Abstract
A ketogenic diet is a potential adjuvant cancer therapy that limits glucose availability to tumours while fuelling normal tissues with ketone bodies. We examined the effect of a low carbohydrate ketogenic diet (LCKD) (80% kcal from fat, ketogenic ratio 1.75:1, w/w) compared to a general hospital diet (GD) on serum metabolic profiles in patients (n = 18, ≥ 19 years old) who underwent pancreatectomy for pancreatobiliary cancer. Serum samples collected preoperatively (week 0) and after the dietary intervention (week 2) were analysed with a nontargeted metabolomics approach using liquid chromatography–tandem mass spectrometry. Serum β-hydroxybutyrate and total ketone levels significantly increased after 2 weeks of LCKD compared to GD (p < 0.05). Principal component analysis score plots and orthogonal partial least squares discriminant analysis also showed significant differences between groups at week 2, with strong validation. In all, 240 metabolites differed between LCKD and GD. Pathways including glycerophospholipid and sphingolipid metabolisms were significantly enriched in the LCKD samples. LCKD decreased C22:1-ceramide levels, which are reported to be high in pancreatic cancer, while increasing lysophosphatidylcholine (18:2), uric acid, citrulline, and inosine levels, which are generally low in pancreatic cancer. Postoperative LCKD might beneficially modulate pancreatic cancer-related metabolites in patients with pancreatobiliary cancer.
Subject terms: Nutrition, Tumour biomarkers
Introduction
Pancreatic cancer causes severe morbidity and has a high mortality rate because its early stage diagnosis is difficult and it presents a high risk of metastasis1. The 5-year survival rate (from 2004 to 2010) of patients diagnosed with pancreatic cancer in the United States was only 7%2. While only 15–20% of patients with pancreatic cancer are eligible for tumour resection, early detection can increase the 5-year survival rate to ≥ 50%3. Surgical treatment for pancreatic cancer commonly leads to malnutrition, which may result in poor surgical outcomes and an increased toxicity of chemo-/radiation therapy; therefore, postoperative nutritional support in patients with pancreatic cancer is important to improve prognosis4. Cancer-related malnutrition has been associated with a low quality of life, poor muscle function, increased length of hospital stay, high mortality, and surgical complications5. La Torre et al. reported that after surgical tumour resection, 88% of patients with pancreatic cancer had a medium-high risk of malnutrition6. These patients had significantly longer recovery times and increased morbidity rates when compared to patients who were at a low risk of malnutrition6.
A ketogenic diet has recently been investigated as an adjuvant cancer treatment7. Allen et al. defined the oral caloric composition of a ketogenic diet as 90% fat, 8% protein, and 2% carbohydrate7. A ketogenic diet is recognized as an effective dietary intervention for neurological diseases, including epilepsy and Alzheimer’s disease, increasing the levels of ketone bodies such as β-hydroxybutyrate, a major ketone body in blood8. The low carbohydrate and high fat composition of a ketogenic diet can limit glucose utilization in cancer cells7. Following ketosis, the levels of the gluconeogenic substrates lactate, pyruvate, and alanine decreased in the blood of cachectic cancer patients while free fatty acids and ketone bodies were increased; thus, ketosis may reduce glucose supply to the tumour while nourishing the patient with alternative energy sources9. Shukla et al. reported that the ketone bodies β-hydroxybutyrate and acetoacetate were associated with inhibited cell growth, reduced glucose uptake, and decreased glutamine and glutamate levels in pancreatic cancer cells; these ketone bodies were also associated with decreased measures of cachexia in patients10. In mice with pancreatic cancer, a ketogenic diet (81% kcal from fat) reduced blood glucose levels, increased ketone levels in the blood and tumour, reduced tumour growth, and alleviated cachexia (45% increase in muscle weight, 20% increase in body weight) in comparison to a control diet10. Schroeder et al. found that 2–5 days of a ketogenic diet in patients with head and neck cancer decreased lactate levels in the tumour, showing that lactate can substitute glucose as an energy source for tumour cells11. Fearon et al. demonstrated that in patients with gastric cancer, lung cancer, or ovarian cancer, a ketogenic diet (44 kcal/kg/day: 70% of energy from medium-chain triglycerides (MCT)) increased body weight by up to 2 kg after 7 days, with no gastrointestinal side effects, whereas a normal diet (55% kcal from carbohydrates and 31% kcal from fat) did not result in any body weight change after 6 days9. Breitkreutz et al. reported that among moderately malnourished patients undergoing chemotherapy for colorectal or gastric cancer, an 8-week fat-enriched liquid diet (66% kcal from fat) in addition to normal meals significantly increased body weight and fat-free mass compared to a normal diet12. Therefore, a ketogenic diet has a potential therapeutic effect on cancer and may improve the postoperative nutritional status of patients with cancer who are at risk of malnutrition.
Metabolomics is a powerful tool used to identify metabolic changes in the body and to analyse full sets of metabolites in cells, tissues, and organisms13. Previous metabolomic analyses have suggested that several blood metabolites are markers for pancreatic cancer. Bathe et al. detected increased levels of glutamate, acetone, and β-hydroxybutyrate using 1H and 2D nuclear magnetic resonance (NMR) spectroscopy14. A metabolomics study using gas chromatography time-of-flight mass spectrometry, liquid chromatography-electrospray ionisation mass spectrometry, and liquid chromatography-tandem mass spectrometry (LC-MS/MS) revealed altered levels of amino acids, fatty acids, bile acids, and lipids such as lysophosphatidylcholine (lysoPC) (18:2), phosphatidylcholine (PC) (34:2), and phosphatidylethanolamine (PE) (26:0) associated with pancreatic cancer15. In addition, gas chromatography-mass spectrometry (GC-MS) detected changes in metabolites of patients with pancreatic cancer, such as increases in lactate and asparagine levels and decreases in urea and saturated fatty acid levels16, as well as reduced serum 1,5-anhydro-D-glucitol and amino acids, including valine, lysine, and tyrosine17. A metabolomics study using flow-injection Fourier transform ion cyclotron resonance mass spectrometry detected changes in serum glycerophospholipids (GPL) in pancreatic cancer patients18. Metabolomics can be used to evaluate the effects of a dietary intervention on metabolic profiles of living organisms19, and metabolic profiles after dietary intervention have been investigated in patients and in mouse models of cancer20. One metabolomics study compared the effects of 6 weeks of a whole grain rye and rye bran-rich diet with a refined white wheat diet in 17 patients with prostate cancer20. However, to the best our knowledge, there are no metabolomics studies on the impact of a ketogenic diet in patients who have undergone pancreatectomy for pancreatobiliary cancer.
The purpose of this pilot study was to examine the postoperative effects of a low-carbohydrate ketogenic diet (LCKD) on the serum metabolic profiles in patients who underwent pancreatectomy for pancreatobiliary cancer, compared to a general hospital diet (GD).
