Abstract
This study was undertaken to investigate the effect of natural bioactive compound thearubigin on neonatal acute lung injury (ALI) using LPS-induced ALI as a model. We also attempted to understand the possible underlying mechanism. The effect of thearubigin on lung wet-to-dry weight ratio, the activity of LDH, lung histopathology, BALF protein levels, the activity of MPO, production and extravasation of cytokines and oxidative stress were studied. The results showed that thearubigin caused a significant reduction in lung inflammation as evident from lung wet-to-dry weight ratio, BALF protein levels and MPO activity and histopathological analysis. It was further observed that the attenuation in inflammation happened due to a significant reduction in cytokine levels in alveolar cavities. Thearubigin also showed strong antioxidant properties as evidenced by reduced levels of oxygen species such as H2O2, MDA and OH ion. Additionally, the antioxidant response element nuclear factor erythroid-2-related factor 2 (Nrf2) pathway was found to be activated in thearubigin-treated group. These results provided a possible mechanism of antioxidant activity of thearubigin in neonatal ALI. Overall, this study showed that thearubigin can be a natural alternative for the treatment of neonatal ALI. However, further studies are required to understand its mechanism antioxidant and anti-inflammatory action.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1986-z) contains supplementary material, which is available to authorized users.
Keywords: Thearubigin, Neonatal rats, ALI, Antioxidant, Anti-inflammatory
Introduction
Acute lung injury (ALI) is a life-threatening situation characterized by pulmonary and capillary edema and acute respiratory failure (Luh and Chiang 2007). Pathological symptoms of ALI include inflammation in alveolar epithelium caused by increased permeability and inflammation of alveolar epithelium (Cheifetz 2016). One of the most common causes of mortality in neonates is ALI (Chakraborty et al. 2010). ALI in newborns is primarily seen in preterm infants (less than 32 weeks) due to the suboptimal production of surfactants in the immature lungs of neonates. Respiratory failure due to ALI in neonates needs to be supported by mechanical ventilation or elevated oxygen levels (Chakraborty et al. 2010).
The lipopolysaccharide (LPS), present in the bacterial cell wall, is an important virulence factor and is a primary factor known to cause pulmonary inflammation (Karmpaliotis et al. 2002). LPS has been reported to cause ALI through several inflammatory mechanisms (Suliman et al. 2003). Thus, LPS has been widely used as an inducer of ALI as a model for the study of lung injury (Ding et al. 2017).
LPS causes the production of proinflammatory cytokines via a pathway dependent on tumor necrosis factor (TNF) (Liu and Lin 2007). The cytokines thus produced include IL-1β, macrophage inflammatory protein 2 (MIP-2), including interleukin (IL)-6. This leads to the amplification of the inflammatory signal (Liu and Lin 2007). Additionally, LPS exposure also causes the generation of cytokines including IL-6 and TNF-α. Elevated levels of cytokines activate a feedback loop leading to amplification of the proinflammatory signal (Li and Verma 2002).
Inflammation and oxidative stress are closely intertwined and are known to play a key role in the pathogenesis of LPS induction of ALI. Leukocytes are recruited as a result of oxidative stress and thus leading to inflammatory signaling (Cachofeiro et al. 2008). The leukocytes also cause induction of ROS, chlorine and nitrogen species. The transcription factor, nuclear factor erythroid-2-related factor 2 (Nrf2), is a key agent for the induction of antioxidant response element genes (Pedruzzi et al. 2012). Host cells use Nrf2 activation in response to oxidative stress signaling. Nrf2 is known to be upregulated by the redox protein thioredoxin (Trx1) (Ago et al. 2006). Moreover, Nrf2 also functions as a master regulator of genes involved in the antioxidant pathways (Deramaudt et al. 2013).
Thearubigin is a polyphenolic bioactive compound extracted from black tea (Maity et al. 2003). Thearubigin has been shown to possess anti-inflammatory properties against inflammatory bowel disease (Maity et al. 2003). However, there are very few reports of thearubigin as a bioactive compound against inflammatory diseases. Moreover, to the best of our knowledge, thearubigin has previously not been reported in the context of neonatal ALI. In this study, the role of thearubigin in neonatal ALI was studied using LPS as a model. We also attempted to study the molecular mechanism of the anti-inflammatory effect of thearubigin in neonatal ALI.
