ABSTRACT
The prevalence of type 2 diabetes (T2D) is increasing worldwide. This complex and multifactorial metabolic condition affects both the quality and expectancy of life in adults. Therefore, appropriate lifestyle strategies are needed in order to reduce the burden of T2D. Dietary patterns characterized by a high consumption of fruits, vegetables, whole grains, legumes, nuts, and seeds, and a minimal consumption of animal products, have been suggested as a dietary approach to prevent and control T2D and related micro- and macrovascular complications. This narrative review summarizes epidemiologic and clinical trial evidence on the role of the most widely studied dietary patterns that emphasize the consumption of plant foods [vegetarian, vegan, Mediterranean, and DASH (Dietary Approaches to Stop Hypertension) diets] in the management of T2D and its complications. Furthermore, their potential underlying mechanisms are discussed. Dietary patterns emphasizing the consumption of plant foods appear to confer beneficial effects on glycemic control in different diabetic populations. Several components of these dietary patterns might confer benefits on glycemia and counterbalance the detrimental effects of animal-based foods. The limited evidence on T2D-related complications makes it difficult to draw solid conclusions.
Keywords: type 2 diabetes, plant-based diets, vegetarian diet, Mediterranean diet, DASH diet
Introduction
Type 2 diabetes (T2D) is an important public health issue worldwide. It is estimated that ∼425 million people had diabetes in 2017, of whom one-third are >65 y old, and this figure is expected to rise to 693 million by 2040 (1), in parallel to the obesity epidemic. Diabetes mellitus, characterized by high blood glucose, is not only among the top 10 causes of death worldwide (2), but is also a potent risk factor for blindness, renal failure, and lower limb amputation, decreasing the quality of life (3).
Efforts need to be made to decrease the incidence of T2D and reduce its complications and mortality. Physical inactivity, sedentary lifestyle, poor diet and nutrition, smoking, and excessive adiposity are the major risk factors for developing T2D (4).
Lifestyle interventions that aim to change diet and the level of physical activity have consistently been shown to reduce T2D risk in the short and long term (5).
The quality of the diet—particularly the consumption of limited amounts of sugar, refined grains, and processed and unprocessed red meat, and large amounts of fresh fruits, vegetables, whole grains, yogurt, and coffee—has proved to be inversely associated with the future risk of T2D (6). Therefore, a dietary pattern with an emphasis on the consumption of plant foods and little animal and processed food may have benefits on the prevention and management of T2D.
Unfortunately, there is no precise definition of a plant-based diet. Strictly speaking, a plant-based diet is based wholly on foods derived from plants, and therefore contains minimal amounts of processed food and encourages the consumption of more fruits, vegetables, whole grains, legumes, nuts, seeds, herbs, and spices and excludes all animal products, e.g, red meat, poultry, fish, eggs, and dairy products.
However, the term plant-based diet has been extensively used to refer not only to vegan diets, which do not include any food from animal sources, but also to other diets such as vegetarian diets, which can include eggs and dairy products, or semivegetarian diets (pescatarian, macrobiotic, etc.), which contain small amounts of meat and fish or other animal products.
Other dietary patterns, which cannot strictly be considered to be plant based, also emphasize the consumption of plant foods and have been extensively studied in various clinical trials. This is the case of the traditional Mediterranean dietary pattern (MedDiet) characterized by a high intake of olive oil, fruit, nuts, vegetables, cereals, herbs, and spices; a moderate intake of fish and poultry; a low intake of dairy products, red meat, processed meats, and sweets; and a moderate intake of wine with meals. Some authors also regard the Dietary Approaches to Stop Hypertension (DASH) diet as plant-based diet because it features high intakes of fruit, vegetables, legumes, and nuts; moderate amounts of low-fat dairy products; and low amounts of animal protein, sweets, and sodium. Table 1 describes several dietary patterns that emphasize the consumption of plant foods.
TABLE 1.
Characteristics of dietary patterns emphasizing the consumption of plant foods
| Dietary pattern | Foods |
|---|---|
| DASH1 diet | Emphasizes fruits, vegetables, and low-fat dairy products, and includes whole grains, poultry, fish, and nuts. Reduced consumption of saturated fat, red meat, sweets, and sodium. |
| Macrobiotic diet | Emphasizes locally grown whole-grain cereals, pulses (legumes), vegetables, seaweed, fermented soy products, and fruit, combined into meals according to the ancient Chinese principle of balance known as yin and yang. |
| Mediterranean diet | Characterized by a high intake of olive oil as the principal source of dietary fat, fruit, nuts, vegetables, and cereals; a moderate intake of fish and poultry; a low intake of dairy products, red meat, processed meats, and sweets; and a moderate intake of wine with meals. |
| Pescatarian diet | Plant-based diet including fish or other seafood, but not the flesh of other animals. |
| Vegan diet | Plant-based diet avoiding all animal foods such as meat (including fish, shellfish, and insects), dairy, eggs, and honey, as well as products such as leather, and those that are tested on animals. |
| Vegetarian diet | Plant-based diet avoiding all animal flesh–based foods and animal-derived products. Some modified versions allow eggs (ovo), dairy products (lacto), or a combination of both. |
DASH, Dietary Approaches to Stop Hypertension.
Previous studies have reviewed the potential beneficial effects of plant-based diets (vegetarian and vegan) on T2D management (7–10). Nonetheless, to the best of our knowledge, no previous review has extended the scope and included the MedDiet and DASH diet, 2 dietary patterns that have been extensively studied and which also emphasize the consumption of plant foods.
In the present narrative review, we summarize the evidence from observational studies and especially clinical trials evaluating the effect of dietary patterns with an emphasis on the consumption of plant foods (vegetarian, vegan, MedDiet, and DASH) on the control and management of T2D and its complications. We also discuss the possible mechanisms involved and the unanswered research questions for future studies.
Literature Search Methods
We performed a literature search of MEDLINE (PubMed). Our search terms combined the exposures—plant-based diets, vegetarian diets, vegan diets, macrobiotic diets, MedDiet, and DASH diet—with several outcomes: insulin resistance and sensitivity, inflammation, obesity, T2D, retinopathy, diabetic nephropathy, and cardiovascular disease (CVD) and mortality. Given that the present article is not a systematic review, we may not have identified all studies, and we must acknowledge a certain publication bias. However, all of the authors conducted the literature search independently.
Dietary Patterns Emphasizing the Consumption of Plant Foods in T2D Incidence and Management
Vegetarian and vegan diets
The first observational study examining associations between vegetarianism and T2D was conducted in the 1960s in a population of 25,698 white adult Seventh-Day Adventists and found a lower prevalence of self-reported diabetes in vegetarians than in nonvegetarians (11). More recently, a cross-sectional analysis of the Adventist Health Study-2 cohort revealed an inverse association between adherence to a vegetarian diet and the prevalence of T2D, which appeared stronger for the vegan (OR: 0.51; 95% CI: 0.40, 0.66) and lacto-ovo-vegetarian diets (OR: 0.54; 95% CI: 0.49, 0.60), followed by the pescovegetarians (OR: 0.70; 95% CI: 0.61, 0.80) and semi-vegetarian diets (OR: 0.76; 95% CI: 0.65, 0.90) (12). A further analysis of the Adventist Health Study-2 revealed that these types of vegetarian diet were associated with a substantially lower incidence of T2D (13). A subsequent study evaluating associations between dietary habits and diabetes prevalence in 4384 Taiwanese Buddhist participants found that a vegetarian diet characterized by complete avoidance of meat and fish was inversely associated with the prevalence of diabetes in men (OR: 0.49; 95% CI: 0.28, 0.89) and menopausal women (OR: 0.25; 95% CI: 0.15, 0.42) (14).
