Abstract
Infants are vulnerable to pertussis infection particularly before initiation of pertussis vaccination. Maternal pertussis vaccination during pregnancy has been introduced in a number of countries in order to confer on young infants indirect protection from the disease through transplacental transfer of maternal antibodies. We reviewed the evidence on the immunogenicity and efficacy of maternal pertussis vaccination during pregnancy. A systematic search of PubMed/MEDLINE, EMBASE, Scopus, Cochrane Database of Systematic Reviews, ProQuest, and Science Direct was undertaken to identify studies published between January 1995 and December 2018. This review was not specific to any particular pertussis vaccine but included applicable data on available pertussis vaccines administered to pregnant women. The search identified 40 publications for inclusion in this review. Vaccination during pregnancy elicited robust maternal immune responses against all vaccine antigens and resulted in high placental transfer of pertussis antibodies to the infant that persisted well beyond delivery. Vaccination during the second or early third trimesters was considered ideal for antibody quantity and functionality. Although blunting of immune responses to some antigens in the primary immunization series was documented in neonates born to women vaccinated during pregnancy, there was no apparent adverse effect on vaccine efficacy. Multiple studies conducted in diverse settings have confirmed the effectiveness of maternal pertussis vaccination during pregnancy in preventing pertussis in infants prior to receipt of their first primary vaccine dose and beyond. These findings collectively underscore the value of maternal pertussis vaccination during pregnancy in protecting vulnerable infants too young to be vaccinated.
Funding Sanofi Pasteur.
Plain Language Summary Plain language summary available for this article.
Keywords: Efficacy, Immunization, Pertussis, Pregnancy
Plain Language Summary
Infants need protection against whooping cough particularly in the first few months after birth. Vaccination of women during pregnancy is recommended in some countries to protect the infant against the disease through the transfer of the mother’s protective antibodies. We reviewed the published evidence on the immunological and clinical benefits of maternal vaccination against whooping cough. Vaccination of pregnant women elicited robust maternal immune responses against whooping cough and resulted in high antibody levels transferred to the infant. Infants born to women who were vaccinated during pregnancy were better protected against whooping cough in the first few months after birth than those whose mothers were not vaccinated. The best time to vaccinate pregnant women was in the late second or early third trimesters. Although the infant’s immune responses to their primary immunizations were reduced in those born to women vaccinated during pregnancy, this did not affect vaccine effectiveness. This review confirms that maternal vaccination against whooping cough during pregnancy protects infants against the disease.
Introduction
Pertussis, whooping cough, results from infection of the upper respiratory tract by Bordetella pertussis. Infection resulting in at least mild disease was universal among children before the introduction of pertussis vaccination. Disease outbreak cycles occurred every 3–5 years, which primed most individuals through repeated exposure, and provided ongoing immunity after the first episode. Young infants were partially spared through indirect protection from maternal antibodies acquired in utero. Although routine pertussis vaccination of infants and young children reduced the incidence by more than 99% [1], the disease still persists today. In addition, there has been a shift in the age-specific disease profile with, in particular, an increased proportion of cases among infants aged < 1 year, adolescents and the elderly [2–7]. This age-specific shift has been attributed to waning of vaccine-induced immunity, and, in young infants, reduced pathogen circulation limiting natural boosting during pregnancy and subsequent passive protection. Infants (in particular those too young to have received the primary immunization series) have the highest rates of pertussis-related morbidity and mortality [6, 8–13].
Older siblings and adult close contacts are common reservoirs for pertussis transmission to young infants [14–16]. Strategies introduced to increase herd immunity have included booster doses for adolescents and close-contact adults. However, widening coverage with pertussis vaccines appears to have had little impact in preventing transmission to young infants or on the resurgence of periodic epidemic peaks in some countries in recent years [11, 13, 17]. Concerns that close contacts continue to remain common reservoirs for disease transmission to young infants have led to vaccination against pertussis during pregnancy [18–23]. Vaccination during pregnancy protects the mother directly, and, more importantly, provides passive protection (via transplacental antibody transfer in utero) for their young infants.
A number of clinical studies assessing the immunogenicity, efficacy and safety of maternal pertussis vaccination during pregnancy have recently been published. As such, it is timely to collate the available evidence and summarize the benefit–risk profile of pertussis vaccination during pregnancy. This will help informed decision-making and identify important gaps in the evidence that may require further investigation. This systematic review focuses on the immunological response to maternal pertussis vaccination during pregnancy as well as on vaccine efficacy in preventing infant pertussis. Maternal immunological responses, placental transfer of antibodies and infant responses to the primary immunization series are summarized. The safety of pertussis vaccination during pregnancy is the focus of an accompanying systematic review (D’Heilly et al., in preparation).
Methods
We conducted a systematic review of the English language literature, following the PRISMA framework [24], to identify studies reporting the immunogenicity and efficacy of pertussis vaccination (tetanus toxoid, reduced-dose diphtheria toxoid, and reduced-dose acellular pertussis [Tdap] vaccine and Tdap-inactivated polio vaccine [IPV]) during pregnancy. The review protocol was registered on PROSPERO (PROSPERO 2016:CRD42016038317) [25].
Our search initially encompassed the period from January 1995 to June 2016, and was later updated to capture relevant studies published between July 2016 and October 2018. Databases searched included PubMed/MEDLINE, EMBASE, Scopus (Elsevier), Cochrane Database of Systematic Reviews, ProQuest, and Science Direct. Reference lists of identified publications were also scanned. ProQuest thesis, Clinicaltrials.gov, Conferences and Congresses, and Trial Trove were searched for unpublished evidence. The search algorithms used were [1]: [(pertussis OR whooping cough) AND (vaccine OR Tdap OR immunization) AND (pregnancy OR pregnant OR pre-partum OR gestation OR maternal)], and [2]: [1] AND (immunogenicity OR immunity OR immune response OR antibody OR antibodies OR effectiveness OR efficacy).
Interventional and observational studies in humans were considered using the Patient Population, Intervention, Comparison, Outcomes, and Setting criteria [26]. The population was pregnant women and their offspring; the interventions were pertussis immunization during pregnancy and the infant series of vaccination; comparison groups were either no vaccination or standard-of-care vaccination, as well as pregnancy or no pregnancy; the outcomes were absolute and relative immunogenicity measures against pertussis vaccine antigens in maternal, umbilical cord, and/or infant blood, as well as infant pertussis antibody responses following each dose of the primary and toddler booster vaccination course, qualitative (clinical symptoms, severity) and quantitative [number, frequencies and relative (e.g., OR, RR)] disease outcome measures, including vaccine efficacy and/or vaccine effectiveness estimates. We excluded reviews, case reports, opinions and letters to editors, meta-analyses, modeling studies, and studies on vaccination program improvement, vaccine uptake increase or health economics, and vaccine acceptability and perception studies.
Selection of publications for inclusion was done in two steps, with two independent reviewers involved at each step. Titles and abstracts of retrieved publications were first screened for relevance. Publications documenting primary research (excluding modeling studies), and specifically dealing with outcomes of pre-partum pertussis vaccination, were retained for full-text review to determined compliance with inclusion and exclusion criteria. Relevant data from included studies were extracted using pre-structured MS Excel forms. Where possible, vaccine brand names are presented to help ascertain the number of pertussis components assessed. A meta-analysis of the immunologic or efficacy/effectiveness findings was not feasible because of heterogeneity in the study designs, vaccines used, measured outcomes, measurement/analysis methods, and background pertussis incidence rates.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
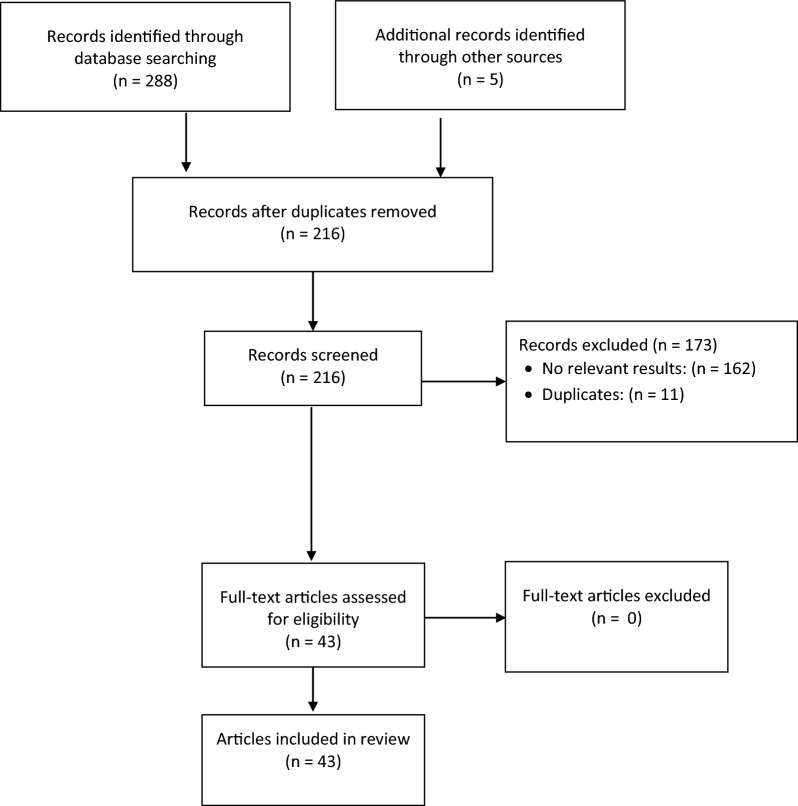
A total of 293 articles were identified, of which 43 articles (summarized in Tables 1 and 2, respectively) were included in this review (Fig. 1).
Table 1.
Characteristics and results of studies included in the immunogenicity systematic review
| References | Study design [setting] | Recruitment/study dates | Inclusion (exclusion) criteria | Interventions (number of participants) | Methods | Results |
|---|---|---|---|---|---|---|
| Gall et al. [44] | Prospective, case–control [University of Louisville Obstetrical Clinic, USA] | Oct 2008–Dec 2009 | PW |
Maternal Tdap (Sanofi Pasteur) during second trimester (n = 52)a No maternal Tdap (n = 52) |
Laboratory serology PT, FHA, pertactin, FIM IgG antibody titers at delivery in maternal and umbilical cord blood |
Neonates of Tdap vs. no Tdap PW: Antibody concentration (EU/mL): PT: 28.22 vs. 11.01 (p < 0.001) FHA: 104.15 vs. 26.83 (p = 0.002) Pertactin: 333.01 vs. 24.70 (p < 0.001) FIM 2/3: 1198.99 vs. 82.83 (p < 0.001) Seroprotection rates (defined as antibody concentrations for PT ≥5 EU/mL; FHA ≥3 EU/mL; Pertactin ≥5 EU/mL; FIM 2/3 ≥5 ELU/mL): PT: 88.5% vs. 40.4%; OR, 11.32 (95% CI, 4.10–31.24; p < 0.0001) FHA: 96.2% vs. 94.2%; OR, 1.53 (95% CI, 0.25–9.56; p = 0.6467) Pertactin: 96.2% vs. 86.5%; OR, 3.89 (95% CI, 0.77–19.70; p = 0.0812) FIM 2/3: 98.1% vs. 84.6%; OR, 9.27 (95% CI, 1.12–77.07; p = 0.0146) Other results: Maternal and umbilical cord antibody levels correlated: Pearson correlation coefficient 0.158 for PT (p = 0.055), 0.165 for FHA (p = 0.045), 0.965 for pertactin (p < 0.001) and 0.293 for FIM 2/3 (p < 0.001) |
| Eberhardt et al. [50] | Prospective, O, noninferiority, with HC [University Hospitals of Geneva, Switzerland] | Jul 2014–May 2015 | PW Tdap-vaccinated after GW 13 and delivering after GW 36 (Known or suspected immune deficiency or immunosuppressive therapy during the past 3mo, known exposure to pertussis [positive PCR/culture], prior pertussis immunization within 5y, major neonatal malformations) |
Maternal Tdap (Boostrix, GSK) during second trimester (13–25 GW) (n = 122) and 68 neonates Maternal Tdap (Boostrix, GSK) during third trimester (≥ 26 GW) (n = 213) and 90 neonates |
ELISA IgG PT, FHA IgG antibody GMCs at delivery in umbilical cord blood |
Neonates of second- vs. third-trimester Tdap: Antibody GMC (EU/mL): PT: 57.1 vs. 31.1 (p < 0.001) FHA: 284.4 vs. 140.2 (p < 0.001) Adjusted GMC ratios: PT: 1.9 (95% CI, 1.4–2.5; p < 0.001) FHA: 2.2 (95% CI, 1.7–3.0; p < 0.001) Seropositivity (PT antibody > 5 EU/mL) rate: 98% vs. 86% (p < 0.001) Seroprotection (defined as PT antibody concentrations > 30 EU/mL at birth) rate: 80% vs. 55% (adjusted OR: 3.7 [95% CI, 2.2–6.5; p < 0.001]) Other results: Optimal GMCs obtained: Vaccination 30–120 days before delivery Vaccination at 13–33 GW should confer infant seropositivity until 3mo of age |
| Gray et al. [31] | Prospective, cohort [USA] | NR |
Tdap vaccination (Immunologic/hematologic disease, immunosuppression, recent blood product use; PW only: multifetal gestation, anemia, vaccination not GW 26–36) |
Tdap (Adacel or Boostrix) during pregnancy (n = 17) Tdap (Adacel or Boostrix) – non-PW (n = 11) |
Magnetic bead multiplex assay FHA, PT, pertactin, FIM 2/3 IgG and IgA responses in maternal blood before (day 0) and 28 ± 7 days (day 28) post-vaccination |
PW vs. non-PW: Median fold-change in IgG antibodies day 0 to day 28: PT: 7.8 vs. 9.5 (p = 0.8) FHA: 13.0 vs. 10.4 (p = 0.4) Pertactin: 6.9 vs. 8.7 (p = 0.4) FIM 2/3: 1.4 vs. 99.8 (p = 0.048)I Median fold-change in IgA antibodies day 0 to day 28: PT: 2.7 vs. 3.8 (p = 0.1) FHA: 3.9 vs. 6.6 (p = 0.4) Pertactin: 2.8 vs. 2.3 (p = 1.0) FIM 2/3: 1.2 vs. 2.2 (p = 0.027)b |
| Ladhani et al. [53] | Prospective, O, single arm, with HC [General practices in the UK (Hertfordshire, Gloucestershire/South London)] | Dec 2012–Jul 2014 |
Infants born to PW vaccinated with Tdap-IPV HC: infants born to unvaccinated PW |
