ABSTRACT
The WHO recommends exclusive breastfeeding of infants for the first 6 mo of life (EBF-6). We reviewed the evidence behind concerns related to this recommendation. The risk of iron deficiency among EBF-6 infants can be significantly reduced if delayed cord clamping is performed in all newborns. At the moment there is no population-level evidence indicating that exclusive breastfeeding for 6 mo compared with <6 mo increases the risk of developing food allergies. Mild to moderate maternal undernutrition may reduce amounts of some nutrients in breast milk but does not directly diminish milk volume. Persistent reports of insufficient milk by women globally are likely to be the result of lack of access to timely lactation counseling and social support rather than primary biological reasons. All newborns should have their growth, hydration status, and development carefully monitored. In instances where formula supplementation is required, it should be done under the guidance of a qualified provider taking into account that early introduction of breast-milk supplements is a risk factor for early termination of exclusive breastfeeding and any breastfeeding. We found no evidence to support changes to the EBF-6 public health recommendation, although variability in inter-infant developmental readiness is recognized. We suggest that infant and young feeding guidelines make clear that complementary foods should be introduced at around 6 mo of age, taking infant developmental readiness into account.
Keywords: exclusive breastfeeding, complementary feeding, food allergies, iron deficiency, anemia, breast milk, policy
Introduction
In 2001, the WHO recommended exclusive breastfeeding (EBF) for the first 6 mo of life (EBF-6), replacing its previous recommendation of EBF for 4-6 mo (1, 2). EBF-6 was reaffirmed in 2012 (3); however, concerns continue to be raised about the feasibility and safety of this recommendation, and some have called for its reexamination (4). The objectives of this Perspective are to: 1) present and discuss the evidence on which the WHO EBF-6 recommendation is based; 2) review evidence behind concerns related to EBF-6 including iron-deficiency anemia, food allergies, and maternal malnutrition; 3) clarify EBF-6 in the context of a public health recommendation compared with an individual-level recommendation; and 4) identify knowledge gaps. The literature reviewed was identified through a scan of review articles, recommendations from professional societies, scientific reports from the WHO, and the National Academies of Science, Engineering and Medicine; the authors’ files; and by eliciting feedback from experts based in academic institutions and at UNICEF, the WHO, and the Bill & Melinda Gates Foundation.
Evidence behind the EBF-6 Recommendation
The 2001 WHO EBF-6 recommendation was based on evidence gathered from a systematic review comparing EBF for 6 mo to EBF for 3-4 mo with continued BF for at least 6 mo, examining the impact of these practices on child health, growth, and development, as well as on maternal health (1, 2). Evidence was gathered from 20 studies in low-income/middle-income countries (LMICs) (n = 9) and high-income countries (n = 11). All but 2 of these studies were observational. The 2 randomized controlled trials (RCTs) were conducted in Honduras and included low-birth-weight (LBW) and adequate-birth-weight term (≥37 wk) infants who were receiving EBF for different durations. In both studies infants were provided with safe and nutritious complementary foods (5, 6). The first of these RCTs found that 1) there was no weight gain or linear-growth benefit for introduction of complementary foods at age 4 mo instead of age 6 mo (7); and 2) EBF-6 (compared with EBF-4) increased the risk of iron deficiency as indicated by low hematocrit, especially among LBW infants (8). The second trial, conducted among LBW infants only, also found no growth advantage during the first year of life for EBF-4 compared with EBF-6 (6). Data from both trials indicated that EBF-6 infants crawled sooner and in the first trial they also walked earlier compared with EBF-4 infants (9). Based on the results from RCTs and observational studies included in this review, it was concluded that: 1) no significant differences were found in growth among EBF-6 children compared with those with other EBF durations; 2) a lower incidence of gastrointestinal infection among EBF-6 infants was found when compared to infants receiving EBF for shorter durations; and 3) there was a potential risk for development of iron-deficiency anemia before age 6 mo, especially among exclusively breastfed infants born with suboptimal iron reserves and among LBW infants (1). In 2012, the WHO commissioned an updated systematic review of the evidence on EBF duration. This updated review included 23 studies, 3 of which were not included in the first review, 11 from LMICs, and 12 from high-income countries (3). This more-recent systematic review, which followed a full guideline review committee process (10), reaffirmed the WHO 2001 EBF-6 recommendation (1). Similar to the findings from the Honduran studies, an RCT conducted in Iceland found no infant growth advantage for EBF-4 compared with EBF-6 infants. Length and head circumference were measured at birth, 6 wk, and 3, 4, 5, 6, 8, 10, and 12 mo of age; and weight and height were measured at 18, 29, and 38 mo (11). An observational analysis of the Promoting Breastfeeding Interventional Trial in Belarus, a country with an adequate sanitation infrastructure, reported that EBF-6 infants had comparable growth and lower risk of gastrointestinal infections when compared to EBF-3 infants, consistent with evidence from low and LMIC settings (12).
Because RCTs are the gold standard for making causal inferences, the scarcity of RCTs underlying the WHO EBF-6 recommendation is seen by some as a weakness. However, the numerous maternal and child health benefits associated with breastfeeding (13–19) make experimental research designs impractical and unlikely to receive ethical approval in many settings.
Concerns about the EBF-6 Recommendation
Iron-deficiency anemia
Iron-deficiency anemia during early infancy has been identified as a risk associated with EBF-6, with potential long-term motor and mental developmental consequences (20). Although iron in human milk is highly bioavailable, breast milk contains relatively small amounts of iron that may not be able to sustain adequate iron status until age 6 mo, particularly among infants who have inadequate iron endowments at birth either because they are born to a mother with iron deficiency or they are preterm or LBW (21–23).
Addressing potential iron deficiency in infants is a challenge because supplementing the mother's diet with iron does not increase iron concentrations in her breast milk (24, 25) and giving iron drops to infants before age 6 mo has been ineffective in settings with weak healthcare systems (26, 27). This has led some to argue that the risk of iron deficiency through EBF-6 implementation outweighs this recommendation's other benefits, and that iron-rich complementary foods should be introduced to infants as early as age 4 mo (8, 11, 28, 29). This risk-benefit assessment does not, however, take into consideration the risk of infant morbidity and mortality associated with unsafe, home-prepared, complementary foods often found in low-income settings (30–32).
The risk for iron deficiency in the first 6 mo of life can be successfully mitigated by delayed umbilical-cord clamping (22, 33, 34) (Figure 1). The amount of iron provided from stores at birth plus intake from breast milk can provide sufficient iron for 6 mo if the exclusively breastfed infant is born at term, normal birth weight, the mother had adequate prenatal iron status, and the infant underwent delayed cord clamping. In contrast, early cord clamping in the same infant would provide sufficient iron for 3 mo only (34). LBW infants also benefit from delayed cord clamping. However, the WHO recommends medicinal iron drops beginning at age 2–3 mo for LBW infants, negating the need for early complementary feeding to reduce iron-deficiency risk (35).
FIGURE 1.
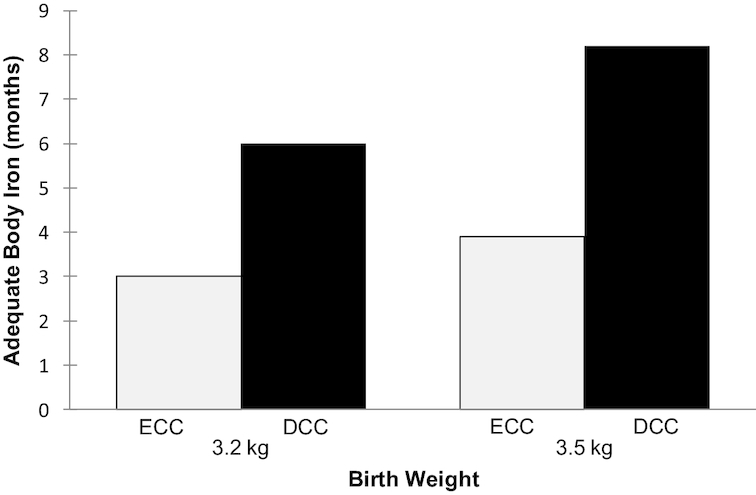
Duration of adequate body iron after birth by birth weight and timing of umbilical-cord clamping. Developed by authors from evidence reported by Chaparro and Lutter (22). ECC, early cord clamping; DCC, delayed umbilical-cord clamping.
The delayed cord clamping recommendation was issued by WHO based on strong evidence, and was subsequently incorporated into the WHO's Essential Newborn Care Course in 2012 (36) and to an official WHO Guideline in 2014 (37).
From the available evidence and WHO recommendations, we conclude that there is no need to change the EBF-6 recommendation based on concerns about iron-deficiency anemia. This condition can be addressed through delayed cord clamping and medicinal iron and does not require infant food introduction before 6 mo.
Food allergies
A food allergy is an adverse immune response to a component of a specific food (38). The majority of food allergies are caused by 8 foods: eggs, milk, peanuts, tree nuts, fish, shellfish, wheat, and soy (38). Between 1997 and 2007 in the United States, the prevalence of food allergies among children aged <18 y increased by 18% (39). In 2016, 6.5% of children in the United States suffered food allergies (40). A population-based parent survey conducted in the United States at around the same time reported a slightly higher prevalence of food allergies among children aged <18 y (7.6%), with peanut allergy being the most commonly reported (41). The HealthNuts cohort study in Australia reported a prevalence of food challenged-confirmed allergies of 11% at age 1 y and of 3.8% at age 4 y (42). The reported prevalence of food allergies varies considerably among countries. For example, a review of studies with adequate diagnosis of food allergies among children aged <6 y reported wide ranges in the following countries: 1.1% to 7.7% in the upper-middle-income countries of Thailand, South Africa, and China (43–45); 1.9% to 10.4% in the high-income countries of Iceland and Denmark (46, 47), the Isle of Wight in England (48), the United Kingdom (49), Norway (50), and Australia (51).
