Abstract
Mass spectrometry (MS) is a well-accepted means for analyzing glycans. Before glycan analysis by MS, several chemical derivatizations are generally carried out. These are classified into three categories; (1) labeling of the reducing end of glycans, (2) permethylation, and (3) sialic acid derivatization. Because sialic acid residues are unstable, they are easily lost during pretreatment and during or after ionization in a mass spectrometer. Sialic acid derivatization can prevent the loss of this residue. Recently, new types of sialic acid derivatization techniques have been developed, which allow straight-forward sialic acid linkage analysis (α2,3-/α2,6-linkages) as well as residue stabilization. This review summarizes the developments in sialic acid derivatization techniques, especially the varied methods of sialic acid linkage-specific derivatization.
Keywords: sialic acid, derivatization, glycan analysis, linkage analysis, mass spectrometry
1. Introduction
Protein glycosylation, which is one of the most common forms of co- or post-translational modifications, plays a crucial role in biological functions such as cellular localization, turnover, and protein quality control.1–4) Thus, protein glycosylation analysis is becoming increasingly important in the field of post-proteomics. Glycosylation can be classified into two categories, N-glycans and O-glycans. N-glycans are attached to asparagine (Asn, N) residues, whereas O-glycans are attached to serine (Ser, S) or threonine (Thr, T) residues. Additionally, several unique types of O-glycosylation have been identified, including O-fucose (Fuc),5) glucose (Glc),6,7) N-acetylglucosamine (GlcNAc),8,9) and mannose (Man)10,11) glycans.
The use of mass spectrometry (MS) in detecting glycans is a relatively new approach; however, MS has now become an indispensable tool for analyzing protein glycosylation. MS is a technique where the mass of ions is determined in the gas phase, and it is necessary to both charge the glycans and then transfer them from the condensed phase to the gas phase via evaporation.12,13) This step is termed “ionization” in MS. Both electrospray ionization (ESI)14) and matrix-assisted laser desorption/ionization (MALDI)15,16) have been mainly used to ionize fragile glycoconjugates, including released glycans, glycopeptides, and glycoproteins. Structural characterization using MS mainly relies on tandem MS (MS/MS). In this technique, analyte ions of interest are selected as precursors and broken down, and the mass of fragment ions are measured. Because the mass of fragment ions reflects the structure of unfragmented original ions, their molecular structure can be estimated. Although a wide variety of ion fragmentation techniques have been developed,17) the collision-induced dissociation technique in which ions are fragmented by collision with an inert gas is the most common, especially in glycan analysis. MS itself may be inherently unsuitable for analyzing isomer-rich glycans, which cannot be distinguished by MS alone. To compensate for the shortcomings of MS in isomeric glycan analysis, ESI-MS is often combined with various chromatographic techniques to separate the glycan isomers.
Although the MALDI technique cannot be directly combined with a chromatographic separation technique, MALDI-MS offers several advantages over ESI-MS. In particular, MALDI-MS facilitates: (i) relatively simple spectral interpretation of the production of singly charged ions; (ii) high-throughput measurements, and (iii) repeated measurements of the same sample. Thus, MALDI-MS has been widely used in a variety of applications for the analysis of glycans and glycopeptides, especially for high-throughput, multi-analyte measurements. By using a disposable MALDI sample plate, the risk of carryover can also be eliminated. However, glycosylation analysis by MS still remains a challenging task because of the low ionization efficiency, the labile nature of residues, and the complicated branched structures involving various linkage isomers. These can be attributed to the highly hydrophilic property of glycans, which potentially disturbs effective desorption from the condensed phase into the gas phase. Enhancing ionization efficiency is an important field of study in achieving highly sensitive MS-based glycosylation analysis.
The presence of sialic acid residues on glycans/glycopeptides offers other analytical difficulties. Sialyl bonds are highly unstable compared with other glycosidic bonds, leading to instantaneous loss of the residues during MS analysis. Strong negative charge retention on the residues also causes quantitative difficulties. The presence of sialyl linkage isomers increases the difficulty of analysis of sialylated glycans.
To facilitate glycan analysis by MS, chemical derivatization is often carried out. The derivatization can mainly be categorized into three types; (1) glycan reducing end labeling, (2) permethylation, and (3) sialic acid derivatization. It is also possible to carry out two or more types of chemical derivatization in a single sample.
The standard method for glycan chemical labeling is reductive amination for the reducing end, in which the glycans are labeled by aromatic hydrocarbons with amine groups in the presence of reductive reagents. This labeling has been developed for high-performance liquid chromatography (HPLC) analysis with ultraviolet or fluorescence detectors. Most of the labeling reagents have an aromatic structure, which increases the hydrophobicity of the glycans; therefore, the reducing end labeling typically enhances ionization efficiency in MS.18)
Permethylation is a reaction in which hydrogens of hydroxyl, amine, and carboxyl groups are replaced by methyl groups. Permethylation can improve sensitivity of MS by increasing ionization efficiency. This may be due to the increased hydrophobicity caused by the incorporation of a large number of methyl groups in a glycan molecule. There are several original and refined reports on the permethylation procedure.19–23)
The need for (3) sialic acid derivatization is described below.
2. Difficulties in analyzing sialylated glycans by mass spectrometry
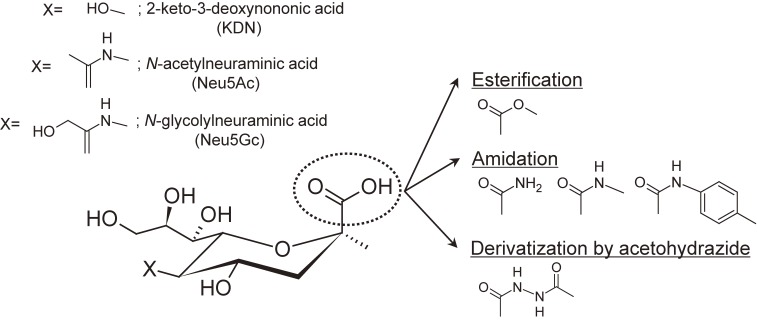
Sialic acids, a family of acidic 9-carbon carbohydrates (Fig. 1), often exist on the non-reducing ends of N-glycans and O-glycans as capping residues of Gal and GalNAc residues via mainly the α2,3-, α2,6-linkages. α2,3-linked sialic acids can be further modified by α2,8- and α2,9-linked sialic acid residues. Sialylated glycans play important roles in various biological processes including viral infection24) and cancer development.25) Serum sialylation changes associated with various types of cancer have been investigated as a potential tumor marker for early and accurate detection.26) This indicates the importance of elucidating glycan structures including sialylation patterns (i.e., the number of sialic acid residues and their linkage types).
Figure 1.
Salic acid families and derivatization scheme (linkage-nonspecific).31–34,36,37)
The presence of sialic acid residues on glycans, however, causes various analytical problems in MS. In many cases, glycan analysis by MS is conducted in positive-ion mode analysis due to their good affinity with alkali-metal cations to form [M + Na]+ or [M + K]+ readily. The presence of strong negative charges on sialic acids generally decrease their ionization efficiency, biasing mass spectrometric quantification. In addition, due to the instability of the residues, sialic acids are readily lost in mass spectrometric steps (i.e., during and/or after the ionization event) as well as in pre-treatment stages. Unfortunately, the loss of sialic acid residues by MALDI-MS is more pronounced than that of ESI-MS, probably due to the higher internal energy of the ions formed by MALDI events compared with the ions formed by ESI.
In MALDI, analyte molecules are mixed with large amounts of “matrix” molecules and then ionized using laser irradiation. The MALDI phenomenon is not fully understood yet; however, the function of the matrix is considered to be as follows; (1) absorption of irradiated laser energy; (2) the main actor causes desorption phenomenon by converting photon energy into kinetic energy; (3) ionizing reagents for analyte molecules (e.g., proton donor/acceptor). Of note, the nature of the matrix molecules impacts the degree of analyte fragmentation, because the internal energy deposited on the analyte molecules depends on the nature of the matrix used.
