ABSTRACT
The sources or types of protein in the diet have long been overlooked regarding their link to cardiometabolic health. The picture is complicated by the fact that animal and plant proteins are consumed along with other nutrients and substances which make up the “protein package” so plant and animal protein come with clear nutrient clusters. This review aimed at deciphering the relation between plant and animal protein and cardiometabolic health by examining different nutritional levels (such as amino acids, protein type, protein foods, protein patterns, and associated overall dietary and nutrient patterns) and varying levels of scientific evidence [basic science, randomized controlled trials (RCTs), observational data]. Plant protein in Western countries is a robust marker of nutrient adequacy of the diet, whereas the contribution of animal protein is highly heterogeneous. Yet recent data from large cohorts have confirmed that total and animal proteins are associated with the risk of cardiovascular disease and diabetes, even when fully adjusting for lifestyle and dietary or nutritional factors. Here again, there is marked variability depending on the type of animal protein. Protein from processed red meat and total red meat on the one hand, and from legumes, nuts, and seeds on the other, are often reported at the extremes of the risk range. RCTs using purified proteins have contributed little to the topic to date, inasmuch as the findings cannot readily be extrapolated to current or near-future diets, but RCTs studying whole protein foods have shown a beneficial effect of pulses. Despite the fact that many of the benefits of plant protein reported in observational or interventional studies may stem from the protein package that they convey and the nutrients that they displace, there are also important indications that protein per se may affect cardiometabolic health via the many amino acids that are present in typically contrasting levels in plant compared with animal proteins.
Keywords: cardiometabolic health, animal protein, plant protein; cardiovascular risk; diabetes; protein sources; protein patterns; amino acids
Introduction
As compared with lipid and carbohydrates, protein is the macronutrient that makes the smallest observed contribution to energy intake and constitutes the lowest proportion of recommended intake (1). Protein also has the specific feature that its dietary reference intake has been set on the basis of a strict nutritional requirement. Further, because protein is found in very large amounts in a limited number of foods, very high intakes are infrequent, and this results in the usual intake ranging from 12% to 20% (of energy intake) in economically developed countries (1, 2). Whereas there is a long-standing debate on the amounts of fat and carbohydrate that are best for long-term health, the issue of protein is more recent, and indeed there are still limited definitive data to address the question.
As with fat and carbohydrates, the question of the total amount of protein may seem outdated and somewhat misplaced: as I will illustrate in this article, the type of protein may matter much more than the total amount. As far as cardiometabolic health is concerned, much progress has been achieved in understanding that the total amount of fat is of much less importance than the type of fatty acids (mostly making a distinction between SFAs and PUFAs) and the foods that convey them (distinguishing basically animal foods from vegetal oils) (3–6). Likewise, total carbohydrates now seems a useless descriptor that should be definitely broken down in order to consider different types of carbohydrates and their sources (so making a distinction in particular between low–glycemic index foods and those containing added sugars) (7, 8). As for proteins, classic distinctions between sources or types of protein are only made on the basis of digestibility and indispensable amino acid contents, and with respect to the primary criterion of the protein requirement. Future efforts to define protein quality need to focus on studying dietary proteins in terms of health-related outcomes, thus encompassing long-term outcomes relative to cardiometabolic health (9). However, this has mostly remained an emerging area of research and, until recently, what mattered in terms of dietary protein was being above the nutritional allowance and of overall “good quality,” which means an ability to supply sufficient indispensable amino acids to renew the body's store of protein (1, 9, 10). Interestingly, by defining protein quality in this way, it becomes dependent on quantity. Therefore, in industrialized countries using varied sources of protein at surfeit levels of intake, this viewpoint cannot take account of the potential influence on health of the type or source of dietary protein. However, the growing importance and urgency of considering dietary protein in terms of sustainability (11–13) means it is now necessary to revise this persistent viewpoint and consider the spectrum of the relation between the type of protein in the diet (animal or plant protein, and different protein sources) and long-term health. In this review, I intend to focus on the relation between animal or plant protein sources and cardiometabolic risk.
The topic of protein sources or types and cardiovascular health can be traced back more than a century. That of animal protein and atherosclerosis was the subject of further study during the 1960s. Considerable progress has been made during the past 10 y and in particular recently, based on an increasing body of evidence from randomized controlled trials (RCTs) and cohort studies. My aim in this article is to review the literature on animal or plant protein and cardiometabolic health by considering varying levels of scientific evidence (basic science, RCTs, observational data). Furthermore, because animal and plant proteins are consumed along with other nutrients and substances which make up the “protein package,” plant and animal protein come with clear nutrient clusters. Therefore, one specific objective of this review was to explore the relation between the intake of animal and plant protein and cardiovascular disease risk by considering different nutritional levels for “protein” (such as amino acids, protein type, protein foods, protein patterns, and their association with overall dietary patterns and clusters of nutrient intakes).
The Protein Package and Dietary Protein Intake as a Marker of Diet Quality
Protein intake goes with the “protein package” and results from complex dietary behaviors
There are 2 reasons for the associations between intakes of proteins of different types and the overall dietary and nutrient patterns seen in individuals. This arises first of all from the nutritional characteristics of the foods containing protein. To take a trivial example, animal protein tends to be accompanied by SFAs, whereas plant protein tends to come with fiber and phenolic compounds. This is usually referred to as the “whole food package,” or to be more specific the “protein package”; this is important if we are to understand the protein issue with respect to long-term health (14). The more intricate and specific the association between a food protein and other nutrients or substances supplied in the food offer, the stronger the relation between their intakes in the diet will tend to be (15, 16). The second reason stems from the overarching behavioral or social factors that govern food choices. In Western countries, specific protein foods may be chosen over other protein foods which they therefore displace and will be accompanied by different associated foods. For instance, a higher protein intake from fish is usually associated with a lower intake of red meat and high-fat dairy products, a higher intake of vegetables, and a lower intake of sweets as has been reported in the United States (17). These dietary features are also associated with more physical activity along with other markers of a health-conscious set of attitudes (such as less cigarette smoking and a greater use of vitamin supplements). Fish intake is also associated with other features of dietary behavior such as eating 3 meals a day more frequently, consuming fewer prepared meals, and more organic foods, and thus implies a more favorable nutrient intake profile and, of course, higher sociodemographic and economic categories, as has been shown in France (16, 18, 19).
Plant and animal protein sources and diet quality: the case of meat eating
It is therefore not surprising that the literature contains numerous reports of associations between protein sources and markers of diet quality. Because of the protein package, animal proteins have long been blamed for their contribution to an inadequate nutrient intake profile, which may explain the association with cardiovascular disease risk (14, 20). This has been reported comprehensively with respect to meat intake in different populations and countries. The relation between meat intake and diet quality has also been considered in studies comparing an omnivorous population qualified as “meat-eaters” with non–meat-eaters who eat fish (pesco-vegetarians) and vegetarians. As discussed by others (21), meat-eaters in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Oxford study (22) had a higher energy intake and a different nutrient intake profile, with a higher qualitative contribution of SFAs and less fiber and PUFAs. However, a simple direct comparison of the nutrient profile remains difficult because “meat-eaters” had also a higher intake of vitamin B-12, vitamin D, zinc, and iodine, as found in the EPIC-Oxford study and other studies on vegetarians (22–24). When considering overall profiles of dietary quality, such as those assessed using dietary quality indexes that are mostly based on food groups (such as Healthy Eating Indexes), the quality is much higher for non–meat-eating diets than in the general population (25). However, any direct comparison between vegetarian and nonvegetarian diets is largely confounded by behavioral factors affecting food choices (26, 27). Finer comparisons have been made with populations displaying less extensive ranges of meat consumption and behaviors than the general population, such as the US Adventists (24, 28), or when comparing high–meat-eaters, low–meat-eaters, poultry-eaters, and fish-eaters (and vegetarians) (29, 30). In more general populations that are almost entirely composed of meat-eaters, meat intake has also been associated with poorer diet quality, as assessed from the profiles of intake of certain important food groups: higher intakes of total meat were associated with lower intakes of vegetables, fruits, and cereals in Europe (20); red meat intake was associated with lower intakes of fruits, whole grains, and nuts in Finland (31); and a lower meat:fish ratio was a strong determinant of diet quality and nutrient profiles in Japan (32).
Protein patterns are strong markers of diet quality
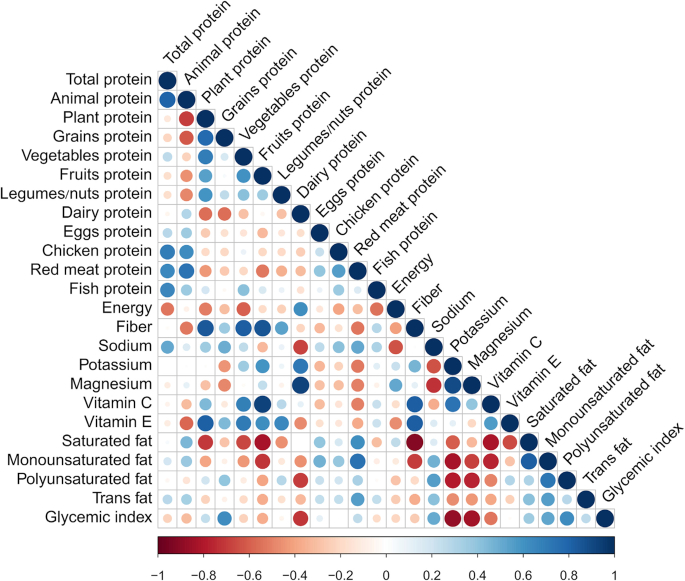
The relation between some specific animal proteins and diet quality indeed extends beyond the case of meat, and a more general link has been reported overall for plant compared with animal proteins. Plant and animal protein intakes, both as a whole and in detail, show strong inverse associations with each other and strong associations with patterns of nutrient intake, as was clearly shown recently by Shang et al. (33) when studying the Melbourne Cohort. In more detail, and as shown in Figure 1, animal protein and total protein intakes were strongly associated, and animal protein was associated with lower intakes of plant proteins (including grains), fiber, and vitamins E and C, and higher intakes of saturated fats. By contrast, plant protein was associated negatively with all animal proteins except fish, positively with fiber and vitamin E, and negatively with energy and SFAs. Indeed, the associations were much stronger regarding the intake of meat protein rather than total animal protein, and contrasted with fish protein when compared to total animal protein. Phillips et al. (34) found in the United States that animal-based protein foods such as meat contributed more to the intake of protein and several “positive” nutrients (notably zinc, vitamin B-12, and highly bioavailable iron) as well as several “negative” nutrients (cholesterol and SFAs) than did plant-based protein foods. Conversely, the authors found that plant-based protein foods contributed more to the intake of other positive nutrients (notably dietary fiber, vitamin E, magnesium, and PUFAs) (34). This confirms that animal-derived nutrients are an important component of the overall pattern of nutrient intake (35). To gain further insight into the nutrient profile associated with animal compared with plant protein intake, we analyzed plant and animal protein intakes and the probabilistic overall nutrient adequacy of the diet of French adults (36, 37). We showed that as a general rule, total plant protein and each type of plant protein intake (cereals, legumes, nuts and grains, etc.) were strongly associated with nutrient adequacy (36, 37). The association between plant or animal protein intake and overall nutrient adequacy was explained by a large set of nutrients, including SFAs, sugars, potassium, sodium, folate, vitamin C, manganese, cholesterol, and fiber (36). It is important to note that this list includes many nutrients that are deemed to be of particular importance to cardiometabolic health, and in particular fiber, SFAs, magnesium, potassium, and sugars. Some nutrients on this list clearly arise from the “protein package” (such as fiber, magnesium, potassium, and SFAs), whereas others (namely sugar) come from a much more complex and indirect association between the nature of protein intake and food choices in the diet. Indeed, it is likely that rather than resulting from a displacement by plant protein foods, a lower sugar intake is due to a smaller contribution of added sugar to the diet of individuals who make healthier choices in general, although we adjusted for potential confounding factors (such as energy, alcohol intake, age, and socioeconomic factors). Unlike the robust association with plant protein intake, we found in this population that there was a marked heterogeneity within the animal protein intake with respect to nutrient intakes and adequacy. This was expected because, as mentioned above, meat intake was not associated with the same protein package and dietary behaviors as fish or dairy intakes. In this respect, we found important contrasts between protein from fish and low-fat dairy (positively associated with the nutrient cluster related to global nutrient adequacy) and protein from processed meat, cheese, and eggs (negatively associated). These associations also varied according to gender, with a negative association between red meat protein and nutrient adequacy in men only (36). Such a difference in gender with respect to meat intake was also reported by Fogelholm et al. (31) who found a positive association between red meat and vegetables in men only, and by Vergnaud et al. (20) who reported an opposite association between the intakes of meat and “sugar and confectionery” or “added fat,” depending on gender. When analyzing protein intake patterns in the general French population, we recently reported that individuals with a protein pattern with the largest contribution from eating pork had low overall scores for nutrient adequacy whereas the population with a dominant contribution from fish protein had the highest scores (16). In this general population, “pork eaters” and “poultry eaters” were the groups with the highest animal:plant protein ratio, whereas fish-eaters had the lowest ratio. Likewise, “milk eaters” (i.e., individuals with high intakes of protein from milk) had a high animal:plant protein intake ratio and their diet displayed relatively good nutrient adequacy (16). This further illustrates the definite heterogeneity within animal proteins regarding their associated clusters of nutrients. By contrast, our analysis showed that plant proteins, whatever their source (i.e., grains, legumes, seeds, and nuts), and gender were consistently associated with nutrient adequacy.