Because food allergies can lead to serious health problems, including anaphylactic reactions and premature death, there is an interest in better understanding of whether, and how, they are related to diverse infant-feeding practices (52, 53). Thirteen reviews and an expert report from the National Academies of Sciences, Engineering and Medicine (52, 53) found a lack of evidence to support the previous recommendations to delay introduction of potentially allergenic foods (specifically peanuts, eggs, fish, and gluten-containing foods) beyond age 12 mo to reduce the risk of allergies. Evidence from high-income countries has led to an interim clinical recommendation indicating that to prevent food allergies, infants at risk of developing them should be exposed to potentially allergenic foods between the ages of 4 and 6 mo (53, 54). This clinical guidance, endorsed by 10 allergy societies/academies from Australia, the United States, Canada, Europe, Japan, and the Middle East, states that such early exposure should be implemented on an individual basis, under the supervision of a qualified healthcare professional. The strongest evidence for this recommendation pertains to peanut and egg allergies based on findings from a single RCT conducted in the United Kingdom (55). The United Kingdom's Learning Early About A Peanut Allergy trial found that exposing infants from age 4 to 11 mo, who were at high risk of peanut allergy, to small tastes of peanut butter reduced the risk of developing allergies to these foods later in life (55). This trial used the term “high risk” for infants with severe eczema or who tested positive for an egg allergy. Interestingly, although it was initially reported that the protective association of peanut exposure was stronger the earlier the introduction took place (specifically, between the ages of 4 and 6 mo) (56), a subsequent analysis showed that protection was significantly higher with peanut introduction between 6 and 11 mo, compared to the challenge between 4 and 6 mo (57). The United Kingdom's Enquiring about Tolerance (EAT) trial focused on EBF infants randomly assigned to introduction of 6 potentially allergenic foods at age 3 mo (compared with age 6 mo): cooked egg, peanut, cow milk, sesame, white fish, and wheat (58). EAT found that the exposed infants were less likely to develop food allergies compared with their counterparts who continued EBF for about 6 mo (59). This protective effect may be the result of “training” the immune system to recognize these foods during a sensitive time in development of the infant's immune system (52). A study limitation of EAT was low compliance with the intervention protocol. However, analysis of the per-protocol group showed that the food-allergy incidence was significantly lower in the intervention group (2.4%) compared with the control group (7.3%, P = 0.01) (58). The EAT intervention most strongly protected against allergy to peanuts (with a dosage similar to that used in the Learning Early About A Peanut Allergy trial) and eggs.
Recommendations on the introduction of potentially allergenic foods in high-income countries remains controversial. The United Kingdom's Scientific Advisory Committee on Nutrition recently concluded (60) that whereas “the available evidence indicates that the deliberate exclusion or delayed introduction of peanut or hen's egg beyond 6 to 12 months of age may increase the risk of allergy to the same foods, the available evidence indicates that allergenic foods such as peanut, hen's egg, gluten, or fish can be introduced from around 6 months of age.” Furthermore, the Scientific Advisory Committee on Nutrition specifically concluded that “there is insufficient evidence to demonstrate that the introduction of peanut or hen's egg into the infant diet before 6 months of age reduces the risk of developing food allergy to any greater extent than introduction from around 6 months of age.”
A recent RCT from Ecuador found that introducing eggs between 6 and 9 mo and feeding 1 daily for 6 mo improved linear growth without increasing the risk of egg allergy (61). Furthermore, a systematic review that included 5 RCTs found that introduction of eggs between ages 4 and 6 mo was associated with a reduction in risk for egg allergies, independent of the level of risk that the infants had for developing them (62).
A previous evidence review concluded that changes to the EBF-6 public health recommendation were not needed because of general concerns about severe food allergy, but individual infants suspected to be at high risk should be assessed by a qualified provider to determine potential risk of developing severe allergy to certain foods and timing for their introduction (52). Recent studies suggest that breastfeeding may protect against development of food allergies and that this relation may be influenced by maternal diet. The Canadian Asthma Primary Prevention Birth Cohort study examined the relation between maternal peanut consumption while breastfeeding, timing of peanut introduction to the child diet, and peanut sensitization at age 7 y. This study found the lowest incidence of peanut sensitization (allergy risk) among mothers who ate peanuts while breastfeeding and introduced peanuts to their child before age 12 mo (63). This finding may have public health implications as a meta-analysis of 15 birth cohorts—mostly located in Europe—found that food sensitization during infancy, assessed by skin prick testing or measuring serum IgE during the first 2 y of life, was associated with eczema in late infancy, wheeze/asthma, and allergies during childhood (64).
Based on findings from the Canadian Healthy Infant Longitudinal Development cohort study, Miliku and Azad (65) concluded that the human milk oligosaccharide (HMO) composition of human milk is associated with development of food sensitization in the first year of life. HMO composition varies between maternal-infant dyads, and may be influenced by maternal diet and other environmental exposures, as well as lactation stage, degree of breastfeeding exclusivity, and genetics (e.g. fucosyltransferase-2 secretor status); and is a strong determinant of the microbiome. Findings on the relation between maternal diet and HMO composition are inconsistent (66), however, and further research is warranted to better understand these relations.
In summary, providing small tastes of potentially allergenic foods under medical supervision to 4 to 6 mo old infants at high risk of developing food allergies (such as infants with a family history of allergies to eggs and peanuts) has been endorsed by several professional allergy societies in high-income countries for the purpose of preventing future food allergies (52–54, 67). However, more recent expert reviews do not support this recommendation (60). Hence, this issue requires further high-quality evidence from diverse settings. Recent studies suggest that maternal diet may modify the impact of early exposure to potentially allergenic foods and subsequent risk of food allergies in the breastfed infant, but this finding also requires further confirmation and better understanding of underlying biological mechanisms.
It is important to note that the aforementioned studies were carried out in populations of infants at risk of developing severe food allergy. There is presently no population-level evidence indicating that EBF for 6 mo compared with <6 mo increases the risk of developing food allergies. Hence, a change to the EBF-6 recommendation for the population at large to prevent food allergies is unwarranted.
Self-reported insufficient milk
Self-reported insufficient milk (SRIM) is the most commonly mentioned reason provided by women the world over for introducing breast-milk substitutes into the infant's diet or for stopping breastfeeding altogether (68–70). Some have hypothesized that SRIM is simply a socially accepted excuse that women give for explaining why they are not practicing what they know is recommended infant-feeding behavior (71). However, others have postulated that SRIM may result from not understanding the lactation process, as women often report SRIM within the first 2 d after birth, a time when only small amounts of colostrum are being produced, and they introduce breast-milk substitutes in response to this (i.e., pre-lacteal feeding) (72–74). SRIM may also be the result of perceptions from healthcare providers about the capacity of women to produce enough milk to satiate their infants (73, 75). The precise proportion of women who cannot produce enough milk for satiating and meeting the nutritional needs of their infants for primary biological reasons remains unknown (76). However, it is likely that this proportion is low because the lactation process is mainly driven by a highly protected infant demand-maternal supply process (77, 78). As the evidence presented below indicates, lack of timely lactation management and social support appear to be the strongest predictors of SRIM and other lactation difficulties (70, 72, 79–81) (Figure 2).
FIGURE 2.
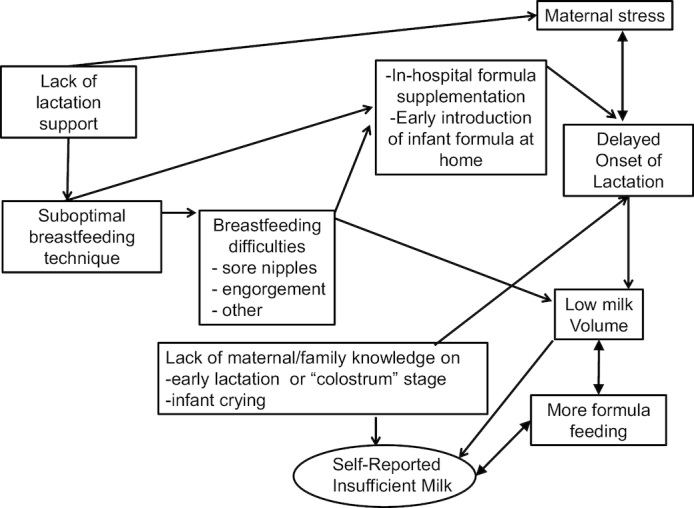
Hypothetical model proposing how lack of access to lactation support can lead to SRIM. Original model prepared by authors from evidence reported by Dewey et al. (96), Segura-Millán et al. (70), Kent (105), Kent et al. (72, 73), Wood et al. (94), Pérez-Escamilla et al. (74), Chapman and Pérez-Escamilla (80), and Giugliani (81).
The evidence suggests that delayed onset of lactation (DOL), i.e., when the copious production of milk does not begin within 72 h postpartum, increases the risk of SRIM and short breastfeeding durations, and can result from pre-lacteal feeds (i.e., foods and fluids other than breast milk given before the onset of lactation or “milk arrival”) that interfere with the infant's suckling process (74). Modifiable risk factors for DOL include maternal stress, maternal obesity, and in-hospital formula supplementation (82–85). Multiparous Guatemalan women with higher salivary amounts of cortisol (a stress hormone) during labor were more likely to have DOL (83). Chen et al. (82) found an association among stress indicators including longer labor duration, maternal exhaustion, increased stress hormones, and DOL. Consistent with these findings, Chapman and Pérez-Escamilla (80) found in a sample of American women that unscheduled cesarean delivery or vaginal delivery with a prolonged second stage (the “pushing” stage) of labor were risk factors for DOL. Living under poverty conditions does not seem to increase the risk of DOL. In fact, DOL appears to happen less frequently in low-income countries, especially in settings where the human birth process is less medicalized (80, 86). By contrast, maternal obesity has been consistently identified as a risk factor for DOL in low-, middle-, and high-income countries (80, 85). Likewise, prenatal insulin profiles denoting poor blood glucose control have been associated with DOL in the United States (87). The importance of early initiation of breastfeeding as a means for reducing interference with normal lactation processes is suggested in data from 51 countries, which indicate that timing of breastfeeding initiation is a significant risk factor for early introduction of supplementary (pre-lacteal) feeds (88).