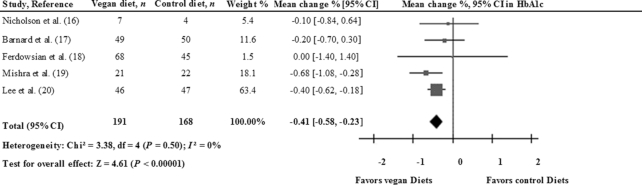
Clinical trials have demonstrated that vegetarian dietary patterns can lead to good glycemic control in diabetes. The results of a meta-analysis (15) assessing the effectiveness of vegetarian diets on glycated hemoglobin (HbA1c) suggest that these diets are beneficial for glycemic control among diabetics. Six randomized controlled trials (RCTs) were included in this analysis, which involved 255 individuals. Five of the studies assessed the effect of a vegan diet, whereas the sixth assessed a lacto-ovo-vegetarian diet. The vegan or vegetarian-like diets resulted in a statistically significant reduction in HbA1c (−0.39 percentage points; 95% CI: −0.62, −0.15; P < 0.01; I2 = 3.0%) compared with control diets. However, to date no meta-analysis of RCTs has analyzed this association separately for those studies conducted on vegan or vegetarian diets. Taking advantage of this gap in the literature, then, we performed a meta-analysis of RCTs evaluating the associations between these diets and glycemic control in T2D. We used the inverse variance method with fixed effect-models to pool between-treatment changes from baseline values or between-treatment end differences in HbA1c. Results were expressed as mean differences and their 95% CIs. The Cochrane Q statistic test was used to assess the heterogeneity, which was quantified by the I2 statistic. Analysis was conducted with Review Manager (RevMan) software version 5.3. Five RTCs (16–20) testing vegan diets reported data on HbA1c, and the pooled estimated effect was −0.41 percentage points (95% CI: −0.58, −0.23; P < 0.01) according to the fixed-effects model (Figure 1) and with no heterogeneity (I2 = 0%). Therefore, these results suggest that a vegan diet could be beneficial for glycemic control in diabetics. However, in most of the studies included in our meta-analysis, participants had either a significantly greater weight loss (16, 18, 19) or abdominal fat reduction (18, 20) than those assigned to the control diet, thus confounding the effect estimates. The net effect of the vegan diet on hyperglycemia induced by T2D should be further examined. In addition to HbA1c, future RCTs should also assess the effectiveness of the vegan diet on insulin resistance.
FIGURE 1.
Forest plot for the change in HbA1c in 5 RCTs of the effects of a vegan diet on diabetics. Pooled risk was estimated with the inverse variance method, with the use of fixed-effects meta-analysis. HbA1c, glycated hemoglobin; RCT, randomized controlled trial.
Mediterranean diet
The association between adherence to the MedDiet and T2D risk has been analyzed in some cross-sectional studies. Results are contradictory, and show either a significant inverse association (21) or no association (22). However, 1 meta-analysis of 8 prospective studies and 1 RCT found that the risk of T2D in those participants in the highest category of adherence to the MedDiet was 19% lower than in those in the lowest (RR: 0.81; 95% CI: 0.73, 0.90; P < 0.01; I2 = 55%) (23). Furthermore, a lower incidence of gestational diabetes mellitus (GDM) was also observed in those pregnant women who adhered closely to a MedDiet (24) or underwent a Mediterranean dietary intervention program (25).
There is also evidence to suggest that adherence to the MedDiet has a beneficial role in glycemic control. An inverse association between adherence to the MedDiet and fasting plasma glucose, insulin, and the HOMA-IR levels has been found in a normoglycemic Greek population (26). On the contrary, HOMA-IR was not found to be significantly modified by the MedDiet, in trials comparing the effect of MedDiets with the American Diabetic Association diet (27) or a low-fat diet (28). As far as the parameters related to glycemic control are concerned, a cross-sectional analysis of 901 Italian adults with T2D showed that the highest MedDiet scores were associated with lower HbA1c levels (P-trend < 0.01) (29). In contrast, in another cross-sectional study conducted in 383 T2D Spanish patients, the degree of adherence to the MedDiet was not significantly associated with HbA1c levels (30). Evidence from RCTs also suggests that the MedDiet can improve glycemic control in the long term in the context of weight loss. The results from 4 meta-analyses showed that the MedDiet had a more favorable effect on HbA1c (ranging from −0.3% to −0.47%) than other diets, mainly low-fat diets (31–34). Therefore, we can conclude that there is strong evidence to suggest that a MedDiet in the context of weight loss is effective in the management of T2D. Future studies are needed to examine the effect of the MedDiet on glycemic control in eucaloric conditions and to clarify its role in diabetes management independently of weight changes.
DASH diet
Several prospective studies have reported inverse associations between adherence to the DASH diet and risk of T2D development. In a recent meta-analysis of 5 prospective studies, a comparison of the highest with the lowest adherence to the DASH dietary pattern revealed an inverse association with T2D incidence (RR: 0.82; 95% CI: 0.74, 0.92; P = 0.03; I2 = 62%) (35). Similar results (RR: 0.80; 95% CI: 0.74, 0.86; P < 0.01; I2 = 61%) were found in the most recent meta-analysis of 8 prospective studies (36). These findings suggest that adherence to the DASH diet has potential for preventing diabetes from developing.
The evidence from RCTs examining the effect of the DASH diet on glycemic control is limited to GDM. Asemi et al. assessed the effect of the DASH diet on glucose tolerance (37) and insulin resistance (38) for 4 wk in a small sample of 32 pregnant women with GDM. Compared with the control diet, the DASH diet had beneficial effects on glucose tolerance (fasting plasma glucose: −7.62 compared with 3.68 mg/dL; P = 0.02; and for serum insulin levels −2.62 compared with 4.32 μIU/mL, P = 0.03) and the HOMA-IR (−0.8 compared with 1.1; P = 0.03). To clarify the role of this dietary pattern in glycemic management during pregnancy, data from larger and well-powered trials are required. Moreover, there is need for evidence based on RCTs to support the benefits of the DASH diet not only in pregnant women but also in other diabetic populations.
Dietary Patterns Emphasizing the Consumption of Plant Foods and Major T2D Complications
Poor T2D control can lead to several important health complications that decrease the quality and expectancy of life and increase the global economic burden. T2D-related complications are classified as microvascular (including nephropathy, retinopathy, and neuropathy) and macrovascular (CVD) (39). Lifestyle modifications, including diet, may play an important role in improving T2D comorbidities. In this regard, dietary patterns emphasizing the consumption of plant foods, because of their intrinsic properties (e.g., high contents of fiber, and antioxidant and anti-inflammatory micronutrients), may help to prevent microvascular and macrovascular complications.
Microvascular Complications
Nephropathy
The main microvascular complication of T2D is nephropathy, which affects ∼40% of diabetic patients and is regarded as the main cause of renal disease (40).
Only 1 cross-sectional study has evaluated the association between adherence to a dietary pattern emphasizing the consumption of plant foods and nephropathy. No differences were found in prevalence in nonvegetarian and vegetarian T2D Indian subjects (41). Findings from prospective studies are along the same lines. In the Atherosclerosis Risk in Communities Study (ARIC), results from the whole cohort showed that those participants in the lowest tertile of adherence to the DASH diet had a 16% (HR: 1.16; 95% CI: 1.07, 1.26; P-trend < 0.001) higher risk of kidney disease development than those in the highest tertile after 23 y of follow-up. However, in the stratified analysis, lower adherence to the DASH diet was only associated with a higher risk of kidney disease in nondiabetic individuals, whereas no association was observed in diabetics (42). Similarly, in the Northern Manhattan Study, a 1-point increase in the MedDiet score was associated with a 17% (OR: 0.83; 95% CI: 0.71, 0.96; P = 0.02) lower risk of incident estimated glomerular filtration rate <60 mL/(min · 1.73 m2) when diabetic and nondiabetic participants were analyzed together, although a significant interaction with diabetes status was observed (P = 0.01). Subsequent stratified analyses showed that a 1-point increase in the MedDiet score was inversely associated with estimated glomerular filtration rate <60 mL/(min · 1.73 m2) (OR: 0.75; 95% CI: 0.63, 0.89; P = 0.001) in nondiabetics, whereas there was a nonsignificant increased risk in diabetics (OR: 1.21; 95% CI: 0.87, 1.69; P = 0.25) (43).
