Abstract
Background
Childhood cancer survivors (CCS) are at increased risk of developing colorectal cancer (CRC) compared to the general population, especially those previously exposed to abdominal or pelvic radiation therapy (APRT). However, the benefits and costs of CRC screening in CCS are unclear. In this study, we evaluated the cost-effectiveness of early-initiated colonoscopy screening in CCS.
Methods
We adjusted a previously validated model of CRC screening in the US population (MISCAN-Colon) to reflect CRC and other-cause mortality risk in CCS. We evaluated 91 colonoscopy screening strategies varying in screening interval, age to start, and age to stop screening for all CCS combined and for those treated with or without APRT. Primary outcomes were CRC deaths averted (compared to no screening) and incremental cost-effectiveness ratios (ICERs). A willingness-to-pay threshold of $100 000 per life-years gained (LYG) was used to determine the optimal screening strategy.
Results
Compared to no screening, the US Preventive Services Task Force’s average risk screening schedule prevented up to 73.2% of CRC deaths in CCS. The optimal strategy of screening every 10 years from age 40 to 60 years averted 79.2% of deaths, with ICER of $67 000/LYG. Among CCS treated with APRT, colonoscopy every 10 years from age 35 to 65 years was optimal (CRC deaths averted: 82.3%; ICER: $92 000/LYG), whereas among those not previously treated with APRT, screening from age 45 to 55 years every 10 years was optimal (CRC deaths averted: 72.7%; ICER: $57 000/LYG).
Conclusions
Early initiation of colonoscopy screening for CCS is cost-effective, especially among those treated with APRT.
With steady improvements in treatment and supportive care, survival of children diagnosed with cancer has greatly improved in recent decades (1). However, with improved survival, childhood cancer survivors (CCS) are at increased risk of developing a second malignancy, in large part related to their treatment (2,3). Abdominal or pelvic radiation therapy (APRT), for example, increases risk of colorectal cancer (CRC) up to 11-fold (3–5), and consequently, some expert panels, such as the Children’s Oncology Group (COG), have recommended a more frequent and early CRC screening among CCS with this exposure (6,7). However, evidence on which to base specific recommendations is limited (4). It is unclear to what extent early screening could produce a clinically meaningful reduction in CRC mortality, whether it is likely to be cost-effective, and what the optimal start age and frequency of screening are. This might in part explain why other expert groups, such as the Scottish Intercollegiate Guidelines Network and the Swedish Working Group for Long-term Follow-up after Childhood Cancer, have not recommended early initiation of CRC screening (8,9). Ideally, effectiveness of CRC screening in CCS would be evaluated in a randomized clinical trial (RCT); however, no such trials are underway or likely to be initiated in the near future, and consequently, evidence to guide clinical practice will need to come from non-RCT sources. In this context, we simulated benefits and costs of CRC screening using a modeling approach and performed a cost-effectiveness analysis to determine which colonoscopy screening strategy may be optimal for this population.
Methods
MISCAN-Colon Model
For this study, we used the Microsimulation Screening Analysis-Colon (MISCAN-Colon) model (Erasmus University Medical Center, Rotterdam, The Netherlands) (10). MISCAN-Colon is a well-established stochastic microsimulation model for CRC that has been used to guide public health policy, including—as part of the Cancer Intervention and Surveillance Modelling Network (CISNET)—decision analyses for the US Preventive Services Task Force (USPSTF) and the American Cancer Society (11,12). The structure and underlying assumptions have been described in previous publications (10,13).
Adaptations of the MISCAN-Colon Model to CCS
We adjusted the existing MISCAN-Colon model used previously for the US general population to reflect CRC and other-cause mortality risk in CCS (Table 1). First, parameters of the model were calibrated to replicate the 4.2-fold higher CRC risk observed in the Childhood Cancer Survivor Study (CCSS) in which 20% of CCS were exposed to APRT (Supplementary Figure 1A, available online) (3), assuming that the higher CRC risk in CCS was caused by a predisposition to develop more adenomas (4,19). Further, the model was adjusted to reflect the increased overall all-cause mortality of CCS as seen in the Surveillance, Epidemiology, and End Results (SEER) program data compared to the US general population (Supplementary Table 1, available online). No differences in CRC stage distribution and survival were assumed compared to the general population. Under these assumptions, the model replicated observed mortality estimates from SEER databases well (1973–2013; Supplementary Figure 1B, available online), suggesting the assumptions are reasonable.
Table 1.
Key modeling assumptions
| Input parameter | Model assumptions |
|
|---|---|---|
| Base-case analyses | Probabilistic sensitivity analyses (CEAF), ranges* | |
| Demography | ||
| All-cause mortality | US lifetables, adjusted using the increased age-specific SMRs observed for CCS in SEER databases: | Log-normal: |
| 25–34, SMR = 5.62 | (5.16; 6.06) | |
| 35–39, SMR = 4.63 | (4.03; 5.31) | |
| 40–44, SMR = 4.02 | (3.50; 4.67) | |
| 45–49, SMR = 3.92 | (3.32; 4.59) | |
| 50–54, SMR = 3.22 | (2.52; 4.07) | |
| 55–99, SMR = 3.43 | (2.01; 5.38) | |
| Natural history | ||
| Adenoma onset | Age-dependent (nonhomogenous Poisson) with more frequent adenoma (assumed after diagnosis of primary cancer, age 15 years) adjusted according to CRC risks observed in CCSS: | Log-normal: |
| All CCS combined: RR = 4.2; | All CCS combined (2.8; 6.1) | |
| CCS with APRT: RR = 8.5; | CCS with APRT (4.5; 14.6) | |
| CCS without APRT: RR = 2.6. | CCS without APRT (1.2; 5.0) | |
| Adenoma progression | ||
| State transitions | Age-dependent | — |
| State durations, y (total) | Exp(λ = 130) | — |
| Cancer progression (preclinical) | ||
| Stage transitions | Age-dependent | — |
| Stage durations, y | Exp(λ = 2.5) | — |
| Colorectal cancer survival | Age-/Stage-/Localization-dependent | — |
| Colonoscopy performance | ||
| Sensitivity, %† | Beta: | |
| Adenomas 0–5 mm | 75 | (68; 82) |
| Adenomas 6–9 mm | 85 | (78; 91) |
| Adenomas ≥10 mm | 95 | (89; 97) |
| Malignant neoplasia | 95 | (89; 97) |
| Specificity, %‡ | 86 | (75; 94) |
| Complete colonoscopy examination, % | 95 | (89; 97) |
| Complication rates, % with polypectomy§ | Age-dependent | |
| Fatal complications‖ | 0.000329 | Relative difference, Log-normal: (−60%; +167%) |
| Without polypectomy | — | |
| Costs, US $¶ | Relative difference, Log-normal: | |
| Colonoscopy | ||
| With polypectomy | 1400 | (−9%; +10%) |
| Without polypectomy | 1700 | (−9%; +10%) |
| Complications | ||
| Serious GI complications# | 11 200 | (−18%; +22%) |
| Other GI complications** | 7600 | (−18%; +22%) |
| Cardiovascular complications†† | 8500 | (−18%; +22%) |
| Per life-year with cancer care | ||
| Initial year, stage I–IV | 36 900–78 200 | (−4%; +4%) |
| Ongoing, stage I–IV | 3100–12 300 | (−11%; +13%) |
| Terminal year (CRC death), stage I–IV | 64 200–88 900 | (−4%; +4%) |
| Terminal year (other causes), stage I–IV | 19 400–50 200 | (−17%; +21%) |
The range for parameter distributions is reported using the 2.5th and 97.5th percentiles. APRT = abdominal or pelvic radiation therapy; CCS = childhood cancer survivors; CCSS= Childhood Cancer Survivors Study; CEAF = cost-effectiveness acceptability frontier; CRC = colorectal cancer; GI = gastrointestinal; RR = relative risk; SEER = Surveillance, Epidemiology, and End Results; SMR = standardized mortality ratio.