PW: Tdap-IPV (Repevax, Sanofi Pasteur) 28–38 GW Infants: DTaP-IPV-Hib (Pediacel, Sanofi Pasteur) + other standard vaccines (n = 141) PW: No Tdap Infants: DTaP-IPV-Hib (Pediacel, Sanofi Pasteur) + other standard vaccines (n = 246, HC) |
ELISA IgG PT, FHA, FIM antibody GMCs in infant blood at ages 2mo (before primary immunization series) and 5mo (3–6wk after third primary immunization) |
Before vs. after immunization in infants of Tdap PW: GMCs: PT: 11.2 vs. 28.8 FHA: 46 vs. 25.5 FIM 2/3: 123.2 vs. 113.9 Fold-ratio: PT: 2.64 (95% CI, 2.12–3.30; p < 0.001) FHA: 0.56 (95% CI, 0.48–0.65; p < 0.001) FIM 2/3: 0.82 (95% CI, 0.59–1.13; p = 0.22) Infants of Tdap vs. no Tdap PW: Fold-ratio: PT: 0.67 (95% CI, 0.58–0.77; p < 0.001) FHA: 0.62 (95% CI, 0.54–0.71; p < 0.001) FIM 2/3: 0.51 (95% CI, 0.42–0.62; p < 0.001) Effect of Tdap timing in PW: PT and FIM 2/3: Not associated with infant pre-immunization antibody concentrations or proportions achieving protective thresholds FHA: 1.08-fold increase (95% CI, 1.03–1.14) per wk pre-delivery (p = 0.002) |
| Gandhi et al. [30] | Retrospective, nested cohort [Ben Taub General Hospital, Houston, TX, USA] | Aug 2011–Aug 2013 |
PW vaccinated with Tdap with sufficient maternal and cord blood samples to allow pertussis IgG antibody assaysc (PW with multiple gestation) |
Tdap (Boostrix, GSK) at 28–32 GW (n = 123) (Normal BMI, n = 29; overweight BMI, n = 54; obese BMI, n = 40)c |
GenWay Bordetella pertussis IgG antibody test kit PT antibody concentrations in maternal and umbilical cord serum at delivery |
Tdap PW with normal BMI (n = 29) vs. overweight BMI (n = 54) vs. obese BMI (n = 40):b Mean maternal pertussis antibody concentration (U/ml): Normal: 167.5 U/mL Overweight: 169.8 Obese 175.5 (NS) Mean fetal pertussis antibody concentration (U/ml): Normal: 182.3 Overweight: 191.4 Obese: 197.7 (NS) Neonatal seroprotection (IgG level ≥ 30U/ml): Normal: 89.7% Overweight: 87.0% Obese: 97.5% (NS) |
| De Schutter et al. [59] | Prospective [Antwerp, Belgium] | Mar 2013–Jun 2014 |
Lactating post-partum women (PW who delivered prematurely or who had received another vaccine or any blood product in the previous mo; controls only: Tdap vaccine in previous 5y) |
Tdap (Boostrix, GlaxoSmithKline Biologicals) during pregnancy (19) Shortly after or at birth (cocoon strategy) (n = 34); < 5y before delivery (n = 9); ≥5y before delivery (n = 12) |
ELISA total sIgA and PT sIgA antibody GMCs in breast milk of lactating women 44–91 days post-partum (median 58 days) |
Tdap during pregnancy vs. shortly after or at birth (cocoon strategy) vs. < 5y before delivery vs. ≥5y before delivery: sIgA antibody GMCs: Total (mg/mL): 0.22 vs. 0.31 vs. 0.29 vs. 0.20 (NS) PT (IU/mL: 0.55 vs. 0.66 vs. 0.51 vs. 0.19 (p = 0.012 during vs. ≥5y; p = 0.001 cocoon vs. ≥5y). Other results: Effect of time since last feed: Total sIgA titer increased linearly with increasing time since last breastfeeding (p = 0.003, R2 value = 0.121) PT sIgA titer: not affected |
| Vilajeliu et al. [39] | Prospective, O [Maternal–Fetal Unit, Hospital Clinic of Barcelona, Spain] | May 2012–Aug 2013 |
PW aged ≥ 18 years, vaccination with DTaP at 20–36 GW pre- and post-vaccination maternal and neonatal determinations available Only one sample collected (from first newborn) if twin pregnancies |
DTap (Triaxis, Sanofi Pasteur MSD, France) at 20–36 GW (n = 132) | ELISA Testkit IgG/IgA PT IgG antibody GMTs in maternal blood before and ≥15 days after vaccination, and newborn blood; antibody titers in infants at 2mo of age estimated by linear interpolation using GMT in newborn samples |
Before vs. after Tdap in PW: PT antibody GMTs (IU/mL): Mean: 7.9 (95% CI 6.8–9.2) vs. 31.1 (95% CI 26.6–36.3) ≥10 IU/mL: 37.1% vs. 90.2% In infants of Tdap PW, birth vs. 2mo: PT antibody GMTs (IU/mL): Mean at birth: 37.8 (95% CI 32.3–44.1) ≥10 IU/mL: 94.7% vs. 66% Other results: Lin’s concordance index rate: Between post-vaccination maternal and newborn samples: 0.8 (95% CI 0.8–0.9) Transplacental transfer ratio: 146.6% Vaccine response: Not significantly affected by maternal age, timing of vaccination (≤37 vs. > 37 GW or time to delivery), maternal history of immune system disorders, twin pregnancy, or newborn sex |
| Abu Raya et al. [28] | Prospective [Obstetrics department, Bnai Zion Medical Center, Haifa, Israel] | Jan–Feb 2015 |
PW with singleton births born ≥ 36 GW (Underlying immunological disease, receipt of immunoglobulins or immunosuppressive drugs within 1y, receipt of blood products 3mo before delivery, receipt of pertussis-containing vaccine within 5y of current pregnancy or after delivery, receipt of any non-Tdap vaccine within 2wk of delivery, neonatal birthweight < 2000 g, documented or suspected pertussis infection after delivery) |
Tdap (Boostrix) at > 20 GW (23.1–37.4 GW; 6–115 days before delivery) (n = 38) No Tdap during pregnancy (n = 10) |
ELISA PT, FHA, pertactin IgG/IgA antibody GMCs in maternal blood at and 9–15mo after delivery |
Tdap vs. no Tdap PW: IgG antibody GMC (IU/mL): PT at delivery: 21.48 vs. 0.77 (p < 0.001) FHA at delivery: 185.95 vs. 12.02 (p < 0.001) Pertactin at delivery: 171.52 vs. 7.77 (p < 0.001) PT at 9–15mo: 11.72 vs. 1.41 (p = 0.007) FHA at 9–15mo: 140.33 (vs. 17.01 (p = 0.007) Pertactin at 9–15mo: 83.74 vs. 5.98 (p < 0.001). IgA antibody GMC (IU/mL): PT at delivery: 3.22 vs. 1.24 (p = 0.002) FHA at delivery: 30.16 vs. 2.42 (p < 0.001) PT at 9–15mo: 2.61 vs. 0.82 (p = 0.001) FHA at 9–15mo: 34.24 vs. 5.34 (p = 0.001) Other results: Tdap, but not no Tdap, PW had a decline in IgG PT, FHA and pertactin GMCs during follow-up (p ≤ 0.001) Neither group of PW had a significant decline in GMCs of IgA to PT between delivery and follow-up (p = 0.10 and p = 0.25, respectively); both groups had an increase in IgA FHA GMCs (p = 0.02 for both) |
| Huygen et al. [36] | Prospective, case–control [Antwerp, Belgium] | Oct 2012–Apr 2013 |
PW aged 18–40y, normal pregnancy, consenting to be immunized during pregnancy (New pregnancy, lost to follow-up) Controls: age-matched non-pregnant women |
Tdap (Boostrix, GSK) in the third trimester (25–32 GW) (n = 18) Tdap (Boostrix, GSK) in non-pregnant women (n = 16) |
ELISA PT, FHA, pertactin IgG antibody GMTs in maternal blood before, and 1mo and 1y after vaccination |
Before vs. 1mo after vs. 1y after Tdap in PW: Antibody GMC (IU/mL): PT: 6.1 vs. 52.7 (p < 0.001d) vs. 26.0 (p < 0.001d, p < 0.01e) FHA: 32.1 vs. 305 (p < 0.001d) vs. 148 (p < 0.001d,e) Pertactin: 59.2 vs. 667 (p < 0.001d) vs. 449 (p < 0.001d, p < 0.01e) Before vs. 1mo after vs. 1y after Tdap in non-PW: Antibody GMC (IU/mL): PT: 11.9 vs. 79.5 (p < 0.001d) vs. 28.3 (NSd, p < 0.05e) FHA: 38.1 vs. 319 (p < 0.001d) vs. 129 (p < 0.01d, p < 0.05e) Pertactin: 78.4 vs. 574 (p < 0.001d) vs. 368 (p < 0.001d, NSe) PW vs. non-PW: Antibody GMC: No significant difference for any antibodies at any time (p > 0.05) Vaccine responsiveness at 1mo: PT: 83.3% (15/18) vs. 81.3% (13/16) FHA: 100% vs. 100% Pertactin: 100% vs. 87.5% (14/16) Vaccine responsiveness at 1y: PT: 11.8% (2/17) vs. 18.2% (2/11) FHA: 94.1% (16/17) vs. 90.9% (10/11) Pertactin: 88.2% (15/17) vs. 63.6% (7/11) |
| Munoz et al. [38] | Randomized, double-blind, placebo-controlled [Three NIH VTEU sites in the USA (Houston, Durham, Seattle)] | Oct 2008–May 2012 |
PW aged 18–45y (Women who had previously received Tdap or any tetanus-containing vaccine within the prior 2 years, PW at high risk for obstetric complications) Non-PW age-matched to PW |
PW: Tdap (Adacel, Sanofi Pasteur) at 30–32 GW Infants: DTaP-IPV-Hib (Pentacel, Sanofi Pasteur) at 2, 4, 6, 12mo (33 PW and infants) PW: Placebo (Tdap [Adacel, Sanofi Pasteur] post-partum) Infants: DTaP-IPV-Hib (Pentacel, Sanofi Pasteur) at 2, 4, 6, 12mo (15 PW and infants) Non-PW: Tdap (Adacel, Sanofi Pasteur) (n = 32) |
ELISA PT, FHA, pertactin, FIM 2/3 IgG antibody levels in maternal/non-PW control blood before and 4wk after vaccination, at delivery (PW only), and 2mo after delivery (PW only); in umbilical cord blood at birth, and in infant blood at ages 2mo (before primary immunization), 7mo, and 13mo |
Tdap vs. no Tdap PW: Antibody concentration (EU/mL): PT before Tdap: 7.9 vs. 9.6 NS) FHA before Tdap: 15.1 vs. 23.2 (NS) Pertactin before Tdap: 8.7 vs. 13.2 (NS) FIM 2/3 before Tdap: 27.2 vs. 36.4 (NS) PT at delivery: 51.0 vs. 9.1 (p < 0.001) FHA at delivery: 184.8 vs. 21.9 (p < 0.001) Pertactin at delivery: 184.5 vs. 12.2 (p < 0.001) FIM 2/3 PT at delivery: 1485.7 vs. 34.9 (p < 0.001) PT 2mo after delivery: 53.1 vs. 66.4 (p < 0.001) FHA 2mo after delivery: 199.8 vs. 270.9 (NS) Pertactin 2mo after delivery: 158.8 vs. 210.1 (NS) FIM 2/3 2mo after delivery: 1274.8 vs. 2910.2 (NS) Infants of Tdap PW vs. of women vaccinated post-partum: Antibody concentration (EU/mL): PT at birth: 68.8 vs. 14.0 (p < 0.001) FHA at birth: 234.2 vs. 25.1 (p < 0.001) Pertactin at birth: 219.0 vs. 14.4 (p < 0.001) FIM 2/3 at birth: 1867.0 vs. 51.8 (p < 0.001) PT at 2mo: 20.6 vs. 5.3 (p < 0.001) FHA at 2mo: 99.1 vs. 6.6 (p < 0.001) Pertactin at 2mo: 71.1 vs. 5.2 (p < 0.001) FIM 2/3 PT at 2mo: 510.4 vs. 12.0 (p < 0.001) PT at 7mo: 64.9 vs. 96.6 (NS) FHA at 7mo: 40.6 vs. 78.6 (p < 0.01) Pertactin at 7mo: 72.3 vs. 77.9 (NS) FIM 2/3 at 7mo: 110.8 vs. 186.5 (NS) PT at 13mo: 80.1 vs. 83.9 (NS) FHA at 13mo: 69.9 vs. 108.9 (NS) Pertactin at 13mo: 203.3 vs. 115.2 (NS) FIM 2/3 at 13mo: 227.4 vs. 358.8 (NS) In infants of Tdap-vaccinated PW: PT, FHA, pertactin and FIM 2/3 concentrations in cord blood were 1.19–1.27 times higher than in maternal serum at delivery, with linear correlation between maternal and infant concentrations Tdap in PW vs. Tdap in non-PW: Antibody concentration (EU/mL): PT before Tdap: 7.9 vs. 17.6 FHA before Tdap: 15.1 vs. 30.1 Pertactin before Tdap: 8.7 vs. 15.4 FIM 2/3 before Tdap: 27.2 vs. 36.8 PT 4wk after Tdap: 56.5 vs. 90.9 FHA 4wk after Tdap: 234.4 vs. 285.6 Pertactin 4wk after Tdap: 205.0 vs. 348.7 FIM 2/3 PT 4wk after Tdap: 1533.2 vs. 1785.1 Other results: Antibody responses to Tdap in PW did not differ from those of non-PW- or PW vaccinated post-partum |
| Abu Raya et al. [27] | Prospective, cohort [Bnai Zion Medical Center, Haifa, Israel] | Nov 2013–May 2014 |
PW with singleton births born ≥ 36 GW (Immunologic disorder, receipt of immunoglobulins within 1y, receipt of immunosuppressive drugs during pregnancy or blood products within 3mo before delivery, documented or suspected pertussis infection within 5y, receipt of pertussis-containing Vaccine within 5y of current pregnancy, any non-Tdap vaccine within 2wk of delivery, neonatal birthweight < 2000 g) |
Tdap (Boostrix, GSK) at >20 GW (n = 61): 23–26 GW (n = 3) 27–36 GW (n = 51) >36 GW (n = 7) No Tdap (n = 20) |
ELISA PT, FHA, pertactin IgG and IgA antibody GMCs in maternal blood and umbilical cord blood at delivery |
Tdap vs. no Tdap PW: IgG antibody GMC (IU/mL): PT at delivery: 16.86 vs. 0.74 (p < 0.001) FHA at delivery: 187.42 vs. 13.42 (p < 0.001) Pertactin at delivery: 166.03 vs. 8.46 (p < 0.001) Tdap (“late” pregnancy) vs. no Tdap PW: IgA antibody GMC (IU/mL): PT at delivery: 3.01 vs. 1.19 (p = 0.001) FHA at delivery: 32.67 vs. 3.95 (p < 0.001) Infants of Tdap PW vs. of women vaccinated post-partum: IgG antibody GMC (IU/mL): PT at birth: 17.81 vs. 1.12 (p < 0.001) FHA at birth: 190.16 vs. 17.13 (p < 0.001) Pertactin at birth: 162.09 vs. 10.62 (p < 0.001) PT at birth Tdap 27–30 GW vs. 31–36 GW vs. > 36 GW: 46.04 vs. 8.69 vs. 21.12 (p < 0.02) FHA at birth Tdap 27–30 GW vs. 31–36 GW vs. > 36 GW: 225.86 vs. 178.31 vs. 138.03 (p < 0.02) Pertactin at birth Tdap 27–30 GW vs. 31–36 GW vs. > 36 GW: 170.77 vs. 155.34 vs. 172.86 (p > 0.49) Other results: Transplacental transfer ratio in Tdap PW for antibodies to PT: 1.3, FHA: 1.08, and pertactin: 1.03 PT and FHA IgG antibody GMCs significantly higher in umbilical cord blood when Tdap at 57–84 days before delivery vs. 1–28 days before delivery (p < 0.03). Estimated IgG PT (p < 0.03), FHA (p < 0.001), and pertactin (p > 0.73) antibody GMTs in infants at 108 days of age higher when Tdap at 27–30 GW vs. 31–36 GW and > 36 GW |
| Abu Raya et al. [58] | Prospective, cohort [Obstetrics department, Bnai Zion Medical Center, Haifa, Israel] | Nov 2013 –Feb 2014 |
PW with singleton births born ≥ 36 GW (Immunologic disorder, receipt of immunoglobulins within 1y, receipt of immunosuppressive drugs during pregnancy or blood products within 3mo before delivery, documented or suspected pertussis infection within 5y, receipt of pertussis-containing Vaccine within 5y of current pregnancy, any non-Tdap vaccine within 2wk of delivery, neonatal birthweight < 2000 g) |
Tdap (Boostrix, GSK) at > 20 GW (n = 25) No Tdap (n = 12) |
ELISA PT, FHA IgA and PT, FHA, pertactin IgG antibody GMCs in colostrum of lactating women at discharge, and in breast milk at 2, 4 and 8wk post-discharge |
Tdap vs. no Tdap: Antibody GMC (EU/mL): PT IgA at discharge: 8.18 vs. 5.17 (p = 0.35) FHA IgA at discharge: 24.12 vs. 6.52 (p = 0.01) PT IgA at 2wk: 1.01 vs. 1.12 (p = 0.72) FHA IgA at 2wk: 3.64 vs. 1.37 (p = 0.02) PT IgA at 4wk: 0.9 vs. 0.8 (p = 0.59) FHA IgA at 4wk: 2.7 vs. 1.54 (p = 0.15) PT IgA at 8wk: 1.01 vs. 1.11 (p = 0.8) FHA IgA at 8wk: 2.22 vs. 0.94 (p = 0.16) PT IgG: not detected in any samples at any time FHA IgG at discharge: 2.19 vs. 1.42 (p = 0.11) FHA IgG at 2wk: 1.44 vs. < 1 (p = 0.38) FHA IgG at 4wk: 1.44 vs. < 1 (p = 0.28) FHA IgG at 8wk: 1.4 vs. < 1 (p > 0.99) Pertactin IgG at discharge: 2.46 vs. < 0.6 (p = 0.03) Pertactin IgG at 2wk: 1.03 vs. < 0.6 (p = 0.12) Pertactin IgG at 4wk: 0.72 vs. < 0.6 (p = 0.4) Pertactin IgG at 8wk: < 0.6 vs. < 0.6 GMCs of PT and FHA IgA declined over 8wk in Tdap-vaccinated PW (p < 0.001); however, pertussis-specific IgA remained measurable at 8wk Pertussis-specific IgA was the predominant pertussis immunoglobulin in colostrum of Tdap-vaccinated women |
