Abstract
Background
Universal varicella vaccination (UVV) for children introduced in Germany in 2004 resulted in a significant overall decline of varicella-related hospitalizations (VRHs). We investigated the incidence of specific types of varicella-related complications (VRCs) in hospitalized children and the impact of UVV on VRCs during the first 7 years of UVV.
Methods
Children < 17 years of age hospitalized with an ICD-10-based (International Classification of Diseases, 10th Revision) discharge diagnosis of varicella were identified as VRH in pediatric hospitals in Bavaria by annual standardized data queries of the hospital databases (2005–2011). For each VRH, the hospitals reported basic demographic data, duration of hospital stay, all diagnostic and procedural codes, and outcome. VRCs were reported overall, per year, and by immune status. Complication rates were calculated as mean number per complication category per hospital and per year; VRC trends over time were assessed by linear regression.
Results
Between 78% (2005) and 61% (2011) of Bavarian hospitals participated and reported a total of 1263 VRHs. Specific VRCs were reported in 954 (76%) children. Complication rates per hospital and year decreased from 6.7 [95% confidence interval (CI): 5.1–8.3] in 2005 to 1.5 (95% CI: 0.8–2.3) in 2011, with the strongest reduction of 90% in children < 5 years of age from 5.3 (95% CI: 4.0–6.6) in 2005 to 0.5 (95% CI: 0.1–0.9) in 2011. Significant decreases were observed for children with upper respiratory tract (URT, by 97%), lower respiratory tract (LRT, by 90%), skin (by 81%), gastrointestinal (by 78%), and neurologic (by 65%) VRCs. Forty-eight children with VRCs were immunocompromised; their annual rate decreased by 87%.
Discussion
Corresponding to increasing varicella vaccination coverage in the population, the incidence of VRC decreased by 77% from 2005 to 2011, with the most substantial decrease in the target group for UVV.
Conclusion
Within 7 years, UVV in Germany led to a decrease of about 77% of all types of VRCs, with the highest reductions observed for VRCs of the respiratory tract.
Electronic supplementary material
The online version of this article (10.1007/s40121-019-00273-6) contains supplementary material, which is available to authorized users.
Keywords: Complication, Hospitalization, Immunocompromised, Pediatric, Post-vaccination period, Varicella
Key Summary Points
| Why Carry out This Study? |
| A decline in burden of pediatric varicella was observed in all countries after implementation of universal varicella vaccination (UVV), including Germany. |
| However, impact of UVV on different varicella-related complication (VRC) categories in children hospitalized with varicella is unknown in Germany to date. |
| What Was Learned from the Study? |
| Complication rates per hospital and year in children < 17 years declined by 77% from 6.7 [95% conficence interval (CI): 5.1–8.3] cases per hospital in 2005 to 1.5 (95% CI 0.8–2.3) cases in 2011. Highest reduction in cases was observed in children below 5 years (by 90%), the target group for UVV. |
| Gastrointestinal complications declined by 78% from 2005 to 2011, neurologic complications by 65%, skin complications by 81%, upper respiratory tract complications by 97% and lower respiratory tract complications by 90%. |
| UVV can significantly reduce the burden of varicella including societal costs. |
Background
The varicella zoster virus (VZV) is highly contagious, usually resulting in a self-limiting, mild, febrile, exanthematous childhood infection in healthy children. Especially immunocompromised children are at risk of severe disease, but even in previously healthy children infections may result in varicella-related hospitalizations (VRH) due to potentially severe varicella-related complications (VRCs) [1–3]. In a German study on varicella-related complications in the pre-vaccination era, neurologic (25.4%), skin-related (23.2%), and gastrointestinal (15.0%) complications were most frequently reported among immunocompetent children < 17 years, whereas in immunocompromised children lower respiratory tract (LRT, 37.5%), hematologic (31.3%), and gastrointestinal (25.0%) complications were most frequently observed [1]. The incidences of VRH [4] and of the different categories of VRCs, however, vary widely among studies and countries, probably reflecting differences in region, varicella vaccination coverage, population structures, and public health systems [1, 5, 6].
In Germany, varicella incidence in the community was estimated as 930 per 100,000 inhabitants during the pre-vaccination era [7] and the burden of VRH as 14.1 per 100,000 children < 16 years of age [1] with an average of five varicella-related fatalities per year [8]. Universal varicella vaccination (UVV) was implemented in Germany for all children aged 11–14 months with a one-dose schedule in 2004 [9] and with a second dose at 15–23 months in 2009 [10]. Monovalent (V) and since 2006 tetravalent measles-mumps-rubella-varicella (MMRV) vaccines are both available in Germany. UVV was well accepted, with one-dose vaccination coverage of 43% for children < 2 years of age born in 2004 to 87% in children born in 2009 and resulted in a strong decline of pediatric VRH overall [11].
In Bavaria, the second largest German Federal State, varicella vaccinations and their impact were studied in the frame of the ‘Bavarian Varicella-Surveillance Project’ [12–18]. Similarly to other regions in Germany until 2011, the coverage for first-dose varicella vaccination in children aged 18–36 months increased in the two observed Bavarian regions to 69% and 83%, respectively [12–14]. Corresponding to the observed increase in coverage, the estimated incidence of pediatric VRH of 13.3 and 16.8 per 100,000 children < 17 years of age in Bavarian hospitals in 2005 and 2006 continuously decreased in the following years to 4.8 in 2011 [16].
Thus far, only limited data are available from Germany and other countries on the magnitude of UVV's impact on different types of VRC in children [19] and are mostly restricted to specific complications, such as the decrease of neurologic VRC published previously [16] by our group. In the current article, we describe all types of VRCs observed in children with VRH within the ‘Bavarian Varicella Surveillance Project’ and compare their decrease during the first 7 years of UVV.
Methods
As part of the ‘Bavarian Varicella Surveillance Project,’ all 37 pediatric hospital wards in Bavaria, covering an annual pediatric population of about 2 million children, were invited to participate [16]. From 2005 to 2011, participating hospitals provided each year's standardized, anonymized data on all children with a varicella-related ICD-10 (International Classification of Diseases, 10th Revision) code.
VRHs were identified by using a pre-specified algorithm with the following inclusion criteria: children < 17 years of age at admission; at least one varicella-related ICD-10 code (B01.0–B01.9) as the main or any secondary diagnosis at discharge; stationary hospitalization for at least 1 day within the observation period from 1 January 2005 to 31 December 2011.