The sensitivity of colonoscopy for the detection of adenomas and CRC within the reach of the endoscope was obtained from a systematic review on miss rates seen in tandem colonoscopy studies (14).
Specificity for colonoscopy is therefore based on an adenoma prevalence study of patients undergoing screening colonoscopy (15).
Age-specific risks for complications of colonoscopy requiring a hospital admission or emergency department visit were obtained from a study by Warren et al. (16).
The mortality rate associated with colonoscopies with a polypectomy was derived by multiplying the risk for a perforation obtained from a study by Warren et al. (16) and by the risk for death given a perforation obtained from a study by Gatto et al. (17).
Costs are presented in 2015 US dollars and include copayments and patient time costs (ie, the opportunity costs of spending time on screening or being treated for a complication or CRC) but do not include travel costs, costs of lost productivity, and unrelated health-care and nonhealth-care costs in added years of life. We assumed that the value of patient time was equal to the median wage rate in 2014: $17.01/h. Cost values were estimated for the year 2014. We assumed that colonoscopies and complications used up 40 and 190 h of patient time, respectively. Patient time costs were already included in the estimates for the costs of LYS with CRC care obtained from a study by Yabroff et al. (18). All costs were adjusted for the year 2015 using the annual average consumer price indexes provided by the US Bureau of Labor Statistics.
Serious GI complications included perforations, gastrointestinal bleeding, or transfusions.
Other GI complications included paralytic ileus, nausea and vomiting, dehydration, or abdominal pain.
Cardiovascular complications included myocardial infarction or angina, arrhythmias, congestive heart failure, cardiac or respiratory arrest, syncope, hypotension, or shock.
Results from CCSS also show that the higher CRC risk seen in CCS varied according to radiation therapy exposure: CCS treated and not treated with APRT were reported to have, respectively, 8.5-fold and 2.6-fold increased risk of CRC compared to the US general population (3). Therefore, we also performed a stratified analysis, developing two additional model versions to take into account differences in CRC risk due to APRT. Because no statistically significant impact on all-cause mortality was observed by APRT (20), we assumed no difference in life expectancy between the two groups, although higher all-cause mortality was evaluated in specific sensitivity analyses described below.
Screening Strategies Simulated and Cost-Effectiveness Analyses
We simulated a cohort of 10 million CCS with first cancer diagnosed at age 15 years for each of three populations described above (CCS all combined, CCS treated, and CCS not treated with APRT). The large simulated cohort size was carried out to guarantee model outcome stability (13), recognizing that it exceeds the actual number of CCS in the United States (21). For each CCS population, we performed a cost-effectiveness analysis simulating benefits and costs under 91 different strategies, including no screening and colonoscopy screening with varying age to start (25, 30, 35, 40, 45, and 50 years), interval (every 3, 5, or 10 years), and age to end (55, 60, 65, 70, and 75 years). Cost-effectiveness analysis was carried out from a modified societal perspective, including patient time costs, but excluding other indirect costs for traveling and time off work (Supplementary Methods, available online) (18,22). Screening effectiveness (ie, number of CRC deaths prevented, relative CRC mortality reduction, and life-years gained [LYG]) and resources (colonoscopies and cost) were computed for each screening strategy, discounting the LYG and cost at the conventional 3% annual discount rate. The number of screening tests needed to prevent a CRC death were calculated by dividing the total number of colonoscopies performed (per 1000) by the number of CRC deaths prevented per 1000 CCS screened. Outcomes are reported per 1000 CCS aged 25 years to allow for generalizability to CCS populations of different sizes. Colonoscopy characteristics and complication rates were based on studies in the general population (Table 1) (14–17). We assumed 100% adherence to screening.
To determine the optimal colonoscopy screening strategy in CCS, we first excluded screening strategies that were more costly and less effective than several other strategies as described elsewhere (23). For all remaining strategies (ie, efficient strategies) we calculated the incremental cost-effectiveness ratio (ICER) as the ratio between additional costs and additional LYG compared to the next less expensive efficient strategy. Of those efficient strategies, we defined as the “optimal” strategy the one that prevented the most CRC deaths with an ICER below the willingness-to-pay threshold of $100 000 per LYG. Strategies with an ICER exceeding $100 000 were considered not cost-effective.
For each CCS population, we also compared the predicted CRC deaths and costs associated with the identified optimal screening strategy against a selected number of alternative strategies: no screening; colonoscopy every 10 years from age 50 to 75 years (colonoscopy screening strategy recommended by the USPSTF for the US general population) (24); and colonoscopy from age 30 to 75 years, every 5 years (recommended by the COG for childhood survivors treated with APRT) (6).