Ideally, RCTs in diabetic populations should evaluate the effect of dietary patterns emphasizing the consumption of plant foods on nephropathy incidence. Nonetheless, this type of study is difficult to carry out because the endpoint takes several years to occur, and the sample needs to be large. Therefore, most of the interventional studies conducted so far have focused on surrogates for nephropathy development and progression, such as the measurement of urinary albumin excretion or serum creatinine concentrations. In an RCT conducted in 22 T2D participants, a vegan diet significantly reduced urinary albumin excretion more than the American Diabetic Association diet (−15.9 mg/24 h and −10.9 mg/24 h, respectively) (44). Similarly, in a nonrandomized clinical trial conducted in 8 participants with type 1 diabetes and an albumin excretion rate >30 μg/min, median fractional albumin clearance significantly decreased from 188 × 10−4 to 87 × 10−4 after a predominantly vegetarian diet (45). Likewise, in a crossover clinical trial involving 17 macroalbuminuric T2D patients, the median (range) of urinary albumin excretion was lower in the lactovegetarian, low-protein diet intervention group [229.3 μg/min (76.6–999.3 μg/min)] and in the replacement of red meat with poultry intervention group [269.4 μg/min (111–1128 μg/min)] than in the group on a usual diet [312.8 μg/min (223.7–1223.7 μg/min)] (46).
To the best of our knowledge, only 1 RCT has evaluated the effect of dietary patterns emphasizing the consumption of plant foods (the MedDiet) on the risk of nephropathy incidence, in a study of 3614 elderly participants with T2D. The results showed that the effect of the MedDiet enriched with nuts (HR: 1.06; 95% CI: 0.72, 1.58) and the MedDiet enriched with extra-virgin olive oil (HR: 1.15; 95% CI: 0.79, 1.67) on the risk of nephropathy incidence in individuals at high risk of CVD was no greater than the effect of the advice to follow a low-fat control diet (47).
Considering that few RCTs with relatively small sample sizes have evaluated the effect of plant-based diets on surrogate markers of kidney disease in T2D patients, and only 1 RCT has assessed the effect of the MedDiet on the risk of diabetic nephropathy, we can conclude that there is insufficient evidence to draw solid conclusions regarding the effect of dietary patterns emphasizing the consumption of plant foods on the prevention of kidney disease in diabetic individuals.
Retinopathy
Diabetic retinopathy strongly correlates with the duration of diabetes and glycemic control, and is regarded as the leading cause of blindness in adults from developed countries (48). Previous research has shown that plant foods (fruits and vegetables) and some nutrients derived from them, such as dietary fiber, are inversely associated with the risk of diabetic retinopathy (49). However, little is known about the association between dietary patterns emphasizing the consumption of plant foods and this diabetes-related complication.
As far as we know, no cross-sectional or prospective studies have evaluated the associations between adherence to dietary patterns emphasizing the consumption of plant foods and the risk of diabetic retinopathy. However, a post-hoc analysis conducted in the framework of the landmark PREvención con DIeta MEDiterránea (PREDIMED) trial has been published. This analysis included 3614 senior participants with T2D at high risk of CVD who were followed for a median of 6 y. The results showed that participants in the MedDiet enriched with extra-virgin olive oil group had a 44% lower risk of diabetic retinopathy (HR: 0.56; 95% CI: 0.32, 0.97) than those in the low-fat control-diet group. Nonetheless, a MedDiet enriched with nuts did not have a greater effect on retinopathy risk than the control diet (HR: 0.63; 95% CI: 0.35, 1.11). When both MedDiet interventions were merged, the relative risk of diabetic retinopathy was 40% lower than for the control group (HR: 0.60; 95% CI: 0.37, 0.96). The subgroup analyses showed that a MedDiet enriched with extra-virgin olive oil reduced the risk of diabetic retinopathy mainly in women, individuals aged ≥70 y, individuals with BMI kg/m2≥30, individuals without hypertension, and individuals with a low adherence to the MedDiet at baseline, although there was no statistical interaction between these categories and the intervention group (47). The results revealed that the MedDiet enriched with mixed nuts had a beneficial effect on diabetic retinopathy only in those individuals without hypertension at baseline.
Although results from this clinical trial show the importance of dietary patterns emphasizing the consumption of plant foods in the prevention of diabetic retinopathy, further studies are warranted in order to better understand the possible role of these dietary patterns in the prevention of diabetic retinopathy.
Neuropathy
According to the American Diabetic Association, neuropathy is a comorbidity of diabetes that affects the nervous system, and which has a wide range of clinical manifestations, foot ulceration being one of the most important. The strategy to prevent diabetic neuropathy should focus on glucose control and lifestyle modifications (50). Little is known about the possible role of dietary patterns emphasizing the consumption of plant foods in the prevention or treatment of this condition. In a small nonrandomized clinical trial, 21 T2D patients with painful diabetic neuropathy received an intervention based on a low-fat, high-fiber, no-cholesterol vegan diet along with exercise (walking every day for >30 min). The results showed that, in <16 d, 17 of the 21 participants completely mitigated their distressingly sharp and burning pain, although numbness persisted (51).
Evidence regarding the effect of dietary patterns emphasizing the consumption of plant foods is scarce and limited to only 1 nonrandomized clinical trial. Additional large, well-designed randomized clinical trials evaluating the degree of neuropathy through the use of direct tests such as the monofilament, vibration, and superficial pain sensation tests are needed to confirm the potential role of these dietary patterns in the prevention and management of this important diabetes-related complication.
Macrovascular Complications
CVD
Diabetes is well recognized as a risk factor for CVD, which in turn is considered to be the leading cause of mortality worldwide (52). To the best of our knowledge, the vast majority of studies published to date have focused on the prevention or management of different CVD risk factors rather than the prevention of CVD itself. Moreover, the research has mostly centered on the MedDiet, and few studies have been conducted strictly in diabetic populations.
In a case-control study conducted in 432 T2D patients, those individuals who adhered closely to the MedDiet (score ≥11) had a 66% (OR: 0.44; 95% CI: 0.24, 0.83) lower risk of having peripheral arterial disease than those who did not adhere closely (score 0–8). The stratified analysis showed that the risk of peripheral arterial disease increased progressively with the duration of the diabetes, and the risk was highest in those with a duration of >10 y (OR: 2.52; 95% CI: 1.45, 4.39) compared with those with ≤5 y (53).
No study has prospectively focused only on diabetic participants. However, several stratified analyses have been conducted depending on diabetes status. For instance, in patients with stable ischemic heart disease and after a median follow-up of 3.7 y, the stratified analysis revealed that a 1-category increase in the MedDiet score was associated with a lower risk of major adverse CVD events in nondiabetic participants, whereas no association was observed in individuals with diabetes (54). Similar results were reported in the ATTICA study (55), in which a significant inverse association between MedDiet adherence and 10 y of CVD risk was reported in nondiabetic participants (RR: 0.96; 95% CI 0.67, 1.00), whereas a nonsignificant inverse association was observed among diabetic participants (RR: 0.88; 95% CI: 0.92, 1.16). However, these results are not in line with those of the PREDIMED study, the only RCT that has assessed the effect of the MedDiet on the risk of major CVD events in a subgroup analysis based on diabetes status (56). Findings showed that those diabetic individuals following the MedDiet had a 29% lower risk of CVD events (HR: 0.71; 95% CI: 0.51, 0.98) than those following a low-fat diet, whereas no effect was reported in nondiabetic participants (HR: 0.73; 95% CI: 0.52, 1.05), after a median follow-up of 4.8 y.
Mortality
Diabetes is considered to be one of the top 10 worldwide leading causes of death (2). The contribution of dietary patterns emphasizing the consumption of plant foods to a reduction in diabetes-associated mortality has also been poorly studied. As far as we know, only 1 prospective study, the MOLI-SANI study, has been conducted exclusively in T2D participants (57). After analyzing data from 1995 individuals, results showed that close adherence to the MedDiet (≥6 points) was inversely associated with all-cause (HR: 0.41; 95% CI: 0.23, 0.73; P-trend < 0.01) and CVD mortality (HR: 0.43; 95% CI: 0.19, 0.99; P-trend < 0.05). Other prospective studies have conducted subgroup analysis of diabetes status at baseline. In a collaborative analysis of 4 prospective studies, the risk of death from ischemic heart disease was lower in vegetarians than in nonvegetarians, and did not differ among subjects with or without CVD or diabetes at baseline (58). In another stratified analysis conducted in a selected subgroup of women with heart failure from the Women's Health Initiative Study (59), compared with low adherence to the MedDiet, high adherence was not associated with a lower risk of death in participants with or without previous history of diabetes. However, DASH diet adherence was inversely associated with a lower risk of mortality only in women without diabetes (HR: 0.76; 95% CI: 0.62, 0.93; P-trend < 0.01) for the fourth compared with the first quartile.
