Abstract
Diabetes and obesity are the most frequently found disease worldwide. Several factors are responsible for obesity, i.e., imbalance in energy expenditure, environmental factors, feeding habit, lifestyle, etc., which can also be responsible for type 2 diabetes mellitus. There are several synthetic drugs available to combat these diseases which have some side effects on sufferers. Therefore, people are shifting towards inexpensive, effective, widely available natural and herbal medicines. Edible mushrooms, which have been used from ancient time to cure these diseases, contain anti-oxidant, fibers, triterpenoids, alkaloid, and other phytochemicals. Comatin, β-glucan, Tremellastin, and Lentinan KS-2 are active chemicals of mushrooms which show great effect on diabetes mellitus and obesity by modulating either cellular function or biochemical pathways. Here, in this review, we have discussed the potential role of edible mushrooms and its biochemicals in control of diabetes and obesity. Using Bioinformatics, we can find the specific targets of theses biochemicals, so that these can be more effective.
Keywords: Edible mushroom, Bioactive compounds, Diabetes mellitus, Insulin, Anti-diabetic
Introduction
The current world’s demography of diabetes is 390 million people. Like diabetes, obesity is also increasing very rapidly; about 105 million obese people were reported in 1975 and 642 million in 2014. The increasing rate of obesity for men was about 3.2% in 1975 while 11% in 2014. For women, it was about 6.4% in 1975 and 15% in 2014. The recent studies reveal that Indian women are more prone to be obese than men. In 2014, approximately 20 million obese women were found in India, and in United States, it was 82 million. A large number of obese were also reported from other countries like the United Kingdom, Canada, China, Russia, Middle-East, etc. (Feeney et al. 2014; Poddar et al. 2013). Diabetes can be of two types, i.e., Type-1 and Type-2; this classification was made in 1936 and in 1988. Type 1 diabetes is an autoimmune disorder, in this β-cells of pancreas destroyed by organism own immune cells whereas Type 2 diabetes is due to resistant of insulin for its receptors present on different cells like hepatocytes, adipocyte, and muscle cells. Insulin is a hormone produced by the β-cells of pancreas, which helps in the removal of extra glucose from blood circulation. Both the type of diabetes causes many harmful effects on metabolism, which is referred as metabolic syndrome (Patlak 2002; Agarwal et al. 2016). Diabetes mellitus can also cause other serious health problems also like cancer, retinopathy, neuropathy, cardiovascular diseases, renal dysfunction, etc. (Hansen et al. 2012; Sobngwi et al. 2012; Chaturvedi et al. 2018, 2019).
Unlike diabetes obesity can be due to alteration in several metabolic pathways among them one is alteration in Hedonic and Homeostatic Pathway, i.e., disturbance in the appetite center in brain result is abnormal eating behavior, second is imbalance in energy expenditure and feeding result is accumulation of excess fat in liver, adipose, and muscle tissues, and third is accumulation of extra lipids in adipose tissue during adipocyte differentiation. Post-translational histone modification, non-coding RNA activity, and DNA methylation are epigenetic mechanism which regulates the above-mentioned metabolic pathways at the level of gene expression (Jaenisch and Bird 2003; Xue and Ideraabdullah 2016).
In early studies, it was considered that obesity and diabetes are entirely different disorder, but studies from the past few decades continuously reveal a direct link between diabetes and obesity. This is because most of the obese are also sufferer of diabetes hypertension, cardiovascular diseases, hyperlipidemia, etc. (Kopelman 2000; Funahashi and Matsuzawa 2007). Therefore, it has become very important to treat diabetes and obesity together. Mushroom can be an elixir for the treatment of both, this is due to presence of plenty of fibers and anti-diabetic compounds in mushrooms; Pleurotusostreatus, Ganodermalucidum, Grifolafrondosa, Lentinusedodes, etc. These mushrooms act as an anti-obesogenic agent by reducing the synthesis of cholesterol, absorption of cholesterol, the extra fiber in mushrooms also reduce the feeding, and kept healthy gastrointestinal tract which helps in reduction of obesity (De Silva et al. 2012). The mushroom, Poria cocos, used as a traditional Chinese medicine which is used alone or in the combination with other herbs to cure diabetes and other disorders related to diabetes (Lindequist et al. 2005). This effect of Poria cocos was due to a triterpenoid called Dehydrotrametanolic Acid which reduces the blood glucose level in diabetic mice (Sato et al. 2002). In this review, we have discussed the roles of edible mushrooms in control of diabetes and obesity (Tables 1 and 2).
Table 1.
Mushrooms having anti-diabetic properties
| S. no. | Species | Extracts/bioactive compounds | Mode of action | References |
|---|---|---|---|---|
| 1 | Agaricus campestris | Aqueous extract of fruiting body | Insulin-releaser and anti-hyperglycemic activity and Facilitate the transportation of 2-deoxyglucose | Gray and Flatt (1998) |
| 2 | Agaricus subrufescens | β-glucans and enzymatically produced oligosaccharides | Improved insulin resistance in type 2 diabetes mellitus through increase in adiponectin concentrations | Niwa et al. (2011) |
| 3 | Agrocybe cylindracea | A glucan (AG-HN1) and a heteroglycan (AG-HN2) isolated from hot-water extract of the fruiting bodies | AG-HN1 showed potent hypoglycemic activity which is higher than AG-HN2 | Kiho et al. (1994) |
| 4 | Astraeus hygrometricus | Ethanolic extract of fruiting bodies and polysaccharide | Reduced the blood glucose level, reduced the plasma glucose, blood glucose, total cholesterol and triglyceride levels | Biswas and Acharya (2013); Kim et al. (2007) |
| 5 | Auricularia auricula-judae | |||
| 6 | Cordyceps militaris | Exo-polymers produced from submerged mycelia cultures | Significantly decreased levels of plasma glucose, total cholesterol, triglyceride and plasma glutamate-pyruvate transaminase (GPT) | Yamac et al. (2009) |
| Aqueous fruiting body extract | Significant reduction of fasting serum glucose levels, increased body glucose disposal rates and glucose utilization in skeletal muscles; amelioration of insulin resistance and improved insulin secretion | Zhang et al. (2006) | ||
| 7 | Coprinus comatus | 4,5- Dihydroxy-2-methoxybenzaldehyde (comatin) | Reduced the high levels of blood glucose concentration and ameliorated the glucose tolerance | Ding et al. (2010); Han et al. (2006) |
| 8 | Cordyceps sinensis | Polysaccharide fraction (CSP-1) | Induce the secretion of insulin from islets of pancreas and reduced the insulin metabolism | Li et al. (2006) |
| 9 | Grifolafrondosa | Glycoprotein extract (SX fraction) | Ameliorated glucose tolerance and showed enhanced sensitivity to exogenous insulin | Preuss et al. (2007) |