The hospitals reported basic demographic data, month and year of hospital admission, duration of hospital stay, all ICD-10 codes (primary and secondary), and all procedural codes (OPS). For each VRH, documented ICD-10 discharge codes indicating VRC were classified using pre-specified diagnosis-related categories of complications [1]: neurologic [16], gastrointestinal, skin, reactive arthritis, lower/upper respiratory tract (LRT/URT), hematologic, systemic bacterial, coagulation complication and related sequelae, and other complications. Codes indicating underlying chronic conditions were allocated to previously described categories [16]. Immunocompromised children were identified by ICD-10 codes indicating malignancies or potentially relevant neoplasms (C00-C97, D37-D48), immunodeficiency disorders (D80-D89) and HIV/AIDS (B20-B24, Z21), chronic juvenile arthritis (M08), nephrotic syndrome (N04), or a code indicating a transplanted organ or tissue (Z94). For further details on the study setting and data collection, see Streng et al. [16, 18].
Ethical Considerations and Data Protection
Approval was obtained from the Bavarian Data Protection Office (Munich) and the Ethics Committees of the Medical Faculties at the University Hospitals in Munich and Würzburg. The participating hospitals reported only anonymized data.
Statistical Analysis
Data were collected from all hospitals using Microsoft Excel tables and were analyzed with SPSS version 25.0 (SPSS Inc., Chicago, IL). Results of descriptive analyses were reported as absolute numbers with percentages or as median values with interquartile range (IQR). Data were compared using Pearson’s Chi2 test or Fisher’s exact test for categorical data and the Mann-Whitney U test for continuous data. Complication rates per age group and per complication category were calculated as mean number of patients per hospital per year to account for the different numbers of participating hospitals per year. The number of patients per age group or per category was divided by the number of participating hospitals in the respective year (between 22 and 29 hospitals per year participated in the study; for further details refer to Supplementary Table 1). Explorative univariate linear regression analyses were employed to assess VRC trends over time. Mean number of VRCs per hospital per year was entered as dependent variable and year of hospitalization as independent variable. Analyses were stratified by age group and by type of complication. Significance level was defined as p < 0.05 (two sided).
Results
Characteristics of Patients with Varicella-Related Hospitalizations (VRH)
From 2005 to 2011, 22–29 hospitals per year (61–78% of all Bavarian pediatric hospitals, representing about 80–87% of pediatric hospital beds) participated in the study. Overall, 1263 children with VRH were reported (Table 1; 54.6% male patients; median age 3 years, IQR 1.0–5.0). Children < 5 years of age represented the largest group of VRH (67.8%), whereas only few children were aged 10–16 years (6.0%). Mean duration of hospital stay was 3 days (IQR: 2.0–6.0). An underlying chronic medical condition was reported in 270 patients (21.4%); most common were malignancies/hematologic disorders/disorders of the immune system (8.9%), and neurologic disorders (3.8%). Of all 1263 children with VRH, 5 (0.4%) showed particularly severe disease requiring treatment in a pediatric intensive care unit (PICU), and 2 patients with fatal outcome were reported, of which 1 was treated at a PICU (Supplementary Table 2).
Table 1.
Characteristics of 1263 children with varicella-related hospitalization (VRH), with and without specific varicella-related complications (VRCs); Bavaria, 2005–2011
| All children hospitalized with varicella (VRH) | Hospitalized varicella patients with specific complications (VRC) | Hospitalized varicella patients without specific complications | p valuea | |
|---|---|---|---|---|
| N = 1263 | N = 954 | N = 309 | ||
| n (%) or median (IQR) | n (%) or median (IQR) | n (%) or median (IQR) | ||
| Demographic data | ||||
| Gender male | 689 (54.6) | 518 (54.3) | 171 (55.3) | 0.793 |
| Age (years) | 3.0 (1.0–5.0) | 3.0 (1.0–5.0) | 4.0 (1.0–5.5) | 0.111 |
| Age groups | ||||
| 0–4 years | 856 (67.8) | 661 (69.3) | 195 (63.1) | 0.114 |
| 5–9 years | 331 (26.2) | 240 (25.2) | 91 (29.4) | |
| 10–16 years | 76 (6.0) | 53 (5.6) | 23 (7.4) | |
| Underlying chronic conditionsb | ||||
| At least one underlying medical condition | 270 (21.4) | 169 (17.7) | 101 (32.7) | < 0.001*** |
| Malignancies/hematologic disorder/disorders of the immune system | 113 (8.9) | 64 (6.7) | 49 (15.9) | < 0.001*** |
| Neurologic disorder | 48 (3.8) | 30 (3.1) | 18 (5.8) | 0.039* |
| Failure to thrive/developmental disorder/preterm birth | 34 (2.7) | 25 (2.6) | 9 (2.9) | 0.840 |
| Cardiovascular/pulmonary disorders | 32 (2.5) | 26 (2.7) | 6 (1.9) | 0.537 |
| Metabolic disorders | 28 (2.2) | 20 (2.1) | 8 (2.6) | 0.657 |
| Ear/nose/throat/eye | 20 (1.6) | 17 (1.8) | 3 (1.0) | 0.436 |
| Congenital malformation | 10 (0.8) | 8 (0.8) | 2 (0.6) | 1.000 |
| Genitourinary disorders | 8 (0.6) | 5 (0.5) | 3 (1.0) | 0.413 |
| Syndromal disease | 7 (0.6) | 5 (0.5) | 2 (0.6) | 0.682 |
| Other comorbidity | 63 (5.0) | 39 (4.1) | 24 (7.8) | 0.015* |
| Hospital stay and outcome | ||||
| Varicella infection/complication main reason for hospital admission (= main diagnostic code) | 1082 (85.7) | 884 (92.7) | 198 (64.1) | < 0.001*** |
| Duration of hospital stay (days) | 3.0 (2.0–6.0) | 4.0 (2.0–6.0) | 2.0 (1.7–4.0) | < 0.001*** |
| Complex intensive care treatment | 5 (0.4) | 5 (0.5) | 0 (0.0) | 0.343 |
| Fatalities | 2 (0.2) | 2 (0.2) | 0 (0.0) | 1.000 |
***Significant at 0.1% level; **significant at 1% level; *significant at 5% level
ap value for comparison of children hospitalized with varicella with and without a specific complication; Pearson’s Chi2 or Fisher’s exact test as appropriate and Mann-Whitney U test for continuous variables
bMultiple nominations possible
Characteristics of Patients with Specific Varicella-Related Complications (VRCs)
Specific complications in varicella patients (VRCs) were reported in 954 of 1263 hospitalized children (75.5%; Table 1); of these 518 (54.3%) were male. Demographic characteristics of children with VRCs were comparable to the 309 (24.5%) children without specific VRCs. Children aged < 5 years made up the largest group in patients either with or without a specific VRC (69.3% vs. 63.1%, p = 0.114), whereas the number of children aged between 10 and 16 years was lowest for both categories (5.6% of patients with VRC vs. 7.4% of patients without VRC). Interestingly, the proportion of children with an underlying medical condition was significantly lower in children with VRC than in children without VRC (17.7% vs. 32.7%, p < 0.001), mainly because of a higher proportion of children with malignancies/disorders of the immune system in the group without VRC. Median duration of hospital stay was longer in children with VRC than in those without VRC (4 days vs. 2 days; p < 0.001). The main reason for hospital admission in children with VRC was the varicella infection (92.7% with a main diagnostic ICD10 code for varicella, B01.0–B01.9) compared with 64.1% (p < 0.001) in children without VRC.