Materials and Methods
Study subjects
This study was approved by and carried out in accordance with the guidelines and regulations of the Severance Hospital Institutional Review Board (Assignment number: 4-2016-0799) in Seoul, Korea. All subjects provided informed consent. Adult patients (≥ 19 years old) with pancreatobiliary cancer (i.e. pancreatic cancer, duodenal cancer, distal bile duct cancer, or ampullary cancer) who underwent pancreaticoduodenectomy or distal pancreatectomy were enrolled at a pancreatic surgery clinic between November 2016 and May 2017. We excluded patients who were pregnant, illiterate, from a foreign country, or who had severe diabetic complications, hyperlipidaemia with cardiovascular complications, or renal insufficiency with a normal glomerular filtration rate < 90%. After screening 47 patients for eligibility, 30 patients voluntarily enrolled and were randomly assigned to receive GD or LCKD. One patient in the GD group was excluded due to postoperative complications, while 11 patients in the LCKD group were excluded due to postoperative complications (n = 4), refusal to consume the diet (n = 5), loss of data (n = 1), and outlying data (n = 1). A total of 18 participants were included in the final analysis (see Supplementary Fig. S1). Body weight and Patient-Generated Subjective Global Assessment (PG-SGA) scores were recorded throughout the study period with the help of a professionally trained dietitian.
Dietary intervention
Once patients resumed a full liquid oral diet after surgery, they were provided either a GD (n = 9) or an LCKD (n = 9). Both diets were equal in energy content (1500 kcal/d) and were provided for 6 or 7 days during the 2-week hospitalization period before chemoradiotherapy was started. The full liquid and soft GD provided 55–65%, 7–20%, and 15–30% of energy from carbohydrate (C), protein (P), and fat (F), respectively. The full liquid and soft LCKD provided 4%, 16%, and 80% of energy from C, P and F, respectively, targeting a ketogenic ratio of 1.75:1 (F:C + P w/w). Diet initiation differed according to the type of surgery. Patients who underwent a pylorus-preserving pancreaticoduodenectomy were provided a clear liquid diet for 5 days and GD or LCKD from postoperative day (POD) 5. Patients who underwent a distal-pancreatectomy were provided a clear liquid diet until POD 1–3, GD or full liquid LCKD until POD 4, and soft diet until discharge. The protocol was adjusted depending on the patients’ conditions; for example, one GD patient received sips of water from POD 9 for 5 days and resumed oral GD until discharge. Dietary intake was measured through a daily 24-hour recall assisted by a professionally trained dietitian.
Sample collection
Blood samples were collected under fasting conditions 3 times in total: the day before surgery (week 0), the day of discharge (week 2), and at the first outpatient visit (week 4). Blood samples were centrifuged at 2,500 rpm for 15 min. The supernatant serum was transferred to a clean tube and stored at −80 °C until analysis.
Quantitative analysis of ketone bodies, insulin, glucose, and TNF-α in blood
The amount of β-hydroxybutyrate (CAS No. 150-83-4, Sigma-Aldrich, USA) in serum was measured by LC-MS/MS using an Ultimate 3000 UHPLC and Q-Extractive Orbitrap Plus (Thermo Fisher Scientific, USA). Standard concentrations ranged from 0.625 to 50 μg/mL. To measure the total ketone (acetoacetate + β-hydroxybutyrate) concentration, the ketone body assay kit (Sigma-Aldrich, USA) was used according to the manufacturer’s instructions. Absorbance was measured at 340 nm using a Tecan GENios multi-functional plate reader (Infinite®F500, TecanGrödig, Austria). Serum insulin levels were measured using an electrochemiluminescence immunoassay with an Insulin Reagent kit (Roche, Germany) and an immunoassay analyser (Roche, Japan). Insulin sensitivity was 0.20 μU/mL. Serum glucose was determined using an enzymatic method (Asan Diagnostics, Korea) according to the manufacturer’s instructions. Serum tumour necrosis factor-α (TNF-α) levels were measured using enzyme-linked immunosorbent assay CyMAX human TNF-α (AbFrontier, Korea) according to the manufacturer’s instructions. The absorbance was measured at 450 nm on a microplate reader (Infinite® 200 PRO, Tecan Trading AG, Switzerland).
Standard sample preparation for LC-MS/MS analysis
Acetaminophen, sulfadimethoxine, terfenadine, and reserpine (Sigma-Aldrich, Canada) were mixed at the same volume ratio to 10 mg/L in 70% acetonitrile (acetonitrile:methanol = 7:3 v/v) and used as internal standards (IS). Pooled serum samples from all patients were used as quality control (QC) samples. Next, 100 μL of each serum sample was mixed with 800 μL of a solvent (methanol:acetone = 7:3 v/v) and 50 μL of IS. After centrifugation, the clear supernatant was lyophilised in a freeze-dryer for 18 hours at −84 °C, and then received 100 μL of 10% methanol. Finally, 90 μL of the sample was collected and 5 μL of each sample was used in the LC-MS/MS analysis.
LC-MS/MS analysis
An Ultimate 3000 UHPLC and Q-Extractive Orbitrap Plus was used for the analysis. The column (2.1 × 150 mm) was packed with C18 stationary 1.7 μm-sized resin. The column oven was maintained at 50 °C. Mobile phase A used 0.1% formic acid in water and mobile phase B used 0.1% formic acid in methanol. The total flow rate was 0.4 mL/min and the elution gradient (A/B, v/v) was changed from 100/0 to 0/100 for 15 min, maintained at 0/100 for 4 min, and then changed back to 100/0 for 2 min. The full scan/dd-MS2 conditions were as follows: FTMS, ESI-positive mode with a mass resolution of 70,000; full scan range: 80–1000 m/z; dd-MS2 (Top 10) resolution of 17,500 with collision energy of 30; flow rate of nitrogen sheath gas and auxiliary gas: 40 (arbitrary units) and 10 (arbitrary units); spray voltage: 3.5 kV; capillary temperature: 320 °C; S- lensRF level: 50; auxiliary gas heater temperature: 300 °C. QC samples were injected into every tenth sample to check data quality and reliability.
Data processing and metabolite identification
The raw LC-MS data files (.raw) were imported to the XCMS online platform (https://xcmsonline.scripps.edu/) for nonlinear alignment of the data in the time domain and for extraction of the peak intensities21. The parameter settings were a 10 sec band width, 15 ppm tolerance for database search, and default values for values not shown. The extracted data included retention time, m/z, and ion intensity. The exact masses of differential ions were verified in online databases, including HMDB (www.hmdb.ca) and MycompoundID (www.mycompoundid.org). The masses and intensities of the query masses were compared with those in the database using a fit score ≥ 0.9. MetaboAnalyst 4.0 (www.metaboanalyst.ca/) was used to conduct hierarchical cluster analysis and draw heat maps to verify the classification ability of the metabolites. Pearson correlation analysis was performed using the same tool. MetaboAnalyst 4.0 is an open access online tool that supports statistical analysis, visualization, and interpretation of metabolomics data22.
