Abstract
BACKGROUND
Gamma Knife radiosurgery (GKRS; Elekta AB) remains a well-established treatment modality for vestibular schwannomas. Despite highly effective tumor control, further research is needed toward optimizing long-term functional outcomes. Whereas dose-rate effects may impact post-treatment toxicities given tissue dose-response relationships, potential effects remain largely unexplored.
OBJECTIVE
To evaluate treatment outcomes and potential dose-rate effects following definitive GKRS for vestibular schwannomas.
METHODS
We retrospectively reviewed 419 patients treated at our institution between 1998 and 2015, characterizing baseline demographics, pretreatment symptoms, and GKRS parameters. The cohort was divided into 2 dose-rate groups based on the median value (2.675 Gy/min). Outcomes included clinical tumor control, radiographic progression-free survival, serviceable hearing preservation, hearing loss, and facial nerve dysfunction (FND). Prognostic factors were assessed using Cox regression.
RESULTS
The study cohort included 227 patients with available follow-up. Following GKRS 2-yr and 4-yr clinical tumor control rates were 98% (95% CI: 95.6%-100%) and 96% (95% CI: 91.4%-99.6%), respectively. Among 177 patients with available radiographic follow-up, 2-yr and 4-yr radiographic progression-free survival rates were 97% (95% CI: 94.0%-100.0%) and 88% (95% CI: 81.2%-95.0%). The serviceable hearing preservation rate was 72.2% among patients with baseline Gardner-Robertson class I/II hearing and post-treatment audiological evaluations. Most patients experienced effective relief from prior headaches (94.7%), tinnitus (83.7%), balance issues (62.7%), FND (90.0%), and trigeminal nerve dysfunction (79.2%), but not hearing loss (1.0%). Whereas GKRS provided effective tumor control independently of dose rate, GKRS patients exposed to lower dose rates experienced significantly better freedom from post-treatment hearing loss and FND (P = .044).
CONCLUSION
Whereas GKRS provides excellent tumor control and effective symptomatic relief for vestibular schwannomas, dose-rate effects may impact post-treatment functional outcomes. Further research remains warranted.
Keywords: Vestibular schwannoma, Acoustic neuroma, Gamma Knife radiosurgery, Stereotactic radiosurgery, Neurosurgery, Radiation oncology, Dose rate
ABBREVIATIONS
- BED
biologically effective dose
- FND
facial nerve dysfunction
- FSRT
fractionated stereotactic radiotherapy
- GKRS
Gamma Knife radiosurgery
- HA
headache
- HL
hearing loss
- LINAC
linear accelerator
- PIV
prescription isodose volume
- SRS
stereotactic radiosurgery
- TTV
treatment target volume
- TND
trigeminal nerve dysfunction
Vestibular schwannomas, also known as acoustic neuromas, are benign Schwann cell tumors of the skull base most commonly arising from the vestibular division of the vestibulocochlear nerve. Vestibular schwannomas represent approximately 11% of nonmalignant central nervous system tumors diagnosed in the United States, with an estimated nationwide incidence of approximately 10 cases per million individuals per year.1 Whereas vestibular schwannomas are benign tumors, potential risks when left untreated include permanent functional deficits, including hearing loss, tinnitus, dizziness, facial pain, numbness or paresthesias, and facial paralysis, as well as hydrocephalus and potentially life-threatening brainstem compression. Accordingly, treatment goals for vestibular schwannomas include local control, symptomatic relief, and effective preservation of existing neurological function while minimizing risks of potential harm (including, but not limited to, postoperative complications, treatment toxicities, and secondary malignancies).
Whereas ongoing surveillance remains appropriate for smaller and minimally symptomatic vestibular schwannomas, approximately 15%-40% of patients will develop local progression without treatment, potentially worsening permanent symptom burden and limiting available therapeutic options.2-4 Whereas no prospective randomized trials have compared potential treatment approaches; both microsurgical resection and stereotactic radiation therapy are associated with excellent local control rates.2 Nationwide, although microsurgery remains the most commonly used approach for vestibular schwannomas, stereotactic radiotherapy has become increasingly popular during recent years as a generally well-tolerated and noninvasive alternative.5-8
Definitive treatment options for vestibular schwannomas involving stereotactic radiotherapy include Gamma Knife radiosurgery (GKRS; Elekta AB), proton beam therapy, and linear accelerator (LINAC)-based treatments using stereotactic radiosurgery (SRS) and fractionated stereotactic radiotherapy (FSRT). Whereas retrospective data suggest excellent tumor control rates across modalities,2,9,10 modern studies have not systematically compared radiotherapeutic treatment approaches beyond retrospective comparisons of LINAC-based SRS and FSRT. However, compared with surgical resection, stereotactic radiotherapy has been associated with both improved short-term hearing preservation rates9 and higher patient-reported post-treatment quality of life.11
The Gamma Knife represents a particularly well-established treatment modality for vestibular schwannomas, backed by almost 50 years of documented clinical experience.12-14 Compared with LINAC-based approaches, GKRS provides unparalleled treatment precision for intracranial tumors.15 During the modern era, GKRS has demonstrated exceptional tumor control rates toward definitive treatment of vestibular schwannomas ranging from approximately 87% to 98%.16-25 But whereas stereotactic radiotherapy maximizes likelihood of short-term hearing preservation compared with microsurgical resection, long-term hearing preservation rates remain lower than desired across treatment approaches, highlighting the importance of continued research toward optimizing current treatment paradigms.9,10
Notably, compared with other forms of external beam radiotherapy, GKRS (Elekta) operates using an alternative mechanism. Specifically, the Gamma Knife delivers radiation using spontaneously emitting radioactive cobalt-60 sources with a half-life of approximately 5.26 years, a measure that reflects the speed of radioactive decay (specifically describing the average time until decay of 50% of radioactive isotopes within given sources). Practically, because of this spontaneous decay, the actual rate of how quickly fixed radiation doses are delivered during GKRS varies across the lifespan of cobalt sources. In radiobiology, dose rate describes the rate of radiation dose delivery, defined as the amount of radiation absorbed by tissues per unit time. Theoretically, lower dose rates allow for more efficient repair of accumulated sublethal DNA damage within both tumors and surrounding normal tissues, which could potentially impact both tumor control and risks for late treatment toxicities. Because the Gamma Knife (Elekta) requires periodic replacement of cobalt-60 sources because of gradual radioactive decay, treatment dose rates during GKRS vary substantially depending on source age. Despite in vitro radiobiological evidence describing dose-rate effects, few studies have evaluated clinical implications of dose rate on treatment outcomes following GKRS. Interestingly, however, research suggests that dose-rate effects may impact both success and durability of pain relief following functional GKRS for trigeminal neuralgia using high prescription doses.26 Based on fundamental differences in dose-response relationships between early- and late-responding tissues,27 we hypothesized that dose-rate effects might impact local control and late toxicity rates following GKRS. For this reason, the purpose of this study was to evaluate both efficacy and potential dose-rate effects on clinical outcomes following definitive GKRS for vestibular schwannomas.
