ABSTRACT
Administration of oropharyngeal colostrum (OPC) is safe, feasible, and potentially beneficial in preterm infants. We aimed to assess the effects of OPC in preterm infants. A systematic review of randomized controlled trials (RCTs) and non-RCTs of OPC administration in preterm infants was conducted. We searched MEDLINE via PubMed and Ovid, EMBASE, the Cochrane Central Register of Controlled Trials, Emcare databases, abstracts of Pediatric Academic Societies meetings, and gray literature in April 2018. Six RCTs (n = 269) and 4 non-RCTs (n = 737) were included. One RCT (n = 40) focused on enteral bovine colostrum and hence was excluded from our review. Five of the 6 RCTs had unclear risk of bias in many domains of assessment. Meta-analysis (random effects model) of RCT data showed no significant difference in ≥stage 2 necrotizing enterocolitis (RR: 0.83; 95% CI: 0.39, 1.75; P = 0.62), late-onset sepsis (RR: 0.78; 95% CI: 0.50, 1.22; P = 0.28), all-cause mortality (RR: 0.74; 95% CI: 0.27, 2.06; P = 0.56); duration of hospital stay (mean difference [MD]: −1.65 d; 95% CI: −10.09, 6.80; P = 0.70), and time to full feeds (MD: −2.86 d; 95% CI: −6.49, 0.77; P = 0.12). Meta-analysis of data from non-RCTs also showed no benefit for any of these outcomes. OPC increased secretory IgA and lactoferrin concentrations (4 RCTs), and had only a transient effect on the oral microbiome (1 RCT). There were no adverse effects (e.g., aspiration) of OPC. The overall quality of evidence (Grades of Recommendation, Assessment, Development, and Evaluation analysis) was very low. Adequately powered RCTs are needed to confirm the nutritional and immunomodulatory benefits of OPC in preterm infants.
Keywords: systematic review, meta-analysis, oropharyngeal colostrum, preterm infants, human colostrum
Introduction
Survival of extremely preterm infants has improved over the last few decades following advances in neonatal intensive care (1, 2). However, necrotizing enterocolitis (NEC) and late-onset sepsis (LOS) continue to be the major contributors to morbidity and mortality despite preventive strategies such as hand hygiene, probiotic supplementation, increased use of mother's own milk (MM), and aseptic precautions for insertion and maintenance of indwelling catheters and long lines (3–5).
Oropharyngeal administration of colostrum (OPC) is a simple, safe, and feasible intervention in infants (6–9). OPC has local immunostimulatory effects and can reduce the risk of sepsis while facilitating enteral feeds (10–12). The potential benefits of OPC relate to its bioactive factors (13). Colostral cytokines stimulate the oropharyngeal-associated lymphoid tissue (OPHALT) by activating T cells with systemic dissemination through cell-to-cell signaling (14). Milk oligosaccharides protect the mucosal barrier and stimulate growth of beneficial bacteria on the mucosa in a prebiotic effect. Secretory immunoglobulin A (sIgA) prevents attachment of pathogens to the mucosal surface. Lactoferrin protects the mucosal barrier by its anti-inflammatory effects and by promoting mucosal healing. Milk antioxidants protect against mucosal injury caused by free radicals and maintain membrane integrity. Intestinal trophic factors have trophic effects on immature intestinal cells. Milk biofactors are absorbed intact into the circulation and protect against NEC (10).
Studies support the potential of OPC to be an effective intervention in preterm infants (15–24). A recent randomized controlled trial (RCT) in preterm very-low-birth-weight (VLBW: birth weight <1500 g) infants evaluated the local and systemic immune effects of OPC, by measuring salivary and urinary concentrations of sIgA and lactoferrin on the first day of starting oropharyngeal colostrum and on the 7th and 21st day of life. A total of 64 preterm infants (mean [±SE] gestation: 29.86 ± 2.02 vs. 30.46 ± 2.5 wk) were randomly allocated to receive 0.2 mL of colostrum or normal saline every 4 h for 7 d. OPC increased salivary lactoferrin concentrations but had no effect on urinary sIgA and lactoferrin (15). Glass et al. have recently compared sIgA concentrations in 30 preterm VLBW infants (mean gestation: 28.4 ± 0.7 vs. 28.5 ± 0.8 wk) randomly allocated to receive oral care with 0.2 mL colostrum or sterile water every 3 h from days 2 to 7. Salivary sIgA was measured on days 2, 7, and 14. OPC increased salivary sIgA by the 7th day of life (16). Sohn et al. randomly allocated 12 VLBW infants (median [IQR] gestation: 27 [25–30] vs. 27 [25–28] wk) to receive 0.2 mL of colostrum every 2 h for 46 h, or routine care. Oral swabs were collected before, and at 46 h and 96 h after starting the intervention. The results showed that OPC influenced colonization of the oral cavity, with differences persisting 48 h after completing the intervention as per the protocol (18). Assessing whether the benefits reported in these small trials translate into clinically important outcomes for preterm infants is important considering that these infants are at the highest risk of mortality and morbidity due to, for example, NEC and LOS. We therefore aimed to conduct a systematic review of studies assessing OPC in preterm infants. The evidence provided by our systematic review is expected to help in guiding research and clinical practice in this field.
Methods and Participants
Guidelines from the Cochrane Neonatal Review Group, Centre for Reviews and Dissemination (25, 26), the PRISMA statement (27), and the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines were followed for conducting and reporting this systematic review and meta-analysis (28).
Eligibility criteria
Types of studies
RCTs, non-RCTs, and studies assessing laboratory-based outcomes of OPC administration in preterm infants were included. Narrative reviews, letters, editorials, and commentaries were excluded, but read to identify potential additional studies.
Types of participants, intervention, and comparison
Studies in preterm (gestation <37 wk) VLBW infants in which clinical and/or laboratory-based outcomes after administration of fresh or frozen OPC started within the first 7 d of life and continued for at least 46 h, were compared with placebo or standard care, were eligible for inclusion in the review.
Clinical (primary) outcomes
These were: NEC ≥ Stage II, all-cause mortality, LOS, time to reach full feeding (TFF), duration of hospital stay, and safety of OPC administration.
Laboratory-based (secondary) outcomes
These were: 1) salivary bioactive protein and immune peptide concentrations (sIgA, lactoferrin, lysozyme, epidermal growth factor, TGF-β, IL-8); 2) urinary bioactive protein concentrations (sIgA, lactoferrin); and 3) oral microbial diversity.