Pathway analysis
MetaboAnalyst 4.0 was used to conduct a pathway analysis, taking the concentrations of the identified metabolites into account with multivariate variable importance in the projection (VIP) value > 1.0 and p < 0.005. Metabolome view plots were generated to allow identification and analysis of the significantly impacted pathways23. Pathways were defined as significantly enriched using cut-offs of p < 0.05 for the adjusted false discovery rate (FDR-adjusted) and > 0.1 for the pathway impact score. The KEGG Pathway database (https://www.kegg.jp/kegg/pathway.html) and SMPDB (http://smpdb.ca/) were used to search the superpathway and pathway comprising each individual metabolite. In the case metabolites were involved in multiple metabolic pathways, the most exposed pathway was indicated.
Statistical analysis
Univariate nonparametric Mann–Whitney U tests were run for all metabolites, and multivariate principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) were performed for all groups using SIMCA version 14.1 (Umetrics Inc., Sweden)24–26. Metabolic peak intensities were log transformed and scaled using Pareto scaling27 prior to multivariate analysis using SIMCA 14.1 (Umetrics, Inc., Sweden)28. Log transformation was used to normalize right-skewed distribution of metabolite intensity values29. Pareto scaling adjusted for the relative importance of large values27. Robustness and validity of the results were assessed with parameters R2X, R2Y, and Q2Y, as well as with a cross-validated analysis of variance (CV-ANOVA). The metabolites were filtered as univariate statistical p-value < 0.0530 and VIP value > 1.0. Information about the patient’s medical and anthropometry measures was expressed as mean ± standard deviation (SD).
Results
Patient characteristics, nutritional intake, and blood biochemistry
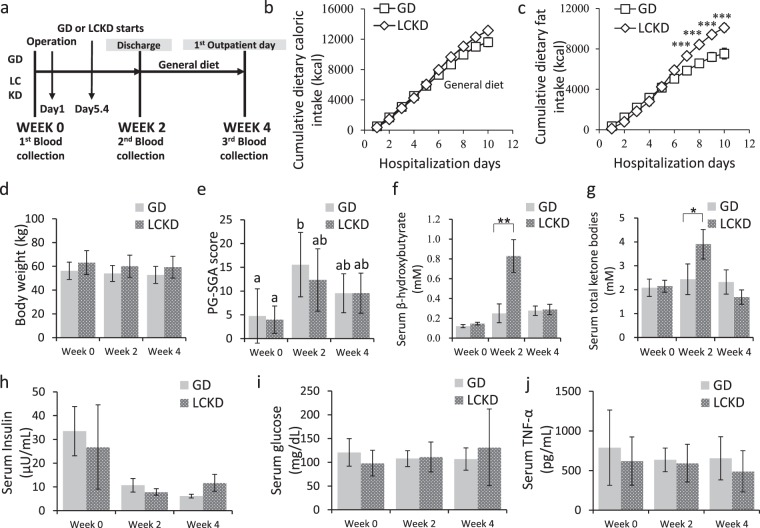
The general characteristics of participants are summarized in Table 1. Average age, male to female ratio, histological cancer type, and surgical operation type were not significantly different between the groups. The timeline of the study is shown in Fig. 1a. The average postoperative days were not different between GD (17.2 ± 11.8 days) and LCKD (13.4 ± 5.5 days) groups. There was no difference between GD and LCKD groups in the cumulative total caloric intake during the hospitalization period (Fig. 1b). However, the cumulative caloric intake of dietary fat was significantly different between the two groups (Fig. 1c). Body weight was not significantly different between GD and LCKD groups during the study period (Fig. 1d). The PG-SGA score indicated that patients were in a poorer nutrition state after surgery, but this was alleviated at week 4 in both groups, and there were no significant differences between groups (Fig. 1e). At week 2, the LCKD group showed significantly higher ketone levels than the GD group, showing that LCKD induced ketone body production (Fig. 1f,g). At week 4, there were no significant differences in ketone levels compared with the baselines either within each group or between the groups (Fig. 1f,g). Serum insulin, glucose, and TNF-α levels were not different between the GD and LCKD groups during the perioperative period (Fig. 1h–j). Changes in blood chemistry, including blood levels of creatinine, pre-albumin, cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglyceride, lipoprotein, transferrin, CEA, and CA19-9, were not significant between the groups during the study period (see Supplementary Table S1).
Table 1.
General characteristics of GD and LCKD groups.
| GD (n = 9) | LCKD (n = 9) | p-value | |
|---|---|---|---|
| Age(year) | 66.3 ± 9.8 | 58.3 ± 7.6 | 0.072 |
| Sex(n(%)) | |||
| Male | 6(66.7) | 5(55.6) | 1.000 |
| Female | 3(33.3) | 4(44.4) | |
| Histological type | |||
| Pancreatic ca. | 2 | 3 | |
| (head/body/tail) | (1/0/1) | (1/2/0) | |
| Ampulla of Vater cancer | 2 | 1 | 0.741 |
| Common bile duct | 5 | 3 | |
| Duodenal cancer | 0 | 1 | |
| NET | 0 | 1 | |
| Surgical operation | |||
| PPPD | 7 | 8 | 1.000 |
| DP | 2 | 1 | |
| BMI (kg/m2) | 22.2 ± 2.7 | 24.0 ± 2.2 | 0.149 |
| Weight (kg) | 56.3 ± 7.3 | 63.1 ± 10.1 | 0.117 |
| CEA | 2.13 ± 1.23 | 1.95 ± 0.63 | 0.693 |
| CA19-9 | 150.6 ± 306.5 | 31.8 ± 33.9 | 0.265 |
| Hospitalization period | 17.4 ± 11.8 | 11.3 ± 2.1 | 0.161 |
| Total oral diet period (day) | 11.9 ± 10.5 | 7.5 ± 4.3 | 0.146 |
| Percentage of energy intake to EER (kcal/kcal %)* | |||
| from total caloric intake (%) | 87.5 ± 16.6 | 89.8 ± 22.4 | 0.807 |
| from meal (%) | 31.9 ± 12.5 | 44.8 ± 18.4 | 0.101 |
| from PN | 57.0 ± 21.1 | 45.9 ± 8.5 | 0.197 |
Values are mean ± standard deviation. p-values were derived from independent Student’s t-tests at baseline. The statistical differences between GD and LCKD groups for histological type were obtained using Fisher’s exact test after crossover analysis.
NET, neuroendocrine tumour; PPPD, pylorus-preserving pancreaticoduodenectomy; DP, distal pancreatectomy; BMI, body mass index; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; EER, estimated energy requirement; PN, parenteral nutrition.
Figure 1.
Nutritional changes and blood profiles in GD and LCKD groups during the study period. (a) Timeline of the study, (b) cumulative total dietary caloric intake, and (c) cumulative total dietary fat intake (kcal) are shown. There were no differences in (d) body weight (kg) via the Mann-Whitney U test. The PG-SGA score is a nutrition assessment tool that identifies malnutrition in hospitalized patients. Higher scores indicate poorer nutritional statuses. Statistical differences in (e) PG-SGA score, serum (f) β-hydroxybutyrate, (g) total ketone bodies, (h) insulin, (i) glucose, and (j) TNF-α levels were analysed between time periods (general linear model) and between groups (Kruskal Wallis). *p < 0.05; **p < 0.01. Different lowercase letters (a, b) over bars represent significant within group differences (Dunnett T3). Mean ± S.E. PG-SGA, Patient-Generated Subjective Global Assessment; TNF-α, tumour necrosis factor-α.