METHODS
Study Design
Experimental protocol and informed consent for this study were approved by our Institutional Review Board. This study was performed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for retrospective cohort studies. We retrospectively reviewed all patients from our medical center who underwent single-fraction radiosurgery using the Leksell Gamma Knife (Elekta) for unilateral vestibular schwannomas between April 1998 and April 2015. During this time, approximately 701 patients underwent definitive treatment for vestibular schwannomas, among whom, 419 (59.8%) received GKRS. Treatment recommendations were performed according to established institutional practices.28 Briefly, GKRS was recommended for smaller and minimally symptomatic tumors (most commonly, for tumors measuring <2.2 cm in maximal diameter14) depending on patient preference, whereas microsurgical resection was recommended for patients presenting with debilitating pretreatment symptoms, larger tumors (typically, maximal diameter >3 cm), or mass effect on surrounding structures. Patients were treated using the Gamma Knife Model B (Elekta) before April 2011 and the Gamma Knife Perfexion system (Elekta) beginning in April 2011. On treatment day, patients underwent application of a Leksell G stereotactic head frame after administration of local anesthetic for immobilization under conscious sedation. Pretreatment volumetric magnetic resonance imaging sequences included 1-mm, axial, T1-weighted, contrast-enhanced images, 1- to 1.5-mm, axial, T2-weighted volume images, and 3-mm, T2, whole-head imaging. Median prescribed SRS dose was 12.0 Gy (range: 11.0-16.8 Gy) to the 50% isodose line. The mean cochlear dose was 4 Gy. GKRS prescription doses above 12 Gy were used only for patients without pretreatment serviceable hearing according to institutional practice. Post-treatment follow-up assessments were performed at approximately 3 to 6 months intervals.
We collected baseline patient demographics (including age, gender, laterality, and pretreatment tumor size according to both maximum diameter and tumor volume; pretreatment tumor grade based on the Samii Classification system,29 and pretreatment symptoms including hearing loss, tinnitus, dizziness, vertigo or disequilibrium, lateralized headache, facial nerve dysfunction [FND], and trigeminal nerve dysfunction [TND]) and dosimetric characteristics (including prescription isodose, maximum, minimum, and mean target doses, prescription isodose volume [PIV], treatment target volume [TTV]), RTOG conformity, selectivity, and homogeneity indices (conformity index, selectivity index, and homogeneity index, respectively)30 and energy index, a proposed measure for target dose homogeneity independent of tumor volume and prescription dose.31 Exclusion criteria included prior surgical resection (60 patients) and lack of available post-treatment follow-up information (118 patients). Given our interest in dose-rate effects, we identified 227 patients who were treated using a consistent GKRS prescription dose of 12 Gy to minimize potential confounding impacts on treatment outcomes. For evaluating clinical dose-rate effects, we divided the study cohort into 2 groups based on the median value for treatment dose rate, 2.675 Gy/min (range: 1.35-3.73 Gy/min). Pretreatment audiometric data were available for 157 patients (69%). Radiographic follow-up was available for 177 patients (78%).
Statistical Analyses
Patient characteristics, treatment parameters, and post-treatment symptomatic outcomes were characterized using descriptive statistics. Clinical and radiographic follow-up durations were determined using the reverse Kaplan-Meier method.32 Radiographic progression was defined as persistently increased maximal tumor diameter by at least 2 mm at 3 years following completion of GKRS. Outcomes examined included clinical tumor control (defined based on freedom from salvage therapy following definitive GKRS), radiographic tumor control (defined based on time from GKRS until development of either asymptomatic or symptomatic radiographic progression), serviceable hearing preservation (defined as maintenance of Gardner-Robertson class I or II hearing for patients with available post-treatment audiometric information), symptomatic hearing preservation (defined based on freedom from patient-reported new or worsened hearing loss), and facial nerve preservation (defined based on freedom from either new or worsened FND). Additional symptomatic outcomes analyzed included lateralized headache, tinnitus, vertigo/dizziness/disequilibrium, TND, and secondary malignancies. Tumor control rates were determined using the Kaplan-Meier product limit method.32 Survival curves were also generated using the Kaplan-Meier method,32 evaluating survival differences between groups using Mantel-Cox log-rank tests. Univariable and multivariable logistic regression and Cox proportional hazards models were used to characterize post-treatment outcomes. Univariable regressions were performed with a threshold P value < .2 to identify potential covariates for multivariable analyses, which were performed using forward conditional modeling. P values < .05 were considered statistically significant without adjustment for multiple comparisons. Data analysis was performed using SPSS Statistics, Version 24 (IBM Corp., Armonk, New York).
RESULTS
Participants and Descriptive Data
Among 227 patients within the study cohort (Table 1), median pretreatment maximal tumor diameter was 1.3 cm (interquartile range (IQR): 0.90-1.70 cm). Before GKRS (Elekta AB), most patients were symptomatic from local tumor burden with most common pretreatment symptoms including hearing loss (90.7%), tinnitus (56.4%), and vertigo, dizziness, or disequilibrium (61.2%). Among 157 patients with available pretreatment audiological evaluations, 68.2% presented with baseline serviceable hearing defined as Gardner-Robertson class I or II functional status (Table 1). All patients underwent GKRS using a prescription dose of 12 Gy most commonly delivered to the 50% isodose line (Table 2). Median durations of clinical and radiographic follow-up were approximately 29.8 months (95% CI: 25.6-34.0 mo) and 29.4 mo (95% CI: 21.6-37.1 mo), respectively.
TABLE 1.
Patient Characteristics
| Baseline characteristics (227 patients) | Value (%) | Range (IQR) |
|---|---|---|
| Age (yr), median | 60 | 27-91 (52-69) |
| Gender | ||
| Male | 111 (48.9%) | |
| Female | 116 (51.1%) | |
| Laterality | ||
| Left | 116 (51.1%) | |
| Right | 111 (48.9%) | |
| Symptomatic presentation | ||
| Hearing loss | 206 (90.7%) | — |
| Tinnitus | 128 (56.4%) | — |
| Dizziness, vertigo, or disequilibrium | 139 (61.2%) | — |
| Facial nerve (CN VII) dysfunction | 9 (4.0%) | — |
| Trigeminal nerve (CN V) dysfunction | 25 (11%) | — |
| Lateralized headache | 21 (9.3%) | — |
| Tumor size | ||
| Volume (cm3), median | 0.43 | 0.01-9.00 (0.16-1.30) |
| Maximal diameter (cm), median | 1.30 | 0.12-3.60 (0.90-1.70) |
| <1 cm | 56 (26.4%) | |
| 1-2 cm | 139 (65.6%) | |
| >2 cm | 17 (8.0%) | |
| Tumor stage (Samii Classification*) | ||
| T1 | 84 (37.7%) | |
| T2 | 41 (18.4%) | |
| T3a | 44 (19.7%) | |
| T3b | 14 (6.3%) | |
| T4a | 39 (17.5%) | |
| T4b | 1 (0.4%) | |
| Pre-GKRS audiometry (n = 157) | ||
| Gardner-Robertson class | ||
| I | 81 (51.6%) | — |
| II | 26 (16.6%) | — |
| III, IV, or V | 50 (31.8%) | — |
| Speech discrimination, %, mean (SD) | 68.5% (34.8) | 0%-100% [44-96] |
| PTA, mean (SD) | 37.4 dB (22.4) | 2-115 dB [20-52] |
| SRT, mean (SD) | 33.0 dB (22.8) | 0-105 dB [15-50] |
*T1: intracanalicular; T2: extending beyond IAC; T3a: occupying cerebellopontine angle; T3b: occupying CPA; contacting brainstem without compression; T4a: brainstem compression; T4b: severe brainstem displacement and/or deformation of fourth ventricle.