| 10 | Ganoderma applanatum | Exo-polymer isolated from submerged mycelial cultures | Reduced the total cholesterol, triglyceride and plasma glucose levels | Yang et al. (2007) |
| 11 | Ganoderma lucidium | Aqueous extract of fruiting bodies (ethylacetate and n-butanol fractions) | Significantly lowers the pre-prandial blood glucose levels | Mohammed et al. (2009); Li et al. (2011a) |
| 12 | Hericiumerinaceus | Methanol extract of fruiting bodies | Reduced the blood glucose levels and lipid levels | Wang et al. (2005) |
| 13 | Laetiporussulphureus var. miniatus | Extra polysaccharide isolated from submerged mycelia culture | Increased anti-genesity of insulin via proliferation and regeneration of β-cells and reduced the plasma glucose levels, total cholesterol | Hwang and Yun (2010); Yang et al. (2002) |
| 14 | Lentinula edodes | |||
| 15 | Lentinusstrigosus | Exo-polysaccharides (EPS) | Reduced the plasma glucose levels, helps in regeneration of pancreatic islets | Yamac et al. (2008) |
| 16 | Phellinus linteus | Exo-polymers from submerged mycelia cultures | Lowered the total cholesterol, GPT (glutamate-pyruvate transaminase) levels, and plasma glucose levels | Ding et al. (2010); Jong-Wonyun et al. (2001) |
| Polysaccharides | Prevents the autoimmune diabetes by regulating the cytokine expression | Kim et al. (2010) | ||
| 17 | Phellinus ribis | Polychlorinated compounds | Therapeutic effects through the enhance PPAR-γ agonistic activity | Lee et al. (2008) |
| 18 | Phellinus badius | Aqueous extract of mycelia and fruiting body | Reduced the cholesterol levels, plasma triglyceride levels and blood glucose levels; marked reduction in the level of aspartate amino-transferase (AST) and alanine amino-transferase (ALT) | Sonawane et al. (2013); Rony et al. (2013) |
| 19 | Phellinus rimosus | |||
| 20 | Pleurotus abalonus | Polysaccharide-peptide complex LB-1b isolated from fruiting bodies | Potent radical scavenging activity with hypoglycaemic effect | Li et al. (2012) |
| 21 | Pleurotus eryngii | Diet rich with mushroom | Worked as insulin sensitizer and showed anti-hyperlipidemic and hypoglycaemic activity | Kim et al. (2010) |
| Powder of freeze-dried fruiting body | ||||
| 22 | Pleurotus pulmonarius | Aqueous extract | Reduced the serum glucose level in diabetes induced mice and improved the oral glucose tolerance | Badole et al. (2008) |
| 23 | Pleurotus citrinopileatus | Polysaccharides | Reduced fasting blood glucose levels | Hu et al. (2006) |
| 24 | Pleurotus ostreatus | Powdered fruiting bodies |
Significantly lower basal and post-prandial glycaemia. Dose-dependent decrease in blood glucose and cholesterol effects |
Chorvathova et al. (1993) |
| Ethanol extract of fruiting bodies | Reduced the blood glucose levels and improve the genetic alterations and sperm abnormalities | Ghaly et al. (2011) | ||
| 25 | Sparassiscrispa | β-glucan | A potent compound having wound healing properties in diabetic patients. Increase the movement of macrophages and fibroblasts | Kwon et al. (2009) |
| Freeze-dried powder of fruiting body | Increased adiponectine level in plasma; reduced the serum triglycerides, blood glucose level and total cholesterol level also | Yamamoto and Kimura (2010) | ||
| 26 | Strophariarugosoannulata | Extracellular polysaccharide (EPS) | Decrease the plasma glucose level, total cholesterol, and triacylglycerol level also; decreased aspartate amino-transferase activity, lipid metabolism regulated by PPAR-γ | He et al. (2012); Kiho et al. (1994); Ma et al. (2013) |
| 27 | Trametesgibbosa | |||
| 28 | Tremella fuciformis | |||
| 29 | Tremella mesenterica | Tremellastin | Reduction of intrinsic blood glucose level in dose-dependent manner and decreased the triglyceride level as well | Elisashvili et al. (2002) |
| 30 | Tremella aurantia | Fruiting bodies containing acidic hetero-polysaccharide and several sugars including glucose | Decreased the elevated level of blood glucose, total cholesterol, triglyceride and lipoperoxide level also | Lo et al. (2006); Kiho et al. (2001) |
Table 2.
Mushrooms having anti-obesity properties
| S. no. | Mushroom | Bioactive compounds/extracts | Mode of action | References |
|---|---|---|---|---|
| 1 | Agaricusbisporus | Dietary fiber | Reduced the total cholesterol level and LDL-mRNA expression | Fukushima et al. (2000) |
| 2 | Ganoderma lucidium | Extracts | Reduced the body weight by modulating the microbiota and reduced the adipogenic transcription factor and prevents the glucose transportation and lipid storage | Delzenne and Bindels (2015) |
| 3 | Hericiumerinaceus | Exo-polymer | Reduced the total cholesterol, LDL, HDL, triglyceride, phospholipids, etc | Yang et al. (2003) |
| 4 | Lentinula edodes | Lentinan KS-2 | Decrease the level of total lipid, LDL, phospholipid and LDL/HDL ratio | Yoon et al. (2011) |
| 5 | Pholiotanameko SW-02 | Polysaccharides | Decreased the blood lipid level, total cholesterol level, liver lipid level | Zheng et al. (2014) |
| 6 | Pleurotus tuber-regium | Polysaccharides | Anti-hyperlipidemic activity and reduced the oxidative stress in obese diabetic rats | Huang et al. (2014) |
| 7 | Tremella fuciformis | Polysaccharides | Decrease the expression of mRNA to prevent the variation of 3T3-L1 adipocytes | Jeong et al. (2008) |
| 8 | Pleurotuseryngii | Powder of fruiting body | Reduced total plasma cholesterol, triglyceride, low-density lipoprotein, total lipid and phospholipids | Alam et al. (2011) |
Neuroendocrine regulation of appetite and obesity
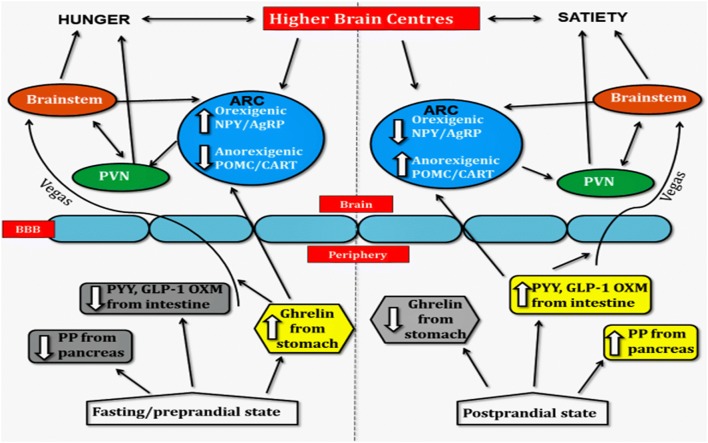
The hunger and satiety are regulated by the complex interaction among neuronal, endocrine, gastrointestinal and adipose tissues (Rui 2013). The leptin hormone of adipocyte decreases the glucose-stimulated secretion of Insulin which causes the induction of brain’s satiety center result is reduction in hunger. Except this, Leptin also inhibits the appetite by binding with orexigenic neuropeptide Y receptors in hypothalamus (Koch et al. 2010; Kelesidis et al. 2010). Leptin stimulates the appetite suppressant, Alpha-melanocyte stimulating hormones (α-MSH), and control the appetite (Benoit et al. 2004). If the receptor for leptin is absent, then it can cause uncontrolled food intake result is obesity. In the absence of insulin, the utilization of glucose from peripheral tissues is inhibited which causes hyperglycemia, i.e., diabetes. During unavailability of glucose, the fat becomes energy source for cells/tissue. Condition of insulin resistance for its receptors can also cause diabetes and obesity. Another hormone Ghrelin secreted from stomach also plays an important role in appetite control and energy balance. The empty stomach releases the ghrelin hormone which stimulates the hypothalamic cells to induce hunger. Presence of food in the gastrointestinal tract activates the vagus nerve to inhibit the hunger center in the brain (Fig. 1). However, hormone cholecystokinin secreted from epithelial cells of the small intestine act upon hypothalamus to reduce the secretion of NPY result is increased in the food intake.
Fig. 1.