| Maertens et al. [46] | Prospective, controlled cohort [Five hospitals in Antwerp, Belgium] | Feb 2012–Sep 2014 | PW who had not received any pertussis-containing vaccine for ≥10y |
PW: Tdap (Boostrix, GSK) at mean 28.6 GW (n = 57) Infants: DTaP-IPV-Hib-HepB (Infanrix hexa, GSK Biologicals) at age 8, 12, and 16wk and 15mo (n = 55) PW: No Tdap (n = 42) Infants: DTaP-IPV-Hib-HepB (Infanrix hexa, GSK Biologicals) at age 8, 12, and 16wk and 15mo (n = 26) |
ELISA PT, FHA, pertactin IgG antibody GMCs in maternal blood before vaccination and at delivery, in umbilical cord blood, and infant blood at 8wk (before primary immunization) and 5mo (28–35 days after third primary immunization dose) |
Tdap vs. no Tdap PW: Antibody GMC (IU/mL): PT before Tdap: 4.5 vs. 7.5 (NS) FHA before Tdap: 21 vs. 17.6 (NS) Pertactin before Tdap: 24 vs. 16.9 (NS) PT at delivery: 31.4 vs. 6.4 (p < 0.001) FHA at delivery: 107 vs. 21.4 (p < 0.001) Pertactin at delivery: 602 vs. 18 (p < 0.001) Infants of Tdap vs. no Tdap PW: Antibody GMC (IU/mL): PT at birth: 100.7 vs. 12.4 (p < 0.001) FHA at birth: 140 vs. 27.5 (p < 0.001) Pertactin at birth: 697 vs. 21 (p < 0.001) PT before primary immunization: 15.5 vs. 1.1 (p < 0.001) FHA before primary immunization: 121 vs. 23 (p < 0.001) Pertactin before primary immunization: 253 vs. 17 (p < 0.001) PT after primary immunization: 29 vs. 54 (p < 0.001) FHA after primary immunization: 65 vs. 54 (NS) Pertactin after primary immunization: 68 vs. 87 (NS) Other results: Transplacental transfer ratio for antibodies to PT: 3.47, FIM: 1.81, and pertactin: 1.24 Umbilical cord antibody titers not affected by vaccination timing by GW (range not defined) |
| Maertens et al. [57] | Prospective, controlled cohort [Five hospitals in Antwerp, Belgium] | Delivery Apr 2012–Apr 2014 | Infants from the study of Maertens et al. (2016) born to PW who had not received any pertussis-containing vaccine for ≥10y |
PW: Tdap (Boostrix, GSK) at 18–34 GW (n = 57) Infants: DTaP-IPV-Hib-HepB (Infanrix hexa, GSK Biologicals) at age 8, 12, and 16wk and 15mo (n = 55) PW: No Tdap (n = 42) Infants: DTaP-IPV-Hib-HepB (Infanrix hexa, GSK Biologicals) at age 8, 12, and 16wk and 15mo (n = 24) |
ELISA PT, FHA, pertactin IgG antibody GMCs in infant blood 1–14 days before and 1mo after the fourth dose of immunization series (booster dose) |
Infants of Tdap vs. no Tdap PW: Antibody GMC (IU/mL): PT before booster immunization: 5.44 vs. 7.27 (p = 0.071) FHA before booster immunization: 14.83 vs. 15.98 (p = 636) Pertactin before booster immunization: 4.44 vs. 7.62 (p = 0.003) PT after booster immunization: 36.29 vs. 56.60 (p = 0.006) FHA after booster immunization: 100.86 vs. 139.42 (NS) Pertactin after booster immunization: 92.73 vs. 81.20 (NS) Other results: Antibody titers for all antigens increased after the fourth (booster) dose |
| Hardy-Fairbanks et al. [33] | Matched cohort [US-based health-care facility] |
Tdap in 2006 Controls: delivery Mar 2008–Feb 2009 |
Infants of DTaP-vaccinated PW Controls: infants of unvaccinated PW (Multiple gestations, serious underlying health issues in PW or infant, infants preterm or needing transfusions/advised not to have blood draws for health reasons) |
PW: Tdap (Adacel, Sanofi Pasteur) in any trimester (first n = 4, second n = 8, third n = 4) Infants: DTaP (multiple brands) (n = 16)f PW: No Tdap (n = 53) Infants: DTaP (multiple brands) (n = 53) |
ELISA PT, FHA, pertactin, FIM 2/3 antibody GMCs/GMTs in maternal serum and umbilical cord blood at delivery and infant serum collected before and 1mo after both primary and booster immunization |
Tdap vs. no Tdap in PW: Antibody concentration (EU/mL) (% with protective concentration): PT at delivery: 14.3 vs. 7.5 (75.0% vs. 55.1%) FHA at delivery: 32.5 vs. 9.6 (100% vs. 66.0%) Pertactin at delivery: 24.4 vs. 6.4 (80.0% vs. 35.8%) FIM 2/3 PT at delivery: 360.3 vs. 17.7 (100% vs. 61.5%) Infants of Tdap vs. no Tdap PW: Antibody concentration (EU/mL) (% with protective concentration): PT at birth: 33.5 vs. 12.6 (100% vs. 71.2%) FHA at birth: 66.1 vs. 15.9 (100% vs. 81.1%) Pertactin at birth: 48.5 vs. 8.9 (80.0% vs. 39.6%) FIM 2/3 PT at birth: 912.9 vs. 25.7 (100% vs. 69.8%) PT before primary immunization: 15.4 vs. 4.8 (83.3% vs. 31.3%) FHA before primary immunization: 41.6 vs. 5.6 (88.9% vs. 43.2%) Pertactin before primary immunization: 32.1 vs. 3.9 (77.8% vs. 17.6%) FIM 2/3 PT before primary immunization: 296.4 vs. 13.0 (100% vs. 58.6%) PT after primary immunization: 56.8 vs. 75.2 (100% vs. 100%) FHA after primary immunization: 61.4 vs. 83.6 (100% vs. 100%) Pertactin after primary immunization: 34.1 vs. 50.7 (93.3% vs. 93.9%) FIM 2/3 PT after primary immunization: 15.0 vs. 10.0 (66.7% vs. 40.0%) PT before booster immunization: 17.6 vs. 14.2 (87.5% vs. 85.2%) FHA before booster immunization: 24.5 vs. 22.7 (100% vs. 85.2%) Pertactin before booster immunization: 11.4 vs. 11.7 (62.5% vs. 48.1%) FIM 2/3 PT before booster immunization: 2.0 vs. 8.3 (0% vs. 37.0%) PT after booster immunization: 64.0 vs. 75.1 (92.3% vs. 100%) FHA after booster immunization: 86.9 vs. 93.2 (100% vs. 100%) Pertactin after booster immunization: 100.2 vs. 105.2 (92.3% vs. 92.3%) FIM 2/3 PT after booster immunization: 2.0 vs. 34.2 (0% vs. 65.4%) Other results: Antibody levels 2.0- to 2.5-fold greater in umbilical cord blood than in maternal blood at delivery |
| Abu Raya et al. [43] | Retrospective, cohort [Bnai Zion Medical Center, Haifa, Israel] | Nov 2013 –May 2014 |
PW with singleton births born ≥ 36 GW (Immunologic disorder, receipt of immunoglobulins within 1y, receipt of immunosuppressive drugs during pregnancy or blood products within 3mo before delivery, documented or suspected pertussis infection within 5y, receipt of pertussis-containing vaccine within 5y of current pregnancy, any non-Tdap vaccine within 2wk of delivery, neonatal birthweight < 2000 g, newborn umbilical cord sera PT IgG < 1 IU) |
Tdap (Boostrix) at 23–38 GW (6 to 115 days before delivery) (n = 52): 23–26 GW (n = 3) 27–36 GW (n = 43) >36 GW (n = 6) No Tdap (n = 8) |
ELISA PT IgG antibody RAI in umbilical cord blood at delivery |
Infants of Tdap PW “late” GW vs. no Tdap PW: PT RAI: 73.77% vs. 50.23% (p < 0.001) Infants of Tdap PW 27–30 GW vs. 31–36 GW vs. > 36 GW:g PT RAI: 79.53% vs. 71.56% (p < 0.04 vs. 27–30 GW) vs. 63.93% (p < 0.02 vs. 27–30 GW) Infants of Tdap PW 57–84 days vs. 29–56 vs. 1–28 days before delivery: PT RAI: 78.53 vs. 71.16 vs. 69.26 (p = 0.127 between groups) RAI of umbilical cord PT IgG increased linearly as function of time between Tdap and delivery (Pearson r = 0.346, p < 0.01) |
| Hoang et al. [35] | Randomized, controlled [Ha Nam province (3 villages), northern Vietnam] | Birth Feb 2013–Oct 2013 | PW |
PW: Tdap (Adacel, Sanofi Pasteur, Canada) at 18–36 GW (n = 52) Infants: DTaP-IPV-Hib-HepB (Infanrix hexa, GSK Biologicals) at age 2, 3, 4mo Tetanus vaccine (IVAC, Vietnam) (n = 51) Infants: DTaP-IPV-Hib-HepB at age 2, 3, 4mo |
ELISA PT, FHA, pertactin IgG antibody GMCs in maternal blood before and 1mo after vaccination, in maternal and umbilical cord blood at delivery, and in infants at 8wk (before primary immunization series) and 1mo after third dose of primary immunization series |
Tdap vs. no Tdap PW: Antibody concentration (EU/mL): PT before Tdap: 8.2 vs. 7.9 (NS) FHA before Tdap: 16.7 vs. 19.1 (NS) Pertactin before Tdap: 6.3 vs. 8.9 (NS) PT after 1mo: 33.1 vs. NR FHA after 1mo: 270 vs. NR Pertactin after 1mo: 229 vs. NR PT at delivery: 17.3 vs. 5.7 (p < 0.001) FHA at delivery: 139 vs. 17.3 (p < 0.001) Pertactin at delivery: 111 vs. 9.4 (p < 0.001) Infants of Tdap PW vs. of women vaccinated post-partum: Antibody concentration (EU/mL): PT at birth: 21 vs. 7.2 (p < 0.001) FHA at birth: 93 vs. 27.6 (p < 0.001) Pertactin at birth: 124 vs. 13.9 (p < 0.001) PT at 8wk: 4.2 vs. 0.8 (p < 0.001) FHA at 8wk: 59 vs. 23.1 (p < 0.001) Pertactin at 8wk: 46 vs. 7.8 (p < 0.001) PT after third DTaP dose: 70 vs. 67 (NS) FHA after third DTaP dose: 77 vs. 66.6 (NS) Pertactin after third DTaP dose: 83 vs. 132.6 (p = 0.006) Transplacental transport rate (cord/maternal titer at delivery): PT: 1.38 vs 1.73 (NS) FHA: 1.04 vs 1.83 (p < 0.001) Pertactin: 1.40 vs 1.73 (NS) |
| Maertens et al. [56] | Randomized, controlled [Ha Nam province (3 villages), northern Vietnam] | Birth Feb 2013–Oct 2013 | PW |
PW: Tdap (Adacel, Sanofi Pasteur, Canada) at 18–36 GW (n = 52) Infants: DTaP-IPV-Hib-HepB (Infanrix hexa, GSK Biologicals) at age 2, 3, 4, 18–25mo (n = 30) Tetanus vaccine (IVAC, Vietnam) (n = 51) Infants: DTaP-IPV-Hib-HepB at age 2, 3, 4, 18–25mo (n = 37) |
ELISA PT, FHA, pertactin IgG antibody GMCs in infants 1mo after third dose of primary immunization series and 1mo after fourth dose of immunization series (booster dose) |
Infants of Tdap PW vs. of women vaccinated post-partum: Antibody concentration (EU/mL): PT after third DTaP dose: 70 vs. 67 (NS) FHA after third DTaP dose: 77 vs. 66.6 (NS) Pertactin after third DTaP dose: 83 vs. 132.6 (p = 0.006) PT after booster DTaP dose: 129.0 vs. 133.7 (NS) FHA after booster DTaP dose: 161.3 vs. 181.7 (NS) Pertactin after booster DTaP dose: 159.0 vs. 187.1 (NS) |
| Healy et al. [41] | Prospective, O [Ben Taub General Hospital, Houston, TX, USA] | Jun 2009–May 2011 | Mother–newborn pairs with delivery at ≥ 37 GW, documented maternal Tdap within previous 2y |
Tdap during pregnancy (n = 19): <20 GW (n = 16) <6 GW (n = 11) Tdap before pregnancy (n = 83) |
ELISA PT, FHA, pertactin, FIM IgG antibody GMCs in maternal and umbilical cord blood at delivery |
Tdap during vs. before pregnancy PW: Antibody GMCs (EU/mL): PT: 10.5 vs 12.8 FHA: 49.3 vs 50.4 Pertactin: 40.4 vs 38.8 FIM: 103.1 vs 132.1 Infants of Tdap during vs. before pregnancy: Antibody GMCs (EU/mL): PT: 17.3 vs. 15.5 FHA: 87.6 vs. 72.9 Pertactin: 70.0 vs. 57.6 FIM: 191.8 vs 173.1 There were no differences in any pertussis-specific IgG antibody GMC in maternal or umbilical cord samples for women immunized before or during early pregnancy Placental transport of maternal pertussis-specific IgG (Tdap during vs. before): PT: 165% vs. 121% FHA: 178% vs. 145% Pertactin: 173% vs. 148% FIM: 186% vs. 131% |
| Villarreal Pérez et al. [40] | Randomized, double-blind, parallel group, placebo-controlled [12 outpatient health centers of the Nuevo Leon Health Services, Mexico] | Sep 2011–Aug 2014 |
PW aged 18–38y, low obstetric risk, normal anatomical ultrasound in second trimester (Psychiatric or severe physical disease, drug or tobacco use, history of severe reactions to any vaccine or febrile illness in the 72 h prior to vaccination, immunization against tetanus and/or pertussis < 2y previously) |
Tdap at 30–32 GW (89 PW and infants) Placebo (78 PW and infants) |
ELISA PT, pertactin IgG antibody GMCs in maternal blood before and > 4wk after vaccination at delivery, umbilical cord blood, infant blood at ages 2, 4, and 6mo |
Tdap vs. no Tdap PW: Antibody GMC (IU/mL): PT before Tdap: 5.93 vs. 7.90 (p = 0.138) Pertactin before Tdap: 8.53 vs. 8.08 (p = 0.908) PT at delivery: 24.04 vs. 7.06 (p = 0.001) Pertactin at delivery: 112.08 vs. 7.16 (p = 0.001) Infants of Tdap vs. no Tdap PW: Antibody GMC (IU/mL): PT at birth: 28.25 vs. 8.02 (p = 0.001) Pertactin at birth: 127.51 vs. 8.07 (p = 0.001) PT at 2mo: 10.95 vs. 6.20 (p = 0.001) Pertactin at 2mo: 71.41 vs. 6.93 (p = 0.001) PT at 4mo: 14.77 vs. 20.45 (p = 0.008) Pertactin at 4mo: 35.35 vs. 5.07 (p = 0.001) PT at 6mo: 49.09 vs. 69.13 (p = 0.007) Pertactin at 6mo: 16.75 vs. 4.51 (p = 0.001) |
| Eberhardt et al. [51] | Prospective, O [University Hospitals of Geneva, Switzerland] | Jul 2014–Feb 2016 | Neonates born before 37 GW with maternal Tdap vaccination in second or third trimester and no recorded pertussis booster within 5y previously |
Tdap vaccine (Boostrix, GSK) at 13–25 GW (n = 37) Tdap vaccine (Boostrix, GSK) at > 26 GW (n = 48) |
ELISA PT, FHA antibody GMCs in umbilical cord blood of preterm neonates |
Infants of Tdap second- vs. third-trimester PW: Antibody GMCs (EU/mL): PT: 41.3 vs. 22.1 (p = 0.024) FHA: 201.1 vs. 120.2 (p = 0.040) Antibody ratios: PT: 1.87 (95% CI, 1.06–3.29; p = 0.032) Adjustedh PT: 2.04 (95% CI, 1.15–3.61; p = 0.016) FHA: 1.67 (95% CI, 1.00–2.81; p = 0.051) Adjustedh FHA: 1.57 (95% CI, 0.93–2.67; p = 0.092) Seronegative rates: 0/37 vs. 11/48 (22.9%; p = 0.002) Infants of Tdap third-trimester PW by birth age (GW): Seronegative rates: GW 30–33: 38% vs. GW 34–36: 20% Other results: 15 days between Tdap and delivery sufficient to observe significantly higher umbilical cord antibody titers |
| Naidu et al. [47] | Prospective, O, cohort [Tertiary obstetric hospital, Melbourne, Australia] | Apr 2014–Sep 2014 |
Healthy PW with singleton pregnancy and Tdap vaccination at 28–36 GW (previous Tdap in current pregnancy, immunosuppression, high risk for preterm delivery) Controls: Unvaccinated PW |
Tdap (trivalent) at 28–32 GW (n = 42) Tdap at 33–36 GW (n = 45) No Tdap (n = 29) |