TABLE 2.
GKRS Treatment Parameters
| GKRS treatment parameters | Value ± SD | Range (IQR) |
|---|---|---|
| Prescribed dose (Gy) | 12 | N/A |
| Prescription isodose (%), median | 50 | 40-90 (50-50) |
| Mean target dose (Gy) | 17.4 ± 4.3 | 12.9-70.2 (16.1-18.2) |
| Maximum target dose (Gy) | 23.5 ± 6.4 | 13.3-100.6 (24.0-24.4) |
| Minimum target dose (Gy) | 9.4 ± 3.0 | 2.7-39.8 (8.3-10.5) |
| Prescription isodose volume/PIV (cm3), median | 0.68 | 0.01-9.20 (0.31-1.95) |
| Treated target volume/TTV (cm3), median | 0.39 | 0.01-5.90 (0.15-1.19) |
| Dose rate (Gy/min), median | 2.675 | 1.35-3.73 (2.05-3.13) |
| Energy index, median | 1.42 | 0-2 (1.32-1.52) |
| Homogeneity index, median | 0.48 | 0.15-2.24 (0.48-0.49) |
| Conformity index, median | 1.88 | 0.94-4.48 (1.43-2.26) |
| Selectivity index, median | 0.54 | 0.22-3.43 (0.44-0.69) |
Outcomes
Following GKRS, 2-yr and 4-yr rates of clinical tumor control were approximately 98% (95% CI: 95.6%-100%) and 96% (95% CI: 91.4%-99.6%), respectively (Figure, Supplemental Digital Content 1). Patients benefited from a mean duration of freedom from salvage therapy lasting approximately 12.9 years (95% CI: 12.4-13.3 years). Radiographic progression-free survival rates were approximately 97% (95% CI: 94.0%-100.0%) at 2 years and 88% (95% CI: 81.2%-95.0%) at 4 years, respectively (Figure, Supplemental Digital Content 2) with a mean radiographic progression-free survival of 10.5 years (95% CI: 9.3-11.6 years). Among patients who initially presented with pretreatment serviceable hearing, the serviceable hearing preservation rate was 72.2%. Univariable and multivariable Cox regression analyses identified no factors that were significantly associated with clinical tumor control (Table 3), radiographic progression-free survival (Table 4) or serviceable hearing preservation (Table, Supplemental Digital Content 3). Two patients (0.9%) were diagnosed with secondary malignancies after a median duration of 4.4 years, although we identified no factors that were significantly associated with development of secondary malignancies (Table, Supplemental Digital Content 4).
TABLE 3.
Univariable and Multivariable Cox Regression for Clinical Progression-Free Survival Following Definitive GKRS for Vestibular Schwannomas
| Univariable regression | Multivariable regression | |||
|---|---|---|---|---|
| Covariate | HR (95% CI) | P value | HR (95% CI) | P value |
| Age | 1.024 (0.946-1.109) | .560 | — | |
| Gender | ||||
| Male | Reference | — | — | |
| Female | 4.596 (0.513-41.185) | .173 | — | .134 (NS) |
| Laterality | ||||
| Left | Reference | — | — | |
| Right | 0.558 (0.093-3.346) | .523 | — | |
| Year of diagnosis | 0.940 (0.741-1.192) | .608 | ||
| Pretreatment serviceable hearing | ||||
| Yes (Gardner-Robertson I or II) | Reference | — | — | |
| No (Gardner-Robertson III, IV, or V) | 2.139 (0.133-34.280) | .591 | — | |
| Pretreatment hearing loss | ||||
| Yes | 0.437 (0.049-3.908) | .459 | — | |
| No | Reference | — | ||
| Pretreatment CN VII dysfunction | ||||
| Yes | 0.045 (0.000-6.7 × 106) | .713 | — | |
| No | Reference | — | ||
| Pretreatment CN V dysfunction | ||||
| Yes | 0.042 (0.000-9.4 × 103) | .613 | — | |
| No | Reference | — | ||
| Tumor size, max. diameter | 2.065 (0.414-10.307) | .377 | — | |
| Tumor size, volume | 0.805 (0.210-3.091) | .752 | — | |
| Tumor grade (Samii classification) | 1.110 (0.626-1.970) | .721 | — | |
| Dose rate, median | ||||
| <2.675 Gy/min | Reference | — | ||
| ≥2.675 Gy/min | 2.522 (0.413-15.394) | .316 | — | |
| Dose rate, continuous | 3.634 (0.782-16.886) | .100 | — | .084 (NS) |
| Prescription isodose | 0.923 (0.772-1.104) | .381 | — | |
| Mean target dose (Gy) | 1.046 (0.906-1.208) | .539 | — | |
| Minimum target dose (Gy) | 1.073 (0.830-1.387) | .591 | — | |
| Maximum target dose (Gy) | 1.033 (0.941-1.134) | .491 | — | |
| RTOG conformity index | 1.219 (0.215-6.897) | .823 | — | |
| Paddick conformity index | 0.667 (0.000-2.4 × 103) | .925 | — | |
| Selectivity index | 0.627 (0.000-1.3 × 103) | .905 | — | |
| Energy index | 26.245 (0.112-6.1 × 103) | .240 | — | |
| RTOG homogeneity index | 4.867 (0.147-161.6) | .376 | — | |
TABLE 4.