Non-targeted metabolomics analysis by LC-MS/MS
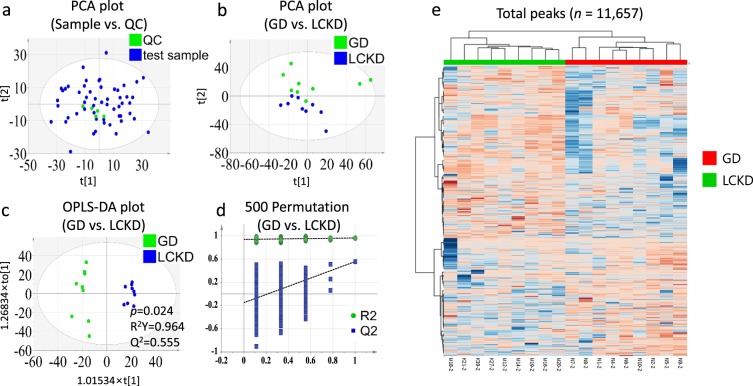
A total of 11,657 ionized compounds in the ESI+ mode were detected using LC-MS/MS. While QC samples were closely clustered (Fig. 2a), results for the GD and LCKD were clearly separated at week 2 in two-dimensional PCA score plot analysis (Fig. 2b). OPLS-DA score plot analysis at each time point (weeks 0, 2, and 4) indicated that baseline profiles between the groups were not different (Table 2). A good classification model was detected only between GD and LCKD groups at week 2 (Table 2 and Fig. 2c). A statistical validation of the OPLS-DA model was performed using 500 permutation tests (Fig. 2d). A heat map of the total peaks retrieved from XCMS is presented in Fig. 2e. These results suggested that a differential metabolite profile between GD and LCKD groups was detected only at week 2.
Figure 2.
PCA score plot, OPLS-DA three-dimensional score plots and validation plot for the metabolic profiling results between GD and LCKD (week 2). PCA score plots compared (a) sample and QC and (b) GD and LCKD groups at week 2. (c) OPLS-DA score plot (three latent variables, p = 0.024, R2Y = 0.964, Q2 = 0.555) and (d) the 500-permutation plot validated GD versus LCKD in the ESI+ mode. All permuted R2 and Q2 values on the left were lower than the point on the right and the Q2 regression line had a negative intercept. (R2 = 0.933959, Q2 = −0.174472). (e) The total peak intensities between GD and LCKD at week 2 are visualized with a hierarchical cluster analysis heat map. OPLS-DA, orthogonal partial least squares discriminant analysis; PCA, principal component analysis; QC, quality control.
Table 2.
OPLS-DA parameters and permutation test for distinguishing GD and LCKD groups.
| Group | OPLS-DA | p-valueb | |||
|---|---|---|---|---|---|
| Componentsa | Q2 | R2 | Q2/R2 | ||
| GD-LCKD | |||||
| Week 0–0 | 5 | 0.470 | 0.943 | 0.50 | 0.055 |
| Week 2–2 | 1 | 0.555 | 0.964 | 0.58 | 0.024 |
| Week 4–4 | 3 | 0.608 | 0.938 | 0.65 | 0.057 |
| GD-GD | |||||
| Week 0–2 | 6 | 0.554 | 0.998 | 0.60 | 0.129 |
| Week 0–4 | 5 | 0.470 | 1.000 | 0.47 | 0.181 |
| Week 2–4 | 3 | 0.569 | 0.999 | 0.57 | 0.174 |
| LCKD-LCKD | |||||
| Week 0–2 | 6 | 0.780 | 1.000 | 0.78 | 0.120 |
| Week 0–4 | — | — | — | — | — |
| Week 2–4 | 7 | 0.649 | 1.000 | 0.65 | 0.525 |
aThe number of components based on Q2 indicates the best classifier of OPLS-DA using a 7-fold cross-validation method.
bp-value was determined by CV-ANOVA of OPLS-DA in SIMCA 14.1. p < 0.05 was considered significant.
CV-ANOVA, cross-validated analysis of variance; OPLS-DA, orthogonal partial least squares discriminant analysis; Q2, predictive capability; R2, goodness of fit. OPLS-DA not performed was shown as “—”.
Differential metabolites between GD and LCKD
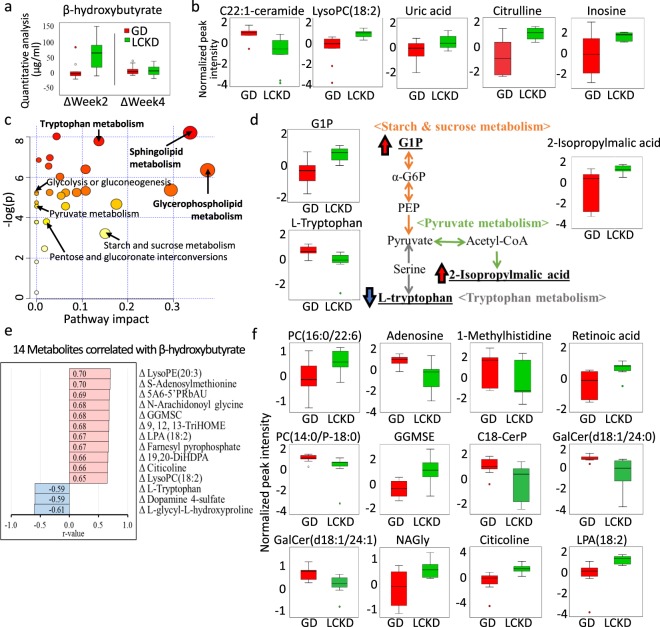
A slight upregulation in β-hydroxybutyrate was detected by targeted quantitative analysis with LCKD at week 2 compared to GD (p = 0.05) (Fig. 3a). To compare the metabolomics data of GD and LCKD at week 2, cut-off values of OPLS-DA VIP score > 1.0 and p < 0.05 were used, resulting in the detection of 588 differential peaks. C22:1-ceramide, a previously reported pancreatic cancer-specific metabolite31, was significantly downregulated while lysoPC(18:2)15,18, uric acid16, citrulline32, and inosine15 were significantly upregulated in the LCKD group at week 2 (Fig. 3b). After excluding drugs (n = 20), xenobiotics (n = 82), a fit score < 0.9 (n = 99), and significantly different metabolites at baseline (n = 147), a total of 240 metabolites were included in further correlation analyses (see Supplementary Table S2).
Figure 3.