Types of Varicella-Related Complications (VRCs)
The most frequent specific VRCs were gastrointestinal complications in 339 (26.8%) of the 1263 varicella patients (Table 2), including mainly patients with dehydration (18.0% of 1263) and enteritis/gastroenteritis (11.1%). Neurologic complications were reported in 228 (18.1%) children; most frequent were febrile convulsion (6.6%), encephalitis/meningitis (5.3%), syncope (2.4%), and cerebral convulsions (2.3%). A varicella-related skin complication occurred in 178 (14.1%) children; most frequent were dermatitis/eczema (5.1%), skin and soft tissue infections (2.9%), and skin abscesses (2.8%). In 21 of 217 (9.7%) children with skin complications, secondary bacterial skin infections were reported (Staphylococcus sp. in 11 and Streptococcus sp. in 10 children). Complications related to the upper respiratory tract (URT) including the ears/nose/throat and eyes affected 190 children (15.0%). The lower respiratory tract (LRT) was affected in 160 (12.7%) children; 6.2% had pneumonia, 5.1% bronchitis, 1.8% respiratory failure, and 1.7% pleural effusion/pyothorax. Reported co-infecting bacterial pathogens in children with LRT were Streptococcus spec. (6 children), Mycoplasma pneumoniae (3), Staphylococcus spec. (1), Haemophilus influenzae (1), and Klebsiella pneumoniae (1). Reported co-infecting respiratory viruses were respiratory syncytial virus (RSV) (9) and influenza virus (1). In 26 (2.1%) children a confirmed or suspected invasive bacterial infection was reported: 18 (1.4%) had sepsis, 8 (0.6%) osteomyelitis, 5 (0.4%) systemic inflammatory response syndrome (SIRS), and 2 (0.2%) necrotizing fasciitis. In children with sepsis, seven were diagnosed with co-infecting Streptococcus species (including four with Streptococcus group A and two with S. pneumoniae) and four with Staphylococcus species (including three with S. aureus). Varicella-specific hematologic complications occurred in 44 (3.5%) children. Coagulation disorders and related sequelae were reported in 21 (1.7%) children and reactive arthritis in 9 (0.7%). The category ‘other complications’ included feeding problems (172, 13.6%) and kidney and urinary tract complications (27, 2.1%) as the most frequent.
Table 2.
Varicella-associated complications (VRC) in 954 (76%) of 1263 hospitalized children; Bavaria, 2005–2011
| Complication category | Number of diagnoses N (% of 1263 patients) |
Number of patients N (% of 1263 patients) |
|---|---|---|
| Gastrointestinal complication | 433 (34.3) | 339 (26.8) |
| Dehydration | 227 (18.0) | |
| Enteritis/gastroenteritis | 140 (11.1) | |
| Constipation | 17 (1.3) | |
| Other diagnoses | 49 (3.9) | |
| Neurologic | 271 (21.5) | 228 (18.1) |
| Febrile convulsion | 83 (6.6) | |
| Encephalitis/meningitis | 67 (5.3) | |
| Syncope | 30 (2.4) | |
| Cerebral convulsion | 29 (2.3) | |
| Facial nerve palsy | 8 (0.6) | |
| Cerebral vasculitis/infarction | 4 (0.3) | |
| Other diagnoses | 50 (4.0) | |
| Skin | 217 (17.2) | 178 (14.1) |
| Dermatitis/eczema/skin eruption | 64 (5.1) | |
| Phlegmon | 36 (2.9) | |
| Abscess | 35 (2.8) | |
| Skin complication due to specific co-infecting pathogen | 21 (1.7) | |
| Impetigo | 16 (1.3) | |
| Urticaria/erythema | 15 (1.2) | |
| Other diagnoses | 30 (2.4) | |
| Upper respiratory tract (URT), ear, nose and throat (ENT), eye | 232 (18.4) | 190 (15.0) |
| Otitis | 51 (4.0) | |
| Conjunctivitis/other affection of conjunctiva | 41 (3.2) | |
| Acute URT infection, unspecified | 40 (3.2) | |
| Tonsillitis | 35 (2.8) | |
| Laryngitis/tracheitis/laryng. spasm | 21 (1.7) | |
| Other diagnoses | 44 (33.5) | |
| Lower respiratory tract (LRT) | 197 (15.6) | 160 (12.7) |
| Pneumonia | 78 (6.2) | |
| Bronchitis | 64 (5.1) | |
| Respiratory failure | 23 (1.8) | |
| Pleural effusion/pyothorax | 22 (1.7) | |
| Other diagnoses | 10 (0.8) | |
| Hematologic complications | 57 (4.5) | 44 (3.5) |
| Thrombocytopenia | 26 (2.1) | |
| Anemia | 15 (1.2) | |
| Other diagnoses | 16 (1.3) | |
| Systemic bacterial | 35 (2.8) | 26 (2.1) |
| Sepsis | 18 (1.4) | |
| Osteomyelitis | 8 (0.6) | |
| SIRS | 5 (0.4) | |
| Necrotizing fasciitis | 2 (0.2) | |
| Other diagnoses | 2 (0.2) | |
| Coagulation and sequelae | 22 (1.7) | 21 (1.7) |
| Reactive arthritis | 9 (0.7) | 9 (0.7) |
| Other complications | 312 (24.3) | 258 (20.4) |
| Feeding problems | 172 (13.6) | |
| Kidney and urinary tract | 27 (2.1) | |