Review methods: search strategy
MEDLINE via PubMed (http://www.ncbi.nlm.nih.gov/pubmed/, 1966–2018) and via Ovid (http://ovidsp.tx.ovid.com, 1946–2018), EMBASE (Excerpta Medica dataBASE) via Ovid (http://ovidsp.tx.ovid.com, 1946–2018), the Cochrane Central Register of Controlled Trials (www.thecochranelibrary.com, through April 2018), Emcare via OVID (http://ovidsp.tx.ovid.com, 1980–2018) databases, and e-abstracts from the Pediatric Academic Society meetings (https://www.pas-meeting.org/about/#past, 2000–2018) were searched in November 2017 and April 2018. Abstracts of other conference proceedings such as the Perinatal Society of Australia and New Zealand, the European Academy of Paediatric Societies, and the British Maternal and Fetal Medicine Society were searched in EMBASE. Google Scholar was searched for articles that might have missed citation in the standard medical databases. Gray literature was searched using the US National Technical Information Service (http://www.ntis.gov/), Open Grey (http://www.opengrey.eu/), Mednar (http://mednar.com/mednar/desktop/en/search.html), the Canadian Agency for Drugs and Technologies in Health (https://www.cadth.ca/resources/finding-evidence/grey-matters), and Trove (http://trove.nla.gov.au/). The reference lists of identified studies and key review articles were searched to identify additional studies. No language restriction was applied. Authors were contacted for additional data and clarification of methods. Only published data were used for those studies, where available.
MEDLINE via PubMed was searched using MeSH (Medical Subject Headings) terms: “Infant, Extremely Premature”[MeSH] OR “Infant, Extremely Low Birth Weight”[MeSH] OR “Infant, Very Low Birth Weight”[MeSH] OR “Infant, Small for Gestational Age”[MeSH] OR “Infant, Premature, Diseases”[MeSH] OR “Infant, Premature”[MeSH] OR “Infant, Newborn, Diseases”[MeSH] OR “Infant, Newborn”[MeSH] OR “Infant, Low Birth Weight”[MeSH] OR “Infant”[MeSH] AND “Colostrum”[MeSH]. Keywords such as colostrum, oral, oropharyngeal were also used with infant OR newborn OR neonate to enhance the comprehensive search. Other databases were searched using similar terms. We searched ClinicalTrials.gov (https://clinicaltrials.gov), the International Clinical Trials Registry Platform (http://www.who.int/ictrp/en/), and BioPortfolio (www.bioportfolio.com) for ongoing RCTs.
Study selection
Abstracts of the citations obtained from the initial broad search were read independently by reviewers HP and GJ to identify potentially eligible studies. Full-text articles of these studies were obtained and assessed independently for eligibility by reviewers HP and GJ, under the predefined eligibility criteria. Differences in opinion were resolved by discussion between HP and GJ to reach consensus. Multiple publications of the same study were excluded to avoid data duplication.
Data extraction
Authors HP and GJ independently extracted the data using a standardized data collection form. Discrepancies were resolved by discussion and consensus among all authors.
Contacting authors
Authors of studies that did not report the relevant outcomes were contacted. Such studies were excluded if there was no response from authors.
Assessment of risk of bias of RCTs
Risk of bias (ROB) was assessed using the Cochrane Risk of Bias Assessment Tool (26). Authors HP and GJ independently assessed the ROB in all domains including random sequence generation, allocation concealment, blinding of intervention and outcome assessors, completeness of follow up, selectivity of reporting, and other potential sources of bias. For each domain, the ROB was assessed as low, high, or unclear based on the Cochrane Collaboration guidelines (26).
Assessment of ROB of non-RCTs
This was assessed using the Newcastle–Ottawa scale for quality assessment of observational studies (29).
Data synthesis and statistical analysis
Meta-analysis was performed using the Review Manager (version 5.3; Cochrane Collaboration, Nordic Cochrane Centre). A random effects model was used. Analysis was also conducted using a fixed effect model to ensure consistency of results and effect of the choice of model on the results. For data not suitable for meta-analysis, results are given in a tabular format (Table 1). Mean difference (MD) and 95% CIs were calculated for continuous variables. RR and 95% CIs were used for binary outcomes.
TABLE 1.
Characteristics of included RCTs and non-RCTs studying effects of oropharyngeal colostrum (OPC) administration
| Study author (year), country, study design | Sample size; colostrum vs. controls | Participants | Protocol for OPC/placebo administration | Specimen collection (for biochemical outcomes) | Biochemical outcomes (colostrum vs. controls) | Clinical outcomes (colostrum vs. controls)1 |
|---|---|---|---|---|---|---|
| Zhang et al. (15) (2017), China, RCT | n = 64; 32 vs. 32 (5 of 32 vs. 4 of 32 excluded, hence analyzed 27 vs. 28) | GA4: 29.86 ± 2.02 vs. 30.46 ± 2.5 wkBW4: 1241 ± 275 vs. 1248 ± 233 g | 0.2 mL of colostrum vs. normal saline every 4 h for 7 d | Urine and saliva collected within 24 h of life (baseline), day 7 and day 21 | Salivary sIgA2,4: at day 7: 31.92 ± 8.69 μg/mL vs. 27.78 ± 5.26 μg/mL; P = 0.04Salivary lactoferrin2,4: 1) at day 7: 5.18 ± 7.07 μg/mL vs. −1.74 ± 4.67 μg/mL (P < 0.001); 2) at day 21: 5.31 ± 9.74 μg/mL vs. −1.17 ± 10.38 μg/mL (P = 0.02)Urinary lactoferrin2: no differencessIgA in urine and saliva2: no differences | NEC ≥ stage II3: 1 vs. 5; P = 0.10Clinical sepsis3: 4 vs. 8; P = 0.61Proven sepsis3: 3 vs. 6; P = 0.17Days starting oral feeding3,4 > 5 mL: 15.38 (10.74) vs. 16.29 (10.13) d; P = 0.75 TFF3,4: 24.71 (11.23) vs. 32.72 (20.11) d; P = 0.09Safety: no adverse effects |
