences, New Delhi, India); Viswanathan Mohan (Madras Diabetes Research Foundation, Chennai, India); and K Srinath Reddy (Public Health Foundation of India, New Delhi, India). The authors’ contributions were as follows—MJO, THTC, PKD, TJK, and GEF: drafted the manuscript; and all authors: critically revised the manuscript for important intellectual content and read and approved the final manuscript.
Notes
Published in a supplement to Advances in Nutrition. This supplement was sponsored by the Harding-Buller Foundation of Ohio. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the sponsors. Publication costs for this supplement were defrayed in part by the payment of page charges. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
Research on the health of vegetarians in EPIC-Oxford is supported by UK Medical Research Council grant MR/M012190/1 and the Wellcome Trust (Livestock, Environment and People: 205212/Z/16/Z). The Adventist Health Study—2 project support was obtained from National Cancer Institute grant 1U01CA152939 (to GEF) and World Cancer Research Fund grant 2009/93 (to GEF). The Tzu Chi Health Study was funded by Buddhist Dalin Tzu Chi General Hospital grant TCRD—I9605-02 for baseline data collection, and Buddhist Tzu Chi Medical Foundation grants TCMMPSP104-08-02, TCMMP105-13-05, and TCMMP106-04-01 for follow-ups. Indian Migration Study research was funded by Wellcome Trust project grant GR070797MF.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: AHS-2, Adventist Health Study–2; EPIC-Oxford, European Prospective Investigation into Cancer and Nutrition–Oxford; IMS, Indian Migration Study; NFHS-3, National Family Health Survey–3; TCHS, Tzu Chi Health Study.
References
- 1. Davey GK, Spencer EA, Appleby PN, Allen NE, Knox KH, Key TJ. EPIC-Oxford: lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31 546 non meat-eaters in the UK. Public Health Nutr. 2003;6(3):259–69. [DOI] [PubMed] [Google Scholar]
- 2. Appleby PN, Thorogood M, Mann JI, Key TJ. The Oxford Vegetarian Study: an overview. Am J Clin Nutr. 1999;70(3 Suppl):525S–31S. [DOI] [PubMed] [Google Scholar]
- 3. Key TJ, Appleby PN, Spencer EA, Travis RC, Roddam AW, Allen NE. Mortality in British vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am J Clin Nutr. 2009;89(5):1613S–19S. [DOI] [PubMed] [Google Scholar]
- 4. Rosell MS, Lloyd-Wright Z, Appleby PN, Sanders TA, Allen NE, Key TJ. Long-chain n-3 polyunsaturated fatty acids in plasma in British meat-eating, vegetarian, and vegan men. Am J Clin Nutr. 2005;82(2):327–34. [DOI] [PubMed] [Google Scholar]
- 5. Gilsing AM, Crowe FL, Lloyd-Wright Z, Sanders TA, Appleby PN, Allen NE, Key TJ. Serum concentrations of vitamin B12 and folate in British male omnivores, vegetarians and vegans: results from a cross-sectional analysis of the EPIC-Oxford cohort study. Eur J Clin Nutr. 2010;64(9):933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crowe FL, Steur M, Allen NE, Appleby PN, Travis RC, Key TJ. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: results from the EPIC-Oxford study. Public Health Nutr. 2011;14(2):340–6. [DOI] [PubMed] [Google Scholar]
- 7. Schmidt JA, Rinaldi S, Scalbert A, Ferrari P, Achaintre D, Gunter MJ, Appleby PN, Key TJ, Travis RC. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: a cross-sectional analysis in the EPIC-Oxford cohort. Eur J Clin Nutr. 2016;70(3):306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sobiecki JG, Appleby PN, Bradbury KE, Key TJ. High compliance with dietary recommendations in a cohort of meat eaters, fish eaters, vegetarians, and vegans: results from the European Prospective Investigation into Cancer and Nutrition-Oxford study. Nutr Res. 2016;36(5):464–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tong TYN, Appleby PN, Bradbury KE, Key TJ. Cross-sectional analyses of participation in cancer screening and use of hormone replacement therapy and medications in meat eaters and vegetarians: the EPIC-Oxford study. BMJ Open. 2017;7(12):e018245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spencer EA, Appleby PN, Davey GK, Key TJ. Diet and body mass index in 38000 EPIC-Oxford meat-eaters, fish-eaters, vegetarians and vegans. Int J Obes Relat Metab Disord. 2003;27(6):728–34. [DOI] [PubMed] [Google Scholar]