Metabolomic analysis of 240 metabolites enriched in LCKD compared to GD. All figures except Fig. 3a represent data at week 2 compared with baseline. (a) Changes in serum β-hydroxybutyrate levels using LC-MS/MS quantitative analysis between baseline and week 2 or 4 are compared in a box plot form. (b) Box plots of significantly altered pancreatic cancer-specific biomarkers. Peaks were normalized using log transformation and Pareto scaling. (c) An overview of pathway analysis using 65 metabolites (VIP > 1.0, p < 0.005) is shown. A total of 33 metabolic pathways were detected by MetaboAnalyst. Significant metabolic pathways (FDR-adjusted < 0.05, pathway impact > 0.1) are labelled in bold and carbohydrate-related pathways are labelled in plain text. (d) An overview of carbohydrate-related metabolic pathways among 240 detected metabolites (p < 0.05). Among the 65 metabolites (VIP > 1.0, p < 0.005) hit in the 3 pathways, those upregulated and downregulated by LCKD are marked with red and blue arrows, respectively. (e) Correlation analysis between the 240 metabolites and β-hydroxybutyrate changes (Pattern Hunter, Pearson). Red and blue colours indicate a positive and negative correlation, respectively. (f) Box plots of the 4 significant metabolites found in both pathway and correlation analyses. LysoPC, lysophosphatidylcholine; PC, phosphatidylcholine; LPA, lysophosphatidic acid; α-G6P, α-D-Glucose-6P; G1P, Glucose-1-phosphate; PEP, 2-phosphoenolpyruvate; 5A6-5′PRbAU, S-Amino-6-(5′phosphoribitylamino)uracil; GGMSC, Gamma-glutamyl-Se-methylselenocysteine; CerP, ceramide phosphate; GalCer, Galabiosylceramide; NAGly, N-Arachidonoyl glycine.
Pathway analysis
For metabolic pathway analysis, we chose a more stringent cut-off value of VIP score > 1.0 and p < 0.005, resulting in 65 differential metabolites for analysis (Fig. 3c, also see Supplementary Table S3). A total of 8 pathways were found to be significantly enriched, including sphingolipid (FDR-adjusted = 0.003, pathway impact = 0.337) and GPL (FDR-adjusted = 0.005, pathway impact = 0.375) metabolic pathways (Fig. 3c, also see Supplementary Table S3). In addition, carbohydrate-related metabolic pathways were also found among 8 differential metabolites (VIP > 1.0, p < 0.05), including glucose-1-phosphate (G1P) and 2-isopropylmalic acid (Fig. 3d).
Correlation analysis
We conducted a correlation analysis for metabolite changes between weeks 0 and 2 with LCKD-induced changes in β-hydroxybutyrate levels (see Supplementary Table S4). Significant positive and negative correlations were detected in in 63 and 7 metabolites, respectively (see Supplementary Table S4). The top 11 upregulated and 3 downregulated metabolites are presented in Fig. 3e. To further investigate the significance of our LCKD in postoperative patients with pancreatic cancer, metabolites found in both pathway and correlation analyses were selected. Among these metabolites, those with literature-based importance were summarized as box plots in Fig. 3f.
Discussion
The data demonstrated that our LCKD was ketogenic and induced metabolic alterations in postoperative patients with pancreatobiliary (periampullary and distal pancreatic) cancer who underwent pancreatectomy. A ketogenic diet is generally high in fat, at a ketogenic ratio of 3:1 to 4:1, F:C + P (w/w)7. A low glycaemic index treatment and a modified Atkins diet with a 1:1 ketogenic ratio are also used in the Johns Hopkins protocol, which induces ketosis by restricting carbohydrates to 10–20 g/day while not restricting protein, fluid, or energy33. Our LCKD targeted a ketogenic ratio of 1.75:1 and a nutritional composition of 15.95 g (4% kcal), 60.00 g (16% kcal), and 132.91 g (80% kcal) of C, P, and F, respectively. To prevent body protein loss, a minimum of 20~25 kcal/kg and 1.0 g of protein per kg was provided, based on the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines34.
LCKD noticeably increased the serum levels of total ketones and β-hydroxybutyrate, suggesting that the ketogenic process was enhanced. In our metabolomics analysis, β-hydroxybutyrate was detected and significantly upregulated by LCKD, but the change was offset at week 4. This supports the ketogenic effect of LCKD. Clinical cancer studies using ketone-producing diets have shown that ketosis may decrease lactate levels in tumour tissues, reduce tumour-associated markers, and improve body weight in cachectic cancer patients and mice9,10. The anticancer effects of ketone bodies have received attention due to their ineffectiveness as an energy source for cancer cells with altered mitochondrial function35. β-hydroxybutyrate supplementation reduced proliferation in metastatic cancer cells and decreased progression and also increased survival in mice35. Recently, a β-hydroxybutyrate-induced reduction of the mTOR oncogenic signalling pathway including c-Myc and its target genes has been reported in pancreatic cancer cells36. A β-hydroxybutyrate treatment of pancreatic cancer cells also reduced motility by affecting epithelial-mesenchymal transition markers, and inhibited inflammatory cytokines36. In addition, β-hydroxybutyrate has been reported to suppress oxidative stress by increasing oxidative stress resistance factors (FOXO3A and MT2) as a part of the inhibitory actions of class I histone deacetylases37. Our LCKD study was the first trial to investigate the effects of a postoperative ketogenic diet in patients with pancreatobiliary cancer who underwent pancreatectomy. Although the 7-day application of LCKD did not prevent body weight loss when compared to the GD, there were significant alterations in serum metabolites, including those associated with pancreatic cancer. This lack of effect of LCKD on body weight might have been due to an insufficient duration of the diet. Previous studies indicated that ketogenic diets lasting 7 days or 8 weeks limited body weight loss in cancer patients that experienced weight loss7,11. A well-designed prospective study on the long-term use of a postoperative ketogenic diet in patients with pancreatic cancer is warranted to evaluate the potential role of a ketogenic diet on nutritional statuses perioperatively and in the long-term.
Significant alterations in serum metabolites by LCKD were observed postoperatively in patients with pancreatobiliary cancer. Among significantly altered metabolites, 48.8%, 27.5%, 10.8%, 8.8%, and 2.9% were involved in the metabolism of lipids, amino acids, nucleotides, cofactors and vitamins, and carbohydrates, respectively. Our pathway analysis suggested that changes might have occurred in the metabolic pathways for sphingolipids, tryptophan, ascorbate and aldarate, GPL, thiamine, cysteine and methionine, retinol, and starch and sucrose.
Sphingolipids participate as bioactive lipids within cancer cell signal transduction networks to regulate tumour growth, proliferation, migration, and metastasis38. LCKD appeared to lower serum levels of C22:1-ceramide, C18-ceramide phosphate, galabiosylceramides, and glucosylceramides in comparison to GD. Previously, significant elevations of specific individual ceramides, which are metabolites of the sphingolipid pathway, were reported in the blood of cancer patients18,31,39,40. Serum C24:6-ceramide18 and serum exosome C22:1-ceramide31 levels were upregulated in pancreatic cancer. C18-ceramide phosphate is a type of ceramide 1-phosphate (C1P) classified as a sphingolipid. C1P is a key regulator in human pancreatic cancer cell migration and invasion41. Galabiosylceramide (d18:1/18:1) was reported to be upregulated in ovarian cancer42. In our study, galabiosylceramide (d18:1/24:0) and galabiosylceramide (d18:1/24:1) levels were found to be lowered by the LCKD. Several glucosylceramides were closely linked to anticancer drug resistance43,44. Unlike the GD, LCKD might elicit changes in blood ceramide levels against the pancreatic cancer-associated dysregulation of blood metabolites.