Sensitivity Analyses
Multiple one-way sensitivity analyses were also carried out to assess the robustness of the results. First, separate analyses were performed for specific subtypes of CCS, including Hodgkin lymphoma (HL) patients (5.7-fold increased CRC risk compared to US general population) (3,4,20,25); Wilms tumor (WT) patients (15.5-fold increased CRC risk, first cancer diagnosis assumed at age 5 years) (3,20); CCS treated with APRT exceeding 30 Gy in doses (10.9-fold increased CRC risk caused by both a higher rate of adenoma incidence and a faster adenoma progression) (5); base case all-cause mortality, as well as variant with up to 2.6-fold higher all-cause mortality (20); and alternative ages at first malignancy (5 or 20 years). Details about assumptions for these CCS subtypes are provided in the Supplementary Methods and Supplementary Figure 2 (available online).
Second, we assessed results under a variety of assumptions regarding CRC risk and other-cause mortality. These assumptions included 28% lower CRC survival following a diagnosis of CRC at regional or distant stage compared to CRCs diagnosed with the same corresponding stage in the US general population (25); different mechanism for the higher CRC risk combining shorter time of adenoma progression to invasive cancer (50% reduced) and higher adenoma incidence (Supplementary Figure 1A, available online); a lower all-cause mortality in older ages (≥65 years), assuming a 2-fold standardized mortality ratio compared to the US general population rather than the last available value from SEER (3.4 at age 65 years; Supplementary Table 1, available online); a higher all-cause mortality rate as observed in CCSS (Supplementary Methods, Supplementary Tables 1 and 2 and Supplementary Figure 3, available online) for CCS treated with APRT (20,26); and higher health-care expenses for conditions unrelated to CRC for CCS with averted CRC incidence or death compared to the US general population (Supplementary Methods, available online) (27).
Multiway probabilistic sensitivity analyses (PSA) were finally performed to assess uncertainty surrounding the optimal choices in the base case analyses, computing cost-effectiveness acceptability frontiers (CEAFs, probabilities that optimal choice is cost-effective) for the optimal strategy and the two neighboring efficient strategies (28). Outcomes were reassessed while randomly varying key model assumptions across 1000 simulations (Table 1).
Results
Optimal Colonoscopy Screening Strategies
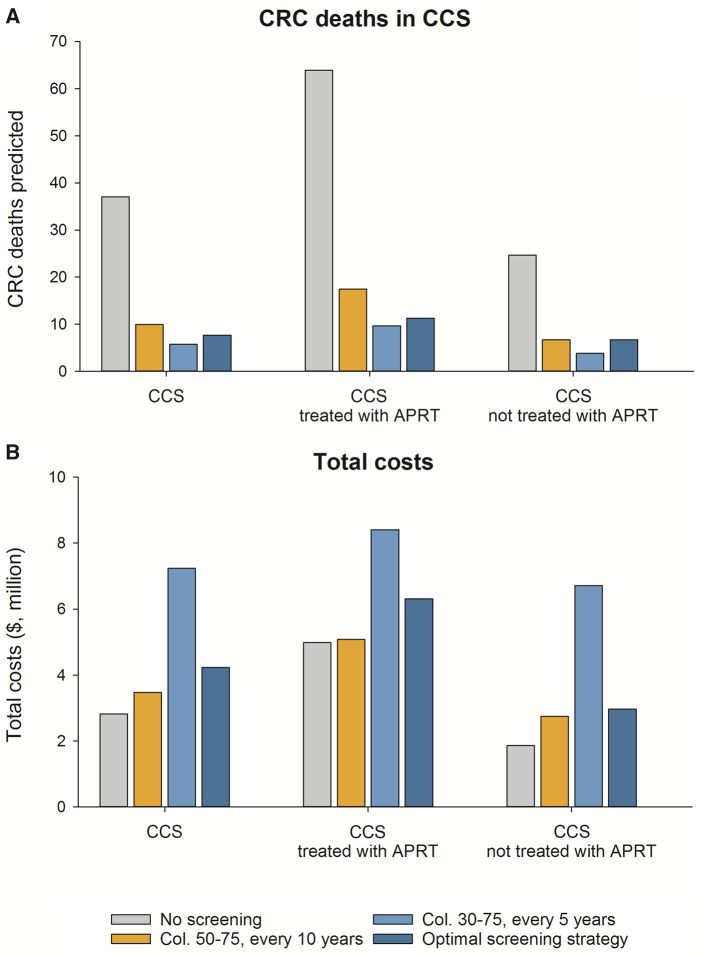
Among all CCS combined (with primary malignancy occurring at age 15 years), 37 per 1000 were predicted to die of CRC without screening (Table 2; modeled outcomes of all evaluated strategies are shown in Supplementary Table 3, available online). Optimal results within acceptable cost levels were achieved with colonoscopy every 10 years from age 40 to 60 years, averting 79.2% of CRC mortality. This strategy required 134 colonoscopies to prevent one CRC death at a total cost of $4.2 million/1000 CCS, for an associated ICER of $67 000 per LYG. With screening employed as currently recommended for the average-risk US general population (USPSTF, colonoscopy from age 50 to 75 years, every 10 years), 73.2% of CRC deaths would be averted compared to no screening. Colonoscopy screening from age 30 to 75 years repeated every 5 years, as suggested by COG for CCS exposed to APRT, was estimated to prevent up to 84.4% of CRC mortality among CCS, but at higher total costs ($7.2 million/1000 CCS; Figure 1).
Table 2.