FIGURE 1.
Partial correlation analysis between intakes of protein according to sources and intakes of nutrients in the Melbourne Cohort study. Pearson correlation analysis was used to calculate age- and gender-adjusted partial correlation coefficients. Blue and red colors indicate positive and negative correlations, respectively, and color intensity and the size of the circle are proportional to the correlation coefficient. Adapted from reference 33 with permision.
Issues when interpreting current and future diet quality in terms of plant compared with animal protein patterns
The complex links between animal:plant protein intake and the overall characteristics of the diet were also found when examining the design of dietary interventional trials. The Dietary Approach to Stop Hypertension (DASH) diet, Omniheart diet, and others such as the “Beef in an Optimal Lean diet” (BOLD) could be compared on the basis of their different levels of plant:animal proteins but neither the composition of these diets, nor their nutrient contents, were indeed simply related to this ratio (38, 39). The diets were indeed designed to increase the amounts of certain nutrients (such as potassium, magnesium, and fiber) and were based on an overall healthy dietary pattern which included fruits and vegetables and low-fat versions of dairy and meat products (40). The levels of plant compared with animal proteins per se were secondary to the design of these diets and the results of these studies cannot be compared using this parameter alone (39).
It remains unclear whether the association between plant protein intake and overall nutrient adequacy can be ascribed mainly to the intrinsic characteristics of the foods that are currently available to compose our diet (i.e., to the “protein package” of the usual protein food groups), or if this might be largely confounded by the healthy behaviors of individuals who purposely adopt a diet containing more plants (i.e., linked to overarching factors of diet quality) (41). Dietary diversity is an overarching factor in diet quality which we found was associated with both overall nutrient adequacy and plant protein intake in French adults (37). When analyzing this relation in more detail, we found that the association between plant protein intake and nutrient adequacy was independent of overall dietary diversity (37). This result further strengthens the view that plant protein intake is a robust marker of the nutritional adequacy of the diet (36). Satija et al. (42) studied the relation between health and overall score of adherence to a plant-based diet or to a healthy plant-based diet (as measured using a plant-based diet index that positively counts plant food groups that are not fruit juices, sweetened beverages, refined grains, potatoes, and sweets or dessert). The authors found that the negative association between a plant-based diet and the risk of diabetes in 3 US cohorts was stronger when the healthy plant-based diet was considered. Similar findings were reported recently regarding the cardiovascular disease (CVD) mortality risk in the general US adult population (43). It is important to note that food groups that contribute negatively to a healthy plant-based diet are not rich in protein. When looking more closely at the data on the relation with protein intake, it can be seen that contrary to other indexes, the healthy plant-based diet index is positively associated with total protein intake (42, 43). This association appears to be the result of a higher intake of plant protein and a moderately lower intake of animal protein. Therefore, plant or animal protein intake appears to be tightly associated with diet quality, even across different types of plant-based or animal-based diets in the general population.
However, what is difficult to infer from the present literature is the extent to which the association between plant protein intake and the intake patterns for nutrients that are important to cardiometabolic health may depend on the characteristics of the plant protein foods that are traditionally available, and whether this may change in the near future when new products come onto the market. This indeed reflects the classic nutrients-foods-patterns complexity (44). As pointed out in the analysis by Satija et al. (42), not all plant foods are healthy but the plant protein foods currently available are mostly good contributors to healthy diets. This is typically the case of legumes, nuts, and seeds, for instance. By contrast, it remains unclear whether the development of new plant protein products to be used as substitutes for meat products by the general population will cause a healthy revision of the plant protein food benchmark; there could be a risk that it will lead to a more heterogeneous relation between plant protein intake and a healthy nutrient intake, and hence long-term health.
Finally, this intricate association between intakes of various protein sources, the overall dietary pattern, and the profile of nutrient intake means that we should be very cautious when analyzing and interpreting observational data. There are indeed 2 types of confusion that should be borne in mind and do not have the same implications. The first concerns the association between the intake of plant or animal proteins, dietary patterns, and overall health-related behaviors. This is indeed a classic pitfall in nutritional epidemiology. Carefully designed models for very large samples are good solutions to overcome this issue using residualization (45), but because the associations with protein intakes are strong, there will always be an important risk of residual confusion. The second type of confusion arises from the fact that when referring to “protein” in the diet we do indeed mean protein-rich products, i.e., protein plus the “protein package,” along with nutrients and other substances that are more or less closely associated with the protein per se. This second issue has implications when interpreting the results of observational studies. It means that it may not be possible to extrapolate the association found for “protein” in a given study to another dietary context (another population not sharing the same dietary background). It also means that it may not be possible to extrapolate in the future if the population changes the nature of the foods conveying their protein, after a change in the food offer. Beyond observational data, this issue also has implications regarding the setting and interpretation of RCTs, as I shall discuss further below.
Plant and Animal Proteins in Observational Studies
Until recently, only a few studies had specifically examined the association between dietary plant protein (compared with animal protein) and the cardiovascular disease or diabetic risk, and they produced a mixed picture. When modelling the data from the Iowa Women's Health Study, Kelemen et al. (46) reported that plant protein could have potentially favorable effects on coronary artery disease mortality when compared with both animal protein and total carbohydrate. Indeed, an inverse association was observed when plant protein replaced either carbohydrate or animal protein, whereas no association was observed when animal protein replaced carbohydrates. When analyzed in the context of low-carbohydrate diets, plant- (compared with animal)-based diets were associated with lower rates of mortality from CVD in US cohorts (47), but there was little evidence for such a contrast in European cohorts (48). This difference could thus be ascribed to countries with different background diets and metabolic statuses of individuals, and different types of dietary substitutions between dietary proteins and carbohydrates (49). Because the models were adjusted to take account of numerous potential confounding factors (such as physical activity, BMI, energy intake, alcohol intake, and smoking) but to take little or no account of the dietary factors associated with animal compared with plant protein intakes, the associations with plant and animal protein could rather have been ascribed to some association with plant-based or animal-based diets, and the amount of animal or plant protein and fat that they conveyed. In other words, plant and animal proteins could in this case be taken as markers of underlying dietary patterns.
More recently, positive findings regarding plant compared with animal proteins and cardiovascular disease risk have been reported by major cohort studies. After combining the large Harvard cohorts, Song et al. (50) reported significant associations between plant compared with animal protein intake and CVD mortality. In models adjusting for lifestyle risk factors and for numerous dietary and fatty acid intakes, the authors found that a 10% increase in energy intake from animal protein would translate into an 8% increase in CVD mortality. Conversely, a 3% increase in energy from plant protein would reduce this risk by 12%. When compared with the energy contribution of animal protein (mean: 14%; 5th percentile: 9%; 95th percentile: 22%) and plant protein (4%; 95% CI: 2%, 6%) in the population, the impact of equally rebalancing plant and animal protein could therefore be important. These associations were confined to participants with ≥1 unhealthy lifestyle factor (based on smoking, heavy alcohol intake, overweight or obesity, and physical inactivity) and were not evident among those without any of these risk factors. In a Mediterranean population at high cardiovascular disease risk, animal protein (and not plant protein) was also associated with a higher risk of CVD events and mortality (51). In the large cohort of the Adventist Health Study 2, we found an association between CVD mortality and animal protein intake, but not total plant protein intake. An 18-g increment of animal protein was associated with a 7% increase in CVD mortality, which is a higher estimate of the risk than that previously found. In the Adventist cohorts, the mean and range of animal protein were lower than in the general population, and the population had a better overall dietary and nutrient pattern, with fewer differences in lifestyle confounding factors across the different types of diet. Therefore, the results could mean that even at low or moderate levels of intake in a population with a good overall health status, an intake of animal protein is associated with a higher CVD risk.
An association between animal or plant protein and type 2 diabetes has also been confirmed by recent studies. In a very large cohort of type 2 diabetics in 8 European countries (EPIC-InterAct), it was found that animal protein was associated with a higher risk of diabetes, after adjusting for dietary and nutrient intakes. The relation tended to be attenuated but persisted after adjustment for BMI and waist circumference, suggesting that the effect of animal protein intake was not entirely mediated by its association with body weight and composition (52, 53), a classical association in the literature (52, 53). Similar results were found in Chinese women, with evidence for mediation by insulin resistance, independently of BMI (54). Genetic susceptibility to diabetes did not change the associations (55). Based on the Melbourne Cohort (21,523 participants), Shang et al. (56) also reported a higher risk of incident diabetes in individuals with a higher animal protein intake. These positive associations appeared to be stronger in individuals with normal baseline plasma glucose, BMI, or blood pressure, and were more marked in men. An association between plant protein intake and diabetes was only found in women. In another recent analysis of US cohort studies that included a large number of diabetics, the relation was confirmed for animal protein, but it was also found that plant protein was inversely associated with risk, irrespective of gender (57). A recent meta-analysis (58) confirmed that the data overall clearly demonstrate an association between total and animal protein intake and diabetes risk, but do not reveal a significant association with plant protein, as reported previously (56). Interestingly, it has been argued that the relation with animal protein may be nonlinear, with important effects only being observed at high intakes, and may only be mediated in part by BMI (59). It was recently found that total and animal protein intake and the animal:plant protein ratio were strongly and positively associated with insulin resistance in a cross-sectional analysis of middle-aged and older people from the Adventist Health Study 2 (60). These results suggest that the effect of animal protein on insulin sensitivity can also occur at the low levels of intake seen in this population.