| Cardiac complications | 21 (1.7) | |
| Other diagnoses | 92 (7.3) |
Multiple nominations per patient possible
Annual Rates of Varicella-Related Complications (VRCs)
During the first year of observation (2005), on average 6.7 [95% confidence interval (CI): 5.1–8.3] patients with any specific VRC were recorded per participating hospital. Supplementary Table 1 provides information on the number of patients with varicella-related hospitalizations and complication categories including the respective rates per hospital and year with confidence intervals. In 2006, the highest average number of patients with VRC was recorded (8.6; 95% CI: 6.7–10.4). During the following years, average numbers of VRC patients decreased continuously, from 8.0 (95% CI: 6.2–9.7) in 2007 to 5.2 (95% CI: 3.9–6.4) in 2008, 3.0 (95% CI: 2.1–3.8) in 2009, 2.5 (95% CI: 1.5–3.6) in 2010 to 1.5 (95% CI: 0.8–2.3) in 2011. Overall, VRCs declined significantly by 76.9% (p = 0.006) from 2005 to 2011, with the highest reduction of average cases per hospital and year in children aged < 5 years by 89.7% from 5.3 (95% CI: 4.0–6.6) in 2005 to 0.5 (95% CI: 0.1–0.9; p = 0.001) in 2011. VRCs in children aged 5–9 years were reduced by 45.1% from 1.2 (95% CI: 0.7–1.8) in 2005 to 0.7 (95% CI: 0.0–2.0; p = 0.109). Only few patients aged 10–16 years were reported each year, with average patient numbers of 0.1 (95% CI: 0.0–0.3) in 2005 and 0.3 (95% CI: 0.1–0.5; p = 0.923) in 2011.
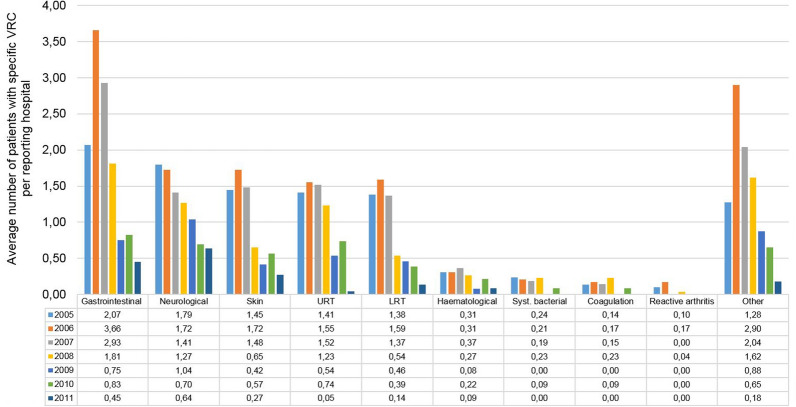
The annual rates for the different types of VRC per hospital and per year are presented in Fig. 1. All the main VRC categories decreased significantly during the 2005 to 2011 observation period. Gastrointestinal complications decreased by 78.0% from an average patient number per hospital of 2.1 (95% CI: 1.4–2.8; 60 patients per 29 hospitals) to 0.5 (95% CI: 0.2–0.7; 10 patients per 22 hospitals) (p = 0.026), neurologic complications by 64.5% from 1.8 (95% CI: 1.2–2.4; 52 patients per 29 hospitals) to 0.6 (95% CI: 0.3–1.0; 14 patients per 22 hospitals) (p < 0.001), and skin complications by 81.2% from 1.4 (95% CI: 0.9–2.0; 42 patients per 29 hospitals) to 0.3 (95% CI 0.0–0.6; 6 patients per 22 hospitals) (p = 0.006). Complications of the upper respiratory tract decreased by 96.8% from 1.4 (95% CI: 0.9–2.0; 41 patients per 29 hospitals) to 0.0 (95% CI: 0.0–0.1; 1 patient per 22 hospitals) (p = 0.006) and of the lower respiratory tract by 90.1% from 1.4 (95% CI: 0.9–1.8; 40 patients per 29 hospitals) to 0.1 (95% CI: 0.0–0.4; 3 patients per 22 hospitals) (p = 0.003). The category ‘other complications’ decreased by 85.7% from 1.3 (95% CI 0.7–1.8; 37 patients per 29 hospitals) to 0.2 (95% CI 0.0–0.4; 4 patients per 22 hospitals) (p = 0.050). Hematologic and systemic bacterial complications, coagulation/sequelae, and reactive arthritis also showed lower complication rates per hospital in 2011 compared with 2005, but the numbers were too low for formal trend analyses. Despite the overall decreasing trend from 2005 to 2011, a conspicuously higher average number of patients per hospital was observed in 2006 and 2007 for the categories ‘gastrointestinal complications’ and ‘other complications’ (mainly feeding problems) compared with all other years.
Fig. 1.
Change in the rate of varicella-related complications (VRCs) in 954 children in 22–29 participating hospitals from 2005 to 2011; average numbers per reporting hospital are provided per year. Multiple nominations of a patient in different VRC categories were possible. Patients with more than one diagnosis within a VRC category were listed only once per category
Varicella-Related Complications in Immunocompromised Children
A subgroup of 93 (7.4%) of the 1263 children with VRH was classified as immunocompromised (Table 3). They were older at hospital admission compared with 1170 immunocompetent children (median age 4 years, IQR: 2.0–6.0 vs. 3 years, IQR: 1.0–5.0; p = 0.006). Varicella infection was less often the primary diagnosis for hospital admission than in immunocompetent children (54.8% vs. to 88.1%, p < 0.001), and their median hospital stay duration was 2 days longer (5 days, IQR: 3.5–9.0 vs. 3 days, IQR: 2.0–6.0; p < 0.001). One immunocompromised child died (Supplementary Table 2). The proportion of immunocompromised children with a specific VRC was lower than in immunocompetent children (51.6% vs. 77.4%, p < 0.001).
Table 3.