Efficient and currently recommended colonoscopy screening strategies among CCS with primary cancer diagnosis at age 15 years
| Screening strategies | Outcomes per 1000 CCS free of diagnosed cancer and aged 25 years in 2017 (3% discounted)* |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| CRC deaths predicted† | CRC mortality† |
LYG‡ | NNS‖ | COLs† | Screening rounds | Total costs ($1000)¶ | ICER ($1000)¶ | ||
| Reduction, %‡ | Δ vs USPSTF, %§ | ||||||||
| All CCS | |||||||||
| No screening | 37.06 | 0.00 | −73.21 | — | — | 95.22 | 0 | 2821.96 | — |
| COL 50–55 y, 10 y | 12.57 | 66.1 | −7.14 | 57.54 | 90.94 | 2227.05 | 1 | 3213.66 | 6.81 |
| COL 50–75 y, 10 y (USPSTF§) | 9.93 | 73.2 | — | 61.45 | 107.67 | 2921.06 | 3 | 3475.59 | Dominated |
| COL 45–55 y, 10 y | 9.25 | 75.0 | 1.82 | 70.92 | 113.89 | 3167.41 | 2 | 3687.14 | 35.38 |
| COL 40–60 y, 10 y (Optimal)# | 7.70 | 79.2 | 6 | 79.01 | 133.70 | 3925.35 | 3 | 4228.77 | 67.00 |
| COL 35–65 y, 10 y | 7.10 | 80.8 | 7.63 | 82.77 | 153.33 | 4593.74 | 4 | 4861.52 | 167.94 |
| COL 35–60 y, 5 y | 6.53 | 82.4 | 9.18 | 86.97 | 192.52 | 5877.77 | 6 | 5856.35 | 237.04 |
| COL 35–65 y, 5 y | 6.20 | 83.3 | 10.06 | 87.37 | 198.18 | 6115.88 | 7 | 5954.34 | 246.17 |
| COL 35–60 y, 3 y | 4.46 | 88.0 | 14.77 | 94.21 | 279.25 | 9103.56 | 9 | 7951.45 | 291.86 |
| COL 35–65 y, 3 y | 4.00 | 89.2 | 16 | 94.81 | 290.93 | 9618.10 | 11 | 8175.32 | 372.07 |
| COL 30–65 y, 3 y | 4.11 | 88.9 | 15.7 | 98.34 | 334.01 | 11005.72 | 12 | 10017.09 | 521.97 |
| COL 30–70 y, 3 y | 3.88 | 89.5 | 16.31 | 98.59 | 343.04 | 11382.16 | 14 | 10168.58 | 617.05 |
| COL 25–70 y, 3 y | 3.55 | 90.4 | 17.21 | 100.87 | 391.99 | 13135.47 | 16 | 12525.52 | 1034.32 |
| COL 25–75 y, 3 y | 3.51 | 90.5 | 17.31 | 100.90 | 394.94 | 13250.27 | 17 | 12566.07 | 1367.87 |
| CCS treated with APRT | |||||||||
| No screening | 63.88 | 0.0 | −72.72 | — | — | 157.74 | 0 | 4980.73 | — |
| COL 50–55 y, 10 y | 19.84 | 68.9 | −3.78 | 108.46 | 59.77 | 2632.19 | 1 | 4881.48 | −0.92 |
| COL 50–75 y, 10 y (USPSTF§) | 17.43 | 72.7 | — | 112.04 | 68.19 | 3167.25 | 3 | 5074.20 | Dominated |
| COL 45–55 y, 10 y | 14.69 | 77.0 | 4.28 | 133.53 | 73.31 | 3606.17 | 2 | 5213.47 | 13.24 |
| COL 40–55 y, 10 y | 13.99 | 78.1 | 5.38 | 146.98 | 80.82 | 4032.13 | 2 | 5557.05 | 25.55 |
| COL 40–60 y, 10 y | 12.25 | 80.8 | 8.1 | 149.70 | 85.26 | 4401.75 | 3 | 5692.82 | 49.89 |
| COL 35–55 y, 10 y | 12.06 | 81.1 | 8.4 | 157.08 | 93.79 | 4860.37 | 3 | 6226.70 | 72.39 |
| COL 35–65 y, 10 y (Optimal)# | 11.30 | 82.3 | 9.59 | 158.02 | 96.78 | 5088.73 | 4 | 6313.29 | 92.14 |
| COL 30–60 y, 10 y | 11.04 | 82.7 | 10 | 163.33 | 105.50 | 5574.53 | 4 | 6981.17 | 125.73 |
| COL 30–70 y, 10 y | 10.73 | 83.2 | 10.49 | 163.62 | 107.47 | 5711.78 | 5 | 7032.62 | 176.32 |
| COL 30–75 y, 5 y (COG)§ | 9.69 | 84.8 | 12.11 | 169.76 | 137.93 | 7474.49 | 10 | 8405.05 | Dominated |
| COL 35–55 y, 3 y | 8.36 | 86.9 | 14.19 | 172.84 | 156.63 | 8696.30 | 7 | 8729.36 | 184.17 |
| COL 35–60 y, 3 y | 7.51 | 88.3 | 15.53 | 174.28 | 164.65 | 9281.40 | 9 | 9009.83 | 193.77 |
| COL 30–60 y, 3 y | 7.21 | 88.7 | 15.99 | 181.77 | 192.22 | 10893.21 | 11 | 10899.11 | 252.48 |
| COL 30–65 y, 3 y | 7.02 | 89.0 | 16.29 | 182.01 | 194.97 | 11086.20 | 12 | 10983.16 | 345.04 |
| COL 25–65 y, 3 y | 6.48 | 89.9 | 17.13 | 186.14 | 223.88 | 12850.47 | 14 | 13323.50 | 566.21 |
| COL 25–70 y, 3 y | 6.32 | 90.1 | 17.38 | 186.30 | 227.74 | 13108.47 | 16 | 13424.69 | 650.5 |
| COL 25–75 y, 3 y | 6.30 | 90.1 | 17.42 | 186.32 | 229.10 | 13191.37 | 17 | 13454.12 | 1467.2 |
| CCS not treated with APRT | |||||||||
| No screening | 24.68 | 0.0 | −72.91 | — | — | 64.68 | 0 | 1860.17 | — |
| COL 50–55 y, 10 y | 9.35 | 62.1 | −10.8 | 35.46 | 126.66 | 1941.75 | 1 | 2436.16 | 16.24 |
| COL 50–75 y, 10 y (USPSTF) § | 6.69 | 72.9 | — | 39.42 | 152.54 | 2744.14 | 3 | 2748.27 | Dominated |
| COL 45–55 y, 10 y (Optimal)# | 6.74 | 72.7 | −0.24 | 44.61 | 160.19 | 2873.73 | 2 | 2961.78 | 57.48 |
| COL 40–60 y, 10 y | 5.54 | 77.6 | 4.64 | 49.69 | 189.58 | 3628.54 | 3 | 3526.29 | 111.00 |
| COL 40–70 y, 10 y | 5.11 | 79.3 | 6.39 | 50.09 | 196.93 | 3853.92 | 4 | 3611.11 | 213.10 |
| COL 35–65 y, 10 y | 5.02 | 79.6 | 6.73 | 52.21 | 218.77 | 4300.97 | 4 | 4163.27 | 261.27 |
| COL 40–65 y, 5 y | 4.49 | 81.8 | 8.89 | 53.12 | 251.06 | 5068.92 | 6 | 4415.31 | 275.64 |
| COL 35–65 y, 5 y | 4.19 | 83.0 | 10.1 | 56.20 | 293.29 | 6009.52 | 7 | 5399.78 | 319.63 |
| COL 35–65 y, 3 y | 2.66 | 89.2 | 16.32 | 61.27 | 433.39 | 9543.18 | 11 | 7721.08 | 458.00 |