Overall, these results on diabetes risk and CVD mortality are generally in line with the findings regarding intermediary endpoints for cardiometabolic health. A relation between the type of protein intake and incident metabolic syndrome was recently documented in the Melbourne Cohort study (33). The authors reported a higher risk with higher total and animal protein intakes and lower plant protein intakes. A higher energy intake from animal protein was associated with increases in systolic blood pressure, waist circumference, and body weight over 11 y, whereas a higher plant protein intake was associated with reductions in waist circumference and weight (33).
Considerable study over many years has focused on the relation between animal or plant protein intake and blood pressure (61). A number of transversal or longitudinal studies reported negative associations between plant protein and blood pressure (62–66). However, total protein intake was shown to be inversely associated with blood pressure in many studies, and some reported inverse associations between animal protein intake and blood pressure or the risk of hypertension (67–69). Some of the heterogeneity affecting these findings could be linked to the background dietary context and the type of animal protein consumed, which would explain the contrasting findings in Asian compared with Western populations. Taken together, however, the difference between the effects of plant or animal protein on blood pressure does not appear to be so marked (70–72), unlike the harder endpoints discussed above.
Beyond Total Plant and Total Animal Protein: The Type of Animal or Plant Protein Matters
It has become clear in the last decade that plant protein and animal protein are overly coarse descriptors of protein intake. When reviewing the epidemiological studies that have attempted to analyze in detail the association between more specific protein sources and some health endpoints, we can see dramatic differences between specific protein sources within the “animal protein” type. In a recent meta-analysis of diabetes risk, the intake of proteins from red meat and processed meat was associated with a higher risk, whereas the intake from dairy products was associated with a lower risk (58). Studies with fully adjusted models of substitution have been helpful in further analyzing this heterogeneity. When modeling the effect of replacing 1 portion of animal protein food with 1 portion of average plant protein food (comprised of whole grains, legumes, peanuts, peanut butter, and other nuts), Malik et al. (57) found that substitution for processed meat was associated with a greater benefit with respect to diabetes risk (a 21% reduction in risk), whereas the association with dairy protein was nonsignificant (Figure 2). Red meat was intermediate in this gradation. Interestingly, high–glycemic index foods such as refined grains and potatoes were also associated with a higher risk when compared with plant protein foods (Figure 2). In the Melbourne Cohort, Shang et al. (33) ascribed the positive effect of animal protein on incident metabolic syndrome and its components to red meat and chicken, and the negative effect of plant protein to legumes and nuts. Red meat was the animal protein which was found to be significantly associated with diabetes risk in a Chinese cohort (54). In the context of low-carbohydrate diets, red and processed meats have been implicated in the positive association between intakes of animal protein and fat and a higher risk of diabetes (73). Because these studies adjusted for BMI, there could be additional effects of meat on diabetes risk beyond those mediated by changes to BMI (74–76). An elucidation of the underlying mechanisms supports the causal role of processed and total red meat consumption in increasing risk of type 2 diabetes (77). These possible mechanisms include the role of SFAs, sodium, advanced glycation end products, nitrates or nitrites for processing, heme iron, trimethylamine N-oxide, branched-chain amino acids, and endocrine disruptor chemicals (77).
FIGURE 2.
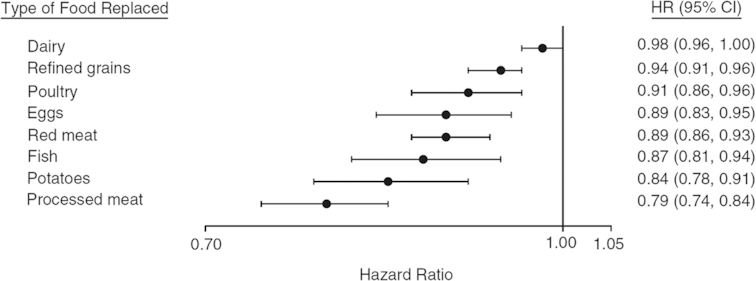
Pooled HRs and 95% CIs for type 2 diabetes associated with replacement of 1 serving of individual animal protein foods (dairy foods, poultry, eggs, red meat, and processed meat), refined grains, and potatoes with 1 serving of vegetable protein foods (composite variable comprised of whole grains, legumes, peanuts, peanut butter, and other nuts) in the Nurses’ Health Study (1984–2008), Nurses’ Health Study II (1991–2009), and the Health Professionals Follow-up Study (1986–2008). The models adjusted for age; family history of diabetes; smoking; alcohol intake; physical activity; race/ethnicity; total energy intake; postmenopausal hormone use (Nurses’ Health Study, Nurses’ Health Study II); oral contraceptive use (Nurses’ Health Study II); intakes of sugar-sweetened beverages, fruit, and vegetables; and BMI. Results were mutually adjusted for other food sources of animal protein, refined grains, and potatoes. Reproduced from reference 57 with permission.
As far as cardiovascular mortality is concerned, and using the same approach of protein source substitution as in the Harvard cohorts, it was found that replacing processed meat by plant protein was the most beneficial (39% reduction in risk), whereas replacing unprocessed meat (17% reduction in risk) or dairy (11% reduction) procured the most modest benefits (50). These findings have clarified earlier reports regarding the association between the risk of coronary heart disease and the consumption of protein food groups in the Nurses’ Health Study in 2010. In this report, Bernstein et al. (78) found that in a model controlling statistically for energy intake, 1 serving/d of nuts was associated with a 30% lower risk when compared with 1 serving/d of red meat. Similarly, when compared with 1 serving/d of red meat, a lower risk was associated with 1 serving/d of low-fat dairy (13% lower risk), poultry (19%), or fish (24%). This was also in line with the recent report from the Prevención con Dieta Mediterránea (PREDIMED) study, where portions of red and processed meats involved the highest risk of incidence of metabolic syndrome, when compared with legumes, poultry and rabbit, fish, or eggs (79). Because intakes of protein from various food groups are indeed highly multicollinear, our first aim was to analyze the underlying structure of the protein pattern in order to identify the most salient component. Therefore, we analyzed the pattern of protein intake in the Adventist Health Study 2 and found 5 protein factors (independent of each other), which we then used in models to analyze cardiovascular mortality Tharrey et al. (80). We found significant and strong associations with 2 of these factors. Comparing the highest with the lowest quintiles of factor scores, we found a 61% higher risk for the “Meat” protein factor and a 40% lower risk for the “Nuts & Seeds” protein factor. These estimates were little influenced by other characteristics of the diet, such as vegetarian dietary patterns or nutrients related to CVD health. These associations were particularly strong among young adults aged 25–44 y (with a 100% higher risk for the “Meat” protein factor and a 60% lower risk for the “Nuts & Seeds” protein factor); see Figure 3.
FIGURE 3.
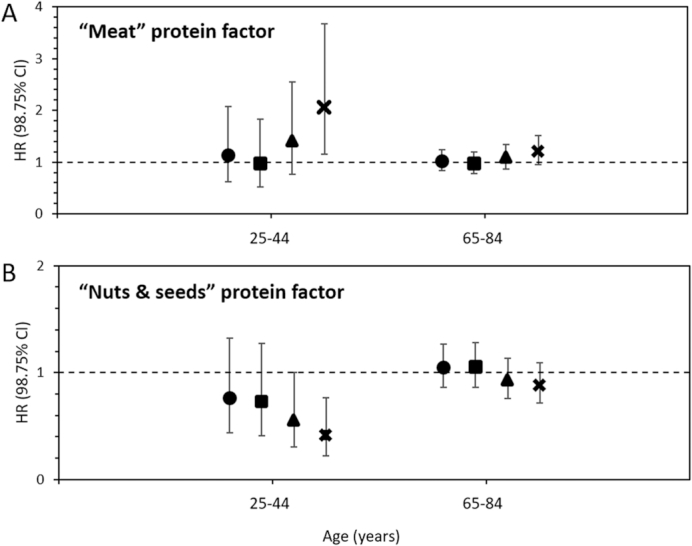
Multivariate-adjusted HR of CVD mortality by quintile of the “Meat” protein factor (A) and “Nuts & Seeds” protein factor (B) in 81,337 participants in the Adventist Health Study 2, presented for 2 age categories. The model was adjusted for age, sex, race, BMI, individual socioeconomic factors, health-related lifestyle, energy intake, and the type of diet in the vegetarian spectrum. Significant awith permission ge interactions were found for the “Meat” and “Nuts & Seeds” protein factors. HRs were estimated at the mean age of each age category. CVD, cardiovascular disease. Adapted from reference 80 with permission.
Finally, although there is a very important body of evidence for their effects on diabetes and CVD risk, it should be kept in mind that red meat, and especially processed meat, are strongly associated with health-oriented behaviors that still make it difficult to disentangle the causal effects of red meat from observational studies (81).
Lessons from Clinical Trials
Clinical trials offer another line of evidence regarding the specific effects of some plant and animal proteins. The literature proposes a large body of studies that have examined the effects of specifically manipulating plant proteins on intermediary endpoints related to cardiometabolic health. The studies can be divided into 2 different types. The first contains studies that manipulate protein in quite a specific manner by using purified proteins that can be included in experimental foods with a closely controlled composition. The second type concerns studies that test the utilization of protein foods as included in individual diets, usually controlling for the macronutrient content of the diets. In a clinical trial, if a “plant protein” is tested using foods that are rich in that protein, the trial will refer to the effect of consuming certain plant protein–rich foods along with the protein package of these foods and not specifically the plant protein. It would be erroneous to assign the results either to a particular protein or to the protein food group in general, i.e., irrespective of variations in the nutrient contents of these protein foods (82). However, the findings of such studies have important implications for public nutrition, because they can be extrapolated to how individuals actually consume dietary proteins or would eat more of a dietary protein if willing to do so, using the protein foods that are available. By contrast, if a trial uses a protein ingredient that is purified (e.g., protein isolate) and used to prepare experimental foods, the results will apply more to “protein” but are less extrapolable to real life. Furthermore, it should be kept in mind that proteins are never pure and they usually convey >10% nutrients and other substances intimately associated with them (notably in the case of plant protein) (83). Even if they are intimately associated, there may be variations in the amounts and nature of the substances involved, thus making the “protein” quite heterogeneous. This has long been discussed regarding the amounts of isoflavones in soy protein, the opinion being that isoflavones may convey a large part of the benefit associated with “soy protein” (84–86). Another point (which might seem a detail and is often overlooked) is that protein ingredients may be isonitrogenous but differ regarding the amount of amino acids they contain, as we discussed several years ago (87). In any case, the more the trial design aims to decipher a specific effect of the protein isolated, the more the results will apply to protein per se, but the less they will readily apply to public nutrition, unless the food industry can rapidly deliver new food products containing purified protein that individuals are prepared to consume. In that case, it is questionable whether such trials are relevant to the actual situation where individuals would incorporate these new protein foods in their diet, because these new protein foods would be expected to replace the other protein foods that they are currently consuming, in a way that has not been analyzed by the design of the RCT.
I do not intend to review all the literature concerning RCTs on specific protein and protein sources and cardiometabolic health, but I would like to discuss certain aspects of this literature.
