ABSTRACT
The gut microbiota is increasingly implicated in the health and metabolism of its human host. The host's diet is a major component influencing the composition and function of the gut microbiota, and mounting evidence suggests that the composition and function of the gut microbiota influence the host's metabolic response to diet. This effect of the gut microbiota on personalized dietary response is a growing focus of precision nutrition research and may inform the effort to tailor dietary advice to the individual. Because the gut microbiota has been shown to be malleable to some extent, it may also allow for therapeutic alterations of the gut microbiota in order to alter response to certain dietary components. This article is the second in a 2-part review of the current research in the field of precision nutrition incorporating the gut microbiota into studies investigating interindividual variability in response to diet. Part I reviews the methods used by researchers to design and carry out such studies as well as analyze the results subsequently obtained. Part II reviews the findings of these studies and discusses the gaps in our current knowledge and directions for future research. The studies reviewed provide the current understanding in this field of research and a foundation from which we may build, utilizing and expanding upon the methods and results they present to inform future studies.
Keywords: gut microbiome, precision nutrition, personalized nutrition, interindividual variability, effect modification, prediction, response, metabolism
Introduction
High rates of obesity, type 2 diabetes, and other chronic conditions related to poor nutrition represent a large burden of disease in the developed world (1). There is now ample evidence to show that certain dietary factors and patterns of dietary intake, such as high intake of refined carbohydrates and low intake of fiber, are associated with higher risk of obesity and associated diseases such as cardiovascular disease and metabolic syndrome (2). Although these general associations are meaningful, increasing attention is being given to the large variation in individual responses to diet. This movement of precision nutrition is growing and becoming more important as we recognize that, although summary statistics of populations can give us an idea of the incidence of chronic diseases, ultimately the processes leading to development and maintenance of disease occur within individuals.
Review outline and scope
This is the second article of a 2-part review covering the research that has been done to investigate the role of the gut microbiota in precision nutrition and its validity as a predictive biomarker for individual metabolic response. Studies included in the current review identify groups of individuals that show different metabolic phenotypes, typically in response to a dietary intervention, which are associated with some aspect of the gut microbiota. The first part of this review details the methods used to conduct and analyze precision nutrition–microbiome studies.
The second part of this review covers the results obtained from these studies. First, the growth of precision nutrition and the background of the role of the gut microbiome in precision nutrition and host metabolic response are summarized. This includes a discussion of the stability and variability of the gut microbiota and of host metabolic response, as well as the potential for modifying host metabolism. The current state of research includes the results of the studies whose methods were discussed in Part I of this review. For clarity, these results have been divided into sections based on the dietary intervention used: fiber; energy restriction and excess; and bioactives, fermented products, and other dietary components. The complexities of understanding the human–microbiome interaction are then presented, including the distinction between association and prediction as well as the relevance and applicability of results. This is followed by a discussion of gaps in the research and future directions such as the issue of generalizing results across dietary and metabolic contexts, the influence of prior dietary practices, factors in food choice beyond nutrition, the contribution of genetics and epigenetics, and the clinical application of this research.
Current Status of Knowledge of the Potential Role of the Gut Microbiome in Precision Nutrition and Variability of Human Metabolism
The gut microbiome in precision nutrition
In recent years, humans have been recognized as supraorganisms, combinations of human and microbe (3). In addition, the gut microbiome has become increasingly recognized as a significant source of variation between individuals. There are significant interindividual differences in microbiome composition, function, and diversity (4, 5). Many intervention and observational studies have documented how variation in the gut microbiome is shaped and influenced by host genetic, epigenetic, and lifestyle factors such as diet (6–10). However, the effect of this variability in the resident gut microbiome on variability in host health and metabolism is less well documented.
The gut microbiota produces a wide variety of metabolites (11, 12) that have the potential to modulate pathways of host metabolism and immunity (13, 14). The most well-known of these metabolites are the SCFAs, which affect functions such as glucose homeostasis and adipose tissue inflammation (13). Given these effects of gut microbial metabolites on host metabolism, it is important to acknowledge that the composition, function, and diversity of the microbiome are associated with an individual's predisposition to a wide variety of diseases (15, 16), many of which are also associated with diet. Taking this a step further, we can hypothesize that the interindividual variability seen in the microbiota may also contribute to variability in the development of disease via impact on the metabolism of dietary components. The extent of the contribution of the microbiome to personalized response and how it can be modified by other environmental and host factors is a growing area of research that is receiving increasing scrutiny (17–21).
Stability and variability of the gut microbiota
If an individual's microbiome remains relatively stable, and this translates to stability in response to diet, then the “optimal” diet for the individual will remain stable as well. However, if the microbiome and dietary response are more flexible, then what is “optimal” may change over time and thus require constant re-evaluation. Although studies have suggested an overall stability of the microbiota over long timescales (22–24), there are still variability and fluctuation in microbiota composition (24–26). One confusing aspect in this research question is how to define stability in the microbiota. Stability can be based on specific taxa (e.g., abundance, presence/absence), enterotype (i.e., compositional grouping), or functions. Each of these metrics affects how we interpret stability and its effect on an individual's dietary response over time. In order to develop an understanding of these processes, we must determine what features of the microbiome affect dietary response and what features may be altered by lifestyle factors such as diet.
Results from the literature of microbiota stability
Some general trends that have been identified by these studies are that interindividual variability in the microbiome is greater than intraindividual variability (23, 24, 26); variability between body sites is greater than within body sites (23, 26), and more specifically, variability within the skin microbiome is greater than in the gut which is greater than in the mouth (23, 26); and variability in taxa is greater than variability in functions (24). When considering taxonomic composition, a large portion of the gut microbiome seems to be relatively stable over long periods of time (22, 25). Broad patterns in microbiota composition such as enterotype have been shown to remain constant in response to dietary intervention (27–30). However, abundances of individual taxa have been shown to be susceptible to changes in diet (10, 24). For example, 1 study has shown that 75–88% of taxa detected in 2 individuals remained present for several months at a time, but that specific taxa such as Clostridium, Ruminococcus, Faecalibacterium prausnitzii, Eubacterium rectale, Eggerthella, Blautia, and Bifidobacteriales can fluctuate in response to daily variation in host diet, particularly fiber (24).
Contrasting results of microbiota stability
However, studies have obtained different and sometimes contrasting results, although this may be due to the different ways in which “stability” is defined. For example, Actinobacteria have been found to be stable over a period of 5 y (31). Studies investigating a shorter time period (∼1 y) found Actinobacteria to be less stable and to fluctuate on short timescales in response to factors such as diet (24, 26). Part of the reason for these seemingly contrasting results may be due to the classification of “stability” based on presence or absence (26, 31) as opposed to changes in relative abundance (24). Two studies found the phylum Bacteroidetes to be more stable than other phyla, whereas Firmicutes was less stable and more susceptible to fluctuations caused by diet (24, 31). However, other studies have suggested that Bacteroidetes are more susceptible to environmental regulation, whereas Firmicutes are more stable and heritable (8, 32). The heritability of bacteria has implications for the stability of these taxa both within individuals as well as between generations. Lastly, Proteobacteria have been found to be less stable in some cases (31), but others have found that there are varying levels of stability within the phylum, with some classes being more stable (i.e., Betaproteobacteria and Deltaproteobacteria) than others (i.e., Gammaproteobacteria and Epsilonproteobacteria) (26). The results of gut microbiome stability must be further investigated and definitions of stability must be standardized. This research will help contribute to our understanding of stability in metabolism and dietary response.
Stability and flexibility of host metabolic response
In addition to the question of the stability of the gut microbiome, the issue of host metabolic flexibility also has implications for whether dietary recommendations for an individual based on personal data have an expiration date and whether we can manipulate response to diet for therapeutic purposes. Several studies have found metabolic phenotype and response to diet to be relatively stable over time and to exhibit greater variability between individuals than within individuals (33–35). Although there is strong variability over short timescales (days) (33, 34), analysis over longer time periods (months, years) and comparison between repeated dietary challenges show stability in the characteristic metabolic signature of individuals (34, 35) as well as response to diet (28, 33, 36). As with microbiome stability, however, metabolic stability is nuanced, with some individuals showing greater stability than others and some diets showing a greater capacity to shift metabolic phenotype (33). Whereas some of these studies have directly analyzed the microbiome (28, 36), others have inferred microbiome contribution indirectly via the presence of metabolites known to be produced by gut bacteria (33–35). The stability of the microbiome and its effect on dietary response raises the question of whether metabolism, and the gut microbiome, can be modified over the long term. The extent to which this is possible is unclear.
Modification of host metabolism
One possible avenue for modifying metabolism via the gut microbiome is through probiotic supplementation. In 1 study, probiotic supplementation altered the function of the microbiota, particularly in relation to carbohydrate metabolism (37), although the effect on microbiota composition was nonsignificant. It is an intriguing possibility that response to certain dietary components could be altered by supplementing with specific probiotics. Indeed, some evidence suggests that supplementation with specific bacterial taxa that have a known role in the production of a certain metabolite can change the metabolic phenotype or response of an individual (38, 39). Possemiers et al. (38) investigated whether production of 8-prenylnaringenin (8-PN) from isoxanthohumol (IX) could be induced or increased by supplementation with a species of bacteria known to be involved in this process (Eubacterium limosum). 8-PN is a potent phytoestrogen that has been used to alleviate menopausal symptoms (40) and its production from IX, present in hops and beer, has been found to exhibit interindividual variability (41). Possemiers et al. (38) indeed found that administration of this bacterium induced 8-PN production from IX in germ-free rats and increased production in germ-free rats transplanted with microbiota from low 8-PN producing individuals. This probiotic approach must be investigated further in terms of its application in humans, the duration of effect, interindividual variability in colonization/function, and its use for production of other metabolites.
Results of Precision Nutrition–Microbiome Studies
Search methods
The articles included in this review were found by searching Google Scholar and PubMed using combinations of relevant keywords such as “precision nutrition,” “gut microbiome,” “inter-individual variability,” “diet,” “response,” and “effect modification.” Additional studies were often found in the citations of articles found via this original search method. Studies had to include a baseline or preintervention microbiota sample that was used to classify or predict response to diet. The majority of studies included a nutritional intervention or challenge, although this included both animal (42–49) and in vitro studies (38, 50, 51) as well as human studies. A few studies analyzing cross-sectional data (52, 53) or drug interventions (54, 55) were included because they contained results pertinent to the gut microbiome–nutrition–metabolism interaction. The heterogeneity of methods and interventions of these studies does not invalidate this review because the goal is to give an overview of the work done on this topic thus far and illustrate general findings, rather than draw scientifically valid conclusions from these data. Results are shown in Table 1.
TABLE 1.