| Glass et al. (16) (2017), USA, RCT | n = 30; 17 vs. 13 | GA4: 28.4 ± 0.7 vs. 28.5 ± 0.8 wkBW4: 1132 ± 64 vs. 1079 ± 59 g | Oral care with 0.2 mL colostrum vs. sterile water every 3 h from day 2 to day 7 of life | Saliva collection at day 2 (before colostrum/water), day 7, and day 14 | Salivary sIgA2 at day 2: no differenceSalivary sIgA2 concentration increased from day 2 to day 7 in intervention vs. controls (no numerical data, only graphical data; P < 0.00001).Salivary sIgA2 at day 7 was higher in the intervention group (only graphical data; P < 0.05).Salivary IgA2 at day 14: changes not sustained | Suspected sepsis3P = 0.58 (P value is for overall sepsis): None: 7 (41%) vs. 6 (46%); 1 episode: 6 (35%) vs. 5 (39%); 2 or more episodes: 4 (24%) vs. 2 (15%)Culture-positive sepsis3 (overall): P = 0.37None: 12 (71%) vs. 10 (77%); 1 episode: 4 (23%) vs. 3 (23%); 2 episodes: 1 (6%) vs. 0NEC stage ≥ II3: 3 (18%) vs. 2 (15%); P = 0.87 (for overall NEC ≥ stage II)Safety: no adverse effects |
| Romano-Keeler et al. (17) (2017), USA, RCT | n = 99; 48 vs. 51 | GA5: 30 (27, 31) vs. 29 (28, 30) wk; P = 0.252BW5: 1272 (988, 1602) vs. 1170 (905, 1340) g; P = 0.121 | 0.2 mL of colostrum every 6 h from 48 h to 5 d of life | Saliva collection at day 1–2, day 8–9, and day 30 of life | OPC2 priming had minimal effects on salivary immune peptide concentrationsPeptides corresponding to lactoferrin, IgA, and lysozyme-C increased whereas S100 A7 and α-defensin-5 decreased at 8–9 days of life vs. 1–2 days of life; P < 0.05No differences between groups at baseline and postinterventionOral bacterial diversity and overall composition3 were not altered by OPC priming. OPC marginally modified oral taxa with decline in streptococcal abundance in OPC group at day 30 of life (P = NS) | Days to discharge3,5: 40 (31, 76) vs. 56 (41, 74) d; P = 0.038Total days intubated3,5: 1 (0, 3) vs. 1 (0, 5) d; P = 0.971Age at feeding initiation3,5 (d): 2 (2, 3) vs. 2 (2, 3); P = 0.671TFF3,5: 11 (8, 15) vs. 11 (9, 19) d (TFF was defined as 100 mL/kg/d); P = 0.391NEC ≥ stage II3: 4% vs. 2%; P = 0.642Culture-proven sepsis3EOS: 0% vs. 2%; P = 0.332LOS: 2% vs. 6%; P = 0.342Safety: no adverse effects |
| Sohn et al. (18) (2016), USA, RCT | n = 12; 6 vs. 6 | GA5: 27 (25, 30) vs. 27 (25, 28) wk; P = NSBW5: 1092 (490, 1350) vs. 1015 (735, 1300) g; P = NS | 0.2 mL colostrum every 2 h for 46 h regardless of enteral feeding status vs. routine care (no colostrum) | Oral swabs collected before initiation (0 h), after completion of protocol (48 h), and at 96 h | At enrollment (hour 0): similar oral microbiota (no numerical data, graphical only)2At 48 h: lower Moraxellaceae (nonpathogenic environmental) vs. controls (t-statistic: −2.91; Satterthwaite Df: 5.36; P = 0.03)At 96 h: lower Staphylococcaceae (colonizer) vs. controls (t-statistic: −3.21; Satterthwaite Df: 9.36, P = 0.01) and a trend toward a greater percentage of Planococcaceae (nonpathogenic environmental) (t-statistic 2.06; Satterthwaite Df: 5.08; P = 0.09)By 96 h: control group (5 of 6 infants): Staphylococcaceae (colonizers) were the dominant organism vs. 1 of 6 infants for colostrum group | NEC ≥ stage II3 = 1, 1 vs. 1, 0 respectively; P = NSBacterial sepsis: early-onset3: 0 vs. 1, late-onset: 0 vs. 2Fungal sepsis3: early-onset: 0 vs. 0, late-onset: 1 vs. 0VAP3 = 1 vs. 0Other pneumonia3: 1 vs. 0BPD3: 3 vs. 2Death3: 0 vs. 1Age at first feeding3,7: 2.5 (1–3) vs. 2 (1–3) dTFF3,7: 17 (14–41) vs. 13 (9–24) dSafety: no adverse effects |
| Lee et al. (19) (2015), Korea, RCT | n = 48; 24 vs. 24 (3 excluded per group hence analyzed 21 vs. 21) | GA6: 26+5 (24+2, 27+4) vs. 26+5 (24+3, 27+1) wkBW6: 830 (701–993) vs. 815 (610–1003) g | 0.2 mL of colostrum or sterile water every 3 h for 72 consecutive hours; commenced at 48 to 96 h after birth, regardless of infant's enteral feeding status | Urine and saliva collected during the first 24 h and at day 8 and day 15 | Salivary bioactive protein concentrations2:1) At 1 wk: mean salivary sIgA: (5.4 vs. 2.1 μg/mL; P = 0 .02); mean salivary EGF: (464.3 vs. 258.4 pg/mL; P = 0.04); salivary TGF-β1 and salivary IL-8 concentrations: only graphical data)2) At 2 wk: salivary concentrations of sIgA and EGF were similar between intervention and placebo groups (only graphical data); mean salivary TGF-β1 (39.2 vs. 69.7 mg/mL; P = 0 .03); mean salivary IL-8 (1.2 vs. 4.9 ng/mL; P = 0.04)Urinary bioactive protein concentrations2:1) At 1 wk: mean urinary sIgA concentration (71.4 vs. 26.5 ng/g creatinine; P = 0.04); mean urinary lactoferrin concentration (3.5 vs. 0.9 mg/g creatinine; P = 0.01); urinary IL-1β concentration: only graphical data2) At 2 wk: mean urinary sIgA concentration (233.8 vs. 48.3 ng/g creatinine; P = 0.006); urinary lactoferrin concentration: only graphical data, mean urinary IL-1β concentration (55.3 vs. 91.8 mg/g creatinine; P = 0.01) | NEC stage ≥ II3: 4 (17%) vs. 6 (25%); P = 0.72BPD3: 15 (63%) vs. 14 (58%); P = 0.58VAP3: 3 (12.5%) vs. 8 (33%); P = 0.17Proven sepsis3: 11 (46%) vs. 14 (58%); P = 0.56Clinical sepsis3: 12 (50%) vs. 22 (92%); P = 0.003IVH grade ≥ 33: 4 (16.7%) vs. 3 (12.5%); P = 0.34Laser surgery for ROP3: 11 (46%) vs. 7 (29%); P = 0.26Postnatal days to reach full feeding3,6: 20 (13–27) vs. 17 (14.3–25.8); P = 0.86Hospital stay3,6 (d): 89 (69.3–109.8) vs. 81.5 (56.5–99); P = 0.44Death3: 3 (12.5%) vs. 6 (25%); P = 0.46Safety: no adverse effects |
