Abstract
The present review reports all management approaches (physical, chemical, and biological) traditionally adopted in mitigating the global impact of harmful cyanobacterial blooms (cyanoHABs). It recognizes that each mitigation strategy shows characteristic associated limitations and notes that no remedial step has provided a sustainable solution to HABs on a global scale. It emphasizes that the putative anthropogenic N&P inputs reduction through improved wastewater treatment and regulation of point and non-point sources—agricultural fertilizers only offer a short term solution. These approaches are rather preventive than curative hence, do not address concerns relating to the recovery of already-eutrophic and hypereutrophic systems. It raises new concerns on the implications of non-agricultural pollutants such as hydrocarbon fractions in bloom accretions often neglected while addressing HAB triggers. It also accesses the global impacts of HABs as it pertains to socio-economic implications in the geographically diverse world. It, therefore, proposes that Integrated Management Intervention involving the merging of two or more mitigation steps be administered across the aquatic continua as a prudent management solution to complement the current N&P dual management paradigm. It stresses that the contemporaneous adoption of management options with both preventive and curative measures is a key to sustainable HAB management. This review provides sufficient advances and current scenarios for approaching cyanoHABs. Further, it advocates that future research perspectives tackle the mitigation design beyond the short-term nutrient regulations and the parochial attention to the point and non-point N&P input sources.
Keywords: Eutrophication, Harmful algal bloom, Climate change, Anthropogenic, Management
Introduction
Excess primary production in the form of harmful algal blooms (HABs) causes several negative shifts in the natural ecological balance of freshwater ecosystems or other coastal zones. The ever-increasing case of nutrient over-enrichment described as eutrophication is the primary inducer. Eutrophication disrupts the integrity of aquatic biodiversity, ecosystem resilience, recreational and fishing activities, water quality deterioration, and ultimately, water crisis (Sunda and Shertzer 2014). Nutrients over-enrichment in freshwaters downstream estuarine, and coastal systems have triggered harmful algal bloom (HAB) outbreaks (Paerl et al. 2018). The problematic trends of phytoplankton blooms are now rapidly intensifying in most regions of the world including developed and developing countries like Japan, Israel. Central and South America, Australia, Europe, Africa, and Asia (Garce and Maso 2006; Hadas et al. 2015; Paerl et al. 2015, 2016, 2018; Paerl and Paul 2012; Sanseverino and Conduto 2016; Watson et al. 2015). Several phytoplankton blooms have been implicated in water ecosystems (Fig. 1), jeopardizing water quality and public health. Bloom-forming taxa, which can produce toxins, generate hypoxia, and disrupt the food web, which are harmful cyanoHABs have attracted peculiar attention (Paerl 2017). HABs have been a decadal environmental dilemma confronting decision-makers and water quality managers. However, mitigation approaches so far have not sufficiently reversed the tide on the global scale (Paerl 2009; Paerl et al. 2018; Watson et al. 2015). One example, massive blooms in Lake Kasumigaura, Japan, compelled the Ministry of Environment of Japan to promote integrated measures for bloom control and water quality conservation. The Kasumigaura bloom has caused substantial clogging of water purification equipment and fisheries gear, which has affected local and socio-economic activities. The emergence of seasonally mediated undesirable odour in Chuzanji Lake, Japan, following the proliferation of chrysophyte (Uroglena) bloom since 1981 has not abated despite concerted and massive interventions from the Japanese Government. Also, the report of the massive proliferation of cyanobacteria under the ice in Baltic Sea basin, Europe, in 2015 following the intense summer heat in August indicates yearly persistence. Furthermore, the Saving Water, Cape Town, South Africa, a report titled “Lake Victoria turning green with algal bloom” which went viral across the geographically sensitive globe stressing that the discharge of indiscriminate effluents in addition to other wastes disposal into Lake Victoria, Africa is promoting heavy bloom of cyanoHABs that have left the system beyond the ecosystem recovery limit (Mchau et al. 2019). Millions of inhabitants of neighbouring Kenya, Tanzania and Kampala region of Uganda have expressed public health concerns and loss of fishery resources in all the three countries. Agricultural fertilizer-rich runoff and warm summer trigger severe incidence of cyanoHABs bloom in Lake Erie, North America which shut down the drinking water system of the Toledo, OH, ca. 500,000 inhabitants (Michalak et al. 2013; Pelley 2016).
Fig. 1.
Examples of water bodies covered by different algal blooms: a left to right: Green water caused by Microcystis aeruginosa and black water caused by Peridiniopsis sp. in Xiangxi tributary, Yangtze River, China (photo credit: Yange et al. 2018a, b). b Left to right: Upper Klamath Lake, USA and Taihu Lake, China (Photo credit: Paerl 2017)
CyanoHABs were reported in Taihu Lake, China, on May 2007 and have reoccurred despite numerous mitigation approaches deployed to arrest the situation during the initial outbreak. During this CyanoHABs outburst, a very large ‘‘cyanobacterial mat’’ inundated and damaged the drinking water plants in the vicinity of Wuxi, a large city in Jiangsu Province with approximately 6 million human inhabitants, leading to a highly and internationally publicized drinking water crisis (Paerl et al. 2011a, b; Xu et al. 2015, 2013). Despite the several bloom mitigation steps, decision-makers, managers, scientists, and individuals are still saddled with the question, what next?
Herein, the present review attempts to access all management strategies that have been conventionally enacted across aquatic systems to mitigating the impacts of cyanoHABs and addressed their characteristic inadequacies. We stress the need for integrated adoption of sustainable approaches while advocating for both preventive and curative measures as a key to a long term recovery of bloom impacted ecosystems. Further, we recognize that short-term solutions have not significantly reduced the cyanoHABs risks on a global scale. It brings into limelight the fate of the non-agricultural based contaminants, e.g., petroleum hydrocarbon and its derivatives in bloom expansion across the aquatic systems, which are rarely considered when factors boosting HABs are enumerated.
The triggers of HABs expansions
There are some conditions and global modifications that can lead to harmful algal bloom expansion in geographical diverse water systems. These conditions, either singly or in concert, can drive to episodic HAB situations. Generally, drivers of HABs expansion are grouped into three broad classes (i) climatic, (ii) anthropogenic, and (iii) hydrodynamic.
Climatic change
Climatic change constitutes a remarkable challenge in evaluating dynamics in HABs intensity, integrity, expansion, frequency, and proliferation. Sea level rise, global warming, changes in the pattern of precipitation both on annual and diurnal scale all impact HABs dominance. These result in intense alterations in freshwaters (lakes and rivers) and ocean circulations, cyclone severity and frequency, upwelling and stratification (Paerl et al. 2018), wind speed and direction (Johnson et al. 2012; Deng et al. 2018) most of which are not subject to anthropogenic control on a regional or large scales. Extreme hydrologic events such as storm-related flooding and droughts (Lin et al. 2017) are essential drivers of bloom expansions. In association with excessive nutrients loads, climatic change and hydrologic modifications support HABs to attain both optimal magnitude and amplitude with persistent dominance over time. For instance, decreasing trends in rainfall can result in episodic increase in nutrient concentrations and to favour stratification and bloom formation in slow recharging lotic systems (Anderson et al. 2015; Anderson 2009; Garce and Maso 2006; Paerl et al. 2016) due to substantially decreased mixing mechanism that persists under this condition. Several studies have predicted that controlling the predominance and expansion of harmful algal blooms, especially cyanoHABs may be more challenging in the future than it is at present considering the increasingly warming climate (Paerl et al. 2016; Wells et al. 2015). Studies have also shown that local hydrologic and major biogeochemical drivers, including runoff and rainfall, internal nutrient recycling, food web dynamics, mixing events, as well as nutrients export from watersheds all of which directly affect HABs proliferations, are altered by climate change. Pollution and eutrophication are generally magnified by rainfall due to nutrient-rich run-off and the transport of contaminants across the freshwater to the marine continuum (Sanseverino and Conduto 2016). The cyanobacteria, which are sometimes referred to as extremophilic algae, can survive a wide range of extreme climatic changes while showing optimal growth at both high temperature and salinity (Paerl and Paul 2012).
Anthropogenic activities
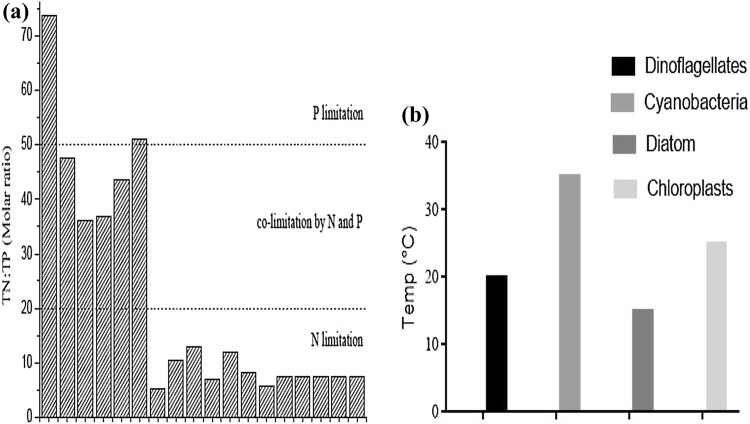
The Anthropocene has been implicated in contributing to greater than 80% eutrophication and HAB events in the freshwater, marine, and other interconnected hydrologic systems. Anthropogenic activities are day-to-day human activities that directly favour the phenomenon of HABs in a freshwater-marine continuum. Notable anthropogenic triggers of HABs are the construction of dams, reservoirs, levees, river diversions, deforestation, watershed and land developments, as well as point and non-point sources N&P inputs. Examples include; the impoundment of the largest hydro-electric project in China, The Three Gorges Dam (TGD) in 2003, which warranted the upstream rivers (e.g., Xiangxi River), tributaries and reservoirs to be severely affected by HABs (Zhou et al. 2009). The largest tributary in the Three Gorges Reservoir (TGR) called Xiangxi Bay (XXB) is currently facing massive phytoplankton bloom (Huang et al. 2012). The XXB is a tributary bay which was formed due to the backwater movement caused by the cumulative impacts of operations of the gigantic hydraulic structures (TGD) over the years. The backwater extended to 40 km when the reservoir was filled to the maximum water level of 175 m during 2010 impoundment (Hayashi et al. 2008; Ji et al. 2017; Jiang et al. 2018; Li et al. 2016; Smith et al. 2016; Yang et al. 2015; Zhang et al. 2016; Zhou et al. 2011; Zhu et al. 2013). Currently, the bay is now characterized as a stable system similar to lakes with some defined limnetic characteristics, including thermal stratification, long residence time and eutrophication (Xu et al. 2011; Huang et al. 2012) all of which are predisposing factors for HABs formation and expansion. The dam storage allows water with high TN/TP from mainstream reaches into XXB while dam discharge allows water with low TN/TP into the mainstream. The periodic water transitions and nutrient fluxes promote HAB expansions (Zhou et al. 2009). Regrettably, this situation is not only confined to China’s TGD. For example, the transition from the -naturally eutrophic to now recognized hyper-eutrophic, Aphanizomenon flos-aquae dominated system of the Upper Klamath Lake USA, has recently been attributed to the significant increase in anthropogenic N&P loadings into the system due to upstream discharge, combined with dam impoundment (Paerl et al. 2018). To address this situation, selective removal of a series of dams along the systems has been prescribed to increase water flow regimes and significantly reduce residence time (Campbell 2005). Although this dam removal operation has been described as the most significant and most robust official endorsement in history (Oliver et al. 2014; Paerl et al. 2018). The Levant basin from the activation of Aswan High Dam on the Nile basin, has been shown to induce eutrophication due to remarkable nutrient over-enrichment in the coastal waters, stimulating increased phytoplankton proliferation with negative impacts on marine life. A recent investigation also posited that the potentially toxic dinoflagellate, Prorocentrum minimum dominates the Nile River in the large Adriatic and Kiel fjord areas. In the landlocked Mediterranean and Baltic Seas, increased