Hormone secreted in gastrointestinal tract showing signaling pathways in fasting/pre-prandial and in post-prandial state. In fasting condition, ghrelin hormone stimulates the vagus nerve by activating the ARC. During post-prandial condition, anarectic, PYY, GLP-1, OXM, and PP hormone act on the brainstem, ARC, and vagus nerve which cause satiety. ARC arcuate nucleus, PVN paraventricular nucleus, NPY/AgRP neuropeptide Y and agouti-related peptide, POMC/CART pro-opiomelanocortin, and cocaine and amphetamine-related transcript, GLP-1 glucagon like peptide-1, PP pancreatic polypeptide, PYY peptide YY, OXM oxyntomodulin
Generally, obese people have low ghrelin level compared to normal people. Rodents treated with a daily dose of ghrelin get fatty; this is probably due to reduced use of fat as an energy source (Tschöp et al. 2000). The persons suffering from anorexia nervosa (eating disorder) have high plasma ghrelin level as compared to normal body persons (Cummings et al. 2002; Germain et al. 2007). These findings reveal that ghrelin has inverse effect on energy consumption. In another pathway, anandamide and neuropeptide Y both are inhibited by the leptin result was activation of α-melanocyte which is a hunger-suppressing peptide which suppresses the hunger. There are many drugs prevailing in the market for the control of obesity, but the use of these drugs also causes several side effects, i.e., cardiovascular disorders. Due to this reason, some of the anti-obesogenic drugs have been removed from the market that is sibutramine which disturbs the nervous system by inhibiting the absorption of serotonin, noradrenaline, and dopamine (Dietrich and Horvath 2012) and reduces the appetite. Rimonabant is another drug that was withdrawn from the market due to their adverse effect. This drug reduces the appetite by blocking cannabinoid receptor 1(Dietrich and Horvath 2012).
Impact of dietary mushroom fibers on obesity and diabetes
Dietary fibers are not digestible by the human gastric enzymes. These fibers help to lower the lipid level in blood and also help to reduce the cholesterol level. This dietary fibers bind with lipids and cholesterol, which inhibit the absorption by the intestine (Anderson 1986; Topping 1991). Many types of research have been demonstrated that dietary fiber can play a supporting role in the prevention of cancer, cardiovascular disease, obesity, and diabetes (Theuwissen and Mensink 2008; Bordonaro and Sartorelli 2008). The mushrooms dietary fibers contain “chitosan” which is also found in crustaceans. After break down of dietary fibers, monomers generated which provide energy in the large intestine after fermentation. This energy is less than the energy released by the carbohydrates. In mushrooms, most of the carbohydrates are non-digestible; these are chitins, β-glucans, and mannans (Brennan 2005). Another DF has been identified in mushroom sclerotia (mushroom sclerotic is a condition in which the mycelia structure becomes compact and remains dormant to overcome unfavorable condition) which is consolidated with chitin and β-glucans and indigestible for humans; therefore, this is considered as a novel DF (AACC Report 2000; Alimentarius C. Report 2009). Lipase is the main enzyme which hydrolyzes dietary triglycerides and releases fatty acids which are further absorbed by the mucosa cells of small intestine. Therefore, most of the anti-obesogenic drugs are designed to inhibit the activity of lipase enzyme by forming a covalent bond at the catalytic site of the enzyme. The most common anti-obesogenic drug is ‘Orlistat’ acts by blocking the activity of lipase (Guerciolini 1997). Bile acids play a major role in digestion and absorption of lipids. Generally, fibers and dietary lipid of mushrooms bind with bile acids and facilitate the digestion and absorption in small intestine. By the enterohepatic circulation, the bile acid transported back to the liver. The enterohepatic circulation is inhibited when dietary fiber and dietary lipid binds with bile acid and increase excretion in feces of bile acids (Martel et al. 2017).
Changes in adipocyte functions due to diabetes and obesity
High-fat diets are linked to obesity, type 2 diabetes mellitus, and weight gain (Wang and Liao 2012). Usually, a normal amount of adipose tissue is used as a source of energy and to regulate body temperature. Body fat consists of two type adipose tissue, white adipose tissue and (WAT), which stores maximum energy, and brown adipose tissue, which is involved in thermogenesis (Enerbäck 2010). After eating the insulin-level raises, this may be responsible for diet-induced thermogenesis. White adipocytes contain triglycerides and cholesteryl esters in the form of large lipid vacuole. When the energy is required, then these triglycerides get hydrolyzed by lipolysis and fatty acids releases which enter the blood and undergo β-oxidation to produce energy. Compared with a normal level of adipocytes in the presence of more lipids, the white adipocytes increase in the number and in size also. Adipocyte release hormones and cytokines (adipokines) play important role in endocrine functions, which regulate many homeostatic processes like immune function, energy levels, satiety, etc Waki and Tontonoz 2007). Hypertrophied adipocytes release more pro-inflammatory adipokines such as tumor necrosis factor (TNF) and IL-6 than normal adipocytes (Waki and Tontonoz 2007). These adipokines impede with insulin signaling and induce chronic inflammation. Insulin resistance increases the demand for insulin which can eventually cause the development of type 2 diabetes mellitus. Many triterpenes from edible mushrooms can activate the peroxide proliferator-activated receptor-γ (PPAR-γ) which regulates glucose metabolism (Sato et al. 2002; Li et al. 2011b). PPAR-γ downregulates the expression of TNF, leptin, and IL-6, and also induces the expression of adiponectin and adipokine which sensitizes the liver and muscle to insulin (Nawrocki et al. 2006; Wang et al. 2014).
β-cells, insulin sensitivity, and role of mushroom-related compounds
β-cells of the pancreas secrete insulin when food is ingested. Pro-inflammatory cytokines released by hypertrophied adipocyte cause β-cell dysfunction, apoptosis, and necrosis which also renders the insulin secretion. The mushrooms compounds like protein, polysaccharides, lipopolysaccharides, and glycoprotein identified as potent immune modulators. These bioactive molecules protect β-cells from pro-inflammatory cytokines by reducing the activation of NF-κB. β-cells of the pancreas are very susceptible to oxidative damage and have poor anti-oxidant capacity (Kajimoto and Kaneto 2004). The bioactive molecules of edible mushrooms protect the β-cells from oxidative damage which is caused by free radicals generated due to oxidative stress and glucotoxicity. Muscles and adipocytes of the body increase the insulin-dependent uptake of glucose and promote energy storage. Insulin also inhibits glucose production by the liver. Adiponectin and GLP-1 hormones sensitize the body for insulin (Cerf 2013; MacDonald et al. 2002; Canfora et al. 2015). In vitro treatment of intestinal endocrine cells with chitosan at a concentration of 200 μg/ml for 2 h cause 1.6-fold increase in secretion of GLP-1 (Liu et al. 2013). Dietary supplements mixed with chitosan given to diabetic rats cause accumulation of GLP-1, leading to improvement of insulin sensitivity (Hsieh et al. 2012).