ELISA PT, FHA, pertactin IgG antibody concentrations in maternal blood before Tdap and umbilical cord blood at delivery; log-transformed because of skewed values |
Infants of Tdap PW 28–32 GW vs. 33–36 GW vs. no Tdap PW: Log transformed antibody concentration: PT: 4.18 vs. 3.50 vs. 2.80 (p < 0.001) FHA: 5.56 vs. 5.03 vs. 4.21 (p < 0.001) Pertactin: 5.83 vs. 5.31 vs. 4.9 (p = 0.001) Infants of Tdap PW 28–32 GW vs. 33–36 GW: Multivariate β coefficient antibody concentration adjusted for maternal pre-Tdap antibody levels: PT: 0.44:1 (p = 0.06) Pertactin: 0.44:1 (p = 0.03) FHA: 0.36 (p = 0.12) Other results: PT (Pearson correlation coefficient = 0.31; p = 0.004), FHA (0.30; p = 0.007), pertactin (0.23; p = 0.04) IgG antibody concentrations and number of wk of exposure linearly correlated |
| Vilajeliu et al. [52] | Prospective, O [Hospital Clinic of Barcelona, Spain] | Nov 2014 | Infants of PW aged ≥ 18y with Tdap vaccination 1–19wk before delivery |
Tdap (Triaxis, Sanofi Pasteur MSD, France) at 21–38 GW (n = 37): 21–26 GW (n = 3) 27–31 GW (n = 17) 32–36 GW (n = 13) 37–40 GW (n = 2) Not reported (n = 2) |
ELISA PT IgG antibody GMCs in umbilical cord blood at birth, and in blood from infants at age 1–2mo; antibody GMCs in infants at 2mo of age estimated by linear interpolation using GMT in umbilical cord and 1–2mo samples |
Infants of Tdap PW 21–26 GW vs. 27–31 GW vs. 32–36 GW vs. 37–40 GW: Antibody GMCs (IU/mL): PT at birth: not reported PT at 1–2mo: 29.9 vs. 52.5 vs. 62.5 vs. 83.7 PT at 2mo: 2.5 vs. 6.8 vs. 8.7 vs. 31.1 Change over time (1–2mo vs. 2mo) in infants of Tdap PW: Antibody GMCs (IU/mL): PT 52.7 vs. 7.5 (p < 0.001) Change in PT IgG antibody GMC by timing of Tdap significant only in infants of PW vaccinated at 27–31 GW (p = 0.001) or 32–36 GW (p = 0.009) Other results: PT IgG GMC not affected by time between Tdap and delivery (p = 0.1964) At 2mo 51.4% of infants estimated to have detectable titers and 29.7% titers ≥10 IU/mL Newborns of PW vaccinated with Tdap at ≥27 GW expected to sustain highest PT IgG antibody GMCs over time (p = 0.0842) |
| Kent et al. [54] | O [Neonatal units, England] | May 2012–May 2014 |
Premature infants (28–35 GW)–medically fit for vaccination, between 7 and 12 weeks of age born to PW eligible for pertussis vaccination in pregnancy (> 28 GW) |
PW: Tdap-IPV (Repevax; Sanofi Pasteur, France) at > 28 GW (n = 31) Infants born prematurely: DTaP-IPV-Hib (Pediacel, Sanofi Pasteur MSD) at age 2, 3, and 4mo + other standard vaccines PW: No Tdap (n = 121) Infants born prematurely: DTaP-IPV-Hib (Pediacel, Sanofi Pasteur MSD) at age 2, 3, and 4mo + other standard vaccines |
ELISA PT, FHA, FIM 2/3 IgG antibody GMCs in infant blood at age 2mo (before primary immunization series), 5mo (1mo after primary immunization series) and 12mo; concentrations were log transformed |
Infants of Tdap vs. no Tdap PW: Antibody GMCs (mcg/mL): PT at 2mo: 3.53 vs. 1.49 (p < 0.001) FHA at 2mo: 17.50 vs. 3.36 (p < 0.001) FIM2/3 at 2mo: 33.58 vs. 4.13 (p < 0.001 PT at 5mo: 37.15 vs. 44.07 (p = 0.35) FHA at 5mo: 23.04 vs. 45.55 (p = 0.003) FIM2/3 at 5mo: 119.55 vs. 135.14 (p = 0.72) PT at 12mo: 8.49 vs. 10.75 FHA at 12mo: 16.44 vs. 19.07 FIM2/3 at 12mo: 25.78 vs. 37.24 Other results: Number of days between maternal Tdap and delivery positively correlated with IgG concentration at 2mo for PT (4% increase in PT concentration per day; p = 0.011) and FHA (7%; p = 0.001), but not FIM2/3 (5%; p = 0.061) |
| Fallo et al. [29] | Prospective, O [D. F. Santojanni Public Hospital, Argentina] |
2011–2012 2013–2014 |
PW aged ≥ 18y, gave birth ≥ 37 GW, had singleton pregnancy, no underlying chronic medical conditions Controls: healthy non-PW aged 18–44y |
PW: Tdap at 24.7 ± 4.8 GW, > 15 days before delivery (105 PW and neonateI) PW: No Tdap (99 PW and neonates) Non-PW: No Tdap (n = 69) |
ELISA PT IgG antibody GMCs in maternal blood and in umbilical cord blood at delivery and in infants at ages 1mo and 2mo |
Tdap vs. no Tdap PW: Antibody GMC (EU/mL): PT: 35.1 vs. 9.8 (p < 0.0001) Antibody GMC < 5 EU/mL: PT: 2.9% vs. 16.1% (p < 0.001) Infants of Tdap vs. no Tdap PW: Antibody GMC (EU/mL): PT at birth: 51.3 vs. 11.6 (p < 0.0003) Antibody GMC < 5 EU/mL: PT: 1.9% vs. 16.1% (p < 0.0003) Placental antibody transfer efficiency: PT: 1.46 vs. 1.18 Infants of Tdap PW 13–19 vs. 20–23 GW vs. 24–27 GW vs. 28–31 vs. 32–36 GW: Antibody GMCs (IU/mL): PT at birth: 41.5 vs. 56.3 vs. 52.2 vs. 45.4 vs. 61.8 Infants of Tdap PW 13–25 vs. 26–36 GW: Antibody GMCs (IU/mL): PT at birth: 53.1 vs. 49.1 Difference between maternal or umbilical cord serum levels by GW at Tdap vaccination (NS) Change over time (birth vs. 1mo vs. 2mo) in infants of Tdap PW: Antibody GMCs (IU/mL): PT: 48.4 vs. 17.7 vs. 11.6 |
| Nadège Caboré et al. [55] | Prospective, controlled cohort [Five hospitals in Antwerp, Belgium]j | Apr 2012–Apr 2014 | Term infants of PW vaccinated with Tdap or unvaccinated |
PW: Tdap (Boostrix, GSK Biologicals) at 22–23 GW Infants: DTaP- IPV-Hib-HepB (Infanrix hexa, GSK Biologicals) 8, 12, 16wk, 15mo (n = 46) PW: No Tdap Infants: DTaP -IPV-Hib-HepB (Infanrix hexa, GSK Biologicals) 8, 12, 16wk, 15mo (n = 24) |
ELISA PT, FHA, pertactin IgG antibody avidity in infant blood before and 1mo after the fourth (booster) immunization dose |
Infants of Tdap vs. no Tdap PW: Geometric mean RAI (%): PT before: 55.40 vs. 59.64 (p = 0.201) FHA before: 47.82 vs. 50.13 (p = 0.761) Pertactin before: 44.13 vs. 46.89 (p = 0.582) PT after: 68.06 vs. 78.65 (p = 0.003) FHA after: 50.51 vs. 58.94 (p = 0.092) Pertactin: 59.05 vs. 64.82 (p = 0.347) |
| Abraham et al. [49] | Prospective, O, cohort [Hofstra University —Northwell Health System–Staten Island University Hospital, USA] | Jul 2015–Feb 2017 | PW aged 18–45y with singleton pregnancy and Tdap vaccination at 27–36 GW (serious underlying disease, history of febrile illness ≤72 h before Tdap, severe reaction to any vaccine, expected delivery < 37 GW, antenatal detection of major birth defect) |
Tdap (Adacel) at 27–30 GW (n = 52) Tdap (Adacel) at 31–35 GW (n = 36) |
ELISA PT, pertactin IgG antibody concentrations in maternal and umbilical cord blood at delivery |
Tdap PW 27–30 vs. 31–35 GW: Antibody concentration (EU/mL): PT: 48.6 vs. 48.6 (p = 0.99) Infants of Tdap PW 27–30 vs. 31–35 GW: Antibody concentration: PT (EU/mL): 92.1 vs. 90.7 (p = 0.95) Pertactin (IU/mL): 798 vs. 730 (p = 0.73) Antibody concentration > 10: PT (EU/mL): 87% vs. 97% (p = 0.13) Pertactin (IU/mL): 98% vs. 100% (p = 0.99) Other results: Umbilical cord vs. maternal PT IgG concentrations: 91.6 vs. 48.6 EU/mL (p < 0.01) and significantly correlated (Pearson correlation coefficient = 0.85; p < 0.01) No correlation between time from Tdap to delivery and maternal serum PT IgG, umbilical cord serum PT IgG, and umbilical cord serum pertactin IgG concentration |
| Fortner et al. [42] | Prospective, O, cohort [Two CDC-funded CISA centers (Vanderbilt University Medical Center, Duke University Health System), USA] | Jul 2014–Jul 2015 |
PW aged 18–45y with singleton pregnancy and Tdap vaccination at 20–33 GW Controls: non-PW aged 18–45y |
PW: Tdap (Adacel, Sanofi Pasteur or Boostrix, GSK) at 20–33 GW (n = 365) Non-PW: Tdap (Adacel, Sanofi Pasteur or Boostrix, GSK) (n = 222) |
ELISA PT, FHA, FIM2/3 k, pertactin IgG antibody GMCs in maternal/non-PW blood before and 28 days post-vaccination |
PW vs. non-PW: Antibody GMCs (IU/mL): PT at day 0: 8.7 vs. 9.6 (p = 0.14) FHA at day 0: 23.9 vs. 29.6 (p = 0.02) FIM2/3 at day 0: 61.4 vs. 98.2 (p < 0.01) Pertactin at day 0: 27.5 vs. 47.9 (p < 0.01) PT at day 28: 43.1 vs. 61.8 (p < 0.01) FHA at day 28: 114.8 vs. 145.0 (p < 0.01) FIM2/3 at day 28: 807.7 vs. 800.3 (p = 0.92) Pertactin at day 28: 261.3 vs. 264.4 (p = 0.89) |
| Halperin et al. [32] | Randomized, single-blind, parallel group, Td-controlled [Centers in Canada (Halifax, Montreal, Ottawa, Calgary, Edmonton, Vancouver)] | Nov 2007–Jun 2011 and Mar 2012–Apr 2014 | Healthy PW aged 18–45y at ≥ 30 GW, with low risk for complications (history of significant medical disorder or, in previous 5y, pertussis or previous Td/Tdap, receipt of high-dose systemic corticosteroids or, within 3mo, blood products or immunoglobulin, except rhesus immunoglobulin or, within 2wk, any vaccine, except influenza, or sensitivity to any component of Td or Tdap) |
PW: Tdap (Adacel, Sanofi Pasteur) at ≥ 30 GW (n = 135) Infants: DTaP-IPV-Hib (Pediacel Sanofi Pasteur) or DTaP-IPV-Hib-HepB (Infanrix hexa) 2, 4, 6, 12mo of age (n = 126) PW: Td (Sanofi Pasteur) at ≥ 30 GW (n = 138) Infants: DTaP-IPV-Hib (Pediacel Sanofi Pasteur) or DTaP-HepB-IPV-Hib (Infanrix hexa) 2, 4, 6, 12mo of age (n = 132) |
ELISA PT, FHA, FIM2/3 l, pertactin IgG antibody concentrations in maternal and umbilical cord blood at delivery, in maternal and infant blood at 2, 4, 6, 7, 12mo post-delivery and in infants 13mo post-delivery |
Tdap PW: Antibody concentrations (EU/mL): PT, FHA, pertactin, and FIM antibodies significantly higher vs. baseline at all postimmunization assessments (no p-values; graphical data) Peak levels reached by 2mo post-delivery then decreased by ≈50%–60% by 12mo Tdap vs. Td PW: Antibody concentrations (IU/mL): PT, FHA, pertactin, and FIM antibodies significantly higher after Tdap at all postimmunization assessments (no p-values; graphical data) Infants of Tdap vs. Td PW: Antibody GMCs (IU/mL): PT at delivery: 54.2 vs. 9.5 (p < 0.001) FHA at delivery: 184.2 vs. 21.4 (p < 0.001) FIM2/3 at delivery: 939.6 vs. 31.5 (p < 0.001) Pertactin at delivery: 294.1 vs. 11.2 (p < 0.001) PT at 2mo: 14.1 vs. 3.6 (p < 0.001) FHA at 2mo: 51.0 vs. 6.1 (p < 0.001) FIM2/3 at 2mo: 220.0 vs. 9.0 (p < 0.001) Pertactin at 2mo: 76.8 vs. 4.4 (p < 0.001) PT at 4mo: 11.7 vs. 11.5 FHA at 4mo: 23.5 vs. 11.3 (p < 0.001) FIM2/3 at 4mo: 89.7 vs. 6.4 (p < 0.001) Pertactin at 4mo: 28.1 vs. 6.6 (p < 0.001) PT at 6mo: 26.5 vs. 46.0 (p < 0.001) FHA at 6mo: 30.0 vs. 54.9 (p < 0.001) FIM2/3 at 6mo: 56.4 vs. 64.5 Pertactin at 6mo: 17.8 vs. 23.3 PT at 7mo: 56.9 vs. 77.3 (p = 0.002) FHA at 7mo: 50.8 vs. 84.0 (p < 0.001) FIM2/3 at 7mo: 90.9 vs. 232.3 (p < 0.001) Pertactin at 7mo: 40.0 vs. 67.9 (p < 0.001) PT at 12mo: 14.4 vs. 17.8 (p = 0.019) FHA at 12mo: 19.9 vs. 35.3 (p < 0.001) FIM2/3 at 12mo: 23.3 vs. 70.7 (p < 0.001) Pertactin at 12mo: 10.3 vs. 13.7 (p = 0.037) PT at 13mo: 55.6 vs. 70.2 (p = 0.016) FHA at 13mo: 69.3 vs. 101.8 (p < 0.001) FIM2/3 at 13mo: 146.4 vs. 349.8 (p < 0.001) Pertactin at 13mo: 114.2 vs. 101.7 Other results: Newborn to maternal antibody ratios were > 1 for PT (1.23), FHA (1.14) in Tdap PW-infant pairs, suggesting active transport of antibody across the placenta Tdap PW newborn antibody levels were noninferior to Td PW infant antibody levels at 6 mo (post–infant primary series dose 2) |
| Healy et al. [45] | Prospective, O, cohort [Pavilion for Women at Texas Children’s Hospital in Houston, TX, USA] | Dec 2013–Mar 2014 | PW delivering ≥37 GW, recorded Tdap at 27–36 GW and ≥14 days before delivery or recorded no Tdap during pregnancy (HIV- or syphilis-positive) |
PW: Tdap (n = 312) PW: No Tdap (n = 314) |
ELISA PT IgG antibody GMCs in umbilical cord blood at delivery |
Infants of Tdap vs. no Tdap PW: Antibody GMCs (IU/mL): PT at delivery: 47.3 vs. 12.9 (GMC ratio: 3.6; p < 0.001) Tdap at 30 GW resulted in the highest PT GMC: 57.3 Tdap at 28–30 GW: PT GMC > 50.0 Tdap at 31–36 GW: PT GMC 48.1–20.0 (decreasing with increasing GW) Infants achieving PT antibody cut-offs: ≥15 IU/mL: 86% vs 37%; difference, 49% (p < 0.001) ≥30 IU/mL: 72% vs 17%; difference, 55% (p < 0.001) ≥40 IU/mL: 59% vs 12%; difference, 47% (p < 0.001) Tdap at 28–31 GW resulted in the highest proportion of infants achieving each cut-off Estimated antibody GMC at age 2mo (IU/mL): PT: 11.8 vs. 3.2 (GMC ratio: 3.7; p < 0.001) Highest for Tdap at 30 GW (then 29, 28, 31, 27, 32, 33, 36, 34, 35 GW) |
| Hincapié-Palacio et al. [34] | O, cohort [17 hospitals in Medellin and metropolitan area of Antioquia, Colombia] | Dec 2015–Apr 2016 | PW delivering ≥37 GW with singleton pregnancy (fever in previous 72 h, admission to intensive care unit, or at an advanced stage of labor at recruitment) |
PW: Tdap (707 PW, 683 infants) PW: No Tdap (254 PW, 245 infants) |
ELISA PT IgG antibody GMCs in maternal and umbilical cord blood at delivery |
Tdap vs. no Tdap PW: Antibody GMCs (IU/mL): PT: 46.9 vs. 7.7 (p = 0.000) PW achieving PT antibody cut-offs: ≥100 IU/mL: 23.6% vs. 3.1% <100 IU/mL: 76.4% vs. 96.9% 41–100 IU/mL: 33.8% vs. 7.5% 5–40 IU/mL: 38.8% vs. 49.2% <5 IU/mL: 3.8% vs. 40.2% Infants of Tdap vs. no Tdap PW: Antibody GMCs (IU/mL): PT: 59.4 vs. 9.9 (p = 0.000) Infants achieving PT antibody cut-offs: ≥100 IU/mL: 30.7% vs. 4.1% <100 IU/mL: 69.3% vs. 95.9% 41–100 IU/mL: 32.5% vs. 9.4% 5–40 IU/mL: 33.7% vs. 51.8% <5 IU/mL: 3.1% vs. 34.7% Pre vs. post Tdap in PW (n = 141) Antibody GMCs (IU/mL): PT: 7.64 vs. 36.13 (p = 0.000) Other results: PT antibody GMC in maternal blood and umbilical cord blood positively correlated (Spearman correlation: 90%; p = 0.000) PT antibody GMC in maternal blood highest when Tdap at 31–36 GW (overall range 20–40 GW) PT antibody GMC in umbilical cord blood highest when Tdap at 26–30 GW (overall range 20–40 GW) Umbilical cord titers higher than maternal titers when Tdap 8–16 weeks before delivery, but lower when Tdap ≤4 weeks before delivery |