fish mortality and release of odoriferous ooze have been linked to the intense proliferation of the Prymnesiomonad, Chrysochromulina polylepis. Again, a significant increase in nutrient loadings and expansion of phytoplankton communities arising from river flow disruption due to the filling of the European Alqueva dam, South of Portugal has been reported to pose a significant threat. These have led to a pronounced water quality deterioration of the upstream reaches and the downstream estuary (Morais et al. 2009). Furthermore, the increasing rates of urbanization and industrialization have put significant pressures on forest resources, rerouting of some rivers, wetland removal, watershed and land development exacerbating eutrophication and HAB dynamics on a regional levels (Castro et al. 2016; Mccarthy et al. 2018; Zhang et al. 2013). Anthropogenic N&P inputs from both point and non-point sources are significant triggers for HABs. Effluents from the wastewater treatment plants and industries are suitable point sources of N&P. The near-total dependence on detergents for laundry services contributes to increasing P supply. Non-point sources from agricultural run-offs, inorganic fertilizer saturated lands due to indiscriminate fertilizer application beyond crop requirement thresholds have also remarkably encouraged HAB proliferation in a freshwater-marine continuum. Agricultural run-off and urban storm waters are potential sources of N-especially NO3 to aquatic systems (Paerl et al. 2015). Other non-agricultural pollutants such as petroleum products (oils) including dual purpose kerosene (Nwankwegu et al. 2016a), diesel (Nwankwegu et al. 2016b), premium motor spirit (Nwankwegu 2016), gasoline (Nwankwegu and Onwosi 2017a), automobile gas oil, (Nwankwegu et al. 2017), spent lubricating oil (Nwankwegu et al. 2018) as well as heavy metals and the recalcitrant hydrocarbon congeners like polycyclic aromatic hydrocarbons (PAHs) (Oladunjoye et al. 2017; Posada-baquero and Martín 2019; Verma and Kuila 2019) also contaminate waterbodies through pipeline vandalism and accidental discharge while they significantly promote the processes that trigger episodic blooms. These hydrocarbon pollutants are usually difficult to deal with upon spill, and the few management options in water environments such as photo-oxidation and the use of dispersants (surfactants) often pose a great threat to aquatic biodiversity thus eco-hazardous. Although bioremediation has been globally advocated as an eco-friendly remedial alternative (Chaudhary and Kim 2019; Daniel et al. 2019) but this technology principally involves three strategies notably; natural attenuation, bio-stimulation (exogenous nutrient addition) and bio-augmentation (seeding of allochthounous rather than autochthonous microbial species) (Nwankwegu and Onwosi 2017b) which are predominantly more appropriate in polluted soil than the water ecosystems. Specifically, bio-stimulation essentially helps to increase ecosystem nutrient budget (N&P) and promotes the factors that exacerbate blooms when deployed across the aquatic systems. The anthropogenic N&P inputs are not the sole sources of nutrient supply and circulation across the interconnected aquatic ecosystems as an enormous supply of N-also come from atmospheric depositions and the activities of planktonic N2-fixers including Anabaena, Aphanizomenon, Nodularia, Nostoc, and Cylindrospermopsis which can carry out nitrogen fixation (Paerl et al. 2011a, b; Watson et al. 2015). Atmospheric depositions make up an essential source of fixable nitrogen for the diazotrophic taxa. The supply of the massive amount of dissolved inorganic nitrogen (DIN) up to 38–155 mg m−2 month−1 in N-deficient Eastern Indian Ocean and the South China Sea has been attributed to atmospheric deposition (Zhou et al. 2018a, b). At present, there are no chemical precipitation mechanisms (either entrapment, binding or complexation) available for N-immobilization in sediment since all the bioavailable forms (NO3 and NH4+) forms are highly soluble in water. In contrast, however, excessive P-supply has been traced to legacy loading from both sediment and geo-genic sources (e.g. rocks). Legacy loading is a hydrodynamic event driving by high temperature and tidal inundations. Internal nutrient loading, especially P-released from sediments, is intensified during the hot seasons (summer) accounting for increased P-availability during hotter seasons (Ding et al. 2018; Zhou et al. 2009). The high affinity of P to humic substances in sediment–water interfaces reduces its availability for phytoplankton uptake. The total nutrient (N&P) ratio has been used as a prudent indicator to evaluate N&P limitation paradigm although, this has been debated over the years by different researchers. For instance, Ding et al. 2018 posited that N-limitation or P-abundance occurs at N&P ratio less than 9 (TN/TP < 9) and P-limitation N-abundance occurs at TN/TP > 22.6. In contrast, other studies stated the N-limitation occurs at TN/TP < 20, co-limitation by N&P at TN/TP = 20 − 50, and P-limitation at TN/TP < 50 (Zhou et al. 2009). However, irrespective of the discrepancies based on TN/TP paradigm, there is one consensus agreement that N&P limitation shift in freshwater e.g. lakes is seasonally mediated and while summer and fall are associated with N-limitation, winter and spring are associated with P-limitation (Morais et al. 2009; Paerl et al. 2011a, b; Xu et al. 2015). Table 1 and Fig. 2a show nutrient limitation dynamics and TN/TP transitions on an annual scale. The TN/TP evaluated from the total nitrogen and phosphorus. Data acquired from different studies (Zhou et al. 2009, 2011; Ding et al. 2018). Initial management protocol stipulates that eutrophic freshwater which is highly P-enriched (i.e. low N: P molar ratio < 15) is more prone to dominance by diazotrophic taxa, while eukaryotic algae and several non-N2-fixers dominate at N: P > 20 (Watson et al. 2015). However, the relationship between nutrient ratios and phytoplankton species dominance lacks consistency and hence is controversial.
Table 1.
Annual dynamic characteristics of Xiangxi tributary bay based on field observations
| Month | Nutrients | ||
|---|---|---|---|
| TN (mg L−1) | TP (mg L−1) | TN/TP | |
| January | 2.92 ± 0.11 | 0.11 ± 0.01 | 26.50 ± 0.25 |
| February | 2.88 ± 0.20 | 0.12 ± 0.03 | 24.00 ± 0.02 |
| March | 2.89 ± 0.01 | 0.12 ± 0.11 | 24.10 ± 0.31 |
| April | 3.25 ± 0.40 | 0.13 ± 0.02 | 25.00 ± 0.12 |
| May | 3.21 ± 0.20 | 0.11 ± 0.05 | 29.20 ± 0.01 |
| June | 2.30 ± 0.13 | 0.13 ± 0.01 | 17.70 ± 0.19 |
| July | 1.86 ± 0.14 | 0.27 ± 0.11 | 6.90 ± 0.19 |
| August | 2.01 ± 0.11 | 0.25 ± 0.03 | 8.04 ± 0.15 |
| September | 2.12 ± 0.40 | 0.28 ± 0.03 | 7.60 ± 0.19 |
| October | 2.51 ± 0.14 | 0.31 ± 0.12 | 8.09 ± 0.19 |
| November | 1.81 ± 0.14 | 0.21 ± 0.10 | 8.61 ± 0.19 |
| December | 2.81 ± 0.20 | 0.19 ± 0.03 | 23.40 ± 0.01 |
Fig. 2.
a The TN and TP molar ratio indicating the forms of nutrient limitation dynamics shift (Zhou et al. 2009). b Temperature optima for maximum growth of major harmful algal bloom (HABs) formers (Paerl et al. 2016)
Hydrodynamic variables
The magnitude of hydrologic forces exerted on freshwaters, and other coastal waters significantly affect phytoplankton integrity, cell aggregations, HABs, and nutrient dynamics (Wang et al. 2017). Aquatic systems are potentially impacted by both advection (horizontal) and vertical fluxes in association with alluvial and fluvial processes. Mixing and flushing are hydrodynamics activities that control the HAB expansions. Adequate mixing and flushing directly affect water temperature and residence time. For example, cyanobacteria prefer it hot and slow and thrive in systems with minimal mixing (thermally stratified) and long residence time. CyanoHABs dominate summer bloom in freshwater ecosystems. Extremely shallow mixing depth during summer in Xiangxi Bay of Three Gorges Reservoir, Yangtze River, Chima has been reported to exacerbate cyanoHABs dominance (Johnson et al. 2012; Mao et al. 2015). The rapid decrease in mixing is associated with spring bloom. However, the bacillariophytes, e.g. diatoms, prefer the system cold, highly mixed, well flushed, and fast, thus can withstand high mixing regimes and short residence time making them potential pre-colonizers in such habitats. Spring diatom blooms of Stephanodiscus hantzschii, Cyclotella meneghiniana in Lake Taihu and Hanjiang River, China have been documented under similar nutrient gradients (Wang et al. 2012). Due to the adaptive ability of diatoms under low light and temperature ranges, they have been implicated as the dominant species in both lotic and lentic systems during winter blooms. Previous studies have corroborated the dependence of diatoms bloom on hydrodynamics wherein, it was shown that under different flow regimes, diatom population exhibit species-specific variations in P-uptake (Wang et al. 2012; Watson et al. 2015). The intense proliferations of specific microscopic algal bloom called “red tide” under calm wind situations in weakly flushed coastal waters were observed in eutrophic and subtropical coastal systems for example in Hong Kong and South China (Wong et al. 2007). Generally, the propensity for cell aggregation or formation of surface scum by planktonic taxa spreading through impacted waters is limited by high mixing and flushing. Wind-driven mixing regimes especially in sediment-rich broad but shallow eutrophic systems like Taihu Lake, China triggers upwelling and massive nutrient release from legacy loading. This could warrant nutrient over-enrichment beyond the eutrophication thresholds (TN = 0.8 mg L−1. TP = 0.07 mg L−1) (Xu et al. 2015). It also has been reported that harmful algal bloom expansions with characteristic dense surface “mat” in large but relatively shallow Lake Winnipeg, North America is associated with intermittent mixing regimes principally driven by wind (Binding et al. 2018). Low mixing and tidal events support both hypoxia and anoxia in freshwater systems (Jalil et al. 2018), both of which intensely encourage cyanoHAB expansions. Vertical and horizontal distributions of materials across concentration gradients are critically altered by wind-induced mixing and circulations and adequately select for the short or long term algal population predominance.
Factors influencing growth and adaptation of Phytoplankton
The tendency for certain taxonomic groups of phytoplankton to maximize growth at a minimal cell loss through absolute aquatic territorial dominance under constantly changing environments is controlled by several factors and adaptive characteristics. The growth influencing factors such as temperature, pH, dissolved oxygen, nutrient, and predation are important physicochemical and biological factors affecting HABs dynamics in the freshwater-marine continuum. Different phytoplankton exhibit peculiar survival mechanism, ranging from habitat modification by toxins production, expressions of physicochemical defense mechanisms, nutrient uptake processes (autotrophic, heterotrophic or mixotrophic), siderophores/chelators secretion for micronutrient assimilations, diazotrophs/N2-fixation, motility (buoyancy), reactive oxygen species (ROS) tolerance (benthic or planktonic) and formation of large protective sheaths.
The optimum temperature requirements for growth vary among phytoplankton taxa (Fig. 2b). The order of phytoplankton temperature –growth optima; cyanobacteria (cyanoHABs) > chlorophytes > dinoflagellates > diatoms has been reported (Paerl et al. 2011a, b, 2016; Watson et al. 2015). Seasonal variations in temperature affect nutrient and oxygen availability, water column stratification as well as phytoplankton dominance and expansions. In thermally stratified systems, the cyanobacteria can move through the water column by altering their buoyancy. Diazotrophic cyanoHABs such as Anabaena, Aphanizomenon, Nostoc, and Nodularia dominate the photic zone while they lead to the decline of other taxa within the euphotic zones. Again, specific blooms of macroalgae have been reported to occur during spring and summer utilizing the low precipitation with high temperatures to maximize dominance in coastal lagoons (Neill et al. 2015). Bloom intensity significantly depletes bottom water O2 and promotes hypoxia. This condition favours the food web disrupting and hypoxia generating cyanoHABs to thrive. In other words, the big issue with dissolved oxygen (DO) is essentially the bottom water hypoxia and anoxia. In the sea of Oman, Southeast Asia, the seasonal decline in dissolved oxygen concentration has been attributed to a dramatic increase in fish kills (Harrison et al. 2017).