Univariable and Multivariable Cox Regression for Radiographic Progression-Free Survival Following Definitive GKRS for Vestibular Schwannomas
| Covariate | Univariable regression | Multivariable regression | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.010 (0.970-1.052) | .634 | N/A | |
| Gender | ||||
| Male | Reference | — | N/A | |
| Female | 1.478 (0.593-3.682) | .401 | N/A | |
| Laterality | ||||
| Left | Reference | — | N/A | |
| Right | 1.036 (0.415-2.583) | .940 | N/A | |
| Year of diagnosis | 1.009 (0.885-1.150) | .896 | N/A | |
| Pretreatment serviceable hearing | ||||
| Yes (Gardner-Robertson I or II) | Reference | — | N/A | |
| No (Gardner-Robertson III, IV, or V) | 1.003 (0.311-3.234) | .997 | N/A | |
| Pretreatment hearing loss | ||||
| Yes | 1.026 (0.231-4.563) | .973 | N/A | |
| No | Reference | |||
| Pretreatment CN VII dysfunction | ||||
| Yes | 0.044 (0.000-97.313) | .426 | N/A | |
| No | Reference | |||
| Pretreatment CN V dysfunction | ||||
| Yes | 1.266 (0.289-5.548) | .754 | N/A | |
| No | Reference | |||
| Tumor size, max. diameter | 0.623 (0.253-1.533) | .303 | N/A | |
| Tumor size, volume | 0.790 (0.381-1.635) | .525 | N/A | |
| Tumor grade (Samii classification) | 1.069 (0.801-1.427) | .651 | N/A | |
| Dose rate, median | ||||
| < 2.675 Gy/min | Reference | |||
| ≥ 2.675 Gy/min | 1.300 (0.518-3.265) | .576 | N/A | |
| Dose rate, continuous | 1.424 (0.716-2.834) | .314 | N/A | |
| Prescription isodose | 0.998 (0.953-1.045) | .926 | N/A | |
| Mean target dose (Gy) | 1.001 (0.912-1.097) | .991 | N/A | |
| Minimum target dose (Gy) | 0.950 (0.762-1.184) | .646 | N/A | |
| Maximum target dose (Gy) | 0.991 (0.913-1.076) | .838 | N/A | |
| RTOG conformity index | 0.908 (0.277-2.983) | .874 | N/A | |
| Paddick conformity index | 3.279 (0.023-469.685) | .639 | N/A | |
| Selectivity index | 3.416 (0.031-377.991) | .609 | N/A | |
| Energy index | 9.072 (0.317-259.60) | .198 | N/A | |
| RTOG homogeneity index | 0.748 (0.036-15.373) | .850 | N/A | |
Patient-reported functional outcomes are provided in Table 5. Encouragingly, after GKRS, most patients experienced effective symptomatic relief from many pretreatment symptoms including lateralized headache (94.7%), tinnitus (83.7%), balance problems (62.7%), FND (90.0%), and TND (79.2%), but not existing hearing loss (1.0%). Overall, 23.8% of patients with functionally intact pretreatment hearing developed new persistent hearing loss, and 24.2% of patients with symptomatic pretreatment hearing loss experienced symptomatic worsening.
TABLE 5.
Symptomatic Outcomes Following Definitive GKRS for Vestibular Schwannomas
| Post-treatment Symptoms | % (Frequency): Transient symptoms | % (Frequency): Persistent symptoms | % (Frequency): Total symptoms (Persistent or transient) |
|---|---|---|---|
| Hearing loss (HL) | |||
| New-onset HL | 14.3% (3/21) | 23.8% (5/21) | 38.1% (8/21) |
| Worsening of prior HL | 3.9% (8/206) | 50/206 (24.2%) | 28.2% (58/206) |
| Improvement of prior HL | — | — | 1.0% (2/206) |
| FND | |||
| New-onset FND | 4.6% (10/217) | 4.1% (9/217) | 8.8% (19/217) |
| Worsening of prior FND | 0.0% (0/10) | 0.0% (0/10) | 0.0% (0/10) |
| Improvement of prior FND | — | — | 90% (9/10) |
| TND | |||
| New-onset TND | 2.5% (5/203) | 4.4% (9/203) | 6.9% (14/203) |
| Worsening of prior TND | (0.0%) 0/24 | 4.2% (1/24) | 4.2% (1/24) |
| Improvement of prior TND | — | — | 79.2% (19/24) |
| Vertigo/dizziness/disequilibrium (V/D/D) | |||
| New-onset V/D/D | 10.8% (10/93) | 16.1% (15/93) | 26.9% (25/93) |
| Worsening of prior V/D/D | 6.0% (8/134) | 6.0% (8/134) | 11.9% (16/134) |
| Improvement of prior V/D/D | — | — | 62.7% (84/134) |
| Tinnitus | |||
| New-onset tinnitus | 1.9% (2/104) | 5.8% (6/104) | 7.7% (8/104) |
| Worsening of prior tinnitus | 0.8% (1/123) | 5.7% (7/123) | 6.5% (8/123) |
| Improvement of prior tinnitus | — | — | 83.7% (103/123) |
| Lateralized headache (HA) | |||
| New-onset HA | 1.0% (2/208) | 2.4% (5/208) | 7/208 (3.4%) |
| Worsening of prior HA | 0.0% (0/19) | 0.0% (0/19) | 0/19 (0.0%) |
| Improvement of prior HA | — | — | 18/19 (94.7%) |
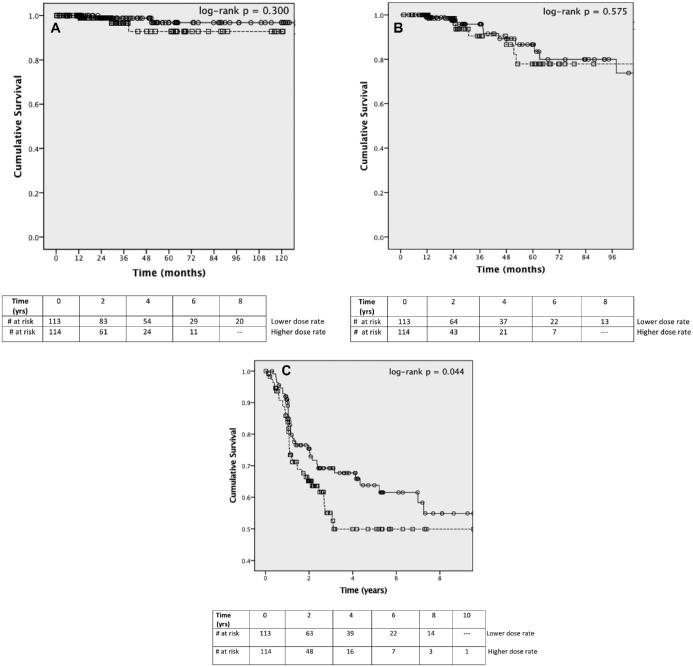
Given our interest in potential dose-rate effects, we specifically evaluated whether dose rate was associated with treatment outcomes following GKRS. Overall, both clinical tumor control (Figure 1A) and radiographic tumor control (Figure 1B) rates were similar between the lower and higher dose-rate groups (log-rank P = .300 and log-rank P = .575, respectively). Dose rate was also not significantly associated with either progressive hearing loss or progressive FND when evaluated as separate functional outcomes (Table, Supplemental Digital Content 5 and Table, Supplemental Digital Content 6, respectively). Interestingly, however, GKRS patients exposed to lower treatment dose rates (< 2.675 Gy/min) experienced significantly better post-treatment survival free from both progressive symptomatic hearing loss and FND (P = .044) (Figure 1C). Multivariable Cox regression confirmed that GKRS exposure to treatment dose rates above 2.675 Gy/min was associated with increased risk of developing progressive post-treatment hearing loss, FND, or both (HR: 2.248, 95% CI: 1.082-4.672; P = .030) (Table 6), whereas larger pretreatment maximal tumor diameter appeared protective (HR: 0.324, 0.155-0.887, P = .003).
FIGURE 1.
Impact of treatment dose rate on post-treatment outcomes for vestibular schwannomas following definitive GKRS (Elekta AB). Solid line = lower dose rate, < 2.675 Gy/min; dashed line = higher dose rate, ≥ 2.675 Gy/min. A, Clinical tumor control. B, Radiographic progression-free survival. C, Post-treatment freedom from progressive hearing loss and FND.
TABLE 6.