Materials and methods
Establishment of the ALI model
Neonatal Sprague–Dawley rats (2–6 days old, weighing 6–12 g) were provided by the animal house of the Hainan Provincial People’s Hospital. The animals were kept under a 12 h by 12-h light and dark circadian cycle and the under controlled conditions of temperature and humidity. The experimental animals were kept with their nursing mothers. The mothers were fed with the standard rat diet and water ad libitum. Thearubigin was isolated from black tea using HSCCC method as described by Stodt et al. (2015). Escherichia coli LPS were procured from Merck (USA). The optimal dose of thearubigin for this study was estimated based on previous reports (Maity et al. 2003) and pilot experiments in our laboratory. The animals were assigned to two sets of six experimental groups of ten pups each viz. (1) PBS (phosphate buffered saline) as control; (2) LPS group (5 mg/kg, administered through intratracheal instillation); (3) (LPS + vehicle 3) LPS + thearubigin (10 mg/kg) group; (4) LPS + thearubigin (20 mg/kg) group; (5) LPS + thearubigin (40 mg/kg) group. Thearubigin was prepared for administration by dissolving in DMSO as vehicle. PBS and thearubigin were intraperitoneally (i.p.) administered 24 h after the LPS challenge for seven consecutive days. The pups were killed on the eighth day. The killing was followed by the collection of lung samples for subsequent experimental analysis. One set of experimental groups was used for BALF and lung edema experiments described below. The other set of experimental groups was used for western blotting, RT-PCR and histopathological analysis.
Evaluation of lung edema
LPS-induced pulmonary edema in the lungs was estimated using the lung wet-to-dry weight ratio (Yang et al. 2005). For the lung wet-to-dry weight ratio estimation, the right lung was excised and rinsed in PBS. The rinsed lung was weighed to determine the wet lung weight. The excised lung was then dried by baking at 80 °C in an oven for 24 h. After heat drying, the lung was weighted again to estimate the dry weight.
Estimation of lactate dehydrogenase (LDH) activity
Bronchoalveolar lavage fluid (BALF) was extracted by lavaging the lungs with sterile PBS by intratracheal instillation repeated four times. The BALF was centrifuged at 1000g for 15 min at 4 °C, and supernatant was collected. The activity of LDH in BALF supernatant was estimated as reported by Lee et al. (2015). LDH estimation kit (Sigma, USA) was used for the estimation of LDH activity according to the manufacturer’s protocol. BALF supernatant (2 ml) was incubated with the reagent-A for 1 min and then reagent-B was added. The absorbance of the reaction product was estimated at every min for 4 min. The LDH activity in U/I was estimated using the standards provided with the kit.
BALF protein concentration and cell counts
Estimation of protein concentration in BALF supernatants was performed following Lee et al. (2010). The total protein concentration was determined by Bradford’s method using the Assay Dye (Bio-Rad, USA). A standard protein curve was generated using BSA protein. The total cell count in the BALF was also estimated by resuspending the cell pellet in 100 μl PBS.
Myeloperoxidase (MPO) activity in lungs
One of the primary features of ALI is an elevated accumulation of neutrophils in the lungs. MPO activity can be used to estimate the neutrophil accumulation in the lungs (Kim et al. 2012). Briefly, the lung tissue of equal weight was homogenized and centrifuged at 13000g for 1 h at 4 °C. The MPO activity in the supernatants was estimated using the ELISA kit (Abbkine, USA) following the manufacturer’s protocol. The absorbance of the reaction product was determined using a spectrophotometer at 450 nm wavelength. The measurements were made in three replicates, and the result was extrapolated to per gram units.
Estimation of lung oxidative stress
The oxidative stress in the lungs of neonatal rats was measured as per Trocha et al. (2014). The lung tissue of equal weight was homogenized (10% w/v) and centrifuged at 13000g for 1 h at 4 °C. Colorimetric assay of supernatants was performed for estimating the concentration of hydrogen peroxide (H2O2) using hydrogen peroxide assay kit (Abcam, USA), malondialdehyde (MDA) using lipid peroxidation (MDA) assay kit (Abcam, USA) and hydroxyl radical (OH) using hydroxyl radical antioxidant capacity assay kit (Eagle, bioscience, USA). All the experimental procedures were carried out following the respective manufacturer’s protocols.
Pulmonary histopathology analysis
The histopathology of LPS-induced ALI pups was performed as per Li et al. (2016). The upper left lobe of one animal randomly selected from each experimental group was excised and fixed in 10% formaldehyde for 1 day. The fixed tissue was dehydrated in gradually increasing ethanol strength. The dehydrated sample was embedded in paraffin wax and sliced to achieve 4-µm sections. The sectioned tissue was de-paraffinized and stained with hemotoxylin–eosin (H&E). The tissue was observed under a light microscope.