GPLs, including PC, PE, PS, phosphatidylinositol, plasmalogen ethanolamine, and plasmalogen choline play structural roles in the lipid bilayers of cell membranes45. The GPL metabolic pathway has been associated with pancreatic cancers46. PC(16:0/22:6) was downregulated in the serum of patients with pancreatic cancer18 but was elevated by our LCKD. PC(14:0/P-18:0), which was upregulated in breast cancer47,48, colon cancer, oesophageal cancer, and stomach cancer tissues48, was lowered by LCKD. Longnecker et al. showed that dietary choline supplementation in rats alleviated pancreatic cancer lesions compared with a choline-free diet49. In our study, LCKD increased serum levels of lysoPC(18:2). Reports on lysoPC(18:2) levels in patients with pancreatic cancer are inconsistent, showing reduced serum lysoPC(18:2)13,18 and increased plasma lysoPC(18:2)15. Moreover, when GPL is degraded, various bioactive lipid mediators are produced, such as phosphatidic acid and lysophosphatidic acid (LPA)50. Plasma LPA was elevated in patients with ovarian cancer51, but LPA 18:2 was significantly downregulated in human colon cancer tissues compared with non-cancerous tissues52. Our LCKD increased LPA 18:2 in patients with pancreatic cancer. Partial reversion of the behaviour of GPL metabolites such as PC(16:0/22:6) and PC(14:0/P-18:0) by LCKD might suggest that LCKD induced some changes in GPL metabolism against pancreatobiliary cancer.
A low carbohydrate and high fat diet is known to reduce glucose and ATP supplies for pancreatic cancer cells, inhibiting their growth and proliferation10. Alternatively, ketogenesis may occur with liver glycogen depletion and fatty acid oxidation53. We searched for LCKD-induced changes in metabolites of the glucose metabolism. An upregulation of G1P was detected in the LCKD group, which might suggest glycogen degradation by the ketogenic diet-induced elevation in ketone bodies. G1P, derived from glycogen through glycogen phosphorylase, plays an important role in glycolysis, pentose synthesis, ATP generation, and fatty acid synthesis54. Another metabolite, 2-isopropylmalic acid, an intermediate of pyruvate metabolism synthesized from acetyl-CoA, was increased by LCKD, although it is downregulated in colon cancer-initiating cells55.
Vitamins and amino acid metabolites were also regulated by LCKD. Adenosine, inosine, L-tryptophan, 1-methylhistidine, and creatinine levels were lowered with LCKD. Serum adenosine was upregulated in pancreatic cancer patients18. Inosine, detected by GC- and LC-MS-based metabolomic analyses, was upregulated in plasma samples from patients with pancreatic ductal adenocarcinoma15. Serum tryptophan was upregulated in a pancreatic cancer mouse xenograft and 1-methylhistidine was upregulated in murine pancreatic tumour tissue46. In patients with pancreatic cancer, the serum creatinine level was found to be elevated using 1H NMR spectroscopy metabolomic profiling56 but was found to be decreased when analysed by GC-MS17. However, retinoic acid, N-arachidonoyl glycine, gamma-glutamyl-Se-methylselenocysteine, citicoline, uric acid, and citrulline levels were elevated with LCKD. The increased level of retinoic acid was associated with cell cycle arrest and a synergistic effect on apoptosis when combined with chemotherapeutic treatment in pancreatic cancer cells57. N-Arachidonoyl glycine exhibited anti-inflammatory effects in rat ear oedema and peritonitis models58. Gamma-glutamyl-Se-methylselenocysteine, an organic selenium compound, showed an anti-cancer effect in a rat model of colon cancer59. A protective effect of citicoline on colitis has been reported in rats, related to its contribution to anti-inflammatory and antioxidant mechanisms60. Uric acid was downregulated in patients with pancreatic cancer compared with healthy participants16. Citrulline was decreased in patients with pancreatic cancer in an LC-MS-based plasma profiling study32. Overall, LCKD might partially provide beneficial effects against pancreatic cancer.
Animal studies have shown increased survival rates with a ketogenic versus non-ketogenic diet; however, few human clinical studies have investigated this61. Anti-cancer effects of ketogenic diets were evidenced in animal studies by a clear reduction in tumour mass61. A ketogenic diet (C:P:F = 0.76%:8.36%:78.8%) reduced tumour growth and improved survival compared to a standard chow diet (composition unknown) in glioma cancer cell-implanted mice (median survival days: ketogenic diet = 25 days, standard diet = 19 days)62. A ketogenic diet supplemented with omega-3 fatty acids and MCTs (C:P:F = 0.2%:13.0%:35.5%) delayed tumour formation in human gastric cancer cell-implanted mice compared with a standard diet (C:P:F = 36.4%:23.8%:7.0%) (g/100 g diet)63. LCKD might provide anti-cancer effects by reducing the proliferation of any residual postoperative cancer cells. Based on the present study, a prospective randomized clinical trial evaluating the impact of LCKD on survival in patients with pancreatobiliary cancers is necessary.
Although the present study was a phase I prospective clinical trial demonstrating the safety and feasibility of the postoperative use of an LCKD and its effects on the metabolomics of patients with pancreatobiliary cancer, there are several limitations to this study. First, the study population is small due to the high exclusion and withdrawal rates. The measurement of ketone bodies might have provided more insight if more frequent and using other sources (e.g. urinary). The present metabolomic analysis was highly validated by the OPLS-DA model, suggesting that our results show significant differences between GD and LCKD. As a novel metabolomics study on the application of a post-surgery dietary intervention in patients with cancer, our results may provide useful data for further research.
In summary, our study successfully demonstrated that LCKD with 80% kcal from fat induced moderate ketosis by increasing postoperative serum total ketones and β-hydroxybutyrate in patients with cancer. In addition, an LCKD might revert some pancreatic cancer metabolite biomarkers, such as C22:1-ceramide, lysoPC (18:2), uric acid, citrulline, and inosine, and might also affect the metabolism of carbohydrates, amino acids, and vitamins. The LCKD used in our study might provide potential benefits to patients with pancreatobiliary cancer who undergo pancreatectomy. Further studies are mandatory to ascertain the effects of the serum metabolite changes induced by LCKD.
Supplementary information
Supplementary Figure S1 and Tables S1, S2, S3, S4
Acknowledgements
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (iPET, 316055-3) and the BK21 Plus Project for Bioactive Nutrition, Yonsei University, Seoul 03722, Republic of Korea.
Author contributions
C.M.K., B.K.Y., M.S. and S.-M.L. wrote the main manuscript text. B.K.Y., M.K. and Y.K. prepared the figures and tables. C.M.K., S.H.L., H.L., S.M.L. and S.-M.L. contributed to project administration and to the acquirement of funding. S.-M.L. and C.M.K. supervised the research. All authors reviewed the manuscript.
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary Information File.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-53287-y.