Univariable and Multivariable Cox Regression for Post-treatment Freedom from Progressive Symptomatic Hearing Loss and FND following GKRS
| Univariable regression | Multivariable regression | |||
|---|---|---|---|---|
| Covariate | HR (95% CI) | P value | HR (95% CI) | P value |
| Age | 0.995 (0.977-1.014) | .613 | — | |
| Gender | ||||
| Male | Reference | — | — | |
| Female | 1.085 (0.696-1.691) | .718 | — | |
| Laterality | ||||
| Left | Reference | — | — | |
| Right | 0.820 (0.527-1.278) | .381 | — | |
| Year of diagnosis | 1.027 (0.974-1.084) | .325 | ||
| Pretreatment serviceable hearing | ||||
| Yes (Gardner-Robertson I or II) | 2.187 (1.098-4.356) | .026* | — | |
| No (Gardner-Robertson III, IV, or V) | Reference | — | — | .247 (NS) |
| Pretreatment hearing loss | ||||
| Yes | 0.805 (0.402-1.613) | .541 | — | |
| No | Reference | — | ||
| Pretreatment CN VII dysfunction | ||||
| Yes | 0.228 (0.032-1.641) | .142 | — | .412 (NS) |
| No | Reference | — | ||
| Pretreatment CN V dysfunction | ||||
| Yes | 0.893 (0.429-1.857) | .761 | — | |
| No | Reference | — | ||
| Tumor size, max. diameter | 0.407 (0.254-0.651) | .000* | 0.324 (0.155-0.677) | .003 |
| Tumor size, volume | 0.633 (0.404-0.994) | .047* | — | .889 (NS) |
| Tumor grade (Samii classification) | 0.753 (0.630-0.898) | .002* | — | .242 (NS) |
| Post-treatment tumor size | ||||
| Decreased | Reference | — | — | .554 (NS) |
| Stable | 2.126 (1.084-4.169) | .028* | — | |
| Increased | 2.619 (1.250-5.487) | .011* | — | |
| Dose rate, median | ||||
| <2.675 Gy/min | Reference | Reference | ||
| ≥2.675 Gy/min | 1.581 (1.008-2.479) | .046* | 2.248 (1.082-4.672) | .030 |
| Dose rate, continuous | 1.145 (0.820-1.600) | .427 | — | |
| Prescription isodose | 0.997 (0.976-1.019) | .800 | — | |
| Mean target dose (Gy) | 1.003 (0.961-1.047) | .881 | — | |
| Minimum target dose (Gy) | 0.953 (0.862-1.053) | .341 | — | |
| Maximum target dose (Gy) | 0.998 (0.966-1.031) | .905 | — | |
| RTOG conformity index | 1.292 (0.795-2.101) | .302 | — | |
| Paddick conformity index | 0.310 (0.034-2.866) | .302 | — | |
| Selectivity index | 0.334 (0.041-2.706) | .304 | — | |
| Energy index | 1.902 (0.540-6.697) | .317 | — | |
| RTOG homogeneity index | 1.004 (0.300-3.353) | .995 | — | |
DISCUSSION
Key Results
Consistent with previous large cohort studies from the modern era,16-25 we found that single-fraction GKRS (Elekta) provided excellent rates of clinical and radiographic tumor control as a definitive treatment modality for vestibular schwannomas. Alongside existing data from other large retrospective studies, these findings provide further support for definitive GKRS as a noninvasive treatment option for smaller vestibular schwannomas, providing durable tumor control with good functional outcomes. Encouragingly, whereas microsurgical resection was initially recommended for patients who presented with debilitating pretreatment symptoms, we found that most patients who underwent GKRS for mildly to moderately symptomatic vestibular schwannomas experienced effective symptomatic relief from prior tinnitus (83.7%), vertigo, dizziness and disequilibrium (62.7%), FND (90.0%), TND (79.2%), and lateralized headache (94.7%) following GKRS, but not existing hearing loss (1.0%). Despite limited availability of post-treatment audiological assessments, GKRS was also associated with a 72.2% serviceable hearing preservation rate within this cohort, consistent with prior estimates ranging from 34% to 86% across varying durations of follow-up,18,21-23,25,33-49 Interestingly, we also observed that vestibular schwannoma patients exposed to lower GKRS treatment dose rates (below 2.675 Gy/min) experienced significantly improved freedom from progressive symptomatic hearing loss and FND following treatment. Collectively, whereas GKRS provided excellent rates of both clinical and radiographic tumor control without evidence of potential dose-rate effects, our results suggest that dose-rate effects might impact cumulative risks for post-treatment toxicities following single-fraction GKRS.
Interpretation
According to classic radiobiology, dose-rate effects play an important role in determining the resulting biologic effects of a given absorbed dose of radiation. Lower dose-rate exposure might result in lower biologically effective doses (BEDs) within both tumor and surrounding normal tissues, which could potentially impact both tumor control and post-treatment toxicities. Whereas dose-rate effects are well-documented in vitro, potential dose-rate effects are much more difficult to evaluate systematically in clinical practice. Practically, evaluating potential effects of treatment dose rate can be difficult given that patients undergoing GKRS in the setting of malignant tumors often have limited life expectancies, complicating routine assessment of late toxicities. Patients receiving GKRS for malignant tumors are also often receiving concurrent systemic treatments (including hormonal, chemotherapeutic, and immunotherapeutic agents), which also modulate local tissue responses and thereby complicate evaluation of radiation-induced treatment toxicities. For these reasons, vestibular schwannomas and other benign intracranial tumors represent an especially appropriate and relevant clinical context for evaluation of potential impacts of dose-rate effects on long-term functional outcomes following GKRS. Whereas GKRS remains an extremely precise treatment modality, providing highly selective dose distributions while minimizing incident radiation exposure within surrounding normal tissue, late toxicities remain an important concern.
Current evidence suggests that dose-rate effects may influence treatment outcomes following brachytherapy for genitourinary and gynecologic tumors.50-54 However, potential dose-rate effects on treatment outcomes following GKRS remain largely uncharacterized. Several studies have evaluated potential impacts of dose-rate effects on treatment outcomes following high-dose functional GKRS for trigeminal neuralgia,26,55,56 with one study suggesting that higher dose-rate exposure might be associated with reduced short-term post-treatment pain intensity and reduced risk of recurrent pain. Whereas potential dose-rate effects on post-treatment toxicities following GKRS remain unknown, anecdotal reports suggest that treatment toxicities may be more common after radioactive source changes. Accordingly, dosimetric analyses for functional GKRS have demonstrated that predicted biologically effective doses for a given prescription dose vary widely across the lifespan of radioactive cobalt-60 sources.57
Theoretically, lower dose rates might reduce late toxicity rates through better preservation of surrounding normal tissues by allowing for more effective sublethal DNA damage repair during treatment. However, lower dose rates could also potentially compromise tumor control by facilitating increased repair of sublethal radiation-induced tumor damage within tumor tissue. Classically, dose-rate effects are considered most relevant within the approximate range of 0.1 Gy/hr to 10 Gy/min.27 Whereas relevance of the traditional linear-quadratic model for SRS remains controversial,58,59 late-responding tissues might theoretically derive most benefit from fractionation of treatment regimens toward reducing late treatment toxicities.60
Excitingly, the Gamma Knife Icon (Elekta AB, Stockholm, Sweden) offers new possibilities for alternative treatment paradigms using fractionated GKRS by facilitating highly reproducible fractionated SRS through a combination of frameless thermoplastic mask-based immobilization, integrated cone-beam computed tomography imaging, and real-time high-definition motion management monitoring. Interestingly, retrospective data have suggested that fractionated SRS may be associated with improved functional hearing preservation rates compared with single-fraction GKRS.61 Prior dosimetric analyses evaluating hypofractionated SRS have also demonstrated exceptional treatment precision with the Gamma Knife (Elekta) compared with other modalities.15 Encouragingly, preliminary data evaluating fractionated GKRS on the Gamma Knife Perfexion (Elekta) using a relocatable immobilization system appears promising.62 Overall, fractionated GKRS represents a promising new approach for benign intracranial tumors toward potentially minimizing risks of late toxicities by combining the exceptional treatment precision of GKRS with the theoretical radiobiological advantages of treatment fractionation.