Cytokine measurements
The levels of TNF-α, IL-1β, MIP-2 and IL-6 in the BALF and sera were measured using ELISA kit (Invitrogen, USA), according to the manufacturer’s instructions.
Western blot analysis
Western blot for Nrf2 and Trx1 was performed to estimate their concentration in BALF. β-Actin was used as an internal control for the estimation of expression. The antibodies for these proteins were procured from R&D systems, USA. The total protein in BALF was estimated using Bradford’s method as mentioned above. Ten micrograms of BALF total protein was loaded in each well. Electrophoretic transfer of proteins was performed on PVDF membranes. After incubation for two hours and washing with Tris-buffered saline which contained 0.2% Tween 20 (TBST) and blocking with 2% nonfat dry-milk, the membranes were incubated with primary antibodies overnight. Finally, the membranes were incubated with horseradish peroxidase substrate. ECL detection system was used to develop the membranes. The relative band intensity was determined using ImageJ software (NIH, USA).
Isolation of total RNA and qRT-PCR
Total macrophage RNA was extracted using E.Z.N.A. HP Total RNA Kit (Omega Bio-Tek, USA). Geneious software (USA) was used for designing of primers for qRT-PCR. The following primers were used for qRT-PCR: Nrf2 (5′-ATTTGTAGATGACCATGAGTCGC-3′, 5′-GAGCTATCGAGTGACTGAGCC-3′), Trx1 (5′-TAGTGGACTTCTCTGCCACG-3′, 5′-TGGCAGTCATCCACGTCTAC-3′); GAPDH (5′-AGTGCCAGCCTCGTCTCATA-3′, 5′-CGTTGATGGCAACAATGTCCA-3′) was used as internal control. Three technical replicates for each biological replicate were used. RNA was quantified using Qubit fluorometer. The following components were added in the PCR master-mix: 1.5 μl cDNA, 1 μl (5 pm/μl) each primer, 5 μl DyNAmo Flash SYBR Green (Thermo) (2X). The PCR was cycled for 42 times with following conditions: 10 m at 95 °C, 40 cycles 22 s at 95 °C, 60 s at 60 °C. ABI prism 7500 was used for the qRT-PCR run. The Ct (threshold cycle) value was normalized and quantified using the Ct value of GADPH. 2−ΔΔCt method (Livak and Schmittgen 2001) was used to calculate the relative expression.
Statistical evaluation
All statistical calculations were depicted in the form of mean ± SEM. ANOVA test was used to compare the results with bonferroni multiple comparison test. The statistical calculations were performed in GraphPad Prism software program with P value ≤ 0.05 for a significant test.
Results
Thearubigin attenuates lung edema and hyperpermeability
The induction of ALI with LPS resulted in a significant increase in lung wet-to-dry weight ratio in comparison with the pups treated with control (Fig. 1a). The protein concentration in BALF was also significantly increased in comparison with the pups treated with control (Fig. 1b). However, a significant reduction of these parameters was seen when the groups treated with thearubigin. Moreover, a dose-dependent decrease in these parameters was observed in the treatment groups.
Fig. 1.