Characteristics of 1263 children with varicella-related hospitalization (VRH) by immune status (Bavaria, 2005–2011)
| Immunocompromised children | Immunocompetent children | p valuea | |
|---|---|---|---|
| N = 93 | N = 1170 | ||
| n (%) or median (IQR) | n (%) or median (IQR) | ||
| Demographic data | |||
| Gender male | 44 (47.3) | 645 (55.1) | 0.089 |
| Age (years) | 4.0 (2.0–6.0) | 3.0 (1.0–5.0) | 0.006** |
| Age groups | |||
| 0–4 years | 58 (62.4) | 798 (68.2) | 0.493 |
| 5–9 years | 29 (31.2) | 302 (25.8) | |
| 10–16 years | 6 (6.5) | 70 (6.0) | |
| Hospital stay and outcome | |||
| With varicella-related complication | 48 (51.6) | 906 (77.4) | < 0.001*** |
| Varicella infection/complication main reason for hospital admission | 51 (54.8) | 1031 (88.1) | < 0.001*** |
| Duration of hospital stay (days) | 5.0 (3.5-9.0) | 3.0 (2.0–6.0) | < 0.001*** |
| Complex intensive care treatment | 0 (0.0) | 5 (0.4) | 0.682 |
| Fatalities | 1 (1.1) | 1 (0.1) | 0.142 |
***Significant at 0.1% level, **significant at 1% level, *significant at 5% level
ap value for comparison of children hospitalized with varicella with and without a specific complication; Pearson’s Chi2 or Fisher’s exact test as appropriate and Mann-Whitney U test for continuous variables
Regarding specific VRCs, immunocompromised children were significantly more frequently reported to have hematologic complications (20.4% vs. 2.1%, p < 0.001), systemic bacterial infections (6.5% vs. 1.7%, p = 0.009), and coagulation disorders and related sequelae (5.4% vs. 1.4%, p = 0.015) than immunocompetent children (Table 4). In contrast, the proportion of gastrointestinal complications was lower in immunocompromised children (16.1% vs. 27.7%, p = 0.015) as well as infections of the URT (5.4% vs. 15.8%, p = 0.004) and neurologic complications (4.3% vs. 19.1%, p < 0.001).
Table 4.
Varicella-related complications (VRCs) in immunocompromised and immunocompetent children hospitalized in Bavaria between January 2005 and December 2011
| VRC in immunocompromised patients | VRC in immunocompetent patients | p valuea | |
|---|---|---|---|
| N = 93 | N = 1170 | ||
| n (%) | n (%) | ||
| At least one complication | 48 (51.6) | 906 (77.4) | < 0.001*** |
| Gastrointestinal complication | 15 (16.1) | 324 (27.7) | 0.015* |
| Neurologic | 4 (4.3) | 224 (19.1) | < 0.001*** |
| Skin | 7 (7.5) | 171 (14.6) | 0.063 |
| URT, ENT, eye | 5 (5.4) | 185 (15.8) | 0.004** |
| LRT | 7 (7.5) | 153 (13.1) | 0.145 |
| Hematologic complications | 19 (20.4) | 25 (2.1) | < 0.001*** |
| Systemic bacterial | 6 (6.5) | 20 (1.7) | 0.009** |
| Coagulation and sequelae | 5 (5.4) | 16 (1.4) | 0.015* |
| Reactive arthritis | 1 (1.1) | 8 (0.7) | 0.499 |
| Other complications | 22 (23.7) | 236 (20.2) | 0.424 |
Patients could appear in more than one complication category, but only once per category
***Significant at 0.1% level, **significant at 1% level, *significant at 5% level
aTwo-sided p value for comparison of complications in immunocompromised and immunocompetent children hospitalized with varicella; Pearson’s Chi2 or Fisher’s exact test as appropriate
The average number of immunocompromised children with VRC decreased by 86.8% from 0.3 to 0.4 per hospital and year in 2005–2008 to 0.1–0.05 in 2009–2011.
Discussion
The ICD-10 surveillance in Bavarian hospitals conducted from 2005 to 2011 included the majority of the Bavarian pediatric clinics (annually 61–78%). Clinical data were available for 1263 children with VRH, including 954 (75.5%) children with specific VRC. This proportion of children with VRC was similar to other studies in European countries [20–24]. Descriptions of specific VRC types in hospitalized children are available from many countries, but were often limited by the monocentric design and low patient numbers, whereas our multicenter study provided solid data from 22 to 29 pediatric hospitals. Furthermore, it is the first study to describe a relevant impact of UVV simultaneously for the various types of VRC.
The incidence of the various types of VRC varies widely among studies from different countries. This is probably due to divergence in sociodemographic aspects and health care systems [25] as well as differences regarding data sources, data collection methodology, diverging definitions of diagnoses allocated to specific VRC categories, presence of UVV programs, and pre-vaccination [1, 20, 22, 24, 26–29] or post-vaccination [19, 30–32] periods observed. In Germany, the present study from the post-vaccination period showed lower proportions of neurologic (18% vs. 25%) [16] and skin complications (14% vs. 23%) but higher proportions of gastrointestinal complications (27% vs. 15%) compared with the pre-vaccination period [1]. Although gastrointestinal complications were the most frequent VRCs during most study years, they showed a conspicuous peak in 2006, a year with an exceptionally strong rotavirus season in Germany [33]. Hence, some of the children with varicella might have been co-infected as the varicella virus and rotavirus show a similar seasonal peak, and both affect mainly children < 5 years of age. Nevertheless, similarly high rates (23%) of patients with gastrointestinal VRC were observed in one of the largest studies on VRC from Canada [19]. Regarding skin complications, our results were in the typical range of 15–25% described by a recent meta-analysis [34]. Furthermore, rates of rare VRC types and for single VRCs corresponded well with other reports from other large studies.
A high impact of UVV with increasing vaccination coverage on VRH incidence has been shown by a multitude of studies in various countries [35–44], but only single studies addressed the impact of UVV on specific types of VRC [19, 31, 45]. Corresponding to the decrease of VRH incidence in Bavaria following UVV [16], during the 7-year period our study showed a significant overall decrease of VRCs by 76.9%, with the main impact on children < 5 years of age.
During the first 3 years of observation, vaccination coverage in the Bavarian population had only increased slowly because of a delay in reimbursement regulations, and VRH remained largely on the pre-vaccination level, with some variation in strength between varicella seasons [12, 14, 15]. Thus, the main impact on VRCs was seen after 2007, with a large decrease observed in the most frequent VRC categories, but also evident for more rare VRC types. Upper respiratory tract VRCs almost disappeared during the observation period. Of note, neurologic complications and lower respiratory tract infections (both usually requiring longer hospital stay) also showed a reduction of 64.5% and 90.1%, respectively.