To prevent the loss of sialic acid residues in MALDI-MS, several “cold” matrices, which induce less fragmentation of glycan ions during MS analysis, have been investigated;27–30) however, this has not been a satisfactory approach because it is difficult to completely stabilize the residues. Furthermore, the presence of carboxyl groups (-COOH) in sialic acids often leads to multiple salt formations (-COONa, -COOK, -COONH4 etc.), depending on the experimental conditions. These salt structures have different masses, thereby one acidic glycan appears as multiple peaks in the mass spectrum. They not only complicate mass spectral interpretation but also reduce sensitivity of acidic glycan analysis.
The use of negative-ion mode might be an alternative approach for sialylated glycan MS analysis; however, the above analytical problems have not been solved. The loss of sialic acid residues and unfavorable salt formation are generally suppressed in the negative-ion mode analysis, but is not completely eliminated. Ionization bias between sialylated and neutral glycans cannot be eliminated, because acidic glycans readily form negatively charged ions (i.e., [M − H]−) compared with neutral glycans.
3. Linkage-nonspecific derivatization
Sialic acid derivatization provides a fundamental solution to the above problems. By neutralizing carboxyl groups, the loss of sialic acid and multiple alkali metal adduction are suppressed. Moreover, sialic acid derivatization inhibits preferential negative ion formation, allowing acidic glycans to possess similar ionization efficiencies as the neutral glycans. For this purpose, various types of derivatization methods have been reported. The established methods are summarized in Table 1 and Fig. 1.
Table 1.
Linkage-nonspecific sialic acid derivatization
| Nucleophile | Condensing reagents or main reagents | Sialic acid derivative | Reaction condition | Publication |
|---|---|---|---|---|
| — | CH3I, DMSO | Methylester | Room Temp., 2 hours | Powell et al., 199631) |
| Ammonium chloride (NH4Cl) | DMT-MM | Amide | 50 ℃, 24 hours | Sekiya et al., 200533) |
| — | MTT | Methylester | 50 ℃, 1∼2 hours | Miura et al., 200732) |
| Acetohydrazide | EDC (pH 2.5) | Amidation by acetohydrazide | Room Temp., 2 hours | Toyoda et al., 200834) |
| Methylamine chloride | PyAOP, NMM | Methylamide | Room Temp., 30 minutes | Liu et al., 201037) |
| p-Toluidine | EDC (pH 4.5) | Amidation by p-toluidine | Room Temp., 4 hours | Shah et al., 201336) |
The most conventional derivatization method is simple esterification. Powell et al. first introduced methyl esterification for stabilizing sialic acids in N-glycans and gangliosides.31) They used methyl iodide for esterifying the carboxyl groups on sialic acids. The sialic acid residue is stable after methyl esterification, irrespective of its linkage to the adjacent monosaccharide residue. Miura et al. presented unique methyl esterification for derivatizing glycans attached to a solid support.32) In general, solid-phase esterification is more difficult than reactions in solution. They employed a triazene derivative, 3-methyl-1-p-tolyltriazene, for efficient and facile methyl esterification of glycans immobilized onto hydrazide beads.
As another type of derivatization, amidation of sialic acid residues using a condensing reagent was introduced by Sekiya et al.33) Condensing reagents couple sialic acid with nucleophiles such as amines and alcohols. They used 4-(4,6-dimethoxy-1,3,5-triazin-2yl)-4-methylmorpholinium chloride (DMT-MM) as a condensing reagent to couple the carboxyl group on the sialic acid with ammonium chloride, forming a simple amide bond. An advantage of amidation over esterification is the stability of the derivative. Ester bonds can decompose in the MS/MS stages of structural analysis, resulting in the neutral loss of alcohol molecules. On the other hand, amides do not decompose during the collisional activation step. By using a stable isotope labeled with 15NH4Cl instead of normal NH4Cl, the m/z change of sialoglycans before and after derivatization could be suppressed to 0.0013 Da.
For these “linkage-nonspecific” derivatizations, the difference in derivatization efficiency between α2,3- and α2,6-linked sialic acids is an important issue. Incomplete derivatization weakens quantification accuracy, reducing analytical usefulness. Toyoda et al. pointed out that the modification of α2,3-linked sialic acids proceeds less efficiently than those at α2,6-linkages, resulting in incomplete derivatization of α2,3-linked sialic acids. To address this issue, they introduced quantitative derivatization for both α2,3- and α2,6-linked sialic acids.34) Amidation by acetohydrazide using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) hydrochloride in acidic conditions (pH 2.5) successfully converted the carboxyl groups on sialic acids to the corresponding acetohydrazide derivatives regardless of linkage type. Quantitative amidation with acetohydrazide has been used in one-pot sialic acid derivatization of glycoproteins on a 96-well format 10 K molecular weight cut-off membrane.35) Shah et al. extended the acidic reaction conditions to derivatize sialic acids by an aromatic amine, p-toluidine.36) The derivatization of sialic acids with p-toluidine made the glycans hydrophobic, allowed stronger retention on C18 columns, and improved ionization of glycans. They also presented an example of relative quantification using both normal and heavy (D9) p-toluidine. Other facial amidation by methylamine (MA) was introduced by Liu et al.37) The methylamidation could derivatize both α2,3- and α2,6-linked sialic acids, probably owing to the use of a powerful condensing reagent, PyAOP. Because the reaction conditions were mild, unstable modifications such as O-acetylations on sialic acids were preserved.38)
4. Linkage-specific derivatization
Although the derivatization methods mentioned above can effectively stabilize sialic acid residues, sialyl linkage isomers cannot be distinguished by these methods. The linkage isomers have identical mass; therefore, mass spectrometric distinction still remains a great challenge, even after the introduction of “linkage-nonspecific” derivatization methods.
The difference in sialyl linkages can be associated with various biological processes. The most famous example is the influenza virus infection.39,40) Avian influenza hemagglutinin preferentially recognizes α2,3-linked sialic acids, whereas human influenza hemagglutinin preferentially recognizes α2,6-linked sialic acids. The recognition process is the first step in animal influenza virus infection. However, a virus variant of avian origin with an altered binding specificity for α2,6-linked sialic acids capped glycans may acquire transmutability in humans, increasing the risk of a pandemic. The epithelial cells of the pig trachea have both α2,3- and α2,6-linked sialic acids, making them a fortuitous intermediate host for viral avian-to-human transmission. Aberrant glycosylation has also been recognized to be one of the signatures of cancer, because glycans participate in many cancer-associated events. Cancer-associated glycosylation changes often involve specific sialic acid linkages.26,41,42)
There are two main approaches in the differentiation of α2,3-/α2,6-linkages. The use of α2,3-sialidases is a traditional approach. Although this method has proven successful, specificity of discrimination directly depends on the specificity of enzymes for the α2,3-linked sialic acid residues. Therefore, the enzymatic reaction conditions need to be precisely controlled. In some cases, not all of the α2,3-linked sialic acids are detached by the enzyme.43) Some sialidase-resistant structures including Sda antigen, Cad antigen, glycolipid GM1 and GM2 structure limit their application. Nevertheless, linkage-specific sialidase treatment followed by MS has been used in the combination with various separation methods such as hydrophilic-interaction liquid chromatography (HILIC)44,45) and ion mobility (IM) spectrometry.46) In MALDI-MS, “on-target” exoglycosidase digestion can be performed. Colangelo et al. optimized “on-target” exoglycosidase digestion conditions so that the digestion completes within 30 minutes at room temperature.47) After digestion, MS can be directly acquired by adding MALDI matrix onto the target.