| COL 35–70 y, 3 y | 2.55 | 89.7 | 16.76 | 61.38 | 441.29 | 9765.71 | 12 | 7809.57 | 792.70 |
| COL 30–70 y, 3 y | 2.50 | 89.9 | 16.95 | 63.77 | 512.39 | 11364.74 | 14 | 9754.06 | 813.27 |
| COL 30–75 y, 3 y | 2.43 | 90.2 | 17.25 | 63.82 | 522.62 | 11628.33 | 16 | 9847.12 | 1727.38 |
| COL 25–75 y, 3 y | 2.27 | 90.8 | 17.88 | 65.03 | 591.86 | 13263.67 | 17 | 12174.87 | 1923.92 |
Full participation in postcolonoscopy surveillance was assumed; we defined low-risk adenoma (LRA) and high-risk adenoma (HRA) patients considering adenoma size (LRA = 1–2 adenomas ≤ 10 mm; HRA = >2 adenomas ≤10 mm or 1 adenoma >10 mm). For HRA and LRA individuals, colonoscopy surveillance was simulated with 3- to 5-year intervals according the US Multi-Society Task Force guidelines. Although high-risk pathologies are strongly correlated with size, approximately 3% of adenomas <10 mm in diameter may harbor these features (29). APRT = abdominal or pelvic radiation therapy; CCS = childhood cancer survivors; COG = Children’s Oncology Group; COL = colonoscopy; CRC = colorectal cancer; ICER = incremental cost-effectiveness ratio (Δ costs/Δ LYGs compared to the previous, less costly efficient strategy); LYG = life-years gained; NNS = number needed to screen; USPSTF = US Preventive Services Task Force.
CRC deaths and number of colonoscopies were not discounted.
Compared with no screening.
USPSTF guideline for average risk screening; COG guideline for screening of survivors with abdominal or pelvic radiation.
NNS indicates the number of screening colonoscopies needed to prevent one colorectal cancer death.
Costs and ICERs in US dollars.
Optimal screening strategy defined as the strategy with highest LYG from screening among those efficient strategies with ICER less than $100k/LYG.
Figure 1.
Colorectal cancer deaths and total costs ($) per 1000 CCS aged 25 years in 2017 under different colonoscopy screening scenarios. Colorectal cancer deaths (A) and total costs (B) are shown for no screening; colonoscopy every 10 years between age 50 and 75 years (US Preventive Task Force’s general population recommended colonoscopy screening strategy); colonoscopy every 5 years between age 30 and 75 years (the Children’s Oncology Group colonoscopy screening indication for CCS treated with APRT); and the corresponding optimal colonoscopy screening strategy suggested by our model (CCS all combined: colonoscopy between age 40 and 60 years every 10 years; CCS treated with APRT: colonoscopy between age 35 and 65 years every 10 years; and CCS not treated with APRT: colonoscopy between age 45 and 55 years every 10 years years). APRT = abdominal or pelvic radiation therapy; CCS = childhood cancer survivor; Col = colonoscopy; CRC = colorectal cancer.
Among CCS treated with APRT, 64 per 1000 CCS were predicted to die of CRC without screening (Supplementary Table 4, available online). The optimal strategy for CCS treated with APRT was screening with colonoscopy from age 35 to 65 years every 10 years, averting 82.3% of CRC deaths (97 colonoscopies needed per CRC death prevented), with an overall cost of $6.3 million/1000 CCS (ICER of $92 000/LYG; Table 2). Screening, as recommended for the general population (USPSTF), prevented 72.7% of CRC deaths compared to no screening, whereas up to 84.8% of CRC deaths were prevented by colonoscopy from age 30 to 75 years repeated every 5 years (COG recommended strategy; total costs per 1000 CCS = $8.4 million; Figure 1).
Even among CCS not treated with APRT (Supplementary Table 5, available online), earlier initiation of screening was cost-effective, with the optimal strategy being colonoscopy screening at age 45 and 55 years repeated every 10 years, averting 72.7% of CRC deaths (160 colonoscopies needed per CRC death prevented; ICER of $57 000 per LYG; Table 2).
Sensitivity Analyses
There were clinically important differences in the optimal screening strategy depending on initial diagnosis and APRT dose. For WT survivors, screening intensively with colonoscopy every 3 years from age 35 to 60 years was optimal, preventing up to 86.2% of CRC deaths (ICER of $73 000 per LYG), whereas among HL survivors, the optimal strategy was colonoscopy from age 40 to 60 years every 10 years, averting 80.2% of CRC mortality (ICER of $51 000 per LYG; Supplementary Table 6 and Supplementary Figure 4, available online). Among CCS exposed to APRT with elevated doses (≥30 Gy, 10.9-fold increased CRC risk, and all-cause mortality as assumed in base case), the optimal screening strategy was colonoscopy from age 35 to 60 years every 5 years (Table 3).
Table 3.