Review of results of precision nutrition studies1
| Citation | Association or prediction | Results | Conclusion |
|---|---|---|---|
| Fiber | |||
| Korpela et al. (56) | Prediction | Microbiota-R: very low or very high baseline abundance of Eubacterium ruminantium and Clostridium felsineum. Microbiota-NR: average abundance of Eubacterium ruminantium and C. felsineum. Cholesterol-R: higher Clostridium sphenoides; decrease in cholesterol; associated with Microbiota-R. Cholesterol-NR: lower Clostridium sphenoides; increase in cholesterol; associated with Microbiota-NR. HOMA-R/NR: baseline abundance of Clostridium clusters XVI and XVIa, Bacilli, and Proteobacteria predicts response (56–79% accuracy). C-reactive protein-R/NR: baseline abundance of Clostridium clusters VI, XI, XIVa, and XVIII predicts response (46–80% accuracy). Diversity, richness, or evenness not associated with responsiveness, nor was P:B ratio. Baseline abundance of Eubacterium ruminantium and Clostridium felsineum predicts Microbiota-R/NR (78–100%). Baseline abundance of Clostridium sphenoides predicts Cholesterol-R/NR (100% accuracy). | Baseline abundances of several bacterial taxa (mostly Firmicutes) are associated with and predictive of microbiota and metabolic response to several dietary interventions related to dietary CHO. The taxa associated with/predictive of response vary based on the response variable in question. |
| Korem et al. (57) | Prediction | R/NR: glycemic response to different bread types predicted by relative abundance of Coprobacter fastidiosus and Lachnospiraceae bacterium 3_1_46FAA (83% accuracy). | Baseline abundances of certain bacterial taxa are predictive of lower glycemic response to different bread types. |
| Smits et al. (44) | Prediction | R: D3 colonized mice, dominated by Clostridiales, higher α-diversity, marked change along principal coordinate (PC) 2 after FOS intervention, and significant changes in taxonomic composition (decreased Clostridiales; increased Bacteroides fragilis, Sutterella spp., Barnesielaceae). NR: D1 and D2 colonized mice, dominated by Bacteroides and Parabacteroides, marginal changes in PC and composition after FOS intervention. Metabolomic features exhibited little change compared with microbiota composition in response to FOS intervention. | Abundance of certain taxa is predictive of changes in microbiota composition and glycoside hydrosylate families in response to FOS intervention, whereas metabolomic features are better predictors of individual donors than of response to diet. |
| Hjorth et al. (27) | Association | R: high P:B ratio, higher body fat loss on new Nordic diet vs. average Danish diet, maintained weight at 1-y follow-up. NR: low P:B ratio, no difference in body fat loss, regained weight at 1-y follow-up. | Baseline enterotype (i.e., P:B ratio) is associated with responsiveness (i.e., fat loss) to high-fiber/wholegrain diet. |
| Roager et al. (28) | Association | R: high P:B ratio, higher TC after intervention and tendency before. NR: low P:B ratio, lower TC after intervention and tendency before. Enterotypes (high-/low-P:B ratio) remained relatively stable over the course of the intervention. | Baseline enterotype (i.e., P:B ratio) is associated with responsiveness (i.e., TC change) to high-fiber/wholegrain diet. |
| Zhao et al. (58) | Association | R/NR: ASP index at day 28 negatively correlated with HbA1c at day 84. | Abundance and diversity of SCFA-producing bacteria, as indicated by the ASP index, at early time point may predict later changes in host metabolic markers in response to a high-fiber diet. Further research must determine whether individuals may be sorted into R and NR to a similar intervention based on ASP index at baseline. |
| Kovatcheva-Datchary et al. (36) | Association | R: high Prevotella; change in microbiota composition (increased Prevotella and P:B ratio, increased methanogens), microbiota function (increased complex polysaccharide fermentation), microbiota diversity (increased), and metabolic response (improved PPGR) with BKB supplementation; higher habitual fiber intake. NR: high Bacteroides; no change in above parameters. No differences in fecal SCFA or breath hydrogen between R and NR. R/NR status stayed stable at 1-y follow-up. Mice colonized with microbiota from R individuals after intervention showed improved glucose tolerance and increased expression of glycogen storage/metabolism genes (no difference when colonized by R microbiota before intervention). Mice colonized with Bacteroides thetaiotaomicron (abundant in NR) vs. Prevotella copri (abundant in R) showed differential SCFA production and improvement in PPGR (both higher with Prevotella copri colonization). | Baseline enterotype (i.e., P:B ratio) is associated with microbiota (i.e., composition and function) and metabolic (i.e., PPGR) response to dietary fiber. Key species may drive this response. |
| Chen et al. (50) | Association | R: Prevotella enterotype, fermented all 3 fibers with similar high total SCFA production, 2–3 times more propionate than NR. NR: Bacteroides enterotype, slower fermentation of the more complicated fiber structures and different total amounts/profiles of SCFA production from different fibers. | Baseline enterotype (i.e., P:B) is associated with the production of SCFAs from different dietary fiber sources. |
| Salonen et al. (59) and Walker et al. (60) | Association | R: lower diversity, greater change in microbiota (lower stability), correlated with prior dietary practices. NR: higher diversity, less change in microbiota (higher stability), not correlated with prior dietary practices. Diet had stronger effect on functional output (SCFA) and insulin sensitivity than on microbiota but no correlation with diversity on these responses noted. | Baseline microbiota diversity is associated with responsiveness (i.e., change in microbiota composition) to dietary fiber. The effect of diversity on metabolic response is unclear. |
| Tap et al. (61) | Association | R: low species richness, low microbiota stability (more change). NR: high species richness, high microbiota stability (less change). Link between diversity of vegetables in participants’ habitual diets and microbiota richness. Link between amounts of some SCFAs (caproate and valerate) and proportions of some genera (Prevotella, Dorea, Coprococcus, Bacteroides). | Baseline species richness is associated with microbiota response (i.e., change in microbiota composition) to fiber. Specific taxa are also associated with metabolic response (i.e., SCFA) but relation to species richness not noted. Diversity is linked to prior dietary practices (i.e., diversity of vegetable intake). |
| Martinez et al. (62) | Association | R/NR: magnitude and direction of microbiota response (i.e., change in abundance of certain taxa) different between RS types and individuals. No shifts were observed in all 10 subjects. Common RS2 response: increased Eubacterium rectale and Ruminococcus bromii. Common RS4 response: increased Bacteroidetes, Parabacteroides distasonis, and Bif. adolescentis. | Different individuals respond differently and different types of RS elicit different changes in the microbiome. Baseline features of the gut microbiome that may contribute to this variability and the metabolic effects of it are unclear. |
| Martinez et al. (63) | Association | R: higher Dialister, lower Coriobacteriaceae; greater improvement in IL-6. NR: lower Dialister, higher Coriobacteriaceae; smaller improvement in IL-6. All treatments increased microbial diversity, Firmicutes:Bacteroidetes ratio, and abundance of the genus Blautia. | Baseline abundances of certain bacterial taxa (i.e., Dialister, Coriobacteriaceae) are associated with immunologic response (i.e., IL-6) to whole grains. |
| Venkataraman et al. (64) | Association | R: enhanced and high butyrate concentration before/during RS; higher Bif. adolescentis and Ruminococcus bromii during RS (no difference before RS); higher Eubacterium rectale before and during RS in high group. NR: low butyrate concentration before/during RS, lower Bif. adolescentis, Ruminococcus bromii, and Eubacterium rectale. RS supplementation increased fecal SCFA and altered microbiota composition in the study population as a whole. | Baseline abundance of certain taxa (i.e., Eubacterium rectale) and metabolites (i.e., butyrate) are associated with responsiveness (i.e., change in microbiota, butyrate production) to RS. |
| Davis et al. (65) | Association | R: increase in Bif. in response to GOS. NR: no increase in Bif. in response to GOS. No taxa or operational taxonomic units (OTUs) significantly different between R and NR. | No baseline abundance of any taxa was associated with microbiota response (i.e., increase in Bif.) to GOS. |
| Bouhnik et al. (66) | Association | R: lower baseline Bif.; greater increase in Bif. NR: higher baseline Bif; smaller increase in Bif. Bifidogenic NDCHs: short-chain fructooligosaccharides, soybean oligosaccharides, GOSs, and RS3. Nonbifidogenic NDCHs: lactulose, long-chain inulin, and isomaltooligosaccharides. | Baseline abundance of Bif. in the gut is associated with microbiota response (i.e., increase in Bif.) to prebiotics. |
| Tuohy et al. (67) | Association | R: low baseline Bif.; greater increase in Bif. NR: high baseline Bif.; smaller increase in Bif. | Baseline abundance of Bif. in the gut is associated with microbiota response (i.e., increase in Bif.) to prebiotics. |
| Eid et al. (68) | Association | R: lower baseline Bacteroides, change in microbiota (lower stability), low baseline fiber intake. NR: higher baseline Bacteroides, no change in microbiota (higher stability), high baseline fiber intake. | Baseline abundance of Bacteroides is associated with microbiota response (i.e., change in composition) to date (polyphenol/fiber) supplementation. This may be connected to prior dietary practices (fiber intake). |
| Tuohy et al. (69) | Association | R: lower baseline Bif; greater increase in Bif. NR: higher baseline Bif; smaller increase in Bif. | Baseline abundance of Bif. in the gut is associated with microbiota response (i.e., increase in Bif.) to prebiotics (i.e., high performance (HP)-inulin). |
| Kolida et al. (70) | Association | R: lower baseline Bif; greater increase in Bif. NR: higher baseline Bif; smaller increase in Bif. | Baseline abundance of Bif. in the gut is associated with microbiota response (i.e., increase in Bif.) to prebiotics (i.e., inulin). |
| de Preter et al. (71) | Association | Microbiota-R1: lower baseline Bif.; greater increase in Bif. Microbiota-NR: higher baseline Bif.; smaller increase in Bif. Metabolite-R: higher baseline 15N (ammonia) and p-cresol; greater decrease in 15N (ammonia) and p-cresol in response to prebiotic intake. Metabolite-NR: lower baseline 15N and p-cresol; smaller decrease. | Baseline abundance of 1) Bif. in the gut as well as 2) baseline metabolite concentrations (i.e., ammonia and p-cresol) are associated with response to prebiotics. The overlap between these 2 features and their effects is unclear. |
| Sonnenburg et al. (51) | Association | R: higher abundance/specificity of genes involved in inulin metabolism (i.e., Bacteroides caccae > Bacteroides thetaiotaomicron > Bacteroides vulgatus); relative increase in abundance. NR: lower specificity/abundance of genes involved in inulin metabolism (i.e., Bacteroides vulgatus); relative decrease in abundance. | Genomic/functional content of microbiota is associated with effect of prebiotic (i.e., inulin) supplementation on microbiota response (i.e., increase/decrease in abundance). |
| Holscher et al. (72) | Association | R: female participants more responsive (i.e., greater shift in Bif.). NR: male participants less responsive (i.e., smaller/no shift in Bif.). Overall, Actinobacteria and Bif. significantly enriched after higher doses of agave inulin. | Gender is associated with microbiota response (i.e., increase in Bif.) to inulin. It is unclear if this is connected to differences in baseline microbiota features in men and women. |
| Fuller et al. (73) | Association | Inulin increased Bif. (effect not dependent on baseline abundance). No association between change in Bif. and allyl mercapturic acid excretion. | Baseline Bif. abundance not associated with prebiotic effect (i.e., increase in Bif.) and no effect observed on ITC metabolism. |
| Energy restriction and excess | |||
| Cotillard et al. (74) | Prediction | R: HGC at baseline; greater improvement in inflammation (hsCRP); higher baseline intake of fruit/veg and fish. NR: LGC at baseline; smaller improvement in inflammation (hsCRP); greater increase in gene richness (remained significantly lower than HGC); lower baseline intake of fruit/veg and fish. Model using 9 differentially abundant species able to distinguish HGC and LGC individuals with 99% accuracy. | Baseline microbiota richness is associated with improvement in inflammatory response (i.e., hsCRP) to energy restriction and remains higher despite a smaller increase in richness during intervention. This may be linked to prior dietary practices. The abundances of certain bacterial taxa are predictive of HGC/LGC status. |
| Shoaie et al. (75) [using data from Cotillard et al. (74)] | Prediction | R: HGC; higher baseline Bif. adolescentis, Faecalibacterium prausnitzii, and Eubacterium rectale; Bacteroides thetaiotaomicron significantly increased, Lactobacillus reuteri and Faecalibacterium prausnitzii significantly decreased after intervention. NR: LGC; lower baseline Bif. adolescentis, Faecalibacterium prausnitzii, and Eubacterium rectale; significant decrease only for Lactobacillus reuteri. Correctly predicted dietary changes made by participants, metabolite concentrations, and contribution of each bacterial species to production of specific metabolites. | Baseline microbiota richness and abundance of certain taxa are associated with microbiota response (i.e., change in abundance of certain taxa) to energy restriction. Community And Systems-level INteractive Optimization (CASINO) can be used to predict metabolic changes and contributions of the microbiota in response to energy restriction and provide advice to alter response (efficacy not evaluated). |