Another approach is by using chromatographic separation followed by retention-time matching. Because retention times are influenced by the linkage type, we can identify the linkage types by comparing with the retention time of a previously analyzed glycan standard. It is also possible to estimate retention time of a new glycan structure; however, not all cases are successful, especially for multiply-sialylated large glycans.
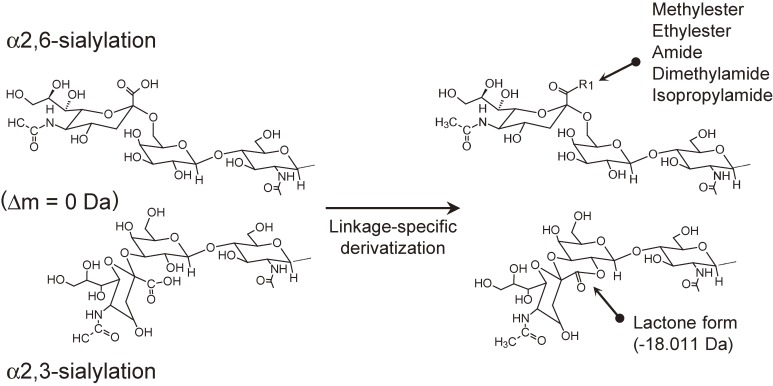
As mentioned above, MS is inherently unsuited for isomer differentiation, including sialic acid linkage analysis. However, MS can differentiate glycan peaks, as long as they possess different mass. Recently, several unique derivatization methods for MS-based α2,3-/α2,6-differentiation have been developed where α2,3- and α2,6-linked sialic acid residues have different masses.48–52) So far, all of the methods utilize the difference in reactivity of the carboxyl groups on the α2,3- and α2,6-linked sialic acid residues. They are based on a common approach: α2,6-linked sialic acids react with external nucleophiles, whereas under the same reaction conditions, α2,3-linked sialic acids form lactones by intramolecular dehydration (Fig. 2). The resulting mass difference allows the direct discrimination of sialyl linkage isomers. For these types of reaction, the reaction conditions should be strictly controlled. In particular, the choice and combination of the condensing reagent(s) and nucleophiles are important factors in the successful distinction of sialyl linkages. The established methods are summarized in Table 2.
Figure 2.
Derivatization scheme of sialic acid linkage-specific derivation.48–52)
Table 2.
Linkage-specific sialic acid derivatization
| (1st step) Linkage-specific derivatization | (2nd step) Lactone stabilization | |||||
|---|---|---|---|---|---|---|
| Nucleophile | Condensing reagents and reaction condition | Derivatives (α2,6-/α2,3-) | Reaction condition for lactones originating from α2,3- | Total reaction time for 2-step derivatization | Publication | Name |
| MeOH | DMT-MM, 80 ℃, 1 hour | Methylester/ Lactone | — | — | Wheeler et al., 200948) | |
| Ammonia | DMT-MM, 60 ℃, 15 hours | Amide/ Lactone | Solid-phase permethylation, room temp., 0.5 hour | Over 15 hours | Alley et al., 201049) | |
| EtOH | EDC+HOBt, 37 ℃, 1∼2 hours | Ethylester/ Lactone | — | — | Reiding et al., 201450) | |
| Lactone cleavage (pH 10, 1 hour) followed by methylamidation by PyAOP (room temp., 0.5 hour) | 2.5 hours | Li et al., 201654) | STSD | |||
| Amidation by p-toluidine, room temp., 3 hours | 5 hours | Yang et al., 201757) | ||||
| Amidation by ethylenediamine (EDA), 37 ℃, 3 hours | 5 hours | Yang et al., 201858) | ||||
| Amidation by EDC+HOBt, 60 ℃, 2 hours | 3 hours | Lageveen-Kammeijer and de Haan et al., 201960) | ||||
| Dimethylamine | EDC+HOBt in DMSO, 60 ℃, 1∼3 hours | Dimethylamide/ Lactone | — | — | de Haan et al., 201551) | |
| Amidation by EDC+HOBt, 60 ℃, 2 hours | 3 hours | Holst et al., 201655) | Double amidation | |||
| Permethylation | 2.5 hours | Jiang et al., 201756) | SSAP | |||
| Isopropylamine | EDC+HOBt in DMSO, room temp., 1∼2 hours | Isopropylamide/ Lactone | Methylamidation by PyAOP, room temp., 1 hour | 2 hours | Nishikaze et al., 201752) | SALSA |
| Aminolysis by methylamine or ethylamine, room temp., Few seconds | 1 hour | Hanamatsu et al., 201859) | Aminolysis-SALSA | |||
| Methylamidation by EDC+ HOBt, 60 ℃, 2 hours | 4 hours | Suzuki et al., 201869) | Modified-SALSA | |||
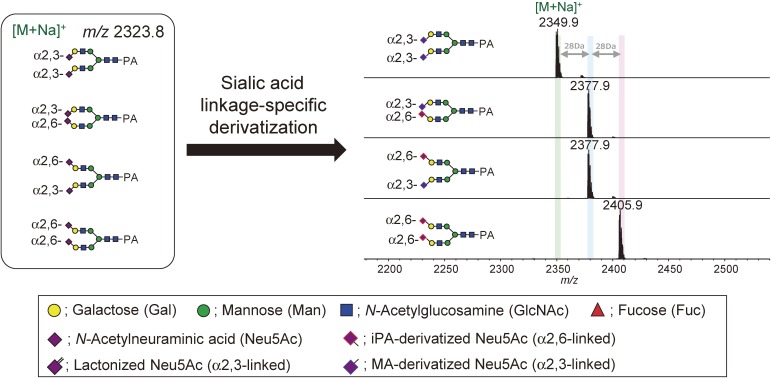
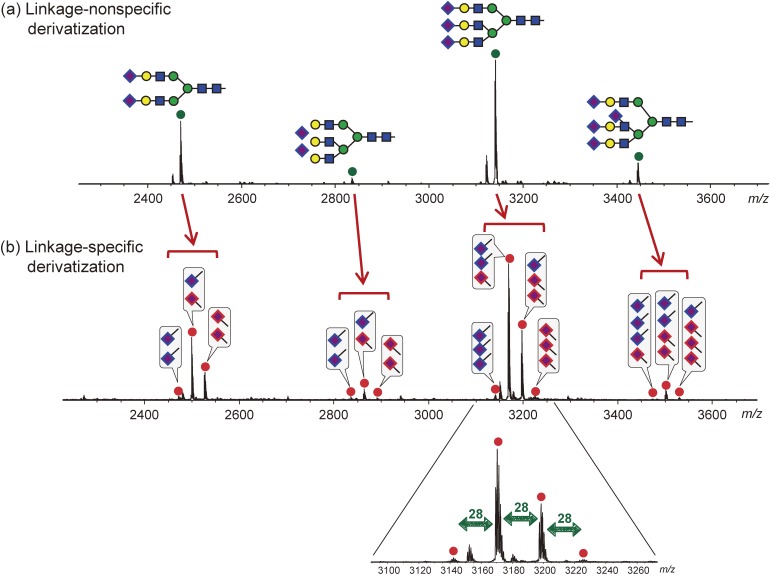
As an example, mass spectra of isomeric glycans after linkage-specific derivatization is shown in Fig. 3. Without derivatization, these isomeric glycans are detected in the mass spectra in identical m/z positions. By performing linkage-specific derivatization, isomeric glycans acquire an m/z change depending on the linkage types and their number.48–52) Figure 4 shows an example comparing the N-glycan mass spectra of bovine fetuin derivatized by linkage-nonspecific or linkage-specific derivatization. Linkage-nonspecific derivatization just stabilizes the sialic acid residues, whereas linkage-specific derivatization additionally provides linkage information by splitting a single peak into multiple peaks depending on the linkage type.
Figure 3.