| Wanlapakorn et al. [48] | Prospective [King Chulalongkorn Memorial Hospital, Bangkok, Thailand] | Apr 2015–Sep 2016 | Healthy PW aged 18–45y, with low risk for complications; infants born after 36 GW and weighing 2500 g | PW: Tdap (Boostrix, GSK Biologicals) at 26–36 GW (n = 297) | ELISA PT, FHA, pertactin IgG antibody GMCs in maternal and umbilical cord blood at delivery |
Tdap PW: Antibody GMCs (IU/mL): PT: 42.9 FHA: 347.4 Pertactin: 125.3 Infants of Tdap PW: Antibody GMCs (IU/mL): PT: 48.6 FHA: 383.0 Pertactin: 128.8 Other results: Ratio umbilical cord/maternal blood > 1 for all pertussis antibody titers (PT: 1.18; FHA: 1.18; pertactin: 1.08) Pertussis antibody GMCs in maternal blood and umbilical cord blood positively correlated (Pearson’s correlation coefficient: 0.85–0.89; p < 0.001 for all) Pertussis antibody levels in umbilical cord blood higher with longer interval between Tdap and delivery (particularly Tdap 2–8 weeks before delivery, but less so Tdap 8–14 weeks before delivery) |
CDC Centers for Disease Control and Prevention, CISA Clinical Immunization Safety Assessment, DTaP diphtheria tetanus acellular pertussis vaccine, ELISA, enzyme-linked immunosorbent assay, EU ELISA units, FHA filamentous hemagglutinin, FIM fimbriae, GMC geometric mean concentration, GMT geometric mean titer, GW gestational weeks (either plus 6/7 days for outer limit or not specified), HC, historic control, HepB hepatitis B vaccine, Hib Haemophilus influenzae type b vaccine, Ig immunoglobulin, IPV inactivated poliovirus vaccine, IU international units, mo, month, NIH VTEU National Institutes of Health Vaccine Treatment Evaluation Unit, NR not reported, NS no significant difference between groups, O observational, OR odds ratio, PT pertussis toxin, PW pregnant women, RAI relative avidity index, Td tetanus and reduced-dose diphtheria vaccine, Tdap tetanus reduced-dose diphtheria and reduced-dose acellular pertussis vaccine, sIg secretory Ig, wk week, y year
aEncouraged timing of vaccination; however, exact timing could not be determined (some patients were vaccinated prior to pregnancy or at referring clinics)
bMore than 50% of non-pregnant women received Adacel, which contains FIM, whereas pregnant subjects most frequently received Boostrix, which contains no FIM (p = 0.33)
cBlood samples had been stored in PeriBank, a biobank that stores specimens collected during the perinatal period. Study groups were stratified by first trimester or pre-pregnancy BMI. BMI was defined as normal (BMI 18–24.9 kg/m2), overweight (BMI 25–29.9 kg/m2), and obese (BMI ≥ 30 kg/m2). Vaccination as per American College of Obstetricians and Gynecologists recommendations of 28–32 GW but exact timing was unknown
dIn comparison with pre-vaccination GMCs
eIn comparison with GMCs at 1 mo
fOnly 5 PW provided maternal serum and cord blood at delivery (first trimester n = 3, second trimester n = 2)
gPW vaccinated at 20–26 GW were excluded from analyses because of the small sample size (n = 3). Newborn umbilical cord RAI of PT IgG was 65.03%, 79.74% and 78.78%, respectively, for these women
hAdjusted for maternal age, gestational, age at birth, parity, and socioeconomic status
iA total of 36 of the 105 enrolled infants provided a blood sample at age 1 mo and 32 provided a blood sample at age 2 mo
jAnalysis of data from a convenience sample of patients enrolled in a previous study (Maertens et al. 2016)
kDetermined in recipients of Adacel only (PW, n = 359; non-PW, n = 205)
lDetermined in recipients of Pediacel only (Td group, n = 68–77; Tdap group n = 64–75)
Table 2.
Characteristics and results of studies included in the effectiveness systematic review
| References | Study design [setting] | Recruitment/study dates | Inclusion (exclusion) criteria | Interventions (number of participants) | Methods | Results |
|---|---|---|---|---|---|---|
| Dabrera et al. [63] | Case–control [General practice in England, Wales] | Oct 2012–Jul 2013 |
Infants aged < 8wk with PCR- or culture-confirmed pertussis infection Controls: infants with no pertussis born consecutively after case from same medical practice |
Cases (n = 58) Controls (n = 55) Tdap at 26–38 GW (n = 49) No Tdap (n = 64) |
Cases: infants aged < 8wk, PCR + or culture confirmed for Bordetella pertussis Controls: infant without pertussis born consecutively at same practice as case VE = 1–OR for maternal vaccination for cases compared with controls (× 100) |
Tdap in PW: Cases: 17% Controls: 71% OR for vaccination in PW: Unadjusted OR: 0.09 (95% CI, 0.03–0.23) VE: Unadjusted VE: 91% (95% CI, 77%–97%) Adjusted VEa: 93% (95% CI, 81%–97%) Other results: Length of hospital stay for cases did not differ between infants of Tdap-vaccinated PW (median 4 days) and infants of unvaccinated PW (3.5 days; p = 0.58) |
| Amirthalingam et al. [60] | Retrospective, cohort [Hospital admissions identified in the Public Health England surveillance database (CPRD)] | Jan 2008–Sep 2013 | Infants with laboratory-confirmed pertussis or whooping cough (ICD-10 codes A37·0, A37·1, A37·8, and A37·9) and know maternal vaccination status |
Cases aged < 3 mo (n = 82) Cases aged < 2 mo (n = 71) National coverage estimates For cases aged < 3 mo: Tdap during pregnancy (≥7 days before birth) (n = 12) No Tdap (n = 70) For cases aged < 2 mo: Tdap during pregnancy (≥7 days before birth) (n = 11) No Tdap (n = 60) |
Cases: infants with laboratory-confirmed pertussis or hospitalized for whooping cough (ICD-10 codes A37·0, A37·1, A37·8, and A37·9) and know maternal vaccination status VE = 1–OR for maternal vaccination for cases compared with estimates of vaccine coverage for the national population of PW (×100) |
Infants aged < 3 mo: Tdap in PW: Cases: 15% Average matched coverage: 62% VE: 91% (95% CI, 84%–95%) Tdap in PW: Cases: 15% Average matched coverage: 49% VE: 84% (95% CI, 71%–93%) Infants aged < 2 mo: Tdap in PW: Cases: 15% Average matched coverage: 61% VE: 90% (95% CI, 82%–95%) Tdap in PW: Cases: 15% Average matched coverage: 49% VE: 82% (95% CI, 67%–90%) |
| Baxter et al. [62] | Retrospective, cohort [Kaiser Permanente of northern California patient-integrated database, USA] | Birth: 2010–2015 |
Neonates born full-term [≥ 37 GW] + enrolled in Kaiser health plan by age 4mo + mothers continuously enrolled in Kaiser during pregnancy [confirmed Tdap status] + mothers born before 1996 [received whole-cell pertussis vaccines for primary series] + cases are PCR positive for pertussis [Missing GW data] |
Newborns: 148,981 Cases aged < 2 mo (n = 17) Cases aged < 12 mo (n = 103) Tdap during pregnancy (≥8 days before birth) (68,168; cases: 1) Tdap during pregnancy (1–7 days before birth) (1521; cases: 1) No Tdap (79,292; cases: 15) Infants: ± DTaP immunization |
Cases: Infants PCR + for pertussis VE = 1 – HR of pertussis in infants of PW vaccinated With Tdap (≥8 days before birth) versus those of unvaccinated PW (×100) |
Infants of Tdap vs. no Tdap PW: Unadjusted pertussis incidence ratio at 2mo: 0.08 (95% CI, 0.00–0.43) Unadjusted pertussis incidence ratio at 1y: 0.35 (95% CI, 0.21–0.55) VE: At 2mo: 91.4% (95% CI, 19.5–99.1; p = 0.032) At 1y: 69.0% (95% CI, 43.6–82.9; p < 0.001) VE (Tdap ± DTaP) at 1y: +0 DTaPb: 87.9% (95% CI, 41.4–97.5; p = 0.009) +1 DTaPc: 81.4% (95% CI, 42.5–94.0; p = 0.004) +2 DTaPc: 6.4% (95% CI, −165.1–66.9; p = 0.901) +3 DTaPc: 65.9% (95% CI, 4.5–87.8; p = 0.041) Other results: Maternal Tdap after pregnancy did not significantly reduce pertussis risk in infants (VE 24.1%) but maternal Tdap before pregnancy did significantly reduce pertussis risk (VE 55.6%; p = 0.007) |
| Romanin et al. [64] | Multi-center, matched case–control [Four hospitals in Argentina] | Sep 2012–Mar 2014 |
Infants ages < 2 mo with confirmed pertussis infection Controls: matched to cases by maternal health district |
Cases (n = 28) Controls (n = 109) Tdap during pregnancy (n = 98) No Tdap during pregnancy (n = 39) |
Cases: infants aged < 2 mo PCR + for pertussis Controls: Infants without pertussis matched to cases maternal health district in 5:1 ratio VE = 1 – OR for vaccination in pregnancy between cases and controls (×100) |
Tdap in PW: Cases: 43% Controls: 79% VE: 73.5% (95% CI, 38.4–88.6) |
| Winter et al. [67] | Retrospective, O, cohort [California Immunization Registry (CAIR), USA] | Birth 2013–2014 |
PW aged 14–44y, recorded Tdap vaccination during pregnancy or within 14 days post-partum (Birth < 27 GW or birth weight < 500 g) |
Newborns: 74,504 Cases aged < 8wk (n = 25) Cases aged ≤12wk (n = 35) Cases < 1 y (n = 119) Controls (35,959) Tdap during pregnancy (42,941): Tdap < 27 GW (6092), 27–36 GW (32,445), > 36 GW (3681) Tdap 0–14 days post-partumd (31,563) |
Cases: infants with clinical case definition for pertussis, or culture or PCR + for Bordetella pertussis VE = 1 – OR for pertussis in infants of women vaccinated during pregnancy compared with post-partum (×100) |
Infants of Tdap PW vs. Tdap post-partum: Pertussis illness: At age < 8wk: 0.02% vs. 0.05% (p = 0.01); OR: 0.36 (95% CI, 0.15–0.89) At age ≤12wk: 0.03% vs. 0.08% (p = 0.01); OR: 0.47 (95% CI, 0.24–0.92) At age < 12mo: 0.14% vs. 0.19% (p = 0.11) VE: At age < 8wk: 63.8% (95% CI, 10.6–85.4) At age ≤12wk: 53.0% (95% CI, 8.2–75.9) Infants of Tdap PW by timing OR for Tdap 27–36 GW vs. Tdap not 27–36 GW: 0.22 (95% CI, 0.08–0.63) VE in Infants of Tdap PW at 27–36 GW: At age < 8wk: 85.4% (95% CI, 33.0–96.7) At age ≤12wk: 71.6% (95% CI, 29.6–88.6) Other results: Infants of women vaccinated at 27–31 GW appeared to have the lowest OR for pertussis at < 8 or ≤12wk |
| Winter et al. [68] |
Retrospective, O, cohort [California Department of Public Health (CDPH), USA] |
Birth Jan 2011–Dec 2015 | Infants aged < 63 days with pertussis |
Infants aged < 63 days with pertussis (n = 420) Tdap during pregnancy (n = 49) No Tdap during pregnancy (n = 371) |
Cases: infants with hospital/ICU admission Controls: infants without hospital/ICU admission VE in infants with pertussis = 1 – OR of hospitalization/ICU admission in infants of PW vaccinated With Tdap versus those of unvaccinated PW (×100) |
Infants of Tdap vs. no Tdap PW: Hospitalization: 43% vs. 73% (p < 0.001); RR: 0.47 (95% CI, 0.35–0.63) Duration of hospital stay: 3 days vs. 6 days (p = 0.02) ICU admission: 13% vs. 30% (p = 0.01); RR: 0.80 (95% CI, 0.70–0.91) Death: 0 vs. 2 (p > 0.99) Adjustede OR for risk of hospitalization: 0.42 (95% CI, 0.20–0.85) Adjustede OR for risk of ICU admission: 0.49 (95% CI, 0.19–1.23) VE for preventing hospitalization: Unadjusted: 72.3 (95% CI, 49.0–85.0) Adjustede: 58.3 (95% CI, 14.9–79.6) VE for preventing hospitalization in infants of Tdap in third trimester PW: Unadjusted: 75.4 (95% CI, 49.8–88.0) Adjustede: 52.1 (95% CI, −0.16–80.3) |
| Amirthalingam et al. [61] | Retrospective, O, case control [General practice in England]f | Birth Oct 2012–Aug 2015 | Infants with maternal Tdap vaccination between 300 days prior to birth and ≤8wk after birth |
Newborns: 72,781 Infants aged < 3mo with pertussis (243) Infants aged < 2mo with pertussis (192) Infants aged < 24mo with pertussis TdaP: Td5aP-IPV (≥8 day before delivery) (n = 172) Td3aP-IPV (≥8 day before delivery) (n = 71) Most commonly ≥8wk before delivery |
Cases: infants with culture, serology/oral fluid testing or PCR + for pertussis Controls: matched population without pertussis VE = 1 – OR for maternal vaccination in cases compared with in the matched population (×100) |
Infants aged < 3 mo: Tdap in PW: Cases: 14.4% Average matched coverage: 64.8% VE: 91% (95% CI, 88%–94%) Tdap in PW: Cases: 14.4% Average matched coverage: 51.8% VE: 85% (95% CI, 78%–89%) Infants aged < 2 mo: Tdap in PW: Cases: 16.1% Average matched coverage: 64.3% VE: 90% (95% CI, 86%–93%) Tdap in PW: Cases: 16.1% Average matched coverage: 51.4% VE: 82% (95% CI, 74%–88%) Other results: VE did not differ significantly by vaccine (Td5aP-IPV vs. Td3aP-IPV) VE against death was estimated to be 95% (95% CI, 79–100) Maternal Tdap continued to offer protection to infants who had received a first (VE: 82% [95% CI, 65–91]) or second (VE: 69% [95% CI, 8–90]) primary DTaP dose; after completion of the primary immunization schedule, protection declined further but remained above 0% |
| Walls et al. [70] | Prospective observational cohort [Canterbury region of New Zealand] | Sep 2012–Nov 2014 | PW who received Tdap vaccine between 28 and 38 GW with ≥1 ultrasound during early pregnancy, adequate prenatal care, ± TIV (fetus with congenital/severe structural/chromosomal abnormalities during prenatal screening) | Tdap (Boostrix, GSK) during pregnancy (403 PW, 408 infants) | Clinical review |
Infants aged up to 12 months: No cases of pertussis; 9 infants exposed to a confirmed case of pertussis DTaP administered on time for first dose: 97.8%; second dose: 98.5%; third dose: 94.2% |
| Skoff et al. [66] | O, case–control [6 US Emerging Infection Program Network states]g | Jan 2011–Dec 2014 | Infants aged ≥2 days and < 2mo residing in catchment area at onset of cough, born in a hospital in state of residence, ≥ 37 GW at birth, not adopted, in foster care or living in a residential care facility |
Cases (n = 240) Controls (n = 535) Tdap before pregnancy (n = 91) Tdap during pregnancy (n = 139; 107 in third trimester) Tdap after pregnancy (n = 264) No Tdap (n = 281) |
Cases: infants aged ≥2 days and < 2mo with clinical case definition for pertussis, or culture or PCR + for pertussis Controls: infants without pertussis hospital-matched to cases and selected by birth certificate VE = 1–OR for vaccination in pregnancy between cases and controls (×100) |