| Kong et al. (76) | Prediction | High-R: cluster A; lost more weight during energy restriction and continued to lose weight during stabilization; lowest fasting insulinemia, highest insulin sensitivity (HOMA-S, HOMA-IR, quantitative insulin-sensitivity check index, McAuley, SIisOGTT, Matsuda indexes); lower systemic inflammation (leukocytes, neutrophils, IL-6); lower adipose inflammation (HAM56+ cells); lowest Lactobacillus/Leuconostoc/Pediococcus. R: cluster B; lost more weight during energy restriction but did not continue to lose weight during stabilization; higher fasting insulinemia, lower insulin sensitivity; lowest systemic and adipose inflammation; lower Lactobacillus/Leuconostoc/Pediococcus. NR: cluster C; lost less weight and rapidly regained weight during the stabilization; highest baseline insulin, IL-6, adipose tissue inflammation (HAM56+ cells), Lactobacillus/Leuconostoc/Pediococcus. Bayesian network analysis identified plasma insulin, IL-6, leukocyte number, and adipose tissue (HAM56+ cells) at baseline as predictors sufficient to characterize the 3 clusters (75.5% accuracy). | Baseline abundances of several bacterial taxa as well as metabolic and inflammatory markers are associated with response (i.e., weight loss) to energy restriction. Baseline metabolic and inflammatory markers (not baseline microbiota composition) are incorporated into the best-fit model to predict cluster status (A, B, C). |
| Griffin et al. (42) | Prediction and association | R: lower CIV, greater diversity, higher abundance of Bacteroides, greater change in CIV with an unrestricted diet (AMER); higher hepatic amino acids and lactate. NR: higher CIV, lower diversity, higher abundance of Ruminococcus, smaller change in CIV with a calorie restricted with adequate nutrition (CRON) diet (greater change when cohoused with CRON mice); higher propionate. CIV: ranges from −1 [completely associated with CRON dietary pattern (DP)] to +1 (completely associated with AMER DP). Lower CIV (CRON-associated), higher CIV (AMER-associated). | Baseline microbiota composition and diversity are associated with (and predictive of) dietary pattern. Microbiota composition and diversity as well as concentrations of certain metabolites are also associated with responsiveness (i.e., change in microbiota composition) to different dietary patterns (i.e., AMER or CRON). |
| Piening et al. (77) | Prediction | IR: higher baseline immune/inflammatory pathways; no detectable Oxalobacter formigenes, no increase in Akkermansia muciniphila in response to weight gain, positive correlation between Eubacterium halli and Parabacteroides as well as between Bacteroides vulgatus and Eubacterium eligens. IS: higher baseline Oxalobacter formigenes, higher Allistipes (using all time points), increase in Akkermansia muciniphila in response to weight gain, negative correlation between Eubacterium halli and Parabacteroides as well as between Bacteroides vulgatus and Eubacterium eligens. IR/IS: random forest and AdaBoost using metabolomics features able to predict IR/IS with 87.5% accuracy, ANOVA showed 8 host metabolites that were differentially associated with the gut microbiota in IR or IS individuals. | Abundances of certain taxa are associated with metabolic status (IR/IS) and response (metabolites) to weight fluctuations. Metabolomic profile is able to predict metabolic status but no attempt made to predict metabolic status or response to treatment using microbiome measures. |
| Santacruz et al. (78) | Association | R: higher weight loss; higher total bacteria (richness), Bacteroides fragilis, Clostridium leptum, and Bif. catenulatum. NR: lower weight loss; higher Clostridium coccoides, Lactobacillus, Bif., Bif. breve, and Bif. bifidum. | Baseline differences in bacterial richness and abundance of certain bacterial taxa are associated with the effect of calorie restriction on weight loss in adolescents. |
| Hjorth et al. (29) | Association | No differences in response between diet groups. P:B ratio remained stable during intervention (although many 0-Prevotella group → low P:B ratio after intervention). R1: high P:B ratio at baseline; higher body weight, BMI; greater weight loss (3.8 kg more than low P:B ratio), greater body fat loss (3.8 kg more than low P:B ratio). R2: 0-Prevotella; greater weight loss (4.47 kg more than low P:B ratio), greater body fat loss (3.41 kg more than low P:B ratio). NR: low P:B ratio; less weight loss and body fat loss compared with high P:B ratio and 0-Prevotella). In adjusted model: low P:B ratio group lost more weight on diet above the median in CHO (%) and dietary fiber (g/10 MJ); high P:B ratio group lost more on diet above the median in CHO (%), fiber (g/10 MJ), and protein (%); 0-Prevotella lost more on diet above the median in CHO (%) and fat (%). Association of fiber intake and weight loss in high P:B ratio group explained entire difference between high and low P:B ratio groups. | Baseline P:B ratio is associated with the effect of calorie restriction on weight loss and body fat loss in adults. Further research is needed to determine why/how Prevotella below the detection limit is associated with different response compared with low P:B ratio. In addition, further research is needed to determine the appropriate cutoffs for high and low P:B ratios when measured using different methodologies. |
| Kreznar et al. (45) | Association | R: B6 mice; susceptible to diet-induced obesity on HF/HS diet; higher abundance of Clostridiaceae (Firmicutes), genes related to membrane transport and lipid metabolism. NR: CAST mice; not susceptible; high Bacteroidaceae (negatively correlated with body weight, fasting plasma insulin, and AUC insulin during oral-glucose-tolerance test). B6-CAST colonized mice recapitulate metabolic phenotypes of donor strains (effect of microbiota) but partially recapitulate strain-specific metabolic responses (effect of genetics and other host factors). | Baseline gut microbiota composition and function are associated with response to HF/HS diet. The final metabolic result is also influenced by other host factors (e.g., genetics). |
| Parks et al. (46) | Association | R: greater body fat growth, higher Lactococcus and Allobaculum (Firmicutes). NR: smaller body fat growth, higher Akkermansia. Mice microbiota clustered according to diet but, within clusters, genetic background had strong effect on microbiota composition and response to diet (i.e., body fat growth). | Baseline abundances of certain taxa (e.g., Akkermansia muciniphila) are associated with response to HF/HS diet. The final metabolic result is also influenced by other host factors (e.g., genetics). |
| Dao et al. (79) | Association | R: higher baseline Akkermansia muciniphila and gene richness; greater improvement in insulin sensitivity and clinical parameters (TC and LDL cholesterol) after calorie restriction; healthier baseline metabolic status (fasting glucose, waist:hip ratio, subcutaneous adipocyte diameter, TG, and body fat distribution). NR: lower baseline Akkermansia muciniphila; smaller improvement in insulin sensitivity and clinical parameters after calorie restriction. Akkermansia muciniphila abundance not associated with dietary intake. | Baseline abundances of certain taxa (i.e., Akkermansia muciniphila) are associated with better metabolic health and response to calorie restriction. This was not associated with any differences in baseline dietary intake. |
| Carmody et al. (47) | Association | R/NR: magnitude and direction of microbiota response (i.e., change in abundance of certain taxa) different between genotypes and affected by prior dietary intake (sequential diet shifts). Overall, HF/HS diet increased the relative abundance of the Firmicutes and Verrucomicrobia and decreased the Bacteroidetes. Bacteroidales (Bacteroidetes) and Clostridiales (Firmicutes) are the main classes of diet-responsive bacteria. | Genetics and prior dietary practices are associated with response of microbiota (i.e., change in composition) to dietary patterns. Whether the impact of these factors is mediated by baseline microbiota composition is unclear. |
| Zou et al. (80) | Association | R: baseline Prevotella enterotype; higher BMI loss; increase in Enterobacter cloacae/hormaechei and Klebsiella oxytoca, decrease in Collinsella aerofaciens; decreased pathways for metabolism of amino sugars, nucleotide sugar, fructose, and mannose. NR: baseline Bacteroides enterotype; lower BMI loss; increase in 7 species including Eubacterium rectale and Prevotella copri, decrease in 3 species (Bacteroides stercoris, Bacteroides coprocola, and Veillonella parvula); increased pathways for propanoate and butanoate metabolism. | Baseline enterotype is associated with BMI loss in response to calorie restriction, with Prevotella enterotype showing a greater loss in BMI. Enterotype is also associated with differences in changes in composition and functional potential of the gut microbiome, although the dietary intervention did result in a measure of convergence in the gut microbiome of the 2 enterotype groups. |
| Muñiz Pedrogo et al. (81) | Association | R: higher baseline Phascolarctobacterium, transposase (COG3328). NR: higher baseline Dialister, CHO metabolism genes. No differences in baseline clinical, biochemical, and demographic characteristics. No differences in α- or β-diversity. | Baseline abundances of CHO metabolism genes and members of the family Veillonellaceae are associated with weight loss success (Phascolarctobacterium) or failure (Dialister) during a lifestyle intervention program. |
| Bioactives, fermented products, and other dietary components | |||
| Faith et al. (43) | Prediction | R/NR: linear model using abundance of taxa in response to isolated nutrients predicts microbiota response to meals with varying concentrations of nutrients (61–62% accuracy). | Changes in abundances of certain taxa in response to isolated nutrients can predict response to novel combinations of isolated nutrients in the context of a simplistic microbial community. The applicability of this model in the context of a complex community and diet (i.e., in humans) is unclear. |
| Zeevi et al. (82) | Prediction | R: Eubacterium rectale and methionine degradation Kyoto Encyclopedia of Genes and Genomes (KEGG) module (M00035) associated with improved PPGR. NR: Parabacteroides distasonis, Bacteroides thetaiotaomicron, Alistipes putredinis, and the Bacteroidetes phylum associated with worse PPGR. Model using microbiome-based features as well as features related to meal content, daily activity, blood parameters, continuous glucose monitor–derived features, and questionnaires able to predict glycemic response (68% accuracy). Model able to prescribe “good” and “bad” diets (lower PPGR on “good” diet for 10/12 participants vs. “expert” recommendation for 8/14 participants). | Numerous characteristics of the microbiome (composition and function) as well as host factors contribute to prediction of PPGRs and can be used to make dietary recommendations to improve PPGR. |
| Mendes-Soares et al. (83) | Prediction | No discussion of specific factors contributing to variability in response. Used and built upon models developed in Zeevi et al. (82) (added abundance of Prevotella and Bacteroides). | Glycemic response is highly variable and is predicted more accurately by a trained model, rather than the calorie or CHO content of the food alone. |
| Le Chatelier et al. (53) | Prediction | R: HGC; less adiposity, weight gain over time, insulin resistance, dyslipidemia; less inflammatory phenotype; higher abundance of butyrate-producing bacteria. NR: LGC; more adiposity, weight gain over time, insulin resistance, dyslipidemia; more inflammatory phenotype; lower abundance of butyrate-producing bacteria. Model using 4 differentially abundant species able to distinguish HGC and LGC individuals (98% accuracy). Model using 9 species able to able to distinguish lean and obese individuals (78% accuracy). | Baseline microbiota richness and abundances of certain bacterial taxa, particularly butyrate-producing bacteria, are associated with and predictive of weight gain/obesity over time. |
| Bennet et al. (84) | Prediction | R: higher Phascolarctobacterium; lower baseline DI score; significant improvement in IBS-SSS (decrease ≥50) in response to low-FODMAP diet. NR: higher Firmicutes (Bacilli and Clostridia) including Clostridium, Ruminococcus gnavus, and Streptococcus; higherBacteroides stercoris, Pseudomonas, Acinetobacter, Desulfitispora, Coprobacillus; higher baseline DI score; no significant decrease in IBS-SSS in response to low-FODMAP diet. Model using baseline and postintervention microbiota profiles predicts R and NR to low-FODMAP diet (54% accuracy). | Baseline abundances of certain bacterial taxa are associated with responsiveness (i.e., improvement in IBS-SSS) to low-FODMAP diet. Baseline microbiota composition is moderately predictive of response to low-FODMAP (but not traditional irritable bowel syndrome) diet. |
| Kolho et al. (55) | Prediction | R: reduction in calprotectin concentrations; higher baseline Bif., Clostridium colinum, Eubacterium rectale, uncultured Clostridiales, and Vibrio; lower Streptococcus mitis. NR: no reduction in calprotectin concentrations; lower baseline Bif., Clostridium colinum, Eubacterium rectale, Clostridiales, and Vibrio; higher Streptococcus mitis. Model using baseline abundance of 9 bacterial groups predicts calprotectin response (85% accuracy). Model using 2 bacterial groups (Clostridium sphenoides and Haemophilus spp.) predicts calprotectin response (above or below 200 µg/g) (88% accuracy). | Baseline abundances of certain bacterial taxa are associated with and predictive of response (i.e., reduction in calprotectin) to anti-TNF-α medication in children with irritable bowel disease. |
| Cho et al. (85) | Association | R: high TMAO production (≥20% increase in response to eggs/beef), lower α-diversity, higher F:B ratio (∼2:1), higher Clostridiales (Clostridiaceae, Lachnospiraceae, Veillonellaceae), no Archaea. NR: low TMAO production, higher α-diversity, lower F:B ratio (1:1), higher Bacteroidales (Bacteroidaceae, Prevotellaceae), Archaea present. | Baseline microbiota diversity and composition, particularly the ratio of abundant phyla (e.g., Firmicutes, Bacteroidetes), are associated with production of TMAO. The effect of this on clinical variables (e.g., lipids, cell count) is not stated. |
| Suez et al. (86) | Association | R: compositional change with NAS consumption; developed significantly poorer glycemic responses with NAS consumption. NR: no compositional change with NAS; no significant effect of NAS on glycemic response. NAS R clustered differently from NR both before and after NAS consumption. | Genetics and prior dietary practices are associated with response of microbiota (i.e., change in composition) to dietary patterns. |