Example of glycan mass spectra after sialic acid linkage-specific derivatization. All pyridylamine (PA)-labeled glycan standards have identical mass before derivatization. Here, sialic acid linkage-specific alkylamidation (SALSA) was adopted to derivatize sialic acid; therefore, the mass difference between α2,3-/α2,6- is 28 Da.52)
Figure 4.
Comparison of N-glycan mass spectra released from bovine Fetuin derivatized by (a) linkage-nonspecific methylamidation37) and (b) linkage-specific alkylamidation (SALSA).52)
Originally, Wheeler et al. introduced linkage-specific methyl esterification using a condensing reagent DMT-MM and methanol as a nucleophile.48) In these derivatization conditions, α2,6-linked sialic acids were converted to methyl esters by the reaction with methanol, whereas α2,3-linked sialic acids formed lactones by intramolecular dehydration. The resulting mass difference obtained from the mass spectra allowed linkage-specific differentiation of the types of sialic acids.
Recently, Reiding et al. reported more practical linkage-specific esterification using a different condensing reagent mixture.50) They concluded that the use of a carbodiimide-based condensing reagent mixture (EDC and HOBt) and ethanol as a nucleophile results in ethyl esterification of α2,6-linked sialic acids and lactonization of α2,3-linked sialic acids. This type of reaction appears to be robust, allowing direct derivatization of PNGaseF-released glycan mixture including detergents, buffer, and deglycosylated proteins without a prior desalting step. This linkage-specific ethyl esterification has been incorporated into automated platforms for glycomics analysis of large scale plasma N-glycans.53) Based on the Reidings’ linkage-specific esterification condition, several amidation versions of linkage-specific derivatization methods have been developed using dimethylamine51) or isopropylamine52) as a nucleophile.
Although the conversion from α2,3-linked sialic acids into lactone forms actually stabilizes the sialic acid residues, the lactone forms are still unstable. In fact, Wheeler et al. pointed out that lactone can be completely degraded within 50 hours by just being dissolved in water.48) This instability can limit the downstream chemical and/or enzymatic treatments.54,55) As pointed out by other researchers,54) lactone forms cannot survive enzymatic digestion for long, resulting in partial delactonization.
5. Linkage-specific derivatization with lactone stabilization
To overcome the limitations caused by lactone instability, two-step derivatization methods have been further developed recently.49,52,54–60) In a two-step reaction, α2,3-/α2,6-discrimination specificity depends on the first reaction; the second step just converts the lactone forms into more stable forms. There are three methods for stabilizing the lactone form: permethylation, lactone cleavage followed by amidation, and direct aminolysis.
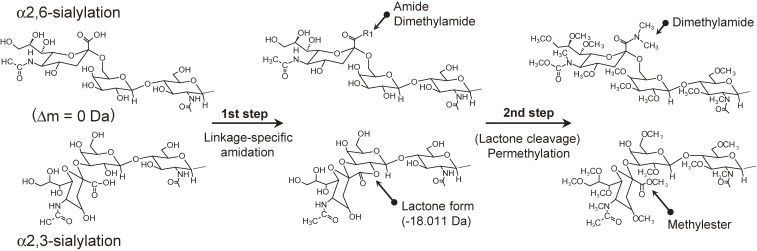
5-1. Linkage-specific derivatization + permethylation.
In general, due to the harsh permethylation reaction conditions, ester bonds are easily cleaved. Therefore, linkage-specific esterification followed by permethylation is not suitable for linkage differentiation. Permethylation can only be combined with linkage-specific amidation (Fig. 5). Alley et al. developed linkage-specific amidation suitable for combination with the permethylation reaction.49) The newly developed linkage-specific amidation requires 15 hours incubation at 60 ℃ with ammonium chloride and DMT-MM. After this, amidated and/or lactonized glycans are permethylated in the solid-phase. By permethylation, amidated α2,6-linked sialic acids are converted to dimethylamidated forms, whereas lactonized α2,3-linked sialic acids are converted to methylesterified forms, allowing m/z distinction by MS. More recently, Jiang et al. combined linkage-specific dimethylamidation with an in-solution permethylation technique. This linkage-specific sialic acid permethylation showed high derivatization specificity for α2,3- and α2,6-sialic acids.56) In the process, linkage-specific dimethylamidation using dimethylamine and EDC+HOBt was first carried out at 60 ℃ for 1 hour, and then the excess dimethyamine was evaporated by vacuum centrifugation for 20 min at 60 ℃. A 10-minute permethylation was finally carried out by adding water, NaOH-dimethyl sulfoxide slurry, and methyl iodide at room temperature. Because the linkage-specific dimethylamidation shared the same reaction solvent of NaOH-dimethyl sulfoxide with permethylation, sample purification between the two steps could be avoided.
Figure 5.
Derivatization scheme of sialic acid linkage-specific derivation combined with permethylation reaction as lactone stabilization step.49,56)
5-2. Linkage-specific derivatization + amidation.
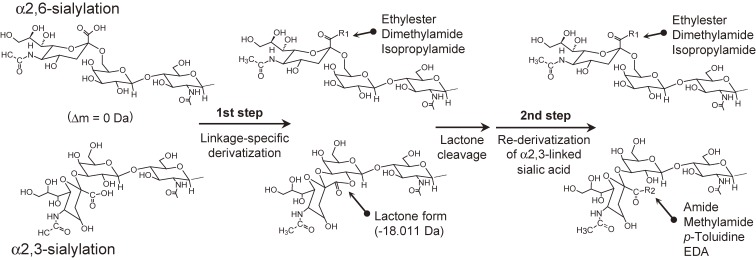
Lactone stabilization can also be achieved by temporary lactone cleavage followed by amidation (Fig. 6). This type of two-step method has been mostly developed for solid-phase reactions (i.e., glycans or glycoproteins bound to a solid support). The methods require different sequential reaction conditions; therefore, by adopting a solid-phase reaction the reaction solution can be changed easily without extensive purification steps such as solid-phase extraction.
Figure 6.
Derivatization scheme of sialic acid linkage-specific derivation combined with temporary lactone cleavage followed by re-derivatization of the carboxyl groups on α2,3-linked sialic acids.52,54,55,57,58,60,69)
Li et al. introduced two-step reactions for linkage-specific derivatization of sialic acids on glycoproteins fixed to a solid support.54) They mixed linkage-specific esterification reported by Reiding et al.50) with linkage-nonspecific methylamidation by PyAOP.37) In the first reaction, α2,6-linked sialic acids were converted to ethylester whereas α2,3-linked sialic acids were converted to lactones. The lactones were then cleaved at mild alkaline condition (pH 10 Tris-HCl solution, 1 hour incubation). Next, original carboxyl groups on α2,3-linked sialic acids were converted to methylamide forms by the reaction with MA using the condensing reagent PyAOP. The final derivatives were as follows; α2,3-linked sialic acid: methylamide, α2,6-linked sialic acid: ethylester. This two-step derivatization (actually, three steps including the lactone cleavage process), requires completely different reaction conditions for each process. Therefore, the use of this two step derivatization method is in essence limited to the analyte derivatization on solid supports. Furthermore, alkali conditions can also partially cleave the ester forms originating from the α2,6-linkages, therefore the lactone cleavage process needs to be strictly controlled. Recently, Yang et al. developed other two-step methods that employ linkage-specific esterification followed by amidation with p-toluidine.57) The final derivatives were as follows; α2,3-linked sialic acid: p-toluidine derivative, α2,6-linked sialic acid: ethylester. Unfortunately, there is an ionization bias between the p-toluidine derivatized form and the ethylester form, so an intensity correction is required for the quantitation using the signal intensities in mass spectra.