- 11. Key TJ, Davey G. Prevalence of obesity is low in people who do not eat meat. BMJ. 1996;313(7060):816–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosell M, Appleby PN, Spencer E, Key TJ. Weight gain over 5 years in 21 966 meat-eating, fish-eating, vegetarian, and vegan men and women in EPIC-Oxford. Int J Obes (Lond). 2006;30(9):1389–96. [DOI] [PubMed] [Google Scholar]
- 13. Bradbury KE, Crowe FL, Appleby PN, Schmidt JA, Travis RC, Key TJ. Serum concentrations of cholesterol, apolipoprotein A-I and apolipoprotein B in a total of 1694 meat-eaters, fish-eaters, vegetarians and vegans. Eur J Clin Nutr. 2013;68(2):178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Appleby PN, Davey GK, Key TJ. Hypertension and blood pressure among meat eaters, fish eaters, vegetarians and vegans in EPIC-Oxford. Public Health Nutr. 2002;5(5):645–54. [DOI] [PubMed] [Google Scholar]
- 15. Crowe FL, Appleby PN, Travis RC, Key TJ. Risk of hospitalization or death from ischemic heart disease among British vegetarians and nonvegetarians: results from the EPIC-Oxford cohort study. Am J Clin Nutr. 2013;97(3):597–603. [DOI] [PubMed] [Google Scholar]
- 16. Appleby PN, Crowe FL, Bradbury KE, Travis RC, Key TJ. Mortality in vegetarians and comparable nonvegetarians in the United Kingdom. Am J Clin Nutr. 2016;103(1):218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Key TJ, Appleby PN, Crowe FL, Bradbury KE, Schmidt JA, Travis RC. Cancer in British vegetarians: updated analyses of 4998 incident cancers in a cohort of 32,491 meat eaters, 8612 fish eaters, 18,298 vegetarians, and 2246 vegans. Am J Clin Nutr. 2014;100(Suppl 1):378S–85S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turney BW, Appleby PN, Reynard JM, Noble JG, Key TJ, Allen NE. Diet and risk of kidney stones in the Oxford cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Epidemiol. 2014;29(5):363–9. [DOI] [PubMed] [Google Scholar]
- 19. Appleby PN, Allen NE, Key TJ. Diet, vegetarianism, and cataract risk. Am J Clin Nutr. 2011;93(5):1128–35. [DOI] [PubMed] [Google Scholar]
- 20. Crowe FL, Appleby PN, Allen NE, Key TJ. Diet and risk of diverticular disease in Oxford cohort of European Prospective Investigation into Cancer and Nutrition (EPIC): prospective study of British vegetarians and non-vegetarians. BMJ. 2011;343:d4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McConnell TJ, Appleby PN, Key TJ. Vegetarian diet as a risk factor for symptomatic gallstone disease. Eur J Clin Nutr. 2017;71(6):731–5. [DOI] [PubMed] [Google Scholar]
- 22. Butler TL, Fraser GE, Beeson WL, Knutsen SF, Herring RP, Chan J, Sabate J, Montgomery S, Haddad E, Preston-Martin S et al.. Cohort profile: the Adventist Health Study-2 (AHS-2). Int J Epidemiol. 2008;37(2):260–5. [DOI] [PubMed] [Google Scholar]
- 23. Jaceldo-Siegl K, Fan J, Sabate J, Knutsen SF, Haddad E, Beeson WL, Herring RP, Butler TL, Bennett H, Fraser GE. Race-specific validation of food intake obtained from a comprehensive FFQ: the Adventist Health Study-2. Public Health Nutr. 2011;14(11):1988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jaceldo-Siegl K, Knutsen SF, Sabate J, Beeson WL, Chan J, Herring RP, Butler TL, Haddad E, Bennett H, Montgomery S et al.. Validation of nutrient intake using an FFQ and repeated 24 h recalls in black and white subjects of the Adventist Health Study-2 (AHS-2). Public Health Nutr. 2010;13(6):812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fraser GE, Jaceldo-Siegl K, Henning SM, Fan J, Knutsen SF, Haddad EH, Sabate J, Beeson WL, Bennett H. Biomarkers of dietary intake are correlated with corresponding measures from repeated dietary recalls and food-frequency questionnaires in the Adventist Health Study-2. J Nutr. 2016;146(3):586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martins MCT, Jaceldo-Siegl K, Orlich M, Fan J, Mashchak A, Fraser GE. A new approach to assess lifetime dietary patterns finds lower consumption of animal foods with aging in a longitudinal analysis of a health-oriented Adventist population. Nutrients. 