Similarly, under intense bloom events, the surface water pH of aquatic systems usually goes up, and as bloom (biomass) decay, pH gradually decreases. Bloom accretion is associated with the release of some gases which compromise water quality and impact odour. When these gases dissolve in the bulk water, they alter the water pH depending on what gas was evolved. In this case, the bloom impacted system with the release of hydrogen sulfide -H2S or methane-CH4 is expected to be more acidic unlike other systems characterized by the release of alkaline gases, e.g. ammonia-NH3. The ambient toxicity of the haptophyte, Prymnesium parvum has been found to increase proportionately with pH such that optimal growth in aquatic systems is inhibited by extremely low pH (Prosser et al. 2012). In effect, extremely low pH can exacerbate the production of secondary metabolites, increasing the mortality of eukaryotic lives by eliciting high BOD conditions in such systems (Visser et al. 2016).
The nutrient is also a principal factor for growth, and different phytoplankton groups show varying preferences to nutrient metabolism. For example, during our recent study in Xiangxi Bay of Three Gorges Reservoir, China. The effect of exogenous nutrients on the relative abundance of major HAB taxa was evaluated in a microcosm bioassay. Our result shows that while N-addition caused the system to become cyanobacteria dominated (relative abundance = 47.15%). The P –addition, on the other hand, led to a chlorophyte-dominated community (Relative abundance RA = 51.88%) as shown in Table 2 (data not published). Significant dominance (relative abundance = 71.61%) of cyanobacteria was seen in cultures with N + P+Si + metal addition. This, however, reflected the nutrients (N, P, and Si) synergy in boosting HABs proliferation and expansion across aquatic systems. Si addition resulted in a sharp increase in the relative abundance (RA = 20.62%) of diatoms compared to other nutrients on the same taxon. Generally, different phytoplankton groups respond differently to the exogenous nutrient supply. In other words, specific nutrient availability can select for which algal species would be dominant during a bloom. Freshwater systems, especially lakes and ponds are classified as oligotrophic, mesotrophic, eutrophic and hyper-eutrophic based on the magnitude of nutrients loading characteristic. Presently, most lakes, e.g. Lake Taihu, China, Upper Klamath Lake, USA, Lake Winnipeg, North America have now transcended to hypereutrophic systems in response to the increasing urbanization, agricultural runoff, industrial and wastewater discharge over the years. Micronutrients are also essential for growth although, some are perhaps not growth-limiting but stimulatory. In other words, eutrophication and bloom can persist without them. However, their presence help to enhance phytoplankton cell morphology and chlorophyll yields (Xu et al. 2015). Iron and silicon have been described as limiting nutrients for algal growth, and different phytoplankton show certain specificities in iron and silicon uptake (Wu and Chou 2003; Xu et al. 2013; Sanseverino and Conduto 2016). Cyanobacteria can sequester iron and the activity of the N2-fixation enzymes, nitrogenases is enhanced by iron availability. The nitrogenases are sensitive to oxygen; thus can be deactivated under aerobic conditions. The cyanoHABs therefore, possess oxygen repelling structure, the heterocyst, which excludes oxygen, helping the N2-fixers to avoid enzyme inactivation. A great challenge to the iron supply in the freshwater-marine continuum is the fact that the biologically utilizable ferrous ion (Fe2+) is unstable and easily oxidized to ferric ion (Fe3+). This phenomenon makes iron less available. However, certain cyanobacteria, e.g., Microcystis spp, the most widely studied and ubiquitous cyanoHABs under freshwater eutrophication, possess iron-binding molecules, siderophores which bind ferric ion species to form the soluble iron-siderophore complexes which mimic Fe2+ thus, utilizable. Again, when the concentration of NO3− is higher than NH4, the most preferred N-form in aquatic systems, cyanoHABs often intensify its Fe requirement for NO3 assimilation (Xu et al. 2013). Natural iron supply from humic substances has been demonstrated to stimulate the growth of marine algal species (Krachler et al. 2019). The Bacillariophyta, diatoms, on the other hand, have obligate silicon (Si) requirements for growth, metabolism, DNA replication, and cell division. Silicon concentration in aquatic systems has a direct relationship with dissolved inorganic nitrogen, DIN and according to Wu and Chou (2003), silicon limitation occurs at a point when Si: DIN = 1:1. This means that in diatom-dominated aquatic systems, primary productivity would be drastically reduced at SI: DIN ≤ 1. An increasing concentration of silicon has been found to result in optimal growth of the diatom, Triparma laevis NIES-2565 (Parmales, Heterokontophyta) during a pilot-scale experiment in artificial seawater (Yamada et al. 2014). Again, habitat exploitation and acclimatization are also crucial for HABs dominance and expansion, for instance, certain freshwater HABs irrespective of their ecosystems hysteresis are known to exhibit certain degrees of salt-tolerance including the diazotrophic taxa, the Cyanophyta such as Dolichospermum, Nodularia, Anabaenopsis, as well as few species of Oscillatoria and Lyngbya. The non-N2-fixers including Phormidium, Microcystis, Oscillatoria, and pico-planktonic Chroococcus and Synechococcus. Some strains of HABs show relative tolerance to saline toxicity e.g., some strains of Microcystis aeruginosa remains unaffected by salinities ≥ 10 (Paerl et al. 2016) thus, can display optimal growth responses under ‘‘mixohaline’’ habitats. The common Baltic Sea bloom-former Nodularia spumigena, thrive in ≥ 20 saline conditions while some Anabaenopsis and Dolichospermum tolerate salinities up to 15. Hence, cyanoHABs are enhanced by the synergistic effects of salinization and nutrient concentrations (Paerl and Paul 2012; Paerl et al. 2016). The biological factors, including predations and competitions also significantly affect HAB expansions. To escape predation or attract preys, several species of phytoplankton have developed some adaptive features ranging from buoyancy, hypoxia generating, food web disrupting to toxin productions (Paerl et al. 2011a, b, 2016; Paerl and Paul 2012). The different taxonomic groups possess distinct adaptive features controlling dominance. The predator–prey relationship, as well as competition, is a common phenomenon in freshwater ecosystems. During this interaction, preys can either be directly ingested by the predators via phagotrophy or release of membrane weakening allergens (dasmotrophy) to incapacitate prey and increase its vulnerability. The chlorophytes can optimize growth under low light and nutrients by mixotrophy and vertical migration. Both intraspecific and interspecific competitions actively control HAB expansions. The Microcystis spp develop toxic blooms, dense surface sums, deoxygenate water column and dominate in summer blooms (Paterson et al. 2017). Interactions between eukaryotic algae and hypoxia generating –toxic cyanobacteria significantly affect bloom dynamics. In Lake Taihu, China, the allelopathic interaction between Microcystis aeruginosa and Chlorella vulgaris, (eukaryotic competitor) caused the former to release linoleic acid and dominated the hyper-eutrophic lake (Song et al. 2017). Again, pheromone secreting cell signaling compound, nitric oxide produced by C. Vulgaris via quorum sensing is utilized by M. aeruginosa in a positive feedback fashion to stimulate toxicity and more linoleic acid release (Song et al. 2017).
Table 2.
Effect of exogenous nutrient on relative abundance (%) of phytoplankton in Xiangxi Bay, China
| Microcosm | Taxonomic group | ||||||
|---|---|---|---|---|---|---|---|
| Eug | Xanth | Cyano | Pyrro | Bacilla | Chloro | Crpto | |
| Control | 0.00 ± 0.00a | 0.21 ± 0.03b | 46.44 ± 2.30d | 0.38 ± 0.20b | 16.75 ± 2.45d | 32.60 ± 3.39d | 3.72 ± 0.20b |
| +N | 0.00 ± 0.00a | 0.09 ± 0.20b | 47.15 ± 2.90d | 0.35 ± 0.01b | 18.46 ± 2.09d | 30.86 ± 2.66d | 2.99 ± 1.21d |
| + P | 0.00 ± 0.00a | 0.03 ± 0.10c | 32.81 ± 1.88d | 0.21 ± 0.20b | 12.63 ± 2.67d | 51.88 ± 4.87d | 2.46 ± 0.31b |
| + Si | 0.00 ± 0.00a | 0.03 ± 0.20b | 49.67 ± 4.02d | 0.10 ± 0.01b | 20.62 ± 3.33d | 26.53 ± 2.11d | 3.02 ± 0.10c |
| + N + P | 0.00 ± 0.00a | 0.06 ± 0.00a | 46.00 ± 3.30d | 0.15 ± 0.20b | 7.53 ± 0.13c | 44.21 ± 3.23d | 2.05 ± 0.20b |
| + N + P+Si | 0.007 ± 0.00a | 0.09 ± 0.20b | 29.79 ± 1.93d | 0.05 ± 0.00a | 16.81 ± 4.22d | 51.31 ± 3.43d | 1.94 ± 0.33b |
| + Metals | 0.05 ± 0.00a | 0.53 ± 0.01b | 8.31 ± 0.12c | 0.32 ± 0.20b | 15.68 ± 2.14d | 74.75 ± 2.76d | 0.37 ± 0.01b |
| + N + P+Si + Metals | 0.00 ± 0.00a | 0.04 ± 0.20b | 71.61 ± 4.11d | 0.10 ± 0.20b | 8.07 ± 1.99d | 17.79 ± 1.01d | 2.38 ± 0.23b |
Values are mean of three replicates ± SD Mean superscripts with different letters differ significantly (P < 0.05) along treatments
Eug euglenophyta, Xanth xanthophyta, Cyano cyanophyta, Pyrro pyrrophyta, Bacilla bacillariophyta, Chloro chlorophyta, Crypto cryptophyta, N nitrogen, P phosphorus, Si silicon, metals (Fe, Zn, Mn, and Cu)
Furthermore, a secondary metabolite, microcarbonin A produced by Microcystis spp caused the inhibition of photosynthetic activities among the autotrophic dinoflagellate, Peridinium gatunense in Lake Kinneret, Sea of Galilee, Israel (Sukenik et al. 2002). The M. aeruginosa also overwhelms other members of its taxon, such as Anabaena, Aphanizomenon, which carry out N2-fixation, which the M. aeruginosa utilizes to dominate. Most filter feeders, e.g., mussels, Daphnia, and Tilapia, which are potential aquatic predators selectively graze other phytoplankton over cyanoHABs. These mechanisms of allelopathy and ecosystem dominance make Microcystis the most toxic alga to both humans and aquatic zooplankton presently recorded.
In terms of nutrient uptake, cyanoHABs can access nutrients at depth in the night by collapsing its intracellular gas vacuole, which lowers buoyancy while it also acts as a potential store for starch granules (Paerl et al. 2015). Although viral and bacterial lysis, low light, sudden cold spells, mixing, and storm events can also cause cynaoHABs collapse (Watson et al. 2015).
Mechanisms of HAB expansions
At present, it is relatively difficult to draw a revolving conclusion on the single mechanism controlling cyanoHAB proliferation and expansion, recalling that the adaptive mechanisms of the majority of phytoplankton groups are still poorly understood (Jonsson et al. 2009; Yang et al. 2018a, b). Several researchers to this effect have different opinions supporting the mechanisms directing HAB formation in the freshwater-marine continuum. The varying worldviews and conclusions have been based on region and the dominant bloom formers. Examples, Yang et al. (2018a, b) classified HABs inducing current into bottom layer intrusive (BLIC), middle layer intrusive (MLIC), and surface layer intrusive (SLIC) and posited that MLIC in association with stable thermal stratification is responsible for the intense HABs proliferation in summer. Again, a bloom is said to occur following the high concentration of phytoplankton populations arising from excess N&P inputs in the presence of physiological mechanisms including compartmentalization and heterocyst formation among the diazotrophic taxa for example that favourably direct cells to fix nitrogen and fertilize the systems (Butler et al. 2000). In essence, while some researchers posit that nutrient over-enrichment remains the principal factor controlling the mechanisms of bloom, HAB expansions and species transitions in aquatic ecosystems (Ajani et al. 2018), others maintain that these conditions though relevant as stated but must be assisted by specific hydrodynamic, hydrological and environmental influences (Huang et al. 2018; Xiaoyi et al. 2016; Zhou et al. 2017). The phytoplankton allelopathy, i.e. the production of toxic metabolites or compounds otherwise referred to allelogens (Table 3) by certain taxonomic groups against their competing taxa in the aquatic system has also gained much attention. Most cyanoHABs belonging to the taxon, Cyanophyta produce several toxins, including the neurotoxin, Beta-methyl-amino-l-alanine (Cox et al. 2005), which targets the motor neurons. Allelopathic interactions ultimately control the bulk of HAB mechanisms and species dominance (Weberg et al. 2008; Scotti et al. 2015; Zuo et al. 2015; Mohamed 2017; Chia et al. 2018; Jiang et al. 2019). Active release of toxins directly affects the survivability of the competing species even within the same taxonomic group (Intraspecific competition). These negative interactions could sufficiently explain the competitive advantage between planktonic cyanobacteria and zooplankton (eukaryotic algae) to initiate persistent bloom. Mechanisms of bloom formation (Fig. 3), therefore, vary with the aquatic systems. Some algal groups have a faster growth rate than others and quickly dominate the system in the presence of materials essential for proliferation. Most marine species also show high ranges of growth rates although, unlike the cyanoHABs, a majority are not motile so rely on hydrodynamic interferences to dominate the marine systems. Most marine species are usually autotrophic or mixotrophic and thus can synthesize their food. The first step in tackling cyanoHAB blooms lies essentially in understanding the bloom species. Different methods have been used in the identification and study of problematic cyanoHAB community structure. In the past, traditional culturing method has been used in cyanobacterial identification from their natural populations (Castenholz 1988). However, over 95% of cyanobacterial species are non-culturable posing a great challenge and bottleneck in the understanding of the bloom community. This necessitated a shift from the morphological to the culture-independent approaches (Pope and Patel 2008; Lee et al. 2014a, b). Table 4 summarizes the different culture-independent molecular methods that have been used to study cyanoHABs across aquatic ecosystems.