Tdap in PW (third trimester): Cases: 7.1% Controls: 16.8% VE: 77.7% (95% CI, 48.3–90.4) Tdap in PW (first or second trimester): Cases: 2.1% Controls: 5.1% VE: 64.3% (95% CI, −13.8–88.8) Other results: VE for Tdap at 27–36 GW: 78.4% (95% CI, 49.8–90.7) VE for preventing pertussis-associated hospitalization: 90.5% (95% CI, 65.2– 97.4) No advantage to infants for maternal Tdap after delivery |
| Becker-Dreps et al. [69] | Retrospective, cohort [US commercial insurance claims database] | Birth 2010–2014 | Mother–infant pairs |
Mother–infant pairs (675,167) Tdap during pregnancy No Tdap |
Cases: infants with pertussis (not defined) aged ≤18 mo Controls: infants without pertussis |
Rate of pertussis Tdap vs. no Tdap in PW: 42% lower HR: 0.58 (95% CI, 0.38–0.89) Other results: No advantage to infants for maternal Tdap < 27 GW (HR: 1.06; 95% CI, 0.53–2.15) Rate of pertussis lower in infants if Tdap in third trimester Protection of infants seen only for periods 0–2 and 0–6 mo post-partum; no effect of Tdap (positive or negative) for 6–18 mo post-partum |
| Hincapié-Palacio et al. [34] | O, cohort [17 hospitals in Medellin and metropolitan area of Antioquia, Colombia] | Dec 2015–Apr 2016 | PW delivering ≥37 GW with singleton pregnancy (fever in previous 72 h, admission to intensive care unit, or at an advanced stage of labor at recruitment) |
PW: Tdap during pregnancy (745 PW, 686 infants) PW: No Tdap during pregnancy (260 PW, 210 infants) |
Follow-up of infants for 6 mo (home visits and symptom diaries); suspected cases confirmed by clinical case definition for pertussis and PCR + , epidemiology-linked to PCR + case or national surveillance guideline clinical criteria |
Infants of Tdap vs. no Tdap PW: Confirmed pertussis: 2 (0.3%) vs. 0 Probable pertussis: 17 (2.5%) vs. 5 (2.4%) Mothers of confirmed cases had PT antibody titers < 100 IU/mL (70 IU/mL and 3.5 IU/mL); umbilical cord blood titers of cases were 93 IU/mL and 4.0 IU/mL, respectively |
| Saul et al. [65] | O, case–control [New South Wales public health units, Australia] | Aug 2015–Aug 2016 | Infants aged < 6mo with pertussis notification (cases, n = 117) or born in a public hospital in the same local health district as case (matched control, n = 117) |
Cases (117) Controls (117) Tdap (Boostrix) during pregnancy (124) No Tdap during pregnancy (110) Pertussis resulting in hospitalization (severe pertussis): Cases (37) Controls (37) |
Cases: infants aged < 6 mo with laboratory-confirmed pertussis Controls: infants without pertussis or cough born in the same local health district within ±3 days of a case VE = 1 – OR for vaccination in pregnancy between cases and controls (×100) |
Infants aged < 6 mo: Tdap in PW: Cases: 44% Controls: 62% VE: 39% (95% CI, −12–66; p = 0.154) Infants aged < 3 mo: Tdap in PW: Cases: 40% Controls: 69% VE: 69% (95% CI, 13–89; p = 0.026) Severe pertussis: Tdap in PW: Cases: 32% Controls: 76% VE: 94% (95% CI, 59–91; p = 0.004) |
CPRD Clinical Practice Research Datalink, DTaP diphtheria tetanus acellular pertussis vaccine, dT3aP-IPV diphtheria–tetanus–3-component acellular pertussis–inactivated polio vaccine, dT5aP-IPV diphtheria–tetanus–5-component acellular pertussis–inactivated polio vaccine, GMT geometric mean titer, GW gestational weeks (either plus 6/7 days for outer limit or not specified), HR hazard ratio, IPV inactivated poliovirus vaccine, ICD International Classification of Diseases, IU international units, mo month, O observational, OR odds ratio, PCR polymerase chain reaction, PCR + , real-time polymerase chain reaction positive, PT pertussis toxin, PW pregnant women, Tdap tetanus reduced-dose diphtheria and reduced-dose acellular pertussis vaccine, TIV, trivalent influenza vaccine, VE vaccine effectiveness, wk week, y year
aAdjusted for sex, geographical region, and birth period
bFrom birth to day 7 after first DTaP dose
cFrom day 8 after DTaP dose to day 7 after next DTaP dose
dThere was no unvaccinated comparison group, and infants of women vaccinated post-partum was used as a surrogate for “no Tdap during pregnancy”. This approach will underestimate VE because infants born to these mothers are at lower risk of pertussis than those born to unvaccinated mothers
eAdjusted for infant chronological and gestational age
fData from primary care data sets [Immform, which measures coverage at national and subnational levels on a monthly basis using data held on computerized records from > 90% of general practices in England, and clinical practice research datalink (CRPD), a sentinel primary care data source representing about 6% of the UK population and including 520 English general practices]
gCalifornia, Connecticut, Minnesota, and New Mexico, and select counties of New York and Oregon
Fig. 1.
PRISMA diagram of results of search strategy
Immune Indicators
Maternal immune responses to pertussis antigens in both the mother and child were considered in this review. However, extrapolation of persisting antibody geometric mean concentrations (GMCs) to protection against pertussis is not possible as there is no agreed immunological correlate of protection.
Maternal Immune Responses
Vaccination with Tdap during pregnancy induces a robust maternal immune response (Table 1) [27–40], irrespective of maternal body mass index [30]. Pertussis antibody levels measured ≥ 1 month after Tdap immunization, or at delivery, were substantially higher against all vaccine antigens [pertussis toxin (PT), filamentous hemagglutinin (FHA), pertactin, and fimbriae (FIM)], in the Tdap-vaccinated group versus Tdap unvaccinated control groups across studies (Table 1). The higher antibody levels in Tdap-vaccinated women persisted through to delivery, and for up to 12–15 months post-delivery in some follow-up studies [28, 32].
Antibody responses to Tdap vaccination during pregnancy appear similar to those in non-pregnant women and women immunized post-partum [36, 38, 41]. However, although antibody GMTs increased for all antigens post-vaccination in both pregnant and non-pregnant women in one study [42], those against PT and FHA were significantly higher in non-pregnant women. The difference in the latter study may have simply reflected that a higher portion of non-pregnant women had received prior Tdap vaccination (65% vs. 53%).
Infant Serological Outcomes—Transfer of Antibodies
Newborn infants of mothers who received Tdap during pregnancy consistently had higher anti-pertussis antibody levels (antibodies to PT, FHA, pertactin and FIM) versus newborns of non-vaccinated mothers (p ≤ 0.002 across antibodies and studies; Table 1) [27, 29, 32, 34, 35, 38, 40, 43–47]. Estimates of cord to maternal blood antibody ratios from studies assessing transplacental antibody transfer ranged from 1.03 to 3.47 across the four pertussis vaccine antigens [27, 29, 32, 33, 35, 38, 39, 41, 46, 48]. A linear positive correlation was found between maternal and newborn’s antibody levels [29, 34, 38, 44, 49]. This correlation may be important for defining the optimal vaccination window during pregnancy since timing may impact the level of antibodies in the mother pre-partum and consequently the level of antibodies transferred to the infant at birth.
The relationship between the timing of Tdap vaccination during pregnancy has been explored, both with respect to gestational week and interval before delivery, and anti-pertussis antibody GMCs in the newborn. A study involving 105 pregnant women found no differences in umbilical cord serum levels and gestational week of Tdap vaccination (13–19 vs. 20–23 vs. 24–27 vs. 28–31 vs. 32–36 weeks) [29]. However, in a larger cohort (335 pregnant women), Eberhardt et al. [50] showed that vaccinating women in the second versus the third trimester resulted in higher umbilical cord antibody levels (p < 0.001) and infant seropositivity rates (PT antibody concentration > 30 EU/mL at birth; p < 0.001). When second trimester vaccination was compared with vaccination at 26–36 weeks gestation, superiority was again shown for second trimester vaccination [50].
Transplacental transfer of anti-pertussis antibodies to the newborns appears more efficient with maternal Tdap immunization earlier in the third trimester than later [27, 34, 43, 45, 47]. The transfer ratio was also found to be positively impacted by earlier Tdap vaccination [27, 34, 45]; for example, transfer ratio estimates fell from 1.12–1.45 across antigens in the group vaccinated between 27–30 weeks to 0.97–0.98 in the group vaccinated after 36 weeks of gestation [27]. The optimal time for vaccination was suggested as 30 gestational weeks, based on umbilical cord antibody GMCs and estimated GMCs in infants aged 2 months [45]. However, pertussis antibody concentrations in umbilical cord blood at delivery did not differ significantly between groups with maternal vaccination at 27–30 gestational weeks or at 31–35 gestational weeks in another study [49].
Although umbilical cord pertussis antibody levels were positively correlated with the interval between vaccination and delivery (a period of about 2–13 weeks of vaccine exposure was considered) [47], this correlation does not seem to extend to pertussis immunization very early in pregnancy. Healy et al. found that, although vaccination before pregnancy or early in pregnancy both resulted in increased antibodies at birth, there was no difference (p ≥ 0.45) between GMCs of newborns in the two groups [41]. Similarly, cord blood PT and FHA antibody GMCs were similar in infants whose mothers were immunized < 2 weeks prior to delivery or not immunized, suggesting that vaccination < 2 weeks prior to delivery was unlikely to effectively protect the newborn [50]. Optimal timing of vaccination, based on umbilical cord antibody GMCs, was determined to be 30–120 days before delivery (range considered < 15– > 150 days) [50], although 15 days was considered sufficient to significantly elevate umbilical cord antibody GMCs in a preterm population [51]. Other studies have suggest that vaccination ≥ 8 weeks before delivery may maximize antibody levels to pertussis antigens in umbilical cord blood [27, 34, 48], with the optimal timing being 57–84 days before delivery (range considered 1–84 days) [27]. Of note, the timing of vaccination may not only affect the quantity of antibodies transferred but also their functionality, since the relative avidity of antibodies in cord blood increased linearly with time between Tdap vaccination and delivery [43]. In contrast, two studies found no correlation between time from Tdap vaccination to delivery (range 3–21 weeks and about 2–14 weeks, respectively) and pertussis antibody concentrations in umbilical cord blood at delivery [39, 49].
Transplacentally acquired antibodies in early infancy decay over time up until receipt of the first dose of the primary pertussis vaccination series [29, 32, 33, 35, 38–40, 46, 52]. Two studies quantified this decay at 58–76% across the pertussis-specific antibodies [29, 38]. Nonetheless, the anti-pertussis antibody levels remained higher through the period before the first pertussis vaccine (generally 8 weeks), with 3.2–24.4-fold higher antibody GMCs persisting in infants of Tdap-vaccinated versus non-vaccinated mothers [32, 33, 35, 38, 40, 45, 46]. Using previously estimated half-life values for maternally acquired antibodies in newborns to model the persistence of anti-pertussis antibodies, it was determined that transferred maternal antibodies would remain detectable at age 2 months in 51–89% of infants born to mothers who received Tdap during pregnancy [39, 41, 52, 53]. These findings suggest that maternal pertussis immunization could bridge the susceptibility gap until the infant receives the first dose of pertussis vaccine.
Infant Immune Responses—Interference with Primary Vaccination
There is a theoretical risk that transplacentally acquired maternal antibodies may blunt the immune responses and thus the protection elicited by the primary diphtheria tetanus acellular pertussis vaccine (DTaP) immunization series. The available studies on the immunological effect found blunting of antibodies to at least one pertussis antigen following all or part of the primary vaccination series among infants whose mothers received pertussis immunization during pregnancy compared with infants of mothers who did not (Table 1) [33, 35, 38, 40, 46, 54]. Whether the blunting effect also persists with the infant booster vaccination is unclear. Some studies suggest that pertussis-specific antibody GMCs either remained lower [32, 55] or did not differ notably [33, 38, 56] 1 month after the booster dose in infants of mothers who received Tdap during pregnancy compared with those of mothers who did not. However, all of the latter studies had small sample sizes, which may have precluded detection of significant differences. In one study, FHA and pertactin antibody levels did not differ 1 month after the booster dose between infants of Tdap-vaccinated versus unvaccinated mothers, but PT antibody levels were significantly lower in the infants of vaccinated mothers [57]. Nonetheless, increases in all relevant pertussis antibody GMCs following the primary and/or booster immunization series are observed in the infants of mother immunized during pregnancy [33, 35, 46, 54, 56, 57].