Briefly, as far as purified protein ingredients are concerned, much has been published on soy protein, and the reader is directed to some interesting reviews and opinions (86, 88, 89). I would like to use this example to consider the amounts (“doses”) of soy protein that could be expected to convey the effects reported in the literature. The effects of soy protein on blood lipids and blood pressure have mostly been observed with high intakes of protein, generally ∼35 g/d (84, 90), whereas the effects of soy protein at intakes <25 g/d are either weak or found statistically nonsignificant; this was discussed recently in a meta-analysis concerning its effects on blood pressure (85). In order to judge the practical implications of such levels of intake, it is worth mentioning that the high soy protein intake of Adventist vegans in the United States averages only 13 g/d (24). Other plant proteins, which have been less studied, may have effects on cholesterol or blood pressure, as was suggested for lupin protein (91–93), but the data are scarce. It may therefore be difficult to determine whether a combination of purified plant proteins in large quantities (∼50 g/d) could result in reproducing some of the findings regarding soy protein at high doses reported in RCTs.
It is more likely that plant protein might benefit cardiometabolic risk factors when consumed as a protein food. This would not be surprising because some components in the plant protein package could favorably replace others that are associated with animal protein (e.g., fatty acid types), but also because some of them have been shown to be effective in modulating cardiometabolic risk factors, such as fiber. In RCTs, the positive effects of plant protein when using plant foods or unpurified protein ingredients have been ascribed to the effects of protein and fiber that are found at high levels in the unrefined ingredients. For instance, this is the case for lupin (94, 95), although there have been some conflicting results (96). Indeed, as shown in the literature when comparing the results with soy products against those obtained with soy protein ingredients, the evidence generally appears much stronger when studying whole plant foods or raw ingredients than purified proteins (97–99). Overall, a recent analysis reported that the evidence available from RCTs suggests that 1–2 servings of plant protein foods instead of animal protein foods decreases LDL cholesterol, non–HDL cholesterol, and apolipoprotein B by ∼4% in adults with and without hyperlipidemia (100). Because of inconsistencies or inaccuracies in the estimates, the overall certainty of the evidence is moderate and suggests that more research will be required to refine these estimates (100). The effect of pulses on cardiovascular disease risk factors in RCTs is reviewed elsewhere in the same issue of this journal (101). In my view, further studies are needed to assess the effects of plant protein on various cardiometabolic risk factors, such as low-grade inflammation, endothelial vascular function, and insulin sensitivity, because there have been some null results (e.g., 101) and the field has not been studied sufficiently. These studies would be better served by the use of different types of plant protein and different types of substitution.
When considering even more general changes to food consumption involving foods rich in plant proteins, a consensus has now been reached that legumes improve cardiometabolic risk factors (as compared with various diets controlled for energy and nutrients). For a full review on legumes and beans and cardiometabolic risk, the reader can refer to the review by Salas-Salvadó in the same issue of this journal (102). By contrast, it is difficult to draw any conclusions from RCTs that have tested the effects of meat consumption because the dietary treatments and results have varied. O'Connor et al.’s recent meta-analysis (103) reported no effect of total red meat intake >0.5 servings/d on blood lipids and blood pressure. It had already been found that substituting red meat for poultry or fish did not affect blood lipids (102) and substituting protein in general for some types of carbohydrates in a healthy diet could benefit CVD risk factors (104, 105). However, this information from RCTs contrasts with observational findings that associated red meat intake with CVD risk factors (106) or CVD events and mortality (107–115).
Approaches that rely on simultaneous changes to several foods in a diet, such as a more food-based or dietary pattern method, are useful to clarify dietary recommendations aimed at achieving a reduction in cardiometabolic risk (116). If trying to summarize the findings of observational and interventional studies, what stands out is that a healthier protein pattern would consist in reducing the consumption of processed and red meats and preferring more protein sourced from legumes, nuts, and seeds. This general conclusion would also fall in line with the common features of generally prudent diets such as the Mediterranean diet (117–119). More data are required from investigating the specific effects of other animal-based protein products (such as chicken and dairy) and other plant-based products (such as grains), and the factors which modulate their effects on cardiometabolic health.
Could Amino Acids Form Part of the Relation?
As I discussed at the beginning of this article, one might consider that a large part of the relation between plant or animal protein intakes and cardiometabolic risk could be ascribed to the large cluster of nutrients and other substances (e.g., phytochemicals) that they convey, either directly or indirectly (39, 120). Indeed, in observational studies, it remains difficult to separate the effects of a specific protein from that of the closely associated “protein package,” and also from other characteristics of the overall diet, as I have argued. It also seems clear that the literature is more conclusive when RCTs involve whole protein plant foods rather than purified proteins. Nonetheless, it should also be considered that the type of protein per se (i.e., the relative amounts of amino acids that it supplies) may affect cardiometabolic risk. Support for this proposal can be found in human and animal studies that manipulated amino acid intakes, and also from an analysis of observational studies. Many studies have reported a significant association with cardiometabolic outcomes, even when fully adjusted models were used, i.e., including dietary or nutrient intakes and despite classic confounding factors related to behaviors and socioeconomic status. For example, this was the case regarding a recent report on plant or animal protein intake and the risk of diabetes, which used a model that, as well as numerous genetic and lifestyle factors, also adjusted for total energy intake; percentages of energy from trans-fats, saturated fats, monounsaturated fats, and polyunsaturated fats; dietary cholesterol; dietary fiber; and the glycemic index (57). Song et al. (50) also recently found significant relations between CVD mortality and plant compared with animal protein intakes when adjusting for dietary intakes (e.g., whole grains, fruits and vegetables, glycemic index) and the intakes of different fatty acids. Likewise, in the Adventist Health Study 2 cohort, we found that the strong association between protein intake factors and CVD mortality was not modified when account was taken of potential confounders, such as the vegetarian diet category and the intake of a series of nutrients considered as being relevant to cardiovascular disease risk (e.g., PUFAs, SFAs, sodium, and vitamins A, C, E, B-6, folate, and B-12) (80).
As well as their utilization for protein synthesis or oxidation, amino acids enter specific metabolic pathways that lead to the synthesis of metabolites which play key roles in physiology and pathophysiology. For this review, I would like to mention a few amino acids that are present at varying levels in plant and animal proteins and have been studied widely for their probable impacts on physiology: arginine, cysteine, and BCAAs.
Arginine provides the substrate for the synthesis of nitric oxide, the key mediator of vascular homeostasis (121–123). Indeed, an impairment of NO production or bioactivity has been largely reported as a central feature associated with cardiometabolic risk, including that of coronary artery disease, stroke, and diabetes (124). As shown by a meta-analysis, arginine supplementation improves endothelial function when its levels are low at baseline (125). The beneficial effects of arginine supplementation on endothelial function can be achieved with a low intake (such as that seen by modifying the amount or type of protein in the diet), as has been demonstrated by its ability to blunt postprandial endothelial function after a high-fat meal (126, 127). The kinetics of arginine bioavailability may also play an important role inasmuch as arginine that is made available slowly—as is the case for dietary, protein-bound arginine—is directed more towards nitric oxide synthesis than arginine that is rapidly available (for example, in a dietary supplement) (128). The potential of arginine-rich proteins to prevent alterations to postprandial endothelial function has been documented (129, 130) and arginine may mediate some of the beneficial effects of arginine-rich proteins in the diet (91, 130). Arginine may also have other benefits on cardiometabolic health (131–135). The benefits of high arginine intake have also been studied for many years in association with lysine intakes, stemming from seminal works on plant and animal proteins and atherosclerosis (136, 137). More recently, in a closely controlled trial, Vega-Lopez et al. (138) found that a low (compared with high) lysine:arginine ratio lowered fasting and postprandial C-reactive protein and lowered postprandial plasma concentrations of TGs in moderately hypercholesterolemic subjects.
Sulfur amino acids, and particularly cysteine, have also been studied for their potential effects on cardiometabolic risk, because of their links with homocysteine (a probable risk factor for CVD) and glutathione (a pivotal molecule in redox homeostasis). The relation between cysteine intake, glutathione metabolism, and cardiometabolic health has been widely studied and reviewed elsewhere (139–141). In brief, dietary cysteine supplementation has been reported to reduce diet-induced oxidative stress and insulin resistance in rats (142). This protective effect was notably accompanied by an alteration of glutathione redox status, which has been reported as being an early marker of atherosclerosis in healthy humans (143). In humans, variations in sulfur amino acid intakes affect fasting plasma free cysteine concentrations and the redox status, as shown by the extracellular cysteine or cysteine redox potential (144). Supplementation with a cysteine donor lowers plasma homocysteine, improves glutathione concentrations and redox status, and lowers blood pressure (145). Cysteine intake has also been inversely associated with the incidence of stroke (146).
BCAAs have been widely studied for their relation with insulin resistance since it was reported that high plasma BCAA concentrations formed part of a metabolic signature correlated with insulin resistance in obese individuals. BCAA concentrations are elevated in obese subjects with insulin resistance or metabolic syndrome (147). They are associated with plasma acylcarnitines and cardiovascular disease risk factors (147–152) and they are predictive of diabetes and CVD (153–155). BCAA intake has been associated with the incidence of insulin resistance and diabetes, although this intake could simply be a marker of total and animal protein intake (156, 157). BCAA supplementation has also been reported to modulate insulin sensitivity, notably in the context of diet-induced obesity (158–160) or in individuals with low baseline amounts of intake (161). These findings remain controversial, because opposite results were reported with leucine alone in mice (162, 163) and high plasma BCAA concentrations resulted from a complex change in their metabolism (150, 164). Importantly, dietary BCAAs largely interplay with fatty acid oxidation, and we recently showed that this could explain why the type of protein in a high-fat meal modulates the extent of postprandial mitochondrial overload and incomplete substrate oxidation (165). However, it remains difficult to ascertain whether dietary and plasma BCAAs contribute to the onset of the dysregulations contributing to cardiometabolic risk, or if a high plasma BCAA concentration is a marker of dysregulated metabolism (166, 167). Recent Mendelian randomization analyses suggested a causal role of BCAA metabolism, which is impaired by insulin resistance, in the etiology of type 2 diabetes (166, 168, 169).
Based on experimental studies in animals or humans, or associations found in epidemiological studies, other amino acids have been studied for their links with cardiovascular disease risk factors and identified as candidates for mediating the effects of protein intakes. For instance, oral glycine was shown to be particularly efficient in potentiating the action of insulin (170, 171). Intakes of glutamic acid have been associated with lower systolic blood pressure, independently of other amino acids (172). Plasma glycine and glutamine are inversely associated with the incidence of type 2 diabetes (153). Other candidate amino acids include histidine, phenylalanine, and tyrosine (135, 173).
It would be interesting to study both the separate and combined effects of amino acid intakes, to identify the most influential amino acids when taken together (with additive or synergetic effects) and try to determine how they might account for the effects of plant proteins. Because amino acid intakes are not independent of each other, and are also associated with other characteristics of the diet, the analysis of observational data needs to take account of this complexity and ways must be found to reduce it to the most salient components. In this regard, Teymoori et al. (174) recently analyzed patterns of amino acid intake using models with multiple dietary or nutritional confounders. They found that an amino acid factor with high loadings from BCAAs, aromatic amino acids, serine, and threonine was strongly associated with the incidence of hypertension in a model adjusting for fatty acid types, calcium, sodium, magnesium, potassium, and fiber. In the study by Jennings et al. (173), the association between the intake of several amino acids and blood pressure was found to depend on whether they originated from plant or animal protein sources, which is indicative of the complexity of this issue. Further analysis of this complex situation is necessary in the context of large cohorts.