In summary, SRIM leads to shortened breastfeeding durations, perhaps as a result of introduction of pre-lacteal feeds in response to DOL or perceptions of breastfeeding inadequacy as a result of infant crying, not feeling breasts full, or not seeing milk flowing from nipple (70, 89, 90). Studies have consistently shown that providing infant formula to neonates before the mother's milk comes in—which can be in response to SRIM—increases the risk for interference with the demand-supply process and hence can shorten breastfeeding duration (74, 91, 92). The evidence suggests that it is likely most cases of SRIM are the result of poor lactation management and social support and could be prevented if addressed antenatally and within the first 2 wk postpartum with skilled counseling and support (70, 72, 93–96). Therefore, for the population at large, maternal concerns about milk supply do not justify revision of the EBF-6 recommendation. This condition does, however, necessitate provision of timely breastfeeding support, particularly as lactation is established. The WHO and UNICEF (97) recommendation to initiate breastfeeding within the first hour of life, and to offer the breast exclusively and on demand to facilitate the establishment of a milk supply, can help to mitigate this problem.
Maternal nutritional status and breast milk quality
The WHO committee issuing the EBF-6 recommendation in 2001 acknowledged the dearth of evidence they had available to assess how EBF impacts maternal nutrition. It also recognized the lack of evidence on human milk meeting all infant nutrient requirements (2). Furthermore, the committee did not have the evidence available to assess the nutrient adequacy of human milk output in the first 6 mo across different populations (98). In fact, to this day, reference standards for human milk volume and composition do not exist, although these are currently being explored (77, 99, 100).
Recognizing these limitations, the WHO guideline development group (98) concluded that: 1) infant protein and energy needs can be met through EBF-6; 2) the adequacy of some nutrients (such as vitamin A and vitamin B-6) depend on maternal diet; 3) calcium quantity in breast milk is tightly regulated and does not depend on maternal intake; 4) human milk is low in iron and by 6 mo is also relatively low in zinc, and the concentrations of these nutrients in breast milk cannot be altered through maternal supplementation; 5) the potential for the EBF-6 infant to become iron-deficient should be considered, especially among LBW/premature infants. These conclusions, which echoed the Institute of Medicine (IOM) 1991 seminal report on human lactation (101), have been reaffirmed over time (25, 102).
Despite decades of research on human lactation, we still lack a fundamental understanding of what is the normal range of breast-milk production and human milk nutrient composition. This is in large part because there are still many uncertainties about infant nutrient requirements during the first 6 mo of life and hence there is a lack of blood thresholds for identifying micronutrient deficiencies (99, 100, 103). Understanding the physiology of lactation sheds light on some of these outstanding questions. Therefore, in the following sections we examine drivers of milk synthesis and nutrient transport.
Human milk synthesis
Lactocytes or milk-producing cells lining the inner walls of the mammary gland alveoli become activated for milk synthesis in response to infant suckling (104). Human milk synthesis involves complex and highly coordinated mechanisms in transport of maternal-blood nutrients into the alveolar lumen (105). The process of milk synthesis takes place via exocytosis, secretion of lipid droplets, transcellular transport through the lactocyte, or paracellular transport through lactocyte junctions. The synthesis of human milk proteins, fat, and lactose involves different lactocyte organelles before secretion into the alveolar lumen (106). The mammary gland can tightly control the concentration of minerals, such as calcium, iron, zinc, and copper, to reduce the risk of nutrient deficiencies or excesses in the infant (107, 108). This process involves numerous protein transporters and steps highly synchronized with each other. Transport of calcium, electrolytes, and water-soluble vitamins occurs through transcellular pathways involving complex protein transport systems working in concert with each other (108). Human milk synthesis is an energy- and nutrient-intense process. Once lactation has been established, mothers produce a mean of 750 g/d breast milk, although there is wide variation among women (from 440 g/d to 1220 g/d) (103), in part driven by infant demand. On average, women need an additional 500 kcal/d to support lactation, compared to 250 kcal/d during the second trimester of pregnancy and 452 kcal/d during the third trimester (103).
Maternal nutrition status and human milk composition and volume
Mild to moderate maternal undernutrition can lead to suboptimal amounts of some key micronutrients in human milk (98). The WHO EBF-6 recommendation could not take human milk nutritional quality fully into account because of a lack of data on breast-milk composition in different settings. Although the evidence is relatively scant, available data show significant variation across populations in both human milk composition and infant response to maternal nutrient supplements (98). Some evidence shows that for some of the nutrients that can be increased in breast milk through maternal supplementation (i.e., group 1 nutrients), very high supplementation would be needed to achieve modest nutrient increases in the infant's status for the corresponding nutrient (see supplementation section below).
Early infant supplementation may be advised on an individualized basis, when 1 or more nutrient deficiencies are detected. Indeed, infant vitamin and mineral supplementation is permitted within the WHO definition of EBF.
Regarding macronutrient composition, evidence suggests uniformity in mean human milk protein, fat, carbohydrate (lactose), and energy concentration across populations, regardless of maternal nutritional status (101, 102). This is particularly true for protein and lactose (109–118) (Table 1).
TABLE 1.
Macronutrient concentrations of mature human milk1
| Term infants mature milk | Country | Study reference | Protein, g/dL | Fat, g/dL | Lactose, g/dL | Energy, kcal/dL |
|---|---|---|---|---|---|---|
| High-income countries2 | Gidrewicz and Fenton (109) | 0.9 (0.6–1.2) | 3.4 (1.6–5.2) | 6.8 ± 0.3 | 68 (50–86) | |
| Fore milk, hind milk3 | Finland | Saarela et al. (110) | 1.1 ± 0.1 (0.1–1.1) | 1.9 ± 0.4, 5.7 ± 2.4 | 7.6 ± 0.5, 7.1 ± 0.2 | 53.1 ± 4.3, 86.3 ± 21.0 |
| United States | Nommsen et al. (111) | 1.2 (0.9–1.5) | 3.6 (2.2–5.0) | 7.4 (7.2–7.7) | 70 (57–83) | |
| Brazil | Gomes et al. (112) | 1.1 (1.1–1.3) | 2.8 (2.0–3.8) | 7.1 (6.7–7.3) | - | |
| Bangladesh | Brown et al. (113) | - | 2.8 ± 0.60 | 7.9 ± 0.4 | 61.0 ± 6 | |
| Israel, <35-y-old women | Lubetzky et al. (114) | 0.9 ± 0.4 (0.2–1.9) | 4.6 ± 1.1 (2.6–7.0) | 5.2 ± 0.7 (4.0–6.7) | 71.5 ± 12.7 (47–100) | |
| Israel, ≥35-y-old women | Lubetzky et al. (114) | 0.9 ± 0.3 (0.5–1.7) | 4.1 ± 1.1 (1.3–6.0) | 5.8 ± 0.9 (4.3–6.9) | 67.9 ± 19.4 (36–90) | |
| Korea | Chang et al. (115) | 1.4 ± 0.3 (0.5–2.9) | 3.0 ± 1.4 (0.2–9.5) | 7.1 ± 0.4 (4.2–9.9) | 61.1 ± 13.1 (43.2–104.2) | |
| Donor samples | Wojcik et al. (116) | 1.2 (0.7–1.7) | 3.2 (1.2–5.2) | 7.8 (6.0–9.6) | 65 (43–87) | |
| Michaelsen et al. (117) | 0.9 (0.6–1.4) | 3.6 (1.8–8.9) | 7.2 (6.4–7.6) | 67 (50–115) | ||
| Saarela et al. (110) | 1.1 ± 0.1 | 3.2 ± 1.1 | 7.3 ± 0.4 | 64.7 ± 10.8 | ||
| Reference standard | American Academy of Pediatrics (118) | 0.9 | 3.5 | 6.7 | (65–70) |
1Values are means ± SDs and/or (range).
2Australia, Canada, France, Finland, Germany, Japan, Italy, the Netherlands, Spain, Sweden, and the United States.
3Fore milk refers to breast milk sampled at beginning of the nursing episode; hind milk refers to milk sampled toward the end of the nursing episode.
This finding is consistent across studies even when different human milk sampling and analysis methods are used to determine macronutrient content; and also, when socioeconomic, nutritional status, and demographic characteristics—including infant age at which milk samples were obtained—vary within the populations assessed.
In the field of human milk composition, vitamins and minerals are classified into 2 groups, depending on whether or not they are responsive to maternal nutritional status (25) (Table 2). Group 1 nutrient deficiencies can be addressed through public-health measures as their concentrations in human milk are negatively affected by maternal depletion and their concentration in breast milk can potentially be increased through changes in maternal diet or via supplementation (98, 103). By contrast, group 2 nutrient amounts in breast milk are unrelated to maternal nutritional status, as seen in studies of undernourished and well-nourished populations in Honduras and Sweden (119). Although long-chain PUFAs, which are crucial for the child's brain development have not been previously listed as group 1 nutrients, it is important that they are recognized as such as their amounts in breast milk are responsive to maternal diet and supplementation (102). Expert panels suggest that adult women ingest ω-3 fatty acid-rich fish sources and/or supplements to consume a minimum of 300–1000 mg DHA/d while pregnant or breastfeeding (120–122).
TABLE 2.