References
- 1.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. The Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J. Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Ansari D, Gustafsson A, Andersson R. Update on the management of pancreatic cancer: surgery is not enough. World J. Gastroenterol. 2015;21:3157. doi: 10.3748/wjg.v21.i11.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karagianni VT, Papalois AE, Triantafillidis JK. Nutritional status and nutritional support before and after pancreatectomy for pancreatic cancer and chronic pancreatitis. Indian J. Surg. Oncol. 2012;3:348–359. doi: 10.1007/s13193-012-0189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur. J. Oncol. Nurs. 2005;9(Suppl 2):S51–63. doi: 10.1016/j.ejon.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 6.La Torre M, et al. Malnutrition and pancreatic surgery: prevalence and outcomes. J. Surg. Oncol. 2013;107:702–708. doi: 10.1002/jso.23304. [DOI] [PubMed] [Google Scholar]
- 7.Allen BG, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963–970. doi: 10.1016/j.redox.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paoli A, Bianco A, Damiani E, Bosco G. Ketogenic diet in neuromuscular and neurodegenerative diseases. BioMed Res. Int. 2014;2014:474296. doi: 10.1155/2014/474296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fearon KC, et al. Cancer cachexia: influence of systemic ketosis on substrate levels and nitrogen metabolism. Am. J. Clin. Nutr. 1988;47:42–48. doi: 10.1093/ajcn/47.1.42. [DOI] [PubMed] [Google Scholar]
- 10.Shukla SK, et al. Metabolic reprogramming induced by ketone bodies diminishes pancreatic cancer cachexia. Cancer & Metabolism. 2014;2:18. doi: 10.1186/2049-3002-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroeder U, et al. Decline of lactate in tumor tissue after ketogenic diet: in vivo microdialysis study in patients with head and neck cancer. Nutr. Cancer. 2013;65:843–849. doi: 10.1080/01635581.2013.804579. [DOI] [PubMed] [Google Scholar]
- 12.Breitkreutz R, et al. Effects of a high-fat diet on body composition in cancer patients receiving chemotherapy: a randomized controlled study. Wien. Klin. Wochenschr. 2005;117:685–692. doi: 10.1007/s00508-005-0455-3. [DOI] [PubMed] [Google Scholar]
- 13.Mehta KY, et al. Metabolomic biomarkers of pancreatic cancer: a meta-analysis study. Oncotarget. 2017;8:68899–68915. doi: 10.18632/oncotarget.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bathe OF, et al. Feasibility of identifying pancreatic cancer based on serum metabolomics. Cancer Epidemiol. Biomar. Prev. 2010;20:140–147. doi: 10.1158/1055-9965.EPI-10-0712. [DOI] [PubMed] [Google Scholar]
- 15.Urayama S, Zou W, Brooks K, Tolstikov V. Comprehensive mass spectrometry based metabolic profiling of blood plasma reveals potent discriminatory classifiers of pancreatic cancer. Rapid Commun. Mass Spectrom. 2010;24:613–620. doi: 10.1002/rcm.4420. [DOI] [PubMed] [Google Scholar]
- 16.Nishiumi S, et al. Serum metabolomics as a novel diagnostic approach for pancreatic cancer. Metabolomics. 2010;6:518–528. doi: 10.1007/s11306-010-0224-9. [DOI] [Google Scholar]
- 17.Kobayashi T, et al. A novel serum metabolomics-based diagnostic approach to pancreatic cancer. Cancer Epidemiol. Biomar. Prev. 2013;22:571–579. doi: 10.1158/1055-9965.EPI-12-1033. [DOI] [PubMed] [Google Scholar]
- 18.Ritchie SA, et al. Metabolic system alterations in pancreatic cancer patient serum: potential for early detection. BMC Cancer. 2013;13:416. doi: 10.1186/1471-2407-13-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rezzi S, Ramadan Z, Fay LB, Kochhar S. Nutritional metabonomics: applications and perspectives. J. Proteome Res. 2007;6:513–525. doi: 10.1021/pr060522z. [DOI] [PubMed] [Google Scholar]
- 20.Moazzami AA, et al. Nuclear magnetic resonance-based metabolomics enable detection of the effects of a whole grain rye and rye bran diet on the metabolic profile of plasma in prostate cancer patients. J. Nutr. 2011;141:2126–2132. doi: 10.3945/jn.111.148239. [DOI] [PubMed] [Google Scholar]
- 21.Smith CA, Want EJ, O’Maille G, Abagyan R, Siuzdak G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006;78:779–787. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
- 22.Chong J, et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018;46:W486–W494. doi: 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Booth SC, Weljie AM, Turner RJ. Computational tools for the secondary analysis of metabolomics experiments. Comput. Struct. Biotechnol. J. 2013;4:e201301003. doi: 10.5936/csbj.201301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trygg J, Holmes E, Lundstedt T. Chemometrics in metabonomics. J. Proteome Res. 2007;6:469–479. doi: 10.1021/pr060594q. [DOI] [PubMed] [Google Scholar]
- 25.Vinaixa M, et al. A guideline to univariate statistical analysis for LC/MS-based untargeted metabolomics-derived data. Metabolites. 2012;2:775–795. doi: 10.3390/metabo2040775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trygg J, Wold S. Orthogonal projections to latent structures (O‐PLS) J. Chemom. 2002;16:119–128. doi: 10.1002/cem.695. [DOI] [Google Scholar]
- 27.van den Berg RA, Hoefsloot HC, Westerhuis JA, Smilde AK, van der Werf MJ. Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics. 2006;7:142. doi: 10.1186/1471-2164-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodacre R, et al. Proposed minimum reporting standards for data analysis in metabolomics. Metabolomics. 2007;3:231–241. doi: 10.1007/s11306-007-0081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Livera AM, et al. Normalizing and integrating metabolomics data. Anal. Chem. 2012;84:10768–10776. doi: 10.1021/ac302748b. [DOI] [PubMed] [Google Scholar]
- 30.Gibbons H, et al. A metabolomics approach to the identification of biomarkers of sugar-sweetened beverage intake. Am. J. Clin. Nutr. 2015;101:471–477. doi: 10.3945/ajcn.114.095604. [DOI] [PubMed] [Google Scholar]
- 31.Tao L, et al. Metabolomics identifies serum and exosomes metabolite markers of pancreatic cancer. Metabolomics. 2019;15:86. doi: 10.1007/s11306-019-1550-1. [DOI] [PubMed] [Google Scholar]
- 32.Fukutake N, et al. A novel multivariate index for pancreatic cancer detection based on the plasma free amino acid profile. PLoS One. 2015;10:e0132223. doi: 10.1371/journal.pone.0132223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y-M, Wang H-S. Medium-chain triglyceride ketogenic diet, an effective treatment for drug-resistant epilepsy and a comparison with other ketogenic diets. Biomed. J. 2013;36:9–15. doi: 10.4103/2319-4170.107154. [DOI] [PubMed] [Google Scholar]