Possible Mechanisms by which Dietary Patterns Emphasizing the Consumption of Plant Foods May Enhance T2D Management
The mechanisms by which the aforementioned dietary patterns emphasizing the consumption of plant foods may modulate glucose and insulin metabolism and confer protection against T2D are still putative, but mainly related to specific components, such as increased intake of fiber, PUFAs and MUFAs, antioxidant and anti-inflammatory micronutrients, and a reduced intake of SFAs, heme iron, sodium, and nitrites and nitrates. Overall, these components promote insulin sensitivity and glycemic control by controlling adiposity and body weight, reducing inflammation and oxidation, and modulating gut microbiota composition and activity. However, alternatives such as vegetarian diets, the MedDiet, and the DASH diet also include minimal amounts of animal-based food. These products contain not only essential nutrients, but also various potential harmful nutrients and nonnutrient components that could counterbalance the beneficial effects of plant-based foods on T2D management. The following paragraphs and Table 2 discuss the mechanisms by which several nutrient and nonnutrient components from plant and animal foods affect T2D management.
TABLE 2.
Mechanisms by which dietary components modulate glycemic control1
| Dietary component | Potential mechanism |
|---|---|
| Harmful components of animal-based diets | |
| SFAs | Changes in cell-membrane composition Glucolipotoxicity Inflammation |
| Heme iron | β-cell dysfunction Increased hepatic glucose production |
| Sodium | Reduced glucose uptake in muscle Reduced glycogen synthase activation in liver Impaired microvascular responsiveness to insulin |
| Nitrites and nitrates | DNA damage and oxidative stress Impaired insulin response |
| Beneficial components of plant-based diets | |
| Carbohydrates and fiber | Stimulation of incretin production Inhibition of α-amylase, α-glucosidase, lipase, and dipeptidyl peptidase 4 Increase in GLUT4 receptors Reduction of inflammation |
| PUFAs | Inhibition of glycolysis and lipogenesis Reduction of oxidative stress and inflammation |
| MUFAs | Preservation of GLUT4 receptors Stimulation of incretin production |
| Vitamin K | Inhibition of inflammation |
| Vitamins C and E | Increased β-cell proliferation Reduction of oxidative stress |
| Vitamin B | Decreased DNA glycation in leukocytes Increase in GLUT4 receptors |
| Magnesium | Increase in GLUT4 receptors Reduction of oxidative stress and inflammation |
| Polyphenols | Inhibition of α-amylase and α-glucosidase Increase in GLUT4 receptors Increased glucose uptake |
GLUT4, glucose transporter type 4.
Carbohydrates and Fiber
A high intake of fruits and vegetables and adherence to dietary patterns emphasizing the consumption of plant foods has been associated with reduced body weight and lower abdominal adiposity. These benefits are partly attributed to a high fiber content and a low glycemic index (GI). The intake of soluble fiber leads to a delay in gastric emptying and a slower absorption of macronutrients, which contributes to a lower postprandial rise in glucose (60, 61). Dietary fiber is resistant to enzymatic digestion in the small intestine but is fermented by the colonic bacteria to produce SCFAs which, in turn, reduce the production of hepatic glucose and stimulate the secretion of incretins (62, 63). Beyond their role as satiety hormones, incretins such as glucagon-like peptide 1 and gastric inhibitory polypeptide both promote β-cell proliferation and stimulate pancreatic insulin secretion, favoring the balance of glucose homeostasis (63, 64).
Higher dietary-fiber intake and low-GI diets have been related to improved inflammation (65), and this could partly explain their effectiveness at lowering insulin resistance and T2D risk. However, 2 other pathways have been proposed to explain how low-GI/low glycemic–load diets reduce T2D risk. First, the chronically increased insulin demand induced by a high-GI diets may result in pancreatic β-cell failure and, as a consequence, impaired glucose tolerance. Second, high-GI diets can directly increase insulin resistance because of their effect on glycemia, free fatty acids, and counter-regulatory hormone secretion (66).
Moreover, the consumption of dietary fiber has also been associated with a lower risk of CVD incidence, which could be mainly explained through its cholesterol- and blood pressure–lowering effects (67).
Fatty Acids
Epidemiologic studies have reported variations in the percentage of energy consumption from different types of fatty acid between vegans and meat eaters (68, 69). Although vegans had the highest intake of PUFAs, meat eaters had the highest intake of SFAs, which have different effects on glucose homeostasis and insulin sensitivity. SFAs have negative effects, whereas PUFAs and MUFAs have a beneficial impact (70). Importantly, some data also demonstrate that beyond the quantity and quality of fat, the PUFA:SFA ratio also has an effect on glucose metabolism (71).
Dietary fatty acids can affect insulin sensitivity, and thereby the metabolic control of T2D, by modifying the fatty acid composition of the cell membrane, which involves changes in membrane fluidity, thickness of volume, and even the stability of membrane proteins such as the insulin receptor (72). It has been shown that the more saturated the cell membrane is, the less fluid it is. Unsaturated fatty acids, such as PUFAs, not only increase cell membrane fluidity but also the number of insulin receptors and their affinity (73). Moreover, it has been reported that PUFAs, in general, could also inhibit hepatic glycolysis and lipogenesis (74, 75), whereas ω-3 (n–3) fatty acids, in particular, might have antioxidant effects (76). Furthermore, substituting α-linolenic acid for SFAs in rats induced beneficial effects on insulin resistance in liver and peripheral tissues (77). Even the conversion of α-linolenic acid to EPA and DHA acids is inefficient (78), especially when linoleic acid is consumed in high amounts, because these long-chain PUFAs exert anti-inflammatory effects by stimulating G protein-coupled receptor 120, a potent insulin sensitizer, thus enhancing systemic insulin sensitivity (79).
MUFAs can exert beneficial effects on glucose and insulin metabolism by modulating incretin responses, gastric emptying, and inflammation. Several intervention studies have shown that substituting MUFAs for SFAs could have beneficial effects on insulin sensitivity. In skeletal muscle from a T2D rat model, the deleterious effect of SFAs on insulin-signaling pathways (including changes in the insulin receptor substrate 1/2, phospho PI 3 kinase p85, and phospho-ERK1/2) disappeared when SFAs were replaced with MUFAs. Moreover, the translocation of glucose transporter type 4 (GLUT4) and insulin-stimulated glucose disposal were preserved with MUFA intervention (80). In animal models, it has also been reported that MUFAs can improve glucose tolerance through enhanced glucagon-like peptide 1 secretion (81).
Vitamins, Minerals, and Trace Elements
Evidence from RCTs suggest that supplementing vitamins that are mainly found in plant foods such as vitamins K (82), C (83), and E (84) might exert beneficial effects on glycemic control in T2D. Vitamin K is suggested to control glucose levels by modulating inflammation and osteocalcin. Animal studies have reported that osteocalcin improves β-cell proliferation and increases insulin expression and insulin secretion. Moreover, osteocalcin also increases adiponectin expression in mice adipocytes (85, 86).
Antioxidant vitamins, such as C and E, which are mainly found in vegetables, fruits, nuts, seeds, and oils, may prevent the glycosylation of hemoglobin by interrupting glycosylation at an early stage in the Maillard reaction (87, 88) or inhibiting the formation of advanced glycation end products (89). These vitamins have also been found to mitigate the β-cell dysfunction caused by oxidative stress (90).
Population-based studies indicate that vegetarians have lower mean intakes than nonvegetarians of zinc and higher intakes of magnesium, copper, and polyphenols (69, 91), all nutrients that have been involved in glucose homeostasis. Magnesium is an essential mineral with established properties as a cofactor for several enzymatic reactions. In muscle cells from rats, an intracellular magnesium deficiency can negatively affect insulin sensitivity because of an alteration in the tyrosine kinase activity of insulin receptors (92). Moreover, the oral administration of magnesium to diabetic rats improves blood glucose levels by increasing GLUT4 mRNA expression (93). It is important to highlight that magnesium can also improve T2D management because of its antioxidant and anti-inflammatory properties (94).