Group 1 and Group 2 nutrients in human milk1
| Nutrient type | |
|---|---|
| Group 1 | Group 2 |
| Thiamin | Calcium |
| Riboflavin | Folate |
| Vitamin B-6 | Iron |
| Vitamin B-12 | Copper |
| Choline | Zinc |
| Retinol | Vitamin K |
| Vitamin A | |
| Vitamin D | |
| Selenium | |
| Iodine | |
| PUFAs | |
Effects of maternal supplementation on human milk quality
When a breastfeeding mother ingests nutrient supplements, complex metabolic processes may allow for only a very small amount of these supplement-derived nutrients to enter the breast milk. Indeed, wide variability exists in how individual infants respond to similar amounts of maternal supplementation, and for some nutrients the amounts of maternal supplementation would need to be exceedingly high to meet infant requirements (123, 124). This variability in response to micronutrient supplementation is a function of the specific micronutrient, amount of supplementation, and maternal nutritional status. Differential responses to various micronutrient supplements are described in the paragraphs that follow.
A 2013 review confirms the presence in breast milk of nutrients that are responsive (group 1) and nonresponsive (group 2) to maternal nutritional status in low- and high-income country settings (123). For group 1 nutrients, earlier studies among thiamine-deficient women in Gambia and India (125, 126) demonstrated strong human milk response to maternal thiamine supplementation. In the Gambia study, both maternal and infant thiamine status improved in <2 wk after supplementation. These 2 studies showed that human milk responds to maternal riboflavin supplementation, and that riboflavin status rapidly improved in both mothers and infants after maternal supplementation was started. Another study in Guatemala showed a relatively small human milk response to vitamin B-12 supplementation among women deficient in this vitamin (127).
With regard to group 2 nutrients, studies in a rural Mexican Otomi population confirmed that maternal folate status is unrelated to folate concentration in human milk, and that folate concentration in breast milk cannot be altered through maternal supplementation (128). EBF infants generally have adequate folate status, however, irrespective of maternal folate concentrations (25). Therefore, folate is a good example of a nutrient that gets prioritized for the mammary-gland usage, for transfer to the infant, if needs be at the expense of maternal folate status.
Among urban Bangladeshi women, the effects of maternal supplementation on human milk content were detected within 2–4 h for thiamin, riboflavin, vitamin A, and vitamin B-6, although the fraction transferred to breast milk ranged from 0.1% to 6.17% (129). Response to vitamin A supplementation varies across studies, perhaps as a function of maternal vitamin A deficiency. Although studies have consistently demonstrated that maternal vitamin A supplementation impacts its presence in breast milk (101, 102), a recent study conducted in the eastern region of Ghana found that daily supplementation with small-quantity lipid-based nutrient supplements covering the recommended daily intake of vitamin A did not increase breast-milk retinol concentrations (130). The authors attribute this finding to the strong likelihood that women already had adequate vitamin A status.
Concentrations of vitamin D in human milk correlate with maternal vitamin D intake and sunlight exposure. The concentration of vitamin D in breast milk, even for healthy lactating women, is low. Breast milk does not provide infants with adequate vitamin D intake, even in populations typically known to be well nourished, and especially when infants are not exposed to sufficient sunlight (124). Because vitamin D synthesis is stimulated by sunlight on the skin and not obtained in significant amounts from the diet, breastfed and formula-fed infants without sufficient exposure and synthesis may require supplementary vitamin D. Studies in Finland and the United States show that very high maternal vitamin D supplementation is needed (≥2000 IU/d) to provide a significant amount of breast-milk vitamin D for the infant (123, 124). In fact, some studies suggest that as much as 6400 IU/d would be needed to bring the infant's vitamin D status to the desired range while simultaneously protecting the vitamin D status of the mother. This amount of supplementation is so high (about 10 times the daily recommended intake for vitamin D) that this explains in part why the public-health measure currently endorsed for improving vitamin D status of all breastfed infants is based on directly supplementing the infant with this vitamin (400 IU/d) (131).
Infant self-regulation of breast-milk intake
Nutrient composition of human milk is a contributing factor to the nutritional status of the EBF infant. However, it is not the only contributor, as infants can self-regulate energy and nutrient intakes by adjusting the volume of milk they consume. The Honduras RCT mentioned previously found that all groups of infants exclusively breastfeeding for 6 mo compared with 4 mo (5) consumed the same mean daily energy intake, indicating that they adjusted their milk-volume intakes in spite of variation in breast-milk fat and energy content. Indeed, an observational analysis of the Honduras trial data documented that infants whose mothers produced higher-energy milk consumed less milk volume compared with infants consuming milk with lower-energy densities (132). By extension, the self-regulation in energy intake also impacts total consumption of other nutrients.
The finding that infant demand drives breast-milk production and subsequent consumption has been reported in multiple studies in high-income countries (133, 134). For example, in a study conducted in the United States among exclusively breastfed infants whose mothers increased their milk supply by expressing additional breast milk for 2 wk, breast-milk intake was driven by infant demand and not by increased maternal breast -ilk production capacity (134). Another study conducted by the same research group in the same population found that residual breast-milk volume, defined as total volume extracted minus mean milk intake in a 24-h period to predict milk volume left in the breast when the infant had completed nursing, was around 100 g/d, and this applied to infants consuming relatively low amounts of breast milk (<650 g/d) (135). A study conducted in the United Kingdom (136) that allowed for mothers to randomly select the breast from which to feed the baby first, found that intake from the second breast was only about 60% of the amount consumed from the first breast.
Stuff and Nichols (137), demonstrated in the United States that energy intake per kg of body weight did not increase after solids were introduced into the diet of exclusively breastfed infants. Nommsen et al. (111) found among 6-mo-old infants from the United States breastfed on demand that solid foods displaced energy intake from breast milk. These findings provide strong evidence that infants have a strong capacity to self-regulate breast-milk intake.
In summary, despite evidence documenting that human milk responds to maternal nutritional status for some key nutrients for which the infants in low-income countries may be compromised, we have gaps in our knowledge about how several nutrients are metabolized and transported in human milk, and this has hindered the development of specific public health recommendations for nursing mothers (99). Questions exist with regard to the nutrient amounts in human milk that provide infants with optimal health and development, while at the same time protecting maternal health and wellbeing (98). The relation between maternal nutritional status and human milk nutrient composition is not straightforward, even for group 1 nutrients (98). Nutrients absorbed by the mother's gastrointestinal tract enter her bloodstream, travel to the breast lactocytes, and cross the lactocytes’ membranes to enter her milk. The suckling infant ingests these nutrients, which are absorbed by the infant's gastrointestinal tract into the bloodstream, and then enter the infant's body tissues. The nutrient concentrations circulating in the infant's blood are therefore a function of the volume of breast milk consumed, the microbiota, and other factors including infections or inflammation (138). Infants have the capacity to self-regulate breast-milk intake as a function of milk energy density, therefore it is difficult to predict nutrient deficiency risk solely based on human milk composition. Hence, currently we do not see a basis for changing the EBF-6 public health recommendation based on concerns about suboptimal maternal dietary intakes.
Infant Developmental Readiness for Complementary Foods
Increasingly, infant-feeding guidelines, especially those from high-income countries, are acknowledging that there is a relatively wide variability on the exact age at which individual infants are ready to start complementary feeding (139–142). From a child-development perspective, this individual-level variability in readiness to start consuming solid foods should be expected, given that in all developmental domains that have been studied—including crawling, walking, and language—children achieve the same developmental milestones at quite different ages, even when living in relatively similar environments (143–145).
Evidence indicates that most infants will be developmentally ready to handle and benefit from the introduction of complementary foods at around age 6 mo (139, 142, 146, 147). However, some infants may be ready earlier or later (146–150). The key signals that caregivers should pay attention to are the infant's ability to sit with little or no help; to munch/chew and swallow soft, solid foods; to have lost the extrusion reflex (the projection of food from the mouth); and to demonstrate interest in food (151). Achieving these developmental milestones strongly correlates with the maturation of the gastrointestinal tract, kidneys, and immune system required to benefit from introducing complementary foods (147, 148).
Discussion
The WHO EBF-6 recommendation issued in 2001 (1, 2) was reaffirmed in 2012 (3), and based on our analysis of the literature today, there is still no basis for changing it. Valid concerns have been expressed regarding how this recommendation may not be optimal for certain subgroups of infants, e.g., those born with low iron stores, at high risk of developing food allergies, or not being able to consume an adequate volume of milk. However, in each instance there are interventions that can be tailored to the needs of specific infants rather than changing the EBF-6 recommendation for the population of infants at large. As shown in this Perspective, the risk of development of iron-deficiency anemia can be greatly reduced, even among those at highest risk (premature and low-birth-weight newborns) through delayed cord clamping, which is a simple and strongly effective intervention that can be readily implemented through proper training of healthcare providers (33, 152). It is important to acknowledge that iron supplements are recommended for these target vulnerable groups, regardless of age of introduction of complementary foods.
The persistent reports of insufficient milk supply by mothers as a reason for supplementing breast milk with infant formula can be explained by the lack of adequate lactation management and social support rather than for primary biological reasons that cannot be properly addressed through sound lactation management. Studies have shown that lactation support during the first days and weeks after birth is central to successful establishment of lactation and to minimizing the risk of subsequent lactation difficulties (77, 78). Indeed, SRIM is most likely a result of interference with the normal lactation processes, beginning at birth and often associated with hospital practices (such as unnecessary separation of mother and baby at birth, cesarean delivery without quality breastfeeding support, and in-hospital supplementation policies) and/or cultural norms that encourage pre-lacteal feeds (such as giving newborns water or other ritual liquids) (70, 72, 73, 106, 153). Therefore, successful implementation of the EBF-6 recommendation relies on having a qualified work force and appropriate social protection policies in place to protect, support, and promote breastfeeding since gestation (154).
This Perspective also reaffirms that women with mild to moderate malnutrition, who comprise the majority of undernourished women in LMICs, can produce enough milk volume provided infant demand is not restricted because milk synthesis is dependent more on infant suckling than on maternal nutritional status itself. Breastfeeding is highly protective against infant morbidity and mortality in settings where infectious diseases and undernutrition are common, and therefore is strongly recommended even though the amounts of some micronutrients in mothers’ milk may be suboptimal (155). It is important to reiterate that whereas some of the micronutrients in milk are affected by maternal diet (most of the vitamins, plus iodine and selenium), others are not (most of the minerals, including iron and zinc).