Effect of mushrooms and its ingredients on gut microbiota
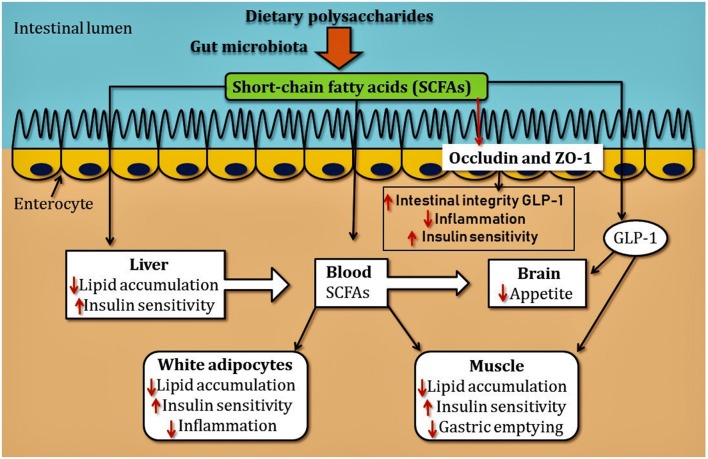
Gut microbiota full of trillions of microbes play very crucial physiological roles, i.e., intestinal cells maintenance, vitamins production, neutralization of pathogens, toxins, drugs, and development of the immune system (Lin et al. 2014). These gut microbes extract energy from the food taken by the individual and it is another factor which can develop obesity and can cause type 2 diabetes (Delzenne et al. 2011; Shen et al. 2013). In an experiment, it is observed that the gut microbiota extracted more energy from food in obese mice than lanky mice (Turnbaugh et al. 2006). The gut microbiota transferred from laky mice to obese mice can improve the sensitivity of insulin (Vrieze et al. 2012). The observation suggests that modulation of gut microbiota could have beneficial effects on obesity and type 2 diabetes mellitus. It was found that the anti-obesogenic effect of Ganoderma lucidum is due to modulation of gut microbiota (Chang et al. 2015; Delzenne and Bindels 2015). The extract of Ganoderma lucidum reduces the body weight and fat accumulation in high-fat-diet mice and also decreases the expression level of pro-inflammatory cytokines TNF, IL-1B, and IL-6. In high-fat diet animals, the expression of the protein occludin and zona occludin protein-1 is responsible for the maintenance of intestinal tight junctions. The expression of these proteins was lower in low-fed animals. The supplementation of G. lucidum extract with anti-obesogenic drugs can restore the physiological level of pro-inflammatory cytokines which maintain the integrity of intestine and prevents the translocation of pro-inflammatory endotoxins (such as lipopolysaccharides) from gut microbiota to blood. In particular, G. Lucidum-induced weight loss are transmissible via horizontal transfer of faces from G. Lucidum-treated mice to HFD-feed mice, this observation shows that these effects are mediated by the gut microbiota. The high-molecular-weight polysaccharides found in G. Lucidum extracts are responsible for the anti-obesogenic effects (Chang et al. 2015). The polysaccharides found in fungus are not digested in small intestine but can digested by bacteria in the large intestine and produce short-chain fatty acids (SCFAS), which induce the secretion of GLP-1 protein in the blood and cause effects on the brain, adipose tissue, muscles, and liver, and also cause delaying gastric emptying that lead to reductions in appetite, lipid deposition, insulin resistance, and inflammation (Fig. 2). GLP-1 has also played an important role in promoting proliferation, inhibition of apoptosis in β-cells (Cornu and Thorens 2009). Shiitake (Lentinula edodes) and button mushrooms are highly rich in polysaccharides which can induce the growth of gut microbiota (Aida et al. 2009). It was reported that treatment of sexual reproductive form (Anamorph) of O. sinensis and Hirsutella modifies the composition of the gut microbiota, and has anti-inflammatory effects, anti-diabetic, and anti-obesogenic effects in HFD-fed mice. Several substances found in mushrooms such as fiber and polysaccharides which have effects on the microbiota of assimilated directly (Chen et al. 2016). The above findings and reports suggest that these substances act as prebiotics and can be used as an anti-diabetic and anti-obesogenic treatment.
Fig. 2.
Dietary polysaccharides are digested only in large intestine with the help of gut microbiota by which short-chain fatty acid (SCFAs) are produced. These SCFAs induced the secretion of GLP-1 (glucagon like protein-1). SCFAs and GLP-1 together act in brain and reduce the appetite and increase the sensitivity of insulin in liver, muscle, and adipocyte, whereas decrease the accumulation of lipids which reduce the inflammation in these organs. SCFAs induce the expression of occluding and zonulaoccludens proteins-1 (ZO-1) in intestinal cells which maintain the integrity of intestine and prevent the release of bacterial endotoxins in bloodstream
Effects of energy expenditure and lipid storage on diabetes and obesity
To reduce the chances of adiposity, there is mainly two strategies which can be followed below. The first strategy is to manage the expenditure of energy and the second is to inhibit the synthesis of lipid. The white adipocytes contain a large number of triglycerides which is hydrolyzed by the lipolysis to produce the β-fatty acids which undergo β-oxidation and release of energy. If these triglycerides are not hydrolyzed, then white adipose tissue will store more triglycerides and cause of obesity. The enhance of energy expenditure is the best way to stop the accumulation of triglycerides storage. A strategy by targeting the AMP-activated protein kinase (AMPK) enzyme (Chen et al. 2012). When ATP is hydrolyzed in ADP and AMP, then ADP and AMP activate the AMPK enzyme in skeletal muscle, which provides the cellular energy by inhibiting the anabolic pathways and activate the catabolic pathways. An example is fatty-acid oxidation which generates the ATP and reduces the storage of lipids. AMPK has an important role in diabetes also, because it releases GLUT4 in the cytosol which organizes in the membrane of muscle and adipose tissues and allows the insulin to entre inside the tissue (Asrafuzzaman et al. 2018), which is good for type-2 diabetes mellitus. The second strategy to reduce obesity is by inhibiting the lipid synthesis. Statins are the anti-obesogenic drug which inhibits the 3-hydroxy-3-methylglutaryl-coenzyme-A reductase (HMG-CoA reductase) enzyme which plays an important role in cholesterol biosynthesis. Statins were first time isolated from fungi. Lovastatin is also isolated first time from Aspergillus terreus, and also found in various mushroom’s mycelial and fruiting body. Some examples are Agaricus, Antrodia, Ganoderma, Ophiocordyceps, and Pleurotus species (Chen et al. 2012).
Understanding of Diabetes and Obesity from different metabolic pathways
There are many mechanisms of loss of function of insulin-producing β-cells. The general pathway of β-cell destruction of is autoimmunity. In autoimmunity disorder, immune effector molecule destructs the β-cells which cause type-1 diabetes. CD8 T cells directly recognize B cells via MHC class 1 protein, whereas CD4 T cells could not directly recognize β-cells, because these cells do not express MHC class II proteins (Siddiqui et al. 2013). CD4 cells recognize antigen-presenting cells that are dendritic cells, macrophages, etc. The death of β-cell is preceded indirectly, because there is not antigen-specific interaction between β-cells and CD4 T cells. CD4 T cells activate the CD8 T cells via the interaction with peptides of antigens presented by MHC class I and cause the destruction of β-cells through perforin and granzyme molecules (Trivedi et al. 2016). There are many other pathways of β-cells destruction which is not discussed in this review. In type 2 diabetes mellitus, the tissue shows resistance against insulin due to the lower expression of the insulin receptor. The low expression of the insulin receptor is the result of impaired phosphorylation of tyrosine and the phosphorylation of tyrosine residue of IRS-1. The attenuation of the regulatory subunit of phosphoinositide-3 kinase (PI3K) with IRS-1 plays an important role in insulin resistance. Due to the attenuation of the catalytic subunit gets deactivated by which the PI3K signaling pathways reduced, this results in the activation of protein kinase Akt and reduces the glucose transportation (Anai et al. 1998). Due to the above events, plasma-free fatty acid increases and the insulin-stimulated glucose uptake decreases (Moller 2001). Due to the development of insulin resistance pro-inflammatory cytokines, release and stimulation of TNF-α production occur which destruct the β-cells (Yuan and Chung 2010). In obesity, obesity-induced inflammation could occur by the activation of TLR4 (Toll-like receptors) that are activated by Gram-negative bacteria. These bacteria can also activate the TLR2 and NOD1/2 which interact with peptidoglycans, lipoteichoic acid, and lipoproteins of Gram-negative bacteria. Moreover, saturated fatty acids and their metabolites could indirectly activate the TLR4 by activating its receptor through DAMPs such as HMGB1. Saturated fatty acid could activate the TLR2 directly, because TLR2 could be the receptor of saturated fatty acid also. By the activation of these receptors, it could cause the generation of cytokines like IL-1β and IL-18, etc., which triggers the other pathways. Some fungi like mushrooms extract contain substances which target the multiple pathways of obesity and diabetes. Polysaccharides of mushrooms could reduce the body weight and deposition of fat. These polysaccharides act by inhibiting the absorption of fat dietary (Chang et al. 2015; Topping 1991). These molecules have synergistic effects on target pathways and molecule involved in these pathways. By targeting these pathways, we could enhance the treatments and could prevent the development of obesity and diabetes (Martel et al. 2017).