Dietary polyphenols come mainly from plant foods, including fruits, vegetables, whole grains, coffee, tea, and nuts. Polyphenols can influence glycemia and T2D via various mechanisms, such as promoting the uptake of glucose in tissues, and thereby improving insulin sensitivity (95). Some polyphenols, such as quercetin or ellagic acid, have proved to be effective at inhibiting α-amylase and α-glucosidase activities, and preventing the lipid peroxidation of pancreatic tissue homogenates (96–99). There is also increasing evidence to suggest that these dietary components have a modulatory role in T2D-related epigenetic processes (100). For instance, resveratrol and other phytochemicals such as curcumin have been shown to act through epigenetic mechanisms that affect the global epigenome (101), but future studies are needed to determine the exact role of these components in DNA methylation.
Heme Iron, Sodium, Nitrites, and Nitrates
Dietary patterns that emphasize the consumption of plant foods are characterized by a low consumption of heme iron, sodium, nitrites, and nitrates, all components that have been associated with insulin resistance.
Iron plays an important role in glucose metabolism due to its pro-oxidant capacity. It catalyzes cellular reactions to produce reactive oxygen species that impair β-cell function through oxidative stress, a process that favors apoptosis and leads to insulin deficiency (66). Moreover, on the one hand, the accumulation of iron in the liver induces hyperinsulinemia, which affects the suppression of hepatic glucose output (67). On the other hand, in muscle, iron stores accentuate free fatty acid oxidation and can hinder glucose disposal (68).
Sodium content is high in processed food such as meats, snacks, sauces, and spreads, and low in processed vegetables (102). Despite the fact that results are not consistent, research appears to indicate that high sodium intake is associated with insulin resistance (103–105). A high-salt diet could induce insulin resistance by reducing insulin-stimulated glucose uptake in muscle (106, 107). High- sodium diets may also produce the loss of microvascular insulin response in skeletal muscle, leading to the insulin resistance state in this tissue. This vascular affection could be the result of enhanced angiotensin II signaling or angiotensin II type 1 receptor levels (108).
Nitrites and nitrates are mainly used for the preservation of meat (109). In the stomach or even within food, they are transformed to nitrosamines by a chemical reaction with amino compounds. Nitrosamines are toxic for cells because they induce DNA damage, lipid peroxidation, oxidative stress (through the production of reactive oxygen species), and the activation of inflammatory signaling pathways, which could lead to insulin resistance (110). In animal models, nitrosamine appears to have toxic effects on pancreatic β-cells and increases the risk of diabetes (111). Moreover, in humans, blood nitrite concentrations have been associated with endothelial dysfunction (112) as well as impaired insulin response (113).
Conclusions
Increasing evidence suggests that dietary patterns emphasizing the consumption of plant foods may be effective not only at preventing the incidence of T2D but also at improving its management (7–10). There is strong evidence from the meta-analysis (15, 31–34) of RCTs to support the notion that these dietary patterns, especially the MedDiet, vegan, and vegetarian diets, improve glycemic control by reducing levels of HbA1c. However, evidence regarding the DASH diet is scarce and limited to gestational diabetes (38).
In terms of microvascular complications, the literature on the efficacy of dietary patterns emphasizing the consumption of plant foods is limited to a few cross-sectional, prospective cohorts and clinical trials with small samples, suggesting an improvement of these conditions. Evidence on nephropathy comes mainly from clinical trials that use surrogates such as the urinary albumin excretion to evaluate its development and progression (44–46). Although results suggest that dietary patterns emphasizing the consumption of plant foods could prevent the development of this diabetes-related complication by reducing the excretion of albumin more than other conventional diets (44–46), these findings are not supported by the PREDIMED study, the only clinical trial that has evaluated the effect of the MedDiet on the risk of nephropathy (47). Data on retinopathy and neuropathy are also scarce: only 1 clinical trial, the PREDIMED study, has evaluated the effect of the MedDiet on retinopathy (and found a beneficial effect) (47); only 1 nonrandomized clinical trial has assessed the effect of a vegan diet on diabetic neuropathy (and found that it seems to improve this condition) (51).
In terms of CVD, results are inconclusive. Whereas data from the stratified analysis of prospective cohort studies showed no association between these dietary patterns and the risk of CVD incidence in participants with T2D (54, 55), results from the PREDIMED clinical trial showed that the MedDiet protects against the development of T2D (56). Finally, as far as mortality is concerned, the only prospective study conducted specifically in patients with T2D showed an inverse association between a dietary pattern emphasizing the consumption of plant foods and the risk of overall and CVD mortality (57).
The beneficial effects of these dietary patterns may be explained by mechanisms specifically related to the increased intake of fiber, PUFAs, MUFAs, and antioxidant and anti-inflammatory micronutrients, and the reduced intake of SFAs, heme iron, sodium, nitrites, and nitrates.
In conclusion, the overall evidence suggests that patients with T2D will benefit from adopting dietary patterns emphasizing the consumption of plant foods. The components of these dietary patterns might confer benefits on glycemia by counterbalancing the detrimental effect of animal foods. However, there is limited evidence on T2D-related complications, which makes it difficult to draw solid conclusions. Additional large, well-conducted randomized clinical trials evaluating the effect of these dietary patterns on the incidence of micro- and macrovascular complications in patients with T2D are needed to confirm their effectiveness and to develop solid evidence-based scientific guidelines for diabetes prevention and management.
Acknowledgments
The authors’ responsibilities were as follows—JS-S and NB-T: developed the overall research plan; JS-S: had primary responsibility for final content; and all authors: conducted the literature search, wrote the article, and read and approved the final manuscript.
Notes
Published in a supplement to Advances in Nutrition. This supplement was sponsored by the Harding-Buller Foundation of Ohio. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the sponsors. Publication costs for this supplement were defrayed in part by the payment of page charges. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
The Centro de Investigación Biomédica en Red de la Fisiopatología de la Obesidad y Nutrición (CIBERobn) is an initiative of the Instituto de Salud Carlos III (ISCIII) of Spain which is supported by funding from the Fondo Europeo de Desarrollo Regional (FEDER).
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; GDM, gestational diabetes mellitus; GI, glycemic index; HbA1c, glycated hemoglobin; MedDiet, Mediterranean Diet; PREDIMED, PREvención DIeta MEDiterránea; RCT, randomized controlled trial; T2D, type 2 diabetes.