Moving forward, improving maternal nutrition should be a top global public health priority, not only to improve the quality of the milk for infant consumption (especially group 1 nutrients), but also to improve the nutrition and wellbeing of the mother herself.
The child-development literature confirms that there is variability in the age at which infants achieve the necessary developmental milestones to be introduced to complementary foods, and that, on average, this happens at around 6 mo. Parents, other caregivers, and health providers should be taught how to identify the developmental cues that signal readiness for complementary feeding.
All newborns should have their growth, hydration status, and development regularly monitored. In the exceptional circumstances where formula supplementation may be called for, it should be done with careful oversight from a qualified provider, keeping in mind that early breastfeeding problems and corresponding introduction of infant formula are major risk factors for the early termination of both exclusive breastfeeding and any breastfeeding (74, 91, 156–160).
Further research is needed to continue developing standards for human milk production, consumption, and composition in diverse settings and at different stages of lactation (77, 99). It is possible that within a population some women are more predisposed (genetically or otherwise) to experience insufficient milk when subjected to risk factors for a suboptimal milk volume. Research is needed to identify at-risk women so they can be offered additional breastfeeding support and close monitoring of their infants’ growth and development.
A limitation of our Perspective is that we did not address the breastfeeding needs and outcomes among women in extreme circumstances such as starvation, or experiencing chronic diseases. Because that scope falls beyond the public health recommendation for EBF-6 and requires a personalized clinical approach, it is important that future reviews focus on those topics.
In conclusion, the 2001 WHO EBF-6 recommendation was issued as a public health recommendation, which acknowledged that tailoring to individual infants’ circumstances would be required (3), and that these needs should be determined by regular growth and developmental monitoring. Existing, evidence-based responsive feeding guidelines provide recommendations on how best to determine when an infant is ready to be introduced to complementary foods based on her/his developmental stage (139). We suggest that infant and young feeding guidelines make clear that complementary foods should be introduced at around age 6 mo, taking infant developmental readiness into account, but that no other modifications be made to the EBF-6 public health recommendation based on the concerns reviewed in this Perspective paper. Research will help refine clinical guidelines on infant feeding in vulnerable populations in the future.
Acknowledgments
The authors’ responsibilities were as follows—RP-E and EP conceptualized the article; RP-E wrote the first draft; all authors critically reviewed several manuscript versions, read and approved the final manuscript.
Notes
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
Supported by the Bill & Melinda Gates Foundation.
Author disclosures: RP-E, GSB, and SS-P, no conflicts of interest. EP works for the Bill & Melinda Gates Foundation, which funded this Perspective.
Abbreviations used: DOL, delayed onset of lactation; EAT, Enquiring about Tolerance; EBF, exclusive breastfeeding; EBF-6, exclusive breastfeeding for 6 mo; HMO, human milk oligosaccharide; LBW, low birth weight; LMIC, low-income/middle-income country; RCT, randomized controlled trial; SRIM, self-reported insufficient milk.
References
- 1. Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2002;(1):CD003517. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. The Optimal Duration of Exclusive Breastfeeding: Report of an Expert Consultation. Geneva; 2001. [Google Scholar]
- 3. Kramer MS, Kakuma R.. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012;(8):CD003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fewtrell M, Wilson DC, Booth I, Lucas A. Six months of exclusive breast feeding: how good is the evidence?. BMJ. 2011;342:c5955. [DOI] [PubMed] [Google Scholar]
- 5. Cohen RJ, Brown KH, Canahuati J, Rivera LL, Dewey KG. Effects of age of introduction of complementary foods on infant breast milk intake, total energy intake, and growth: a randomised intervention study in Honduras. Lancet. 1994;344:288–93. [DOI] [PubMed] [Google Scholar]
- 6. Dewey KG, Cohen RJ, Brown KH, Rivera LL. Age of introduction of complementary foods and growth of term, low-birth-weight, breast-fed infants: a randomized intervention study in Honduras. Am J Clin Nutr. 1999;69:679–86. [DOI] [PubMed] [Google Scholar]
- 7. Cohen RJ, Brown KH, Canahuati J, Rivera LL, Dewey KG. Determinants of growth from birth to 12 months among breast-fed Honduran infants in relation to age of introduction of complementary foods. Pediatrics. 1995;96:504–10. [PubMed] [Google Scholar]
- 8. Dewey KG, Cohen RJ, Rivera LL, Brown KH. Effects of age of introduction of complementary foods on iron status of breast-fed infants in Honduras. Am J Clin Nutr. 1998;67:878–84. [DOI] [PubMed] [Google Scholar]
- 9. Dewey KG, Cohen RJ, Brown KH, Rivera LL. Effects of exclusive breastfeeding for four versus six months on maternal nutritional status and infant motor development: results of two randomized trials in Honduras. J Nutr. 2001;131(2):262–7. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization. WHO Handbook for Guideline Development. 2nd Edition Geneva: World Health Organization;2014. [Google Scholar]
- 11. Jonsdottir OH, Thorsdottir I, Hibberd PL, Fewtrell MS, Wells JC, Palsson GI, Lucas A, Gunnlaugsson G, Kleinman RE. Timing of the introduction of complementary foods in infancy: a randomized controlled trial. Pediatrics. 2012;130:1038–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kramer MS, Guo T, Platt RW, Sevkovskaya Z, Dzikovich I, Collet JP, Shapiro S, Chalmers B, Hodnett E, Vanilovich I et al.. Infant growth and health outcomes associated with 3 compared with 6 mo of exclusive breastfeeding. Am J Clin Nutr. 2003;78:291–5. [DOI] [PubMed] [Google Scholar]
- 13. Horta BL, Victora CG. Short-term Effects of Breastfeeding: A Systematic Review on the Benefits of Breastfeeding on Diarrhoea and Pneumonia Mortality. Geneva: World Health Organization; 2013. [Google Scholar]
- 14. Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:30–7. [DOI] [PubMed] [Google Scholar]
- 15. Sankar MJ, Sinha B, Chowdhury R, Bhandari N, Taneja S, Martines J, Bahl R. Optimal breastfeeding practices and infant and child mortality: a systematic review and meta-analysis. Acta Paediatr. 2015;104:3–13. [DOI] [PubMed] [Google Scholar]
- 16. Horta BL, Loret de Mola C, Victora CG. Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr. 2015;104:14–9. [DOI] [PubMed] [Google Scholar]
- 17. Victora CG, Horta BL, Loret de Mola C, Quevedo L, Pinheiro RT, Gigante DP, Goncalves H, Barros FC. Association between breastfeeding and intelligence, educational attainment, and income at 30 years of age: a prospective birth cohort study from Brazil. Lancet Glob Health. 2015;3:e199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Victora CG, Bahl R, Barros AJD, França GVA, Horton S, Krasevec J, Murch S, Sankar MJ, Walker N, Rollins NC. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–90. [DOI] [PubMed] [Google Scholar]
- 19. Chowdhury R, Sinha B, Sankar MJ, Taneja S, Bhandari N, Rollins N, Bahl R, Martines J. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:96–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yakoob MY, Lo CW. Nutrition (Micronutrients) in child growth and development: A systematic review on current evidence, recommendations and opportunities for further research. J Dev Behav Pediatr. 2017;38:665–79. [DOI] [PubMed] [Google Scholar]
- 21. Domellof M, Braegger C, Campoy C, Colomb V, Decsi T, Fewtrell M, Hojsak I, Mihatsch W, Molgaard C, Shamir R et al.. Iron requirements of infants and toddlers. J Pediatr Gastroenterol Nutr. 2014;58:119–29. [DOI] [PubMed] [Google Scholar]
- 22. Chaparro C, Lutter C.. Beyond Survival: Integrated Delivery Care Practices for Longterm Maternal and Infant Nutrition, Health and Development. Washington DC: Pan American Health Organization; 2007. [Google Scholar]
- 23. Greer FR. How Much Iron is Needed for Breastfeeding Infants?. Curr Pediatr Rev. 2015;11:298–304. [DOI] [PubMed] [Google Scholar]
- 24. Allen LH. Micronutrient research, programs, and policy: From meta-analyses to metabolomics. Adv Nutr. 2014;5:344S–51S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Allen LH. B vitamins in breast milk: relative importance of maternal status and intake, and effects on infant status and function. Adv Nutr. 2012;3:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lutter CK. Iron deficiency in young children in low-income countries and new approaches for its prevention. J Nutr. 2008;138:2523–8. [DOI] [PubMed] [Google Scholar]
- 27. Dewey KG, Domellof M, Cohen RJ, Landa Rivera L, Hernell O, Lonnerdal B. Iron supplementation affects growth and morbidity of breast-fed infants: results of a randomized trial in Sweden and Honduras. J Nutr. 2002;132:3249–55. [DOI] [PubMed] [Google Scholar]