In silico analysis for culinary mushrooms as an anti-diabetic potential
The Human Genome Project (HGP) completion has resulted in an increasing number of targets for drug discovery, which has also become a major challenge for pharmaceutical companies as it requires high cost input and a much large amount of time (Dangi-Garimella 2014). However, the emerging role of bioinformatics tools has made it relatively easier, by involving techniques such as molecular docking and virtual screening (Haan et al. 2015). However, the computer-aided drug designing methods play a very crucial role in the designing of novel anti-diuretic agents (Makheswari and Sudarsanam 2012). The molecular simulation models have proposed to the study of the physiology of diabetes. On the other hand, the computer-assisted drug development process saves costs for several pharma companies (Ramanathan et al. 2010). With the help of in silico analysis, we can identify the drug targets for diseased candidates. Edible mushroom compounds have a very complex structure, so based on the chemical organization, these compounds can be classified in two categories—high-molecular-weight (HMW) compounds and low-molecular-weight (LMW) compounds (Vitak et al. 2017). However, high-molecular-weight compounds play a very crucial role as an oral-diabetic drug such as Guanide, isolated from the Pleurotus species which have an anti-hypoglycemic property (Patel et al. 2012). Nowadays, in silico approach is used to assess the medicinal mushroom LMW as well as HMW compounds as potential drug agents which act as an activator of insulin receptor even as reduce the risk of harmful effect of diabetes. With the help of ligand-based virtual screening, we can investigate to generate the novel activator of insulin receptor. Some of the docking programs are listed below (Table 3).
Table 3.
Type of docking programs used to regulate for protein–ligand interactions
| S.n | Program | Algorithm | URL |
|---|---|---|---|
| 1. | 3D-DOCK | Global FFT; Rescoring: residue potential. Refinement-field: side chain multicopy. | http://www.bmm.icnet.uk/docking/ |
| 2. | HEX | Global: Fourier correlation of spherical harmonics | http://www.biochem.abdn.ac.uk/hex/ |
| 3. | GRAMM | Global: FFT clustering and rescoring | http://vakser.compbio.ku.edu/resources/gramm/grammx/ |
| 4. | PPD | Global: geometric hashing; rescoring, multiple filters | ftp://flash62.bioc.columbia.edu/pub/other |
| 5. | DOT | Global: FFT for shape complementarity and approximate Poisson–Boltzmann electrostatics | http://www.sdsc.edu/CCMS/DOT |
| 6. | AUTODOCK | Grid-based empirical potential flexible docking via Monte Carlo search and incremental construction | http://autodock.scripps.edu/ |
Conclusion
Diabetes and obesity both are the most prevailing disease worldwide at present time. These two diseases together create a complex condition and the therapy presents, are a need for more attention to the development of more effective therapy. There are many drugs floating in the market, but these drugs have some side effects which may cause another disease. Plants and edible mushrooms have been used from ancient time to cure these diseases. Due to great nutritional value, edible mushrooms are now becoming a powerful weapon against malnutrition and diseases. Various bioactive compounds of edible mushrooms can help to prevent obesity and diabetes. The bioactive compounds of edible mushrooms can be used with other therapy for the more effective results. Only a few compounds of edible mushrooms are known. There are many other compounds still to be identified in edible mushrooms, and its extraction and purification are also required. In silico approach has lightened a new path to develop more effective drugs, so that it can cure diabetes more efficiently.
Acknowledgements
SKD is thankful to the University Grants Commission (UGC), New Delhi, for granting research fellowship. SKD also acknowledge DBT-New Delhi facilities of Centre of Biotechnology, University of Allahabad, Prayagraj, India.
Authors’ contribution
SKD contributed in writing, drawing the figures and tables in this review article. VKC, DM, AB, and AT designed all the content of the article. MPS drafted and reviewed the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declared no conflict of interest with respect to the research, authorship, and publication of this article.
References
- Agarwal S, Vaseem H, Kushwaha A, Gupta KK, Maurya S, Chaturvedi VK, Pathak RK, Singh MP. Yield, biological efficiency and nutritional value of Pleurotussajor-caju cultivated on floral and agro-waste. Cell Mol Biol. 2016;62:1–5. doi: 10.4172/1165-158X.1000130. [DOI] [Google Scholar]
- Aida F, Shuhaimi M, Yazid M, Maaruf A. Mushroom as a potential source of prebiotics: a review. Trends Food Sci Technol. 2009;20:567–575. [Google Scholar]
- Alam N, Yoon KN, Lee JS, Cho HJ, Shim MJ, Lee TS. Dietary effect of Pleurotuseryngii on biochemical function and histology in hypercholesterolemic rats. Saudi J Biol Sci. 2011;18:403–409. doi: 10.1016/j.sjbs.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anai M, Funaki M, Ogihara T, Terasaki J, Inukai K, Katagiri H, Fukushima Y, Yazaki Y, Kikuchi M, Oka Y. Altered expression levels and impaired steps in the pathway to phosphatidylinositol 3-kinase activation via insulin receptor substrates 1 and 2 in Zucker fatty rats. Diabetes. 1998;47:13–23. doi: 10.2337/diab.47.1.13. [DOI] [PubMed] [Google Scholar]
- Anderson JW (1986) Dietary fiber in nutrition management of diabetes. In: Dietary fiber. Springer, pp 343–360
- Asrafuzzaman M, Rahman MM, Mandal M, Marjuque M, Bhowmik A, Rokeya B, Hassan Z, Faruque MO. Oyster mushroom functions as an antihyperglycaemic through phosphorylation of AMPK and increased expression of GLUT4 in type 2 diabetic model rats. J Taibah Univ Med Sc. 2018;13:465–471. doi: 10.1016/j.jtumed.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badole SL, Patel NM, Thakurdesai PA, Bodhankar SL. Interaction of aqueous extract of Pleurotus pulmonarius (Fr.) Quel-Champ. with glyburide in alloxan induced diabetic mice. J Evid Based Complement Altern Med. 2008;5:159–164. doi: 10.1093/ecam/nem010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin as adiposity signals. Recent Prog Horm Res. 2004;59:267–286. doi: 10.1210/rp.59.1.267. [DOI] [PubMed] [Google Scholar]
- Biswas G, Acharya K. Hypoglycemic activity of ethanolic extract of Astraeus hygrometricus (Pers.) Morg. in alloxan-induced diabetic mice. Int J Pharm Pharm Sci. 2013;5:391–394. [Google Scholar]
- Bordonaro M, Sartorelli AC. Fiber, cancer stem cells and the Wnt signalling continuum. Chin J Cancer. 2008;27:1–4. [PubMed] [Google Scholar]
- Brennan CS. Dietary fibre, glycaemic response, and diabetes. Mol Nutr Food Res. 2005;49:560–570. doi: 10.1002/mnfr.200500025. [DOI] [PubMed] [Google Scholar]
- Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11:577. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol (Lausanne) 2013;4:37. doi: 10.3389/fendo.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-J, Lin C-S, Lu C-C, Martel J, Ko Y-F, Ojcius DM, Tseng S-F, Wu T-R, Chen Y-YM, Young JD. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi: 10.1038/ncomms8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi VK, Agarwal S, Gupta KK, Ramteke PW, Singh MP. Medicinal mushroom: boon for therapeutic applications. 3 Biotech. 2018;8:334. doi: 10.1007/s13205-018-1358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi VK, Singh A, Dubey SK, Hetta HF, John J, Singh MP. Molecular mechanistic insight of hepatitis B virus mediated hepatocellular carcinoma. Microb Pathol. 2019;128:184–194. doi: 10.1016/j.micpath.2019.01.004. [DOI] [PubMed] [Google Scholar]
- Chen S-Y, Ho K-J, Hsieh Y-J, Wang L-T, Mau J-L. Contents of lovastatin, γ-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. LWT-Food Sci Technol. 2012;47:274–278. [Google Scholar]
- Chen F, Wen Q, Jiang J, Li H-L, Tan Y-F, Li Y-H, Zeng N-K. Could the gut microbiota reconcile the oral bioavailability conundrum of traditional herbs? J Ethnopharmacol. 2016;179:253–264. doi: 10.1016/j.jep.2015.12.031. [DOI] [PubMed] [Google Scholar]
- Chorvathova V, Bobek P, Ginter E, Klvanova J. Effect of the oyster fungus on glycaemia and cholesterolaemia in rats with insulin-dependent diabetes. Physiol Res. 1993;42:175. [PubMed] [Google Scholar]
- Cornu M, Thorens B. GLP-1 protects β-cells against apoptosis by enhancing the activity of an IGF-2/IGF1-receptor autocrine loop. Islets. 2009;1:280–282. doi: 10.4161/isl.1.3.9932. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Weigle DS, Frayo RS, Breen PA, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- Dangi-Garimella S. The role of bioinformatics in diabetes drug development and precision medicine. Am J Manag Care. 2014;20:E1. [PubMed] [Google Scholar]
- De Silva DD, Rapior S, Hyde KD, Bahkali AH. Medicinal mushrooms in prevention and control of diabetes mellitus. Fungal Divers. 2012;56:1–29. [Google Scholar]
- Delzenne NM, Bindels LB. Gut microbiota: Ganoderma lucidum, a new prebiotic agent to treat obesity? Nat Rev Gastroenterol Hepatol. 2015;12:553. doi: 10.1038/nrgastro.2015.137. [DOI] [PubMed] [Google Scholar]
- Delzenne NM, Neyrinck AM, Bäckhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol. 2011;7:639. doi: 10.1038/nrendo.2011.126. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Horvath TL. Limitations in anti-obesity drug development: the critical role of hunger-promoting neurons. Nat Rev Drug Discov. 2012;11:675. doi: 10.1038/nrd3739. [DOI] [PubMed] [Google Scholar]
- Ding Z, Lu Y, Lu Z, Lv F, Wang Y, Bie X, Wang F, Zhang K. Hypoglycaemic effect of comatin, an antidiabetic substance separated from Coprinus comatus broth, on alloxan-induced-diabetic rats. Food Chem. 2010;121:39–43. [Google Scholar]
- Elisashvili V, Wasser SP, Tan K-K. Hypoglycemic, interferonogenous, and immunomodulatory activity of Tremellastin from the submerged culture of Tremella mesenterica Retz.: Fr. (Heterobasidiomycetes) Int J Med Mushrooms. 2002;4:1–13. [Google Scholar]
- Enerbäck S. Brown adipose tissue in humans. Int J Obes (Lond) 2010;34:S43. doi: 10.1038/ijo.2010.183. [DOI] [PubMed] [Google Scholar]
- Feeney MJ, Dwyer J, Hasler-Lewis CM, Milner JA, Noakes M, Rowe S, Wach M, Beelman RB, Caldwell J, Cantorna MT. Mushrooms and health summit proceedings. J Nutr. 2014;144:1128S–1136S. doi: 10.3945/jn.114.190728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima M, Nakano M, Morii Y, Ohashi T, Fujiwara Y, Sonoyama K. Hepatic LDL receptor mRNA in rats is increased by dietary mushroom (Agaricusbisporus) fiber and sugar beet fiber. J Nutr. 2000;130:2151–2156. doi: 10.1093/jn/130.9.2151. [DOI] [PubMed] [Google Scholar]
- Funahashi T, Matsuzawa Y. Metabolic syndrome: clinical concept and molecular basis. Ann Med e. 2007;39:482–494. doi: 10.1080/07853890701491026. [DOI] [PubMed] [Google Scholar]
- Germain N, Galusca B, Le Roux CW, Bossu C, Ghatei MA, Lang F, Bloom SR, Estour B. Constitutional thinness and lean anorexia nervosa display opposite concentrations of peptide YY, glucagon-like peptide 1, ghrelin, and leptin. Am J Clin Nutr. 2007;85:967–971. doi: 10.1093/ajcn/85.4.967. [DOI] [PubMed] [Google Scholar]
- Ghaly IS, Ahmed ES, Booles HF, Farag IM, Nada SA. Evaluation of antihyperglycemic action of oyster mushroom (Pleurotusostreatus) and its effect on DNA damage, diabetic patients in Denmark. Diabetologia. 2011;55:294–302. [Google Scholar]
- Gray A, Flatt P. Insulin-releasing and insulin-like activity of Agaricus campestris (mushroom) J Endocrinol. 1998;157:259–266. doi: 10.1677/joe.0.1570259. [DOI] [PubMed] [Google Scholar]
- Guerciolini R. Mode of action of orlistat. Int J Obes Relat Metab Disord. 1997;12–23:21. [PubMed] [Google Scholar]
- Haan H, Den Fassihi A, Soto-iniesta J, Vegara-meseguer J, Montoro S, Pérez-sánchez H. Application of modern drug discovery techniques in the context of diabetes mellitus and atherosclerosis. Drug Des. 2015;4(1):1–2. doi: 10.4172/2169-0138.1000e125. [DOI] [Google Scholar]
- Han C, Yuan J, Wang Y, Li L. Hypoglycemic activity of fermented mushroom of Coprinus comatus rich in vanadium. J Trace Elem Med Biol. 2006;20:191–196. doi: 10.1016/j.jtemb.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Hansen M, Jensen M, Carstensen B. Causes of death among diabetic patients in Denmark. Diabetologia. 2012;55:294–302. doi: 10.1007/s00125-011-2383-2. [DOI] [PubMed] [Google Scholar]
- He P, Geng L, Wang J, Xu C. Characterization and bioactivities of exopolysaccharides produced by the wine cap culinary-medicinal mushroom, Strophariarugosoannulata 2#(higher basidiomycetes) Int J Med Mushrooms. 2012;14(4):365–376. doi: 10.1615/intjmedmushr.v14.i4.40. [DOI] [PubMed] [Google Scholar]
- Hsieh Y-L, Yao H-T, Cheng R-S, Chiang M-T. Chitosan reduces plasma adipocytokines and lipid accumulation in liver and adipose tissues and ameliorates insulin resistance in diabetic rats. J Med Food. 2012;15:453–460. doi: 10.1089/jmf.2011.1882. [DOI] [PubMed] [Google Scholar]
- Hu S-H, Wang J-C, Lien J-L, Liaw E-T, Lee M-Y. Antihyperglycemic effect of polysaccharide from fermented broth of Pleurotuscitrinopileatus. Appl Microbiol Biotechnol. 2006;70:107–113. doi: 10.1007/s00253-005-0043-5. [DOI] [PubMed] [Google Scholar]
- Huang H-Y, Korivi M, Yang H-T, Huang C-C, Chaing Y-Y, Tsai Y-C. Effect of Pleurotus tuber-regium polysaccharides supplementation on the progression of diabetes complications in obese-diabetic rats. Chin J Physiol. 2014;57:198–208. doi: 10.4077/CJP.2014.BAC245. [DOI] [PubMed] [Google Scholar]