Optimal colonoscopy screening strategies in specific parameter uncertainty analyses and patient subgroups
| Base-case analysis | Optimal screening strategy |
||
|---|---|---|---|
| CCS all combined | CCS treated with APRT | CCS not treated with APRT | |
| Age 40–60 years, every 10 years | Age 35–65 years, every 10 years | Age 45–55 years, every 10 years | |
| Specific CCS subpopulation analyses* | |||
| Hodgkin lymphoma survivors | Unchanged | — | — |
| Wilms tumor survivors (primary malignancy at age 5 years) | Age 35–60, every 3 years | — | — |
| CCS treated with APRT at high doses (≥30 Gy) and all-cause mortality as in base-case analysis | — | Age 35–60, every 5 years | — |
| CCS treated with APRT at high doses (≥30 Gy) and up to 2.6-fold increase in all-cause mortality† | — | Age 35–55, every 10 years | — |
| CCS with primary malignancy at age 5 years | Age 40–55, every 10 years | Age 35–55, every 10 years | Unchanged |
| CCS with primary malignancy at age 20 years | Age 40–70, every 10 years | Unchanged | Age 45–65, every 10 years |
| Parameter uncertainty analyses* | |||
| 1.37-fold lower CRC survival‡ | Unchanged | Unchanged | Unchanged |
| CRC risk due to a combination of higher adenoma onset and faster adenoma progression | Unchanged | Unchanged | Age 45–65, every 10 years |
| Lower all-cause mortality in older ages (≥65 years) | Unchanged | Unchanged | Age 45–65, every 10 years |
| Higher all-cause mortality according to Mertens et al., 2008 (26)§ | Age 40—55, every 10 years | Age 35—55, every 10 years | Unchanged |
| Higher-than-average health-care expenses for conditions unrelated to CRC‖ | Age 45—55, every 10 years | Age 40—60, every 10 years | Unchanged |
When age at primary cancer diagnosis was not mentioned, results were estimated assuming first cancer malignancy occurring at age 15 years. CCS = childhood cancer survivors; APRT = abdominal-pelvic radiation therapy; CRC = colorectal cancer.
Compared to age-specific other-cause mortality assumed in the base case analysis for CCS, more details are reported in the Supplementary Methods (available online) (personal information provided by Armstrong et al., 2016) (20).
In CCS diagnosed with CRC at regional or distant stage.
For CCS treated with APRT, age-specific other-cause mortality was assumed up to 1.6-fold increase compared to other-cause mortality assumed for CCS all combined (20, 26).
Higher hospitalization costs due to the higher probability of being hospitalized seen in CCS compared to US general population (Supplementary Methods, available online) (27).
Age at primary cancer diagnosis did not influence optimal start age or screening interval, although it did affect optimal stop age. For primary cancer diagnoses at ages 5 or 20 years, optimal stop ages were 55 and 70, respectively.
The optimal screening strategy remained the same as the base case for most alternative assumptions concerning CRC risk and other-cause mortality (Table 3). Assuming a higher all-cause mortality for all CCS combined and CCS treated with APRT (when based on CCSS data), the age to stop screening was reduced to age 55 years. Screening with colonoscopy every 10 years from age 45 to 65 years was optimal in CCS not treated with APRT when we assumed a lower all-cause mortality or a different mechanism for the increased CRC risk (higher adenoma onset and faster adenoma progression). Assuming higher health-care expenses unrelated to CRC, the optimal starting age for all CCS and those treated with APRT shifted 5 years later to age 45 and 40 years, respectively.
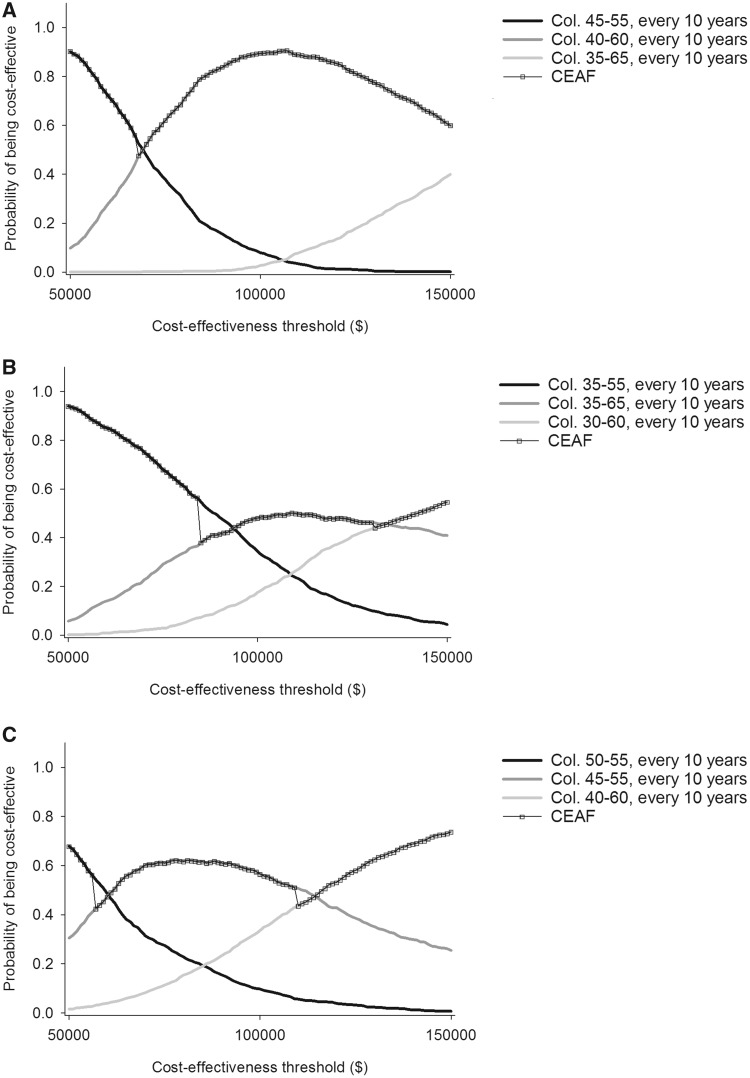
Among the screening strategies included in our PSA, the optimal screening strategy for all CCS combined was robust to model parameter uncertainty. The optimal strategy from the base case remained optimal in almost 90% of replicated scenarios (CEAF = 89.4%, at WT patients of $100 000 per LYG; Figure 2A). Among CCS with APRT exposure, optimal screening was relatively sensitive to parameter uncertainty. Screening every 10 years from age 35 to 65 years (model recommended strategy) remained optimal in 48.1% of the PSA scenarios (Figure 2B), whereas colonoscopy from age 35 to 55 years every 10 years was optimal in 34.3%. Repeating screening every 10 years from age 30 to 60 years was cost-effective in only 17.6%. Results were sensitive also in CCS not previously treated with APRT: colonoscopy every 10 years during 45–55 and 40–60 years were optimal in 56.6% and 33.6% of PSA replications, respectively (Figure 2C).