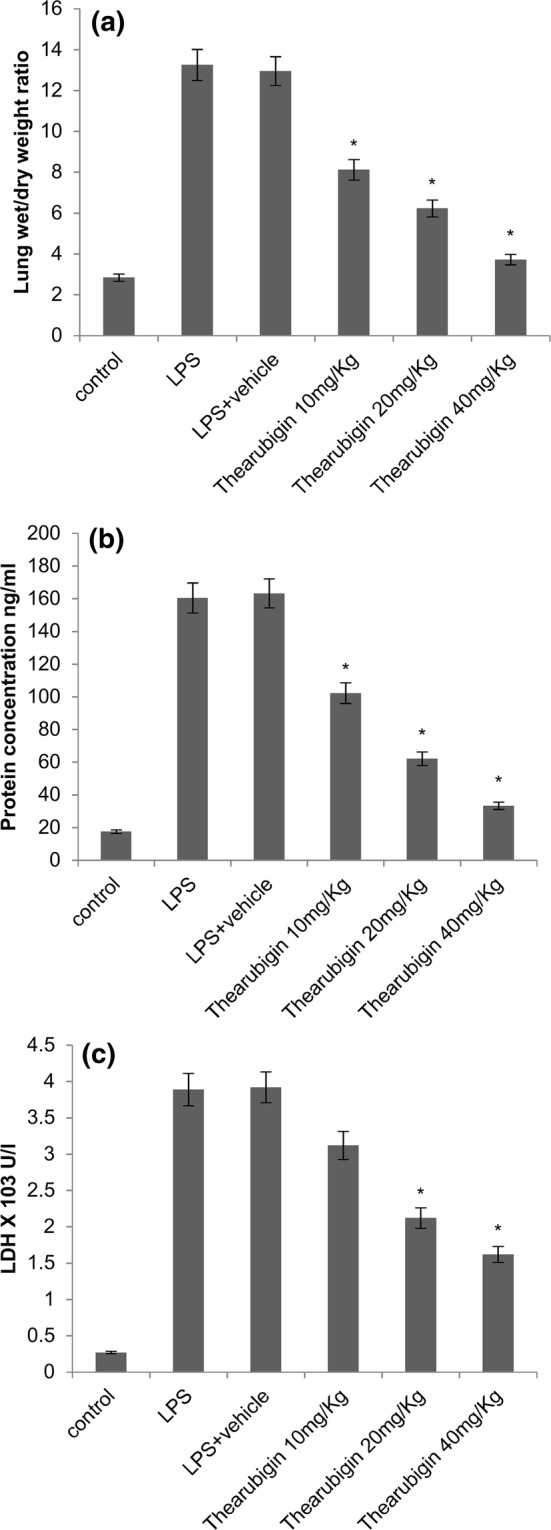
Effect of thearubigin treatment on a lung wet-to-dry weight ratio, b LDH levels and c protein concentration in BALF. Data are presented as the mean ± standard error (n = 10). *P < 0.05 compared with the LPS-induced ALI group. ALI acute lung injury, LPS lipopolysaccharide, LDH lactate dehydrogenase, BALF bronchoalveolar lavage fluid
In addition, we observed a significant increase in the LDH levels upon treatment with LPS (Fig. 1c). The thearubigin-treated animals showed a significant reduction of LDH levels dose-dependently.
Thearubigin reduces MPO activity and neutrophil count
The activity of MPO and counts of neutrophils were determined in the lung tissue homogenates. The counts of neutrophils were also estimated in the BALF of experimental animals. A significant increase in the MPO activity was observed in the LPS-induced animals (Fig. 2a). Moreover, the count of neutrophils was also significantly increased in the BALF of LPS-treated animals. Treatment with thearubigin resulted in significant attenuation of MPO activity. Moreover, a significant reduction in the counts of neutrophils was observed in BALF (Fig. 2b). These reductions were found to be dose-dependent at all the studied concentrations.
Fig. 2.
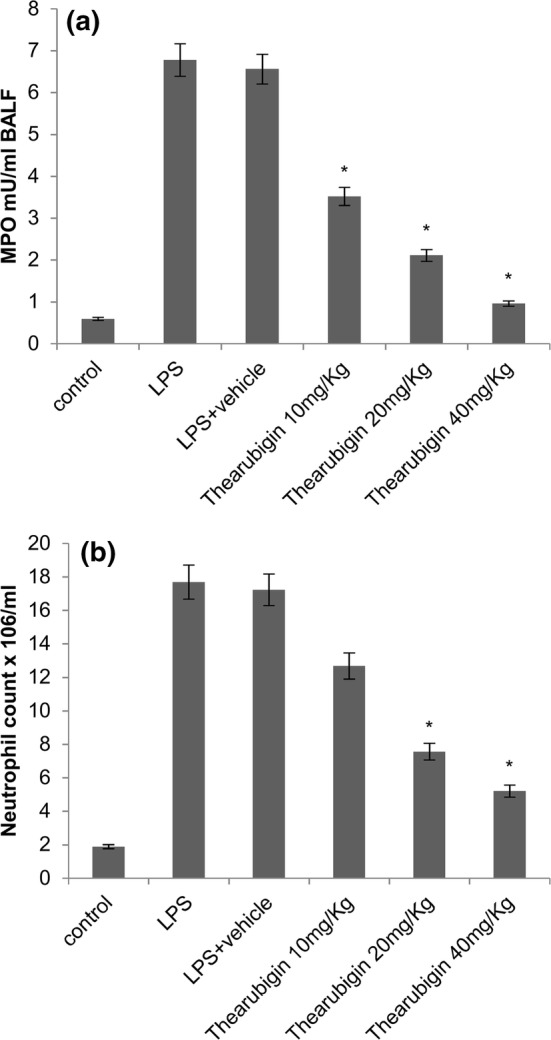
Effect of thearubigin treatment on a MPO activity in the lung tissue homogenates, b neutrophil count in the BALF. Data are presented as the mean ± standard error (n = 10). *P < 0.05 compared with the LPS-induced ALI group. ALI acute lung injury, LPS lipopolysaccharide, LDH lactate dehydrogenase, BALF bronchoalveolar lavage fluid
Thearubigin attenuates oxidative stress in LPS-induced ALI
The oxidative stress due to LPS exposure was measured by estimating the concentrations of H2O2, MDA and OH ion in the experimental animals (Fig. 3). LPS exposure caused a significant upregulation in the levels of H2O2, MDA and OH ion. Thearubigin treatment had a strong downregulating effect on these markers of oxidative stress in a dose-dependent manner in the case of H2O2 and MDA. However, the levels of OH ion were similar in 20 mg/Kg and 40 mg/Kg groups. This may have happened because a control group level of OH ion was already achieved with 20 mg/Kg dose, and therefore, a lower level is not expected with a higher dose (Fig. 3c).