Although it is much too soon to draw any definite conclusions, the amino acids identified in the literature that I have reviewed here as possibly being detrimental to cardiometabolic health are found at higher levels in animal protein and their intakes are contributed to a greater degree by animal protein intake. These are mostly BCAAs and aromatic amino acids—indispensable amino acids (175). Conversely, the amino acids identified as being potentially beneficial are mainly found at higher levels in plant proteins. These are mostly arginine, cysteine, glutamine/glutamate, and glycine—nonindispensable amino acids (173). These findings warrant further study to understand patterns of amino acid intake in relation to cardiometabolic health. This research may indeed change our vision of amino acids as being classified as “indispensable” or “nonindispensable,” and drive attempts to shift our understanding of protein quality for human health.
Conclusion
Plant protein in Western countries is a robust marker of nutrient adequacy of the diet, whereas the contribution of animal protein largely varies according to animal source. Plant and animal proteins are indeed consumed with other nutrients and substances that make up the “protein package” and this may explain their relation to cardiometabolic health in the current dietary patterns. Yet recent data from large cohorts have confirmed that total and animal proteins are associated with the risk of CVD and diabetes, even in models that are largely adjusted for lifestyle and dietary or nutritional factors. Here again, there is marked variability depending on the type of animal protein. Proteins from processed red meat and total red meat on the one hand, and from legumes, nuts, and seeds on the other, are often reported at the extremes of the risk range. RCTs using purified proteins have contributed little to the topic to date, inasmuch as the findings cannot readily be extrapolated to current or near-future diets, but RCTs studying whole protein foods have shown a beneficial effect of pulses. Despite the fact that many of the benefits of plant protein reported in observational or interventional studies may stem from the protein package that they convey and the nutrients that they displace, there are also important indications that protein per se may affect cardiometabolic health via the many amino acids that are present at typically contrasting levels in plant compared with animal proteins.
Acknowledgments
I thank Prof. Jean-François Huneau for interesting discussions on this topic. The sole author had responsibility for all parts of the manuscript.
Notes
Published in a supplement to Advances in Nutrition. This supplement was sponsored by the Harding-Buller Foundation of Ohio. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the sponsors. Publication costs for this supplement were defrayed in part by the payment of page charges. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
The author reported no funding received for this study.
Author disclosures: The author report no conflicts of interest.
Abbreviations used: CVD, cardiovascular disease; EPIC, European Prospective Investigation into Cancer and Nutrition; RCT, randomized controlled trial.
References
- 1. Mariotti F. Protein intake throughout life and current dietary recommendations. In: Dardevet D, editor. Molecular Nutrition of Amino Acids and Proteins. London: Academic Press; 2016. pp. 13–25. [Google Scholar]
- 2. Gerbens-Leenes PW, Nonhebel S, Krol MS. Food consumption patterns and economic growth. Increasing affluence and the use of natural resources. Appetite. 2010;55(3):597–608. [DOI] [PubMed] [Google Scholar]
- 3. Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JG et al.. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017;136(3):e1–e23. [DOI] [PubMed] [Google Scholar]
- 4. Chen M, Li Y, Sun Q, Pan A, Manson JE, Rexrode KM, Willett WC, Rimm EB, Hu FB. Dairy fat and risk of cardiovascular disease in 3 cohorts of US adults. Am J Clin Nutr. 2016;104(5):1209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zong G, Li Y, Wanders AJ, Alssema M, Zock PL, Willett WC, Hu FB, Sun Q. Intake of individual saturated fatty acids and risk of coronary heart disease in US men and women: two prospective longitudinal cohort studies. BMJ. 2016;355:i5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kris-Etherton PM, Fleming JA. Emerging nutrition science on fatty acids and cardiovascular disease: nutritionists’ perspectives. Adv Nutr. 2015;6(3):326S–37S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Augustin LS, Kendall CW, Jenkins DJ, Willett WC, Astrup A, Barclay AW, Bjorck I, Brand-Miller JC, Brighenti F, Buyken AE et al.. Glycemic index, glycemic load and glycemic response: an International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr Metab Cardiovasc Dis. 2015;25(9):795–815. [DOI] [PubMed] [Google Scholar]
- 8. Liu AG, Ford NA, Hu FB, Zelman KM, Mozaffarian D, Kris-Etherton PM. A healthy approach to dietary fats: understanding the science and taking action to reduce consumer confusion. Nutr J. 2017;16(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. FAO. Dietary Protein Quality Evaluation in Human Nutrition. Report of an FAO Expert Consultation. 31 March–2 April, 2011, Auckland, New Zealand. Food and Nutrition Paper 92. Rome, Italy: FAO; 2013. [PubMed] [Google Scholar]
- 10.Food and Agriculture Organization/World Health Organization/United Nations University. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation (2002: Geneva, Switzerland). WHO Technical Report Series, No 935. Geneva, Switzerland: WHO; 2007. [Google Scholar]
- 11. Nelson ME, Hamm MW, Hu FB, Abrams SA, Griffin TS. Alignment of healthy dietary patterns and environmental sustainability: a systematic review. Adv Nutr. 2016;7(6):1005–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Boer J, Aiking H. On the merits of plant-based proteins for global food security: marrying macro and micro perspectives. Ecol Econ. 2011;70(7):1259–65. [Google Scholar]
- 13. Cleveland DA, Gee Q. Plant-based diets for mitigating climate change. In: Mariotti F, editor. Vegetarian and Plant-Based Diets in Health and Disease Prevention. San Diego, CA: Academic Press; 2017. pp. 135–56. [Google Scholar]
- 14. Hu FB. Plant-based foods and prevention of cardiovascular disease: an overview. Am J Clin Nutr. 2003;78(3 Suppl):544S–51S. [DOI] [PubMed] [Google Scholar]
- 15. Cifelli CJ, Houchins JA, Demmer E, Fulgoni VL. Increasing plant based foods or dairy foods differentially affects nutrient intakes: dietary scenarios using NHANES 2007–2010. Nutrients. 2016;8(7):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gavelle E, Huneau JF, Mariotti F. Patterns of protein food intake are associated with nutrient adequacy in the general French adult population. Nutrients. 2018;10(2):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ascherio A, Rimm EB, Stampfer MJ, Giovannucci EL, Willett WC. Dietary intake of marine n-3 fatty acids, fish intake, and the risk of coronary disease among men. N Engl J Med. 1995;332(15):977–82. [DOI] [PubMed] [Google Scholar]
- 18. Escalon H, Bossard C, Beck F. Baromètre Santé Nutrition 2008. Saint Denis, France: Baromètres santé, INPES; 2009. [Google Scholar]
- 19. Touvier M, Kesse-Guyot E, Mejean C, Estaquio C, Peneau S, Hercberg S, Castetbon K. Variations in compliance with recommendations and types of meat/seafood/eggs according to sociodemographic and socioeconomic categories. Ann Nutr Metab. 2010;56(1):65–73. [DOI] [PubMed] [Google Scholar]
- 20. Vergnaud AC, Norat T, Romaguera D, Mouw T, May AM, Travier N, Luan J, Wareham N, Slimani N, Rinaldi S et al.. Meat consumption and prospective weight change in participants of the EPIC-PANACEA study. Am J Clin Nutr. 2010;92(2):398–407. [DOI] [PubMed] [Google Scholar]
- 21. Petersen KS, Flock MR, Richter CK, Mukherjea R, Slavin JL, Kris-Etherton PM. Healthy dietary patterns for preventing cardiometabolic disease: the role of plant-based foods and animal products. Curr Dev Nutr. 2017;1(12):117.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sobiecki JG, Appleby PN, Bradbury KE, Key TJ. High compliance with dietary recommendations in a cohort of meat eaters, fish eaters, vegetarians, and vegans: results from the European Prospective Investigation into Cancer and Nutrition-Oxford study. Nutr Res. 2016;36(5):464–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gilsing AM, Crowe FL, Lloyd-Wright Z, Sanders TA, Appleby PN, Allen NE, Key TJ. Serum concentrations of vitamin B12 and folate in British male omnivores, vegetarians and vegans: results from a cross-sectional analysis of the EPIC-Oxford cohort study. Eur J Clin Nutr. 2010;64(9):933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rizzo NS, Jaceldo-Siegl K, Sabate J, Fraser GE. Nutrient profiles of vegetarian and nonvegetarian dietary patterns. J Acad Nutr Diet. 2013;113(12):1610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Conrad Z, Karlsen M, Chui K, Jahns L. Diet quality on meatless days: National Health and Nutrition Examination Survey (NHANES), 2007–2012. Public Health Nutr. 2017;20(9):1564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Allès B, Baudry J, Méjean C, Touvier M, Péneau S, Hercberg S, Kesse-Guyot E. Comparison of sociodemographic and nutritional characteristics between self-reported vegetarians, vegans, and meat-eaters from the NutriNet-Santé study. Nutrients. 2017;9(9):1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Niva M, Vainio A, Jallinoja P. Barriers to increasing plant protein consumption in Western populations. In: Mariotti F, editor. Vegetarian and Plant-Based Diets in Health and Disease Prevention. San Diego, CA: Academic Press; 2017. pp. 157–71. [Google Scholar]
- 28. Orlich MJ, Jaceldo-Siegl K, Sabate J, Fan J, Singh PN, Fraser GE. Patterns of food consumption among vegetarians and non-vegetarians. Br J Nutr. 2014;112(10):1644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bradbury KE, Tong TYN, Key TJ. Dietary intake of high-protein foods and other major foods in meat-eaters, poultry-eaters, fish-eaters, vegetarians, and vegans in UK Biobank. Nutrients. 2017;9(12):1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Walsh S, Hebbelinck M, Deriemaeker P, Clarys P. Dietary patterns in plant-based, vegetarian, and omnivorous diets. In: Mariotti F, editor. Vegetarian and Plant-Based Diets in Health and Disease Prevention. San Diego, CA: Academic Press; 2017. pp. 175–96. [Google Scholar]
- 31. Fogelholm M, Kanerva N, Mannisto S. Association between red and processed meat consumption and chronic diseases: the confounding role of other dietary factors. Eur J Clin Nutr. 2015;69(9):1060–5. [DOI] [PubMed] [Google Scholar]
- 32. Okubo H, Sasaki S, Murakami K, Takahashi Y; Freshmen in Dietetic Courses Study II Group . The ratio of fish to meat in the diet is positively associated with favorable intake of food groups and nutrients among young Japanese women. Nutr Res. 2011;31(3):169–77. [DOI] [PubMed] [Google Scholar]