References
- 1. International Diabetes Federation. IDF Diabetes Atlas. 8th edn Brussels: International Diabetes Federation; 2017. [Google Scholar]
- 2. World Health Organization, editor. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva: WHO; 2009. [Google Scholar]
- 3. Luscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Circulation. 2003;108:1655–61. [DOI] [PubMed] [Google Scholar]
- 4. Hu FB, Satija A, Manson JE. Curbing the diabetes pandemic: the need for global policy solutions. JAMA. 2015;313:2319–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Diabetes Association. 5. Prevention or delay of type 2 diabetes: standards of medical care in diabetes—2018. Diabetes Care. 2018;41:S51–4. [DOI] [PubMed] [Google Scholar]
- 6. Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383:1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kahleova H, Pelikanova T. Vegetarian diets in the prevention and treatment of type 2 diabetes. J Am Coll Nutr. 2015;34:448–58. [DOI] [PubMed] [Google Scholar]
- 8. Pawlak R. Vegetarian diets in the prevention and management of diabetes and its complications. Diabetes Spectr. 2017;30:82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barnard ND, Katcher HI, Jenkins DJ, Cohen J, Turner-McGrievy G. Vegetarian and vegan diets in type 2 diabetes management. Nutr Rev. 2009;67:255–63. [DOI] [PubMed] [Google Scholar]
- 10. McMacken M, Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol. 2017;14:342–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Snowdon DA, Phillips RL. Does a vegetarian diet reduce the occurrence of diabetes?. Am J Public Health. 1985;75:507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tonstad S, Butler T, Yan R, Fraser GE. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care. 2009;32:791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tonstad S, Stewart K, Oda K, Batech M, Herring RP, Fraser GE. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr Metab Cardiovasc Dis. 2013;23:292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chiu THT, Huang H-Y, Chiu Y-F, Pan W-H, Kao H-Y, Chiu JPC, Lin M-N, Lin C-L. Taiwanese vegetarians and omnivores: dietary composition, prevalence of diabetes and IFG. PLoS One. 2014;9:e88547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yokoyama Y, Barnard ND, Levin SM, Watanabe M. Vegetarian diets and glycemic control in diabetes: a systematic review and meta-analysis. Cardiovasc Diagn Ther. 2014;4:373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nicholson AS, Sklar M, Barnard ND, Gore S, Sullivan R, Browning S. Toward improved management of NIDDM: a randomized, controlled, pilot intervention using a lowfat, vegetarian diet. Prev Med (Baltim). 1999;29:87–91. [DOI] [PubMed] [Google Scholar]
- 17. Barnard ND, Cohen J, Jenkins DJ, Turner-McGrievy G, Gloede L, Green A, Ferdowsian H. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr. 2009;89:1588S–96S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferdowsian HR, Barnard ND, Hoover VJ, Katcher HI, Levin SM, Green AA, Cohen JL. A multicomponent intervention reduces body weight and cardiovascular risk at a GEICO corporate site. Am J Health Promot. 2010;24:384–7. [DOI] [PubMed] [Google Scholar]
- 19. Mishra S, Xu J, Agarwal U, Gonzales J, Levin S, Barnard ND. A multicenter randomized controlled trial of a plant-based nutrition program to reduce body weight and cardiovascular risk in the corporate setting: the GEICO study. Eur J Clin Nutr. 2013;67:718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee Y-M, Kim S-A, Lee I-K, Kim J-G, Park K-G, Jeong J-Y, Jeon J-H, Shin J-Y, Lee D-H. Effect of a brown rice based vegan diet and conventional diabetic diet on glycemic control of patients with type 2 diabetes: a 12-week randomized clinical trial. PLoS One. 2016;11:e0155918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Panagiotakos DB, Pitsavos C, Chrysohoou C, Stefanadis C. The epidemiology of Type 2 diabetes mellitus in Greek adults: the ATTICA study. Diabet Med. 2005;22:1581–8. [DOI] [PubMed] [Google Scholar]
- 22. Ortega E, Franch J, Castell C, Goday A, Ribas-Barba L, Soriguer F, Vendrell J, Casamitjana R, Bosch-Comas A, Bordiú E et al.. Mediterranean diet adherence in individuals with prediabetes and unknown diabetes: the Di@bet.es Study. Ann Nutr Metab. 2013;62:339–46. [DOI] [PubMed] [Google Scholar]
- 23. Schwingshackl L, Missbach B, König J, Hoffmann G. Adherence to a Mediterranean diet and risk of diabetes: a systematic review and meta-analysis. Public Health Nutr. 2015;18:1292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tobias DK, Zhang C, Chavarro J, Bowers K, Rich-Edwards J, Rosner B, Mozaffarian D, Hu FB. Prepregnancy adherence to dietary patterns and lower risk of gestational diabetes mellitus. Am J Clin Nutr. 2012;96:289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Assaf-Balut C, García de la Torre N, Durán A, Fuentes M, Bordiú E, del Valle L, Familiar C, Ortolá A, Jiménez I, Herraiz MA et al.. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): a randomized controlled trial: the St. Carlos GDM prevention study. PLoS One. 2017;12:e0185873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Panagiotakos DB, Tzima N, Pitsavos C, Chrysohoou C, Zampelas A, Toussoulis D, Stefanadis C. The association between adherence to the Mediterranean diet and fasting indices of glucose homoeostasis: the ATTICA Study. J Am Coll Nutr. 2007;26:32–8. [DOI] [PubMed] [Google Scholar]
- 27. Elhayany A, Lustman A, Abel R, Attal-Singer J, Vinker S. A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: a 1-year prospective randomized intervention study. Diabetes, Obes Metab. 2010;12:204–9. [DOI] [PubMed] [Google Scholar]
- 28. Lasa A, Miranda J, Bulló M, Casas R, Salas-Salvadó J, Larretxi I, Estruch R, Ruiz-Gutiérrez V, Portillo MP. Comparative effect of two Mediterranean diets versus a low-fat diet on glycaemic control in individuals with type 2 diabetes. Eur J Clin Nutr. 2014;68:767–72. [DOI] [PubMed] [Google Scholar]
- 29. Esposito K, Maiorino MI, Di Palo C, Giugliano D; Campanian Postprandial Hyperglycemia Study Group . Adherence to a Mediterranean diet and glycaemic control in type 2 diabetes mellitus. Diabet Med. 2009;26:900–7. [DOI] [PubMed] [Google Scholar]
- 30. Díez-Espino J, Buil-Cosiales P, Serrano-Martínez M, Toledo E, Salas-Salvadó J, Martínez-González MÁ. Adherence to the Mediterranean diet in patients with type 2 diabetes mellitus and HbA1c level. Ann Nutr Metab. 2011;58:74–8. [DOI] [PubMed] [Google Scholar]
- 31. Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97:505–16. [DOI] [PubMed] [Google Scholar]
- 32. Carter P, Achana F, Troughton J, Gray LJ, Khunti K, Davies MJ. A Mediterranean diet improves HbA1c but not fasting blood glucose compared to alternative dietary strategies: a network meta-analysis. J Hum Nutr Diet. 2014;27:280–97. [DOI] [PubMed] [Google Scholar]
- 33. Huo R, Du T, Xu Y, Xu W, Chen X, Sun K, Yu X. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr. 2015;69:1200–8. [DOI] [PubMed] [Google Scholar]
- 34. Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D. A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open. 2015;5:e008222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jannasch F, Kröger J, Schulze MB. Dietary patterns and type 2 diabetes: a systematic literature review and meta-analysis of prospective studies. J Nutr. 2017;147:1174–82. [DOI] [PubMed] [Google Scholar]
- 36. Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2018;118:74–100.e11. [DOI] [PubMed] [Google Scholar]
- 37. Asemi Z, Tabassi Z, Samimi M, Fahiminejad T, Esmaillzadeh A. Favourable effects of the Dietary Approaches to Stop Hypertension diet on glucose tolerance and lipid profiles in gestational diabetes: a randomised clinical trial. Br J Nutr. 2013;109:2024–30. [DOI] [PubMed] [Google Scholar]
- 38. Asemi Z, Samimi M, Tabassi Z, Sabihi S, Esmaillzadeh A. A randomized controlled clinical trial investigating the effect of DASH diet on insulin resistance, inflammation, and oxidative stress in gestational diabetes. Nutrition. 2013;29:619–24. [DOI] [PubMed] [Google Scholar]
- 39. Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26:77–82. [Google Scholar]
- 40. Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–76. [DOI] [PubMed] [Google Scholar]