- 28. Dube K, Schwartz J, Mueller M, Kalhoff H, Kersting M. Iron intake and iron status in breastfed infants during the first year of life. Clin Nutr. 2010;29:773–8. [DOI] [PubMed] [Google Scholar]
- 29. Chantry C, Howard C, Auinger P. Full breastfeeding duration and risk for iron deficiency in U.S. infants. Breastfeed Med. 2007;2:63–73. [DOI] [PubMed] [Google Scholar]
- 30. Issaka A, Agho K, Page A, Burns P, Stevens G, Dibley M. The problem of suboptimal complementary feeding practices in West Africa: what is the way forward?. Matern Child Nutr. 2015;11(Suppl 1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simondon K. Early breast-feeding cessation and infant mortality in low-income countries: workshop summary. Adv Exp Med Biol. 2009;639:319–29. [DOI] [PubMed] [Google Scholar]
- 32. Imdad A, Yakoob M, Bhutta Z. Impact of maternal education about complementary feeding and provision of complementary foods on child growth in developing countries. BMC Public Health. 2011;11(Suppl 3):S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blouin B, Penny ME, Casapia M, Aguilar E, Silva H, Joseph SA, Creed-Kanashiro HM, Maheu-Giroux M, Gyorkos TW. Effect of a two-component intervention to change hospital practice from early to delayed umbilical cord clamping in the Peruvian Amazon. Rev Panam Salud Publica. 2011;29:322–8. [PubMed] [Google Scholar]
- 34. Dewey KG, Chaparro CM.. Session 4: Mineral metabolism and body composition iron status of breast-fed infants. Proc Nutr Soc. 2007;66:412–22. [DOI] [PubMed] [Google Scholar]
- 35. Yang Z, Lonnerdal B, Adu-Afarwuah S, Brown KH, Chaparro CM, Cohen RJ, Domellof M, Hernell O, Lartey A, Dewey KG. Prevalence and predictors of iron deficiency in fully breastfed infants at 6 mo of age: comparison of data from 6 studies. Am J Clin Nutr. 2009;89:1433–40. [DOI] [PubMed] [Google Scholar]
- 36. World Health Organization. Essential Newborn Care Course. Geneva; 2010. [Google Scholar]
- 37. World Health Organization. Guideline: Delayed Umbilical Cord Clamping for Improved Maternal and Infant Health and Nutrition Outcomes. Geneva; 2014. [PubMed] [Google Scholar]
- 38. Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH et al.. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-Sponsored Expert Panel report. J Am Acad Dermatol. 2011;64:175–92. [DOI] [PubMed] [Google Scholar]
- 39. Branum AM, Lukacs SL.. Food allergy among U.S. children: trends in prevalence and hospitalizations. NCHS Data Brief. 2008;(10):1–8. [PubMed] [Google Scholar]
- 40. NCHS. National Health Interview Survey, 2017. Atlanta: Centers for Disease Control and Prevention; 2017. [Google Scholar]
- 41. Gupta RS, Warren CM, Smith BM, Blumenstock JA, Jiang J, Davis MM, Nadeau KC. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics. 2018;142:e20181235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peters RL, Koplin JJ, Gurrin LC, Dharmage SC, Wake M, Ponsonby AL, Tang MLK, Lowe AJ, Matheson M, Dwyer T et al.. The prevalence of food allergy and other allergic diseases in early childhood in a population-based study: HealthNuts age 4-year follow-up. J Allergy Clin Immunol. 2017;140:145–53..e8. [DOI] [PubMed] [Google Scholar]
- 43. Connett GJ, Gerez I, Cabrera-Morales EA, Yuenyongviwat A, Ngamphaiboon J, Chatchatee P, Sangsupawanich P, Soh SE, Yap GC, Shek LP et al.. A population-based study of fish allergy in the Philippines, Singapore and Thailand. Int Arch Allergy Immunol. 2012;159:384–90. [DOI] [PubMed] [Google Scholar]
- 44. Basera W, Botha M, Gray CL, Lunjani N, Watkins AS, Venter C, Allen KJ, Hlela C, Zar HJ, Levin ME. The South African Food Sensitisation and Food Allergy population-based study of IgE-mediated food allergy: validity, safety, and acceptability. Ann Allergy Asthma Immunol. 2015;115:113–9. [DOI] [PubMed] [Google Scholar]
- 45. Hu Y, Chen J, Li H. Comparison of food allergy prevalence among Chinese infants in Chongqing, 2009 versus 1999. Pediatr Int. 2010;52:820–4. [DOI] [PubMed] [Google Scholar]
- 46. Kristinsdottir H, Clausen M, Ragnarsdottir HS, Halldorsdottir IH, McBride D, Beyer K, Sigurdardottir ST. [Prevalence of food allergy in Icelandic infants during first year of life]. Laeknabladid. 2011;97:11–8. [DOI] [PubMed] [Google Scholar]
- 47. Eller E, Kjaer HF, Host A, Andersen KE, Bindslev-Jensen C. Food allergy and food sensitization in early childhood: results from the DARC cohort. Allergy. 2009;64:1023–9. [DOI] [PubMed] [Google Scholar]
- 48. Venter C, Maslin K, Patil V, Kurukulaaratchy R, Grundy J, Glasbey G, Twiselton R, Dean T, Arshad SH. The prevalence, natural history and time trends of peanut allergy over the first 10 years of life in two cohorts born in the same geographical location 12 years apart. Pediatr Allergy Immunol. 2016;27:804–11. [DOI] [PubMed] [Google Scholar]
- 49. Grimshaw KE, Bryant T, Oliver EM, Martin J, Maskell J, Kemp T, Clare Mills EN, Foote KD, Margetts BM, Beyer K et al.. Incidence and risk factors for food hypersensitivity in UK infants: results from a birth cohort study. Clin Transl Allergy. 2015;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Namork E, Faeste CK, Stensby BA, Egaas E, Lovik M. Severe allergic reactions to food in Norway: a ten year survey of cases reported to the food allergy register. Int J Environ Res Public Health. 2011;8:3144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, Ponsonby AL, Wake M, Tang ML, Dharmage SC et al.. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127:668–76..e1-2. [DOI] [PubMed] [Google Scholar]
- 52. National Academies of Engineering Sciences and Medicine. Finding a Path to Safety in Food Allergies. Washington DC; 2017. [Google Scholar]
- 53. Fleischer D, Sicherer S, Greenhawt M, Campbell D, Chan E, Muraro A, Halken S, Katz Y, Ebisawa M, Eichenfield L et al.. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants. Allergy. 2015;70:1193–5. [DOI] [PubMed] [Google Scholar]
- 54. American Academy of Allergy, Asthma and Immunology. Primary Prevention of Allergic Disease through Nutritional Interventions. Parent Prevention Guidelines; 2015. [Google Scholar]
- 55. Toit GD, Roberts G, Sayre P, Bahnson H, Radulovic S, Santos A, Brough H, Phippard D, Basting M, Feeney M et al.. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Du Toit G, Sayre PH, Roberts G, Sever ML, Lawson K, Bahnson HT, Brough HA, Santos AF, Harris KM, Radulovic S et al.. Effect of Avoidance on Peanut Allergy after Early Peanut Consumption. N Engl J Med. 2016;374:1435–43. [DOI] [PubMed] [Google Scholar]
- 57. Greenhawt M, Fleischer DM, Chan ES, Venter C, Stukus D, Gupta R, Spergel JM. LEAPing through the looking glass: secondary analysis of the effect of skin test size and age of introduction on peanut tolerance after early peanut introduction. Allergy. 2017;72:1254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Perkin MR, Logan K, Tseng A, Raji B, Ayis S, Peacock J, Brough H, Marrs T, Radulovic S, Craven J et al.. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. N Engl J Med. 2016;374:1733–43. [DOI] [PubMed] [Google Scholar]
- 59. Perkin MR, Logan K, Marrs T, Radulovic S, Craven J, Flohr C, Lack G. Enquiring About Tolerance (EAT) study: Feasibility of an early allergenic food introduction regimen. J Allergy Clin Immunol. 2016;137:1477–86..e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Scientific Advisory Committee on Nutrition (SACN). Feeding in the First Year of Life. Public Health England; 2018. [Google Scholar]
- 61. Iannotti LL, Lutter CK, Stewart CP, Gallegos Riofrio CA, Malo C, Reinhart G, Palacios A, Karp C, Chapnick M, Cox K et al.. Eggs in early complementary feeding and child growth: A randomized controlled trial. Pediatrics. 2017;140:e20163459. [DOI] [PubMed] [Google Scholar]
- 62. Ierodiakonou D, Garcia-Larsen V, Logan A, Groome A, Cunha S, Chivinge J, Robinson Z, Geoghegan N, Jarrold K, Reeves T et al.. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: A systematic review and meta-analysis. JAMA. 2016;316:1181–92. [DOI] [PubMed] [Google Scholar]
- 63. Pitt TJ, Becker AB, Chan-Yeung M, Chan ES, Watson WTA, Chooniedass R, Azad MB. Reduced risk of peanut sensitization following exposure through breast-feeding and early peanut introduction. J Allergy Clin Immunol. 2018;141(2):620–25. [DOI] [PubMed] [Google Scholar]
- 64. Alduraywish SA, Lodge CJ, Campbell B, Allen KJ, Erbas B, Lowe AJ, Dharmage SC. The march from early life food sensitization to allergic disease: a systematic review and meta-analyses of birth cohort studies. Allergy. 2016;71(1):77–89. [DOI] [PubMed] [Google Scholar]
- 65. Miliku K, Robertson B, Sharma AK, Subbarao P, Becker AB, Mandhane PJ, Turvey SE, Lefebvre DL, Sears MR, Bode L et al.. Human milk oligosaccharide profiles and food sensitization among infants in the CHILD Study. Allergy. 2018;73:2070–3. [DOI] [PubMed] [Google Scholar]
- 66. Jorgensen JM, Arnold C, Ashorn P, Ashorn U, Chaima D, Cheung YB, Davis JC, Fan YM, Goonatilleke E, Kortekangas E et al.. Lipid-based nutrient supplements during pregnancy and lactation did not affect human milk oligosaccharides and bioactive proteins in a randomized trial. J Nutr. 2017;jn252981.(Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fewtrell M, Wilson D, Booth I, Lucas A. Six months of exclusive breast feeding: how good is the evidence?. BMJ. 2010;13:c5955. [DOI] [PubMed] [Google Scholar]