A recent literature review summarized the annual societal costs of varicella in the pre-vaccination era at 187.5 million euros, of which 18% were direct medical costs [46]. Hospitalizations due to (severe) varicella complications are the main contributors to the direct medical costs of varicella [47]. For Europe, it has been estimated that about 18,200 to 23,500 VRHs occur each year, the majority in children [4]. Thus, the decrease observed in both frequent and severe VRCs due to UVV suggested a considerable reduction not only of direct medical costs but of societal costs as well.
During the 7-year observation period, we found some decline of VRHs in immunocompromised children, suggesting herd protection effects, but analyses were limited by the low patient numbers. The analyzed data sets did not contain information regarding possible preemptive use of acyclovir. Nevertheless, about half of the immunocompromised children with varicella were admitted without a specific VRC. Hence, it is likely that these VRHs were preventive measures rather than indicated because of a severe course of varicella disease. Although VRCs occurred less frequently, their overall severity was higher in immunocompromised patients, with a higher occurrence of severe bacterial and hematologic complications. Neurologic complications occurred less frequently in immunocompromised patients, similarly as observed in our pre-vaccination study [1]. For the subgroup of oncologic patients, differences in the frequencies of specific VRCs were discussed previously [18].
Two fatal cases occurred, both in children with severe chronic conditions, one of them explicitly attributed to a diagnosis of varicella pneumonia. One child was aged < 11 months and therefore not indicated yet for varicella vaccination. In the other child, varicella vaccination was contraindicated. Children with contraindications or newborn/infants are likely to benefit from indirect vaccination effects in populations with high vaccination coverage.
Limitations of this study include lack of information on (permanent) sequelae for children with VRC. Nevertheless, as the overall numbers of VRC decreased, it is likely that the rate of (permanent) sequelae attributed to varicella decreased correspondingly to the increasing vaccination rates. The hospital databases did not include information on varicella vaccination, and therefore we could not identify possible varicella breakthrough cases. However, breakthrough varicella cases usually have a milder course of disease than in unvaccinated children [35, 48] and therefore rarely result in hospitalization. A recent literature review outlined that only about 0–4% of VRHs were breakthrough varicella cases, based on results from 12 studies in 9 countries [49]. Available treatment data were limited and not sufficiently detailed to evaluate treatment effects during the course of varicella disease. Furthermore, verification of diagnoses and coding via medical record review was not feasible in our study, which is a previously discussed limitation of pseudonymized hospital discharge-based data [1, 16, 36]. This may potentially lead to overestimation of specific VRCs (including children with multiple hospital admissions) or underestimation (children with late-stage complications not attributed to the varicella infection during the allocation of ICD-10 codes).
Conclusion
In the first 7 years following the introduction of UVV in Germany, we observed a substantial decline in VRC—about 76.9% overall—in both previously healthy and immunocompromised children of all age groups, but this was especially strong in children < 5 years of age. The impact affected all types of VRCs, with the highest reductions for VRCs of the respiratory tract.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
The ‘Bavarian Varicella Surveillance Project’ was supported by an unrestricted research grant from GlaxoSmithKline (GSK) Vaccines (Rixensart, Belgium) from 2005 to 2011. We acknowledge support for the Rapid Service Fee by the Deutsche Forschungsgemeinschaft and Open Access Publication Fund of Bielefeld University.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Andrea Streng, Veit Grote, and Johannes G. Liese developed the study. Andrea S treng and Johannes G. Liese supervised the study. Andrea Streng, Christine Hagemann, and Johannes G. Liese analyzed the data and interpreted the results. Christine Hagemann drafted the manuscript, and Veit Grote and Alexander Krämer provided valuable input regarding the analysis.
Disclosures
Andrea Streng received further research grants, support for conference attendance, and honoraria for expert meetings from both manufacturers of the varicella vaccines, GSK and Sanofi Pasteur MSD, and from manufacturers of other vaccines. Johannes G. Liese received further research grants, support for conference attendance, and honoraria for expert meetings from both manufacturers of the varicella vaccines, GSK and Sanofi Pasteur MSD, and from manufacturers of other vaccines. Christine Hagemann, Alexander Krämer, and Veit Grote declare no conflict of interest.
Compliance with Ethics Guidelines
Approval was obtained from the Bavarian Data Protection Office (Munich) and the Ethics Committees of the Medical Faculties at the University Hospitals in Munich and Würzburg. The participating hospitals reported pseudonymized data.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available because of data protection regulations and contracts with the participating hospitals including guarantees to all participants that individual patient data would be analyzed solely by the investigators at the Department of Pediatrics, University Hospital of Würzburg, and would not be forwarded to any third party.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.9994730.
References
- 1.Liese JG, Grote V, Rosenfeld E, Fischer R, Belohradsky BH, von Kries, Rüdiger and the ESPED Varicella Study Group. The burden of varicella complications before the introduction of routine varicella vaccination in Germany. Pediatr Infect Dis J. 2008;27:125–9. [DOI] [PubMed]
- 2.Borte M, Heininger U, Liese JG, Sauerbrei A, Siedler A. Varizellen Zoster. In: Deutsche Gesellschaft für Pädiatrische Infektiologie e.V. (DGPI), editor. DGPI Handbuch: Infektionen bei Kindern und Jugendlichen. 6th ed. s.l.: Georg Thieme Verlag KG. 2013;582–7.
- 3.Robert Koch-Institut. Ratgeber Infektionskrankheiten: 20. Folge: Varizellen, Herpes Zoster. Epid Bull. 2000;46:365–9.