- 33. Shang X, Scott D, Hodge A, English DR, Giles GG, Ebeling PR, Sanders KM. Dietary protein from different food sources, incident metabolic syndrome and changes in its components: an 11-year longitudinal study in healthy community-dwelling adults. Clin Nutr. 2017;36(6):1540–8. [DOI] [PubMed] [Google Scholar]
- 34. Phillips SM, Fulgoni VL 3rd, Heaney RP, Nicklas TA, Slavin JL, Weaver CM. Commonly consumed protein foods contribute to nutrient intake, diet quality, and nutrient adequacy. Am J Clin Nutr. 2015;101(6):1346S–52S. [DOI] [PubMed] [Google Scholar]
- 35. Pisa PT, Pedro TM, Kahn K, Tollman SM, Pettifor JM, Norris SA. Nutrient patterns and their association with socio-demographic, lifestyle factors and obesity risk in rural South African adolescents. Nutrients. 2015;7(5):3464–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Camilleri GM, Verger EO, Huneau JF, Carpentier F, Dubuisson C, Mariotti F. Plant and animal protein intakes are differently associated with nutrient adequacy of the diet of French adults. J Nutr. 2013;143(9):1466–73. [DOI] [PubMed] [Google Scholar]
- 37. Bianchi CM, Egnell M, Huneau JF, Mariotti F. Plant protein intake and dietary diversity are independently associated with nutrient adequacy in French adults. J Nutr. 2016;146(11):2351–60. [DOI] [PubMed] [Google Scholar]
- 38. Swain JF, McCarron PB, Hamilton EF, Sacks FM, Appel LJ. Characteristics of the diet patterns tested in the optimal macronutrient intake trial to prevent heart disease (OmniHeart): options for a heart-healthy diet. J Am Diet Assoc. 2008;108(2):257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Richter CK, Skulas-Ray AC, Champagne CM, Kris-Etherton PM. Plant protein and animal proteins: do they differentially affect cardiovascular disease risk?. Adv Nutr. 2015;6(6):712–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miller ER 3rd, Erlinger TP, Appel LJ. The effects of macronutrients on blood pressure and lipids: an overview of the DASH and OmniHeart trials. Curr Atheroscler Rep. 2006;8(6):460–5. [DOI] [PubMed] [Google Scholar]
- 41. Grosso G, Micek A, Godos J, Pajak A, Sciacca S, Galvano F, Boffetta P. Health risk factors associated with meat, fruit and vegetable consumption in cohort studies: a comprehensive meta-analysis. PLoS One. 2017;12(8):e0183787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, Willett WC, Manson JE, Sun Q, Hu FB. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med. 2016;13(6):e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim H, Caulfield LE, Rebholz CM. Healthy plant-based diets are associated with lower risk of all-cause mortality in US adults. J Nutr. 2018;148(4):624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tapsell LC, Neale EP, Satija A, Hu FB. Foods, nutrients, and dietary patterns: interconnections and implications for dietary guidelines. Adv Nutr. 2016;7(3):445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. York R. Residualization is not the answer: rethinking how to address multicollinearity. Soc Sci Res. 2012;41(6):1379–86. [DOI] [PubMed] [Google Scholar]
- 46. Kelemen LE, Kushi LH, Jacobs DR Jr, Cerhan JR. Associations of dietary protein with disease and mortality in a prospective study of postmenopausal women. Am J Epidemiol. 2005;161(3):239–49. [DOI] [PubMed] [Google Scholar]
- 47. Fung TT, van Dam RM, Hankinson SE, Stampfer M, Willett WC, Hu FB. Low-carbohydrate diets and all-cause and cause-specific mortality: two cohort studies. Ann Intern Med. 2010;153(5):289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lagiou P, Sandin S, Lof M, Trichopoulos D, Adami HO, Weiderpass E. Low carbohydrate–high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. BMJ. 2012;344:e4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Willett WC. Low-carbohydrate diets: a place in health promotion?. J Intern Med. 2007;261(4):363–5. [DOI] [PubMed] [Google Scholar]
- 50. Song M, Fung TT, Hu FB, Willett WC, Longo VD, Chan AT, Giovannucci EL. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern Med. 2016;176(10):1453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hernández-Alonso P, Salas-Salvadó J, Ruiz-Canela M, Corella D, Estruch R, Fitó M, Arós F, Gómez-Gracia E, Fiol M, Lapetra J et al.. High dietary protein intake is associated with an increased body weight and total death risk. Clin Nutr. 2016;35(2):496–506. [DOI] [PubMed] [Google Scholar]
- 52. van Nielen M, Feskens EJ, Mensink M, Sluijs I, Molina E, Amiano P, Ardanaz E, Balkau B, Beulens JW, Boeing H et al.. Dietary protein intake and incidence of type 2 diabetes in Europe: the EPIC-InterAct case-cohort study. Diabetes Care. 2014;37(7):1854–62. [DOI] [PubMed] [Google Scholar]
- 53. Lin Y, Mouratidou T, Vereecken C, Kersting M, Bolca S, de Moraes AC, Cuenca-Garcia M, Moreno LA, Gonzalez-Gross M, Valtuena J et al.. Dietary animal and plant protein intakes and their associations with obesity and cardio-metabolic indicators in European adolescents: the HELENA cross-sectional study. Nutr J. 2015;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li J, Sun C, Liu S, Li Y. Dietary protein intake and type 2 diabetes among women and men in northeast China. Sci Rep. 2016;6:37604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li SX, Imamura F, Schulze MB, Zheng J, Ye Z, Agudo A, Ardanaz E, Aune D, Boeing H, Dorronsoro M et al.. Interplay between genetic predisposition, macronutrient intake and type 2 diabetes incidence: analysis within EPIC-InterAct across eight European countries. Diabetologia. 2018;61(6):1325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shang X, Scott D, Hodge AM, English DR, Giles GG, Ebeling PR, Sanders KM. Dietary protein intake and risk of type 2 diabetes: results from the Melbourne Collaborative Cohort Study and a meta-analysis of prospective studies. Am J Clin Nutr. 2016;104(5):1352–65. [DOI] [PubMed] [Google Scholar]
- 57. Malik VS, Li Y, Tobias DK, Pan A, Hu FB. Dietary protein intake and risk of type 2 diabetes in US men and women. Am J Epidemiol. 2016;183(8):715–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tian S, Xu Q, Jiang R, Han T, Sun C, Na L. Dietary protein consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Nutrients. 2017;9(9):982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen GC, Zhang Z, van Dam RM, Qin LQ. Nonlinear relation between animal protein intake and risk of type 2 diabetes: a dose-response meta-analysis of prospective studies. Am J Clin Nutr. 2017;105(4):1014–6. [DOI] [PubMed] [Google Scholar]
- 60. Azemati B, Rajaram S, Jaceldo-Siegl K, Sabate J, Shavlik D, Fraser GE, Haddad EH. Animal-protein intake is associated with insulin resistance in Adventist Health Study 2 (AHS-2) Calibration Substudy participants: a cross-sectional analysis. Curr Dev Nutr. 2017;1(4):e000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Elliott P. Protein intake and blood pressure in cardiovascular disease. Proc Nutr Soc. 2003;62(2):495–504. [DOI] [PubMed] [Google Scholar]
- 62. Elliott P, Stamler J, Dyer AR, Appel L, Dennis B, Kesteloot H, Ueshima H, Okayama A, Chan Q, Garside DB et al.. Association between protein intake and blood pressure: the INTERMAP study. Arch Intern Med. 2006;166(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Altorf-van der Kuil W, Engberink MF, Vedder MM, Boer JM, Verschuren WM, Geleijnse JM. Sources of dietary protein in relation to blood pressure in a general Dutch population. PLoS One. 2012;7(2):e30582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang YF, Yancy WS Jr, Yu D, Champagne C, Appel LJ, Lin PH. The relationship between dietary protein intake and blood pressure: results from the PREMIER study. J Hum Hypertens. 2008;22(11):745–54. [DOI] [PubMed] [Google Scholar]
- 65. Stamler J, Liu K, Ruth KJ, Pryer J, Greenland P. Eight-year blood pressure change in middle-aged men: relationship to multiple nutrients. Hypertension. 2002;39(5):1000–6. [DOI] [PubMed] [Google Scholar]
- 66. Tielemans SM, Kromhout D, Altorf-van der Kuil W, Geleijnse JM. Associations of plant and animal protein intake with 5-year changes in blood pressure: the Zutphen Elderly Study. Nutr Metab Cardiovasc Dis. 2014;24(11):1228–33. [DOI] [PubMed] [Google Scholar]
- 67. Umesawa M, Sato S, Imano H, Kitamura A, Shimamoto T, Yamagishi K, Tanigawa T, Iso H. Relations between protein intake and blood pressure in Japanese men and women: the Circulatory Risk in Communities Study (CIRCS). Am J Clin Nutr. 2009;90(2):377–84. [DOI] [PubMed] [Google Scholar]
- 68. Liu R, Dang S, Yan H, Wang D, Zhao Y, Li Q, Liu X. Association between dietary protein intake and the risk of hypertension: a cross-sectional study from rural western China. Hypertens Res. 2013;36(11):972–9. [DOI] [PubMed] [Google Scholar]
- 69. Iseki K, Iseki C, Itoh K, Sanefuji M, Uezono K, Ikemiya Y, Fukiyama K, Kawasaki T. Estimated protein intake and blood pressure in a screened cohort in Okinawa, Japan. Hypertens Res. 2003;26(4):289–94. [DOI] [PubMed] [Google Scholar]
- 70. Altorf-van der Kuil W, Engberink MF, Brink EJ, van Baak MA, Bakker SJ, Navis G, van ’t Veer P, Geleijnse JM. Dietary protein and blood pressure: a systematic review. PLoS One. 2010;5(8):e12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tielemans SM, Altorf-van der Kuil W, Engberink MF, Brink EJ, van Baak MA, Bakker SJ, Geleijnse JM. Intake of total protein, plant protein and animal protein in relation to blood pressure: a meta-analysis of observational and intervention studies. J Hum Hypertens. 2013;27(9):564–71. [DOI] [PubMed] [Google Scholar]
- 72. Pedersen AN, Kondrup J, Borsheim E. Health effects of protein intake in healthy adults: a systematic literature review. Food Nutr Res. 2013;57:21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. de Koning L, Fung TT, Liao X, Chiuve SE, Rimm EB, Willett WC, Spiegelman D, Hu FB. Low-carbohydrate diet scores and risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93(4):844–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Halkjaer J, Olsen A, Overvad K, Jakobsen MU, Boeing H, Buijsse B, Palli D, Tognon G, Du H, van der A DL et al.. Intake of total, animal and plant protein and subsequent changes in weight or waist circumference in European men and women: the Diogenes project. Int J Obes (Lond). 2011;35(8):1104–13. [DOI] [PubMed] [Google Scholar]
- 75. Bujnowski D, Xun P, Daviglus ML, Van Horn L, He K, Stamler J. Longitudinal association between animal and vegetable protein intake and obesity among men in the United States: the Chicago Western Electric Study. J Am Diet Assoc. 2011;111(8):1150–5..e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Smith JD, Hou T, Ludwig DS, Rimm EB, Willett W, Hu FB, Mozaffarian D. Changes in intake of protein foods, carbohydrate amount and quality, and long-term weight change: results from 3 prospective cohorts. Am J Clin Nutr. 2015;101(6):1216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tonstad S, Clifton P. Vegetarian diets and the risk of type 2 diabetes. In: Mariotti F, editor. Vegetarian and Plant-Based Diets in Health and Disease Prevention. San Diego, CA: Academic Press; 2017. pp. 355–67. [Google Scholar]