- 41. Viswanathan V, Cle A, Snehalatha C, Varadharani M, Nair B, Jayaraman M, Ramachandran A. Prevalence of albuminuria among vegetarian and non-vegetarian south Indian diabetic patients. Indian J Nephrol. 2002;12:73–6. [Google Scholar]
- 42. Rebholz CM, Crews DC, Grams ME, Steffen LM, Levey AS, Miller ER, Appel LJ, Coresh J. DASH (Dietary Approaches to Stop Hypertension) diet and risk of subsequent kidney disease. Am J Kidney Dis. 2016;68:853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khatri M, Moon YP, Scarmeas N, Gu Y, Gardener H, Cheung K, Wright CB, Sacco RL, Nickolas TL, Elkind MS. The association between a Mediterranean-style diet and kidney function in the Northern Manhattan Study cohort. Clin J Am Soc Nephrol. 2014;9:1868–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barnard ND, Cohen J, Jenkins DJA, Turner-McGrievy G, Gloede L, Jaster B, Seidl K, Green AA, Talpers S. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29:1777–83. [DOI] [PubMed] [Google Scholar]
- 45. Jibani MM, Bloodworth LL, Foden E, Griffiths KD, Galpin OP. Predominantly vegetarian diet in patients with incipient and early clinical diabetic nephropathy: effects on albumin excretion rate and nutritional status. Diabet Med. 1991;8:949–53. [DOI] [PubMed] [Google Scholar]
- 46. de Mello VDF, Zelmanovitz T, Perassolo MS, Azevedo MJ, Gross JL. Withdrawal of red meat from the usual diet reduces albuminuria and improves serum fatty acid profile in type 2 diabetes patients with macroalbuminuria. Am J Clin Nutr. 2006;83:1032–8. [DOI] [PubMed] [Google Scholar]
- 47. Díaz-López A, Babio N, Martínez-González MA, Corella D, Amor AJ, Fitó M, Estruch R, Arós F, Gómez-Gracia E, Fiol M et al.. Mediterranean diet, retinopathy, nephropathy, and microvascular diabetes complications: a post hoc analysis of a randomized trial. Diabetes Care. 2015;38:2134–41. [DOI] [PubMed] [Google Scholar]
- 48. Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL, Wykoff CC, Gardner TW. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wong MYZ, Man REK, Fenwick EK, Gupta P, Li L-J, van Dam RM, Chong MF, Lamoureux EL. Dietary intake and diabetic retinopathy: a systematic review. PLoS One. 2018;13:e0186582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pop-Busui R, Boulton AJM, Feldman EL, Bril V, Freeman R, Malik RA, Sosenko JM, Ziegler D. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:136–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Crane MG, Sample C. Regression of diabetic neuropathy with total vegetarian (vegan) diet. J Nutr Med. 1994;4:431–9. [Google Scholar]
- 52. World Health Organization. WHO | Cardiovascular Diseases (CVDs) [Internet]. WHO; 2017[cited 27 March, 2018]. Available from: http://www.who.int/mediacentre/factsheets/fs317/en/. [Google Scholar]
- 53. Ciccarone E, Di Castelnuovo A, Salcuni M, Siani A, Giacco A, Donati MB, De Gaetano G, Capani F, Iacoviello L; Gendiabe Investigators . A high-score Mediterranean dietary pattern is associated with a reduced risk of peripheral arterial disease in Italian patients with type 2 diabetes. J Thromb Haemost. 2003;1:1744–52. [DOI] [PubMed] [Google Scholar]
- 54. Stewart RAH, Wallentin L, Benatar J, Danchin N, Hagström E, Held C, Husted S, Lonn E, Stebbins A, Chiswell K et al.. Dietary patterns and the risk of major adverse cardiovascular events in a global study of high-risk patients with stable coronary heart disease. Eur Heart J. 2016;37:1993–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Panagiotakos DB, Georgousopoulou EN, Pitsavos C, Chrysohoou C, Skoumas I, Pitaraki E, Georgiopoulos GA, Ntertimani M, Christou A, Stefanadis C et al.. Exploring the path of Mediterranean diet on 10-year incidence of cardiovascular disease: the ATTICA study (2002–2012). Nutr Metab Cardiovasc Dis. 2015;25:327–35. [DOI] [PubMed] [Google Scholar]
- 56. Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J et al.. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. [DOI] [PubMed] [Google Scholar]
- 57. Bonaccio M, Di Castelnuovo, Costanzo A, Persichillo S, De Curtis M, Donati A, de Gaetano MB, Iacoviello G L; MOLI-SANI Study Investigators . Adherence to the traditional Mediterranean diet and mortality in subjects with diabetes. Prospective results from the MOLI-SANI study. Eur J Prev Cardiol. 2016;23:400–7. [DOI] [PubMed] [Google Scholar]
- 58. Key TJ, Fraser GE, Thorogood M, Appleby PN, Beral V, Reeves G, Burr ML, Chang-Claude J, Frentzel-Beyme R, Kuzma JW et al.. Mortality in vegetarians and nonvegetarians: detailed findings from a collaborative analysis of 5 prospective studies. Am J Clin Nutr. 1999;70:516S–24S. [DOI] [PubMed] [Google Scholar]
- 59. Levitan EB, Lewis CE, Tinker LF, Eaton CB, Ahmed A, Manson JE, Snetselaar LG, Martin LW, Trevisan M, Howard BV et al.. Mediterranean and DASH diet scores and mortality in women with heart failure: the Women's Health Initiative. Circ Heart Fail. 2013;6:1116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hopping BN, Erber E, Grandinetti A, Verheus M, Kolonel LN, Maskarinec G. Dietary fiber, magnesium, and glycemic load alter risk of type 2 diabetes in a multiethnic cohort in Hawaii. J Nutr. 2010;140:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dikeman CL, Fahey GC. Viscosity as related to dietary fiber: a review. Crit Rev Food Sci Nutr. 2006;46:649–63. [DOI] [PubMed] [Google Scholar]
- 62. Lovejoy JC. The impact of nuts on diabetes and diabetes risk. Curr Diab Rep. 2005;5:379–84. [DOI] [PubMed] [Google Scholar]
- 63. Heppner KM, Perez-Tilve D. GLP-1 based therapeutics: simultaneously combating T2DM and obesity. Front Neurosci. 2015;9:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bodnaruc AM, Prud'homme D, Blanchet R, Giroux I. Nutritional modulation of endogenous glucagon-like peptide-1 secretion: a review. Nutr Metab. 2016;13:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Buyken AE, Goletzke J, Joslowski G, Felbick A, Cheng G, Herder C, Brand-Miller JC. Association between carbohydrate quality and inflammatory markers: systematic review of observational and interventional studies. Am J Clin Nutr. 2014;99:813–33. [DOI] [PubMed] [Google Scholar]
- 66. Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC. Glycemic index, glycemic load, and chronic disease risk—a meta-analysis of observational studies. Am J Clin Nutr. 2008;87:627–37. [DOI] [PubMed] [Google Scholar]
- 67. Dahl WJ, Stewart ML. Position of the Academy of Nutrition and Dietetics: health implications of dietary fiber. J Acad Nutr Diet. 2015;115:1861–70. [DOI] [PubMed] [Google Scholar]
- 68. Clarys P, Deliens T, Huybrechts I, Deriemaeker P, Vanaelst B, De Keyzer W, Hebbelinck M, Mullie P. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients. 2014;6:1318–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sobiecki JG, Appleby PN, Bradbury KE, Key TJ. High compliance with dietary recommendations in a cohort of meat eaters, fish eaters, vegetarians, and vegans: results from the European Prospective Investigation into Cancer and Nutrition-Oxford study. Nutr Res. 2016;36:464–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rivellese AA, Lilli S. Quality of dietary fatty acids, insulin sensitivity and type 2 diabetes. Biomed Pharmacother. 2003;57:84–7. [DOI] [PubMed] [Google Scholar]
- 71. Riserus U, Willet W, Hu F. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009;48:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Slater SJ, Kelly MB, Yeager MD, Larkin J, Ho C, Stubbs CD. Polyunsaturation in cell membranes and lipid bilayers and its effects on membrane proteins. Lipids. 1996;31(Suppl):S189–92. [DOI] [PubMed] [Google Scholar]
- 73. Cascio G, Schiera G, Di Liegro I. Dietary fatty acids in metabolic syndrome, diabetes and cardiovascular diseases. Curr Diabetes Rev. 2012;8:2–17. [DOI] [PubMed] [Google Scholar]
- 74. Clarke SD. Polyunsaturated fatty acid regulation of gene transcription: a molecular mechanism to improve the metabolic syndrome. J Nutr. 2001;131:1129–32. [DOI] [PubMed] [Google Scholar]
- 75. Dentin R, Benhamed F, Pégorier J-P, Foufelle F, Viollet B, Vaulont S, Girard J, Postic C. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J Clin Invest. 2005;115:2843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Giordano E, Visioli F. Long-chain omega 3 fatty acids: molecular bases of potential antioxidant actions. Prostaglandins Leukot Essent Fat Acids. 2014;90:1–4. [DOI] [PubMed] [Google Scholar]