Table 3.
Dominant HAB genera, their characteristic allelopathic materials, harmful toxins, site and mechanism of actions for ecosystem dominance (Weberg et al. 2008; Scotti et al. 2015; Zuo et al. 2015)
| Allelogens | Toxigenic group | HAB genera | Taxa | Site of action | Mechanism/mode of action |
|---|---|---|---|---|---|
| Linoleic acid | Metabolite | Microcystis | Cyanophyta | Membrane lysisa | Growth inhibition via increased membrane permeability, initiation of toxicity through habitat modification e.g. pH reduction and ecosystem dominance |
| Microcarbin (A) | Metabolite | Microcyctis | Cyanophyta | Photo-membranesb Inactivation/damage | Inhibition of photosynthetic activity in autotrophic/mixotrophic HABs and eukaryotic algae |
| Lyngbyatoxin | Neurotoxin | Lyngbya | Cyanophyta | Protein kinase Ca | Inhibition of phosphorylation of of hydroxyl group by inactivation of the enzyme PKC (EC.2.7.11.13) |
| Nodularin | Hepatotoxin | Nodularia | Cyanophyta | Protein phosphatase 1 and 2a | Inhibition of RNA splicing, protein synthesis, membrane receptor/channels in PP1 and disruption of oncogenic signaling cascade protein (RAF, MEK, AKT) of PP2 |
| Microcystin | Hepatotoxin | Microcystis Aphanizomenon Nostoc Anabaena | Cyanophyta | Protein phosphatase 1 and 2Aa | Inhibition of cell growth signaling through serine/threonine malfunctions |
| Saxitoxin | Neurotoxin | Alexandrium Cylindrospermopsis | Dinoflagellate Cyanophyta | Na, K, Ca electrochemical channelsc | Paralytic shellfish poisoning (PSP). and sodium voltage gated blockade initiation |
| Aeruginosin | Hepatotoxin | Microcystis viridis | Cyanophyta | Polypeptide networka | Inhibition of enzymes peptidic proteases |
| Palytoxin | Neurotoxin | Ostreopsis | Dinoflagellete | Respiratory tract impairmenta | Asphyxiation via aerosolized particles inhalation |
| Anatoxin-a | Neurotoxin | Planktothrix Oscillatoria | Cyanophyta | Nicotinic acetyl choline receptora | Acute neurotoxicity and transmembrane deficiency |
| Cylindrospermopsin | Cytotoxin | Clylindrospermopsis Anabaena, Lyngbya Aphanozomenon | Cyanophyta | Protein and glutathione receptors | Inhibition of protein synthesis necrosis, membrane proliferation and lipid infiltration |
| Beta-methyl-amino-l-alanine | Neurotoxin | All cyanobacteria | Cyanophyta | Motor neurons | Induces neurodegenerative disorders; oxidative stress, depletion of glutathione and attacks ion channel protein localized in nerve cells e.g. NMDA and AMPA receptors |
NMDA N-methyl-d-aspartate, AMPA α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
aTargets all range of phytoplankton populations and humans
bOnly target autotrophs and mixotrophs
cMost phytoplankton and humans but principal vector is fish
Fig. 3.
Conceptual illustrations of bloom mechanism and the several physicochemical and biological interactions exacerbating HABs proliferation and expansions on a geographically diverse scale
Table 4.
Different culture-independent molecular methods deployed for cyanoHAB identification
| Technology/ method | Sample | Identified cyanobacteria/sepcies | References |
|---|---|---|---|
| mMetagenomic Complete genomic sequencing | Cyanobacteria impacted freshwater | Aphanizomenon Cylindrospermopsis | Pope and Patel. (2008) |
| mPolyphasic Microscopy/DNA base analyses | Australian freshwater | Chroococcales Oscillatoriales Nostocales | Lee et al. (2014a, b) |
| aLC HPLC Mass-spectroscopy | Bloom surface scum | Cyano toxins | Msagati et al. (2006) |
| mImaging-driven technique Integrated florescence signature | Bloom water sample | Anabaena flos-aquae, Microcystis aeruginosa Ankistrodesmus | Jin et al. (2017) |
| Molecular | Bloom scum | Nostoc | Kust et al. (2018) |
LC liquid chromatography, HPLC high performance liquid chromatography
mMolecular, aAnalytical
The socioeconomic implications of HABs
The impacts of harmful algal bloom under changing climates and globally increasing anthropogenic nutrient loading create synergistic interactions that negatively affect the freshwater-marine continuum. These adverse influences also result in significant alterations in the biological and physicochemical characteristics of these interconnected systems. Consequently, significant socio-economic implications have been attributed to HAB impacts on a geographically global scale with huge effects on the main sectors including tourism, recreational activities, public health, commercial fisheries, monitoring and management (Hoagland et al. 2009; Manganelli 2015; Sanseverino and Conduto 2016). According to the Florida Department of Health, the red tide in Sarasota County reported a total of 54% respiratory, 19% pneumonia, and 40% gastrointestinal illnesses. This culminated in hospital costs of 0.5 to 4 million USD and an average annual loss of 22 million USD on both medical expenses and lost works days due to illnesses (Alcock 2017).
Broadly, tourism and recreation impacts include; a rapid reduction in the recreational experiences of the beach tourists, total fish harvesting/fishing closures concerning recreational fishers, all of which are substantial economic losses and damage to tourism industries. The public health impacts are indeed the medical expenses arising from HABs’ allelopathy and intoxication, including the cost of transportation to the hospital, hospitalization cost, and a significant decrease in daily productivity. Commercial fisheries’ impacts include; remarkable reduction in the consumers’ demand for fish due to fear of shellfish poisoning (SFP) (sometimes plays a significant role in people’s psychology), increased fish mortality, commercial-scale fish closures, fish scarcity, and general price increase. The monitoring and management implications centrally include; water quality deterioration and filter clogging, increased water sampling, intensified actions to identify factors exacerbating bloom and its detriments in terms of time, energy and cost, the extra cost of water treatment for toxins removal, and approaches developed to destroy HABs may be exorbitant. However, certain cyanobacteria, the bulk of which are not among the harmful bloom formers are used as potentials food additives (Siah et al. 2013; Vinogradova et al. 2011). Blue-green algae supplemented foods (BGAS) e.g. Aphanizomenon flos aquae, Spirulina and chlorella have been found useful in weight reduction and elevating moods among people with depressions (Sanseverino and Conduto 2016) but since these edible taxa are usually harvested from natural environment where the potentially toxic counterparts including the Microcystis aeruginosa, Cylindrospermopsis, Lyngbya, Phormidium, Planktothrix, and Raphidiopsis also thrive, risk of food toxicity cannot be presumably eliminated. The evidence of increased human health risks through the consumption of neurotoxin (cyanotoxin) contaminated foods has been recently reported (Manolidi et al. 2019; Vichi et al. 2012).
Management of HABs
Over the years, HABs management has attracted quite pretty much attention, and different approaches have been regularly evaluated and applied in practical situations to mitigating the globally increasing bloom events. Mitigation strategies such as physical, biological, and chemical have been proposed by researchers (Garce and Maso 2006; Schindler et al. 2008; Glibert 2017; Harrison et al. 2017; Ji et al. 2017; McGowan et al. 2017; Paterson et al. 2017; Jiang et al. 2019). Regrettably, each mitigation approach (Table 5) is confronted with challenges and inadequacies, making most management strategies ultimately system-specific and in most cases, almost difficult and rarely applicable. The physical approaches and their intrinsic limitations include; (1) application of ultrasonic treatment involving sound wave frequency ≥ 20 kHz (Park et al. 2017) targeted at disrupting the planktonic bloom taxa through mutation and algal inactivation. Provision of induced vertical mixing (Lundgren et al. 2013). The vertical mixing is aimed at reducing both hypoxia, and thermal stratification in the aquatic systems, e.g. wind-induced flow velocity has been applied to reduce thermal stratification and hypoxia at the Meiling Bay of Lake Taihu (Jalil et al. 2018). The technology is not cost-effective and thus, cannot be applied in large aquatic ecosystems (freshwater-marine continuum). Apart from this, the technology, if adopted even in small but shallow freshwater would result in bloom exacerbation through sediment burst and concomitant nutrient release from legacy stores (2) promoting hypolimnion oxygenation and surface flushing assisting to destroy the phytoplankton biomass integrity and tendencies to form cell assemblage (biofilm). The approach is tedious and does not guarantee sufficient success as preponderance of bloom formers in addition to being buoyant and planktonic also possess heterocyst which aids in excluding oxygen and increases the survivability of these groups in oxygen surplus hypereutrophic systems e.g. the diazotrophic cyanobacteria including Anabaena, Aphanizomenon, Nostoc, Nodularia are often potential survivors under these scenarios (3) optimizing the competitive abilities of rooted and submerged macrophytes by deliberating lowering lakes and reservoirs. This strategy would have proven relatively effective both in terms of cost and time, but lake manipulations through lowering may not generally represent a reliable and feasible option. Again, the approach is limited to lakes and reservoirs, other freshwater and marine systems are not considered for this kind of intervention (4) weather conditions forecasting. The efficient bloom management mostly relies in part on the accurate predictions of weather and climatic variables, including autumn–winter and spring–summer transitions. Sustainable control of bloom has been attributed to managers’ abilities to forecast future while backing up forecast results with remedial actions (Paerl et al. 2011a, b, 2016). However, under the constantly changing climate especially the extremes in rainfall (precipitations) and droughts substantially impacting the aquatic systems, information from forecast often do not provide a reliable and prudent solution for sustainable bloom mitigation (5) physical removal of dense surface scums by skimming, physically scooping of dense surface scums, concentrating and conversion of toxins into innocuous by-products/materials. This approach though cost-effective but can only be applied in reservoirs (≤ 40 ha) and not in freshwater systems as large as ~ 2500 ha e.g. Taihu Lake, China, and Lake Erie, USA. Again, this strategy can predispose individuals to direct contact with toxic HABs since cyanotoxins can pose serious health threats with both acute and chronic manifestations through oral, dermal, and inhalation exposures (Chia et al. 2018; Funari et al. 2017). Clarification, coagulation, filtration, and floatation are also traditional physical methods. These are too complex, expensive and possess the tendency to worsen water pollution (Rajasekhar et al. 2012).
Table 5.