Figure 2.
Model cost-effectiveness acceptability frontiers (CEAFs) for childhood cancer survivors (CCS) with primary cancer diagnosed at age 15 years. Results are shown for (A) all CCS; (B) CCS treated with pelvic or abdominal radiation; and (C) CCS not treated with pelvic or abdominal radiation. Uncertainty was assessed in a selected number of efficient screening strategies (the study’s optimal screening strategy, the corresponding previous less costly, and the corresponding subsequent more costly strategies). CCS = childhood cancer survivors; CEAF = cost-effectiveness acceptability frontiers; Col. = colonoscopy.
Discussion
This study provides, to our knowledge, the first evidence that the early initiation of colonoscopy screening among CCS is cost-effective and supplies the first quantitative basis for personalizing screening recommendations based on initial diagnosis and APRT dose. Colonoscopy every 10 years starting at age 35 and 45 years was predicted to produce substantial improvements in LYG and CRC mortality, respectively, among CCS treated and not treated with APRT at an acceptable cost (ICER of, respectively, $92 000 and $57 000 per LYG). Our cost-effectiveness analysis also shows that, among WT survivors, the CCS with the highest risk of developing CRC, colonoscopy screening starting at age 35 years and repeated every 3 years may be appropriate.
Recent studies have shown that CCS develop CRC more frequently and at a younger age than the general population (3–5), with the magnitude of the risk comparable to those with a family history of CRC (two or more first-degree relatives with CRC) (30). It is not surprising therefore that our results indicate to start screening at age 40 years for all CCS combined (recommended starting age for individuals with family history of CRC) (31). APRT is associated with a substantial increase in CRC risk (3) and, therefore, CCS previously treated with APRT appear to additionally benefit from an even earlier introduction of screening from age 35 years.
Despite their lower risk, early screening initiation at age 45 years was cost-effective and optimal among CCS not treated with APRT. Whether this start age should be considered “early” is controversial (12). Recently updated screening guidelines from the American Cancer Society recommend that individuals at average risk of CRC start regular screening at age 45 years, whereas the USPSTF recommends starting at age 50 years (24,32). Our results suggest that CCS treated without APRT should start screening at the earlier age.
The optimal screening frequency varied depending on past clinical circumstances. Overall, among survivors treated with APRT, screening was ideally repeated every 10 years, whereas colonoscopy repeated every 3 years was optimal in WT survivors. Shorter intervals are unlikely to be beneficial for other subgroups. The recommendations for high-risk subgroups in our study, such as WT survivors, were based on limited data. These patients merit further study, because WT is relatively common among childhood cancer diagnoses and WT survivors treated with APRT are at high risk of CRC (3). An epidemiologic evaluation of WT survivors undergoing screening might clarify whether CRC or high-risk polyps arise among those undergoing screening at more infrequent (every 5–10 year) intervals.
This study should be interpreted with caution considering the following limitations. First, our model assumptions regarding all-cause mortality in CCS were based on SEER data (1973–2013), which has very limited follow-up beyond 45 years after initial cancer diagnosis. Sensitivity analyses using different data sources (20,33) indicated that optimal stopping ages are sensitive to the source used. As prudently recommended for individuals with cystic fibrosis (34), we would suggest to decide the optimal cessation age of screening considering individual life expectancy (screening should not be indicated in CCS with a life expectancy of less than 10 years). Second, the biology underpinning the causes of the higher risk is still unclear. We assumed that the higher risk of CRC shown in CCS was caused by an increased incidence of adenomas (19), while faster progression from adenoma to CRC may also play a role, as suggested by our model validation in HL survivors (4). However, our results were not sensitive to this assumption. Third, we assumed full adherence to screening, diagnostic, and surveillance tests, because this provides unbiased estimates for optimal screening strategies. However, high-risk CCS may not attend screening as recommended (35), and therefore, population impact of screening will be lower than our estimates. Still, our results provide useful insight into the potential benefits of CRC screening among CCS. Fourth, we simulated only colonoscopy screening with data from the general population (14–17). Colonoscopy is the preferred screening option in other populations at high risk of CRC, and available literature does not suggest a higher rate of colonoscopy complications in CCS (4). Fecal immunochemical tests or fecal occult blood testing may be valid alternatives, although their diagnostic performances are still to be established in CCS.
Despite its limitations, this study has important clinical implications. Starting colonoscopy screening from age 30 years, as recently recommended by the COG (6), is unlikely to be the most cost-effective strategy for screening CCS treated with APRT. Our findings suggest that under most clinically plausible scenarios, commencing screening at age 35 years would be the most cost-effective approach, supporting indirectly COG’s previous colonoscopy screening recommendations (7). CCS not previously treated with APRT may also benefit from prompt introduction of colonoscopy screening at age 45 years. Future empirical research should further elucidate which patients have particularly high risk for CRC, such as potentially those treated with alkylating agent chemotherapy or CCS with a family history of CRC (5,19,36,37).
In conclusion, this study shows that under a range of plausible clinical scenarios, early initiation of CRC screening is cost-effective and will prevent most of the CRC deaths expected among CCS. These findings mark an important contribution to the current debate by clinicians, researchers, and policy makers about the appropriateness and necessity of an early CRC screening among these cancer survivors.
Funding
This study was funded by the Pediatric Oncology Group of Ontario and the Cancer Intervention and Surveillance Modeling Network consortium (CISNET, grant U01CA199335).
Notes
Affiliations of authors: Department of Public Health, Erasmus MC, Rotterdam, the Netherlands (AG, RGSM, IL-V); Stanford University School of Medicine, Stanford, CA (RGSM); Department of Radiation Oncology, Princess Margaret Cancer Centre, University of Toronto, Toronto, ON, Canada (HK, SA, DCH); Department of Medicine, Duke University School of Medicine, Durham, NC (KCO); Duke Cancer Institute, Durham, NC (KCO); Pediatric Oncology Group of Ontario, Toronto, ON, Canada (DCH).