- 4.Riera-Montes M, Bollaerts K, Heininger U, Hens N, Gabutti G, Gil A, et al. Estimation of the burden of varicella in Europe before the introduction of universal childhood immunization. BMC Infect Dis. 2017;17:353. doi: 10.1186/s12879-017-2445-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozzola E, Bozzola M. Varicella complications and universal immunization. J Pediatr (Rio J). 2016;92:328–330. doi: 10.1016/j.jped.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Kalies H, Grote V, Schmitt H-J, von Kries R. Immunisation status of children in Germany: temporal trends and regional differences. Eur J Pediatr. 2006;165:30–36. doi: 10.1007/s00431-005-1758-0. [DOI] [PubMed] [Google Scholar]
- 7.Wagenpfeil S, Neiss A, Banz K, Wutzler P. Empirical data on the varicella situation in Germany for vaccination decisions. Clin Microbiol Infect. 2004;10:425–430. doi: 10.1111/j.1469-0691.2004.00853.x. [DOI] [PubMed] [Google Scholar]
- 8.Grote V, von Kries R, Springer W, Hammersen G, Kreth HW, Liese J. Varicella-related deaths in children and adolescents-Germany 2003–2004. Acta Paediatr. 2008;97:187–192. doi: 10.1111/j.1651-2227.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- 9.Koch-Institut Robert. Begründung der STIKO für eine allgemeine Varizellenimpfung. Epid Bull. 2004;49:421–3. [Google Scholar]
- 10.Koch-Institut Robert. Begründung einer zweiten Varizellenimpfung im Kindesalter. Epid Bull. 2009;32:328–36. [Google Scholar]
- 11.Siedler A, Dettmann M. Hospitalization with varicella and shingles before and after introduction of childhood varicella vaccination in Germany. Hum Vaccin Immunother. 2014;10:3594–3600. doi: 10.4161/hv.34426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagemann C, Seeger K, Krämer A, Liese JG, Streng A. Entwicklung der Varizellen-Impfraten und mögliche Einflussfaktoren auf die Impfentscheidung der Eltern im Raum München in den Jahren 2009–2011 nach Einführung der allgemeinen Varizellenimpfung. Gesundheitswesen. 2017;79:286–295. doi: 10.1055/s-0042-100726. [DOI] [PubMed] [Google Scholar]
- 13.Hagemann C, Streng A, Kraemer A, Liese JG. Heterogeneity in coverage for measles and varicella vaccination in toddlers—analysis of factors influencing parental acceptance. BMC Public Health. 2017;17:724. doi: 10.1186/s12889-017-4725-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Streng A, Seeger K, Grote V, Liese JG. Varicella vaccination coverage in Bavaria (Germany) after general vaccine recommendation in 2004. Vaccine. 2010;28:5738–5745. doi: 10.1016/j.vaccine.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Streng A, Grote V, Carr D, Hagemann C, Liese JG. Varicella routine vaccination and the effects on varicella epidemiology—results from the Bavarian Varicella Surveillance Project (BaVariPro), 2006–2011. BMC Infect Dis. 2013;13:303. doi: 10.1186/1471-2334-13-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Streng A, Grote V, Rack-Hoch A, Liese JG. Decline of neurologic varicella complications in children during the first seven years after introduction of universal varicella vaccination in Germany, 2005–2011. Pediatr Infect Dis J. 2017;36:79–86. doi: 10.1097/INF.0000000000001356. [DOI] [PubMed] [Google Scholar]
- 17.Streng A, Liese JG. Decline of varicella vaccination in German surveillance regions after recommendation of separate first-dose vaccination for varicella and measles-mumps-rubella. Vaccine. 2014;32:897–900. doi: 10.1016/j.vaccine.2013.12.065. [DOI] [PubMed] [Google Scholar]
- 18.Streng A, Wiegering V, Liese JG. Varicella in pediatric oncology patients in the post-vaccine era—analysis of routine hospital data from Bavaria (Germany), 2005–2011. Pediatr Hematol Oncol. 2016;33:468–479. doi: 10.1080/08880018.2016.1245805. [DOI] [PubMed] [Google Scholar]
- 19.Tan B, Bettinger J, McConnell A, Scheifele D, Halperin S, Vaudry W, Law B. The effect of funded varicella immunization programs on varicella-related hospitalizations in IMPACT centers, Canada, 2000–2008. Pediatr Infect Dis J. 2012;31:956–963. doi: 10.1097/INF.0b013e318260cc4d. [DOI] [PubMed] [Google Scholar]
- 20.Bonhoeffer J, Baer G, Muehleisen B, Aebi C, Nadal D, Schaad UB, Heininger U. Prospective surveillance of hospitalisations associated with varicella-zoster virus infections in children and adolescents. Eur J Pediatr. 2005;164:366–370. doi: 10.1007/s00431-005-1637-8. [DOI] [PubMed] [Google Scholar]
- 21.Bozzola E, Quondamcarlo A, Krzysztofiak A, Pandolfi E, Lancella L, Tozzi A. Haematological complications in otherwise healthy children hospitalized for varicella. Vaccine. 2011;29:1534–1537. doi: 10.1016/j.vaccine.2010.12.095. [DOI] [PubMed] [Google Scholar]
- 22.van Lier A, van der Maas NAT, Rodenburg GD, Sanders EAM, de Melker HE. Hospitalization due to varicella in the Netherlands. BMC Infect Dis. 2011;11:85. doi: 10.1186/1471-2334-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blumental S, Sabbe M, Lepage P. Varicella paediatric hospitalisations in Belgium: a 1-year national survey. Arch Dis Child. 2016;101:16–22. doi: 10.1136/archdischild-2015-308283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glode Helmuth I, Broccia MD, Glenthøj JP, Harder K, Jensen L, von Linstow M-L, et al. Children hospitalized with varicella in Denmark: sensitivity of the National Patient Register. Pediatr Infect Dis J. 2017;36:31–35. doi: 10.1097/INF.0000000000001347. [DOI] [PubMed] [Google Scholar]
- 25.Bozzola E, Gattinara GC, Bozzola M, Mirante N, Masci M, Rossetti C, et al. Varicella associated pneumoniae in a pediatric population. Ital J Pediatr. 2017;43:49. doi: 10.1186/s13052-017-0366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gowin E, Wysocki J, Michalak M. Don’t forget how severe varicella can be-complications of varicella in children in a defined Polish population. Int J Infect Dis. 2013;17:e485–e489. doi: 10.1016/j.ijid.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 27.Popescu CP, Ceausu E, Florescu SA, Chirita D, Ruta S. Complications of varicella in unvaccinated children from Romania, 2002–2013: a retrospective study. Pediatr Infect Dis J. 2016;35:211–212. doi: 10.1097/INF.0000000000000969. [DOI] [PubMed] [Google Scholar]