Summary of HABs mitigation strategies, associated inadequacies and relative functions across the freshwater–marine continuum (Sanseverino and Conduto 2016; Glibert 2017; Harrison et al. 2017; McGowan et al. 2017; Park et al. 2017)
| Treatment | Category | Application spectrum | Measures | Function | Inadequacy |
|---|---|---|---|---|---|
| Sonication (Sound wave ≥ 20 kHz) | Physical | N | RCurative | Disruption of algal cells, discourages cell assemblage, induces mutation and sterility among algae | High frequency soundwave can trigger cell lysis and release of toxins into the bulk water phase, dead algal cells remain in water and aggrandize pollution |
| Vertical mixing | Physical | N | RCurative | Epilimnion/hypolimnion Oxygenation/disrupts thermal stratification | Intensifies burst of rich sediments, re suspension and high nutrient releases |
| Weather forecasting | Physical | B | Preventive | Provides early signs of imminent HABs episodes | Constantly changing climate makes predictions most times inexact and unreliable |
| Skimming | Physical | N | RCurative | Attempts reduction of dense surface sum, sparingly allowing surface Water–atmosphere interactions | Increased the chances of human—toxin contacts via oral, dermal and inhalation exposures |
| Dredging | Physical | N | QCurative | Ensures absolute elimination of factors exacerbating HABs proliferations/expansions. | May initiate short term water deterioration but disappears after a short time |
| Multiple nutrients input reductions pollution | Physical | B | Preventive | Maintains nutrients level across aquatic systems below | Approach pays less attention to aquatic nutrient over |
| Filter feeders allelopathy, deployment | Biological | N | RCurative | Directly ingest HABs | High chances of allelopathy, shellfish poisoning, gill suffocation/massive fish kills |
| Flocculation/Sedimentation | Chemical | N | RCurative | Kill algae cells by chemical precipitation/some are directly toxic to algae | Chemical-cell lysis/algal toxin releases increase the risks of aquatic pollution |
Preventive This means strategies only ensures control of subsequent pollution but not the current state of problems
N narrow spectrum/system selective, only applicable in small aquatic systems such as ponds and reservoirs (≤ 50 ha)
B Broad spectrum/applicable to all systems, thus, can be deployed across the diverse freshwater–marine continuum
RRelatively curative/short term
QAbsolutely curative especially in multiple deployments and dredged sediment discharged ex situ hence, considers the state of contemporary pollution
The biological bloom management paradigm often includes maximizing all the ecosystem predations and competitions, especially those that negatively affect HABs survival and expansions. Predation mediated interaction involving the quagga mussel (Dreis-sena rostriformis bugensis) has been applied to significantly reduce phytoplankton biomass while restoring clear water characteristics in the shallow hypereutrophic urban pond, Netherlands (Waajen et al. 2016). Still, this approach is limited by the narrow application spectrum thus cannot be applied in both lakes and large reservoirs (> 100 ha). Furthermore, the phytoplankton proliferations proceed faster than the reproductive rates of the bivalves and in some cases generate hypoxia and acute toxicity that kill the predators, which in turn provide more food for the HABs species expansions through putrefaction. Example, in Fjordic coastlines, Scottish Clyde Sea, high cell density (2840 cells L−1) of D. acuta in water column triggered high Shellfish toxicity (601 ± 237 µg OA eq/kg shellfish flesh) which resulted in the ban of fish harvesting in the area (Paterson et al. 2017). Preservation of herbivorous zooplankton via population reduction of their potential predators including the zooplanktivorous and piscivorous fish, adequately helps to reduce HABs proliferations and expansion. Filter feeders such as Tilapia and Daphnia can directly ingest large populations of phytoplankton, but it is limited to a few grazers in aquatic systems. Besides, the incidence of gill suffocation by the toxigenic species like the dinoflagellates, Karenia brevis possesses a significant threat. The use of algaecidal microbial species further provides an insight into the exploitation of predator–prey interaction to control HABs. Algaecidal Streptomyces, U3 (1 µg mL−1 crude extract) has been used to eliminate > 70% harmful alga, Heterosigma akashiwo in three days under laboratory conditions (Yu et al. 2018). However, the fast-growing actinomycete, when seeded into freshwater-marine continuum would worsen water quality deterioration through its ability to produce active compounds highly resistant to a wide range of environmental factors thus does not constitute a reliable biocontrol measure. Again, HABs monitoring via the use of biosensors to rapidly and continuously estimate algal cell densities and toxin levels at PON (Point of Needs) has also been proposed (Mcpartlin et al. 2017). The so-called use of a biosensor, though, could be promising, but this device is only recommended for small systems detection of likely bloom outbreak and thus cannot be applicable in larger aquatic systems actively facing intense bloom. A study has also explored the potentials of some parasitic protozoa and fungi to reduce algal cell density in aquatic systems through parasitism, e.g., Chytrid parasite has been shown to play an essential role in checking toxins production in phytoplankton (Gleason et al. 2015). Although, this can adversely affect aquatic food web structure as the zoosporic fungi of the phylum Chytridiomycota is associated with severe mycosis and has been linked to global amphibian mass extinction. Generally, biological manipulations ultimately involve altering the aquatic food web while exacerbating pressures on HABs grazing by the potential predators and subsequently ridding the aquatic ecosystems of these deleterious phytoplankton groups.
Chemical treatments for HABs, such as the use of titanium dioxide to trigger the release and degradation of microcystin produced by M. aeruginosa, have been reported (Chang et al. 2018). Application of sophorolipids (Sun et al. 2004) and absorbents materials, e.g. modified and natural clays (Pan et al. 2006; Yu et al. 2017), which aggregate algal cell by flocculation ensuring their massive sedimentations and consequent removal from impacted waters. Greater than 90% cells of Alexandrium tamarense and paralytic shellfish toxins were effectively removed by 0.25 g L−1 of modified clay under laboratory conditions. The use of chemical oxidants such as chlorine (Cl), potassium permanganate (KMnO4), and ozone (O3) in cyanoHABs control in the laboratory resulted in average cell lysis of > 80% (Fan et al. 2013).
However, these techniques could be useful in short term consideration and small aquatic systems but make the process adequately tedious and cost-ineffective. Again, some chemicals possess selective toxicity to specific phytoplankton taxa, e.g., a study has recently shown that while H2O2 causes dramatic cyanobacteria inactivation, the growth of chlorophytes is usually promoted under the same condition (Yang et al. 2018a, b). In Ran Yi Tan Reservoir, China, 10 mg L−1 K2FeO4 caused dramatic membrane inactivation and cell lysis of cyanobacteria, M. aeruginosa (Yang et al. 2018a, b). Other chemical treatments include the use of Phoslock, copper sulfate, alum (hydrated aluminum sulfate), Ca (OH)2 (lime), which yield CaCO3 (carbonate), ferric and ferrous chloride. (Yu et al. 2017; Paerl et al. 2018). Several studies have also reported the use of hydrogen peroxide (H2O2) (Barrington et al. 2013; Burson et al. 2014; Dra et al. 2007; Randhawa et al. 2012; Zhou et al. 2018a, b).
The toxicity of H2O2 to M. aeruginosa through P450 inhibition has been recently studied (Wang et al. 2018). However, the use of chemical formulations in water ecosystem reclamation could be exceptionally costly and limited to small systems. Apart from this, some of the chemical precipitants e.g.FeCl3 under changing water conditions can dissociate to liberate Fe2+, which can boost the expansion of the N2-fixers through ‘nitrogenases’ reactivation in iron-limiting condition thus intensifying bloom. Again, copper though, a potent algaecide but could become problematic. The physical (colour) characteristic of water can be compromised by a massive influx of copper into aquatic systems. Copper sulfate causes the release of endotoxins into the bulk water phase through massive algal cell lysis. The toxicity of copper to HABs has been attributed to the dynamics of copper ions and not the total copper concentrations, and the sensitivity of HABs to copper ion has been shown to vary considerably with different phytoplankton species (Schoffman et al. 2016). Complexation, precipitation by DOM, and adsorption by suspended material are instrumental in regulating the chemical speciation of anthropogenic copper into the aquatic systems. In small lakes and hatchery ponds, barley straws can be utilized to suppress HAB proliferation through the release of algistatic phenolic compounds into freshwater upon oxidation of barley straws (Islami and Filizadeh 2012). Generally, chemical control has shown massive success in small systems but are still challenged by the full range of sensitive environmental concerns. The potential lethality of some chemicals also raises considerable concern. The chemical treatment is associated with several adverse residual effects.
The recent paradigm for N-reduction principally involves denitrification and the globally advocated anthropogenic N&P dual management (Paerl et al. 2011a, b; Xu et al. 2015). However, denitrification is a natural process, usually slow and potentially dependent on seasons. The N&P dual management is a preventive intervention targeted only on subsequent anthropogenic nutrients inputs, which do not consider the state and fate of systems already facing hyper-eutrophy. On this account, Paerl et al. 2018 stated that the only suitable and relatively long term N and P control measure remains the adoption of the 4R stewardships (Right rate, Right time, Right place and Right source) in nitrogen based-fertilizer management and improved wastewater treatment as well as the total ban of P-related detergents for P-regulations. Still, this did not consider the accidental discharge of non-agricultural products like oils that do not pay attention to quantity exposures, and it is impossible to ensure absolute adherence to the above conditions. Any mitigation design that could not address all these contingencies lacks sustainability and, therefore, not efficient in guaranteeing long term solutions. The importance of creating buffer zones and cover crops to help deal with nutrient over-enrichment through run-offs break/check has been advised (Lee et al. 2014a, b). Greater than 50% N&P input reductions can be achieved by planting riparian buffers (Paerl et al. 2016, 2018). High efficient N and P removal (≥ 75% removal efficiency) through optimization in anaerobic-anoxic –aerobic constructed wetland and water quality improvement beyond nutrient strategies had also been reported (Christianson et al. 2018; Roe-sosa et al. 2019). Although, shortcomings also exist as most freshwater and marine systems do not have sufficient arable lands to kick-off large scale construction of wetlands, buffer zones, and cover crops. Again, the significant N and P removal reported in most successful attempts were performed under controlled conditions and not in the globally and naturally distributed aquatic systems with geometric nutrient increase per day.
From the Foregoing, it becomes empirically evident that though each strategy has shown potentially effective results in a wide range of microcosm, mesocosm, and pilot-scale studies, but the actual field outcome is different. In the light of these limitations, however, which have made none of the remedial options necessarily the best, the last option that would need to be tried is the exploitation and adoption of more than one mitigation approaches into a single treatment protocol while utilizing their functions in concert. It is also important to diversify concerns to the non-agriculturally-related contaminants like oil which are continually drained into the waters. In the present study, this technology has been described as Integrated Management Intervention (IMI), as shown in Fig. 4. The integrated mitigation design awakens new scientific consciousness to targeting bloom control beyond the traditional association of N&P inputs regulation. Since every management approach is associated with unique function and shortcoming, a combination of strategies would provide an insight into successfully by-passing the inadequacies while exploiting their optimum remedial functions to dealing with HABs across the freshwater-marine continuum. For example, anthropogenic N&P inputs reduction (preventive) significantly reduces the global influx of nutrients into the diverse aquatic systems but does not address the current state of nutrient over-enrichment across water bodies. Sediment dredging (curative) substantially eliminates nutrient bulk, making the system relatively mesotrophic, but there could be cases of nutrient reloading to exacerbate pollution, eutrophication, and more HAB events if stringent anthropogenic nutrient regulation is not enacted across the continuum following a dredging operation or if dredged sediments are not disposed ex situ. Again, the use of chemical treatments such as H2O2 (curative) selectively inactivates the threatening HAB taxa through cell lysis, which are released into the bulk water phase. These can accumulate over time, compromising water quality and public health if not dredged, making water treatment processes ultimately expensive and tedious. This evidently indicates that the success of a remedial step usually depends on the others towards achieving a lasting solution and the outcome of a single strategy without complementary deployments often short-lived. An effective management decision to dealing with HABs should be one that considers potential remedial outcome beyond a short term solution. It, therefore, becomes extremely relevant to consider the integration/merging of these three remedial options as a single management ideology to effectively deal with phytoplankton proliferation and expansions on a geographically global scale. The proposition/new consideration here is, therefore, that these three remedial steps be merged as a single mitigation design through an integrated management intervention to create a novel and sustainable solution to HAB outbreaks.
Fig. 4.