- 78. Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122(9):876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Becerra-Tomas N, Babio N, Martinez-Gonzalez MA, Corella D, Estruch R, Ros E, Fito M, Serra-Majem L, Salaverria I, Lamuela-Raventos RM et al.. Replacing red meat and processed red meat for white meat, fish, legumes or eggs is associated with lower risk of incidence of metabolic syndrome. Clin Nutr. 2016;35(6):1442–9. [DOI] [PubMed] [Google Scholar]
- 80. Tharrey M, Mariotti F, Mashchak A, Barbillon P, Delattre M, Fraser GE. Patterns of plant and animal protein intake are strongly associated with cardiovascular mortality: the Adventist Health Study-2 cohort. Int J Epidemiol. 2018;47(5):1603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Boutron-Ruault M-C, Mesrine S, Pierre F. Meat consumption and health outcomes. In: Mariotti F, editor. Vegetarian and Plant-Based Diets in Health and Disease Prevention. San Diego, CA: Academic Press; 2017. pp. 197–214. [Google Scholar]
- 82. Garvey WT. The conundrum of whole foods versus macronutrient composition in assessing effects on insulin sensitivity. Am J Clin Nutr. 2015;101(6):1109–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mariotti F, Bos C, Huneau JF. When the effect of dairy “protein” on weight gain cannot be solely ascribed to protein. Obesity (Silver Spring). 2010;18(5):863; author reply: 864. [DOI] [PubMed] [Google Scholar]
- 84. Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88(1):38–50. [DOI] [PubMed] [Google Scholar]
- 85. Kou T, Wang Q, Cai J, Song J, Du B, Zhao K, Ma Y, Geng B, Zhang Y, Han X et al.. Effect of soybean protein on blood pressure in postmenopausal women: a meta-analysis of randomized controlled trials. Food Funct. 2017;8(8):2663–71. [DOI] [PubMed] [Google Scholar]
- 86. Ramdath DD, Padhi EM, Sarfaraz S, Renwick S, Duncan AM. Beyond the cholesterol-lowering effect of soy protein: a review of the effects of dietary soy and its constituents on risk factors for cardiovascular disease. Nutrients. 2017;9(4):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mariotti F, Tomé D, Patureau-Mirand P. Converting nitrogen into protein—beyond 6.25 and Jones’ factors. Crit Rev Food Sci Nutr. 2008;48:177–84. [DOI] [PubMed] [Google Scholar]
- 88. Vega-Lopez S, Lichtenstein AH. Dietary protein type and cardiovascular disease risk factors. Prev Cardiol. 2005;8(1):31–40. [DOI] [PubMed] [Google Scholar]
- 89. Akhlaghi M, Zare M, Nouripour F. Effect of soy and soy isoflavones on obesity-related anthropometric measures: a systematic review and meta-analysis of randomized controlled clinical trials. Adv Nutr. 2017;8(5):705–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dong JY, Tong X, Wu ZW, Xun PC, He K, Qin LQ. Effect of soya protein on blood pressure: a meta-analysis of randomised controlled trials. Br J Nutr. 2011;106(3):317–26. [DOI] [PubMed] [Google Scholar]
- 91. Bahr M, Fechner A, Kiehntopf M, Jahreis G. Consuming a mixed diet enriched with lupin protein beneficially affects plasma lipids in hypercholesterolemic subjects: a randomized controlled trial. Clin Nutr. 2015;34(1):7–14. [DOI] [PubMed] [Google Scholar]
- 92. Bahr M, Fechner A, Kramer J, Kiehntopf M, Jahreis G. Lupin protein positively affects plasma LDL cholesterol and LDL:HDL cholesterol ratio in hypercholesterolemic adults after four weeks of supplementation: a randomized, controlled crossover study. Nutr J. 2013;12:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Weisse K, Brandsch C, Zernsdorf B, Nkengfack Nembongwe GS, Hofmann K, Eder K, Stangl GI. Lupin protein compared to casein lowers the LDL cholesterol:HDL cholesterol-ratio of hypercholesterolemic adults. Eur J Nutr. 2010;49(2):65–71. [DOI] [PubMed] [Google Scholar]
- 94. Belski R, Mori TA, Puddey IB, Sipsas S, Woodman RJ, Ackland TR, Beilin LJ, Dove ER, Carlyon NB, Jayaseena V et al.. Effects of lupin-enriched foods on body composition and cardiovascular disease risk factors: a 12-month randomized controlled weight loss trial. Int J Obes (Lond). 2011;35(6):810–9. [DOI] [PubMed] [Google Scholar]
- 95. Lee YP, Mori TA, Puddey IB, Sipsas S, Ackland TR, Beilin LJ, Hodgson JM. Effects of lupin kernel flour–enriched bread on blood pressure: a controlled intervention study. Am J Clin Nutr. 2009;89(3):766–72. [DOI] [PubMed] [Google Scholar]
- 96. Hodgson JM, Lee YP, Puddey IB, Sipsas S, Ackland TR, Beilin LJ, Belski R, Mori TA. Effects of increasing dietary protein and fibre intake with lupin on body weight and composition and blood lipids in overweight men and women. Int J Obes (Lond). 2010;34(6):1086–94. [DOI] [PubMed] [Google Scholar]
- 97. Liu ZM, Chen YM, Ho SC. Effects of soy intake on glycemic control: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2011;93(5):1092–101. [DOI] [PubMed] [Google Scholar]
- 98. Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M; American Heart Association Nutrition Committee . Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113(7):1034–44. [DOI] [PubMed] [Google Scholar]
- 99. Matthan NR, Jalbert SM, Ausman LM, Kuvin JT, Karas RH, Lichtenstein AH. Effect of soy protein from differently processed products on cardiovascular disease risk factors and vascular endothelial function in hypercholesterolemic subjects. Am J Clin Nutr. 2007;85(4):960–6. [DOI] [PubMed] [Google Scholar]
- 100. Li SS, Blanco Mejia S, Lytvyn L, Stewart SE, Viguiliouk E, Ha V, de Souza RJ, Leiter LA, Kendall CWC, Jenkins DJA et al.. Effect of plant protein on blood lipids: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2017;6(12):e006659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Teunissen-Beekman KF, van Baak MA. The role of dietary protein in blood pressure regulation. Curr Opin Lipidol. 2013;24(1):65–70. [DOI] [PubMed] [Google Scholar]
- 102. Maki KC, Van Elswyk ME, Alexander DD, Rains TM, Sohn EL, McNeill S. A meta-analysis of randomized controlled trials that compare the lipid effects of beef versus poultry and/or fish consumption. J Clin Lipidol. 2012;6(4):352–61. [DOI] [PubMed] [Google Scholar]
- 103. O'Connor LE, Kim JE, Campbell WW. Total red meat intake of ≥0.5 servings/d does not negatively influence cardiovascular disease risk factors: a systemically searched meta-analysis of randomized controlled trials. Am J Clin Nutr. 2017;105(1):57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER 3rd, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM et al.. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294(19):2455–64. [DOI] [PubMed] [Google Scholar]
- 105. Te Morenga L, Mann J. The role of high-protein diets in body weight management and health. Br J Nutr. 2012;108(Suppl 2):S130–8. [DOI] [PubMed] [Google Scholar]
- 106. Schwingshackl L, Schwedhelm C, Hoffmann G, Knuppel S, Iqbal K, Andriolo V, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of hypertension: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. 2017;8(6):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes – an updated review of the evidence. Curr Atheroscler Rep. 2012;14(6):515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. 2010;121(21):2271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang X, Lin X, Ouyang YY, Liu J, Zhao G, Pan A, Hu FB. Red and processed meat consumption and mortality: dose-response meta-analysis of prospective cohort studies. Public Health Nutr. 2016;19(5):893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. 2012;172(7):555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Abete I, Romaguera D, Vieira AR, Lopez de Munain A, Norat T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: a meta-analysis of cohort studies. Br J Nutr. 2014;112(5):762–75. [DOI] [PubMed] [Google Scholar]
- 112. Chen GC, Lv DB, Pang Z, Liu QF. Red and processed meat consumption and risk of stroke: a meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2013;67(1):91–5. [DOI] [PubMed] [Google Scholar]
- 113. Kaluza J, Wolk A, Larsson SC. Red meat consumption and risk of stroke: a meta-analysis of prospective studies. Stroke. 2012;43(10):2556–60. [DOI] [PubMed] [Google Scholar]
- 114. Bechthold A, Boeing H, Schwedhelm C, Hoffmann G, Knuppel S, Iqbal K, De Henauw S, Michels N, Devleesschauwer B, Schlesinger S et al.. Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr. 2017:1–20. [DOI] [PubMed] [Google Scholar]
- 115. Kim K, Hyeon J, Lee SA, Kwon SO, Lee H, Keum N, Lee JK, Park SM. Role of total, red, processed, and white meat consumption in stroke incidence and mortality: a systematic review and meta-analysis of prospective cohort studies. J Am Heart Assoc. 2017;6(9):e005983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kris-Etherton PM, Etherton TD, Carlson J, Gardner C. Recent discoveries in inclusive food-based approaches and dietary patterns for reduction in risk for cardiovascular disease. Curr Opin Lipidol. 2002;13(4):397–407. [DOI] [PubMed] [Google Scholar]
- 117. Salas-Salvadó J, Guasch-Ferré M, Lee CH, Estruch R, Clish CB, Ros E. Protective effects of the Mediterranean diet on type 2 diabetes and metabolic syndrome. J Nutr. 2016;146(4):920S–7S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Katz DL, Meller S. Can we say what diet is best for health?. Annu Rev Public Health. 2014;35:83–103. [DOI] [PubMed] [Google Scholar]
- 119. Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169(7):659–69. [DOI] [PubMed] [Google Scholar]
- 120. Mariotti F, Huneau JF. Plant and animal protein intakes are differentially associated with large clusters of nutrient intake that may explain part of their complex relation with CVD risk. Adv Nutr. 2016;7(3):559–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006;147(Suppl 1):S193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Juonala M, Viikari JS, Laitinen T, Marniemi J, Helenius H, Ronnemaa T, Raitakari OT. Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: the Cardiovascular Risk in Young Finns study. Circulation. 2004;110(18):2918–23. [DOI] [PubMed] [Google Scholar]
- 123. Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S et al.. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126(6):753–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Reriani MK, Lerman LO, Lerman A. Endothelial function as a functional expression of cardiovascular risk factors. Biomark Med. 2010;4(3):351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Bai Y, Sun L, Yang T, Sun K, Chen J, Hui R. Increase in fasting vascular endothelial function after short-term oral L-arginine is effective when baseline flow-mediated dilation is low: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2009;89(1):77–84. [DOI] [PubMed] [Google Scholar]
- 126. Borucki K, Aronica S, Starke I, Luley C, Westphal S. Addition of 2.5 g L-arginine in a fatty meal prevents the lipemia-induced endothelial dysfunction in healthy volunteers. Atheroscler Suppl. 2009;205(1):251–4. [DOI] [PubMed] [Google Scholar]
- 127. Deveaux A, Pham I, West SG, Andre E, Lantoine-Adam F, Bunouf P, Sadi S, Hermier D, Mathe V, Fouillet H et al.. L-arginine supplementation alleviates postprandial endothelial dysfunction when baseline fasting plasma arginine concentration is low: a randomized controlled trial in healthy overweight adults with cardiometabolic risk factors. J Nutr. 2016;146(7):1330–40. [DOI] [PubMed] [Google Scholar]