- 34.Arends J, et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017;36:11–48. doi: 10.1016/j.clnu.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Poff A, Ari C, Arnold P, Seyfried T, D’Agostino D. Ketone supplementation decreases tumor cell viability and prolongs survival of mice with metastatic cancer. Int. J. Cancer. 2014;135:1711–1720. doi: 10.1002/ijc.28809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shukla SK, Chaika NV, Singh PK. Beta-hydroxybutyrate inhibits oncogenic signaling and cellular motility in pancreatic cancer cells [abstract] Proceedings of the American Association for Cancer Research Annual Meeting 2018; April 14-18, 2018; Chicago, IL. Philadelphia (PA): AACR. Cancer Res. 2018;78(Suppl 13):3557. doi: 10.1158/1538-7445.AM2018-3557. [DOI] [Google Scholar]
- 37.Shimazu T, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guri Y, et al. mTORC2 promotes tumorigenesis via lipid synthesis. Cancer Cell. 2017;32:807–823 e812. doi: 10.1016/j.ccell.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Knapp P, Bodnar L, Błachnio-Zabielska A, Świderska M, Chabowski A. Plasma and ovarian tissue sphingolipids profiling in patients with advanced ovarian cancer. Gynecol. Oncol. 2017;147:139–144. doi: 10.1016/j.ygyno.2017.07.143. [DOI] [PubMed] [Google Scholar]
- 40.Karahatay S, et al. Clinical relevance of ceramide metabolism in the pathogenesis of human head and neck squamous cell carcinoma (HNSCC): attenuation of C18-ceramide in HNSCC tumors correlates with lymphovascular invasion and nodal metastasis. Cancer Letters. 2007;256:101–111. doi: 10.1016/j.canlet.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rivera I-G, et al. Ceramide 1-phosphate regulates cell migration and invasion of human pancreatic cancer cells. Biochem. Pharmacol. 2016;102:107–119. doi: 10.1016/j.bcp.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Yang W, et al. Identification of potential biomarkers and metabolic profiling of serum in ovarian cancer patients using UPLC/Q-TOF MS. Cell. Physiol. Biochem. 2018;51:1134–1148. doi: 10.1159/000495492. [DOI] [PubMed] [Google Scholar]
- 43.Wang T, Wei J, Wang N, Ma J-L, Hui P-P. The glucosylceramide synthase inhibitor PDMP sensitizes pancreatic cancer cells to MEK/ERK inhibitor AZD-6244. Biochem. Biophys. Res. Commun. 2015;456:821–826. doi: 10.1016/j.bbrc.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 44.Jiang Y, et al. Combinatorial therapies improve the therapeutic efficacy of nanoliposomal ceramide for pancreatic cancer. Cancer Biol. Ther. 2011;12:574–585. doi: 10.4161/cbt.12.7.15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farooqui AA, Horrocks LA, Farooqui T. Glycerophospholipids in brain: their metabolism, incorporation into membranes, functions, and involvement in neurological disorders. Chem. Phys. Lipids. 2000;106:1–29. doi: 10.1016/S0009-3084(00)00128-6. [DOI] [PubMed] [Google Scholar]
- 46.Zhan B, et al. Identification and causes of metabonomic difference between orthotopic and subcutaneous xenograft of pancreatic cancer. Oncotarget. 2017;8:61264–61281. doi: 10.18632/oncotarget.18057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ide Y, et al. Human breast cancer tissues contain abundant phosphatidylcholine (36∶1) with high stearoyl-CoA desaturase-1 expression. PloS One. 2013;8:e61204. doi: 10.1371/journal.pone.0061204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo S, Wang Y, Zhou D, Li Z. Significantly increased monounsaturated lipids relative to polyunsaturated lipids in six types of cancer microenvironment are observed by mass spectrometry imaging. Sci. Rep. 2014;4:5959. doi: 10.1038/srep05959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Longnecker DS, Chandar N, Sheahan DG, Janosky JE, Lombardi B. Preneoplastic and neoplastic lesions in the pancreas of rats fed choline-devoid or choline-supplemented diets. Toxicol. Pathol. 1991;19:59–65. doi: 10.1177/019262339101900107. [DOI] [PubMed] [Google Scholar]
- 50.Tveteraas IH, et al. Lysophosphatidic acid induces both EGFR-dependent and EGFR-independent effects on DNA synthesis and migration in pancreatic and colorectal carcinoma cells. Tumour Biol. 2016;37:2519–2526. doi: 10.1007/s13277-015-4010-1. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, et al. High resolution mass spectrometry coupled with multivariate data analysis revealing plasma lipidomic alteration in ovarian cancer in Asian women. Talanta. 2016;150:88–96. doi: 10.1016/j.talanta.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 52.Kitamura C, et al. The component changes of lysophospholipid mediators in colorectal cancer. Tumor Biol. 2019;41:1010428319848616. doi: 10.1177/1010428319848616. [DOI] [PubMed] [Google Scholar]
- 53.J. D. McGarry A, Foster DW. Regulation of Hepatic Fatty Acid Oxidation and Ketone Body Production. Annu. Rev. Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- 54.Lee WP, et al. Metabolic sensitivity of pancreatic tumour cell apoptosis to glycogen phosphorylase inhibitor treatment. Br. J. Cancer. 2004;91:2094. doi: 10.1038/sj.bjc.6602243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen K-Y, et al. A metabolic signature of colon cancer initiating cells. Conf. Proc. IEEE. Eng. Med. Biol. Soc. 2014;2014:4759–4762. doi: 10.1109/EMBC.2014.6944688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.OuYang D, Xu J, Huang H, Chen Z. Metabolomic profiling of serum from human pancreatic cancer patients using 1 H NMR spectroscopy and principal component analysis. Appl. Biochem. Biotech. 2011;165:148–154. doi: 10.1007/s12010-011-9240-0. [DOI] [PubMed] [Google Scholar]
- 57.Herreros-Villanueva M, Er T-K, Bujanda L. Retinoic acid reduces stem cell–like features in pancreatic cancer cells. Pancreas. 2015;44:918–924. doi: 10.1097/MPA.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 58.Takenouchi R, Inoue K, Kambe Y, Miyata A. N-arachidonoyl glycine induces macrophage apoptosis via GPR18. Biochem. Biophys. Res. Comm. 2012;418:366–371. doi: 10.1016/j.bbrc.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 59.Steinbrenner H, Speckmann B, Sies H. Toward understanding success and failures in the use of selenium for cancer prevention. Antioxid. Redox Signal. 2013;19:181–191. doi: 10.1089/ars.2013.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ek RO, et al. Protective effects of citicoline on TNBS-induced experimental colitis in rats. Int. J. Clin. Exp. Med. 2014;7:989. [PMC free article] [PubMed] [Google Scholar]
- 61.Lv M, Zhu X, Wang H, Wang F, Guan W. Roles of caloric restriction, ketogenic diet and intermittent fasting during initiation, progression and metastasis of cancer in animal models: a systematic review and meta-analysis. PloS One. 2014;9:e115147. doi: 10.1371/journal.pone.0115147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stafford P, Abdelwahab MG, Preul MC, Rho JM, Scheck AC. The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutr. Metab. 2010;7:74. doi: 10.1186/1743-7075-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Otto C, et al. Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer. 2008;8:122. doi: 10.1186/1471-2407-8-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1 and Tables S1, S2, S3, S4
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Supplementary Information File.