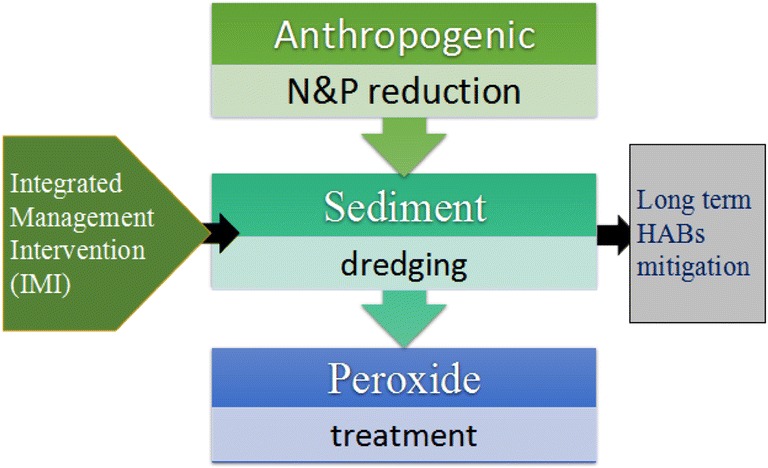
A typical Integrated Management (IMI) design to mitigating HAB across the geographically diverse regions
Concluding remarks
The glaring evidence from literature has strongly demonstrated that ecosystem resilience to HAB impacts is grossly challenged by increasing anthropogenic activities and constantly changing climate. This review is a delineation of management options conventionally adopted in response to the episodic impacts of cyanoHABs on a global scale. It, therefore, concludes that the lack of mitigation steps is not the central problem currently confronting water managers and scientists thus advocated that the strategy and rationale for selection and adoption of existing remedial options be reviewed. It notes that such adoption should take into account the mitigation designs with both curative and preventive measures to effectively tackle the already impacted aquatic systems and at the same time prevent future HAB outbreaks, respectively stating that reliance on a single measure can only deliver a parochial solution to the increasing HAB events with high tendency for resurgence. Further, it underscores that all management options are potentially effective under short term consideration, but no single remedial step can guarantee sustainable solutions to HABs at large. To this effect, we proposed an integrated management intervention (IMI) as a key to circumventing the situation. Based on both application and implication, this paradigm is fundamentally a new remedial convention where future research should be directed. Although it involves the combinations of already existing mitigation steps, this approach points to the promising potentials to deciphering the limitations that characterize single deployments and represents a strategy that can deliver a broad solution to HABs, which has not been considered before. Finally, it states that the non-nutrient pollutants including oil/hydrocarbon and its congeners also be recognized as potential triggers of HABs as all efforts are heavily focused on N&P inputs reduction while warning that there could be possible transition from nutrient-related to non-nutrient-related triggers of HABs in future if both factors do not simultaneously attract adequate attention.
Acknowledgements
The research was supported by National key research and development plan (2016YFC0401703), and Chinese National Science Foundation (5177090079, 51579071, 51379061, 41323001, 51539003, 41330751, 51279192); National Science Funds for Creative Research Groups of China (No. 51421006); the program of Dual Innovative Talents Plan and Innovative Research Team in Jiangsu Province; the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the Fundamental Research Funds for the Central Universities.
Compliance with ethical standards
Conflict of interest
Authors declare that there are no conflicts of interest.
Human and animal rights
This study did not involve human and/or animal participants.
Informed consent
Authors declare that this study did not involve human or animal participation.
References
- Ajani PA, Larsson ME, Woodcock S, Rubio A, Farrell H, Brett S, Murray SA. Bloom drivers of the potentially harmful dinoflagellate Prorocentrum minimum (Pavillard) Schiller in a south eastern temperate Australian estuary. Estuar Coast Shelf Sci. 2018;215:161–171. [Google Scholar]
- Alcock F (2017) An assessment of floridA red tide: causes, consequences and Management Strategies. New College of Florida. Marine Policy Institute at Mote Marine Laboratory technical report
- Anderson DM. Approaches to monitoring, control and management of harmful algal blooms (HABs) Ocean Coast Manag. 2009;52:342–347. doi: 10.1016/j.ocecoaman.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CR, Moore SK, Tomlinson MC, Silke J, Cusack CK. Living with harmful algal blooms in a changing world in: strategies for modeling and mitigating their effects in coastal marine ecosystems. In: Anderson CR, editor. Coastal and marine hazards, risks, and disasters. Amsterdam: Elsevier; 2015. pp. 496–561. [Google Scholar]
- Barrington DJ, Reichwaldt ES, Ghadouani A. The use of hydrogen peroxide to remove cyanobacteria and microcystins from waste stabilization ponds and hypereutrophic systems. Ecol Eng. 2013;50:86–94. [Google Scholar]
- Binding CE, Greenberg TA, Mccullough G, Watson SB, Page E. An analysis of satellite-derived chlorophyll and algal bloom indices on Lake Winnipeg. J Great Lakes Res. 2018;44:436–446. [Google Scholar]
- Burson A, Matthijs HCP, Bruijne W De, Talens R, Hoogenboom R, Gerssen A, Visser PM, Stomp M, Steur K, Scheppingen Y Van, Huisman J. Termination of a toxic Alexandrium bloom with hydrogen peroxide. Harmful Algae. 2014;31:125–135. doi: 10.1016/j.hal.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Butler B, Terlizzi D, Ferrier D (2000) Barley straw : a potential method of algae control in ponds. In: Water Quality Workbook Series., MaryLand Sea grant Extension program, pp 1–3
- Campbell SG. Predicting the thermal effects of Dam removal on the Klamath River. Environ Manag. 2005;34:856–874. doi: 10.1007/s00267-004-0269-5. [DOI] [PubMed] [Google Scholar]
- Castenholz RW. Culturing methods for cyanobacteria. Method Enzymol. 1988;167(1988):68–93. [Google Scholar]
- Castro NO, Domingos P, Moser GAO. National and international public policies for the management of harmful algal bloom events. A case study on the Brazilian coastal zone Ministry of Environment. Ocean Coast Manag. 2016;128:40–51. [Google Scholar]
- Chang C, Huo X, Lin T. Exposure of Microcystis aeruginosa to hydrogen peroxide and titanium dioxide under visible light conditions: modeling the impact of hydrogen peroxide and hydroxyl radical on cell rupture and microcystin degradation. Water Res. 2018;141:217–226. doi: 10.1016/j.watres.2018.05.023. [DOI] [PubMed] [Google Scholar]
- Chaudhary DK, Kim J. New insights into bioremediation strategies for oil-contaminated soil in cold environments. Int Biodeter Biodegrad. 2019;142:58–72. [Google Scholar]
- Chia MA, Jankowiak JG, Kramer BJ, Goleski JA, Huang I, Zimba PV, Bittencourt-oliveira C, Gobler CJ. Succession and toxicity of Microcystis and Anabaena (Dolichospermum) blooms are controlled by nutrient-dependent allelopathic interactions. Harmful Algae. 2018;74:67–77. doi: 10.1016/j.hal.2018.03.002. [DOI] [PubMed] [Google Scholar]
- Christianson R, Christianson L, Wong C, Helmers M, Mcisaac G, Mulla D, Mcdonald M. Beyond the nutrient strategies: common ground to accelerate agricultural water quality improvement in the upper Midwest. J Environ Manag. 2018;206:1072–1080. doi: 10.1016/j.jenvman.2017.11.051. [DOI] [PubMed] [Google Scholar]
- Cox PA, Banack SA, Murch SJ, Rasmussen U, Tien G, Bidigare RR, Metcalf JS, Morrison LF, Codd GA, Bergman B. Diverse taxa of cyanobacteria produce BMAA, a neurotoxic amino acid. Proc Natl Acad Sci (USA) 2005;102:5074–5078. doi: 10.1073/pnas.0501526102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J, Garcia-velasco N, Urionabarrenetxea E, Soto M, Álvarez A, Alejandra M. Evaluation of the e ff ectiveness of a bioremediation process in experimental soils polluted with chromium and lindane. Ecotoxicol Environ Saf. 2019;181:255–263. doi: 10.1016/j.ecoenv.2019.06.019. [DOI] [PubMed] [Google Scholar]
- Deng J, Paerl H, Qin B, Zhang Y, Zhu G, Jeppesen E, Cai Y, Xu H. Climatically-modulated decline in wind speed may affect eutrophication in shallow lakes. Sci Total Environ. 2018;645:1361–1370. doi: 10.1016/j.scitotenv.2018.07.208. [DOI] [PubMed] [Google Scholar]
- Ding S, Chen M, Gong M, Fan X, Qin B, Xu H, Gao SS, Jin Z, Tsang DCW, Zhang C. Internal phosphorus loading from sediments causes seasonal nitrogen limitation for harmful algal blooms. Sci Total Environ. 2018;625:872–884. doi: 10.1016/j.scitotenv.2017.12.348. [DOI] [PubMed] [Google Scholar]
- Dra M, Mars B, Lek Ä. Combined exposure to hydrogen peroxide and light selective effects on Cyanobacteria, Green Algae, and Diatoms. Environ Sci Technol. 2007;41:309–314. doi: 10.1021/es060746i. [DOI] [PubMed] [Google Scholar]
- Fan J, Ho L, Hobson P, Brookes J. Evaluating the effectiveness of copper sulphate, chlorine, potassium permanganate, hydrogen peroxide and ozone on cyanobacterial cell integrity. Water Res. 2013;47:5153–5164. doi: 10.1016/j.watres.2013.05.057. [DOI] [PubMed] [Google Scholar]
- Funari E, Manganelli M, Buratti FM, Testai E. Cyanobacteria blooms in water: Italian guidelines to assess and manage the risk associated to bathing and recreational activities. Sci Total Environ. 2017;598:867–880. doi: 10.1016/j.scitotenv.2017.03.232. [DOI] [PubMed] [Google Scholar]
- Garce E, Maso M. Harmful microalgae blooms (HAB); problematic and conditions that induce them. Mar Pollut Bull. 2006;53:620–630. doi: 10.1016/j.marpolbul.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Gleason FH, Jephcott TG, Upper FCK, Karpov SA, Guillou L, Ogtrop FFVAN, Pascal B. Potential roles for recently discovered chytrid parasites in the dynamics of harmful algal blooms. Fungal Biol Rev. 2015;29:20–33. [Google Scholar]
- Glibert PM. Eutrophication, harmful algae and biodiversity—challenging paradigms in a world of complex nutrient changes. Mar Pollut Bull. 2017;124:591–606. doi: 10.1016/j.marpolbul.2017.04.027. [DOI] [PubMed] [Google Scholar]
- Hadas O, Kaplan A, Sukenik A. Long-term changes in cyanobacteria populations in Lake Kinneret (Sea of Galilee), Israel: an eco-physiological outlook. Life. 2015;5:418–431. doi: 10.3390/life5010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Piontkovski S, Al-hashmi K. Understanding how physical-biological coupling in fl uences harmful algal blooms, low oxygen and fi sh kills in the Sea of Oman and the Western Arabian Sea. Mar Pollut Bull. 2017;114:25–34. doi: 10.1016/j.marpolbul.2016.11.008. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Murakami S, Xu KQ, Watanabe M. Effect of the Three Gorges Dam Project on flood control in the Dongting Lake area, China, in a 1998-type flood. J Hydro Environ Res. 2008;2:148–163. [Google Scholar]
- Hoagland P, Jin D, Polansky LY, Kirkpatrick B, Kirkpatrick G, Fleming LE, Reich A, Watkins SM, Ullmann SG, Backer LC. The costs of respiratory illnesses arising from florida gulf coast Karenia brevis Blooms. Environ Health Perspect. 2009;117:1239–1243. doi: 10.1289/ehp.0900645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YL, Huang GH, Liu DF, Zhu H, Sun W. Simulation-based inexact chance-constrained nonlinear programming for eutrophication management in the Xiangxi Bay of Three Gorges Reservoir. J Environ Manag. 2012;108:54–65. doi: 10.1016/j.jenvman.2012.04.037. [DOI] [PubMed] [Google Scholar]
- Huang J, Zhang Y, Huang Q, Gao J. When and where to reduce nutrient for controlling harmful algal blooms in large eutrophic lake Chaohu, China. Ecol Indic. 2018;89:808–817. [Google Scholar]