- Hwang HS, Yun JW. Hypoglycemic effect of polysaccharides produced by submerged mycelial culture of Laetiporussulphureus on streptozotocin-induced diabetic rats. Biotechnol Bioprocess Eng. 2010;15:173–181. [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33:245. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jeong H-J, Yoon S-J, Pyun Y-R. Polysaccharides from edible mushroom hinmogi (Tremella fuciformis) inhibit differentiation of 3T3-L1 adipocytes by reducing mRNA expression of PPARγ, C/EBPα, and leptin. Food Sci Biotechnol. 2008;17:267–273. [Google Scholar]
- Jong-Wonyun JW, Lee YS, Song CH. A preliminary study on the hypoglycemic effect of the exo-polymers produced by five different medicinal mushrooms. J Microbiol Biotechnol. 2001;11:167–171. [Google Scholar]
- Kajimoto Y, Kaneto H. Role of oxidative stress in pancreatic β-cell dysfunction. Ann N Y Acad Sci. 2004;1011:168–176. doi: 10.1007/978-3-662-41088-2_17. [DOI] [PubMed] [Google Scholar]
- Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med. 2010;152:93–100. doi: 10.1059/0003-4819-152-2-201001190-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiho T, Sobue S, Ukai S. Structural features and hypoglycemic activities of two polysaccharides from a hot-water extract of Agrocybecylindracea. Carbohydr Res. 1994;251:81–87. doi: 10.1016/0008-6215(94)84277-9. [DOI] [PubMed] [Google Scholar]
- Kiho T, Kochi M, Usui S, Hirano K, Aizawa K, Inakuma T. Antidiabetic effect of an acidic polysaccharide (TAP) from Tremella aurantia and its degradation product (TAP-H) Biol Pharm Bull. 2001;24:1400–1403. doi: 10.1248/bpb.24.1400. [DOI] [PubMed] [Google Scholar]
- Kim SK, Hong UP, Kim JS, Kim CH, Lee KW, Choi SE, Park KH, Lee MW. Antidiabetic effect of Auricularia auricula mycelia in streptozotocin-induced diabetic rats. Nat Prod Sci. 2007;13:390–393. [Google Scholar]
- Kim J-I, Kang M-J, Im J, Seo Y-J, Lee Y-M, Song J-H, Lee J-H, Kim M-E. Effect of king oyster mushroom (Pleurotuseryngii) on insulin resistance and dyslipidemia in db/db mice. Food Sci Biotechnol. 2010;19:239–242. [Google Scholar]
- Koch A, Weiskirchen R, Zimmermann HW, Sanson E, Trautwein C, Tacke F. Relevance of serum leptin and leptin-receptor concentrations in critically ill patients. Mediators Inflamm. 2010;2010:1–9. doi: 10.1155/2010/473540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- Kwon A-H, Qiu Z, Hashimoto M, Yamamoto K, Kimura T. Effects of medicinal mushroom (Sparassiscrispa) on wound healing in streptozotocin-induced diabetic rats. Am J Surg. 2009;197:503–509. doi: 10.1016/j.amjsurg.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Lee I-K, Lee J-H, Yun B-S. Polychlorinated compounds with PPAR-γ agonistic effect from the medicinal fungus Phellinus ribis. Bioorg Med Chem Lett. 2008;18:4566–4568. doi: 10.1016/j.bmcl.2008.07.034. [DOI] [PubMed] [Google Scholar]
- Li S, Zhang G, Zeng Q, Huang Z, Wang Y, Dong T, Tsim K. Hypoglycemic activity of polysaccharide, with antioxidation, isolated from cultured Cordyceps mycelia. Phytomedicine. 2006;13:428–433. doi: 10.1016/j.phymed.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Li F, Zhang Y, Zhong Z. Antihyperglycemic effect of Ganoderma lucidum polysaccharides on streptozotocin-induced diabetic mice. Int J Mol Sci. 2011;12:6135–6145. doi: 10.3390/ijms12096135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T-H, Hou C-C, Chang CL-T, Yang W-C. Anti-hyperglycemic properties of crude extract and triterpenes from Poriacocos. J Evid Based Complement Altern Med. 2011;2011:1–8. doi: 10.1155/2011/128402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Li L, Fang JC, Wong JH, Ng TB, Jiang Y, Wang CR, Zhang NY, Wen TY, Qu LY. Isolation and identification of a novel polysaccharide–peptide complex with antioxidant, anti-proliferative and hypoglycaemic activities from the abalone mushroom. Biosci Rep. 2012;32:221–228. doi: 10.1042/BSR20110012. [DOI] [PubMed] [Google Scholar]
- Lin C-S, Chang C-J, Lu C-C, Martel J, Ojcius D, Ko Y-F, Young J, Lai H-C. Impact of the gut microbiota, prebiotics, and probiotics on human health and disease. Biomed J. 2014;37:259–68. doi: 10.4103/2319-4170.138314. [DOI] [PubMed] [Google Scholar]
- Lindequist U, Niedermeyer THJ, Jülich W-D. The pharmacological potential of mushrooms. Evid Based Complement Altern Med. 2005;2(3):285–299. doi: 10.1093/ecam/neh107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SH, Huang YW, Wu CT, Chiu CY, Chiang MT. Low molecular weight chitosan accelerates glucagon-like peptide-1 secretion in human intestinal endocrine cells via a p38-dependent pathway. J Agric Food Chem. 2013;61:4855–4861. doi: 10.1021/jf305410k. [DOI] [PubMed] [Google Scholar]
- Lo H-C, Tsai F-A, Wasser SP, Yang J-G, Huang B-M. Effects of ingested fruiting bodies, submerged culture biomass, and acidic polysaccharide glucuronoxylomannan of Tremella mesenterica Retz.: Fr. on glycemic responses in normal and diabetic rats. Life Sci J. 2006;78:1957–1966. doi: 10.1016/j.lfs.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Ma Y, Mao D, Geng L, Wang Z, Xu C. Production, fractionation, characterization of extracellular polysaccharide from a newly isolated Trametesgibbosa and its hypoglycemic activity. Carbohydr Polym. 2013;96:460–465. doi: 10.1016/j.carbpol.2013.04.019. [DOI] [PubMed] [Google Scholar]
- MacDonald PE, El-kholy W, Riedel MJ, Salapatek AMF, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002;51:434–442. doi: 10.2337/diabetes.51.2007.s434. [DOI] [PubMed] [Google Scholar]
- Makheswari U, Sudarsanam D. A review on bio informatics for diabetic mellitus. Int J Pharm Sci Res. 2012;3:389. [Google Scholar]
- Martel J, Ojcius DM, Chang C-J, Lin C-S, Lu C-C, Ko Y-F, Tseng S-F, Lai H-C, Young JD. Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nat Rev Endocrinol. 2017;13:149. doi: 10.1038/nrendo.2016.142. [DOI] [PubMed] [Google Scholar]
- Mohammed A, Adelaiye A, Bakari A, Mabrouk M. Anti-diabetic and some haematological effects of ethylacetate and n-butanol fractions of Ganoderma lucidum aqueous extract in alloxan-induced diabetic Wistar rats. Int J Med Med Sci. 2009;1:530–535. [Google Scholar]
- Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414:821. doi: 10.1038/414821a. [DOI] [PubMed] [Google Scholar]
- Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor γ agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- Niwa A, Tajiri T, Higashino H. Ipomoea batatas and Agarics blazei ameliorate diabetic disorders with therapeutic antioxidant potential in streptozotocin-induced diabetic rats. J Clin Biochem Nutr. 2011;2011:1101050064. doi: 10.3164/jcbn.10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel Y, Naraian R, Singh V. Medicinal properties of Pleurotus species (oyster mushroom): a review. World J Fungal Plant Biol. 2012;3:1–12. [Google Scholar]
- Patlak M. New weapons to combat an ancient disease: treating diabetes. FASEB J. 2002;16:1853e. [PubMed] [Google Scholar]