Holst et al. developed a linkage-specific double amidation method for obtaining sialyl linkage-specific N-glycan MS images from formalin-fixed, paraffin-embedded tissues.55) MS imaging of N-glycan requires sialic acid derivatization followed by glycan release by PNGaseF. Because lactone forms cannot survive this enzymatic treatment step, they introduced an additional amidation step for stabilization of the lactone form. Their procedure was relatively simple; 1 hour linkage-specific amidation by dimethylamine followed by the direct addition of ammonia solution and 2 hour incubation at 60 ℃. An additional ammonia adduction step initiated the hydrolysis of the lactones and the subsequent stable amidation of the α2,3-linked sialic acids. They actually developed the method for on-tissue derivatization; however, the method is also suited to in-solution derivatization of sialic acids. Lageveen-Kammeijer et al. combined above lactone stabilization using ammonia with linkage-specific esterification using EDC + HOBt/ethanol. This two-step derivatization can be carried out as a one-pot reaction. Excess reagents can be removed using a single HILIC-solid-phase extraction clean-up step, offering simple handling over other two-step derivatization methods.60)
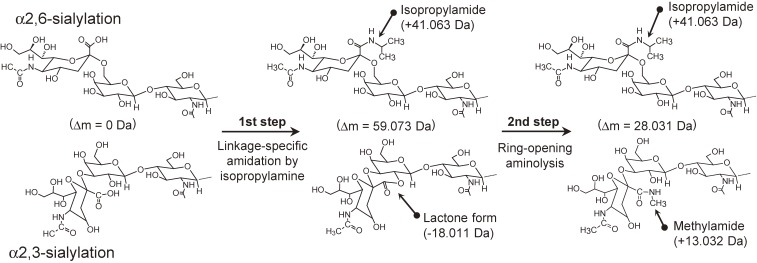
Our group has developed a similar but more reliable amidation-based linkage-specific derivatization, named sialic acid linkage-specific alkylamidation (SALSA).52) SALSA converts carboxyl groups on sialic acid residues into alkylamide forms with different alkyl chain lengths in a linkage-specific manner, allowing direct discrimination of sialyl linkages based on the difference in m/z values. We employed isopropylamine (iPA) as the nucleophile for linkage-specific derivatization. The use of iPA offers selective conversion for distinguishing α2,3- and α2,6-linked sialic acids by converting them into lactone and isopropylamide forms. Lactone forms are then stabilized by amidation with MA. Methylamidation using a condensing reagent PyAOP appeared to directly convert lactonized α2,3-sialic acids to their methylamide forms; however, employing a lactone cleavage step before methylamidation improved conversion efficiency, especially for highly sialylated N-glycans containing α2,3-linkages. The final derivatives were as follows; α2,3-linked sialic acid: methylamide, α2,6-linked sialic acid: isopropylamide. We combined derivatization and a one-pot glycan purification technique, glycoblotting.61–63)
5-3. Linkage-specific derivatization + ring-opening aminolysis.
As described above, several researchers have tried to stabilize lactone forms by temporary lactone cleavage followed by amidation. We proposed a more straightforward approach. The second lactone stabilization process was replaced by direct amidation of the lactones, the so-called ring-opening aminolysis reaction (Fig. 7).59) It should be noted that the aminolysis of lactone is nearly instantaneous; therefore, no reaction time is required. Furthermore, this reaction does not require condensing reagents. Primary amine and hydrazine analogues can be used for ring-opening aminolysis, without causing unfavorable hydrolysis side-reaction.
Figure 7.
Derivatization scheme of aminolysis-SALSA.59)
We also demonstrated that the SALSA technique combined with ring-opening aminolysis (termed aminolysis-SALSA) can successfully distinguish serum glycosphingolipid (GLS)-glycan isomers in MS.59) The glycan structure in GLS is known to contain α2,3-, α2,6-, and additional α2,8-linked sialic acid. The glycan moiety was first released from GLS by EGCase I, and then the glycan moiety was captured by the hydrazide beads. Sialic acids were derivatized sequentially by aminolysis-SALSA on the beads. It was confirmed that α2,8-linked sialic acids were derivatized by MA by aminolysis-SALSA. Although α2,3- and α2,8-linked sialic acid residues had identical mass even after aminolysis-SALSA, α2,8-linked sialic acid could be distinguished from α2,3-linked sialic acids by MS/MS analysis. Because α2,8-linked sialic acid residues are attached to other sialic acid residues to form polysialic acid structure, a characteristic neutral loss consisting of two or more sialic acid residues can be found in the MS/MS spectra. The characteristic neutral loss is less common in the MS/MS spectra of glycan with only α2,3-linked sialic acid residues.59)
6. Sialic acid derivatization on glycopeptides
Compared with the sialic acid derivatization of (released) glycans, the derivatization of glycopeptides remains a challenging task, mostly due to the difficulties in ensuring uniformity in the reactions occurring on the peptide moiety. At present, only a few reports are available, indicating that this is an ongoing research area.
Quantitative derivatization by acetohydrazide has been used to derivatize glycopeptides as well. Because the derivatization can cap carboxyl groups on both sialic acids and on the peptide backbone, other acidic groups such as sulfate groups can emerge, allowing the enrichment of sulfated glycopeptides.64) Methylamidation by PyAOP also shows highly effective and specific reactivity for carboxyl groups on glycopeptides. Methylamidation suppresses preferential loss of sialic acid residues during MS analysis and enhances important glycan fragments in negative-ion MS/MS conditions.65) Note that these derivatization techniques are linkage-nonspecific; therefore, sialyl linkages cannot be distinguished.
Linkage-specific sialic acid derivatization on glycopeptides is more challenging than the linkage-nonspecific derivatization techniques mentioned above. de Haan et al. reported an application of linkage-specific derivatization using dimethylamine for IgG Fc glycopeptide derivatization.51) The derivatization was successful, showing no side reactions affecting the peptide backbone. Because the report focused on only the derivatization of IgG Fc glycopeptides, other glycopeptide derivatizations were not shown. General use of linkage-specific dimethylamidation for the derivatization of a wide variety of glycopeptides needs to be optimized to ensure uniformity in the reactions occurring on the peptide moiety.
Recently, Yang et al. developed a linkage-specific derivatization approach for glycoproteomics.58) Glycoproteins were first immobilized on a solid support and then the sialic acids (and side chains on acidic amino acid residues) were derivatized in a linkage-specific manner. They used Reiding’s linkage-specific ethylesterification followed by lactone stabilization by amidation with ethylenediamine (EDA). Glycopeptides were released from the solid support by trypsin digestion. Derivatization by EDA enhanced ionization efficiency, achieving special suitability for the production of multiply charged ions. Although sialic acid derivatization before trypsin digestion can suppress the reaction complexity, the derivatization conditions seem to have room for improvement in suppressing cross derivatization (i.e. ethylester or EDA derivative) for side chains on acidic amino acid residues.
7. Sialic acid derivatization for liquid chromatography MS or capillary electrophoresis MS
Although the advantage of sialic acid derivatization is mostly emphasized in MALDI-MS analysis, the neutralization of sialic acids also has several benefits in chromatographic separation using liquid chromatography (LC) and capillary electrophoresis (CE). However, the number of reports is limited so far.
Tousi et al. reported the combination of linkage-specific methylesterification using DMT-MM and nano-HILIC-MS.66) Linkage-specific charge neutralization of sialic acids not only allows discrimination of α2,3-/α2,6-sialyl linkages by MS, but also improves the preceding HILIC separation by increasing hydrophobicity and altering the selectivity of the glycan analytes. Linkage-nonspecific methylamidation has been applied to nano-LC analysis of sialoglycans.67) Improvements in separation and peak shape were achieved in porous carbon graphitic columns.