- Islami HR, Filizadeh Y. Use of barley straw to control nuisance freshwater algae. J Am Water Works Assoc. 2012;103:111–118. [Google Scholar]
- Jalil A, Li Y, Du W, Wang W, Wang J, Gao X, Khan S, Pan B, Acharya K. The role of wind fi eld induced fl ow velocities in destrati fi cation and hypoxia reduction at Meiling Bay of large shallow Lake Taihu, China. Environ Pollut. 2018;232:591–602. doi: 10.1016/j.envpol.2017.09.095. [DOI] [PubMed] [Google Scholar]
- Ji D, Wells SA, Yang Z, Liu D, Huang Y, Ma J, Berger CJ. Impacts of water level rise on algal bloom prevention in the tributary of Three Gorges Reservoir, China. Ecol Eng. 2017;98:70–81. [Google Scholar]
- Jiang H, Qiang M, Fan Q, Zhang M. Scientific research driven by large-scale infrastructure projects: A case study of the Three Gorges Project in China. Technol Forecast Soc Change. 2018;134:61–71. [Google Scholar]
- Jiang M, Zhou Y, Wang N, Xu L, Zheng Z, Zhang J. Allelopathic effects of harmful algal extracts and exudates on bio fi lms on leaves of Vallisneria natans. Sci Total Environ. 2019;655:823–830. doi: 10.1016/j.scitotenv.2018.11.296. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Liu L, Liu D, Johnson DM, Yi Z, Huang Y. Effects of vertical mixing on phytoplankton blooms in Xiangxi Bay of Three Gorges Reservoir: implications for management. Water Res. 2012;46:2121–2130. doi: 10.1016/j.watres.2012.01.029. [DOI] [PubMed] [Google Scholar]
- Jonsson PR, Pavia H, Toth G. Formation of harmful algal blooms cannot be explained by allelopathic interactions. Proc Natl Acad Sci (USA) 2009;106:11177–11182. doi: 10.1073/pnas.0900964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krachler R, Krachler R, Valda A, Keppler BK. Natural iron fertilization of the coastal ocean by “blackwater rivers. Sci Total Environ. 2019;656:952–958. doi: 10.1016/j.scitotenv.2018.11.423. [DOI] [PubMed] [Google Scholar]
- Kust A, Urajová P, Hrouzek P, Long D, Lenka Š, Klára Ř, Lep O, Luke A, Mare J. Toxicon A new microcystin producing Nostoc strain discovered in broad toxicological screening of non-planktic. Nostocaceae (cyanobacteria) 2018;150:66–73. doi: 10.1016/j.toxicon.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Lee E, Ryan UM, Monis P, Mcgregor GB, Bath A, Gordon C, Paparini A. Science direct polyphasic identification of cyanobacterial isolates from Australia. Water Res. 2014;59:248–261. doi: 10.1016/j.watres.2014.04.023. [DOI] [PubMed] [Google Scholar]
- Lee SO, Kim S, Kim M, Lim KJ, Jung Y. The effect of hydraulic characteristics on algal bloom in an artificial Seawater Canal: a case study in Songdo City, South Korea. Water. 2014;6:399–413. [Google Scholar]
- Li C, Yang SY, Lian EG, Yang CF, Deng K, Liu ZF. Damming effect on the Changjiang (Yangtze River) river water cycle based on stable hydrogen and oxygen isotopic records. J Geochem Explor. 2016;165:125–133. [Google Scholar]
- Lin Q, Wu Z, Singh VP, Sadeghi SHR, He H, Lu G. Correlation between hydrological drought, climatic factors, reservoir operation, and vegetation cover in the Xijiang Basin, South China. J Hydrol. 2017;549:512–524. [Google Scholar]
- Lundgren VM, Roelke DL, Grover JP, Brooks BW, Prosser KN, Scott WC, Laws CA, Umphres GD. Interplay between ambient surface water mixing and manipulated hydraulic flushing: implications for harmful algal bloom mitigation. Ecol Eng. 2013;60:289–298. [Google Scholar]
- Manganelli M. Blooms of toxic microorganisms in aquatic environments: marine microalgae and freshwater cyanobacteria. A brief review with a particular focus on the Italian situation in Italy. Rendiconti Lincei. 2015;27:135–143. [Google Scholar]
- Manolidi K, Triantis TM, Kaloudis T, Hiskia A. Neurotoxin BMAA and its isomeric amino acids in cyanobacteria and cyanobacteria-based food supplements. J Hazard Mater. 2019;365:346–365. doi: 10.1016/j.jhazmat.2018.10.084. [DOI] [PubMed] [Google Scholar]
- Mao J, Jiang D, Dai H. Spatial e temporal hydrodynamic and algal bloom modelling analysis of a reservoir tributary embayment. J Hydro Environ Res. 2015;9:200–215. [Google Scholar]
- Mccarthy FMG, Riddick NL, Volik O, Danesh DC, Krueger AM. Algal palynomorphs as proxies of human impact on freshwater resources in the Great Lakes region. Anthropocene. 2018;21:16–31. [Google Scholar]
- McGowan JA, Deyle ER, Ye H, Carter ML, Perretti CT, Seger KD, de Verneil A, Sugihara G. Predicting coastal algal blooms in southern California. Ecology. 2017;98:1419–1433. doi: 10.1002/ecy.1804. [DOI] [PubMed] [Google Scholar]
- Mchau GJ, Makule E, Machunda R, Gong YY, Kimanya M. Harmful algal bloom and associated health risks among users of Lake Victoria freshwater: Ukerewe Island. Tanzania: J Water Health; 2019. [DOI] [PubMed] [Google Scholar]
- Mcpartlin DA, Loftus JH, Crawley AS, Silke J, Murphy CS, Kennedy RJO. ScienceDirect Biosensors for the monitoring of harmful algal blooms. Curr Opin Biotechnol. 2017;45:164–169. doi: 10.1016/j.copbio.2017.02.018. [DOI] [PubMed] [Google Scholar]
- Michalak AM, Anderson EJ, Beletsky D, Boland S, Bosch NS, Bridgeman TB, Chaf JD, Cho K, Confesor R, Dalo I, Liu X, Mcwilliams MR, Moore MR, Posselt DJ, Richards RP, Scavia D, Steiner AL, Verhamme E, Wright DM, Zagorski MA. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc Natl Acad Sci (USA) 2013;110:6448–6452. doi: 10.1073/pnas.1216006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed ZA. Macrophytes-cyanobacteria allelopathic interactions and their implications for water resources management—a review. Limnologica. 2017;63:122–132. [Google Scholar]
- Morais P, Chícharo MA, Chícharo L. Changes in a temperate estuary during the filling of the biggest European dam. Sci Total Environ. 2009;407:2245–2259. doi: 10.1016/j.scitotenv.2008.11.037. [DOI] [PubMed] [Google Scholar]
- Msagati TAM, Siame BA, Shushu DD. Evaluation of methods for the isolation, detection and quantification of cyanobacterial hepatotoxins. Aquat Toxicol. 2006;78:382–397. doi: 10.1016/j.aquatox.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Neill KO, Schreider M, Mcarthur L, Schreider S. Changes in the water quality characteristics during a macroalgal bloom in a coastal lagoon. Ocean Coast Manag. 2015;118:32–36. [Google Scholar]
- Nwankwegu AS. Sawdust assisted bioremediation of PMS hydrocarbon impacted agricultural soil in NigerDelta Nigeria. Int J Pharma Biosci. 2016;7:47–57. [Google Scholar]
- Nwankwegu AS, Onwosi CO. Bioremediation of gasoline contaminated agricultural soil by bioaugmentation. Environ Technol Innov. 2017;7:1–11. [Google Scholar]
- Nwankwegu AS, Onwosi CO. Microbial cell immobilization: a renaissance to bioaugmentation inadequacies. A review. Environ Technol Rev. 2017;6:186–198. [Google Scholar]
- Nwankwegu AS, Onwosi CO, Orji MU, Anaukwu CG, Okafor UC, Azi F, Martins PE. Reclamation of DPK hydrocarbon polluted agricultural soil using a selected bulking agent. J Environ Manag. 2016;172:136–142. doi: 10.1016/j.jenvman.2016.02.032. [DOI] [PubMed] [Google Scholar]
- Nwankwegu AS, Orji MU, Onwosi CO. Studies on organic and in-organic biostimulants in bioremediation of diesel-contaminated arable soil. Chemosphere. 2016;162:148–156. doi: 10.1016/j.chemosphere.2016.07.074. [DOI] [PubMed] [Google Scholar]
- Nwankwegu AS, Onwosi CO, Azi F, Azumini P, Anaukwu CG, Nwankwegu AS, Onwosi CO, Azi F, Azumini P. Use of rice Husk as bulking agent in bioremediation of automobile gas oil impinged agricultural soil. Soil Sediment Contam. 2017;26:96–144. [Google Scholar]
- Nwankwegu AS, Li Y, Jiang L, Lai Q, Shenglin W. Kinetic modelling of total petroleum hydrocarbon in spent lubricating petroleum oil impacted soil under different treatments. Environ Technol. 2018;26:1–10. doi: 10.1080/09593330.2018.1498543. [DOI] [PubMed] [Google Scholar]
- Oladunjoye G, Miyauchi K, Huang Y, Chien M. Biotechnological remedies for the estuarine environment polluted with heavy metals and persistent organic pollutants. Int Biodeter Biodegrad. 2017;119:614–625. [Google Scholar]
- Oliver AA, Dahlgren RA, Deas ML. The upside-down river: reservoirs, algal blooms, and tributaries affect temporal and spatial patterns in nitrogen and phosphorus in the. J Hydrol. 2014;519:164–176. [Google Scholar]
- Paerl HW. Controlling eutrophication along the freshwater-Marine continuum: Dual nutrient (N and P) reductions are essential. Estuar Coast. 2009;32:593–601. [Google Scholar]
- Paerl HW. Controlling cyanobacterial harmful blooms in freshwater ecosystems. Microb Biotechnol. 2017;10:1106–1110. doi: 10.1111/1751-7915.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl HW, Paul VJ. Climate change: links to global expansion of harmful cyanobacteria. Water Res. 2012;46:1349–1363. doi: 10.1016/j.watres.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Paerl HW, Hall NS, Calandrino ES. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci Total Environ. 2011;409:1739–1745. doi: 10.1016/j.scitotenv.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Paerl HW, Xu H, McCarthy MJ, Zhu G, Qin B, Li Y, Gardner WS. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): the need for a dual nutrient (N&P) management strategy. Water Res. 2011;45:1973–1983. doi: 10.1016/j.watres.2010.09.018. [DOI] [PubMed] [Google Scholar]
- Paerl HW, Xu H, Hall NS, Rossignol KL, Joyner R, Zhu G, Qin B. Nutrient limitation dynamics examined on a multi-annual scale in Lake Taihu, China: implications for controlling eutrophication and harmful algal blooms. J Freshw Ecol. 2015;30:37–41. [Google Scholar]
- Paerl HW, Gardner WS, Havens KE, Joyner AR, McCarthy MJ, Newell SE, Qin B, Scott JT. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae. 2016;54:213–222. doi: 10.1016/j.hal.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Paerl HW, Otten TG, Kudela R. Mitigating the expansion of harmful algal blooms across the freshwater-to-marine continuum. Environ Sci Technol. 2018;52:5519–5529. doi: 10.1021/acs.est.7b05950. [DOI] [PubMed] [Google Scholar]
- Pan G, Zhang M, Chen H, Zou H, Yan H. Removal of cyanobacterial blooms in Taihu Lake using local soils. I. Equilibrium and kinetic screening on the flocculation of Microcystis aeruginosa using commercially available clays and minerals. Envrion Pollut. 2006;141:195–200. doi: 10.1016/j.envpol.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Park J, Church J, Son Y, Kim K, Hyoung W. Recent advances in ultrasonic treatment: challenges and field applications for controlling harmful algal blooms (HABs) Ultrason Sonochem. 2017;38:323–326. doi: 10.1016/j.ultsonch.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Paterson RF, Mcneill S, Mitchell E, Adams T, Swan SC, Clarke D, Miller PI, Bresnan E, Davidson K. Environmental control of harmful dino fl agellates and diatoms in a fjordic system. Hamful Algal. 2017;69:1–17. doi: 10.1016/j.hal.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Pelley J. Taming toxic algae blooms. ACS Cent Sci. 2016;2:270–273. doi: 10.1021/acscentsci.6b00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope PB, Patel BKC. Metagenomic analysis ofa freshwater toxic cyanobacteria bloom. FEMS Microbiol Ecol. 2008;64:9–27. doi: 10.1111/j.1574-6941.2008.00448.x. [DOI] [PubMed] [Google Scholar]