Fig. 3.
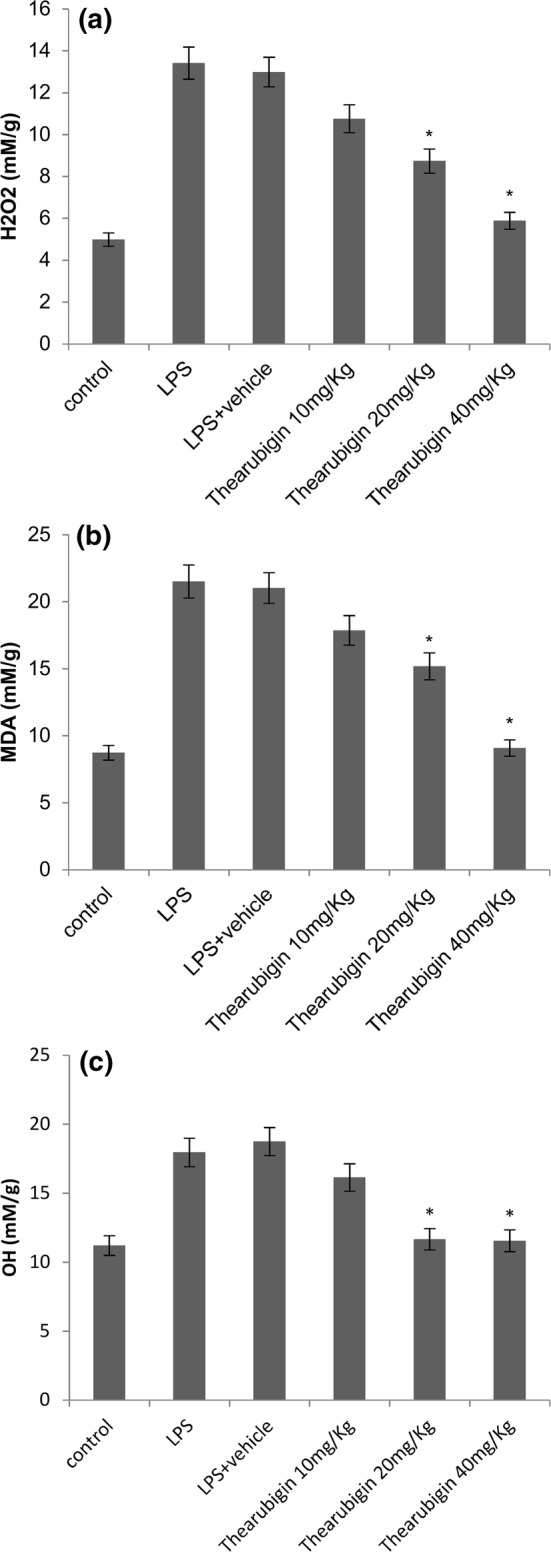
Effect of thearubigin treatment on oxidative stress on the levels of a H2O2, b hydroxyl ion and c MDA in the homogenized lung tissue. Data are presented as the mean ± standard error (n = 10). *P < 0.05 compared with the LPS-induced ALI group. MDA malondialdehyde, ALI acute lung injury, LPS lipopolysaccharide
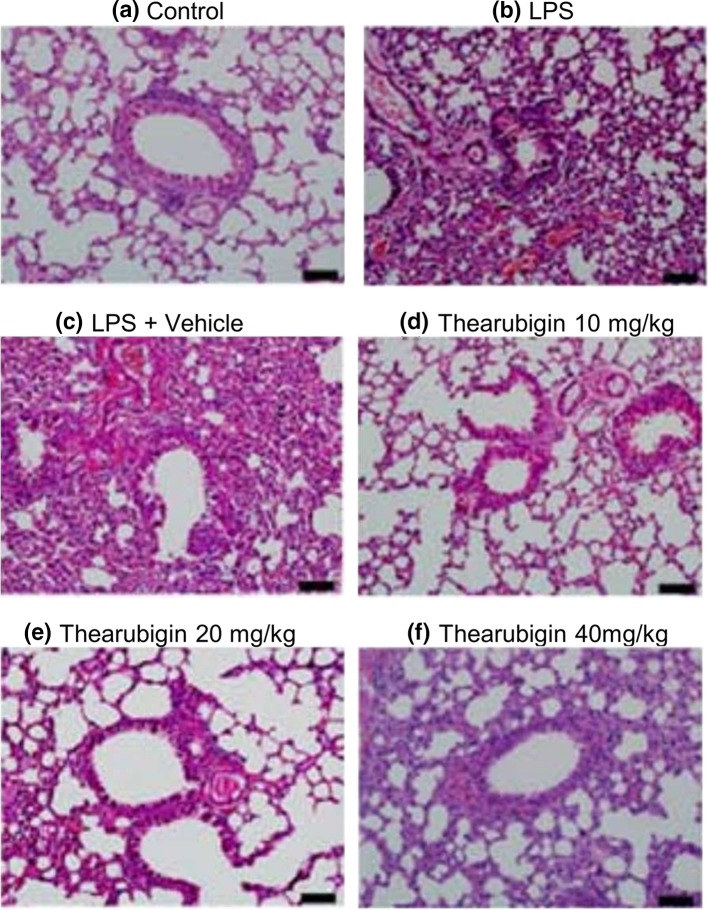
Thearubigin prevents histopathological changes in LPS-induced ALI
The examination of pulmonary histopathology was performed after the commencement of the experiment. The tissue treated with control or vehicle had a normal appearance, and no changes in pulmonary histopathology were observed (Fig. 4). ALI induction with LPS caused extensive and severe damage to the pulmonary tissue. It was observed that LPS exposure caused large-scale intrusion of inflammatory cells into the lung interstitium. Moreover, the inflammatory cells were also seen migrating to cavities into alveoli. Additionally, stromal hemorrhagia, wall thickening of alveoli, the collapse of alveoli and edema were also observed. Treatment with thearubigin