2017;9(10):1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Orlich MJ, Singh PN, Sabate J, Jaceldo-Siegl K, Fan J, Knutsen S, Beeson WL, Fraser GE. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern Med. 2013;173(13):1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tantamango-Bartley Y, Fraser GE, Jaceldo-Siegl K, Fan J. Vegetarian diets and the incidence of cancer in a low-risk population. Cancer Epidemiol Biomarkers Prev. 2013;22(2):286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Orlich MJ, Singh PN, Sabate J, Fan J, Sveen L, Bennett H, Knutsen SF, Beeson WL, Jaceldo-Siegl K, Butler TL et al.. Vegetarian dietary patterns and the risk of colorectal cancers. JAMA Intern Med. 2015;175(5):767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tantamango-Bartley Y, Knutsen SF, Jaceldo-Siegl K, Fan J, Mashchak A, Fraser GE. Independent associations of dairy and calcium intakes with colorectal cancers in the Adventist Health Study-2 cohort. Public Health Nutr. 2017;20(14):2577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tantamango-Bartley Y, Knutsen SF, Knutsen R, Jacobsen BK, Fan J, Beeson WL, Sabate J, Hadley D, Jaceldo-Siegl K, Penniecook J et al.. Are strict vegetarians protected against prostate cancer?. Am J Clin Nutr. 2016;103(1):153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Penniecook-Sawyers JA, Jaceldo-Siegl K, Fan J, Beeson L, Knutsen S, Herring P, Fraser GE. Vegetarian dietary patterns and the risk of breast cancer in a low-risk population. Br J Nutr. 2016;115(10):1790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tharrey M, Mariotti F, Mashchak A, Barbillon P, Delattre M, Fraser GE. Patterns of plant and animal protein intake are strongly associated with cardiovascular mortality: the Adventist Health Study-2 cohort. Int J Epidemiol. 2018;47(5):1603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fraser GE. A comparison of first event coronary heart disease rates in two contrasting California populations. J Nutr Health Aging. 2005;9(1):53–8. [PubMed] [Google Scholar]
- 35. Pettersen BJ, Anousheh R, Fan J, Jaceldo-Siegl K, Fraser GE. Vegetarian diets and blood pressure among white subjects: results from the Adventist Health Study-2 (AHS-2). Public Health Nutr. 2012;15(10):1909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tonstad S, Stewart K, Oda K, Batech M, Herring RP, Fraser GE. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr Metab Cardiovasc Dis. 2013;23(4):292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jaceldo-Siegl K, Haddad E, Knutsen S, Fan J, Lloren J, Bellinger D, Fraser GE. Lower C-reactive protein and IL-6 associated with vegetarian diets are mediated by BMI. Nutr Metab Cardiovasc Dis. 2018;28(8):787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fraser GE, Katuli S, Anousheh R, Knutsen SF, Herring P, Fan J. Vegetarian diets and cardiovascular risk factors in black members of the Adventist Health Study-2. Public Health Nutr. 2015;18(3):537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kahleova H, Lloren JI, Mashchak A, Hill M, Fraser GE. Meal frequency and timing are associated with changes in body mass index in Adventist Health Study 2. J Nutr. 2017;147(9):1722–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fraser GE. Associations between diet and cancer, ischemic heart disease, and all-cause mortality in non-Hispanic white California Seventh-day Adventists. Am J Clin Nutr. 1999;70(3 Suppl):532S–8S. [DOI] [PubMed] [Google Scholar]