| Rodriguez et al. (20) (2011), USA, RCT | n = 15; 9 vs. 6 | GA5: 25.97 ± 1 vs. 26.77 ± 0.97 wk, P = 0.148BW5: 776.11 ± 231.73 vs. 940.83 ± 181.34 g, P = 0.168 | 0.2 mL of oral colostrum within 48 h, and 2-hourly >48 hMinimal enteral feeds started after study protocol completed | Baseline tracheal aspirate specimen immediately after birth, before surfactant administration. Tracheal aspirate, urine, and blood specimens collected within 6 h of completion of protocol | When analyzing pre-/post-treatment separately, no significant differences were found in sIgA, lactoferrin, and IL-10 concentrations2Colostrum group: serum IL-10 significantly decreased from pre- to post-treatment (median: 108.4 to 57.4; P = 0.039)Urine lactoferrin2 significantly increased from pre- to post-treatment (median: 0.17 to 0.69; P = 0.027)Placebo group: no changes in serum IL-10 and urine lactoferrin were observed | TFF3,5: n = 7 vs. 6, 14.29 ± 5.74 vs. 24.17 ± 8.66 d; P = 0.032Time to full per oral feeds3,5 (d): n = 7 vs. 6, 68.86 ± 19.33 vs. 55.83 ± 12.97; P = 0.124Time to full per oral feeds since start of enteral feeds3,5 (d): n = 7 vs. 6, 62.43 ± 17.58 vs. 49.83 ± 12.86; P = 0.125Hospital stay3,5 (d): n = 7 vs. 6, 101.43 ± 44.26 vs. 85.33 ± 32.96; P = 0.780RDS3: 9 (100%) vs. 6 (100%); P = NABacteremia3: 3 (33.33%) vs. 0 (0%); P = 0.229Pneumonia3: 3 (33.33%) vs. 0 (0%); P = 0.229NEC (stage not specified)3: 0 (0%) vs. 0 (0%)BPD3: 2 (22.22%) vs. 1 (16.67%); P = 1Death3: 2 (22.22%) vs. 0 (0%); P = 0.486Safety: no adverse effects |
| Snyder et al. (21) (2017), USA, non-RCT | n = 218; 133 vs. 85 | GA6: 28 (26–30) vs. 28 (26–30) wk; P = 0.69BW6: 1025 (805–1275) vs. 1060 (817–1345) g; P = 0.50 | BW < 1000 g (n = 51): 0.1 mL of buccal colostrum 2–4-hourly for 5 dBW 1000–1500 g (n = 82): received 0.2 ml buccal colostrum every 2–4 hours for 5 d vs. historical controls | No biochemical outcomes | — | Day of commencement of first feed2,6: 1 (1–2) vs. 2 (1–3); P = 0.22Day regained BW2,6: 9 (7–12) vs. 10 (8–13); P = 0.85TFF2,6: 15 (12–23) vs. 12 (9–19) d; P = 0.29Central line (d)2,6: 10 (6–19) vs. 11 (7–19); P = 0.30Medical NEC2: 9 (7%) vs. 6 (7%); P = 0.88Abdominal surgery for SIP2: 4 (3%) vs. 5 (6%); P = 0.27Abdominal surgery for surgical NEC2: 6 (5%) vs. 4 (5%); P = 0.85Suspected sepsis2 = 10 (8%) vs. 3 (4%); P = 0.32VAP2: 3 (2%) vs. 0(0%); P = 0.19ROP1 ≥ stage 2: 12 (9%) vs. 11 (13%); P = 0.51IVH2 (grade III or IV): 7 (5%) vs. 4 (5%); P = 0.66BPD2: 20 (15%) vs. 18 (21%) P = 0.41Feasible2Safety: no adverse effects |
| Seigel et al. (22) (2013), USA, non-RCT | n = 369; 89 vs. 280 | GA6: 26 (25–27) vs. 25 (24–27) wk; P = 0.22BW6: 820 (700–910) vs. 750 (628–860) g; P = 0.004 | 0.2 mL of fresh colostrum every 4 h for 5 d beginning in the first 48 postnatal h vs. historical controls | No biochemical outcomes | — | Time of commencing enteral feeds2,6: 4 (2–6) vs. 6 (3–11) d; P < 0.001TFF2,6(defined as 100 mL/kg/d): 25 (17–34) vs. 29 (19–40) d; P = 0.09Time to regain BW2,6: 14 (11–19) vs. 15 (11–23) d; P = 0.21Length of stay2,6 (d): 79 (48–125) vs. 69 (37–109); P = 0.14Medical NEC2: 6 (7%) vs. 17 (6%); P = 0.80Surgical abdominal pathology2: 14 (16%) vs. 44 (16%); P = 0.86Surgical NEC2: 4 (4%) vs. 19 (7%); P = 0.62SIP2: 10 (11%) vs. 25 (10%); P = 0.53Mortality (all-cause)2: 13 (15%) vs. 55 (20%); P = 0.35Safety: no adverse effects |
| Martin Alvarez et al. (23) (2016), Spain, non-RCT | n = 38; total 5 abandoned; analyzed 17 vs. 16 | GA (mean): 29.82 vs. 29.88 wk; P = 0.95BW (mean): 1259.41 vs. 1346.87 g; P = 0.47 | 0.2 mL of colostrum every 4 h starting in first 24 h, for 15 d vs. historical controls | Serum immune peptides measured at birth, 3, 15, and 30 d of life | Increase in serum sIgA concentrations found in both groups (birth vs. 30th day concentrations)2Colostrum (mean): 5.84 vs. 30.34, P = 0.001; control (mean): 12.48 vs. 22.48; P = 0.001sIgA statistically increased in colostrum group compared with the control group at 1 month of age (mean): 30.34 vs. 22.48; P = 0.026TNF-α2 (pg/mL) higher in controls (P < 0.01); IL-6 and IL-8 lower in colostrum group (P < 0.01); lactoferrin2 higher in colostrum group (P < 0.01) | Body weight3 (mean) (g):Day 3: 1145.71 vs. 1230; P = 0.48Day 15: 1338 vs. 1400; P = 0.67Day 30: 1802 vs. 1830; P = 0.89Enteral nutrition volume3 (mean) (mL/kg/d):Day 3: 45.86 vs. 50.56; P = 0.63Day 15: 136.50 vs. 175.13; P = 0.35Day 30: 152.29 vs. 174.64; P = 0.35Safety: no adverse effects |
| Hariharan et al. (24) (2017), India, non-RCT | n = 112; 56 vs. 56 | GA4: 28.7 ± 0.4 vs. 29.1 ± 0.4 wk; P = NSBW4: 970 ± 23 vs. 986 ± 32 g; P = NS | 0.2 mL colostrum every 6 h until 30 wk corrected age or until oral feeds, vs. historical controls | No biochemical outcomes | — | Gram-negative sepsis2: 4 vs. 9; P < 0.05Ventilator ± inotrope support3 (n): 25 vs. 27; P = NSFeed intolerance episodes3: 28 vs. 41, P < 0.05Stage 2/3 NEC3: 1 vs 1; P = NSTFF3 (d): 17.5 vs. 26.6; P < 0.05Death3: 2 vs. 2Safety: no adverse effects |
1Values for sepsis, NEC, VAP, pneumonia, BPD and death denote number of cases (percentage of total cases) unless otherwise specified.