Khatri et al. used Reiding’s linkage-specific ethyl esterification for capping sialic acids on released glycans and glycopeptides to develop a microfluidics-based CE-MS system.68) If sialic acids are esterified, migration in CE and peak shape are less affected by the negatively charged sialic acid residues. They also pointed out the detection of unesterified forms of glycans and multiple backbone modifications of glycopeptides. Recently, Lageveen-Kammeijer et al. developed a highly sensitive CE/MS glycan analysis platform adopting a sequential derivatization technique. The negative charges of sialic acids are first neutralized by two-step sialic acid linkage-specific derivatization. The reducing end of the glycan is then labeled by Girard reagent P to give permanent positive charges. As a result, all neutral and sialylated glycans have uniform charge, enabling efficient ionization in positive ion mode. By optimizing the ionization conditions, they achieved attomole level glycan analysis sensitivity with excellent reproducibility.60)
Sialic acid linkage-specific alkylamidation has been applied and optimized for LC/MS analysis of PA-labeled glycans.69) Although the influence of sialic acid derivatization on the retention behavior in normal-phase and reverse-phase HPLC has not been mentioned, linkage-specific alkylamidation may accomplish identification of sialyl Lewis X structures in MS/MS structural analysis.
8. Application of linkage-specific sialic acid derivatization
Sialic acid derivatization was originally developed for glycan analysis by MALDI-MS, and a major application is in the field of high-throughput, multiple analyte glycan profiling measurements such as in cohort studies. Many large-scale serum/plasma N-glycan profiling studies have been conducted to find disease-related glycosylation changes.70–73) Using sialic acid linkage-specific derivatization, MALDI-MS based high-throughput analysis can identify new aspects for evaluation.
Other than high-throughput analyses, linkage-specific derivatization also offers the advantage of detailed analysis of glycans. Multi-dimensional HPLC analysis of PA-labeled glycans is recognized to be a powerful tool for full-characterization of the glycan structure.74,75) However, these methods occasionally fail in determining sialyl linkages on multiply-sialylated large glycans. Nonomura et al. used linkage-specific derivatization to determine sialyl linkages on HPLC-separated PA-glycans from mouse cochlea.76) Sialic acid linkage-specific chemical derivatization allows direct determination of sialyl linkages even in the absence of corresponding glycan standards.
9. Conclusions
MS has now become an essential device for post-translational modification analysis of proteins, including the analysis of glycosylation. To increase sensitivity and obtain more detailed structural information, several chemical modifications are generally carried out as pretreatment steps before analyzing glycans with MS. This review summarizes the methods for the modification of carboxylic acid groups on sialic acid residues. Sialic acid derivatization methods were originally developed to compensate for the disadvantages of MALDI-MS analysis of sialoglycans, such as ionization bias and sialic acid loss. However, recently developed sialic acid linkage-specific derivatization techniques have unlocked new possibilities for glycan analysis by MS. The value of sialic acid modification in LC/MS or CE/MS analysis is beginning to be recognized and their use will continue to expand.
Although glycan analysis by MS inherently involves difficulty in isomer differentiation, this is being overcome by many technical developments such as combinations with chromatographic techniques and the novel derivatization techniques mentioned above. These technical developments will contribute to the discovery of new disease biomarkers and functions of glycoproteins and glycolipids.
Profile
Takashi Nishikaze was born in Kagawa prefecture in 1980. He graduated from Yokohama City University in 2003. His doctoral study was focused on the mass spectrometric analysis of biomolecules. After receiving his Ph.D. degree in 2008, he joined the laboratory of glycobiology in The Noguchi Institute as a researcher and entered the world of glycan analysis. In 2011, he moved to Shimadzu Corporation. He has been developing methods for highly sensitive and informative glycan analysis by mass spectrometry as a member of Koichi Tanaka Mass Spectrometry Research Laboratory in Shimadzu Corporation. He received MSSJ Research Award from Mass Spectrometry Society of Japan in 2017.
References
- 1).Apweiler R., Hermjakob H., Sharon N. (1999) On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. Biophys. Acta 1473, 4–8. [DOI] [PubMed] [Google Scholar]
- 2).Parodi A.J. (1999) Reglucosylation of glycoproteins and quality control of glycoprotein folding in the endoplasmic reticulum of yeast cells. Biochim. Biophys. Acta 1426, 287–295. [DOI] [PubMed] [Google Scholar]
- 3).Fukuda M., Sasaki H., Fukuda M.N. (1989) Structure and role of carbohydrate in human erythropoietin. Adv. Exp. Med. Biol. 271, 53–67. [DOI] [PubMed] [Google Scholar]
- 4).Arnold J.N., Wormald M.R., Sim R.B., Rudd P.M., Dwek R.A. (2007) The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 25, 21–50. [DOI] [PubMed] [Google Scholar]
- 5).Moloney D.J., Lin A.I., Haltiwanger R.S. (1997) The O-linked fucose glycosylation pathway: Evidence for protein-specific elongation of O-linked fucose in Chinese hamster ovary cells. J. Biol. Chem. 272, 19046–19050. [DOI] [PubMed] [Google Scholar]
- 6).Takeuchi H., Kantharia J., Sethi M.K., Bakker H., Haltiwanger R.S. (2012) Site-specific O-glucosylation of the Epidermal Growth Factor-like (EGF) repeats of notch: Efficiency of glycosylation is affected by proper folding and amino acid sequence of individual EGF repeats. J. Biol. Chem. 287, 33934–33944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Yu H., Takeuchi H. (2019) Protein O-glucosylation: Another essential role of glucose in biology. Curr. Opin. Struct. Biol. 56, 64–71. [DOI] [PubMed] [Google Scholar]
- 8).Hart G.W., Slawson C., Ramirez-Correa G., Lagerlof O. (2011) Cross talk between O-GlcNAcylation and phosphorylation: Roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 80, 825–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Hu P., Shimoji S., Hart G.W. (2010) Site-specific interplay between O-GlcNAcylation and phosphorylation in cellular regulation. FEBS Lett. 584, 2526–2538. [DOI] [PubMed] [Google Scholar]
- 10).Lommel M., Strahl S. (2009) Protein O-mannosylation: Conserved from bacteria to humans. Glycobiology 19, 816–828. [DOI] [PubMed] [Google Scholar]
- 11).Endo T. (2019) Mammalian O-mannosyl glycans: Biochemistry and glycopathology. Proc. Jpn. Acad. Ser. B 95, 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Nishikaze T., Takayama M. (2006) Cooperative effect of factors governing molecular ion yields in desorption/ionization mass spectrometry. Rapid Commun. Mass Spectrom. 20, 376–382. [DOI] [PubMed] [Google Scholar]
- 13).Nishikaze T., Takayama M. (2007) Study of factors governing negative molecular ion yields of amino acid and peptide in FAB, MALDI and ESI mass spectrometry. Int. J. Mass Spectrom. 268, 47–59. [Google Scholar]