Limitations
Although these findings are interesting, important limitations should be considered given our retrospective study design, including, but not limited to, inherent selection biases associated with nonrandomized treatment assignments and attrition biases reflecting differential losses of patients to follow-up over time. Additional limitations include the lack of post-treatment audiometric reports for most patients within this cohort (limiting statistical power for detection of predictive factors for serviceable hearing preservation) and shorter than desired clinical and radiographic follow-up durations. We also acknowledge the fact that we did not control for potential effects of Gamma Knife model on study outcomes as an important limitation, given our institutional practice change during the study period. Regarding statistical analyses, we also did not perform adjustment for multiple comparisons, which also represents a noteworthy limitation given increased chance for potential false positives. Although intriguing that GKRS dose rate correlated with post-treatment freedom from progressive symptomatic toxicities, it is important to note that calibrated dose rates may not reflect actual in vivo dose rates within target volumes and surrounding tissues. Even within individual treatment plans, absorbed dose rates may vary tremendously given variations in 3-dimensional treatment volumes and patient anatomy. Moreover, even given a constant prescription dose and calibration dose rate, varying treatment times might still impact dose rate effects.63,64 Finally, it is important to note that GKRS treatment dose rate was not significantly associated with post-treatment freedom from progressive symptomatic hearing loss and progressive FND when analyzed as a continuous variable. Given that fact, along with the fact that rates of hearing loss and FND were not significantly associated with dose-rate group when analyzed as separate variables, effect sizes may be fairly small. For all of these reasons, further research remains needed toward clarifying potential impacts of dose-rate effect on both individual and cumulative post-treatment toxicity rates following GKRS.
CONCLUSION
Despite these limitations, our findings provide further support for the efficacy of GKRS (Elekta AB) as a definitive treatment modality for vestibular schwannomas, whereas highlighting important questions in the field of radiosurgery regarding potential toxicities. Indeed, whereas single-fraction GKRS provides excellent local control rates for vestibular schwannomas and remains a longstanding and well-validated treatment approach, our findings highlight the importance of further research concerning potential dose-rate effects on long-term functional outcomes after GKRS. Indeed, whereas single-fraction GKRS remains an excellent treatment option for vestibular schwannomas and other benign intracranial tumors, future research combined with technologic advances such as the Gamma Knife Icon (Elekta) will be essential toward further optimization of long-term functional outcomes.
Disclosures
Dr Wang reports personal fees and non-financial support from AbbVie, personal fees from AstraZeneca, personal fees and non-financial support from Elekta, personal fees and non-financial support from Merck, personal fees from Doximity, personal fees from Wolters Kluwer, personal fees and non-financial support from Novocure, personal fees and non-financial support from RTOG Foundation, personal fees from Cancer Panels, outside the submitted work. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Supplemental Digital Content 1. Figure. Clinical tumor control for vestibular schwannomas after definitive GKRS.
Supplemental Digital Content 2. Figure. Radiographic progression-free survival for vestibular schwannomas after definitive GKRS.
Supplemental Digital Content 3. Table. Univariable and multivariable regression examining Gardner-Robertson serviceable hearing preservation after definitive GKRS.
Supplemental Digital Content 4. Table. Univariable and multivariable regression examining post-treatment secondary malignancies after definitive GKRS.
Supplemental Digital Content 5. Table. Univariable and multivariable Cox regression for post-treatment freedom from progressive symptomatic hearing loss following GKRS.
Supplemental Digital Content 6. Table. Univariable and multivariable Cox regression for post-treatment freedom from progressive FND following GKRS.
REFERENCES
- 1. Ostrom QT, Gittleman H, Xu J et al.. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Int J Radiat Oncol Biol Phys. 2016;18(May):1265-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murphy ES, Suh JH. Radiotherapy for vestibular schwannomas: a critical review. Int J Radiat Oncol Biol Phys. 2011;79(4):985-997. [DOI] [PubMed] [Google Scholar]
- 3. Bakkouri WEl, Kania RE, Guichard J-P, Lot G, Herman P, Huy PTB. Conservative management of 386 cases of unilateral vestibular schwannoma: tumor growth and consequences for treatment. J Neurosurg. 2009;110(4):662-669. [DOI] [PubMed] [Google Scholar]
- 4. Smouha EE, Yoo M, Mohr K, Davis RP. Conservative management of acoustic neuroma: a meta-analysis and proposed treatment algorithm. Laryngoscope. 2005;115(3):450-454. [DOI] [PubMed] [Google Scholar]
- 5. Babu R, Sharma R, Bagley JH, Hatef J, Friedman AH, Adamson C. Vestibular schwannomas in the modern era: epidemiology, treatment trends, and disparities in management. J Neurosurg. 2013;119(1):121-130. [DOI] [PubMed] [Google Scholar]
- 6. Patel J, Vasan R, van Loveren H, Downes K, Agazzi S. The changing face of acoustic neuroma management in the USA: analysis of the 1998 and 2008 patient surveys from the acoustic neuroma association. Br J Neurosurg. 2014;28(1):20-24. [DOI] [PubMed] [Google Scholar]
- 7. Gal TJ, Shinn J, Huang B. Current epidemiology and management trends in acoustic neuroma. Otolaryngol Head Neck Surg. 2010;142(5):677-681. [DOI] [PubMed] [Google Scholar]
- 8. Carlson ML, Marston AP, Glasgow AE et al.. Racial differences in vestibular schwannoma. Laryngoscope. 2016;126(9):2128-2133. [DOI] [PubMed] [Google Scholar]