| Kang et al. (30) | Association | R: Bacteroides enterotype (E1); response to both low and high CAP; greater response to CAP. NR: Prevotella enterotype (E2); response to only high CAP; lower response to high CAP. Response: increased ratio of Firmicutes to Bacteroidetes and abundance of Faecalibacterium and Ruminococcaceae, decreased Bacteroidetes. Increased GLP-1, GIP, and butyrate; decreased ghrelin. Enterotype groups remained relatively stable during intervention. No change in other measured metabolic or clinical outcome measures. | Baseline enterotype (i.e., P:B ratio) is associated with microbiota response (i.e., change in composition) to dietary CAP. The effect of this on clinical outcomes is unclear. |
| Possemiers et al. (87) | Association | High, moderate, and low O-DMA, equol, END, ENL, or 8-prenylnaringenin producers. END-R: lower Clostridium coccoides–Eubacterium rectale. O-DMA-R: higher methanogens. Equol R: higher sulfate-reducing bacteria. | Baseline abundances of certain groups of gut bacteria are associated with production of bioactive estrogen metabolites from food components. |
| Hullar et al. (52) | Association | R: Higher ENL production; higher microbiota diversity and abundance of certain bacterial taxa (Moryella spp., Acetanaerobacterium spp., Fastidiosipila spp., and Streptobacillus spp.). NR: Lower/no ENL production; lower diversity and abundance of taxa above. | Baseline microbiota diversity and abundances of certain bacterial taxa are associated with production of bioactive estrogen metabolites from food components. |
| Romo-Vaquero et al. (88) | Association | R/NR: Coriobacteriaceae discriminated between UMs and correlated with TC, LDL, and ApoA-1; Euryarchaeota higher in UM-B; Methanobacteriaceae, Synergistaceae, Coriobacteriaceae, Clostridiaceae, Enterobacteriaceae, and Clostridialesincertae sedis XI more abundant in UM-B; Lachnospiraceae and Eubacteriaceae more abundant in UM-A and UM-0; diversity/richness at genus level lower in UM-0 than in UM-A and UM-B (higher in UM-B at family and phylum levels); P:B and F:B ratios similar among UMs. | Abundances of an assortment of individual bacterial taxa, such as Coriobacteriaceae, as well as diversity and richness are associated with polyphenol metabolizing phenotype and CVD risk factors, such as TC and LDL cholesterol. |
| Li et al. (89) | Association | R/NR: ex vivo fermentation of high- and low-ITC excretors showed differences in glucosinolate metabolism but terminal restriction fragment length polymorphism showed no statistically significant differences in microbiota composition. | Differences in glucosinolate metabolism and ITC excretion are not directly linked to microbiota composition. Links to microbiome functional genes should be further investigated. |
| Zmora et al. (90) | Association | R: permissive (significant increase in absolute abundance of probiotic strains); lower baseline levels of probiotic strains in the lower gastrointestinal mucosa (not in stools); in the ilea: enrichment in immune-related pathways; after probiotics: ceca enriched in pathways related to dendritic cells, antigen presentation, and ion transport. NR: resistant (no significant increase in absolute abundance of probiotic strains); higher baseline levels of probiotic strains; in the stomach: increased abundance of genes related to adaptive and innate immune responses, inflammation and T cell activation, and differentiation; in the ilea: enrichment in genes elated to digestion, metabolism, and xenobiotics metabolism; after probiotics: enrichment of pathways associated with responses to exogenous stimuli, innate immune activation, and antibacterial defense (especially to Gram+ bacteria). | Baseline abundance of probiotic bacteria in the gut mucosa is associated with persistence of probiotic bacteria in the gut and affects functional response to probiotics at the level of pathway activation. Further research must confirm the metabolic effects of the permissive vs. resistant phenotype. |
| Zhang et al. (48) | Association | Rat-R: permissive (longer persistence of FMP strains); higher Lachnospiraceae; greater variation induced by FMP. Rat-NR: resistant (shorter persistence of FMP strains). 43 OTUs distinguish the gut microbiota of resistant and permissive rats. Human-R: Lactococcus carriers; less Lactococcus shedding (longer persistence); higher interindividual variation (β-diversity). Human-NR: Lactococcus noncarriers; greater Lactococcus shedding (shorter persistence); less interindividual variation (β-diversity). Lactococcus carriers differ in baseline abundance of several taxa (only Lachnospiraceae in common with rats). | Baseline microbiota composition (e.g., abundance of Lactococcus) is associated with persistence of probiotic bacteria in the gut. |
| Senan et al. (91) | Association | R: no change in TC or <1.72 mg/dL; greater increase in Lactobacilli; lower baseline Firmicutes, Clostridium, and Shigella; higher α-diversity and abundance of Eubacterium and Burkholderia. NR: elevation in TC ≥2.509 mg/dL; decrease or smaller increase in Lactobacilli; lower Proteobacteria; higher Escherichia, Crucella, and Campylobacter. | Baseline microbiota composition and diversity are associated with response (i.e., change in lipid metabolism) to probiotic supplementation. Disclaimers: Only 16/59 classified as R and NR; no significant reduction in TC concentrations on probiotic, some significant reductions in other lipid parameters (LDL, TC:HDL, LDL:HDL) but also seen in placebo group. |
| Veiga et al. (49) | Association | R: improvement in colitis score; higher recovery of live Bif. lactis; lower cecal pH; higher SCFA; increase in lactate-consuming, butyrate-producing bacteria; lower baseline Bifidobacteriaceae, Porphyromonadaceae, Prevotellaceae, and Staphylococcaceae; higher Lachnospiraceae. NR: no improvement in colitis score; lower recovery of live Bif. lactis; higher cecal pH; lower SCFA; elevated baseline representation of Lactobacillaceae. | Baseline microbiota composition is associated with response (i.e., reduced colitis) to probiotic supplementation. |
| Volokh et al. (92) | Association | R: lower baseline abundance of LFTs; stronger increase in levels of LFTs, more change in microbiota composition (lower microbiota stability). NR: higher baseline abundance of LFTs (especially Bacteroidaceae); weaker increase in levels of LFTs, less change in microbiota composition (higher microbiota stability). | Baseline abundance of lactose-fermenting bacteria is associated with microbiota response to probiotic dairy supplementation. The potential effect on the host is unclear. |
| Mobini et al. (93) | Association | R: higher baseline α-diversity and abundance of Euryarchaeota; significant reduction in HbA1c, increased DCA. NR: lower baseline α-diversity and abundance of Euryarchaeota; no significant reduction in HbA1c or increase in DCA. | Baseline α-diversity and abundances of certain taxa, not probiotic taxa, are associated with response to probiotic supplementation. |
| Chumpitazi et al. (94) | Association | R: higher abundance of 21 OTUs (e.g., taxa with greater saccharolytic activity: Bacteroides, Ruminococcaceae, Faecalibacterium prausnitzii, Erysipilotrichaceae) and 3 KEGG gene pathways (2 related to FODMAP CHO metabolism). NR: higher abundance of 4 OTUs (e.g., Turicibacter). No differences in α- or β-diversity. | Baseline abundances of certain bacterial taxa and functions are associated with response to low-FODMAP diet. |
| Spencer et al. (95) | Association | R: higher baseline abundance of Gammaproteobacteria and Erysipelotrichia; lower LF/SF after choline depletion. NR: lower baseline abundance of Gammaproteobacteria and Erysipelotrichia; higher LF/SF after choline depletion. Microbiota features in addition to phosphatidylethanolamine N-methyltransferase single nucleotide polymorphism resulted in best correlation with change in LF/SF. | Baseline abundances of certain bacterial taxa as well as genotype are associated with impacts of choline deficiency on LF content. |
1AMER, unrestricted diet; ASP, active SCFA producer; Bif., Bifidobacterium; BKB, barley kernel bread; CAP, capsaicin; CASINO, Community And Systems-level INteractive Optimization; CHO, carbohydrate; CIV, community indicator value; CRON, calorie restricted with adequate nutrition; DCA, deoxycholic acid; DI, dysbiosis index; DP, dietary pattern; END, enterodiol; ENL, enterolactone; F:B ratio, ratio of Firmicutes to Bacteroides; FMP, fermented milk product; FODMAP, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols; FOS, fermentable oligosaccharide; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; GOS, galactooligosaccharide; HbA1c, glycated hemoglobin; HF/HS, high-fat high-sugar; HGC, high gene count; HOMA, homeostasis model assessment; HOMA-S, homeostasis model assessment of insulin sensitivity; HP, high performance; hsCRP, high-sensitivity C-reactive protein; IBS-SSS, irritable bowel syndrome symptom severity score; IR, insulin resistant; IS, insulin sensitive; ITC, isothiocyanate; KEGG, Kyoto Encyclopedia of Genes and Genomes; LF, liver fat; LFT, lactose-fermenting microbial taxon; LGC, low gene count; NAS, noncaloric artificial sweetener; NDCH, nondigestible carbohydrate; NR, nonresponders; O-DMA, O-desmethylangolensin; OTU, operational taxonomic units; P:B ratio, ratio of Prevotella to Bacteroides; PC, principal coordinate; PPGR, postprandial glucose response; R, responders; RS, resistant starch; SF, spleen fat; SIisOGTT, simple index assessing insulin sensitivity derived from oral-glucose-tolerance test; TC, total cholesterol; TMAO, trimethylamine-N-oxide; UM, urolithin metabotype.
Response to fiber interventions: the role of the microbiota
In the context of a fiber-type intervention, features that have been highlighted as associated with response are the ratio of Prevotella to Bacteroides (P:B ratio) or enterotype (27, 28, 36, 50); diversity and richness (59, 61); functional gene content within groups of taxa (51); the abundance and diversity of SCFA-producing bacteria (58); and abundance of certain groups of taxa such as Bifidobacteria (65–67, 69–71), Bacteroides (68), Ruminococci (60), Dialister and Coriobacteriaceae (63), Eubacterium (56, 64), Clostridium (56), and Coprobacter fastidiosusand Lachnospiraceae (57) (Figure 1).
FIGURE 1.
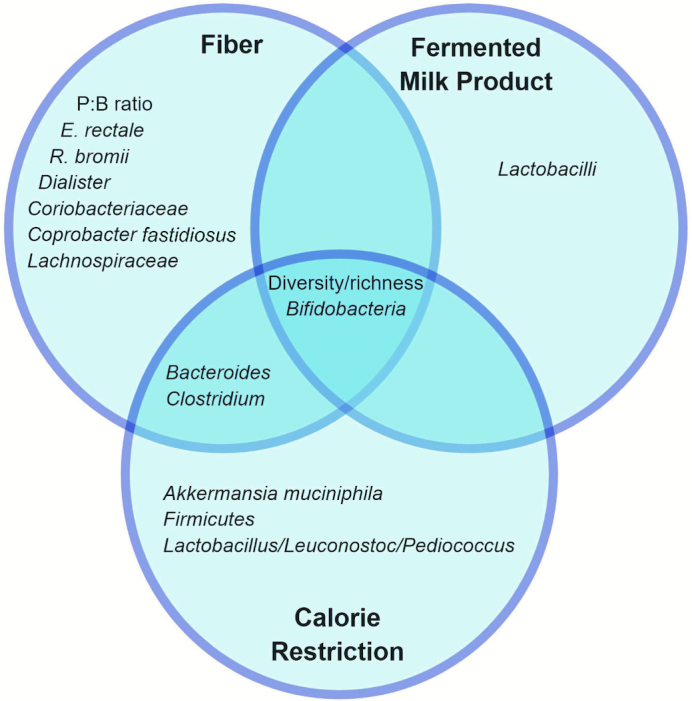
Shared associations between dietary components and the microbiota. Different microbiota features (e.g., taxa and diversity) play different roles and may be involved with response to different dietary components. Identifying what microbiota features are associated with response to which dietary components can help us identify potential pathways and networks as well as understand the full picture of individual metabolic response to diet. P:B ratio, ratio of Prevotella to Bacteroides.
The P:B ratio or enterotype as well as the abundance of Bifidobacteria are among the most common features associated with response to fiber intake, regardless of the outcome being investigated. The Prevotella enterotype has been associated with diets higher in carbohydrate and fiber, whereas the Bacteroides enterotype has been associated with diets higher in protein and animal fat (22, 96). Both the dominance of Prevotella in the overall gut microbiota community, as shown by enterotype, as well as individual Prevotella species, such as P. copri (36), have been shown to associate with the response to fiber intake.
Likewise, Bifidobacteria are one of the most well-known probiotic bacteria and have been shown to ferment a variety of carbohydrate and fiber compounds to produce bioactive metabolites that affect host health, such as acetic and lactic acids, B vitamins, and antimicrobial molecules (72).
Species of Eubacterium, such as E. rectale (62, 64) and E. ruminantium (56), and Ruminococcus, such as R. bromii (62, 64), have also been tied to the fermentation of fiber, particularly resistant starch, and the production of metabolites such as butyrate, which has been shown to have many functions (97). Because SCFAs are the main products of fiber fermentation, an index of active SCFA producers (the ASP index) has also been developed and has been shown to associate with improved response to a high-fiber intervention (58). Associations such as these make sense: if the bacteria capable of metabolizing the beneficial dietary component being ingested are present, then the beneficial effects of said dietary component will follow. But what about the beneficial effects of restricting energy intake or protection against excess of unhealthy dietary components?
Response to energy restriction and excess interventions: role of the microbiota
In the context of an energy-restricted diet, more responsive or higher weight-loss groups were characterized by higher bacterial richness or diversity (42, 74, 78), a higher P:B ratio (29, 80), as well as abundance of specific taxa such as higher Bacteroides, Clostridium leptum, Phascolarctobacterium, and Bifidobacteria catenulatum and lower Dialister and Lactobacillus/Leuconostoc/Pediococcus (42, 76, 78, 81) (Figure 1). Similarly, when consuming a high-fat, high-sugar, or weight-gain diet, resistance to diet-induced obesity or better metabolic profile was associated with higher Akkermansia (46, 77) and Bacteroidaceae (45) and lower Firmicutes (45–47).
When focusing on fiber, the dominant bacterial groups were Prevotella and Bacteroides. However, when the question shifts to energy or fat intake, conversation seems to be more focused on the ratio of Bacteroidetes to Firmicutes, especially as this balance has been associated with obesity (98). Akkermansia muciniphila has also become a common name associated with higher weight loss and improvement in metabolic parameters (79) or less weight gain (46) on energy-restricted and high-fat diets, respectively.
Whereas diversity had a greater effect on the stability of microbiota composition in response to a fiber intervention, diversity and richness seem to have a greater effect on the host metabolic response to energy and fat intake. A higher baseline diversity and richness of the gut microbiota results in greater weight loss and improvement in insulin sensitivity, clinical parameters, and inflammation (74, 78, 79).