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. All authors disclose no conflicts of interest. Contributors AG, DCH, and IL-V were responsible for study coordination. AG, DCH, and IL-V were responsible for the study design and first version of the manuscript. AG, DCH, IL-V, and RGSM drafted the final manuscript. All authors gave critical revisions on intellectual content of the manuscript and approved the final manuscript. DCH and ILV contributed to this work equally.
Supplementary Material
References
- 1. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 9 Regs Limited Use, Nov 2002 Sub (1973–2000). National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch. Released April 2003, based on the November 2002 submission.
- 2. Mertens AC, Yasui Y, Neglia JP, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2001;1913:3163–3172. [DOI] [PubMed] [Google Scholar]
- 3. Henderson TO, Oeffinger KC, Whitton J, et al. Secondary gastrointestinal cancer in childhood cancer survivors. Ann Intern Med. 2012;15611:757–766. W-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Daly PE, Samiee S, Cino M, et al. High prevalence of adenomatous colorectal polyps in young cancer survivors treated with abdominal radiation therapy: results of a prospective trial. Gut. 2017;6610:1797–1801. [DOI] [PubMed] [Google Scholar]
- 5. Nottage K, McFarlane J, Krasin MJ, et al. Secondary colorectal carcinoma after childhood cancer. J Clin Oncol. 2012;3020:2552–2558. [DOI] [PubMed] [Google Scholar]
- 6.Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancer. Version 5.0 (October 2018). http://www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf. Accessed November 26, 2018.
- 7.Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancer. Version 4.0 (October 2013). http://www.survivorshipguidelines.org/pdf/LTFUGuidelines_40.pdf. Accessed May 5, 2017.
- 8. Scottish Intercollegiate Guidelines Network (SIGN). Long term follow up of survivors of childhood cancer. Edinburgh: SIGN, 2013. (SIGN publication no. 132) (March 2013). http://www.sign.ac.uk. Accessed May 5, 2017.
- 9. Swedish Working Group for Long-term Follow-up after Childhood Cancer (SALUB). Version 5.0 (2010). http://www.blf.net/onko/page6/page14/files/Salub_5_2010_Eng.pdf. Accessed July 28, 2017.
- 10. Vogelaar I, van Balegooijen M, Zauber AG, et al. Model profiler of the MISCAN-Colon microsimulation model for colorectal cancer. Department of Public Health, Erasmus MC, Netherlands; 2004. http://cisnet.flexkb.net/mp/pub/cisnet_colorectal_sloankettering_profile.pdf.
- 11. Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US preventive services task force. JAMA. 2016;31523:2595–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peterse EFP, Meester RGS, Siegel RL, et al. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer. 2018;12414:2964–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gini A, Zauber AG, Cenin DR, et al. Cost effectiveness of screening individuals with cystic fibrosis for colorectal cancer. Gastroenterology. 2018;1543:556–567.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Rijn JC, Reitsma JB, Stoker J, et al. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;1012:343–350. [DOI] [PubMed] [Google Scholar]
- 15. Schroy PC 3rd, Coe A, Chen CA, et al. Prevalence of advanced colorectal neoplasia in white and black patients undergoing screening colonoscopy in a safety-net hospital. Ann Intern Med. 2013;1591:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;15012:849–857. W152. [DOI] [PubMed] [Google Scholar]
- 17. Gatto NM, Frucht H, Sundararajan V, et al. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst. 2003;953:230–236. [DOI] [PubMed] [Google Scholar]
- 18. Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;1009:630–641. [DOI] [PubMed] [Google Scholar]
- 19. Teepen JC, Kok JL, van Leeuwen FE, et al. Colorectal adenomas and cancers after childhood cancer treatment: a DCOG-LATER record linkage study. J Natl Cancer Inst. 2018;1107:758–767. [DOI] [PubMed] [Google Scholar]
- 20. Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;3749:833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;664:271–289. [DOI] [PubMed] [Google Scholar]
- 22. Leffler DA, Kheraj R, Garud S, et al. The incidence and cost of unexpected hospital use after scheduled outpatient endoscopy. Arch Intern Med. 2010;17019:1752–1757. [DOI] [PubMed] [Google Scholar]
- 23. Mark DH. Visualizing cost-effectiveness analysis. JAMA. 2002;28718:2428–2429. [DOI] [PubMed] [Google Scholar]
- 24. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2016;31523:2564–2575. [DOI] [PubMed] [Google Scholar]
- 25. Youn P, Li H, Milano MT, et al. Long-term survival among Hodgkin’s lymphoma patients with gastrointestinal cancer: a population-based study. Ann Oncol. 2013;241:202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;10019:1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;35515:1572–1582. [DOI] [PubMed] [Google Scholar]
- 28. Barton GR, Briggs AH, Fenwick EA.. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI). Value Health. 2008;115:886–897. [DOI] [PubMed] [Google Scholar]
- 29. Turner KO, Genta RM, Sonnenberg A.. Lesions of all types exist in colon polyps of all sizes. Am J Gastroenterol. 2018;1132:303–306. [DOI] [PubMed] [Google Scholar]
- 30. Reulen RC, Frobisher C, Winter DL, et al. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA. 2011;30522:2311–2319. [DOI] [PubMed] [Google Scholar]
- 31. Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;583:130–160. [DOI] [PubMed] [Google Scholar]
- 32. Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;684:250–281. [DOI] [PubMed] [Google Scholar]
- 33. Greenberg ML, Barnett H, Williams J. Atlas of childhood cancer in Ontario, 1985–2004 (January 2015). http://www.pogo.ca/research-data/pogo-atlas/. Accessed March 1, 2018.
- 34. Hadjiliadis D, Khoruts A, Zauber AG, et al. Cystic fibrosis colorectal cancer screening consensus recommendations. Gastroenterology. 2018;1543:736–745.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nathan PC, Ness KK, Mahoney MC, et al. Screening and surveillance for second malignant neoplasms in adult survivors of childhood cancer: a report from the childhood cancer survivor study. Ann Intern Med. 2010;1537:442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Eggermond AM, Schaapveld M, Janus CP, et al. Infradiaphragmatic irradiation and high procarbazine doses increase colorectal cancer risk in Hodgkin lymphoma survivors. Br J Cancer. 2017;1173:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schaapveld M, Aleman BM, van Eggermond AM, et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N Engl J Med. 2015;37326:2499–2511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.