- 14).Fenn J.B., Mann M., Meng C., Wong S., Whitehouse C.M. (1989) Electrospray ionization for mass spectrometry of large biomolecules. Science 246, 64–71. [DOI] [PubMed] [Google Scholar]
- 15).Tanaka K., Waki H., Ido Y., Akita S., Yoshida Y., Yoshida T. (1988) Protein and polymer analyses up to m/z 100 000 by laser ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2, 151–153. [Google Scholar]
- 16).Karas M., Hillenkamp F. (1988) Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal. Chem. 60, 2299–2301. [DOI] [PubMed] [Google Scholar]
- 17).Dodds E.D. (2012) Gas-phase dissociation of glycosylated peptide ions. Mass Spectrom. Rev. 31, 666–682. [DOI] [PubMed] [Google Scholar]
- 18).Harvey D.J. (2000) Electrospray mass spectrometry and fragmentation of N-linked carbohydrates derivatized at the reducing terminus. J. Am. Soc. Mass Spectrom. 11, 900–915. [DOI] [PubMed] [Google Scholar]
- 19).Hakomori S. (1964) A rapid permethylation of glycolipid, and polysaccharide catalyzed by methylsulfinyl carbanion in dimethyl sulfoxide. J. Biochem. 55, 205–208. [PubMed] [Google Scholar]
- 20).Kang P., Mechref Y., Klouckova I., Novotny M.V. (2005) Solid-phase permethylation of glycans for mass spectrometric analysis. Rapid Commun. Mass Spectrom. 19, 3421–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Jeong H.-J., Kim Y.-G., Yang Y.-H., Kim B.-G. (2012) High-throughput quantitative analysis of total N-glycans by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal. Chem. 84, 3453–3460. [DOI] [PubMed] [Google Scholar]
- 22).Kang P., Mechref Y., Novotny M.V. (2008) High-throughput solid-phase permethylation of glycans prior to mass spectrometry. Rapid Commun. Mass Spectrom. 22, 721–734. [DOI] [PubMed] [Google Scholar]
- 23).Hu Y., Borges C.R. (2017) A spin column-free approach to sodium hydroxide-based glycan permethylation. Analyst 142, 2748–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Wasik B.R., Barnard K.N., Parrish C.R. (2016) Effects of sialic acid modifications on virus binding and infection. Trends Microbiol. 24, 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Pearce O.M.T., Läubli H. (2015) Sialic acids in cancer biology and immunity. Glycobiology 26, 111–128. [DOI] [PubMed] [Google Scholar]
- 26).Zhang Z., Wuhrer M., Holst S. (2018) Serum sialylation changes in cancer. Glycoconj. J. 35, 139–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Papac D.I., Wong A., Jones A.J.S. (1996) Analysis of acidic oligosaccharides and glycopeptides by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal. Chem. 68, 3215–3223. [DOI] [PubMed] [Google Scholar]
- 28).Laremore T.N., Zhang F., Linhardt R.J. (2007) Ionic liquid matrix for direct UV-MALDI-TOF-MS analysis of dermatan sulfate and chondroitin sulfate oligosaccharides. Anal. Chem. 79, 1604–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Fukuyama Y., Nakaya S., Yamazaki Y., Tanaka K. (2008) Ionic liquid matrixes optimized for MALDI-MS of sulfated/sialylated/neutral oligosaccharides and glycopeptides. Anal. Chem. 80, 2171–2179. [DOI] [PubMed] [Google Scholar]
- 30).Fukuyama Y., Funakoshi N., Takeyama K., Hioki Y., Nishikaze T., Kaneshiro K., et al. (2014) 3-Aminoquinoline/p-Coumaric acid as a MALDI matrix for glycopeptides, carbohydrates, and phosphopeptides. Anal. Chem. 86, 1937–1942. [DOI] [PubMed] [Google Scholar]
- 31).Powell A.K., Harvey D.J. (1996) Stabilization of sialic acids in N-linked oligosaccharides and gangliosides for analysis by positive ion matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun. Mass Spectrom. 10, 1027–1032. [DOI] [PubMed] [Google Scholar]
- 32).Miura Y., Shinohara Y., Furukawa J., Nagahori N., Nishimura S.-I. (2007) Rapid and simple solid-phase esterification of sialic acid residues for quantitative glycomics by mass spectrometry. Chemistry 13, 4797–4804. [DOI] [PubMed] [Google Scholar]
- 33).Sekiya S., Wada Y., Tanaka K. (2005) Derivatization for stabilizing sialic acids in MALDI-MS. Anal. Chem. 77, 4962–4968. [DOI] [PubMed] [Google Scholar]
- 34).Toyoda M., Ito H., Matsuno Y., Narimatsu H., Kameyama A. (2008) Quantitative derivatization of sialic acids for the detection of sialoglycans by MALDI MS. Anal. Chem. 80, 5211–5218. [DOI] [PubMed] [Google Scholar]
- 35).Gil G.-C., Iliff B., Cerny R., Velander W.H., Van Cott K.E. (2010) High throughput quantification of N-glycans using one-pot sialic acid modification and matrix assisted laser desorption ionization time-of-flight mass spectrometry. Anal. Chem. 82, 6613–6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Shah P., Yang S., Sun S., Aiyetan P., Yarema K.J., Zhang H. (2013) Mass spectrometric analysis of sialylated glycans with use of solid-phase labeling of sialic acids. Anal. Chem. 85, 3606–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Liu X., Qiu H., Lee R.K., Chen W., Li J. (2010) Methylamidation for sialoglycomics by MALDI-MS: A facile derivatization strategy for both α2,3- and α2,6-linked sialic acids. Anal. Chem. 82, 8300–8306. [DOI] [PubMed] [Google Scholar]
- 38).Wu Z., Li H., Zhang Q., Liu X., Zheng Q., Li J. (2017) Characterization of O-acetylation in sialoglycans by MALDI-MS using a combination of methylamidation and permethylation. Sci. Rep. 7, 46206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Suzuki Y. (2006) Highly pathogenic avian influenza viruses and their sialo-sugar receptors. Trends Glycosci. Glycotechnol. 18, 153–155. [Google Scholar]
- 40).Suzuki Y. (2005) Sialobiology of influenza: Molecular mechanism of host range variation of influenza viruses. Biol. Pharm. Bull. 28, 399–408. [DOI] [PubMed] [Google Scholar]
- 41).Ruhaak L.R., Miyamoto S., Lebrilla C.B. (2013) Developments in the identification of glycan biomarkers for the detection of cancer. Mol. Cell. Proteomics 12, 846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Mechref Y., Hu Y., Garcia A., Hussein A. (2012) Identifying cancer biomarkers by mass spectrometry-based glycomics. Electrophoresis 33, 1755–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Hanzawa K., Suzuki N., Natsuka S. (2017) Structures and developmental alterations of N-glycans of zebrafish embryos. Glycobiology 27, 228–245. [DOI] [PubMed] [Google Scholar]
- 44).Tao S., Huang Y., Boyes B.E., Orlando R. (2014) Liquid chromatography-selected reaction monitoring (LC-SRM) approach for the separation and quantitation of sialylated N-glycans linkage isomers. Anal. Chem. 86, 10584–10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Mancera-Arteu M., Giménez E., Barbosa J., Sanz-Nebot V. (2016) Identification and characterization of isomeric N-glycans of human alfa-acid-glycoprotein by stable isotope labelling and ZIC-HILIC-MS in combination with exoglycosidase digestion. Anal. Chim. Acta 940, 92–103. [DOI] [PubMed] [Google Scholar]
- 46).Guttman M., Lee K.K. (2016) Site-specific mapping of sialic acid linkage isomers by ion mobility spectrometry. Anal. Chem. 88, 5212–5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Colangelo J., Orlando R. (1999) On-target exoglycosidase digestions/MALDI-MS for determining the primary structures of carbohydrate chains. Anal. Chem. 71, 1479–1482. [DOI] [PubMed] [Google Scholar]
- 48).Wheeler S.F., Domann P., Harvey D.J. (2009) Derivatization of sialic acids for stabilization in matrix-assisted laser desorption/ionization mass spectrometry and concomitant differentiation of α(2→3)- and α(2→6)-isomers. Rapid Commun. Mass Spectrom. 23, 303–312. [DOI] [PubMed] [Google Scholar]