Response to bioactives, fermented products, and other dietary components: role of the microbiota
In the context of other dietary interventions, enterotype or ratios of predominant phyla (30, 83, 85), abundance of other specific taxa (43, 49, 52, 55, 82–84, 87, 91, 93–95), abundance of probiotic bacteria (90, 92), abundance of bacterial functions (82), and bacterial diversity and richness (53, 85, 93) have also been found to be associated with response. An additional study by Suez et al. (86) found that responders and nonresponders to an artificial sweetener intervention clustered separately but did not indicate which taxa may have contributed to this clustering.
Kang et al. (30) found subjects with a Bacteroides-dominated enterotype to be more responsive (i.e., change in microbiota composition; increased glucagon-like peptide-1, gastric inhibitory peptide, and butyrate) to dietary capsaicin, responding to lower doses and having an overall higher response than those with a Prevotella-dominated enterotype. Gu et al. (54) also showed that the Bacteroides enterotype predicted greater responsiveness to the antidiabetic drug Acarbose, with individuals in this category showing greater improvements in C peptide, fasting glucose, insulin, and HOMA-IR than individuals with a Prevotella enterotype. Individuals with a Bacteroides enterotype also showed greater changes in microbiota composition in response to Acarbose with a decrease in Bacteroides and concomitant increase in Bifidobacterium after treatment, leading to a hypothesis that individuals with a Bacteroides enterotype will respond better to interventions that are bifidogenic (21). The studies by Kang et al. (30) and Gu et al. (54) demonstrate the importance of dietary or treatment context because a Prevotella enterotype, rather than the Bacteroides enterotype, was generally found to be more responsive in the context of a fiber intervention.
The gut microbiota is involved in the metabolism of dietary polyphenols into bioactive metabolites (99). Possemiers et al. (87) and Hullar et al. (52) both identified taxa associated with production of phytoestrogens (e.g., O-desmethylangolensin, equol, enterodiol, enterolactone, and 8-PN) from dietary precursors, with individuals ranging from low to high production. Although both studies noted significant associations, such as microbiota diversity and the abundance of groups such as methanogens and sulfate-reducing bacteria, there is little overlap in their results. An analysis of multiple dietary interventions (100–102) by Romo-Vaquero et al. (88) investigating the question of the role of the microbiome in the urolithin metabotype (UM) also found a variety of bacterial taxa associated with metabotype group (UM-A, UM-B, and UM-0). UM-A metabotype individuals produce urolithin A, UM-B produce isourolithin A and urolithin B, and UM-0 individuals do not produce final urolithins. It is worth noting that an earlier report by this group (103) showed little overlap in bacterial taxa identified as associated with urolithin production. In addition, despite earlier findings of the role of the gut microbiota in glucosinolate metabolism (104), Li et al. (89) found no differences in microbiota composition between high and low excretors of bioactive isothiocyanates and also found that, upon second feeding of glucosinolate-rich broccoli, differences in excretion between these 2 groups disappeared. These results beg the question of whether the methods used to investigate microbiota effect modifiers were comprehensive enough or whether the results observed are just different pieces of the puzzle.
This same lack of overlap is demonstrated by the findings of studies investigating the response to fermented milk product or probiotic consumption (48, 49, 90–93), although some findings such as the abundance of Lactobacilli and Bifidobacteria have been replicated. However, these studies were looking at different response variables as well as different probiotic strains, suggesting that factors influencing the response to probiotics are targeted to the specific strain or response in question. With respect to probiotic supplementation, it has also been suggested that presence of bacteria in stool may not be representative of the luminal and mucosal environment and thus may not be an adequate marker of probiotic colonization (90). Further research in the area of probiotic supplementation must identify accurate, noninvasive methods of determining the extent to which bacteria are able to colonize subjects. This will allow for better identification of the benefits of probiotics in the individuals in which they are able to colonize as well as the development of personalized probiotics.
It is worth noting that the response is not always positive. For instance, in the case of trimethylamine-N-oxide (TMAO), a risk factor for atherosclerosis, it has been found that individuals with a higher ratio of Firmicutes to Bacteroidetes and lower α-diversity were more responsive (i.e., produced more TMAO) to foods containing TMAO precursors (i.e., choline and carnitine) (85). In this case, it would be more beneficial to determine how to decrease this response in individuals.
Coming to a Complete Understanding of the Human Supraorganism
Association compared with prediction
As mentioned in Part I of this review, studies can be divided into association and prediction studies, based on their use and analysis of the data to complete the 2 steps in the process towards the development of precision nutrition recommendations: 1) identifying associations between microbiome features and dietary responsiveness and 2) predicting and validating individuals’ response to dietary interventions and/or advice. Although features may be found to be associated with various measures of response, this is not the same as being predictive of response. Although prediction studies frequently identify several features of the microbiome that are individually associated with response to diet, the model that provides the best fit of the data and the best prediction of response often does not include all of these features. This may be due to collinearity of the microbiota variables. For instance, Kong et al. (76) identify Lactobacillus/Leuconostoc/Pediococcus abundance as significantly associated with groups of metabolic phenotypes but ultimately do not include this feature in their predictive model. Similarly, Le Chatelier et al. (53) start with 58 differentially abundant taxa and end with only 4 in their model, whereas Kolho et al. (55) start with 9 differentially abundant taxa and use only 2 in their predictive model. The results obtained from association studies must be used to guide future investigation and development of predictive models that can inform individual dietary recommendations.
Relevance and applicability of results
Extrapolation from model systems and linking the gut microbiota to host response
In addition, a challenge of research using mice or in vitro models is to predict clinically relevant indicators of health and metabolism and validate that these effects occur in the complex system that is the human supraorganism. For example, although Faith et al. (43) elegantly demonstrated the ability to predict the response of a microbial community in response to varying concentrations of nutrients using a linear model, the 10-strain community used was a simplistic representation of the enormously complex and diverse community that inhabits the human gut. The responses of the microbiome were not connected with host features, such as host genetic or epigenetic features, or host metabolic indicators. Thus, results from this study require extrapolation to infer potential effects on human health. Other studies have also noted differences in responsiveness, but define “response” only as a change in the microbiota or a change in a particular species (48, 62, 65, 66, 69, 75). It is important to make the link between observed responses and the implications for host health and metabolism in order to determine what features of the microbiota should be given consideration when providing recommendations.
Building a network
Not only is it important to connect microbiota features and observed responses, it is also important to build a network by making links between different observed responses and their associated microbiota features so the generalizability of certain features of the microbiota may be determined. Within the context of the same dietary intervention, different microbiota features may predict different response variables. For instance, Korpela et al. (56) found that different groups of bacteria predicted the response of cholesterol (Clostridium sphenoides), homeostasis model assessment (Clostridium clusters XVI and XVIa, Bacilli, and Proteobacteria), and C-reactive protein (Clostridium clusters VI, XI, XIVa, and XVIII) in the context of a fiber intervention. Conversely, 1 microbiota feature may also predict the response of multiple metabolic outcomes (Figure 2). For instance, the P:B ratio or enterotype has been found to be associated with body fat loss and body composition (27), total cholesterol (28), glucose tolerance/insulin sensitivity, incretin and gut hormone concentrations (30), glycogen storage/metabolism (36), and SCFA production (50), whereas bacterial diversity and richness have been associated with SCFA production (42), total and LDL cholesterol (91), body composition (53), production of metabolites such as TMAO (85) and enterolactone (52), inflammation (53, 74), insulin sensitivity (53, 59), microbiota stability (42, 44, 59–61), and the persistence of probiotic bacteria (48). It should be noted, however, that the directions of some of these effects may depend on dietary context and should not be viewed as fixed. Building a network also means identifying commonalities between microbiota features such as the fact that many bacteria that are associated with a certain response are SCFA producers (58) or, conversely, that many of the responses affected by a certain microbiota feature are all involved in or stem from a certain pathway. This will allow us to develop mechanistic hypotheses, which can then be investigated using in vitro or animal model systems.
FIGURE 2.
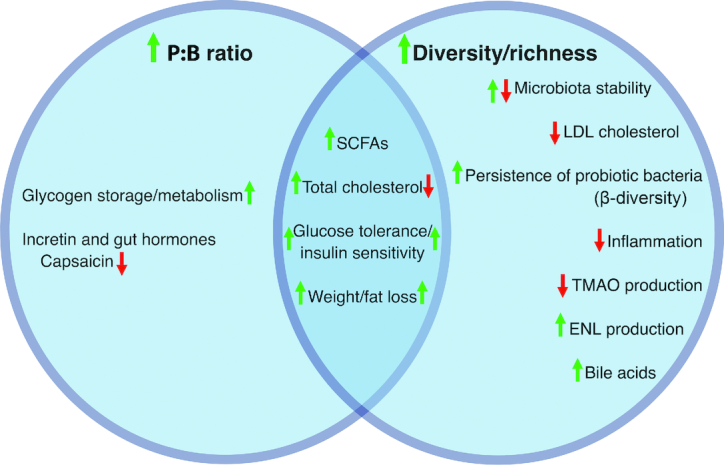
Shared associations between metabolic response and the microbiota. Different microbiota features may be associated with different metabolic responses to dietary interventions. Identifying what responses are associated with certain microbiota features can help identify what factors researchers should focus on based on their primary outcome as well as inform personalized recommendations from clinicians based on the symptoms of the patient. ENL, enterolactone; P:B ratio, ratio of Prevotella to Bacteroides; TMAO, trimethylamine-N-oxide.
Inconsistency in results
However, results have not always been consistent, even within the same type of intervention, and it is important to understand why results are not always replicated. For instance, Korpela et al. (56) observed that neither P:B ratio nor diversity were associated with responsiveness to fiber interventions, despite measuring some of the same outcome variables (e.g., total cholesterol, glucose/insulin) as studies that did find these aspects of the microbiome to influence response to fiber (28, 36). Similarly, Fuller et al. (73) found no effect of baseline abundance of Bifidobacterium on the enrichment of this taxa by a fiber intervention, a common finding by others (67, 69–71). In addition, Chumpitazi et al. (94) and Bennet et al. (84) both found differences among irritable bowel syndrome patients in the efficacy of a low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols diet at decreasing symptom severity and found taxa associated with responder status. However, some of the taxonomic groups associated with responders in Chumpitazi et al. (94) were associated with nonresponders in Bennet et al. (84) (e.g., Clostridia and Bacteroides). These studies raise the question of how specific or universal certain microbiome features are in terms of their association with certain dietary factors or with certain metabolic responses.
Gaps in the Research and Future Directions
Applicability in different dietary and response contexts
As detailed above, different groups of taxa are associated with different dietary components and with different measures of metabolic response. However, a focus only on certain nutrients is an overly simplistic view of nutrition. Foods are composed of complex matrices of nutrients, which interact with one another and with the consumer to produce the final metabolic outcome. In addition, meals and overall dietary patterns are composed of a multitude of food components that combine to create a vast web of nutrients. Therefore, bacterial taxa associated with or involved in the metabolism of certain nutrients may not be affected by or predictive of the response to other dietary nutrients, to broader dietary patterns, or even to the same nutrients when combined with other nutrients and food types. For example, Sawicki et al. (105) discuss the methodology of studies investigating the effect of fiber on the gut microbiota and human health, identifying high-fiber, whole-diet interventions as a gap in the research. Many studies investigating fiber simply supplement with a specific type of fiber in a standard form and dose. However, this then begs the question of whether effects may differ between individuals based on the content of the remainder of their diet. In addition, in Christensen et al. (21), differences in effects of fiber interventions based on the gut microbial enterotype of the individual are shown but differences between different types of fiber are an area of investigation that is identified as needing further research. This complexity may contribute to the lack of universality in the association of certain taxa with response both between and within types of dietary interventions. It is important to acknowledge this limitation as well as explore the applicability of different features of the microbiome in the context of other nutrients or broad dietary patterns as well as on different measures of responsiveness.
Prior dietary practices and metabolic flexibility
Some features of the microbiome that are associated with response are also dependent on long-term or prior dietary practices (22, 36, 47, 59), such as fiber intake. Thus, studies should always survey participants’ dietary habits to determine any effect of baseline intake on the microbiota and how this may prime the response to a specific dietary intervention.
Metabolic flexibility was discussed at the beginning of this article when considering the longevity of an individual's metabolic response. However, metabolic flexibility may also refer to the short-term ability of an individual to adapt their metabolism to available fuel sources (106), which may have implications for their response to diets of different compositions. This flexibility may be influenced by factors such as physical activity, age, and diet and it is unclear whether or to what extent this flexibility can be restored once an inflexible state has been established as a result of obesity, diabetes, or aging (106). What stimulus is needed to significantly shift metabolism, the duration of this stimulus and its effect, and the magnitude of this effect are all important questions that must be answered, especially with respect to the effects of sustained shifts in diet. In addition, the answers to these questions will certainly differ based on the starting point of the individual in terms of their physical health as well as their age, genetics, epigenetics, and microbiome because this has already been demonstrated to an extent in mice (46).
The gut microbiome is a factor that is completely absent from these investigations and it is therefore of interest to determine whether certain features of the gut microbiome may confer greater metabolic flexibility on the host and if this can be utilized to manipulate metabolic flexibility or tendencies. In addition, as detailed below, metabolism and the microbiome are also influenced by genetics and epigenetics that remain stable throughout most of an individual's lifetime, such as lactase persistence status or persistent epigenetic marks that are established in utero (107, 108). These factors may limit the potential effects of the microbiome and its predictive ability, at least when it is considered in isolation.