A prospective cohort study conducted following implementation of the program of immunization of pregnant women in the UK with Tdap-IPV examined the issue of immunological blunting from a broader perspective, exploring the effect on the whole range of vaccines administered to infants in the first few months after birth [53]. Immunological responses to pertussis, diphtheria, tetanus, Haemophilus influenzae type B, pneumococcal, and meningococcal vaccines in infants of vaccinated mothers were compared with historical data from infants vaccinated before program implementation. Although infants born to vaccinated mothers achieved a 2.64-fold increase (p < 0.001) in PT antibodies 1 month after completion of the primary immunization program relative to GMCs before the first primary immunization dose, GMCs for antibodies to FHA and FIM were lower than pre-dose GMCs. In addition, response to all three pertussis antigens was blunted by 33–49% in comparison with the historical data (p < 0.001 for all). In the absence of a pertussis vaccine booster dose in the UK national immunization program, the study could not identify whether this blunting persisted with the booster in second year of life.
Antibody Transfer in Breast Milk
Limited data were identified evaluating the potential for antibody transfer through breast milk and it is unclear whether Tdap vaccination during pregnancy meaningfully affects pertussis antibody concentrations in colostrum or breast milk [58, 59]. While, the value of transfer of breast milk immunoglobulin (Ig)A for clinical protection remains unclear, the data are indicative that secretory antibodies generated by the vaccine are also likely to have been transferred in utero to the fetus and contribute to protection. Abu Raya et al. (2014) found that GMCs of IgA to PT were similar in the colostrum and breast milk of Tdap-vaccinated and unvaccinated women at weeks 2, 4 and 8 post-partum [58]. However, IgA to FHA was significantly higher in colostrum and breast milk from vaccinated women at 2 weeks. IgA to both pertussis antigens significantly declined over the 8-week study period. In the study by De Schutter et al. (2015), women vaccinated during pregnancy (p = 0.012) or at, or shortly after, birth (p = 0.001) had higher levels of secretory IgA to PT in breast milk at a median of about 58 days post-partum versus women not vaccinated for at least 5 years before delivery [59].
Effectiveness Measures
In infants aged < 2 or < 3 months, the effectiveness of pertussis immunization during pregnancy ranges from 53 to 93% in preventing pertussis (Table 2) [60–67]. In addition, Tdap during pregnancy reduces pertussis-related hospitalization in infants, with vaccine effectiveness estimated to be 58–94% [65, 66, 68]. Among hospitalized infants with pertussis, hospital stays were shorter in those whose mothers were vaccinated versus those of unvaccinated mothers (median 3 vs. 6 days; p = 0.02) and, notably, no infants of vaccinated mothers had seizures, required intubation, or died [68]. However, a much smaller study reported that the duration of hospitalization did not differ between infants whose mothers received pertussis vaccination and those of unvaccinated mothers (median 4 vs. 3.5 days; p = 0.58) [63]. Vaccine effectiveness against pertussis-related death was estimated to be 95% in one UK-based study [61].
Vaccine effectiveness was affected by the time between vaccination and delivery, and the gestational week at vaccination. Indeed, effectiveness appeared higher if maternal vaccination occurred during gestational weeks 27–36 than vaccination outside this period during pregnancy [66, 67]. No reduction in pertussis rates were observed in infants of mothers who received Tdap at < 27 gestational weeks in one study with infant follow-up to 18 months [69]. The protection afforded by vaccination prior to pregnancy or post-partum also appears at best low [62, 66]. In the UK program of maternal immunization, effectiveness was similar in infants aged < 2–3 months whose mothers were vaccinated 7–27 days before delivery and in those whose mothers were vaccinated earlier in pregnancy (≥ 28 days before delivery), but lower in those whose mothers were vaccinated between 6 days before and 13 days after delivery (91% vs. 91% vs. 38–43%, respectively) [60, 61].
Three studies investigated the clinical impact of potential immunological blunting of the infant’s response to their primary immunization series [61, 62, 70]. Two studies reported that the relative effectiveness of maternal immunization after each of the three doses of the primary series waned but remained positive, indicating the absence of a negative effect of maternal immunization on the protection afforded by the primary immunization, regardless of any immunological blunting (Table 2 [61, 62]). In the third study, which followed 408 infants of women vaccinated during pregnancy in New Zealand (94.2–98.5% received each of the three infant DTaP doses on time), no infant developed pertussis over a period of up to 12 months despite nine infants having contact with a confirmed case, and there being a high rate of pertussis in the community [70].
Discussion
The concept of “cocooning” was previously considered an appropriate intervention for preventing disease among infants too young to be vaccinated. Over time, it was realized that the broad vaccination coverage required to achieve acceptable protection of the newborn was unrealistic, at least beyond the parents and siblings. Given the resurgence of pertussis, a number of countries have introduced maternal pertussis vaccinations during pregnancy as a strategy to confer protection in young infants.
Our review confirms that maternal pertussis vaccination during pregnancy elicits robust and durable responses to all vaccine antigens similar to those observed in non-pregnant women. Moreover, maternal immunization results in efficient transplacental transfer of anti-pertussis antibodies to the fetus, demonstrated by higher antibody GMCs in cord blood than maternal blood. Pertussis-specific antibodies persisted in the infant and reduced the risk of disease during the period before childhood pertussis vaccination. Although a minimum interval of 2 weeks between maternal vaccine administration and delivery appears to be required, longer intervals, including vaccination during the second trimester or early in the third trimester, lead to higher antibody concentrations in newborn infants and allow for maturation of the immune response, thus improving the quality (i.e., avidity) of antibodies transferred to the fetus [43].
The available evidence suggests that maternal Tdap vaccine effectiveness is maintained during the infant’s primary pertussis vaccination series. It is encouraging that no negative impact on the effectiveness of the primary DTaP series was reported. However, maternal pertussis immunization causes a relative blunting of the infants’ immune response to pertussis antigens after primary immunization with acellular pertussis-component vaccines, although the specific antigen(s) affected varied across studies. Nevertheless, long term consequences of this blunting effect remain to be determined and may take several years to establish. Another question raised may be the potential consequences of blunting on other diseases and in other settings. In addition to reporting the immunogenicity of pertussis vaccine, a UK study identified blunting of responses to other antigens, particularly diphtheria toxoid or diphtheria protein-conjugated vaccines, although most infants (97.7% and 84.4%, respectively) achieved protective antibody concentrations [53]. Short- and long-term clinical consequences need to be ascertained, particularly as the strategy of immunization during pregnancy expands globally and may be introduced in settings where diphtheria remains a public health concern. It is also important to understand whether blunting occurs in infants receiving whole-cell pertussis primary series and whether there are associated short- and long-term consequences.
Additional research will also be needed to clarify whether the findings of this review are applicable to other pertussis vaccines currently in development. Genetically detoxified pertussis toxin-based vaccines are being developed to enhance immunogenicity against PT, both quantitatively and qualitatively. The impact of these vaccines on maternal immunization will need to be studied as part of their research and development. Finally, it should be noted that the overwhelming majority of pregnant women receiving pertussis vaccination were originally primed with whole-cell pertussis vaccines. Whole-cell pertussis vaccine priming induces a robust immune response after subsequent booster doses than does acellular pertussis vaccine priming. It therefore seems reasonable to question, as cohorts of women of child-bearing age shift from whole-cell pertussis vaccine to acellular pertussis vaccine priming, whether their responses to pertussis vaccination in pregnancy will remain robust enough to sufficiently protect the newborn.
Our systematic review has a number of limitations. The heterogeneity in immunological endpoints, the diagnostic assays, seroconversion thresholds, and criteria applied between the studies limit their comparability. In addition, the protective threshold of pertussis-specific antibodies remains to be established, which limits the interpretation of the antibody GMCs presented. A substantial number of studies reviewed were of small sample size, potentially affecting the external validity of the evidence, as well as the power of these studies to detect associations. The restriction to articles published in the English language may have caused some international studies to be overlooked. The strength of this review lies in our adherence to established methods for conducting systematic reviews, including extensive literature searching methods across several databases, and a wide inclusive publication date range. It is reassuring that, despite these limitations, the generally concordant findings between studies supports our conclusions.
There have been four recent reviews assessing the effectiveness and safety of pertussis vaccination in pregnancy [71–74]. Gkentzi et al. [71], McMillan et al. [72] and Furuta et al. [74] all performed reviews of the literature published up to May 2016, and Campbell et al. through to April 2017 [73]. Our review includes several more recently published studies not captured in the earlier reviews, reflecting the high general interest in maternal pertussis vaccination during pregnancy. The findings in the current review and our accompanying safety review (D’Heilly et al., preparation) are in concordance with the earlier reviews.
Conclusion
In conclusion, this systematic review provides evidence that maternal pertussis immunization with Tdap during pregnancy provides sufficient maternally-derived pertussis antibodies in infants, and protects infants aged < 2 or < 3 months against pertussis. Vaccine effectiveness was not adversely affected by the relative blunting of the infants’ immune response to some, but not all, pertussis antigens after primary immunization with acellular pertussis-component vaccines. However, there remains a need to better characterize the impact of maternal pertussis vaccination, so as to determine how it will evolve as the wider maternal population is exposed to pertussis vaccines during pregnancy, and how it will affect the epidemiology of the disease. Nonetheless, a number of countries now recommend maternal pertussis immunization as part of their overall pertussis control program.
Acknowledgements
Funding
This review and the journal’s rapid service fee were funded by Sanofi Pasteur.
Medical Writing and Editorial Assistance
Editorial assistance with the preparation of the manuscript was provided by Caroline Spencer on behalf of inScience Communications, Springer Healthcare, UK, and was funded by Sanofi Pasteur. The authors also thank Burnedette Rose Hill for editorial assistance and manuscript coordination on behalf of Sanofi-Pasteur.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Charlotte Switzer is an employee of Sanofi Pasteur. Caroline D’Heilly is an employee of Sanofi Pasteur. Denis Macina is an employee of Sanofi Pasteur.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.9742709.
References
- 1.Hewlett EL, Edwards KM. Clinical practice. Pertussis–not just for kids. N Engl J Med. 2005;352(12):1215–1222. doi: 10.1056/NEJMcp041025. [DOI] [PubMed] [Google Scholar]
- 2.Celentano LP, Massari M, Paramatti D, Salmaso S, Tozzi AE. Resurgence of pertussis in Europe. Pediatr Infect Dis J. 2005;24(9):761–765. doi: 10.1097/01.inf.0000177282.53500.77. [DOI] [PubMed] [Google Scholar]
- 3.McGirr AA, Tuite AR, Fisman DN. Estimation of the underlying burden of pertussis in adolescents and adults in Southern Ontario, Canada. PLoS ONE. 2013;8(12):e83850. doi: 10.1371/journal.pone.0083850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mink CA, Sirota NM, Nugent S. Outbreak of pertussis in a fully immunized adolescent and adult population. Arch Pediatr Adolesc Med. 1994;148(2):153–157. doi: 10.1001/archpedi.1994.02170020039006. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal S, Strebel P, Cassiday P, Sanden G, Brusuelas K, Wharton M. Pertussis infection among adults during the 1993 outbreak in Chicago. J Infect Dis. 1995;171(6):1650–1652. doi: 10.1093/infdis/171.6.1650. [DOI] [PubMed] [Google Scholar]
- 6.Skoff TH, Hadler S, Hariri S. The epidemiology of nationally reported pertussis in the United States, 2000–2016. Clin Infect Dis. 2018. [DOI] [PubMed]
- 7.Gutierrez-Trujillo G, Perez-Enriquez LR, Gonzalez-Garcia A, Coreno-Juarez MO, Ramirez-Rosales G, Grajales-Muniz C. Preventable diseases by vaccination: coverage and impact. Rev Med Inst Mex Seguro Soc. 2006;44(Suppl 1):S97–S109. [PubMed] [Google Scholar]
- 8.Pertussis epidemic–Washington (2012) Morb Mortal Wkly Rep. 2012;61(28):517–22. [PubMed]
- 9.Carlsson RM, von Segebaden K, Bergstrom J, Kling AM, Nilsson L. Surveillance of infant pertussis in Sweden 1998–2012; severity of disease in relation to the national vaccination programme. Euro Surveill. 2015;20(6). [DOI] [PubMed]
- 10.Haberling DL, Holman RC, Paddock CD, Murphy TV. Infant and maternal risk factors for pertussis-related infant mortality in the United States, 1999 to 2004. Pediatr Infect Dis J. 2009;28(3):194–198. doi: 10.1097/INF.0b013e31818c9032. [DOI] [PubMed] [Google Scholar]
- 11.Public Health England. Laboratory confirmed cases of pertussis reported to the enhanced pertussis surveillance programme in England: annual report 2013. 2014.
- 12.Straney L, Schibler A, Ganeshalingham A, Alexander J, Festa M, Slater A, et al. Burden and outcomes of severe pertussis infection in critically ill infants. Pediatr Crit Care Med. 2016;17(8):735–742. doi: 10.1097/PCC.0000000000000851. [DOI] [PubMed] [Google Scholar]
- 13.Winter K, Harriman K, Zipprich J, Schechter R, Talarico J, Watt J, et al. California pertussis epidemic, 2010. J Pediatr. 2012;161(6):1091–1096. doi: 10.1016/j.jpeds.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 14.Bisgard KM, Pascual FB, Ehresmann KR, Miller CA, Cianfrini C, Jennings CE, et al. Infant pertussis: who was the source? Pediatr Infect Dis J. 2004;23(11):985–989. doi: 10.1097/01.inf.0000145263.37198.2b. [DOI] [PubMed] [Google Scholar]
- 15.Wendelboe AM, Njamkepo E, Bourillon A, Floret DD, Gaudelus J, Gerber M, et al. Transmission of Bordetella pertussis to young infants. Pediatr Infect Dis J. 2007;26(4):293–299. doi: 10.1097/01.inf.0000258699.64164.6d. [DOI] [PubMed] [Google Scholar]
- 16.Skoff TH, Kenyon C, Cocoros N, Liko J, Miller L, Kudish K, et al. Sources of infant pertussis infection in the United States. Pediatrics. 2015;136(4):635–641. doi: 10.1542/peds.2015-1120. [DOI] [PubMed] [Google Scholar]
- 17.Abu-Raya B, Bettinger JA, Vanderkooi OG, Vaudry W, Halperin SA, Sadarangani M. Burden of children hospitalized with pertussis in Canada in the acellular pertussis vaccine era, 1999–2015. J Pediatr Infect Dis Soc. 2018. [DOI] [PMC free article] [PubMed]
- 18.Frere J, De Wals P, Ovetchkine P, Coic L, Audibert F, Tapiero B. Evaluation of several approaches to immunize parents of neonates against B pertussis. Vaccine. 2013;31(51):6087–6091. doi: 10.1016/j.vaccine.2013.09.043. [DOI] [PubMed] [Google Scholar]
- 19.Lim GH, Deeks SL, Crowcroft NS. A cocoon immunisation strategy against pertussis for infants: does it make sense for Ontario? Euro Surveill. 2014;19(5):20688. doi: 10.2807/1560-7917.ES2014.19.5.20688. [DOI] [PubMed] [Google Scholar]
- 20.Rossmann Beel E, Rench MA, Montesinos DP, Healy CM. Acceptability of immunization in adult contacts of infants: possibility of expanding platforms to increase adult vaccine uptake. Vaccine. 2014;32(22):2540–2545. doi: 10.1016/j.vaccine.2014.03.056. [DOI] [PubMed] [Google Scholar]
- 21.Simonetti A, Martini I, Bonomo G, D’Avino R, Puggina P, Vairo U, et al. Improving adherence rates to a cocooning program: a pilot experience in Italy. Hum Vaccin Immunother. 2013;9(5):1142–1145. doi: 10.4161/hv.23795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skowronski DM, Janjua NZ, Tsafack EP, Ouakki M, Hoang L, De Serres G. The number needed to vaccinate to prevent infant pertussis hospitalization and death through parent cocoon immunization. Clin Infect Dis. 2012;54(3):318–327. doi: 10.1093/cid/cir836. [DOI] [PubMed] [Google Scholar]
- 23.Urwyler P, Heininger U. Protecting newborns from pertussis—the challenge of complete cocooning. BMC Infect Dis. 2014;14:397. doi: 10.1186/1471-2334-14-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Switzer C, Macina D. Safety and effectiveness of pertussis immunization during pregnancy. Available at: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42016038317. Last accessed 16 February 2019.