- Poddar KH, Ames M, Hsin-Jen C, Feeney MJ, Wang Y, Cheskin LJ. Positive effect of mushrooms substituted for meat on body weight, body composition, and health parameters. A 1-year randomized clinical trial. Appetite. 2013;71:379–387. doi: 10.1016/j.appet.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Preuss HG, Echard B, Bagchi D, Perricone NV, Zhuang C. Enhanced insulin-hypoglycemic activity in rats consuming a specific glycoprotein extracted from maitake mushroom. Mol Cell Biochem. 2007;306:105–113. doi: 10.1007/s11010-007-9559-6. [DOI] [PubMed] [Google Scholar]
- Ramanathan K, Karthick H, Arun N. Structure based drug designing for diabetes mellitus. J Proteomics Bioinform. 2010;3:310–313. doi: 10.4172/jpb.1000157. [DOI] [Google Scholar]
- Rony KA, Ajith TA, Mathew J, Janardhanan KK. The medicinal cracked-cap polypore mushroom Phellinus rimosus (higher Basidiomycetes) attenuates alloxan-induced hyperglycemia and oxidative stress in rats. Int J Med Mushrooms. 2013;15:287–300. doi: 10.1615/intjmedmushr.v15.i3.60. [DOI] [PubMed] [Google Scholar]
- Rui L. Brain regulation of energy balance and body weight. Rev Endocr Metab Disord. 2013;14:387–407. doi: 10.1007/s11154-013-9261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Tai T, Nunoura Y, Yajima Y, Kawashima S, Tanaka K. Dehydrotrametenolic acid induces preadipocyte differentiation and sensitizes animal models of noninsulin-dependent diabetes mellitus to insulin. Biol Pharm Bull. 2002;25:81–86. doi: 10.1248/bpb.25.81. [DOI] [PubMed] [Google Scholar]
- Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med. 2013;34:39–58. doi: 10.1016/j.mam.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Siddiqui AA, Siddiqui SA, Ahmad S, Siddiqui S, Ahsan I, Sahu K. Diabetes: mechanism, pathophysiology and management: a review. Int J Drug Dev Res. 2013;5:1–23. [Google Scholar]
- Sobngwi E, Ndour-Mbaye M, Boateng KA, Ramaiya KL, Njenga EW, Diop SN, Mbanya J-C, Ohwovoriole AE. Type 2 diabetes control and complications in specialised diabetes care centres of six sub-Saharan African countries: the Diabcare Africa study. Diabetes Res Clin Pract. 2012;95:30–36. doi: 10.1016/j.diabres.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Sonawane HB, Ghole V, Garad S, Bapat G, Bhosle S, Apte K. Hypoglycemic effect of Phansomba (Phellinus Badius Berk ex. Cooke) G. Cunn. on Alloxan-induced diabetic rats. J Nat Rem. 2013;13:29–34. [Google Scholar]
- Theuwissen E, Mensink RP. Water-soluble dietary fibers and cardiovascular disease. Physiol Behav. 2008;94:285–292. doi: 10.1016/j.physbeh.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Topping DL. Soluble fiber polysaccharides: effects on plasma cholesterol and colonic fermentation. Nutr Rev. 1991;49:195–203. doi: 10.1111/j.1753-4887.1991.tb03021.x. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Graham KL, Krishnamurthy B, Fynch S, Slattery RM, Kay TW, Thomas HE. Perforin facilitates beta cell killing and regulates autoreactive CD8 + T-cell responses to antigen in mouse models of type 1 diabetes. Immunol Cell Biol. 2016;94:334–341. doi: 10.1038/icb.2015.89. [DOI] [PubMed] [Google Scholar]
- Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Vitak T, Yurkiv B, Wasser S, Nevo E, Sybirna N. Effect of medicinal mushrooms on blood cells under conditions of diabetes mellitus. World J Diabet. 2017;8:187. doi: 10.4239/wjd.v8.i5.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(913–916):e917. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- Waki H, Tontonoz P. Endocrine functions of adipose tissue. Annu Rev Pathol Mech Dis. 2007;2:31–56. doi: 10.1146/annurev.pathol.2.010506.091859. [DOI] [PubMed] [Google Scholar]
- Wang C-Y, Liao JK. A mouse model of diet-induced obesity and insulin resistance. In: mTOR. Totowa: Springer; 2012. pp. 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC, Hu SH, Wang JT, Chen KS, Chia YC. Hypoglycemic effect of extract of Hericiumerinaceus. J Sci Food Agric. 2005;85:641–646. [Google Scholar]
- Wang L, Waltenberger B, Pferschy-Wenzig E-M, Blunder M, Liu X, Malainer C, Blazevic T, Schwaiger S, Rollinger JM, Heiss EH. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): a review. Biochem Pharmacol. 2014;92:73–89. doi: 10.1016/j.bcp.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Ideraabdullah FY. An assessment of molecular pathways of obesity susceptible to nutrient, toxicant and genetically induced epigenetic perturbation. J Nutr Biochem. 2016;30:1–13. doi: 10.1016/j.jnutbio.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamac M, Kanbak G, Zeytinoglu M, Bayramoglu G, Senturk H, Uyanoglu M. Hypoglycemic effect of Lentinusstrigosus (Schwein.) Fr. crude exopolysaccharide in streptozotocin-induced diabetic rats. J Med Food. 2008;11:513–517. doi: 10.1089/jmf.2007.0551. [DOI] [PubMed] [Google Scholar]
- Yamac M, Zeytinoglu M, Kanbak G, Bayramoglu G, Senturk H. Hypoglycemic effect of crude exopolysaccharides produced by Cerrena unicolor, Coprinus comatus, and Lenzitesbetulina isolates in streptozotocin-induced diabetic rats. Pharm Biol. 2009;47:168–174. [Google Scholar]
- Yamamoto K, Kimura T. Dietary Sparassiscrispa (Hanabiratake) ameliorates plasma levels of adiponectin and glucose in type 2 diabetic mice. J Health Sci. 2010;56:541–546. [Google Scholar]
- Yang B-K, Kim D-H, Jeong S-C, Das S, Choi YS, Shin JS, Lee SC, Song CH. Hypoglycemic effect of a Lentinusedodesexo-polymer produced from a submerged mycelial culture. Bio Sci Biotechnol Biochem. 2002;66:937–942. doi: 10.1271/bbb.66.937. [DOI] [PubMed] [Google Scholar]
- Yang B-K, Park J-B, Song C-H. Hypolipidemic effect of an exo-biopolymer produced from a submerged mycelial culture of Hericiumerinaceus. Biosci Biotechnol Biochem. 2003;67:1292–1298. doi: 10.1271/bbb.67.1292. [DOI] [PubMed] [Google Scholar]
- Yang BK, Jung YS, Song CH. Hypoglycemic effects of Ganoderma applanatum and Collybiaconfluensexo-polymers in streptozotocin-induced diabetic rats. Phytother Res Int J Devot Pharmacol Toxicol Eval Nat Product Deriv. 2007;21:1066–1069. doi: 10.1002/ptr.2214. [DOI] [PubMed] [Google Scholar]
- Yoon KN, Lee JS, Kim HY, Lee KR, Shin PG, Cheong JC, Yoo YB, Alam N, Ha TM, Lee TS. Appraisal of antihyperlipidemic activities of Lentinuslepideus in hypercholesterolemic rats. Mycobiology. 2011;39:283–289. doi: 10.5941/MYCO.2011.39.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H-D, Chung S-H. Protective effects of fermented ginseng on streptozotocin-induced pancreatic β-cell damage through inhibition of NF-κB. Int J Mol Med. 2010;25:53–58. [PubMed] [Google Scholar]
- Zhang G, Huang Y, Bian Y, Wong JH, Ng T, Wang H. Hypoglycemic activity of the fungi Cordyceps militaris, Cordyceps sinensis, Tricholomamongolicum, and Omphalialapidescens in streptozotocin-induced diabetic rats. Appl Microbiol Biotechnol. 2006;72:1152–1156. doi: 10.1007/s00253-006-0411-9. [DOI] [PubMed] [Google Scholar]
- Zheng L, Zhai G, Zhang J, Wang L, Ma Z, Jia M, Jia L. Antihyperlipidemic and hepatoprotective activities of mycelia zinc polysaccharide from Pholiotanameko SW-02. Int J Biol Macromol. 2014;70:523–529. doi: 10.1016/j.ijbiomac.2014.07.037. [DOI] [PubMed] [Google Scholar]