Contribution of genetics and epigenetics
Contribution of genetics and epigenetics to personalized response
Currently, few studies have combined microbiome, genetic, epigenetic, metabolomic, and clinical markers in a comprehensive attempt at developing precision nutrition models. Genetics and epigenetics also contribute to an individual's metabolic phenotype and may therefore contribute to limits on metabolic flexibility and the microbiome. For example, Atkinson et al. (109) showed that individuals with higher copy numbers of salivary α-amylase gene (AMY1) digest starchy foods faster and show higher postprandial responses and lower breath hydrogen excretion than individuals with fewer copy numbers. Although these are both significant factors in prediction of dietary response, they are beyond the scope of this review and have already been comprehensively reviewed elsewhere (110–112). These factors, as well as others, also influence an individual's unique fingerprint of dietary response (Figure 3) and must be taken into account when designing predictive models or defining the limits to which the microbiome can be used as a definitive predictor of response. For example, the models used in Zeevi et al. (82) and Mendes-Soares et al. (83) incorporate both microbiota features and other individual features such as glycated hemoglobin, age, sex, BMI, physical activity, and many others, although host genetic data were not included.
FIGURE 3.
Contribution to the fingerprint of personalized response. In addition to the microbiota, other individual factors such as genetics, epigenetics, and lifestyle factors contribute to an individual's personalized response to diet.
Contribution of genetics and epigenetics to the microbiota and response to diet
In addition, genetics and epigenetics are not independent of the microbiome and this interaction must also be taken into account. An individual's genetics has a role in determining their microbiome, particularly, although not surprisingly, with respect to genes involved in immunity and metabolism, the most well-known and consistent association being that of variants in the lactase (LCT) gene region with the abundance of Bifidobacteria (7–9, 32). A study by O'Connor et al. (113) investigated the interaction between genetics, diet, and the microbiome as it concerned cardiometabolic response to atherogenic nutrients. This study did identify certain taxa that were significantly associated with genetic strain, diet, or both as well as cardiometabolic phenotypes associated with strain, diet, and taxa before or after the diet intervention. However, it remained unclear how all of these factors could be integrated to define or predict response. In addition, the applicability of these taxa as well as the relevant genes that affect this gene–microbiome–diet interaction must be identified and confirmed in humans. Although some studies such as Rothschild et al. (114) indicate that the contribution of genetics to the microbiome is small (1.9–8.1%), it is agreed that this field requires more research.
In addition to genetics, early-life environmental exposures and epigenetic programming in utero that may persist throughout the individual's lifetime add another layer of regulation of the microbiome (107, 115). However, this is not a one-sided relation. The gut microbiome has been shown to shape both genetic and epigenetic expression and development (116). Mechanisms by which this occurs include regulation of microRNA production, which regulates host gene expression as well as feedback that regulates microbiota composition, or production of metabolites involved in epigenetic processes, which can act as substrates used for epigenetic modification or modify the activity of enzymes involved in epigenetic modifications (117, 118). The limits of this effect and how diet may be used to modulate this interaction are unclear.
Limits of genetic and epigenetic effects on the microbiota and response to diet
Despite the influence of genetics and epigenetics, it has been found that diet affects the microbiota in certain ways regardless of genotype. For instance, studies have shown that the microbiota of different genetic strains of mice fed either a high-fat/high-sucrose diet or an unpurified/low-fat, high–plant polysaccharide diet cluster by diet, illustrating the robust reproducible effect of diet on the microbiome (46, 47). However, these studies also demonstrate that the genetic background of the mice can affect the plasticity of the microbiome, with strains clustering separately within each diet cluster and some showing a greater shift in microbiome composition than others. In addition, many twin studies have shown that, although genetics does contribute to similarity in microbiome composition, lifestyle factors such as diet and differential acquisition of epigenetic marks have a significant effect as well (119, 120). Furthermore, there is a wealth of evidence that early-life and neonatal exposures have long-lasting effects on metabolism (121, 122) and the gut microbiome (115, 123), and the extent to which these can be mitigated or change later in life requires further investigation.
Metabolomics and the microbiome
Metabolites, although more indicative of the result of the interaction between the microbiome, genetics, and epigenetics, are often informative and can also be used to predict responsiveness. Although some metabolomic studies have identified microbial metabolites that are associated with or predictive of response to diet or drugs (33, 124), they have not always directly interrogated the composition or function of the gut microbiome. Likewise, some studies have found differences in baseline clinical characteristics associated with response to intervention (125–128), particularly measures of insulin and glucose metabolism (129), but again have failed to incorporate the gut microbiome. This integration is crucial to the success of the efficacy of these models because each of these components contributes significantly to the individual's health and metabolic response to diet.
Clinical and specialty applications
As discussed above, a complete, or at least adequate, understanding of the human-microbiome supraorganism requires the integration of a multitude of factors and understanding how they interact to produce an individual's metabolic phenotype. That being said, it is also crucial to identify those factors (i.e., genetics, microbiome, etc.) and subsets of factors (i.e., specific genes, bacterial taxa, etc.) that contribute the most to an individual's metabolic phenotype and to develop methods to easily measure these characteristics in a clinical setting. If this is not done, even the best model will fail to have any meaningful impact because it will not be accessible to the majority of individuals who would benefit from personalized dietary advice. It is therefore crucial to increase not only the efficacy of such models but also their efficiency and accessibility. This will require both mechanistic as well as technological advances in the field in order to identify significant factors and make their identification in a clinical setting affordable and practicable.
Although the research thus far has focused primarily on response to diet with respect to improvement and maintenance of general health and well-being, this approach can be utilized for a whole spectrum of health concerns from nutrient supplementation in malnourished populations to optimization of performance in athletes. The efficacy of nutrient supplementation interventions in food-insecure populations is mixed, with some trials showing modest overall effects and others showing no significant effects (130). The potential of the gut microbiota to modify the effects of these interventions is a line of research that has not yet been pursued but may be relevant, especially when considering supplementation in infants. Early gut microbiota composition in children has been shown to predict later growth and health outcomes (131, 132), underscoring the importance of early microbiota composition in development. Furthermore, the infant diet, primarily composed of breast milk or formula, has a distinct effect on the assembly and development of the infant microbiota (123, 133–135). Therefore, it may be of interest to determine if certain taxa or patterns of microbiota composition may modify the effects of micro- or macronutrient supplementation in these populations and determine whether the addition of pre- or probiotics to supplements may act as adjuvants to bolster their effect.
Another potential application of this area of research is in the field of athletic performance nutrition. Athletes are individuals for whom optimal performance is crucial, and optimal nutrition is critical in achieving this aim. Therefore, future research in the field of precision nutrition should also explore outcomes such as muscle protein synthesis, glycogen formation, fuel utilization, and inflammatory markers such as creatine kinase and the responses of different individual athletes to different protein sources (e.g., whey, casein, and soy), carbohydrates, and fats in combination with their training regime. As mentioned, optimal performance is of paramount importance to athletes, making them extremely motivated to capitalize on any factors that could enhance their fitness and performance, thus making them prime targets for precision nutrition.
Beyond nutrition
It is also important to remember that “food is not only nutrition” (136), meaning that food is more than just a combination of nutrients. Food is an important social, cultural, and personal aspect of individuals’ lives and these factors must also be taken into account when providing personalized dietary advice. The best diet is useless if not followed, and adherence is much more likely when the food is accessible, palatable, and culturally appropriate. Although these qualitative factors are not as much of an issue when simply trying to determine how an individual will quantitatively respond to diet, they become much more important when ultimately devising dietary recommendations. Therefore, it is important to take into account factors such as ethnicity, socioeconomic status, religion, and lifestyle when tailoring dietary advice to the individual.
Conclusion
Although studies are beginning to address the questions of precision nutrition and the role of the gut microbiome, we are far from gaining conclusive or comprehensive answers. Although the answers provided by the studies outlined in this review are parts of the puzzle, they also show us how many pieces are still missing: enterotypes, diversity, richness, specific taxa, and functions have all been found to have some association with different responses in different dietary contexts. The field of precision nutrition is still in its infancy, although the rate at which it is developing resembles more the growth spurt of a gangly teenager. Growing pains are to be expected and an abundance of frustration is unavoidable, as with any typical teenager. However, as we nurture and invest in this endeavor, the field will develop and mature, becoming more coherent and useful over time.
Acknowledgments
The authors’ responsibilities were as follows—RLH: performed the literature review and conceived and composed the manuscript; MM, NLK, and MEK: provided content and formatting advice and edited the final manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by the University of California Innovation Institute for Food and Health, Agricultural Research Service CRIS Projects 2032-51530-022-00D and 2032-53000-001-00D, Arcadia Biosciences and Ardent Mills. The funding agencies did not participate in study design, data collection, or interpretation of the data. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity provider and employer. Figures were created using BioRender (https://biorender.com).
Author disclosures: RLH, MEK, MM, and NLK, no conflicts of interest.
Abbreviations used: IX, isoxanthohumol; P:B ratio, ratio of Prevotella to Bacteroides; TC, total cholesterol; TMAO, trimethylamine-N-oxide; UM, urolithin metabotype; 8-PN, 8-prenylnaringenin.