- 128. Deveaux A, Fouillet H, Petzke KJ, Hermier D, Andre E, Bunouf P, Lantoine-Adam F, Benamouzig R, Mathe V, Huneau JF et al.. A slow- compared with a fast-release form of oral arginine increases its utilization for nitric oxide synthesis in overweight adults with cardiometabolic risk factors in a randomized controlled study. J Nutr. 2016;146(7):1322–9. [DOI] [PubMed] [Google Scholar]
- 129. Westphal S, Taneva E, Kastner S, Martens-Lobenhoffer J, Bode-Boger S, Kropf S, Dierkes J, Luley C. Endothelial dysfunction induced by postprandial lipemia is neutralized by addition of proteins to the fatty meal. Atheroscler Suppl. 2006;185(2):313–9. [DOI] [PubMed] [Google Scholar]
- 130. Magne J, Huneau JF, Tsikas D, Delemasure S, Rochette L, Tome D, Mariotti F. Rapeseed protein in a high-fat mixed meal alleviates postprandial systemic and vascular oxidative stress and prevents vascular endothelial dysfunction in healthy rats. J Nutr. 2009;139(9):1660–6. [DOI] [PubMed] [Google Scholar]
- 131. Jobgen W, Meininger CJ, Jobgen SC, Li P, Lee MJ, Smith SB, Spencer TE, Fried SK, Wu G. Dietary L-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J Nutr. 2009;139(2):230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Tan B, Yin Y, Liu Z, Li X, Xu H, Kong X, Huang R, Tang W, Shinzato I, Smith SB et al.. Dietary L-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids. 2009;37(1):169–75. [DOI] [PubMed] [Google Scholar]
- 133. Wu Z, Satterfield MC, Bazer FW, Wu G. Regulation of brown adipose tissue development and white fat reduction by L-arginine. Curr Opin Clin Nutr Metab Care. 2012;15(6):529–38. [DOI] [PubMed] [Google Scholar]
- 134. Roberts LD. Does inorganic nitrate say NO to obesity by browning white adipose tissue?. Adipocyte. 2015;4(4):311–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Niu YC, Feng RN, Hou Y, Li K, Kang Z, Wang J, Sun CH, Li Y. Histidine and arginine are associated with inflammation and oxidative stress in obese women. Br J Nutr. 2012;108(1):57–61. [DOI] [PubMed] [Google Scholar]
- 136. Debry G. Experimental data on animals. In: Dietary Proteins and Atherosclerosis. Boca Raton, FL: CRC Press; 2004. pp. 9–126. [Google Scholar]
- 137. Debry G. Data on atherosclerosis. In: Dietary Proteins and Atherosclerosis. Boca Raton, FL: CRC Press; 2004. pp. 159–190. [Google Scholar]
- 138. Vega-Lopez S, Matthan NR, Ausman LM, Harding SV, Rideout TC, Ai M, Otokozawa S, Freed A, Kuvin JT, Jones PJ et al.. Altering dietary lysine:arginine ratio has little effect on cardiovascular risk factors and vascular reactivity in moderately hypercholesterolemic adults. Atheroscler Suppl. 2010;210(2):555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37(1):1–17. [DOI] [PubMed] [Google Scholar]
- 140. McPherson RA, Hardy G. Clinical and nutritional benefits of cysteine-enriched protein supplements. Curr Opin Clin Nutr Metab Care. 2011;14(6):562–8. [DOI] [PubMed] [Google Scholar]
- 141. Yin J, Ren W, Yang G, Duan J, Huang X, Fang R, Li C, Li T, Yin Y, Hou Y et al.. L-cysteine metabolism and its nutritional implications. Mol Nutr Food Res. 2016;60(1):134–46. [DOI] [PubMed] [Google Scholar]
- 142. Blouet C, Mariotti F, Azzout-Marniche D, Mathe V, Mikogami T, Tome D, Huneau JF. Dietary cysteine alleviates sucrose-induced oxidative stress and insulin resistance. Free Radic Biol Med. 2007;42(7):1089–97. [DOI] [PubMed] [Google Scholar]
- 143. Ashfaq S, Abramson JL, Jones DP, Rhodes SD, Weintraub WS, Hooper WC, Vaccarino V, Harrison DG, Quyyumi AA. The relationship between plasma levels of oxidized and reduced thiols and early atherosclerosis in healthy adults. J Am Coll Cardiol. 2006;47(5):1005–11. [DOI] [PubMed] [Google Scholar]
- 144. Jones DP, Park Y, Gletsu-Miller N, Liang Y, Yu T, Accardi CJ, Ziegler TR. Dietary sulfur amino acid effects on fasting plasma cysteine/cystine redox potential in humans. Nutrition. 2011;27(2):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Hildebrandt W, Sauer R, Bonaterra G, Dugi KA, Edler L, Kinscherf R. Oral N-acetylcysteine reduces plasma homocysteine concentrations regardless of lipid or smoking status. Am J Clin Nutr. 2015;102(5):1014–24. [DOI] [PubMed] [Google Scholar]
- 146. Larsson SC, Håkansson N, Wolk A. Dietary cysteine and other amino acids and stroke incidence in women. Stroke. 2015;46(4):922–6. [DOI] [PubMed] [Google Scholar]
- 147. Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA et al.. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Boulet MM, Chevrier G, Grenier-Larouche T, Pelletier M, Nadeau M, Scarpa J, Prehn C, Marette A, Adamski J, Tchernof A. Alterations of plasma metabolite profiles related to adipose tissue distribution and cardiometabolic risk. Am J Physiol Endocrinol Metab. 2015;309(8):E736–46. [DOI] [PubMed] [Google Scholar]
- 149. Rauschert S, Uhl O, Koletzko B, Hellmuth C. Metabolomic biomarkers for obesity in humans: a short review. Ann Nutr Metab. 2014;64(3–4):314–24. [DOI] [PubMed] [Google Scholar]
- 150. Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10(12):723–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Polakof S, Remond D, Bernalier-Donadille A, Rambeau M, Pujos-Guillot E, Comte B, Dardevet D, Savary-Auzeloux I. Metabolic adaptations to HFHS overfeeding: how whole body and tissues postprandial metabolic flexibility adapt in Yucatan mini-pigs. Eur J Nutr. 2018;57(1):119–35. [DOI] [PubMed] [Google Scholar]
- 152. Yang R, Dong J, Zhao H, Li H, Guo H, Wang S, Zhang C, Wang S, Wang M, Yu S et al.. Association of branched-chain amino acids with carotid intima-media thickness and coronary artery disease risk factors. PLoS One. 2014;9(6):e99598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Guasch-Ferré M, Hruby A, Toledo E, Clish CB, Martínez-González MA, Salas-Salvadó J, Hu FB. Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care. 2016;39(5):833–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ruiz-Canela M, Guasch-Ferré M, Toledo E, Clish CB, Razquin C, Liang L, Wang DD, Corella D, Estruch R, Hernáez A et al.. Plasma branched chain/aromatic amino acids, enriched Mediterranean diet and risk of type 2 diabetes: case-cohort study within the PREDIMED trial. Diabetologia. 2018;61(7):1560–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Ruiz-Canela M, Toledo E, Clish CB, Hruby A, Liang L, Salas-Salvadó J, Razquin C, Corella D, Estruch R, Ros E et al.. Plasma branched-chain amino acids and incident cardiovascular disease in the PREDIMED trial. Clin Chem. 2016;62(4):582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Isanejad M, LaCroix AZ, Thomson CA, Tinker L, Larson JC, Qi Q, Qi L, Cooper-DeHoff RM, Phillips LS, Prentice RL et al.. Branched-chain amino acid, meat intake and risk of type 2 diabetes in the Women's Health Initiative. Br J Nutr. 2017;117(11):1523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zheng Y, Li Y, Qi Q, Hruby A, Manson JE, Willett WC, Wolpin BM, Hu FB, Qi L. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int J Epidemiol. 2016;45(5):1482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Balage M, Dupont J, Mothe-Satney I, Tesseraud S, Mosoni L, Dardevet D. Leucine supplementation in rats induced a delay in muscle IR/PI3K signaling pathway associated with overall impaired glucose tolerance. J Nutr Biochem. 2011;22(3):219–26. [DOI] [PubMed] [Google Scholar]
- 159. Cummings NE, Williams EM, Kasza I, Konon EN, Schaid MD, Schmidt BA, Poudel C, Sherman DS, Yu D, Arriola Apelo SI et al.. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol. 2018;596(4):623–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15(5):606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Gojda J, Rossmeislova L, Strakova R, Tumova J, Elkalaf M, Jacek M, Tuma P, Potockova J, Krauzova E, Waldauf P et al.. Chronic dietary exposure to branched chain amino acids impairs glucose disposal in vegans but not in omnivores. Eur J Clin Nutr. 2017;71(5):594–601. [DOI] [PubMed] [Google Scholar]
- 162. Macotela Y, Emanuelli B, Bang AM, Espinoza DO, Boucher J, Beebe K, Gall W, Kahn CR. Dietary leucine – an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One. 2011;6(6):e21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56(6):1647–54. [DOI] [PubMed] [Google Scholar]
- 164. She P, Olson KC, Kadota Y, Inukai A, Shimomura Y, Hoppel CL, Adams SH, Kawamata Y, Matsumoto H, Sakai R et al.. Leucine and protein metabolism in obese Zucker rats. PLoS One. 2013;8(3):e59443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Pujos-Guillot E, Brandolini-Bunlon M, Fouillet H, Joly C, Martin JF, Huneau JF, Dardevet D, Mariotti F. Metabolomics reveals that the type of protein in a high-fat meal modulates postprandial mitochondrial overload and incomplete substrate oxidation in healthy overweight men. J Nutr. 2018;148(6):876–84. [DOI] [PubMed] [Google Scholar]
- 166. Wang Q, Holmes MV, Davey Smith G, Ala-Korpela M. Genetic support for a causal role of insulin resistance on circulating branched-chain amino acids and inflammation. Diabetes Care. 2017;40(12):1779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Gannon NP, Schnuck JK, Vaughan RA. BCAA metabolism and insulin sensitivity – dysregulated by metabolic status?. Mol Nutr Food Res. 2018;62(6):e1700756. [DOI] [PubMed] [Google Scholar]
- 168. Lotta LA, Scott RA, Sharp SJ, Burgess S, Luan J, Tillin T, Schmidt AF, Imamura F, Stewart ID, Perry JR et al.. Genetic predisposition to an impaired metabolism of the branched-chain amino acids and risk of type 2 diabetes: a Mendelian randomisation analysis. PLoS Med. 2016;13(11):e1002179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Mahendran Y, Jonsson A, Have CT, Allin KH, Witte DR, Jorgensen ME, Grarup N, Pedersen O, Kilpelainen TO, Hansen T. Genetic evidence of a causal effect of insulin resistance on branched-chain amino acid levels. Diabetologia. 2017;60(5):873–8. [DOI] [PubMed] [Google Scholar]
- 170. Gannon MC, Nuttall JA, Nuttall FQ. The metabolic response to ingested glycine. Am J Clin Nutr. 2002;76(6):1302–7. [DOI] [PubMed] [Google Scholar]
- 171. Gannon MC, Nuttall FQ. Amino acid ingestion and glucose metabolism—a review. IUBMB Life. 2010;62(9):660–8. [DOI] [PubMed] [Google Scholar]
- 172. Stamler J, Brown IJ, Daviglus ML, Chan Q, Kesteloot H, Ueshima H, Zhao L, Elliott P. Glutamic acid, the main dietary amino acid, and blood pressure: the INTERMAP Study (International Collaborative Study of Macronutrients, Micronutrients and Blood Pressure). Circulation. 2009;120(3):221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Jennings A, MacGregor A, Welch A, Chowienczyk P, Spector T, Cassidy A. Amino acid intakes are inversely associated with arterial stiffness and central blood pressure in women. J Nutr. 2015;145(9):2130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Teymoori F, Asghari G, Mirmiran P, Azizi F. Dietary amino acids and incidence of hypertension: a principle component analysis approach. Sci Rep. 2017;7(1):16838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Schmidt JA, Rinaldi S, Ferrari P, Carayol M, Achaintre D, Scalbert A, Cross AJ, Gunter MJ, Fensom GK, Appleby PN et al.. Metabolic profiles of male meat eaters, fish eaters, vegetarians, and vegans from the EPIC-Oxford cohort. Am J Clin Nutr. 2015;102(6):1518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]