- 9. Carlson ML, Vivas EX, McCracken DJ et al.. Congress of neurological surgeons systematic review and evidence-based guidelines on hearing preservation outcomes in patients with sporadic vestibular schwannomas. Neurosurgery. 2018;82(2):E35-E39. [DOI] [PubMed] [Google Scholar]
- 10. Germano IM, Sheehan J, Parish J et al.. Congress of neurological surgeons systematic review and evidence-based guideline on the role of radiosurgery and radiation therapy in the management of patients with vestibular schwannomas. Neurosurgery. 2018;79(5):E627-E629. [Google Scholar]
- 11. Carlson ML, Oystein VT, Driscoll CL et al.. Long-term quality of life in patients with vestibular schwannoma: an international multicenter cross-sectional study comparing microsurgery, stereotactic radiosurgery, observation and nontumor controls. J Neurosurg. 2015;2(April):1-10. [DOI] [PubMed] [Google Scholar]
- 12. Leksell L. A note on the treatment of acoustic tumours. Acta Chir Scand. 1971;137(8):763-765. [PubMed] [Google Scholar]
- 13. Flickinger JC, Dade Lunsford L, Linskey ME, Duma CM, Kondziolka D. Gamma knife radiosurgery for acoustic tumors: multivariate analysis of four year results. Radiother Oncol. 1993;27(2):91-98. [DOI] [PubMed] [Google Scholar]
- 14. Kondziolka D, Lunsford LD, McLaughlin MR, Flickinger JC. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med. 1998;339(20):1426-1433. [DOI] [PubMed] [Google Scholar]
- 15. Dong P, Pérez-Andújar A, Pinnaduwage D et al.. Dosimetric characterization of hypofractionated Gamma Knife radiosurgery of large or complex brain tumors versus linear accelerator–based treatments. J Neurosurg. 2016;125(Suppl 1):97-103. [DOI] [PubMed] [Google Scholar]
- 16. Hasegawa T, Kida Y, Kato T, Iizuka H, Kuramitsu S, Yamamoto T. Long-term safety and efficacy of stereotactic radiosurgery for vestibular schwannomas: evaluation of 440 patients more than 10 years after treatment with Gamma Knife surgery. J Neurosurg. 2013;118(3):557-565. [DOI] [PubMed] [Google Scholar]
- 17. Boari N, Bailo M, Gagliardi F et al.. Gamma Knife radiosurgery for vestibular schwannoma: clinical results at long-term follow-up in a series of 379 patients. J Neurosurg. 2014;121(December):123-142. [DOI] [PubMed] [Google Scholar]
- 18. Chopra R, Kondziolka D, Niranjan A, Lunsford LD, Flickinger JC. Long-term follow-up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2007;68(3):845-851. [DOI] [PubMed] [Google Scholar]
- 19. Murphy ES, Barnett GH, Vogelbaum MA et al.. Long-term outcomes of Gamma Knife radiosurgery in patients with vestibular schwannomas. J Neurosurg. 2011;114(2):432-440. [DOI] [PubMed] [Google Scholar]
- 20. Wangerid T, Bartek J, Svensson M, Förander P. Long-term quality of life and tumour control following gamma knife radiosurgery for vestibular schwannoma. Acta Neurochir (Wien). 2014;156(2):389-396. [DOI] [PubMed] [Google Scholar]
- 21. Lunsford LD, Niranjan A, Flickinger JC et al.. Radiosurgery of vestibular schwannomas: summary of experience in 829 cases. J Neurosurg. 2005;102(Suppl):195-199. [PubMed] [Google Scholar]
- 22. Yomo S, Carron R, Thomassin J-M, Roche P-H, Régis J. Longitudinal analysis of hearing before and after radiosurgery for vestibular schwannoma. J Neurosurg. 2012;117(5):877-885. [DOI] [PubMed] [Google Scholar]
- 23. Hasegawa T, Kida Y, Kato T, Iizuka H, Yamamoto T. Factors associated with hearing preservation after Gamma Knife surgery for vestibular schwannomas in patients who retain serviceable hearing. J Neurosurg. 2011;115(6):1078-1086. [DOI] [PubMed] [Google Scholar]
- 24. Pollock BE, Link MJ, Foote RL. Failure rate of contemporary low-dose radiosurgical technique for vestibular schwannoma clinical article. J Neurosurg. 2009;111(4):840-844. [DOI] [PubMed] [Google Scholar]
- 25. Flickinger JC., Kondziolka D, Niranjan A, Maitz A, Voynov G, Lunsford LD. Acoustic neuroma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2004;60(1):225-230. [DOI] [PubMed] [Google Scholar]
- 26. Lee JYK, Sandhu S, Miller D, Solberg T, Dorsey JF, Alonso-Basanta M. Higher dose rate Gamma Knife radiosurgery may provide earlier and longer-lasting pain relief for patients with trigeminal neuralgia. J Neurosurg. 2015;123(4):961-968. [DOI] [PubMed] [Google Scholar]
- 27. Hall EJ, Brenner DJ. The dose-rate effect revisited: radiobiological considerations of importance in radiotherapy. Int J Radiat Oncol Biol Phys. 1991;21(6):1403-1414. [DOI] [PubMed] [Google Scholar]
- 28. Haque R, Wojtasiewicz TJ, Gigante PR et al.. Efficacy of facial nerve–sparing approach in patients with vestibular schwannomas. J Neurosurg. 2011;115(5):917-923. [DOI] [PubMed] [Google Scholar]
- 29. Samii M, Matthies C.. Management of 1000 vestibular schwannomas (acoustic neuromas): surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery. 1997;40(1):11-23. [DOI] [PubMed] [Google Scholar]
- 30. Shaw E, Kline R, Gillin M et al.. Radiation therapy oncology group: radiosurgery quality assurance guidelines. Int J Radiat Oncol. 1993;27(5):1231-1239. [DOI] [PubMed] [Google Scholar]
- 31. Yomo S, Tamura M, Carron R, Porcheron D, Régis J. A quantitative comparison of radiosurgical treatment parameters in vestibular schwannomas: the Leksell Gamma Knife Perfexion versus Model 4C. Acta Neurochir (Wien). 2010;152(1):47-55. [DOI] [PubMed] [Google Scholar]
- 32. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. [Google Scholar]
- 33. Baschnagel AM, Chen PY, Bojrab D et al.. Hearing preservation in patients with vestibular schwannoma treated with Gamma Knife surgery. J Neurosurg. 2013;118(3):571-578. [DOI] [PubMed] [Google Scholar]
- 34. Breivik CN, Nilsen RM, Myrseth E et al.. Conservative management or gamma knife radiosurgery for vestibular schwannoma: tumor growth, symptoms, and quality of life. Neurosurgery. 2013;73(1):48-56. [DOI] [PubMed] [Google Scholar]
- 35. Carlson ML, Jacob JT, Pollock BE et al.. Long-term hearing outcomes following stereotactic radiosurgery for vestibular schwannoma: patterns of hearing loss and variables influencing audiometric decline. J Neurosurg. 2013;118(3):579-587. [DOI] [PubMed] [Google Scholar]
- 36. Combs SE, Welzel T, Kessel K et al.. Hearing preservation after radiotherapy for vestibular schwannomas is comparable to hearing deterioration in healthy adults and is accompanied by local tumor control and a highly preserved quality of life (QOL) as patients’ self-reported outcome. Radiother Oncol. 2013;106(2):175-180. [DOI] [PubMed] [Google Scholar]