2Primary outcome for study.
3Secondary outcome for study.
4Denotes values represented as mean ± SD.
5Denotes values represented as median quartiles.
6Denotes values represented as median (IQR).
7Denotes values represented as median (range).
BPD, bronchopulmonary dysplasia; BW, birth weight; EGF, epidermal growth factor; EOS, early-onset sepsis; GA, gestational age; IVH, intraventricular hemorrhage; LOS, late-onset sepsis; NA, not available; NEC, necrotizing enterocolitis; NS, not specified; OPC, oropharyngeal colostrum; RCT, randomized controlled trial; RDS, respiratory distress syndrome; ROP, retinopathy of prematurity; sIgA: secretory immunoglobin A; SIP, spontaneous intestinal perforation; TFF, time to reach full feeds; VAP, ventilator-associated pneumonia.
Heterogeneity
Clinical heterogeneity was assessed and reported by summarizing characteristics such as the study population, and the dose and duration of OPC. Statistical heterogeneity was estimated using the I2 statistic. An I2 statistic >50% was considered indicative of substantial heterogeneity (26).
Publication bias
This was assessed by visual inspection of the funnel plot (30).
Summary-of-findings table
The key information about the quality of evidence, the magnitude of the effect of the intervention, and the sum of available data on the main outcome was presented in the summary-of-findings table according to the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) guidelines (31).
The quality of evidence was assessed using GRADE-pro Guideline Development Tool consideration of the following factors: number of studies, study design, ROB, inconsistency, indirectness of evidence, imprecision, and other considerations. Under the heading “other considerations” the following aspects were assessed: publication bias, presence or absence of large effect, plausible confounding, and dose–response gradient (31).
Results
Selection, characteristics, and quality of studies
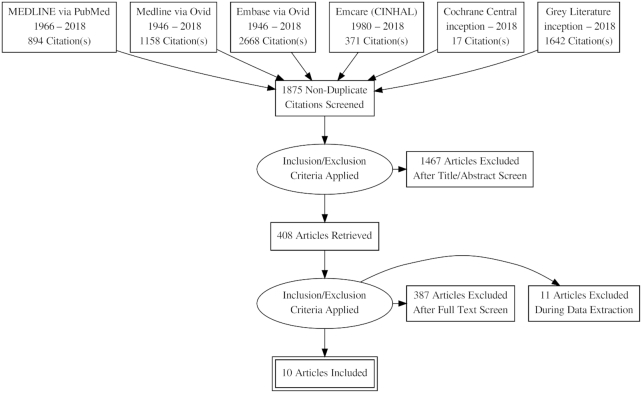
Of the 3347 citations that were identified after an initial broad search, 1875 nonduplicate citations were screened, of which 1467 articles were excluded after title/abstract screen. This left 408 articles to be read to identify if they were relevant. Of these, 398 were excluded, which left 6 RCTs (15–20) and 4 non-RCTs (21–24) for inclusion in the review. Figure 1 shows the flow diagram of the study selection process.
FIGURE 1.
Flow diagram showing study selection process.
Six RCTs (15–20) assessed human colostrum as an intervention, including 5 (15–17, 19, 20) that assessed biochemical outcomes, and 1 that assessed clinical and biochemical outcomes (18). The RCT by Juhl et al. was excluded because it assessed enteral bovine colostrum (32).
In 3 of the 4 included non-RCTs (21, 22, 24) the primary focus was on clinical outcomes, whereas it was on biochemical outcomes in the fourth study (23). The characteristics of the included studies are shown in Table 1. Rodriguez et al. have completed a large multicenter trial across 5 large neonatal units in the United States [Clinical Trials Identifier Number (CTIN): NCT02116699]. They recruited 622 extremely premature infants (birth weight <1250 g) to compare the effects of MM with those of a placebo, for reducing the incidence of LOS (primary outcome). The secondary outcomes included NEC, mortality, and assessment of the pathways for the benefits of MM including enhancement of gut microbiota and reduced oxidative stress (33). The results of this trial are awaited. In addition there are ongoing RCTs from Egypt (CTIN: NCT03513146), France (CTIN: NCT02650167), and Brazil (CTIN: NCT02912585).
Summary of characteristics and outcomes of included studies
The 10 included studies (6 RCTs and 4 non-RCTs) had differences in the intervention (dose, duration of colostrum supplementation) as well as population characteristics (e.g., gestation range: 24+2 to 32+5 wk; birth-weight range: 701–1602 g). The total sample size was 1005, with a larger contribution from non-RCTs than from RCTs (73.4% vs. 26.7%). The median sample size of RCTs was 39 and that of non-RCTs was 165. The details of gestation and birth weight are given in Table 1. The protocol for administering OPC in the 6 included RCTs (15–20) varied from 0.2 mL colostrum commenced within 48 h to a maximum of 7 d of life; and the duration of OPC administration varied from 46 h to a maximum of a week of life. Sterile water (16, 19, 20) or normal saline (15) was used as a control. Specimens such as saliva (16, 17), urine and saliva (15, 19), tracheal aspirates (20), serum immune peptides (17), and oral swabs (18) were collected at different time points, usually prior to initiation and after completion of the OPC protocol. The primary and secondary outcomes in the included studies varied (Table 1).
ROB of included studies
The results of the ROB assessment are shown in Tables 2 and 3. Five of the 6 included RCTs had a moderate risk of bias, and 3 of the 4 non-RCTs had a low risk of bias.
TABLE 2.
ROB assessment for RCTs
| Study ID | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias1 |
|---|---|---|---|---|---|---|---|
| Zhang et al. (15) | Low risk | High risk | Low risk | Unclear risk | Unclear risk | Low risk | Low risk |
| Glass et al. (16) | Low risk | Unclear risk | High risk | High risk | Low risk | Low risk | Unclear risk |
| Romano-Keeler et al. (17) | High risk | High risk | High risk | High risk | Low risk | Low risk | Low risk |
| Sohn et al. (18) | Unclear risk | Low risk | High risk | High risk | Low risk | Low risk | Unclear risk |
| Lee et al. (19) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Rodriguez et al. (20) | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
1Other bias: 1) The conduct of the study is affected by interim results (e.g., recruiting additional participants from a subgroup showing more benefit). 2) There is deviation from the study protocol in a way that does not reflect clinical practice (e.g., post hoc stepping-up of doses to exaggerated concentrations). 3) There is prerandomization administration of an intervention that could enhance or diminish the effect of a subsequent, randomized intervention. 4) Inappropriate administration of an intervention (or co-intervention). 5) Contamination (e.g., participants pooling drugs). 6) Occurrence of “null bias” due to interventions being insufficiently well delivered or overly wide inclusion criteria for participants. 7) An insensitive instrument is used to measure outcomes (which can lead to underestimation of both beneficial and harmful effects). 8) Selective reporting of subgroups. 9) Fraud. RCT, randomized controlled trial; ROB, risk of bias.