- 68. Sun K, Chen M, Yin Y, Wu L, Gao L. Why Chinese mothers stop breastfeeding: Mothers' self-reported reasons for stopping during the first six months. J Child Health Care. 2017;21:353–63. [DOI] [PubMed] [Google Scholar]
- 69. Brown CR, Dodds L, Legge A, Bryanton J, Semenic S. Factors influencing the reasons why mothers stop breastfeeding. Can Journal Public Health. 2014;105:e179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Segura-Millan S, Dewey KG, Perez-Escamilla R. Factors associated with perceived insufficient milk in a low-income urban population in Mexico. J Nutr. 1994;124:202–12. [DOI] [PubMed] [Google Scholar]
- 71. Hill PD. Insufficient milk supply syndrome. NAACOGS Clin Issu Perinat Womens Health Nurs. 1992;3:605–12. [PubMed] [Google Scholar]
- 72. Kent JC, Gardner H, Geddes DT. Breastmilk production in the first 4 weeks after birth of term infants. Nutrients. 2016;8:e756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kent JC, Prime DK, Garbin CP. Principles for maintaining or increasing breast milk production. J Obstet Gynecol Neonatal Nurs. 2012;41:114–21. [DOI] [PubMed] [Google Scholar]
- 74. Pérez-Escamilla R, Segura-Millan S, Canahuati J, Allen H. Prelacteal feeds are negatively associated with breast-feeding outcomes in Honduras. J Nutr. 1996;126:2765–73. [DOI] [PubMed] [Google Scholar]
- 75. Gremmo-Feger G. [An update on lactation physiology and breastfeeding]. Arch Pediatr. 2013;20(9):1016–21. [DOI] [PubMed] [Google Scholar]
- 76. Gatti L. Maternal perceptions of insufficient milk supply in breastfeeding. J Nurs Scholarsh. 2008;40:355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Boss M, Gardner H, Hartmann P. Normal Human Lactation: closing the gap. F1000Res. 2018;7:doi:10.12688/f1000research.14452.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Boss M, Hartmann P. How breastfeeding works: Anatomy and physiology of human lactation. Breastfeeding and Breast Milk- from Biochemistry to Impact. Stuttgart, Germany: Thieme; 2018:39–77. [Google Scholar]
- 79. Dewey KG. Maternal and fetal stress are associated with impaired lactogenesis in humans. J Nutr. 2001;131:3012S–15S. [DOI] [PubMed] [Google Scholar]
- 80. Chapman DJ, Perez-Escamilla R.. Identification of risk factors for delayed onset of lactation. J Am Diet Assoc. 1999;99(4):450–4. [DOI] [PubMed] [Google Scholar]
- 81. Giugliani ER.[Common problems during lactation and their management]. J Pediatr (Rio J). 2004;80:S147–54. [DOI] [PubMed] [Google Scholar]
- 82. Chen DC, Nommsen-Rivers L, Dewey KG, Lonnerdal B. Stress during labor and delivery and early lactation performance. Am J Clin Nutr. 1998;68:335–44. [DOI] [PubMed] [Google Scholar]
- 83. Grajeda R, Pérez-Escamilla R.. Stress during labor and delivery is associated with delayed onset of lactation among urban Guatemalan women. J Nutr. 2002;132:3055–60. [DOI] [PubMed] [Google Scholar]
- 84. Nommsen-Rivers L. Does insulin explain the relation between maternal obesity and poor lactation outcomes? An overview of the literature. Adv Nutr. 2016;7:407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nommsen-Rivers L, Chantry C, Peerson J, Cohen R, Dewey K. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am J Clin Nutr. 2010;92:574–84. [DOI] [PubMed] [Google Scholar]
- 86. Otoo G, Marquis G, Sellen D, Chapman D, Pérez-Escamilla R. HIV-negative status is associated with very early onset of lactation among Ghanaian women. J Hum Lact. 2010;26:107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nommsen-Rivers L, Dolan L, Huang B. Timing of stage II lactogenesis is predicted by antenatal metabolic health in a cohort of primiparas. Breastfeed Med. 2012;7:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. UNICEF, World Health Organization. Capture the Moment – Early Initiation of Breastfeeding: The Best Start for Every Newborn. New York; 2018. [Google Scholar]
- 89. Brownell E, Howard C, Lawrence R, Dozier A. Delayed onset lactogenesis II predicts the cessation of any or exclusive breastfeeding. J Pediatr. 2012;161:608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chapman D, Pérez-Escamilla R.. Does delayed perception of the onset of lactation shorten breastfeeding duration?. J Hum Lact. 1999;15:107–11. [DOI] [PubMed] [Google Scholar]
- 91. Chantry C, Dewey K, Peerson J, Wagner E, Nommsen-Rivers L. In-hospital formula use increases early breastfeeding cessation among first-time mothers intending to exclusively breastfeed. J Pediatr. 2014;164:1339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Woolridge M. Breastfeeding: Physiology into practice. In: Davies D, ed. Nutrition in Child Health: London: Royal College of Physicians of London; 1995:13–31. [Google Scholar]
- 93. Wagner E, Chantry C, Dewey K, Nommsen-Rivers L. Breastfeeding concerns at 3 and 7 days postpartum and feeding status at 2 months. Pediatrics. 2013;132:e865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wood NK, Sanders EA, Lewis FM, Woods NF, Blackburn ST. Pilot test of a home-based program to prevent perceived insufficient milk. Women Birth. 2017; 30(6):472–80. [DOI] [PubMed] [Google Scholar]
- 95. Riddle SW, Nommsen-Rivers LA.. Low milk supply and the pediatrician. Curr Opin Pediatr. 2017;29(2):249–56. [DOI] [PubMed] [Google Scholar]
- 96. Dewey KG, Nommsen-Rivers LA, Heinig MJ, Cohen RJ. Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics. 2003;112:607–19. [DOI] [PubMed] [Google Scholar]
- 97. UNICEF and World Health Organization. The Global Strategy for Infant and Young Child Feeding. Geneva; 2003. [Google Scholar]
- 98. Butte N, Lopez-Alarcon M, Garza C. Nutrient Adequacy of Exclusive Breastfeeding for the Term Infant During the First Six Months of Life. Geneva: World Health Organization; 2002. [Google Scholar]
- 99. Allen LH, Dror DK.. Introduction to Current Knowledge on Micronutrients in Human Milk: Adequacy, Analysis, and Need for Research. Adv Nutr. 2018;9:275S–77S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Dror DK, Allen LH.. Overview of Nutrients in Human Milk. Adv Nutr. 2018;9:278S–94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Institute of Medicine (IOM). Nutrition during lactation. In: Subcommittee on Nutrition During Lactation. Washington DC: Food and Nutrition Board, National Academy of Sciences; 1991. [Google Scholar]
- 102. Valentine CJ, Wagner CL.. Nutritional management of the breastfeeding dyad. Pediatr Clin North Am. 2013;60:261–74. [DOI] [PubMed] [Google Scholar]
- 103. Kent J, Mitoulas L, Cox D, Owens R, Hartmann P. Breast volume and milk production during extended lactation women. Exp Physiol. 1999;84:435–47. [PubMed] [Google Scholar]
- 104. Newton M, Newton NR.. The let-down reflex in human lactation. J Pediatr. 1948;33(6):698–704. [DOI] [PubMed] [Google Scholar]
- 105. Kent J. How breastfeeding works. J Midwifery Womens Health. 2007;52:564–70. [DOI] [PubMed] [Google Scholar]
- 106. Neville MC, Morton J.. Physiology and endocrine changes underlying human lactogenesis II. J Nutr. 2001;131:3005S–08S. [DOI] [PubMed] [Google Scholar]
- 107. Lonnerdal B. Trace element transport in the mammary gland. Annu Rev Nutr. 2007;27:165–77. [DOI] [PubMed] [Google Scholar]
- 108. Montalbetti N, Dalghi MG, Albrecht C, Hediger MA. Nutrient transport in the mammary gland: calcium, trace minerals and water soluble vitamins. J Mammary Gland Biol Neoplasia. 2014;19:73–90. [DOI] [PubMed] [Google Scholar]
- 109. Gidrewicz DA, Fenton TR.. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014;14:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Saarela T, Kokkonen J, Koivisto M. Macronutrient and energy contents of human milk fractions during the first six months of lactation. Acta Paediatr. 2005;94:1176–81. [DOI] [PubMed] [Google Scholar]
- 111. Nommsen LA, Lovelady CA, Heinig MJ, Lonnerdal B, Dewey KG. Determinants of energy, protein, lipid, and lactose concentrations in human milk during the first 12 mo of lactation: the DARLING Study. Am J Clin Nutr. 1991;53:457–65. [DOI] [PubMed] [Google Scholar]
- 112. Gomes MJC, Silva FCF, Oliveira FCC, Rodrigues Júnior PH, Veloso GSS, Perrone IT, Priore SE, Franceschini SCC, Fernandes de Carvalho A. Teor de macronutrientes, ferro e cálcio no leite materno de nutrizes do 1° ao 6° mês pós-parto. XVII Encontro Latino Americano de Iniciação Científica, XIII Encontro Latino Americano de Pós- Graduação e III Encontro de Iniciação à Docência – Universidade do Vale do Paraíba; 2013. [Google Scholar]
- 113. Brown KH, Akhtar NA, Robertson AD, Ahmed MG. Lactational capacity of marginally nourished mothers: relationships between maternal nutritional status and quantity and proximate composition of milk. Pediatrics. 1986;78:909–19. [PubMed] [Google Scholar]
- 114. Lubetzky R, Sever O, Mimouni FB, Mandel D. Human Milk Macronutrients Content: Effect of Advanced Maternal Age. Breastfeed Med. 2015;10:433–6. [DOI] [PubMed] [Google Scholar]
- 115. Chang N, Jung JA, Kim H, Jo A, Kang S, Lee SW, Yi H, Kim J, Yim JG, Jung BM. Macronutrient composition of human milk from Korean mothers of full term infants born at 37–42 gestational weeks. Nutr Res Pract. 2015;9:433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wojcik KY, Rechtman DJ, Lee ML, Montoya A, Medo ET. Macronutrient analysis of a nationwide sample of donor breast milk. J Am Diet Assoc. 2009;109:137–40. [DOI] [PubMed] [Google Scholar]