- Posada-baquero R, Martín ML. Implementing standardized desorption extraction into bioavailability-oriented bioremediation of PAH-polluted soils. Sci Total Environ. 2019;696:1–7. [Google Scholar]
- Prosser KN, Valenti TW, Hayden NJ, Neisch MT, Hewitt NC, Umphres GD, Gable GM, Grover JP, Roelke DL, Brooks BW. Low pH preempts bloom development of a toxic haptophyte. Harmful Algae. 2012;20:156–164. [Google Scholar]
- Rajasekhar P, Fan L, Nguyen T, Roddick FA. A review of the use of sonication to control cyanobacterial blooms. Water Res. 2012;46:4319–4329. doi: 10.1016/j.watres.2012.05.054. [DOI] [PubMed] [Google Scholar]
- Randhawa V, Thakkar M, Wei L. Applicability of hydrogen peroxide in brown tide control—culture and microcosm studies. PLoS ONE. 2012;7:1–11. doi: 10.1371/journal.pone.0047844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roé-sosa A, Rangel-peraza JG, Rodríguez-mata AE, Pat-espadas A, Bustos-terrones Y, Diaz-peña I, Manh C. Emulating natural wetlands oxygen conditions for the removal of N and P in agricultural wastewaters. J Environ Manag. 2019;236:351–357. doi: 10.1016/j.jenvman.2019.01.114. [DOI] [PubMed] [Google Scholar]
- Sanseverino I, Conduto D (2016) Algal bloom and its economic impact. EUR 27905 EN, Italy, pp 1–52. 10.2788/660478
- Schindler DW, Hecky RE, Findlay DL, Stainton MP, Parker BR, Paterson MJ, Beaty KG, Lyng M, Kasian SEM. Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proc Natl Acad Sci (USA) 2008;105:11254–11258. doi: 10.1073/pnas.0805108105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffman H, Lis H, Shaked Y, Keren N, Bibby T. Iron—nutrient Interactions within Phytoplankton. Front Plant Sci. 2016;7:1–12. doi: 10.3389/fpls.2016.01223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti T, Mimura M, Wakano JY. Avoiding toxic prey may promote harmful algal blooms. Ecol Complex. 2015;21:157–165. [Google Scholar]
- Siah C, Pham TX, Park Y, Kim B, Sun M, Kang I, Lee J. Edible blue-green algae reduce the production of pro-in fl ammatory cytokines by inhibiting NF- κ B pathway in macrophages and splenocytes. Biochim Biophys Acta. 2013;1830:2981–2988. doi: 10.1016/j.bbagen.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ND, Morozova GS, Pérez-Arlucea M, Gibling MR. Dam-induced and natural channel changes in the Saskatchewan River below the E.B. Campbell Dam, Canada. Geomorphology. 2016;269:186–202. [Google Scholar]
- Song H, Lavoie M, Fan X, Tan H, Liu G, Xu P, Fu Z, Paerl HW, Qian H. Allelopathic interactions of linoleic acid and nitric oxide increase the competitive ability of Microcystis aeruginosa. ISME. 2017;11:1865–1876. doi: 10.1038/ismej.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukenik A, Eshkol R, Livne A, Hadas O, Israel M, Rom M, Tchernov D, Vardi A, Kaplan A. Inhibition of growth and photosynthesis of the dinoflagellate Peridinium gatunense by Microcystis sp. (cyanobacteria): a novel allelopathic mechanism. Limnol Oceanogr. 2002;47:1656–1663. [Google Scholar]
- Sun X, Choi J, Kim E. A preliminary study on the mechanism of harmful algal bloom mitigation by use of sophorolipid treatment. J Exp Mar Biol Ecol. 2004;304:35–49. [Google Scholar]
- Sunda WG, Shertzer KW. Positive feedbacks between bottom-up and top-down controls promote the formation and toxicity of ecosystem disruptive algal blooms: a modeling study. Harmful Algae. 2014;39:342–356. [Google Scholar]
- Verma S, Kuila A. Bioremediation of heavy metals by microbial process. Environ Technol Innov. 2019;14:1–11. [Google Scholar]
- Vichi S, Lavorini P, Funari E, Scardala S, Testai E. Contamination by Microcystis and microcystins of blue—green algae food supplements (BGAS) on the italian market and possible risk for the exposed population. Food Chem Toxicol. 2012;50:4493–4499. doi: 10.1016/j.fct.2012.09.029. [DOI] [PubMed] [Google Scholar]
- Vinogradova T, Danaher M, Baxter A, Moloney M, Victory D, Haughey SA. Rapid surface plasmon resonance immunobiosensor assay for microcystin toxins in blue-green algae food supplements. Talanta. 2011;84:638–643. doi: 10.1016/j.talanta.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Visser PM, Verspagen JMH, Sandrini G, Stal LJ, Matthijs HCP, Davis TW, Paerl HW, Huisman J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae. 2016;54:145–159. doi: 10.1016/j.hal.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Waajen GWAM, Van Bruggen NCB, Dionisio LM. Biomanipulation with quagga mussels (Dreissena rostriformis bugensis) to control harmful algal blooms in eutrophic urban ponds. Ecol Eng. 2016;90:141–150. [Google Scholar]
- Wang P, Shen H, Xie P. Can hydrodynamics change phosphorus strategies of diatoms? Nutrient levels and diatom blooms in lotic and lentic ecosystems. Microb Ecol. 2012;63:369–382. doi: 10.1007/s00248-011-9917-5. [DOI] [PubMed] [Google Scholar]
- Wang C, Feng T, Wang P, Hou J, Qian J. Understanding the transport feature of bloom-forming Microcystis in a large shallow lake: a new combined hydrodynamic and spatially explicit agent-based modelling approach. Ecol Eng. 2017;343:25–38. [Google Scholar]
- Wang J, Chen Z, Chen H, Wen Y. Effect of hydrogen peroxide on Microcystic aeruginosa: role of cytochromes P450. Sci Total Environ. 2018;626:211–218. doi: 10.1016/j.scitotenv.2018.01.067. [DOI] [PubMed] [Google Scholar]
- Watson SB, Whitton BA, Higgins SN, Paerl HW, Brooks BW, Wehr JD. Freshwater Algae of North America. Introduction and overview. North America: Elsevier Inc.; 2015. Harmful Algal Blooms; pp. 873–920. [Google Scholar]
- Weberg M, Salomon PS, Grane E. Harmful algal blooms of allelopathic microalgal species: the role of eutrophication. Harmful Algae. 2008;8:94–102. [Google Scholar]
- Wells ML, Trainer VL, Smayda TJ, Karlson BSO, Trick CG, Kudela RM, Ishikawa A, Bernard S, Wulff A, Anderson DM, Cochlan WP. Harmful algal blooms and climate change: learning from the past and present to forecast the future. Harmful Algae. 2015;49:68–93. doi: 10.1016/j.hal.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KTM, Lee JHW, Hodgkiss IJ. A simple model for forecast of coastal algal blooms. Estuar Coast Shelf Sci. 2007;74:175–196. [Google Scholar]
- Wu J, Chou T. Silicate as the limiting nutrient for phytoplankton in a subtropical eutrophic estuary of Taiwan. Estuar Coast Shelf Sci. 2003;58:155–162. [Google Scholar]
- Xiaoyi W, Junyang Y, Yan S, Tingli S, Li W, Jiping X. Research on hybrid mechanism modeling of algal bloom formation in urban lakes and reservoirs. Ecol Model. 2016;332:67–73. [Google Scholar]
- Xu Y, Zhang M, Wang L, Kong L, Cai Q. Changes in water types under the regulated mode of water level in Three Gorges Reservoir, China. Quat Int. 2011;244:272–279. [Google Scholar]
- Xu H, Zhu G, Qin B, Paerl HW. Growth response of Microcystis spp. to iron enrichment in different regions of Lake Taihu, China. Hydrobiologia. 2013;700:187–202. [Google Scholar]
- Xu H, Paerl HW, Qin B, Zhu G, Hall NS, Wu Y. Determining critical nutrient thresholds needed to control harmful cyanobacterial blooms in eutrophic Lake Taihu, China. Environ Sci Technol. 2015;49:1051–1059. doi: 10.1021/es503744q. [DOI] [PubMed] [Google Scholar]
- Yamada K, Yoshikawa S, Ichinomiya M, Kuwata A, Kamiya M. Effects of silicon-limitation on growth and morphology of triparma laevis NIES-2565 (Parmales, Heterokontophyta) PLoS ONE. 2014;9:2–9. doi: 10.1371/journal.pone.0103289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Liu D, Huang Y, Yang Z, Ji D, Song L. Isotope analysis of the nutrient supply in Xiangxi Bay of the three gorges reservoir. Ecol Eng. 2015;77:65–73. [Google Scholar]
- Yang Z, Buley RP, Fernandez-figueroa EG, Barros MUG, Rajendran S, Wilson AE. Hydrogen peroxide treatment promotes chlorophytes over toxic cyanobacteria in a hyper-eutrophic aquaculture pond. Environ Pollut. 2018;240:590–598. doi: 10.1016/j.envpol.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Yang Z, Xu P, Liu D, Ma J, Ji D, Cui Y. Hydrodynamic mechanisms underlying periodic algal blooms in the tributary bay of a subtropical reservoir. Ecol Eng. 2018;120:6–13. [Google Scholar]
- Yu Z, Song X, Cao X, Liu Y. Mitigation of harmful algal blooms using modified clays : theory, mechanisms, and applications. Harmful Algae. 2017;69:48–64. doi: 10.1016/j.hal.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Yu X, Cai G, Wang H, Hu Z, Zheng W, Lei X, Zhu X, Chen Y, Chen Q, Din H, Xu H, Tian Y, Fu L, Zheng T. Fast-growing algicidal Streptomyces sp. U3 and its potential in harmful algal bloom controls. J Hazard Mater. 2018;341:138–149. doi: 10.1016/j.jhazmat.2017.06.046. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hu W, Gu K, Li Q, Zheng D, Zhai S. An improved ecological model and software for short-term algal bloom forecasting. Environ Model Softw. 2013;48:152–162. [Google Scholar]
- Zhang W, Yuan J, Han J, Huang C, Li M. Impact of the Three Gorges Dam on sediment deposition and erosion in the middle Yangtze River: a case study of the Shashi Reach. Hydrol Res. 2016;47:175–186. [Google Scholar]
- Zhou G, Bi Y, Zhao X, Chen L, Hu Z. Algal growth potential and nutrient limitation in spring in Three-Gorges Reservoir, China. Fresnius Environ Bull. 2009;18:1642–1647. [Google Scholar]
- Zhou G, Zhao X, Bi Y, Liang Y, Hu J, Yang M, Mei Y, Zhu K, Zhang L, Hu Z. Phytoplankton variation and its relationship with the environment in Xiangxi Bay in spring after damming of the Three-Gorges, China. Environ Monit Assess. 2011;176:125–141. doi: 10.1007/s10661-010-1571-8. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Yu R, Zhou M. Resolving the complex relationship between harmful algal blooms and environmental factors in the coastal waters adjacent to the Changjiang River estuary. Harmful Algae. 2017;62:60–72. doi: 10.1016/j.hal.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Li L, Huang L, Guo L, Song L. Combining hydrogen peroxide addition with sunlight regulation to control algal blooms. Environ Sci Pollut Res. 2018;25:2239–2247. doi: 10.1007/s11356-017-0659-x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Hu B, Zhao W, Cui D, Tan L, Wang J. E ff ects of increasing nutrient disturbances on phytoplankton community structure and biodiversity in two tropical seas. Mar Pollut Bull. 2018;135:239–248. doi: 10.1016/j.marpolbul.2018.07.033. [DOI] [PubMed] [Google Scholar]
- Zhu K, Bi Y, Hu Z. Responses of phytoplankton functional groups to the hydrologic regime in the Daning River, a tributary of Three Gorges Reservoir, China. Sci Total Environ. 2013;450–451:169–177. doi: 10.1016/j.scitotenv.2013.01.101. [DOI] [PubMed] [Google Scholar]
- Zuo S, Fang Z, Yang S, Wan K. Effect of allelopathic potential from selected aquatic macrophytes on algal interaction in the polluted water. Biochem Syst Ecol. 2015;61:133–138. [Google Scholar]