TABLE 3.
ROB assessment of non-RCTs (using Newcastle–Ottawa scale)1
| Domain | Details | Snyder et al. (21) | Seigel et al. (22) | Martin Alvarez et al. (23) | Hariharan et al. (24) |
|---|---|---|---|---|---|
| Selection | Representativeness of exposed cohort | 1 | 1 | 1 | 1 |
| Selection of nonexposed cohort | 1 | 1 | 1 | 1 | |
| Ascertainment of exposure | 1 | 1 | 1 | N/A | |
| Demonstration that outcome of interest was not present at start of study | 1 | 1 | 1 | 1 | |
| Comparability | Study controls | 1 | 1 | 1 | 1 |
| Study controls for additional factors | 1 | 1 | 1 | 1 | |
| Outcome | Assessment of outcome | 1 | 1 | 1 | 1 |
| Was follow-up long enough for outcomes to occur? | 1 | 1 | 1 | 1 | |
| Adequacy of follow-up of cohorts | 2 | 2 | 2 | N/A | |
| Total score (10) | 10 | 10 | 10 | 7 |
1N/A, despite adequate attempts to contact the authors, this information was not available; RCT, randomized controlled trial; ROB, risk of bias.
Publication bias
The risk of publication bias could not be assessed considering the small number of studies.
Outcomes
Summary results
Meta-analysis using a random effects model, separately for the 6 RCTs (n = 268) and 4 non-RCTs (n = 737), showed effects of OPC on various outcomes (Supplemental Table 1).
Meta-analysis of data from RCTs
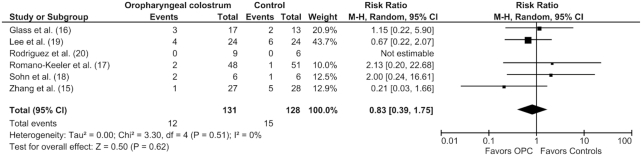
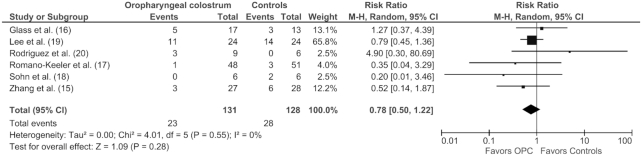
OPC administration showed no differences in the risk of NEC ≥ Stage II (12 of 131 vs. 15 of 128; RR: 0.83; 95% CI: 0.39, 1.75; P = 0.62) (Figure 2), LOS (23 of 131 vs. 28 of 128; RR: 0.78; 95% CI: 0.50, 1.22; P = 0.28) (Figure 3), all-cause mortality (6 of 104 vs. 7 of 100; RR: 0.74; 95% CI: 0.27, 2.06; P = 0.56), duration of hospital stay (MD: −1.65 d; 95% CI: −10.09, 6.80; P = 0.70), and TFF (MD: −2.86 d; 95% CI: −6.49, 0.77; P = 0.12). The results were similar using a fixed effect model, indicating the robustness of the results (Supplemental Table 1).
FIGURE 2.
Forest plot illustrating the overall risk ratio of necrotizing enterocolitis risk in preterm infants administered oropharyngeal colostrum compared with controls in randomized controlled trials. There was no significant difference in the 2 groups. This analysis was conducted using a random effects model. M-H, Mantel–Haenszel test; OPC, oropharyngeal colostrum.
FIGURE 3.
Forest plot illustrating the overall risk ratio of late-onset sepsis in preterm infants administered oropharyngeal colostrum compared with controls in randomized controlled trials. There was no significant difference in the 2 groups. This analysis was conducted using a random effects model. M-H, Mantel–Haenszel test; OPC, oropharyngeal colostrum.
Meta-analysis of data from non-RCTs
Non-RCTs did not show beneficial effects of OPC on NEC ≥ Stage II (26 of 278 vs. 47of 421; OR: 0.90; 95% CI: 0.52, 1.56; P = 0.71), LOS (4 of 56 vs. 9 of 56; OR: 0.90; 95% CI: 0.52, 1.56; P = 0.71), mortality (25 of 298 vs. 60 of 439; OR: 0.91; 95% CI: 0.52, 1.60; P = 0.74), duration of hospital stay (MD: 12.33 d; 95% CI: −1.27, 25.93; P = 0.08), and TFF (MD: −0.23 d; 95% CI: −7.42, 6.96; P = 0.95). Only Seigel et al. reported longer duration of hospital stay in the OPC group (22). No adverse effects of OPC administration (e.g., aspiration pneumonia) were reported (Supplemental Table 1).
Biochemical outcomes
OPC administration improved the concentrations of salivary lactoferrin (15) and sIgA (16), reduced clinical sepsis by inhibiting proinflammatory cytokines (19), and had a maturational immunostimulatory effect on the intestinal epithelium (20). Sohn et al. reported only transient changes in the oral microbiome following administration of buccal colostrum in preterm infants. These included significantly reduced colonization with Moraxellaceae and Staphylococcaceae in the OPC-treated group compared with the control group at 48 and 96 h, respectively (Table 1) (18). Romano-Keeler et al. reported no effect of OPC on salivary immune peptides, or on the oral microbiome (17).
GRADE analysis
The overall quality of evidence was deemed very low in view of the small sample size, unclear ROB in most of the included studies, variable difference in effect size estimates, and wide CIs with minimal overlap (Supplemental Table 2).
Discussion
Our systematic review of 6 RCTs (n = 268) and 4 non-RCTs (n = 737) showed that OPC has the potential to improve outcomes in high-risk preterm infants.
The OPHALT system constitutes a vital part of the preterm infant's immune system. Commencement of feeding within 24 h of delivery with mother's colostrum has become standard practice in most neonatal units (34). Trophic feeds improve gastrointestinal blood flow and gut motility, while reducing dysbiosis. Infants receiving trophic feeds have improved feeding tolerance and weight gain, with reduced LOS and hospital stay (35).