- 41. Chiu TH, Huang HY, Chen KJ, Wu YR, Chiu JP, Li YH, Chiu BC, Lin CL, Lin MN. Relative validity and reproducibility of a quantitative FFQ for assessing nutrient intakes of vegetarians in Taiwan. Public Health Nutr. 2014;17(7):1459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chiu TH, Huang HY, Chiu YF, Pan WH, Kao HY, Chiu JP, Lin MN, Lin CL. Taiwanese vegetarians and omnivores: dietary composition, prevalence of diabetes and IFG. PloS One. 2014;9(2):e88547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chiu TH, Lin MN, Pan WH, Chen YC, Lin CL. Vegetarian diet, food substitution, and nonalcoholic fatty liver. Tzu Chi Med J. 2018;30(2):102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen YC, Chiou C, Lin MN, Lin CL. The prevalence and risk factors for gallstone disease in Taiwanese vegetarians. PloS One. 2014;9(12):e115145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chiu TH, Pan WH, Lin MN, Lin CL. Vegetarian diet, change in dietary patterns, and diabetes risk: a prospective study. Nutr Diabetes. 2018;8(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ebrahim S, Kinra S, Bowen L, Andersen E, Ben-Shlomo Y, Lyngdoh T, Ramakrishnan L, Ahuja RC, Joshi P, Das SM et al.. The effect of rural-to-urban migration on obesity and diabetes in India: a cross-sectional study. PLoS Med. 2010;7(4):e1000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bowen L, Ebrahim S, De Stavola B, Ness A, Kinra S, Bharathi AV, Prabhakaran D, Reddy KS. Dietary intake and rural-urban migration in India: a cross-sectional study. PloS One. 2011;6(6):e14822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shridhar K, Dhillon PK, Bowen L, Kinra S, Bharathi AV, Prabhakaran D, Reddy KS, Ebrahim S. Nutritional profile of Indian vegetarian diets – the Indian Migration Study (IMS). Nutr J. 2014;13:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Agrawal S, Millett CJ, Dhillon PK, Subramanian SV, Ebrahim S. Type of vegetarian diet, obesity and diabetes in adult Indian population. Nutr J. 2014;13:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Satija A, Hu FB, Bowen L, Bharathi AV, Vaz M, Prabhakaran D, Reddy KS, Ben-Shlomo Y, Davey Smith G, Kinra S et al.. Dietary patterns in India and their association with obesity and central obesity. Public Health Nutr. 2015;18(16):3031–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shridhar K, Dhillon PK, Bowen L, Kinra S, Bharathi AV, Prabhakaran D, Reddy KS, Ebrahim S, Indian Migration Study Group . The association between a vegetarian diet and cardiovascular disease (CVD) risk factors in India: the Indian Migration Study. PloS One. 2014;9(10):e110586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shridhar K, Satija A, Dhillon PK, Agrawal S, Gupta R, Bowen L, Kinra S, Bharathi AV, Prabhakaran D, Srinath Reddy K et al.. Association between empirically derived dietary patterns with blood lipids, fasting blood glucose and blood pressure in adults - the India migration study. Nutr J. 2018;17(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fraser GE, Shavlik DJ. Ten years of life: is it a matter of choice?. Arch Intern Med. 2001;161(13):1645–52. [DOI] [PubMed] [Google Scholar]
- 54. Lee JW, Morton KR, Walters J, Bellinger DL, Butler TL, Wilson C, Walsh E, Ellison CG, McKenzie MM, Fraser GE. Cohort profile: the Biopsychosocial Religion and Health Study (BRHS). Int J Epidemiol. 2009;38(6):1470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Orlich MJ, Jaceldo-Siegl K, Sabate J, Fan J, Singh PN, Fraser GE. Patterns of food consumption among vegetarians and non-vegetarians. Br J Nutr. 2014;112(10):1644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bradbury KE, Tong TYN, Key TJ. Dietary intake of high-protein foods and other major foods in meat-eaters, poultry-eaters, fish-eaters, vegetarians, and vegans in UK Biobank. Nutrients. 