- 37. Han JH, Kim DG, Chung H-T et al.. Hearing preservation in patients with unilateral vestibular schwannoma who undergo stereotactic radiosurgery. Cancer. 2012;118(21):5441-5447. [DOI] [PubMed] [Google Scholar]
- 38. Hasegawa T, Kida Y, Kobayashi T, Yoshimoto M, Mori Y, Yoshida J. Long-term outcomes in patients with vestibular schwannomas treated using gamma knife surgery: 10-year follow up. J Neurosurg. 2005;102(1):10-16. [DOI] [PubMed] [Google Scholar]
- 39. Iwai Y, Yamanaka K, Shiotani M et al.. Radiosurgery for acoustic neuromas: results of low-dose treatment. Neurosurgery. 2003;53(2):282-288. [DOI] [PubMed] [Google Scholar]
- 40. Jacob JT, Carlson ML, Driscoll CL, Link MJ. Volumetric analysis of tumor control following subtotal and near-total resection of vestibular schwannoma. Laryngoscope. 2016;126(8):1877-1882. [DOI] [PubMed] [Google Scholar]
- 41. Kim YH, Kim DG, Han JH et al.. Hearing outcomes after stereotactic radiosurgery for unilateral intracanalicular vestibular schwannomas: implication of transient volume expansion. Int J Radiat Oncol Biol Phys. 2013;85(1):61-67. [DOI] [PubMed] [Google Scholar]
- 42. Kim JW, Kim DG, Paek SH et al.. Efficacy of corticosteroids in hearing preservation after radiosurgery for vestibular schwannoma: a prospective study. Stereotact Funct Neurosurg. 2011;89(1):25-33. [DOI] [PubMed] [Google Scholar]
- 43. Litvack ZN, Noren G, Chougule PB, Zheng Z. Preservation of functional hearing after gamma knife surgery for vestibular schwannoma. J Neurosurg. 2005;102(suppl):204-206. [DOI] [PubMed] [Google Scholar]
- 44. Massager N, Lonneville S, Delbrouck C, Benmebarek N, Desmedt F, Devriendt D. Dosimetric and clinical analysis of spatial distribution of the radiation dose in Gamma Knife radiosurgery for vestibular schwannoma. Int J Radiat Oncol Biol Phys. 2011;81(4):511-518. [DOI] [PubMed] [Google Scholar]
- 45. Myrseth E, Møller P, Pedersen PH, Lund-Johansen M. Vestibular schwannoma: surgery or gamma knife radiosurgery? A prospective, nonrandomized study. Neurosurgery. 2009;64(4):654-661. [DOI] [PubMed] [Google Scholar]
- 46. Pollock BE. Management of vestibular schwannomas that enlarge after stereotactic radiosurgery: treatment recommendations based on a 15 year experience. Neurosurgery. 2006;58(2):241-246. [DOI] [PubMed] [Google Scholar]
- 47. Régis J, Carron R, Park MC et al.. Wait-and-see strategy compared with proactive Gamma Knife surgery in patients with intracanalicular vestibular schwannomas. J Neurosurg. 2010;113(December):105-111. [DOI] [PubMed] [Google Scholar]
- 48. Tamura M, Carron R, Yomo S et al.. Hearing preservation after gamma knife radiosurgery for vestibular schwannomas presenting with high-level hearing. Neurosurgery. 2009;64(2):289-296. [DOI] [PubMed] [Google Scholar]
- 49. Sun S, Liu A.. Long-term follow-up studies of Gamma Knife surgery with a low margin dose for vestibular schwannoma. J Neurosurg. 2012;117(December):57-62. [DOI] [PubMed] [Google Scholar]
- 50. Zaorsky NG, Davis BJ, Nguyen PL et al.. The evolution of brachytherapy for prostate cancer. Nat Rev Urol. 2017;14(7):415-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pos FJ, Horenblas S, Lebesque J et al.. Low-dose-rate brachytherapy is superior to high-dose-rate brachytherapy for bladder cancer. Int J Radiat Oncol Biol Phys. 2004;59(3):696-705. [DOI] [PubMed] [Google Scholar]
- 52. Stewart AJ, Viswanathan AN. Current controversies in high-dose-rate versus low-dose-rate brachytherapy for cervical cancer. Cancer. 2006;107(5):908-915. [DOI] [PubMed] [Google Scholar]
- 53. Liu R, Wang X, Ma B et al.. High dose rate versus low dose rate intracavity brachytherapy for locally advanced uterine cervix cancer. Cochrane Database Syst Rev. 2010;(7):CD007563 doi:10.1002/14651858.CD007563.pub2 [DOI] [PubMed] [Google Scholar]
- 54. Viani GA, Manta GB, Stefano EJ, De Fendi LI. Brachytherapy for cervix cancer: low-dose rate or high-dose rate brachytherapy - a meta-analysis of clinical trials. J Exp Clin Cancer Res. 2009;28(1):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Arai Y, Kano H, Lunsford LD et al.. Does the Gamma Knife dose rate affect outcomes in radiosurgery for trigeminal neuralgia? J Neurosurg. 2010;113(December):168-171. [DOI] [PubMed] [Google Scholar]
- 56. Balamucki CJ, Stieber VW, Ellis TL et al.. Does dose rate affect efficacy? The outcomes of 256 gamma knife surgery procedures for trigeminal neuralgia and other types of facial pain as they relate to the half-life of cobalt. J Neurosurg. 2006;105(5):730-735. [DOI] [PubMed] [Google Scholar]
- 57. Kann BH, Yu JB, Stahl JM et al.. The impact of cobalt-60 source age on biologically effective dose in high-dose functional Gamma Knife radiosurgery. J Neurosurg. 2016;125(Suppl 1):154-159. [DOI] [PubMed] [Google Scholar]
- 58. Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol. 2008;18(4):240-243. [DOI] [PubMed] [Google Scholar]
- 59. Brenner DJ. The linear-quadratic model is an appropriate methodology for determining isoeffective doses at large doses per fraction. Semin Radiat Oncol. 2008;18(4):234-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hall EJ, Brenner DJ. The radiobiology of radiosurgery: rationale for different treatment regimes for AVMs and malignancies. Int J Radiat Oncol Biol Phys. 1993;25(2):381-385. [DOI] [PubMed] [Google Scholar]
- 61. Andrews DW, Suarez O, Goldman HW et al.. Stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of acoustic schwannomas: comparative observations of 125 patients treated at one institution. Int J Radiat Oncol Biol Phys. 2001;50(5):1265-1278. [DOI] [PubMed] [Google Scholar]
- 62. McTyre E, Helis CA, Farris M et al.. Emerging indications for fractionated Gamma Knife radiosurgery. Neurosurgery. 2016;80(2):210-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Niranjan A, Gobbel G, Novotny J Jr, Bhatnagar J, Fellows W, Lunsford LD. Impact of decaying dose rate in gamma knife radiosurgery: in vitro study on 9L rat gliosarcoma cells. J Radiosurg SBRT. 2012;1(4):1-8. [PMC free article] [PubMed] [Google Scholar]
- 64. Hopewell JW., Millar WT., Paddick I, Lindquist C. Impact of decaying dose-rate in gamma knife radiosurgery. J Radiosurgery SBRT. 2013;2(3):251-253. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.