References
- 1. Bauer UE, Briss PA, Goodman RA, Bowman BA. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014;384(9937):45–52. [DOI] [PubMed] [Google Scholar]
- 2. Liu S. Intake of refined carbohydrates and whole grain foods in relation to risk of type 2 diabetes mellitus and coronary heart disease. J Am Coll Nutr. 2002;21(4):298–306. [DOI] [PubMed] [Google Scholar]
- 3. Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449(7164):804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP et al.. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koppel N, Balskus EP. Exploring and understanding the biochemical diversity of the human microbiota. Cell Chem Biol. 2016;23(1):18–30. [DOI] [PubMed] [Google Scholar]
- 6. De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107(33):14691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benson AK, Kelly SA, Legge R, Ma F, Low SJ, Kim J, Zhang M, Oh PL, Nehrenberg D, Hua K et al.. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci U S A. 2010;107(44):18933–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT et al.. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonder MJ, Kurilshikov A, Tigchelaar EF, Mujagic Z, Imhann F, Vila AV, Deelen P, Vatanen T, Schirmer M, Smeekens SP et al.. The effect of host genetics on the gut microbiome. Nat Genet. 2016;48(11):1407–12. [DOI] [PubMed] [Google Scholar]
- 10. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA et al.. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–7. [DOI] [PubMed] [Google Scholar]
- 12. Lamichhane S, Sen P, Dickens AM, Oresic M, Bertram HC. Gut metabolome meets microbiome: a methodological perspective to understand the relationship between host and microbe. Methods. 2018;149:3–12. [DOI] [PubMed] [Google Scholar]
- 13. Janssen AW, Kersten S. Potential mediators linking gut bacteria to metabolic health: a critical view. J Physiol. 2017;595(2):477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee WJ, Hase K. Gut microbiota–generated metabolites in animal health and disease. Nat Chem Biol. 2014;10(6):416–24. [DOI] [PubMed] [Google Scholar]
- 15. Zhang Y-J, Li S, Gan R-Y, Zhou T, Xu D-P, Li H-B. Impacts of gut bacteria on human health and diseases. Int J Mol Sci. 2015;16(4):7493–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu Rev Med. 2011;62:361–80. [DOI] [PubMed] [Google Scholar]
- 17. Healey GR, Murphy R, Brough L, Butts CA, Coad J. Interindividual variability in gut microbiota and host response to dietary interventions. Nutr Rev. 2017;75(12):1059–80. [DOI] [PubMed] [Google Scholar]
- 18. Bashiardes S, Godneva A, Elinav E, Segal E. Towards utilization of the human genome and microbiome for personalized nutrition. Curr Opin Biotechnol. 2017;51:57–63. [DOI] [PubMed] [Google Scholar]
- 19. Adalsteinsdottir SA, Magnusdottir OK, Halldorsson TI, Birgisdottir BE. Towards an individualized nutrition treatment: role of the gastrointestinal microbiome in the interplay between diet and obesity. Curr Obes Rep. 2018;7(4):289–93. [DOI] [PubMed] [Google Scholar]
- 20. Sonnenburg JL, Bäckhed F. Diet–microbiota interactions as moderators of human metabolism. Nature. 2016;535(7610):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Christensen L, Roager HM, Astrup A, Hjorth MF. Microbial enterotypes in personalized nutrition and obesity management. Am J Clin Nutr. 2018;108(4):645–51. [DOI] [PubMed] [Google Scholar]
- 22. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R et al.. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326(5960):1694–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. David LA, Materna AC, Friedman J, Campos-Baptista MI, Blackburn MC, Perrotta A, Erdman SE, Alm EJ. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014;15(7):R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davenport ER, Mizrahi-Man O, Michelini K, Barreiro LB, Ober C, Gilad Y. Seasonal variation in human gut microbiome composition. PLoS One. 2014;9(3):e90731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caporaso JG, Lauber CL, Costello EK, Berg-Lyons D, Gonzalez A, Stombaugh J, Knights D, Gajer P, Ravel J, Fierer N. Moving pictures of the human microbiome. Genome Biol. 2011;12(5):R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hjorth MF, Roager HM, Larsen TM, Poulsen SK, Licht TR, Bahl MI, Zohar Y, Astrup A. Pre-treatment microbial Prevotella-to-Bacteroides ratio, determines body fat loss success during a 6-month randomized controlled diet intervention. Int J Obes (Lond). 2018;42(3):580–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roager HM, Licht TR, Poulsen SK, Larsen TM, Bahl MI. Microbial enterotypes, inferred by the Prevotella-to-Bacteroides ratio, remained stable during a 6-month randomized controlled diet intervention with the new Nordic diet. Appl Environ Microbiol. 2014;80(3):1142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hjorth M, Blædel T, Bendtsen L, Lorenzen JK, Holm JB, Kiilerich P, Roager HM, Kristiansen K, Larsen LH, Astrup A. Prevotella-to-Bacteroides ratio predicts body weight and fat loss success on 24-week diets varying in macronutrient composition and dietary fiber: results from a post-hoc analysis. Int J Obes (Lond). 2019;43(1):149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kang C, Zhang Y, Zhu X, Liu K, Wang X, Chen M, Wang J, Chen H, Hui S, Huang L et al.. Healthy subjects differentially respond to dietary capsaicin correlating with specific gut enterotypes. J Clin Endocrinol Metab. 2016;101(12):4681–9. [DOI] [PubMed] [Google Scholar]
- 31. Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL et al.. The long-term stability of the human gut microbiota. Science. 2013;341(6141):1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goodrich JK, Davenport ER, Waters JL, Clark AG, Ley RE. Cross-species comparisons of host genetic associations with the microbiome. Science. 2016;352(6285):532–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heinzmann SS, Merrifield CA, Rezzi S, Kochhar S, Lindon JC, Holmes E, Nicholson JK. Stability and robustness of human metabolic phenotypes in response to sequential food challenges. J Proteome Res. 2012;11(2):643–55. [DOI] [PubMed] [Google Scholar]
- 34. Assfalg M, Bertini I, Colangiuli D, Luchinat C, Schafer H, Schutz B, Spraul M. Evidence of different metabolic phenotypes in humans. Proc Natl Acad Sci U S A. 2008;105(5):1420–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bernini P, Bertini I, Luchinat C, Nepi S, Saccenti E, Schäfer H, Schütz B, Spraul M, Tenori L. Individual human phenotypes in metabolic space and time. J Proteome Res. 2009;8(9):4264–71. [DOI] [PubMed] [Google Scholar]
- 36. Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Bjorck I, Backhed F. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 2015;22(6):971–82. [DOI] [PubMed] [Google Scholar]
- 37. McNulty NP, Yatsunenko T, Hsiao A, Faith JJ, Muegge BD, Goodman AL, Henrissat B, Oozeer R, Cools-Portier S, Gobert G. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci Transl Med. 2011;3(106):106ra106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Possemiers S, Rabot S, Espín JC, Bruneau A, Philippe C, González-Sarrías A, Heyerick A, Tomás-Barberán FA, De Keukeleire D, Verstraete W. Eubacterium limosum activates isoxanthohumol from hops (Humulus lupulus L.) into the potent phytoestrogen 8-prenylnaringenin in vitro and in rat intestine. J Nutr. 2008;138(7):1310–16. [DOI] [PubMed] [Google Scholar]
- 39. Routy B, Le Chatelier E, Derosa L, Duong CP, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–7. [DOI] [PubMed] [Google Scholar]
- 40. Heyerick A, Vervarcke S, Depypere H, Bracke M, De Keukeleire D. A first prospective, randomized, double-blind, placebo-controlled study on the use of a standardized hop extract to alleviate menopausal discomforts. Maturitas. 2006;54(2):164–75. [DOI] [PubMed] [Google Scholar]
- 41. Bolca S, Possemiers S, Maervoet V, Huybrechts I, Heyerick A, Vervarcke S, Depypere H, De Keukeleire D, Bracke M, De Henauw S. Microbial and dietary factors associated with the 8-prenylnaringenin producer phenotype: a dietary intervention trial with fifty healthy post-menopausal Caucasian women. Br J Nutr. 2007;98(5):950–9. [DOI] [PubMed] [Google Scholar]
- 42. Griffin NW, Ahern PP, Cheng J, Heath AC, Ilkayeva O, Newgard CB, Fontana L, Gordon JI. Prior dietary practices and connections to a human gut microbial metacommunity alter responses to diet interventions. Cell Host Microbe. 2017;21(1):84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Faith JJ, McNulty NP, Rey FE, Gordon JI. Predicting a human gut microbiota's response to diet in gnotobiotic mice. Science. 2011;333(6038):101–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smits SA, Marcobal A, Higginbottom S, Sonnenburg JL, Kashyap PC. Individualized responses of gut microbiota to dietary intervention modeled in humanized mice. mSystems. 2016;1(5):00098–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kreznar JH, Keller MP, Traeger LL, Rabaglia ME, Schueler KL, Stapleton DS, Zhao W, Vivas EI, Yandell BS, Broman AT et al.. Host genotype and gut microbiome modulate insulin secretion and diet-induced metabolic phenotypes. Cell Rep. 2017;18(7):1739–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parks BW, Nam E, Org E, Kostem E, Norheim F, Hui ST, Pan C, Civelek M, Rau CD, Bennett BJ et al.. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab. 2013;17(1):141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carmody RN, Gerber GK, Luevano JM Jr, Gatti DM, Somes L, Svenson KL, Turnbaugh PJ. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 2015;17(1):72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang C, Derrien M, Levenez F, Brazeilles R, Ballal SA, Kim J, Degivry MC, Quere G, Garault P, van Hylckama Vlieg JE et al.. Ecological robustness of the gut microbiota in response to ingestion of transient food-borne microbes. ISME J. 2016;10(9):2235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Veiga P, Gallini CA, Beal C, Michaud M, Delaney ML, DuBois A, Khlebnikov A, van Hylckama Vlieg JE, Punit S, Glickman JN. Bifidobacterium animalis subsp. lactis fermented milk product reduces inflammation by altering a niche for colitogenic microbes. Proc Natl Acad Sci U S A. 2010;107(42):18132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen T, Long W, Zhang C, Liu S, Zhao L, Hamaker BR. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci Rep. 2017;7(1):2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sonnenburg ED, Zheng H, Joglekar P, Higginbottom SK, Firbank SJ, Bolam DN, Sonnenburg JL. Specificity of polysaccharide use in intestinal Bacteroides species determines diet-induced microbiota alterations. Cell. 2010;141(7):1241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hullar MA, Lancaster SM, Li F, Tseng E, Beer K, Atkinson C, Wahala K, Copeland WK, Randolph TW, Newton KM et al.. Enterolignan-producing phenotypes are associated with increased gut microbial diversity and altered composition in premenopausal women in the United States. Cancer Epidemiol Biomarkers Prev. 2015;24(3):546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S et al.. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–6. [DOI] [PubMed] [Google Scholar]
- 54. Gu Y, Wang X, Li J, Zhang Y, Zhong H, Liu R, Zhang D, Feng Q, Xie X, Hong J. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat Commun. 2017;8(1):1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kolho KL, Korpela K, Jaakkola T, Pichai MV, Zoetendal EG, Salonen A, de Vos WM. Fecal microbiota in pediatric inflammatory bowel disease and its relation to inflammation. Am J Gastroenterol. 2015;110(6):921–30. [DOI] [PubMed] [Google Scholar]
- 56. Korpela K, Flint HJ, Johnstone AM, Lappi J, Poutanen K, Dewulf E, Delzenne N, de Vos WM, Salonen A. Gut microbiota signatures predict host and microbiota responses to dietary interventions in obese individuals. PLoS One. 2014;9(6):e90702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Korem T, Zeevi D, Zmora N, Weissbrod O, Bar N, Lotan-Pompan M, Avnit-Sagi T, Kosower N, Malka G, Rein M et al.. Bread affects clinical parameters and induces gut microbiome-associated personal glycemic responses. Cell Metab. 2017;25(6):1243–53..e5. [DOI] [PubMed] [Google Scholar]
- 58. Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359(6380):1151–6. [DOI] [PubMed] [Google Scholar]
- 59. Salonen A, Lahti L, Salojarvi J, Holtrop G, Korpela K, Duncan SH, Date P, Farquharson F, Johnstone AM, Lobley GE et al.. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014;8(11):2218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, Brown D, Stares MD, Scott P, Bergerat A et al.. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5(2):220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tap J, Furet JP, Bensaada M, Philippe C, Roth H, Rabot S, Lakhdari O, Lombard V, Henrissat B, Corthier G et al.. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environ Microbiol. 2015;17(12):4954–64. [DOI] [PubMed] [Google Scholar]
- 62. Martinez I, Kim J, Duffy PR, Schlegel VL, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS One. 2010;5(11):e15046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Martinez I, Lattimer JM, Hubach KL, Case JA, Yang J, Weber CG, Louk JA, Rose DJ, Kyureghian G, Peterson DA et al.. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2013;7(2):269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Venkataraman A, Sieber JR, Schmidt AW, Waldron C, Theis KR, Schmidt TM. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome. 2016;4(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Davis LM, Martinez I, Walter J, Goin C, Hutkins RW. Barcoded pyrosequencing reveals that consumption of galactooligosaccharides results in a highly specific bifidogenic response in humans. PLoS One. 2011;6(9):e25200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bouhnik Y, Raskine L, Simoneau G, Vicaut E, Neut C, Flourié B, Brouns F, Bornet FR. The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: a double-blind, randomized, placebo-controlled, parallel-group, dose-response relation study. Am J Clin Nutr. 2004;80(6):1658–64. [DOI] [PubMed] [Google Scholar]
- 67. Tuohy KM, Kolida S, Lustenberger AM, Gibson GR. The prebiotic effects of biscuits containing partially hydrolysed guar gum and fructo-oligosaccharides – a human volunteer study. Br J Nutr. 2007;86(3):341–8. [DOI] [PubMed] [Google Scholar]
- 68. Eid N, Osmanova H, Natchez C, Walton G, Costabile A, Gibson G, Rowland I, Spencer JP. Impact of palm date consumption on microbiota growth and large intestinal health: a randomised, controlled, cross-over, human intervention study. Br J Nutr. 2015;114(8):1226–36. [DOI] [PubMed] [Google Scholar]
- 69. Tuohy KM, Finlay RK, Wynne AG, Gibson GR. A human volunteer study on the prebiotic effects of HP-inulin—faecal bacteria enumerated using fluorescent in situ hybridisation (FISH). Anaerobe. 2001;7(3):113–18. [Google Scholar]