- 117. Michaelsen KF, Skafte L, Badsberg JH, Jorgensen M. Variation in macronutrients in human bank milk: influencing factors and implications for human milk banking. J Pediatr Gastroenterol Nutr. 1990;11:229–39. [DOI] [PubMed] [Google Scholar]
- 118. American Academy of Pediatrics. Pediatric Nutrition Handbook. 6th ed, 2009. [Google Scholar]
- 119. Domellof M, Lonnerdal B, Dewey KG, Cohen RJ, Hernell O. Iron, zinc, and copper concentrations in breast milk are independent of maternal mineral status. Am J Clin Nutr. 2004;79:111–15. [DOI] [PubMed] [Google Scholar]
- 120. Carlson SE. Docosahexaenoic acid supplementation in pregnancy and lactation. Am J Clin Nutr. 2009;89:678S–84S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Koletzko B, Cetin I, Brenna JT. Dietary fat intakes for pregnant and lactating women. Br J Nutr. 2007;98:873–77. [DOI] [PubMed] [Google Scholar]
- 122. Simopoulos A, Leaf A, Salem NJ. Workshop on the essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. J Am Coll Nutr. 1999;18:487–89. [DOI] [PubMed] [Google Scholar]
- 123. Thiele DK, Senti JL, Anderson CM. Maternal vitamin D supplementation to meet the needs of the breastfed infant: a systematic review. J Hum Lact. 2013;29(2):163–70. [DOI] [PubMed] [Google Scholar]
- 124. Dawodu A, Tsang RC. Maternal vitamin D status: effect on milk vitamin D content and vitamin D status of breastfeeding infants. Adv Nutr. 2012;3:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Prentice AM, Roberts SB, Prentice A, Paul AA, Watkinson M, Watkinson AA, Whitehead RG. Dietary supplementation of lactating Gambian women. I. Effect on breast-milk volume and quality. Hum Nutr Clin Nutr. 1983;37:53–64. [PubMed] [Google Scholar]
- 126. Deodhar AD, Hajalakshmi R, Ramakrishnan CV. Studies on human lactation. iii. Effect of dietary vitamin supplementation on vitamin contents of breast milk. Acta Paediatr. 1964;53:42–8. [DOI] [PubMed] [Google Scholar]
- 127. Deegan KL, Jones KM, Zuleta C, Ramirez-Zea M, Lildballe DL, Nexo E, Allen LH. Breast milk vitamin B-12 concentrations in Guatemalan women are correlated with maternal but not infant vitamin B-12 status at 12 months postpartum. J Nutr. 2012;142:112–6. [DOI] [PubMed] [Google Scholar]
- 128. Khambalia A, Latulippe ME, Campos C, Merlos C, Villalpando S, Picciano MF, O'Connor DL. Milk folate secretion is not impaired during iron deficiency in humans. J Nutr. 2006;136:2617–24. [DOI] [PubMed] [Google Scholar]
- 129. Hampel D, Shahab-Ferdows S, Islam M, Peerson J, Allen L. Vitamin concentrations in human milk vary with time within feed, circadian rhythm, and single-dose supplementation. J Nutr. 2017;147:603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Klevor M, Haskell M, Lartey A, Adu-Afarwuah S, Zeilani M, Dewey K. Lipid-based nutrient supplements providing approximately the recommended daily intake of vitamin A do not increase breast milk retinol concentrations among Ghanaian Women. J Nutr. 2016;146:335–42. [DOI] [PubMed] [Google Scholar]
- 131. Wagner CL, Greer FR, American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition . Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–52. [DOI] [PubMed] [Google Scholar]
- 132. Pérez-Escamilla R, Cohen RJ, Brown KH, Rivera LL, Canahuati J, Dewey KG. Maternal anthropometric status and lactation performance in a low-income Honduran population: evidence for the role of infants. Am J Clin Nutr. 1995;61:528–34. [DOI] [PubMed] [Google Scholar]
- 133. De Carvalho M, Robertson S, Friedman A, Klaus M. Effect of frequent breast-feeding on early milk production and infant weight gain. Pediatrics. 1983;72:307–11. [PubMed] [Google Scholar]
- 134. Dewey KG, Lonnerdal B.. Infant self-regulation of breast milk intake. Acta Paediatr Scand. 1986;75(6):893–8. [DOI] [PubMed] [Google Scholar]
- 135. Dewey KG, Heinig MJ, Nommsen LA, Lonnerdal B. Maternal versus infant factors related to breast milk intake and residual milk volume: the DARLING study. Pediatrics. 1991;87:829–37. [PubMed] [Google Scholar]
- 136. Woolridge MW, Baum JD.. The regulation of human milk flow. In Lindblad IBS, ed. Perinatal Nutrition’. San Diego: Academic Press, 1988:243–57. [Google Scholar]
- 137. Stuff JE, Nichols BL.. Nutrient intake and growth performance of older infants fed human milk. J Pediatr. 1989;115:959–68. [DOI] [PubMed] [Google Scholar]
- 138. Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. 2013;60(1):49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Pérez-Escamilla R, Segura-Pérez S, Lott M. Feeding Guidelines for Infants and Young Toddlers: A Responsive Parenting Approach. [Internet]. Healthy Eating Research-Robert Wood Johnson Foundation, 2017. Available from: http://healthyeatingresearch.org. [Google Scholar]
- 140. Prell C, Koletzko B.. Breastfeeding and Complementary Feeding. Dtsch Arztebl Int. 2016;113:435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. American Academy of Pediatrics. Complementary feeding. In: Kleinman R, Greer F, eds. Pediatric Nutrition. American Academy of Pediatrics. Committee on Nutrition; 2013. [Google Scholar]
- 142. Fewtrell M, Bronsky J, Campoy C, Domellof M, Embleton N, Fidler Mis N, Hojsak I, Hulst JM, Indrio F, Lapillonne A et al.. Complementary feeding: A position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2017;64:119–32. [DOI] [PubMed] [Google Scholar]
- 143. Black M, Walker S, Fernald L, Andersen C, DiGirolamo A, Lu C, McCoy DC, Fink G, Shawar Y, Shiffman J et al.. Early childhood development coming of age: science through the life course. Lancet. 2017;389:77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Feigelman S. The first year. In Kliegman R, Stanton B, Geme JSeds. Nelson Textbook of Pediatrics. Philadelphia, PA: Elsevier; 2016. [Google Scholar]
- 145. Marcdante K, Kliegman R. Normal development. In: Marcdante K, Kliegman R, eds. Nelson Essentials of Pediatrics. Philadelphia, PA: Elsevier Saunders; 2015. [Google Scholar]
- 146. Brown A, Lee M.. An exploration of experiences of mothers following a baby-led weaning style: developmental readiness for complementary foods. Matern Child Nutr. 2013;9:233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Outteridge J. The digestive system and nutrition. In Peate I, Gormley-Fleming, eds. Fundamentals of Children's Anatomy and Physiology: A Textbook for Nursing and Healthcare Students. UK: John Wiley and Sons; 2015:256–81. [Google Scholar]
- 148. Carruth BR, Skinner JD.. Feeding behaviors and other motor development in healthy children (2-24 months). J Am Coll Nutr. 2002;21:88–96. [DOI] [PubMed] [Google Scholar]
- 149. Rochat P, Goubet N.. Development of sitting and reaching in five to six month old infants. Infant Behav Dev. 1995;18:53–68. [Google Scholar]
- 150. Rochat P. Mouthing and grasping in neonates; evidence for the early detection of what hard or soft substances afford for action. Infant Behav Dev. 1987;10:435–49. [Google Scholar]
- 151. McNally J, Hugh-Jones S, Caton S, Vereijken C, Weenen H, Hetherington M. Communicating hunger and satiation in the first 2 years of life: a systematic review. Matern Child Nutr. 2016;12:205–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Gyorkos TW, Maheu-Giroux M, Blouin B, Creed-Kanashiro H, Casapia M, Aguilar E, Silva H, Joseph SA, Penny ME. A hospital policy change toward delayed cord clamping is effective in improving hemoglobin levels and anemia status of 8-month-old Peruvian infants. J Trop Pediatr. 2012;58:435–40. [DOI] [PubMed] [Google Scholar]
- 153. Dykes F, Williams C.. Falling by the wayside: a phenomenological exploration of perceived breast-milk inadequacy in lactating women. Midwifery. 1999;15:232–46. [DOI] [PubMed] [Google Scholar]
- 154. Perez-Escamilla R, Curry L, Minhas D, Taylor L, Bradley E. Scaling up of breastfeeding promotion programs in low- and middle-income countries: the “breastfeeding gear” model. Adv Nutr. 2012;3:790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Victora CG, Bahl R, Barros AJ, Franca GV, Horton S, Krasevec J, Murch S, Sankar MJ, Walker N, Rollins NC et al.. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–90. [DOI] [PubMed] [Google Scholar]
- 156. Sinha B, Chowdhury R, Sankar MJ, Martines J, Taneja S, Mazumder S, Rollins N, Bahl R, Bhandari N. Interventions to improve breastfeeding outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:114–34. [DOI] [PubMed] [Google Scholar]
- 157. Perez-Escamilla R, Segura-Millan S, Pollitt E, Dewey KG. Determinants of lactation performance across time in an urban population from Mexico. Soc Sci Medicine. 1993;37:1069–78. [DOI] [PubMed] [Google Scholar]
- 158. O'Sullivan E, Perrine C, Rasmussen K. Early Breastfeeding Problems Mediate the Negative Association between Maternal Obesity and Exclusive Breastfeeding at 1 and 2 Months Postpartum. J Nutr. 2015;145:2369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Wagner EA, Chantry CJ, Dewey KG, Nommsen-Rivers LA. Breastfeeding concerns at 3 and 7 days postpartum and feeding status at 2 months. Pediatrics. 2013;132:e865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Matias SL, Nommsen-Rivers LA, Creed-Kanashiro H, Dewey KG. Risk factors for early lactation problems among Peruvian primiparous mothers. Matern Child Nutr. 2010;6:120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