Colostrum is rich in immunomodulatory biofactors, which are essential to stimulate the preterm infant's defense system (13). The oropharynx of breast-fed infants becomes filled with colostrum, which activates the OPHALT system (14). Critically sick, extremely preterm infants receiving trophic feeds via an orogastric tube are deprived of the benefits of OPC. Priming the oral mucosa with small amounts of colostrum at regular intervals seems to be a simple solution to address this issue. The safety and feasibility of administering OPC in preterm infants have been demonstrated in numerous studies (6–9). Various investigators have assessed the immunomodulatory benefits of OPC by measuring biochemical parameters such as sIgA, lactoferrin, and changes in the microbial milieu, and whether these translate to a reduction in neonatal morbidity (15–20).
Key results of previous studies in this field need to be discussed. Glass et al. reported increased concentrations of secretory IgA on day 7 in OPC-supplemented infants, suggesting an immunomodulatory effect of the intervention (16). There was no effect on clinical outcomes. Zhang et al. reported increased concentrations of salivary lactoferrin in OPC-supplemented infants but no effect on clinical outcomes (15). Lee et al. reported increased concentrations of urinary sIgA and lactoferrin at 1 wk, and reduced LOS in infants supplemented with OPC (19). Rodriguez et al. reported no effect on urinary IgA or lactoferrin, but reduced TFF in OPC-supplemented infants (20). Romano-Keeler et al. reported no difference in oral microbiota but reduced hospital stay in the OPC-supplemented infants (17). In contrast, Sohn et al. reported improved oral microbiota with OPC, but no effect on clinical outcomes (18). Sohn et al. and Lee et al. showed no increase in the incidence of ventilator-associated pneumonia with OPC administration (18, 19). Overall, these data suggest that administration of OPC is feasible and safe in preterm infants but evidence of improvement in clinically important outcomes is inadequate.
The strengths and limitations of our study need to be discussed. To our knowledge this is the first systematic review of OPC in preterm infants using a comprehensive methodology. Overall, the results showed minimal statistical heterogeneity. The limitations include small sample size, clinical heterogeneity, lack of blinding, and the fact that clinical outcomes were not the primary focus of 5 of the 6 included RCTs. Large, well-designed, and adequately powered RCTs are thus necessary to assess the effects of OPC on clinically important outcomes (e.g., NEC, LOS) in preterm infants. The results of the large multicenter RCT (n = 600) by Rodriguez et al. will be important in this context (33).
Although our review focused on oropharyngeal human colostrum, there is also some evidence concerning the effects of bovine colostrum as an enteral supplement (36, 37). The Pre Colos 2017 (Phase A and B) and Juhl 2018 (Phase C) studies assessed the safety and tolerance of enteral bovine colostrum in preterm infants (32, 38). Feed intolerance, surgical NEC, and mortality did not increase, and the median [IQR] TFF (120 mL/kg/d) was reduced, but not by a statistically significant amount (15 [12–20] vs. 22 [15–44] d; P = 0.097), in the group receiving bovine colostrum compared with the control group. The elevated plasma tyrosine concentrations (>200 μmol/L on day 7) observed in the bovine colostrum group (8 of 21 vs. 2 of 19 controls) were probably related to higher enteral protein intake (32).
In summary, the results of our systematic review indicate that the evidence for effects of OPC on clinically important outcomes (e.g., LOS, NEC) in preterm infants is limited and of low quality. Adequately powered RCTs are needed to address this issue.
Supplementary Material
Acknowledgments
We thank James Wynn (17), Futing Ji (15), Nancy Rodriguez (20), and Per Torp Sangild (32) for providing further information about their studies and clarifying methods and results. Nehad Nasef was contacted regarding the RCT from Egypt (CTIN: NCT03513146), but further information was not available because the study was not completed.
The authors' contributions were as follows—HP: independent literature search and writing the first and final drafts of the manuscript; HP, GJ, and SP: data interpretation and supervision; GJ: rechecking the literature search results and interpretation of data, handling the meta-analysis software, and supervising the first and final draft; SP: concept and design, rechecking and interpreting data, acting as a referee author in case of discrepancy between other authors, and supervising the first and the final draft of the manuscript.
All authors: read and approved the final manuscript.
Notes
The authors reported no funding or sponsorship received for this study.
Author disclosures: HP, GA-J, SP, no conflicts of interest.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: CTIN, Clinical Trials Identifier Number; GRADE, Grades of Recommendation, Assessment, Development and Evaluation; LOS, late-onset sepsis; MD, mean difference; MeSH, Medical Subject Headings; MM, mother's own milk; NEC, necrotizing enterocolitis; OPC, oropharyngeal colostrum; OPHALT, oropharyngeal-associated lymphoid tissue; RCT, randomized controlled trial; ROB, risk of bias; RR, risk ratio; sIgA, secretory immunoglobulin A; TFF, time to reach full feeds; VLBW, very low birth weight.