2017;9(12):1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Teixeira Martins MC, Jaceldo-Siegl K, Fan J, Singh P, Fraser GE. Short- and long-term reliability of adult recall of vegetarian dietary patterns in the Adventist Health Study-2 (AHS-2). J Nutr Sci. 2015;4:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, Willett WC, Manson JE, Sun Q, Hu FB. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med. 2016;13(6):e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Satija A, Bhupathiraju SN, Spiegelman D, Chiuve SE, Manson JE, Willett W, Rexrode KM, Rimm EB, Hu FB. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. adults. J Am Coll Cardiol. 2017;70(4):411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rizzo NS, Jaceldo-Siegl K, Sabate J, Fraser GE. Nutrient profiles of vegetarian and nonvegetarian dietary patterns. J Acad Nutr Diet. 2013;113(12):1610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Arnold F, Parasuraman S, Arokiasamy P, Kothari M. Nutrition in India. National Family Health Survey (NFHS-3), India, 2005–06. Mumbai, India and Calverton, MD: International Institute for Population Sciences and Macro International; 2009. [Google Scholar]
- 62. Whitton C, Nicholson SK, Roberts C, Prynne CJ, Pot GK, Olson A, Fitt E, Cole D, Teucher B, Bates B et al.. National Diet and Nutrition Survey: UK food consumption and nutrient intakes from the first year of the rolling programme and comparisons with previous surveys. Br J Nutr. 2011;106(12):1899–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Key TJ, Appleby PN, Rosell MS. Health effects of vegetarian and vegan diets. Proc Nutr Soc. 2006;65(1):35–41. [DOI] [PubMed] [Google Scholar]
- 64. Misra A, Singhal N, Sivakumar B, Bhagat N, Jaiswal A, Khurana L. Nutrition transition in India: secular trends in dietary intake and their relationship to diet-related non-communicable diseases. J Diabetes. 2011;3(4):278–92. [DOI] [PubMed] [Google Scholar]
- 65. Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70(1):3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li M, Shi Z. Dietary pattern during 1991–2011 and its association with cardio metabolic risks in Chinese adults: the China Health and Nutrition Survey. Nutrients. 2017;9(11):1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rivera-Andrade A, Luna MA. Trends and heterogeneity of cardiovascular disease and risk factors across Latin American and Caribbean countries. Prog Cardiovasc Dis. 2014;57(3):276–85. [DOI] [PubMed] [Google Scholar]
- 68. Mehio Sibai A, Nasreddine L, Mokdad AH, Adra N, Tabet M, Hwalla N. Nutrition transition and cardiovascular disease risk factors in Middle East and North Africa countries: reviewing the evidence. Ann Nutr Metab. 2010;57(3–4):193–203. [DOI] [PubMed] [Google Scholar]
- 69. International Institute for Population Sciences (IIPS) and ICF. National Family Health Survey (NFHS-4), India, 2015–16. [Internet] Mumbai, India: International Institute for Population Sciences; 2017; [cited 9 May, 2018]. Available from: http://rchiips.org/NFHS/factsheet_NFHS-4.shtml. [Google Scholar]
- 70. Jaacks LM, Kapoor D, Singh K, Narayan KM, Ali MK, Kadir MM, Mohan V, Tandon N, Prabhakaran D. Vegetarianism and cardiometabolic disease risk factors: differences between South Asian and US adults. Nutrition. 2016;32(9):975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sinha R, Cross AJ, Graubard BI, Leitzmann MF, Schatzkin A. Meat intake and mortality: a prospective study of over half a million people. Arch Intern Med. 2009;169(6):562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. 2012;172(7):555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rohrmann S, Overvad K, Bueno-de-Mesquita HB, Jakobsen MU, Egeberg R, Tjonneland A, Nailler L, Boutron-Ruault MC, Clavel-Chapelon F, Krogh V et al.. Meat consumption and mortality – results from the European Prospective Investigation into Cancer and Nutrition. BMC Med. 2013;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Song M, Fung TT, Hu FB, Willett WC, Longo VD, Chan AT, Giovannucci EL. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern Med. 2016;176(10):1453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lee JE, McLerran DF, Rolland B, Chen Y, Grant EJ, Vedanthan R, Inoue M, Tsugane S, Gao YT, Tsuji I et al.. Meat intake and cause-specific mortality: a pooled analysis of Asian prospective cohort studies. Am J Clin Nutr. 2013;98(4):1032–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tian S, Xu Q, Jiang R, Han T, Sun C, Na L. Dietary protein consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Nutrients. 2017;9(9):982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Villegas R, Shu XO, Gao YT, Yang G, Cai H, Li H, Zheng W. The association of meat intake and the risk of type 2 diabetes may be modified by body weight. Int J Med Sci. 2006;3(4):152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Odegaard AO, Koh WP, Butler LM, Duval S, Gross MD, Yu MC, Yuan JM, Pereira MA. Dietary patterns and incident type 2 diabetes in Chinese men and women: the Singapore Chinese Health Study. Diabetes Care. 2011;34(4):880–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Erber E, Hopping BN, Grandinetti A, Park SY, Kolonel LN, Maskarinec G. Dietary patterns and risk for diabetes: the Multiethnic Cohort. Diabetes Care. 2010;33(3):532–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wallin A, Di Giuseppe D, Orsini N, Patel PS, Forouhi NG, Wolk A. Fish consumption, dietary long-chain n-3 fatty acids, and risk of type 2 diabetes: systematic review and meta-analysis of prospective studies. Diabetes Care. 2012;35(4):918–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fung TT, Stampfer MJ, Manson JE, Rexrode KM, Willett WC, Hu FB. Prospective study of major dietary patterns and stroke risk in women. Stroke. 2004;35(9):2014–9. [DOI] [PubMed] [Google Scholar]
- 82. Rashidkhani B, Åkesson A, Lindblad P, Wolk A. Major dietary patterns and risk of renal cell carcinoma in a prospective cohort of Swedish women. J Nutr. 2005;135(7):1757–62. [DOI] [PubMed] [Google Scholar]
- 83. Villegas R, Yang G, Gao YT, Cai H, Li H, Zheng W, Shu XO. Dietary patterns are associated with lower incidence of type 2 diabetes in middle-aged women: the Shanghai Women's Health Study. Int J Epidemiol. 2010;39(3):889–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nanri A, Mizoue T, Shimazu T, Ishihara J, Takachi R, Noda M, Iso H, Sasazuki S, Sawada N, Tsugane S. Dietary patterns and all-cause, cancer, and cardiovascular disease mortality in Japanese men and women: the Japan Public Health Center-Based Prospective Study. PloS One. 2017;12(4):e0174848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Qi L, Cornelis MC, Zhang C, van Dam RM, Hu FB. Genetic predisposition, Western dietary pattern, and the risk of type 2 diabetes in men. Am J Clin Nutr. 2009;89(5):1453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010;375(9712):408–18. [DOI] [PubMed] [Google Scholar]
- 88. Yabe D, Seino Y, Fukushima M, Seino S. β cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep. 2015;15(6):602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ohn JH, Kwak SH, Cho YM, Lim S, Jang HC, Park KS, Cho NH. 10-year trajectory of β-cell function and insulin sensitivity in the development of type 2 diabetes: a community-based prospective cohort study. Lancet Diabetes Endocrinol. 2016;4(1):27–34. [DOI] [PubMed] [Google Scholar]
- 90. Villegas R, Shu XO, Gao YT, Yang G, Elasy T, Li H, Zheng W. Vegetable but not fruit consumption reduces the risk of type 2 diabetes in Chinese women. J Nutr. 2008;138(3):574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Villegas R, Gao YT, Yang G, Li HL, Elasy TA, Zheng W, Shu XO. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women's Health Study. Am J Clin Nutr. 2008;87(1):162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Odegaard AO, Koh WP, Yuan JM, Gross MD, Pereira MA. Dietary patterns and mortality in a Chinese population. Am J Clin Nutr. 2014;100(3):877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]