- 26.O’Connor D, Green S, Higgins JPT. Chapter 5: Defining the review question and developing criteria for including studies. Cochrane handbook for systematic reviews of interventions. Version 5.0.0 ed2008 ed2008.
- 27.Abu Raya B, Srugo I, Kessel A, Peterman M, Bader D, Gonen R, et al. The effect of timing of maternal tetanus, diphtheria, and acellular pertussis (Tdap) immunization during pregnancy on newborn pertussis antibody levels—a prospective study. Vaccine. 2014;32(44):5787–5793. doi: 10.1016/j.vaccine.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 28.Abu Raya B, Srugo I, Kessel A, Peterman M, Vaknin A, Bamberger E. the decline of pertussis-specific antibodies after tetanus, diphtheria, and acellular pertussis immunization in late pregnancy. J Infect Dis. 2015;212(12):1869–1873. doi: 10.1093/infdis/jiv324. [DOI] [PubMed] [Google Scholar]
- 29.Fallo AA, Neyro SE, Manonelles GV, Lara C, Hozbor D, Zintgraff J, et al. Prevalence of pertussis antibodies in maternal blood, cord serum, and infants from mothers with and those without Tdap booster vaccination during pregnancy in Argentina. J Pediatric Infect Dis Soc. 2018;7(1):11–17. doi: 10.1093/jpids/piw069. [DOI] [PubMed] [Google Scholar]
- 30.Gandhi M, Devaraj S, Sangi-Haghpeykar H, Mastrobattista J. The effect of body mass index on post-vaccination maternal and neonatal pertussis antibody levels. J Reprod Immunol. 2015;112:34–37. doi: 10.1016/j.jri.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Gray L, Quataert S, Secor-Socha S, Thornburg L, Grzesik K, Muthukrishnan G, et al. Antibody responses to pertussis vaccination in pregnant versus non-pregnant subjects. Reprod Sci. 2016;23(Suppl 1):82A. [Google Scholar]
- 32.Halperin SA, Langley JM, Ye L, MacKinnon-Cameron D, Elsherif M, Allen VM, et al. A randomized controlled trial of the safety and immunogenicity of tetanus, diphtheria, and acellular pertussis vaccine immunization during pregnancy and subsequent infant immune response. Clin Infect Dis. 2018;67(7):1063–1071. doi: 10.1093/cid/ciy244. [DOI] [PubMed] [Google Scholar]
- 33.Hardy-Fairbanks AJ, Pan SJ, Decker MD, Johnson DR, Greenberg DP, Kirkland KB, et al. Immune responses in infants whose mothers received Tdap vaccine during pregnancy. Pediatr Infect Dis J. 2013;32(11):1257–1260. doi: 10.1097/INF.0b013e3182a09b6a. [DOI] [PubMed] [Google Scholar]
- 34.Hincapie-Palacio D, Hoyos MC, Ochoa J, Montoya N, Garcia D, Osorio E. Effect of maternal immunization against pertussis in Medellin and the metropolitan area, Colombia, 2016–2017. Vaccine. 2018;36(27):3984–3991. doi: 10.1016/j.vaccine.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 35.Hoang HT, Leuridan E, Maertens K, Nguyen TD, Hens N, Vu NH, et al. Pertussis vaccination during pregnancy in Vietnam: results of a randomized controlled trial of pertussis vaccination during pregnancy. Vaccine. 2016;34(1):151–159. doi: 10.1016/j.vaccine.2015.10.098. [DOI] [PubMed] [Google Scholar]
- 36.Huygen K, Cabore RN, Maertens K, Van Damme P, Leuridan E. Humoral and cell mediated immune responses to a pertussis containing vaccine in pregnant and nonpregnant women. Vaccine. 2015;33(33):4117–4123. doi: 10.1016/j.vaccine.2015.06.108. [DOI] [PubMed] [Google Scholar]
- 37.Maertens K, Hoang TT, Nguyen TD, Cabore RN, Duong TH, Huygen K, et al. The effect of maternal pertussis immunization on infant vaccine responses to a booster pertussis-containing vaccine in Vietnam. Clin Infect Dis. 2016;63(suppl 4):S197–S204. doi: 10.1093/cid/ciw551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munoz FM, Bond NH, Maccato M, Pinell P, Hammill HA, Swamy GK, et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. JAMA. 2014;311(17):1760–1769. doi: 10.1001/jama.2014.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vilajeliu A, Gonce A, Lopez M, Costa J, Rocamora L, Rios J, et al. Combined tetanus-diphtheria and pertussis vaccine during pregnancy: transfer of maternal pertussis antibodies to the newborn. Vaccine. 2015;33(8):1056–1062. doi: 10.1016/j.vaccine.2014.12.062. [DOI] [PubMed] [Google Scholar]
- 40.Villarreal Pérez JZ, Ramírez Aranda JM, de la O Cavazos M, Zamudio Osuna MJ, Perales Dávila J, Ballesteros Elizondo MR, et al. Randomized clinical trial of the safety and immunogenicity of the Tdap vaccine in pregnant Mexican women. Hum Vaccin Immunother. 2017;13(1):128–35. [DOI] [PMC free article] [PubMed]
- 41.Healy CM, Rench MA, Baker CJ. Importance of timing of maternal combined tetanus, diphtheria, and acellular pertussis (Tdap) immunization and protection of young infants. Clin Infect Dis. 2013;56(4):539–544. doi: 10.1093/cid/cis923. [DOI] [PubMed] [Google Scholar]
- 42.Fortner KB, Swamy GK, Broder KR, Jimenez-Truque N, Zhu Y, Moro PL, et al. Reactogenicity and immunogenicity of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant and nonpregnant women. Vaccine. 2018;36(42):6354–6360. doi: 10.1016/j.vaccine.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abu Raya B, Bamberger E, Almog M, Peri R, Srugo I, Kessel A. Immunization of pregnant women against pertussis: the effect of timing on antibody avidity. Vaccine. 2015;33(16):1948–1952. doi: 10.1016/j.vaccine.2015.02.059. [DOI] [PubMed] [Google Scholar]
- 44.Gall SA, Myers J, Pichichero M. Maternal immunization with tetanus-diphtheria-pertussis vaccine: effect on maternal and neonatal serum antibody levels. Am J Obstet Gynecol. 2011;204(4):334. doi: 10.1016/j.ajog.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 45.Healy CM, Rench MA, Swaim LS, Smith EOB, Sangi-Haghpeykar H, Mathis MH, et al. Association between third-trimester Tdap immunization and neonatal pertussis antibody concentration. JAMA. 2018;320(14):1464–1470. doi: 10.1001/jama.2018.14298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maertens K, Cabore RN, Huygen K, Hens N, Van Damme P, Leuridan E. Pertussis vaccination during pregnancy in Belgium: results of a prospective controlled cohort study. Vaccine. 2016;34(1):142–150. doi: 10.1016/j.vaccine.2015.10.100. [DOI] [PubMed] [Google Scholar]
- 47.Naidu M, Wallace E, Giles M. Pertussis vaccine: when is the optimal time to vaccinate during pregnancy? Br J Obstet Gynaecol. 2015;122:365. [Google Scholar]
- 48.Wanlapakorn N, Maertens K, Chaithongwongwatthana S, Srimuan D, Suratannon N, Vongpunsawad S, et al. Assessing the reactogenicity of Tdap vaccine administered during pregnancy and antibodies to Bordetella pertussis antigens in maternal and cord sera of Thai women. Vaccine. 2018;36(11):1453–1459. doi: 10.1016/j.vaccine.2018.01.059. [DOI] [PubMed] [Google Scholar]
- 49.Abraham C, Pichichero M, Eisenberg J, Singh SI. Third-trimester maternal vaccination against pertussis and pertussis antibody concentrations. Obstet Gynecol. 2018;131(2):364–369. doi: 10.1097/AOG.0000000000002438. [DOI] [PubMed] [Google Scholar]
- 50.Eberhardt CS, Blanchard-Rohner G, Lemaitre B, Boukrid M, Combescure C, Othenin-Girard V, et al. Maternal immunization earlier in pregnancy maximizes antibody transfer and expected infant seropositivity against pertussis. Clin Infect Dis. 2016;62(7):829–836. doi: 10.1093/cid/ciw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eberhardt CS, Blanchard-Rohner G, Lemaitre B, Combescure C, Othenin-Girard V, Chilin A, et al. Pertussis antibody transfer to preterm neonates after second- versus third-trimester maternal immunization. Clin Infect Dis. 2017;64(8):1129–1132. doi: 10.1093/cid/cix046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vilajeliu A, Ferrer L, Munros J, Gonce A, Lopez M, Costa J, et al. Pertussis vaccination during pregnancy: antibody persistence in infants. Vaccine. 2016;34(33):3719–3722. doi: 10.1016/j.vaccine.2016.05.051. [DOI] [PubMed] [Google Scholar]
- 53.Ladhani SN, Andrews NJ, Southern J, Jones CE, Amirthalingam G, Waight PA, et al. Antibody responses after primary immunization in infants born to women receiving a pertussis-containing vaccine during pregnancy: single arm observational study with a historical comparator. Clin Infect Dis. 2015;61(11):1637–1644. doi: 10.1093/cid/civ695. [DOI] [PubMed] [Google Scholar]
- 54.Kent A, Ladhani SN, Andrews NJ, Matheson M, England A, Miller E, et al. Pertussis antibody concentrations in infants born prematurely to mothers vaccinated in pregnancy. Pediatrics. 2016;138(1). [DOI] [PubMed]
- 55.Cabore RN, Maertens K, Dobly A, Leuridan E, Van Damme P, Huygen K. Influence of maternal vaccination against diphtheria, tetanus, and pertussis on the avidity of infant antibody responses to a pertussis containing vaccine in Belgium. Virulence. 2017;8(7):1245–1254. doi: 10.1080/21505594.2017.1296998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maertens K, Hoang TT, Nguyen TD, Caboré RN, Duong TH, Huygen K, et al. The effect of maternal pertussis immunization on infant vaccine responses to a booster pertussis-containing vaccine in Vietnam. Clin Infect Dis. 2016;63(Suppl 4):S197–S204. doi: 10.1093/cid/ciw551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maertens K, Cabore RN, Huygen K, Vermeiren S, Hens N, Van Damme P, et al. Pertussis vaccination during pregnancy in Belgium: follow-up of infants until 1 month after the fourth infant pertussis vaccination at 15 months of age. Vaccine. 2016;34(31):3613–3619. doi: 10.1016/j.vaccine.2016.04.066. [DOI] [PubMed] [Google Scholar]
- 58.Abu Raya B, Srugo I, Kessel A, Peterman M, Bader D, Peri R, et al. The induction of breast milk pertussis specific antibodies following gestational tetanus-diphtheria-acellular pertussis vaccination. Vaccine. 2014;32(43):5632–5637. doi: 10.1016/j.vaccine.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 59.De Schutter S, Maertens K, Baerts L, De Meester I, Van Damme P, Leuridan E. Quantification of vaccine-induced antipertussis toxin secretory IgA antibodies in breast milk: comparison of different vaccination strategies in women. Pediatr Infect Dis J. 2015;34(6):e149–e152. doi: 10.1097/INF.0000000000000675. [DOI] [PubMed] [Google Scholar]
- 60.Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Donegan K, et al. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet. 2014;384(9953):1521–1528. doi: 10.1016/S0140-6736(14)60686-3. [DOI] [PubMed] [Google Scholar]
- 61.Amirthalingam G, Campbell H, Ribeiro S, Fry NK, Ramsay M, Miller E, et al. Sustained effectiveness of the maternal pertussis immunization program in England 3 years following introduction. Clin Infect Dis. 2016;63(suppl 4):S236–S243. doi: 10.1093/cid/ciw559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baxter R, Bartlett J, Fireman B, Lewis E, Klein NP. Effectiveness of vaccination during pregnancy to prevent infant pertussis. Pediatrics. 2017;139(5). [DOI] [PubMed]
- 63.Dabrera G, Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, et al. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012–2013. Clin Infect Dis. 2015;60(3):333–337. doi: 10.1093/cid/ciu821. [DOI] [PubMed] [Google Scholar]
- 64.Romanin V, Acosta AM, Sagradini S, Briere E, Sanchez SM, Sevilla ME, et al. Effectiveness of Tdap vaccination during pregnancy in preventing infant pertussis in a country with whole-cell pertussis vaccines during childhood: preliminary results of a case–control study in Argentina. Open Forum Infect Dis. 2015;2(Suppl 1):S447. [Google Scholar]
- 65.Saul N, Wang K, Bag S, Baldwin H, Alexander K, Chandra M, et al. Effectiveness of maternal pertussis vaccination in preventing infection and disease in infants: the NSW public health network case–control study. Vaccine. 2018;36(14):1887–1892. doi: 10.1016/j.vaccine.2018.02.047. [DOI] [PubMed] [Google Scholar]
- 66.Skoff TH, Blain AE, Watt J, Scherzinger K, McMahon M, Zansky SM, et al. Impact of the US maternal tetanus, diphtheria, and acellular pertussis vaccination program on preventing pertussis in infants < 2 months of age: a case–control evaluation. Clin Infect Dis. 2017;65(12):1977–1983. doi: 10.1093/cid/cix724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winter K, Nickell S, Powell M, Harriman K. Effectiveness of prenatal versus post-partum tetanus, diphtheria, and acellular pertussis vaccination in preventing infant pertussis. Clin Infect Dis. 2017;64(1):3–8. doi: 10.1093/cid/ciw634. [DOI] [PubMed] [Google Scholar]
- 68.Winter K, Cherry JD, Harriman K. Effectiveness of prenatal tetanus, diphtheria, and acellular pertussis vaccination on pertussis severity in infants. Clin Infect Dis. 2017;64(1):9–14. doi: 10.1093/cid/ciw633. [DOI] [PubMed] [Google Scholar]
- 69.Becker-Dreps S, Butler AM, McGrath LJ, Boggess KA, Weber DJ, Li D, et al. Effectiveness of prenatal tetanus, diphtheria, acellular pertussis vaccination in the prevention of infant pertussis in the US. Am J Prev Med. 2018;55(2):159–166. doi: 10.1016/j.amepre.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walls T, Graham P, Petousis-Harris H, Hill L, Austin N. Infant outcomes after exposure to Tdap vaccine in pregnancy: an observational study. BMJ Open. 2016;6(1):e009536. doi: 10.1136/bmjopen-2015-009536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gkentzi D, Katsakiori P, Marangos M, Hsia Y, Amirthalingam G, Heath PT, et al. Maternal vaccination against pertussis: a systematic review of the recent literature. Arch Dis Child Fetal Neonatal Ed. 2017;102(5):F456–F463. doi: 10.1136/archdischild-2016-312341. [DOI] [PubMed] [Google Scholar]
- 72.McMillan M, Clarke M, Parrella A, Fell DB, Amirthalingam G, Marshall HS. Safety of tetanus, diphtheria, and pertussis vaccination during pregnancy: a systematic review. Obstet Gynecol. 2017;129(3):560–573. doi: 10.1097/AOG.0000000000001888. [DOI] [PubMed] [Google Scholar]
- 73.Campbell H, Gupta S, Dolan GP, Kapadia SJ, Kumar Singh A, Andrews N, et al. Review of vaccination in pregnancy to prevent pertussis in early infancy. J Med Microbiol. 2018;67(10):1426–1456. doi: 10.1099/jmm.0.000829. [DOI] [PubMed] [Google Scholar]
- 74.Furuta M, Sin J, Ng ESW, Wang K. Efficacy and safety of pertussis vaccination for pregnant women—a systematic review of randomised controlled trials and observational studies. BMC Pregnancy Childbirth. 2017;17(1):390. doi: 10.1186/s12884-017-1559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]