- 28.Jackson MA, Burry VF, Olson LC. Complications of varicella requiring hospitalization in previously healthy children. Pediatr Infect Dis J. 1992;11:441–445. doi: 10.1097/00006454-199206000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Miranda-Choque E, Candela-Herrera J, Díaz-Pera J, Farfán-Ramos S, Muñoz-Junes EM, Escalante-Santivañez IR. Varicela complicada en un hospital pediátrico de referencia, Perú 2001–2011. Rev Peru Med Exp Salud Publica. 2013;30:45–48. doi: 10.1590/S1726-46342013000100009. [DOI] [PubMed] [Google Scholar]
- 30.Marshall HS, McIntyre P, Richmond P, Buttery JP, Royle JA, Gold MS, et al. Changes in patterns of hospitalized children with varicella and of associated varicella genotypes after introduction of varicella vaccine in Australia. Pediatr Infect Dis J. 2013;32:530–537. doi: 10.1097/INF.0b013e31827e92b7. [DOI] [PubMed] [Google Scholar]
- 31.Arlant LHF, Garcia MCP, Avila Aguero ML, Cashat M, Parellada CI, Wolfson LJ. Burden of varicella in Latin America and the Caribbean: findings from a systematic literature review. BMC Public Health. 2019;19:528. doi: 10.1186/s12889-019-6795-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elbaz M, Paret G, Yohai AB, Halutz O, Grisaru-Soen G. Immunisation led to a major reduction in paediatric patients hospitalised because of the varicella infection in Israel. Acta Paediatr. 2016;105:e161–e166. doi: 10.1111/apa.13320. [DOI] [PubMed] [Google Scholar]
- 33.Koch-Institut Robert. Epidemiologie der Rotavirus-Erkrankungen in Deutschland im Zeitraum von 2001 bis 2011. Epid Bull. 2012;44:441–9. [Google Scholar]
- 34.Bozzola E, Bozzola M, Krzysztofiak A, Tozzi AE, El Hachem M, Villani A. Varicella skin complications in childhood: a case series and a systematic review of the literature. Int J Mol Sci. 2016;17:688. doi: 10.3390/ijms17050688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baxter R, Ray P, Tran TN, Black S, Shinefield HR, Coplan PM, et al. Long-term effectiveness of varicella vaccine: a 14-year, prospective cohort study. Pediatrics. 2013;131:e1389–e1396. doi: 10.1542/peds.2012-3303. [DOI] [PubMed] [Google Scholar]
- 36.Davis MM, Patel MS, Gebremariam A. Decline in varicella-related hospitalizations and expenditures for children and adults after introduction of varicella vaccine in the United States. Pediatrics. 2004;114:786–792. doi: 10.1542/peds.2004-0012. [DOI] [PubMed] [Google Scholar]
- 37.Reynolds MA, Watson BM, Plott-Adams KK, Jumaan AO, Galil K, Maupin TJ, et al. Epidemiology of varicella hospitalizations in the United States, 1995–2005. J Infect Dis. 2008;197(Suppl 2):6. doi: 10.1086/522146. [DOI] [PubMed] [Google Scholar]
- 38.Seward JF. Varicella disease after introduction of Varicella Vaccine in the United States, 1995–2000. JAMA. 2002;287(5):606–611. doi: 10.1001/jama.287.5.606. [DOI] [PubMed] [Google Scholar]
- 39.Sheridan SL, Quinn HE, Hull BP, Ware RS, Grimwood K, Lambert SB. Impact and effectiveness of childhood varicella vaccine program in Queensland, Australia. Vaccine. 2017;35:3490–3497. doi: 10.1016/j.vaccine.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Spoulou V, Alain S, Gabutti G, Giaquinto C, Liese J, Martinon-Torres F, Vesikari T. Implementing universal varicella vaccination in Europe: the path forward. Pediatr Infect Dis J. 2019;38:181–188. doi: 10.1097/INF.0000000000002233. [DOI] [PubMed] [Google Scholar]
- 41.Varela FH, Pinto LA, Scotta MC. Global impact of varicella vaccination programs. Hum Vaccin Immunother. 2019;15:645–657. doi: 10.1080/21645515.2018.1546525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.García Comas L, Latasa Zamalloa P, Alemán Vega G, Ordobás Gavín M, Arce Arnáez A, Rodero Garduño I, et al. Descenso de la incidencia de la varicela en la Comunidad de Madrid tras la vacunación infantil universal. Años 2001–2015. Aten Primaria. 2018;50:53–9. [DOI] [PMC free article] [PubMed]
- 43.Heywood AE, Wang H, Macartney KK, McIntyre P. Varicella and herpes zoster hospitalizations before and after implementation of one-dose varicella vaccination in Australia: an ecological study. Bull World Health Organ. 2014;92:593–604. doi: 10.2471/BLT.13.132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah SS, Wood SM, Luan X, Ratner AJ. Decline in varicella-related ambulatory visits and hospitalizations in the United States since routine immunization against varicella. Pediatr Infect Dis J. 2010;29:199–204. doi: 10.1097/INF.0b013e3181bbf2a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez AS, Zhang J, Brown C, Bialek S. Varicella-related hospitalizations in the United States, 2000–2006: the 1-dose varicella vaccination era. Pediatrics. 2011;127:238–245. doi: 10.1542/peds.2010-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Damm O, Witte J, Wetzka S, Prosser C, Braun S, Welte R, Greiner W. Epidemiology and economic burden of measles, mumps, pertussis, and varicella in Germany: a systematic review. Int J Public Health. 2016;61:847–860. doi: 10.1007/s00038-016-0842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horn J, Damm O, Kretzschmar ME, Karch A, Siedler A, Ultsch B, et al. Mathematische Modellierung der Effekte des Varizellen-Impfprogramms in Deutschland: Abschlussbericht, Version 1.2. Braunschweig, Bielefeld, Utrecht, Berlin; 16.09.2014.
- 48.Andrade AL, da Silva Vieira MA, Minamisava R, Toscano CM, Lima Souza MB, Fiaccadori F, et al. Single-dose varicella vaccine effectiveness in Brazil: a case-control study. Vaccine. 2018;36:479–483. doi: 10.1016/j.vaccine.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 49.Leung J, Broder KR, Marin M. Severe varicella in persons vaccinated with varicella vaccine (breakthrough varicella): a systematic literature review. Expert Rev Vaccines. 2017;16:391–400. doi: 10.1080/14760584.2017.1294069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available because of data protection regulations and contracts with the participating hospitals including guarantees to all participants that individual patient data would be analyzed solely by the investigators at the Department of Pediatrics, University Hospital of Würzburg, and would not be forwarded to any third party.