- 77. Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, Kraegen EW. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes. 1991;40:280–9. [DOI] [PubMed] [Google Scholar]
- 78. Saunders AV, Davis BC, Garg ML. Omega-3 polyunsaturated fatty acids and vegetarian diets. Med J Aust. 2013;199:S22–6. [DOI] [PubMed] [Google Scholar]
- 79. Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Moon JH, Lee JY, Kang SB, Park JS, Lee BW, Kang ES, Ahn CW, Lee HC, Cha BS. Dietary monounsaturated fatty acids but not saturated fatty acids preserve the insulin signaling pathway via IRS-1/PI3K in rat skeletal muscle. Lipids. 2010;45:1109–16. [DOI] [PubMed] [Google Scholar]
- 81. Rocca AS, LaGreca J, Kalitsky J, Brubaker PL. Monounsaturated fatty acid diets improve glycemic tolerance through increased secretion of glucagon-like peptide-1. Endocrinology. 2001;142:1148–55. [DOI] [PubMed] [Google Scholar]
- 82. Manna P, Kalita J. Beneficial role of vitamin K supplementation on insulin sensitivity, glucose metabolism, and the reduced risk of type 2 diabetes: a review. Nutrition. 2016;32:732–9. [DOI] [PubMed] [Google Scholar]
- 83. Ashor AW, Werner AD, Lara J, Willis ND, Mathers JC, Siervo M. Effects of vitamin C supplementation on glycaemic control: a systematic review and meta-analysis of randomised controlled trials. Eur J Clin Nutr. 2017;71:1371–80. [DOI] [PubMed] [Google Scholar]
- 84. Xu R, Zhang S, Tao A, Chen G, Zhang M. Influence of vitamin E supplementation on glycaemic control: a meta-analysis of randomised controlled trials. PLoS One. 2014;9:e95008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates β cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A. 2008;105:5266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY et al.. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Subratty AH, Aukburally N, Jowaheer V, Joonus N. Vitamin C and urea inhibit the formation of advanced glycation end products in vitro. Nutr Food Sci. 2010;40:456–65. [Google Scholar]
- 88. Ceriello A, Giugliano D, Quatraro A, Donzella C, Dipalo G, Lefebvre PJ. Vitamin E reduction of protein glycosylation in diabetes. New prospect for prevention of diabetic complications? Diabetes Care. 1991;14:68–72. [DOI] [PubMed] [Google Scholar]
- 89. Minamiyama Y, Takemura S, Bito Y, Shinkawa H, Tsukioka T, Nakahira A, Suehiro S, Okada S. Supplementation of α-tocopherol improves cardiovascular risk factors via the insulin signalling pathway and reduction of mitochondrial reactive oxygen species in type II diabetic rats. Free Radic Res. 2008;42:261–71. [DOI] [PubMed] [Google Scholar]
- 90. Jin L, Xue H-Y, Jin L-J, Li S-Y, Xu Y-P. Antioxidant and pancreas-protective effect of aucubin on rats with streptozotocin-induced diabetes. Eur J Pharmacol. 2008;582:162–7. [DOI] [PubMed] [Google Scholar]
- 91. Farmer B. Nutritional adequacy of plant-based diets for weight management: observations from the NHANES. Am J Clin Nutr. 2014;100(Suppl 1):365S–8S. [DOI] [PubMed] [Google Scholar]
- 92. Suárez A, Pulido N, Casla A, Casanova B, Arrieta FJ, Rovira A. Impaired tyrosine-kinase activity of muscle insulin receptors from hypomagnesaemic rats. Diabetologia. 1995;38:1262–70. [DOI] [PubMed] [Google Scholar]
- 93. Solaimani H, Soltani N, MaleKzadeh K, Sohrabipour S, Zhang N, Nasri S, Wang Q. Modulation of GLUT4 expression by oral administration of Mg(2+) to control sugar levels in STZ-induced diabetic rats. Can J Physiol Pharmacol. 2014;92:438–44. [DOI] [PubMed] [Google Scholar]
- 94. Rosique-Esteban N, Guasch-Ferré M, Hernández-Alonso P, Salas-Salvadó J. Dietary magnesium and cardiovascular disease: a review with emphasis in epidemiological studies. Nutrients. 2018;10:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Guasch-Ferré M, Merino J, Sun Q, Fitó M, Salas-Salvadó J. Dietary polyphenols, Mediterranean diet, prediabetes, and type 2 diabetes: a narrative review of the evidence. Oxid Med Cell Longev. 2017;2017:6723931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ahad A, Ganai AA, Mujeeb M, Siddiqui WA. Ellagic acid, an NF-κB inhibitor, ameliorates renal function in experimental diabetic nephropathy. Chem Biol Interact. 2014;219:64–75. [DOI] [PubMed] [Google Scholar]
- 97. Meng Y, Su A, Yuan S, Zhao H, Tan S, Hu C, Deng H, Guo Y. Evaluation of total flavonoids, myricetin, and quercetin from Hovenia dulcis Thunb. as inhibitors of α-amylase and α-glucosidase. Plant Foods Hum Nutr. 2016;71:444–9. [DOI] [PubMed] [Google Scholar]
- 98. Kang I, Buckner T, Shay NF, Gu L, Chung S. Improvements in metabolic health with consumption of ellagic acid and subsequent conversion into urolithins: evidence and mechanisms. Adv Nutr. 2016;7:961–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yoshimura Y, Nishii S, Zaima N, Moriyama T, Kawamura Y. Ellagic acid improves hepatic steatosis and serum lipid composition through reduction of serum resistin levels and transcriptional activation of hepatic ppara in obese, diabetic KK-Ay mice. Biochem Biophys Res Commun. 2013;434:486–91. [DOI] [PubMed] [Google Scholar]
- 100. Paluszczak J, Krajka-Kuźniak V, Baer-Dubowska W. The effect of dietary polyphenols on the epigenetic regulation of gene expression in MCF7 breast cancer cells. Toxicol Lett. 2010;192:119–25. [DOI] [PubMed] [Google Scholar]
- 101. Link A, Balaguer F, Goel A. Cancer chemoprevention by dietary polyphenols: promising role for epigenetics. Biochem Pharmacol. 2010;80:1771–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ni Mhurchu C, Capelin C, Dunford EK, Webster JL, Neal BC, Jebb SA. Sodium content of processed foods in the United Kingdom: analysis of 44,000 foods purchased by 21,000 households. Am J Clin Nutr. 2011;93:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Donovan DS, Solomon CG, Seely EW, Williams GH, Simonson DC. Effect of sodium intake on insulin sensitivity. Am J Physiol Metab. 1993;264:E730–4. [DOI] [PubMed] [Google Scholar]
- 104. Baudrand R, Campino C, Carvajal CA, Olivieri O, Guidi G, Faccini G, Vöhringer PA, Cerda J, Owen G, Kalergis AM et al.. High sodium intake is associated with increased glucocorticoid production, insulin resistance and metabolic syndrome. Clin Endocrinol (Oxf). 2014;80:677–84. [DOI] [PubMed] [Google Scholar]
- 105. Kim YM, Kim SH, Shim YS. Association of sodium intake with insulin resistance in Korean children and adolescents: the Korea National Health and Nutrition Examination Survey 2010. J Pediatr Endocrinol Metab. 2018;31:117–25. [DOI] [PubMed] [Google Scholar]
- 106. Ogihara T, Asano T, Ando K, Chiba Y, Sekine N, Sakoda H, Anai M, Onishi Y, Fujishiro M, Ono H et al.. Insulin resistance with enhanced insulin signaling in high-salt diet-fed rats. Diabetes. 2001;50:573–83. [DOI] [PubMed] [Google Scholar]
- 107. Ogihara T, Asano T, Ando K, Sakoda H, Anai M, Shojima N, Ono H, Onishi Y, Fujishiro M, Abe M et al.. High-salt diet enhances insulin signaling and induces insulin resistance in Dahl salt-sensitive rats. Hypertension. 2002;40:83–9. [DOI] [PubMed] [Google Scholar]
- 108. Premilovac D, Richards SM, Rattigan S, Keske MA. A vascular mechanism for high-sodium-induced insulin resistance in rats. Diabetologia. 2014;57:2586–95. [DOI] [PubMed] [Google Scholar]
- 109. Honikel K-O. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008;78:68–76. [DOI] [PubMed] [Google Scholar]
- 110. de la Monte SM, Neusner A, Chu J, Lawton M. Epidemilogical trends strongly suggest exposures as etiologic agents in the pathogenesis of sporadic Alzheimer's disease, diabetes mellitus, and non-alcoholic steatohepatitis. J Alzheimers Dis. 2009;17:519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tong M, Neusner A, Longato L, Lawton M, Wands JR, de la Monte SM. Nitrosamine exposure causes insulin resistance diseases: relevance to type 2 diabetes mellitus, non-alcoholic steatohepatitis, and Alzheimer's disease. J Alzheimers Dis. 2009;17:827–44. [PMC free article] [PubMed] [Google Scholar]
- 112. Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, Balzer J, Zotz RB, Scharf RE, Willers R et al.. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med. 2006;40:295–302. [DOI] [PubMed] [Google Scholar]
- 113. Pereira EC, Ferderbar S, Bertolami MC, Faludi AA, Monte O, Xavier HT, Pereira TV, Abdalla DSP. Biomarkers of oxidative stress and endothelial dysfunction in glucose intolerance and diabetes mellitus. Clin Biochem. 2008;41:1454–60. [DOI] [PubMed] [Google Scholar]