- 49).Alley W.R., Novotny M.V. (2010) Glycomic analysis of sialic acid linkages in glycans derived from blood serum glycoproteins. J. Proteome Res. 9, 3062–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Reiding K.R., Blank D., Kuijper D.M., Deelder A.M., Wuhrer M. (2014) High-throughput profiling of protein N-glycosylation by MALDI-TOF-MS employing linkage-specific sialic acid esterification. Anal. Chem. 86, 5784–5793. [DOI] [PubMed] [Google Scholar]
- 51).de Haan N., Reiding K.R., Haberger M., Reusch D., Falck D., Wuhrer M. (2015) Linkage-specific sialic acid derivatization for MALDI-TOF-MS profiling of IgG glycopeptides. Anal. Chem. 87, 8284–8291. [DOI] [PubMed] [Google Scholar]
- 52).Nishikaze T., Tsumoto H., Sekiya S., Iwamoto S., Miura Y., Tanaka K. (2017) Differentiation of sialyl linkage isomers by one-pot sialic acid derivatization for mass spectrometry-based glycan profiling. Anal. Chem. 89, 2353–2360. [DOI] [PubMed] [Google Scholar]
- 53).Vreeker G.C.M., Nicolardi S., Bladergroen M.R., Van Der Plas C.J., Mesker W.E., Tollenaar R.A.E.M., et al. (2018) Automated plasma glycomics with linkage-specific sialic acid esterification and ultrahigh resolution MS. Anal. Chem. 90, 11955–11961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Li H., Gao W., Feng X., Liu B.-F., Liu X. (2016) MALDI-MS analysis of sialylated N-glycan linkage isomers using solid-phase two step derivatization method. Anal. Chim. Acta 924, 77–85. [DOI] [PubMed] [Google Scholar]
- 55).Holst S., Heijs B., de Haan N., van Zeijl R.J.M., Briaire-de Bruijn I.H., van Pelt G.W., et al. (2016) Linkage-specific in situ sialic acid derivatization for N-glycan mass spectrometry imaging of formalin-fixed paraffin-embedded tissues. Anal. Chem. 88, 5904–5913. [DOI] [PubMed] [Google Scholar]
- 56).Jiang K., Zhu H., Li L., Guo Y., Gashash E., Ma C. (2017) Sialic acid linkage-specific permethylation for improved profiling of protein glycosylation by MALDI-TOF MS. Anal. Chim. Acta 981, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Yang S., Jankowska E., Kosikova M., Xie H., Cipollo J. (2017) Solid-phase chemical modification for sialic acid linkage analysis: Application to glycoproteins of host cells used in influenza virus propagation. Anal. Chem. 89, 9508–9517. [DOI] [PubMed] [Google Scholar]
- 58).Yang S., Wu W.W., Shen R.-F., Bern M., Cipollo J. (2018) Identification of sialic acid linkages on intact glycopeptides via differential chemical modification using IntactGIG-HILIC. J. Am. Soc. Mass Spectrom. 29, 1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Hanamatsu H., Nishikaze T., Miura N., Piao J., Okada K., Sekiya S., et al. (2018) Sialic acid linkage specific derivatization of glycosphingolipid glycans by ring-opening aminolysis of lactones. Anal. Chem. 90, 13193–13199. [DOI] [PubMed] [Google Scholar]
- 60).Lageveen-Kammeijer G.S.M., de Haan N., Mohaupt P., Wagt S., Filius M., Nouta J., et al. (2019) Highly sensitive CE-ESI-MS analysis of N-glycans from complex biological samples. Nat. Commun. 10, 2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Nishimura S.-I., Niikura K., Kurogochi M., Matsushita T., Fumoto M., Hinou H., et al. (2005) High-throughput protein glycomics: Combined use of chemoselective glycoblotting and MALDI-TOF/TOF mass spectrometry. Angew. Chem. Int. Ed. 44, 91–96. [DOI] [PubMed] [Google Scholar]
- 62).Shimaoka H., Kuramoto H., Furukawa J., Miura Y., Kurogochi M., Kita Y., et al. (2007) One-pot solid-phase glycoblotting and probing by transoximization for high-throughput glycomics and glycoproteomics. Chemistry 13, 1664–1673. [DOI] [PubMed] [Google Scholar]
- 63).Furukawa J., Shinohara Y., Kuramoto H., Miura Y., Shimaoka H., Kurogochi M., et al. (2008) Comprehensive approach to structural and functional glycomics based on chemoselective glycoblotting and sequential tag conversion. Anal. Chem. 80, 1094–1101. [DOI] [PubMed] [Google Scholar]
- 64).Toyoda M., Narimatsu H., Kameyama A. (2009) Enrichment method of sulfated glycopeptides by a sulfate emerging and ion exchange chromatography. Anal. Chem. 81, 6140–6147. [DOI] [PubMed] [Google Scholar]
- 65).Nishikaze T., Kawabata S., Tanaka K. (2014) In-depth structural characterization of N-linked glycopeptides using complete derivatization for carboxyl groups followed by positive- and negative-ion tandem mass spectrometry. Anal. Chem. 86, 5360–5369. [DOI] [PubMed] [Google Scholar]
- 66).Tousi F., Bones J., Hancock W.S., Hincapie M. (2013) Differential chemical derivatization integrated with chromatographic separation for analysis of isomeric sialylated N-glycans: A nano-hydrophilic interaction liquid chromatography-MS platform. Anal. Chem. 85, 8421–8428. [DOI] [PubMed] [Google Scholar]
- 67).Zhang Q., Feng X., Li H., Liu B.-F., Lin Y., Liu X. (2014) Methylamidation for isomeric profiling of sialylated glycans by nanoLC-MS. Anal. Chem. 86, 7913–7919. [DOI] [PubMed] [Google Scholar]
- 68).Khatri K., Klein J.A., Haserick J.R., Leon D.R., Costello C.E., Mccomb M.E., et al. (2017) Micro fluidic capillary electrophoresis — Mass spectrometry for analysis of monosaccharides, oligosaccharides, and glycopeptides. Anal. Chem. 89, 6645–6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).Suzuki N., Abe T., Natsuka S. (2018) Quantitative LC-MS and MS/MS analysis of sialylated glycans modified by linkage-specific alkylamidation. Anal. Biochem. 567, 117–127. [DOI] [PubMed] [Google Scholar]
- 70).Reiding K.R., Vreeker G.C.M., Bondt A., Bladergroen M.R., Hazes J.M.W., van der Burgt Y.E.M., et al. (2018) Serum protein N-glycosylation changes with rheumatoid arthritis disease activity during and after pregnancy. Front. Med. 4, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71).Clerc F., Novokmet M., Dotz V., Reiding K.R., de Haan N., Kammeijer G.S.M., et al. (2018) Plasma N-glycan signatures are associated with features of inflammatory bowel diseases. Gastroenterology 155, 829–843. [DOI] [PubMed] [Google Scholar]
- 72).Ferrantelli E., Farhat K., Ederveen A.L.H., Reiding K.R., Beelen R.H.J., van Ittersum F.J., et al. (2018) Effluent and serum protein N-glycosylation is associated with inflammation and peritoneal membrane transport characteristics in peritoneal dialysis patients. Sci. Rep. 8, 979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Dotz V., Lemmers R.F.H., Reiding K.R., Hipgrave Ederveen A.L., Lieverse A.G., Mulder M.T., et al. (2018) Plasma protein N-glycan signatures of type 2 diabetes. Biochim. Biophys. Acta 1862, 2613–2622. [DOI] [PubMed] [Google Scholar]
- 74).Takahashi N., Nakagawa H., Fujikawa K., Kawamura Y., Tomiya N. (1995) Three-dimensional elution mapping of pyridylaminated N-linked neutral and sialyl oligosaccharides. Anal. Biochem. 226, 139–146. [DOI] [PubMed] [Google Scholar]
- 75).Natsuka S., Masuda M., Sumiyoshi W., Nakakita S. (2014) Improved method for drawing of a glycan map, and the first page of glycan atlas, which is a compilation of glycan maps for a whole organism. PLoS One 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76).Nonomura Y., Sawamura S., Hanzawa K., Nishikaze T., Okuda S., Miyoshi E., et al. (2019) Characterisation of N-glycans in the epithelial-like tissue of the rat cochlea. Sci. Rep. 9, 1551. [DOI] [PMC free article] [PubMed] [Google Scholar]