References
- 1. Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, Kinney M, Lawn J; Born Too Soon Preterm Birth Action Group. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(Suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Helenius K, Sjors G, Shah PS, Modi N, Reichman B, Morisaki N, Kusuda S, Lui K, Darlow BA, Bassler D et al.. Survival in very preterm infants: an international comparison of 10 National Neonatal Networks. Pediatrics. 2017;140(6):e20171264 doi:10.1542/peds.2017-1264. [DOI] [PubMed] [Google Scholar]
- 3. Battersby C, Santhalingam T, Costeloe K, Modi N. Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2018;103:F182–F9. [DOI] [PubMed] [Google Scholar]
- 4. Sun J, Marwah G, Westgarth M, Buys N, Ellwood D, Gray PH. Effects of probiotics on necrotizing enterocolitis, sepsis, intraventricular hemorrhage, mortality, length of hospital stay, and weight gain in very preterm infants: a meta-analysis. Adv Nutr. 2017;8:749–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sinha AK, Murthy V, Nath P, Morris JK, Millar M. Prevention of late onset sepsis and central-line associated blood stream infection in preterm infants. Pediatr Infect Dis J. 2016;35:401–6. [DOI] [PubMed] [Google Scholar]
- 6. Rodriguez NA, Meier PP, Groer MW, Zeller JM, Engstrom JL, Fogg L. A pilot study to determine the safety and feasibility of oropharyngeal administration of own mother's colostrum to extremely low-birth-weight infants. Adv Neonatal Care. 2010;10:206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Montgomery DP, Baer VL, Lambert DK, Christensen RD. Oropharyngeal administration of colostrum to very low birth weight infants: results of a feasibility trial. Neonatal Intensive Care. 2010;23:27–9. [Google Scholar]
- 8. Thibeau S, Boudreaux C. Exploring the use of mothers' own milk as oral care for mechanically ventilated very low-birth-weight preterm infants. Adv Neonatal Care. 2013;13:190–7. [DOI] [PubMed] [Google Scholar]
- 9. McFadden B. Oral colonization in the preterm neonate: effect of oral care. In: Proquest Dissertations and Theses. Vol. 117 Denton (TX): Texas Woman's University; 2012. [Google Scholar]
- 10. Rodriguez NA, Caplan MS. Oropharyngeal administration of mother's milk to prevent necrotizing enterocolitis in extremely low-birth-weight infants: theoretical perspectives. J Perinat Neonatal Nurs. 2015;29:81–90. [DOI] [PubMed] [Google Scholar]
- 11. Gephart SM, Weller M. Colostrum as oral immune therapy to promote neonatal health. Adv Neonatal Care. 2014;14:44–51. [DOI] [PubMed] [Google Scholar]
- 12. Rodriguez NA, Meier PP, Groer MW, Zeller JM. Oropharyngeal administration of colostrum to extremely low birth weight infants: theoretical perspectives. J Perinatol. 2009;29:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lonnerdal B. Bioactive proteins in human milk—potential benefits for preterm infants. Clin Perinatol. 2017;44:179–91. [DOI] [PubMed] [Google Scholar]
- 14. Bocci V. Absorption of cytokines via oropharyngeal-associated lymphoid tissues. Does an unorthodox route improve the therapeutic index of interferon?. Clin Pharmacokinet. 1991;21:411–7. [DOI] [PubMed] [Google Scholar]
- 15. Zhang Y, Ji F, Hu X, Cao Y, Latour JM. Oropharyngeal colostrum administration in very low birth weight infants: a randomized controlled trial. Pediatr Crit Care Med. 2017;18:869–75. [DOI] [PubMed] [Google Scholar]
- 16. Glass KM, Greecher CP, Doheny KK. Oropharyngeal administration of colostrum increases salivary secretory IgA levels in very low-birth-weight infants. Am J Perinatol. 2017;34:1389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Romano-Keeler J, Azcarate-Peril MA, Weitkamp JH, Slaughter JC, McDonald WH, Meng S, Latuga MS, Wynn JL. Oral colostrum priming shortens hospitalization without changing the immunomicrobial milieu. J Perinatol. 2017;37:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sohn K, Kalanetra KM, Mills DA, Underwood MA. Buccal administration of human colostrum: impact on the oral microbiota of premature infants. J Perinatol. 2016;36:106–11. [DOI] [PubMed] [Google Scholar]
- 19. Lee J, Kim HS, Jung YH, Choi KY, Shin SH, Kim EK, Choi JH. Oropharyngeal colostrum administration in extremely premature infants: an RCT. Pediatrics. 2015;135:e357–66. [DOI] [PubMed] [Google Scholar]
- 20. Rodriguez NA, Groer MW, Zeller JM, Engstrom JL, Fogg L, Du H, Caplan M. A randomized controlled trial of the oropharyngeal administration of mother's colostrum to extremely low birth weight infants in the first days of life. Neonatal Intensive Care. 2011;24:31–5. [Google Scholar]
- 21. Snyder R, Herdt A, Mejias-Cepeda N, Ladino J, Crowley K, Levy P. Early provision of oropharyngeal colostrum leads to sustained breast milk feedings in preterm infants. Pediatr Neonatol. 2017;58:534–40. [DOI] [PubMed] [Google Scholar]
- 22. Seigel JK, Smith PB, Ashley PL, Cotten CM, Herbert CC, King BA, Maynor AR, Neill S, Wynn J, Bidegain M. Early administration of oropharyngeal colostrum to extremely low birth weight infants. Breastfeed Med. 2013;8:491–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin Alvarez E, Jimenez Cabanillas MV, Pena Caballero M, Serrano Lopez L, Kajarabille N, Diaz Castro J, Ochoa Herrera JJ, Maldonado Lozano J. Efectos de la administración de calostro orofaríngeo en recién nacidos prematuros sobre los niveles de inmunoglobulina A. Nutr Hosp. 2016;33:95. [DOI] [PubMed] [Google Scholar]
- 24. Hariharan D, Veluswami G, Balasubramanium L, Kannappan V. Oropharyngeal breastmilk administration in extreme prematurity reduces gram negative sepsis and feed intolerance. J Pediatr Gastroenterol Nutr. 2017;64:784–5. [Google Scholar]
- 25. Centre for Reviews and Dissemination. 2009. CRD's guidance for undertaking reviews in health care [Internet]. CRD; [cited April 16, 2018 and November 23, 2018]. Available from: https://www.york.ac.uk/crd/SysRev/!SSL!/WebHelp/SysRev3.htm. [Google Scholar]
- 26. Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0. Cambridge (UK): The Cochrane Collaboration; 2011. [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 29. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- 30. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rucker G, Harbord RM, Schmid CH et al.. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 31. Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, Brozek J, Norris S, Meerpohl J, Djulbegovic B et al.. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013;66:158–72. [DOI] [PubMed] [Google Scholar]
- 32. Juhl SM, Ye X, Zhou P, Li Y, Iyore EO, Zhang L, Jiang P, van Goudoever JB, Greisen G, Sangild PT. Bovine colostrum for preterm infants in the first days of life: A randomized controlled pilot trial. J Pediatr Gastroenterol Nutr. 2018;66:471–8. [DOI] [PubMed] [Google Scholar]
- 33. Rodriguez NA, Vento M, Claud EC, Wang CE, Caplan MS. Oropharyngeal administration of mother's colostrum, health outcomes of premature infants: study protocol for a randomized controlled trial. Trials. 2015;16:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Klingenberg C, Embleton ND, Jacobs SE, O'Connell LA, Kuschel CA. Enteral feeding practices in very preterm infants: an international survey. Arch Dis Child Fetal Neonatal Ed. 2012;97:F56–61. [DOI] [PubMed] [Google Scholar]
- 35. McClure RJ. Trophic feeding of the preterm infant. Acta Paediatr Suppl. 2001;90:19–21. [DOI] [PubMed] [Google Scholar]
- 36. Thapa BR. Therapeutic potentials of bovine colostrums. Indian J Pediatr. 2005;72:849–52. [DOI] [PubMed] [Google Scholar]
- 37. Rathe M, Muller K, Sangild PT, Husby S. Clinical applications of bovine colostrum therapy: a systematic review. Nutr Rev. 2014;72:237–54. [DOI] [PubMed] [Google Scholar]
- 38. Li Y, Juhl SM, Ye X, Shen RL, Iyore EO, Dai Y, Sangild PT, Greisen GO. A stepwise, pilot study of bovine colostrum to supplement the first enteral feeding in preterm infants (Precolos): Study protocol and initial results. Front Pediatr. 2017;5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.