- 70. Kolida S, Meyer D, Gibson GR. A double-blind placebo-controlled study to establish the bifidogenic dose of inulin in healthy humans. Eur J Clin Nutr. 2007;61(10):1189–95. [DOI] [PubMed] [Google Scholar]
- 71. de Preter V, Vanhoutte T, Huys G, Swings J, Rutgeerts P, Verbeke K. Baseline microbiota activity and initial bifidobacteria counts influence responses to prebiotic dosing in healthy subjects. Aliment Pharmacol Ther. 2008;27(6):504–13. [DOI] [PubMed] [Google Scholar]
- 72. Holscher HD, Bauer LL, Gourineni V, Pelkman CL, Fahey GC Jr, Swanson KS. Agave inulin supplementation affects the fecal microbiota of healthy adults participating in a randomized, double-blind, placebo-controlled, crossover trial. J Nutr. 2015;145(9):2025–32. [DOI] [PubMed] [Google Scholar]
- 73. Fuller Z, Louis P, Mihajlovski A, Rungapamestry V, Ratcliffe B, Duncan AJ. Influence of cabbage processing methods and prebiotic manipulation of colonic microflora on glucosinolate breakdown in man. Br J Nutr. 2007;98(2):364–72. [DOI] [PubMed] [Google Scholar]
- 74. Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N et al.. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500(7464):585–8. [DOI] [PubMed] [Google Scholar]
- 75. Shoaie S, Ghaffari P, Kovatcheva-Datchary P, Mardinoglu A, Sen P, Pujos-Guillot E, de Wouters T, Juste C, Rizkalla S, Chilloux J et al.. Quantifying diet-induced metabolic changes of the human gut microbiome. Cell Metab. 2015;22(2):320–31. [DOI] [PubMed] [Google Scholar]
- 76. Kong LC, Wuillemin PH, Bastard JP, Sokolovska N, Gougis S, Fellahi S, Darakhshan F, Bonnefont-Rousselot D, Bittar R, Dore J et al.. Insulin resistance and inflammation predict kinetic body weight changes in response to dietary weight loss and maintenance in overweight and obese subjects by using a Bayesian network approach. Am J Clin Nutr. 2013;98(6):1385–94. [DOI] [PubMed] [Google Scholar]
- 77. Piening BD, Zhou W, Contrepois K, Rost H, Gu Urban GJ, Mishra T, Hanson BM, Bautista EJ, Leopold S, Yeh CY et al.. Integrative personal omics profiles during periods of weight gain and loss. Cell Syst. 2018;6(2):157–70..e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Santacruz A, Marcos A, Warnberg J, Marti A, Martin-Matillas M, Campoy C, Moreno LA, Veiga O, Redondo-Figuero C, Garagorri JM et al.. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity (Silver Spring). 2009;17(10):1906–15. [DOI] [PubMed] [Google Scholar]
- 79. Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L et al.. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65(3):426–36. [DOI] [PubMed] [Google Scholar]
- 80. Zou H, Wang D, Ren H, Fang C, Shi Z, Zhang P, Chen P, Wang J, Yang H, Cai K et al.. Nonobese subjects of Bacteroides and Prevotella enterotypes responded differentially to calorie restriction intervention. bioRxiv. 2019:514596. [Google Scholar]
- 81. Muñiz Pedrogo DA, Jensen MD, Van Dyke CT, Murray JA, Woods JA, Chen J, Kashyap PC, Nehra V. Gut microbial carbohydrate metabolism hinders weight loss in overweight adults undergoing lifestyle intervention with a volumetric diet. Mayo Clin Proc. 2018;93(8):1104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M et al.. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079–94. [DOI] [PubMed] [Google Scholar]
- 83. Mendes-Soares H, Raveh-Sadka T, Azulay S, Edens K, Ben-Shlomo Y, Cohen Y, Ofek T, Bachrach D, Stevens J, Colibaseanu D. Assessment of a personalized approach to predicting postprandial glycemic responses to food among individuals without diabetes. JAMA Network Open. 2019;2(2):e188102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bennet SMP, Bohn L, Storsrud S, Liljebo T, Collin L, Lindfors P, Tornblom H, Ohman L, Simren M. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut. 2018;67(5):872–81. [DOI] [PubMed] [Google Scholar]
- 85. Cho CE, Taesuwan S, Malysheva OV, Bender E, Tulchinsky NF, Yan J, Sutter JL, Caudill MA. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: a randomized controlled trial. Mol Nutr Food Res. 2017;61(1):1600324. [DOI] [PubMed] [Google Scholar]
- 86. Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A et al.. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521):181–6. [DOI] [PubMed] [Google Scholar]
- 87. Possemiers S, Bolca S, Eeckhaut E, Depypere H, Verstraete W. Metabolism of isoflavones, lignans and prenylflavonoids by intestinal bacteria: producer phenotyping and relation with intestinal community. FEMS Microbiol Ecol. 2007;61(2):372–83. [DOI] [PubMed] [Google Scholar]
- 88. Romo-Vaquero M, Cortés‐Martín A, Loria‐Kohen V, Ramírez‐de‐Molina A, García‐Mantrana I, Collado MC, Espín JC, Selma MV. Deciphering the human gut microbiome of urolithin metabotypes: association with enterotypes and potential cardiometabolic health implications. Mol Nutr Food Res. 2019;63(4):1800958. [DOI] [PubMed] [Google Scholar]
- 89. Li F, Hullar MA, Beresford SA, Lampe JW. Variation of glucoraphanin metabolism in vivo and ex vivo by human gut bacteria. Br J Nutr. 2011;106(3):408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, Kotler E, Zur M, Regev-Lehavi D, Brik RB-Z. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018;174(6):1388–405..e21. [DOI] [PubMed] [Google Scholar]
- 91. Senan S, Prajapati JB, Joshi CG, Sreeja V, Gohel MK, Trivedi S, Patel RM, Pandya H, Singh US, Phatak A et al.. Geriatric respondents and non-respondents to probiotic intervention can be differentiated by inherent gut microbiome composition. Front Microbiol. 2015;6:944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Volokh O, Klimenko N, Berezhnaya Y, Tyakht A, Nesterova P, Popenko A, Alexeev D. Human gut microbiome response induced by fermented dairy product intake in healthy volunteers. Nutrients. 2019;11(3):547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mobini R, Tremaroli V, Ståhlman M, Karlsson F, Levin M, Ljungberg M, Sohlin M, Bertéus Forslund H, Perkins R, Bäckhed F. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2017;19(4):579–89. [DOI] [PubMed] [Google Scholar]
- 94. Chumpitazi BP, Cope JL, Hollister EB, Tsai CM, McMeans AR, Luna RA, Versalovic J, Shulman RJ. Randomised clinical trial: gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Aliment Pharmacol Ther. 2015;42(4):418–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140(3):976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107(33):14691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost F, Brummer RJ. The role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27(2):104–19. [DOI] [PubMed] [Google Scholar]
- 98. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. [DOI] [PubMed] [Google Scholar]
- 99. Bolca S, Van de Wiele T, Possemiers S. Gut metabotypes govern health effects of dietary polyphenols. Curr Opin Biotechnol. 2013;24(2):220–5. [DOI] [PubMed] [Google Scholar]
- 100. Selma MV, González-Sarrías A, Salas-Salvadó J, Andrés-Lacueva C, Alasalvar C, Örem A, Tomás-Barberán FA, Espín JC. The gut microbiota metabolism of pomegranate or walnut ellagitannins yields two urolithin-metabotypes that correlate with cardiometabolic risk biomarkers: comparison between normoweight, overweight-obesity and metabolic syndrome. Clin Nutr. 2018;37(3):897–905. [DOI] [PubMed] [Google Scholar]
- 101. González‐Sarrías A, García‐Villalba R, Romo-Vaquero M, Alasalvar C, Örem A, Zafrilla P, Tomás‐Barberán FA, Selma MV, Espín JC. Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight‐obese individuals consuming pomegranate: a randomized clinical trial. Mol Nutr Food Res. 2017;61(5):1600830. [DOI] [PubMed] [Google Scholar]
- 102. Cortés-Martín A, García-Villalba R, González-Sarrías A, Romo-Vaquero M, Loria-Kohen V, Ramírez-de-Molina A, Tomás-Barberán F, Selma M, Espín J. The gut microbiota urolithin metabotypes revisited: the human metabolism of ellagic acid is mainly determined by aging. Food Funct. 2018;9(8):4100–6. [DOI] [PubMed] [Google Scholar]
- 103. Romo-Vaquero M, García-Villalba R, González-Sarrías A, Beltrán D, Tomás-Barberán FA, Espín JC, Selma MV. Interindividual variability in the human metabolism of ellagic acid: contribution of Gordonibacter to urolithin production. J Funct Foods. 2015;17:785–91. [Google Scholar]
- 104. Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev. 1998;7(12):1091–100. [PubMed] [Google Scholar]
- 105. Sawicki CM, Livingston KA, Obin M, Roberts SB, Chung M, McKeown NM. Dietary fiber and the human gut microbiota: application of evidence mapping methodology. Nutrients. 2017;9(2):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Storlien L, Oakes ND, Kelley DE. Metabolic flexibility. Proc Nutr Soc. 2004;63(2):363–8. [DOI] [PubMed] [Google Scholar]
- 107. Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey L. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105(44):17046–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Comerford KB, Pasin G. Gene–dairy food interactions and health outcomes: a review of nutrigenetic studies. Nutrients. 2017;9(7):710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Atkinson FS, Hancock D, Petocz P, Brand-Miller JC. The physiologic and phenotypic significance of variation in human amylase gene copy number. Am J Clin Nutr. 2018;108(4):737–48. [DOI] [PubMed] [Google Scholar]
- 110. Ortega A, Berna G, Rojas A, Martin F, Soria B. Gene-diet interactions in type 2 diabetes: the chicken and egg debate. Int J Mol Sci. 2017;18(6):1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ramos-Lopez O, Milagro FI, Allayee H, Chmurzynska A, Choi MS, Curi R, De Caterina R, Ferguson LR, Goni L, Kang JX et al.. Guide for current nutrigenetic, nutrigenomic, and nutriepigenetic approaches for precision nutrition involving the prevention and management of chronic diseases associated with obesity. J Nutrigenet Nutrigenomics. 2017;10(1–2):43–62. [DOI] [PubMed] [Google Scholar]
- 112. Wang DD, Hu FB. Precision nutrition for prevention and management of type 2 diabetes. Lancet Diabetes Endocrinol. 2018;6(5):416–26. [DOI] [PubMed] [Google Scholar]
- 113. O'Connor A, Quizon PM, Albright JE, Lin FT, Bennett BJ. Responsiveness of cardiometabolic-related microbiota to diet is influenced by host genetics. Mamm Genome. 2014;25(11–12):583–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N et al.. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210–15. [DOI] [PubMed] [Google Scholar]
- 115. Indrio F, Martini S, Francavilla R, Corvaglia L, Cristofori F, Mastrolia SA, Neu J, Rautava S, Russo Spena G, Raimondi F et al.. Epigenetic matters: the link between early nutrition, microbiome, and long-term health development. Front Pediatr. 2017;5:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sook Lee E, Ji Song E, Do Nam Y. Dysbiosis of gut microbiome and its impact on epigenetic regulation. J Clin Epigenet. 2017;3(2):100048. [Google Scholar]
- 117. Dalmasso G, Nguyen HT, Yan Y, Laroui H, Charania MA, Ayyadurai S, Sitaraman SV, Merlin D. Microbiota modulate host gene expression via microRNAs. PLoS One. 2011;6(4):e19293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hullar MA, Fu BC. Diet, the gut microbiome, and epigenetics. Cancer J. 2014;20(3):170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suner D, Cigudosa JC, Urioste M, Benitez J et al.. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102(30):10604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Xie H, Guo R, Zhong H, Feng Q, Lan Z, Qin B, Ward KJ, Jackson MA, Xia Y, Chen X et al.. Shotgun metagenomics of 250 adult twins reveals genetic and environmental impacts on the gut microbiome. Cell Syst. 2016;3(6):572–84..e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8(4):253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118(2):511–21. [DOI] [PubMed] [Google Scholar]
- 124. Clayton TA, Baker D, Lindon JC, Everett JR, Nicholson JK. Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proc Natl Acad Sci U S A. 2009;106(34):14728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Westerman K, Reaver A, Blander G, Nogal B, Ward C, Benard T, Katz D, Blumberg J. An algorithm-based personalized nutrition platform improves metabolic biomarkers. J Acad Nutr Diet. 2017;117(9):A99. [Google Scholar]
- 126. O'Donovan CB, Walsh MC, Nugent AP, McNulty B, Walton J, Flynn A, Gibney MJ, Gibney ER, Brennan L. Use of metabotyping for the delivery of personalised nutrition. Mol Nutr Food Res. 2015;59(3):377–85. [DOI] [PubMed] [Google Scholar]
- 127. Lefevre M, Champagne CM, Tulley RT, Rood JC, Most MM. Individual variability in cardiovascular disease risk factor responses to low-fat and low-saturated-fat diets in men: body mass index, adiposity, and insulin resistance predict changes in LDL cholesterol. Am J Clin Nutr. 2005;82(5):957–63. [DOI] [PubMed] [Google Scholar]
- 128. Gower BA, Bergman R, Stefanovski D, Darnell B, Ovalle F, Fisher G, Sweatt SK, Resuehr HS, Pelkman C. Baseline insulin sensitivity affects response to high-amylose maize resistant starch in women: a randomized, controlled trial. Nutr Metab (Lond). 2016;13(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hjorth MF, Zohar Y, Hill JO, Astrup A. Personalized dietary management of overweight and obesity based on measures of insulin and glucose. Annu Rev Nutr. 2018;38:245–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, Webb P, Lartey A, Black RE; The Lancet Nutrition Interventions Review Group. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost?. Lancet. 2013;382(9890):452–77. [DOI] [PubMed] [Google Scholar]
- 131. Kalliomäki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87(3):534–8. [DOI] [PubMed] [Google Scholar]
- 132. Azad MB, Konya T, Guttman DS, Field CJ, Sears MR, HayGlass KT, Mandhane PJ, Turvey SE, Subbarao P, Becker AB et al.. Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy. 2015;45(3):632–43. [DOI] [PubMed] [Google Scholar]
- 133. De Leoz ML, Kalanetra KM, Bokulich NA, Strum JS, Underwood MA, German JB, Mills DA, Lebrilla CB. Human milk glycomics and gut microbial genomics in infant feces show a correlation between human milk oligosaccharides and gut microbiota: a proof-of-concept study. J Proteome Res. 2015;14(1):491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lewis ZT, Totten SM, Smilowitz JT, Popovic M, Parker E, Lemay DG, Van Tassell ML, Miller MJ, Jin YS, German JB et al.. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome. 2015;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. LoCascio RG, Ninonuevo MR, Freeman SL, Sela DA, Grimm R, Lebrilla CB, Mills DA, German JB. Glycoprofiling of bifidobacterial consumption of human milk oligosaccharides demonstrates strain specific, preferential consumption of small chain glycans secreted in early human lactation. J Agric Food Chem. 2007;55(22):8914–19. [DOI] [PubMed] [Google Scholar]
- 136. Kohlmeier M, De Caterina R, Ferguson LR, Gorman U, Allayee H, Prasad C, Kang JX, Nicoletti CF, Martinez JA. Guide and position of the International Society of Nutrigenetics. /Nutrigenomics on personalized nutrition: part 2 - ethics, challenges and endeavors of precision nutrition. J Nutrigenet Nutrigenomics. 2016;9(1):28–46. [DOI] [PubMed] [Google Scholar]