showed significant attenuation of these symptoms. The experimental group treated with 20 mg/kg and 40 mg/kg of thearubigin showed large-scale decrease in the inflammatory cells.
Fig. 4.
Effect of thearubigin treatment on pulmonary histopathology. a PBS-treated healthy control group, b LPS-induced ALI group, c vehicle-treated ALI group, d 10 mg/kg thearubigin-treated ALI group, e 20 mg/kg thearubigin-treated ALI group, and f 40 mg/kg thearubigin-treated ALI group. ALI acute lung injury, LPS lipopolysaccharide. Scale bar indicates 20 μm
Thearubigin attenuates proinflammatory cytokine levels
ELISA was used to determine the levels of proinflammatory cytokines such as TNF-α, IL-1β, MIP-2 and IL-6 in serum and BALF of the experimental animals. The levels of cytokines were significantly upregulated with induction by LPS in serum (Supplementary Fig. 1) and BALF (Supplementary Fig. 2). Thearubigin treatment caused a significant downregulation of the cytokines in comparison with the control groups under study.
Effects of thearubigin treatment on the levels of Nrf2 and Trx1
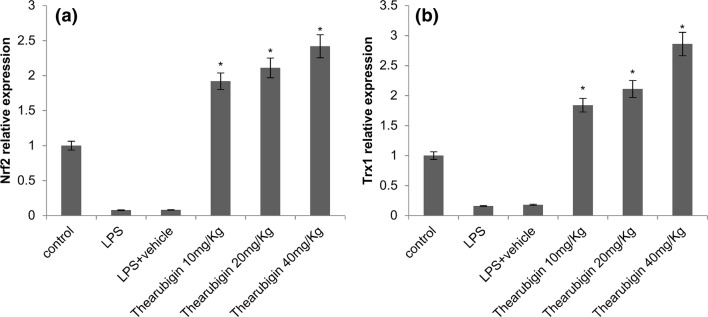
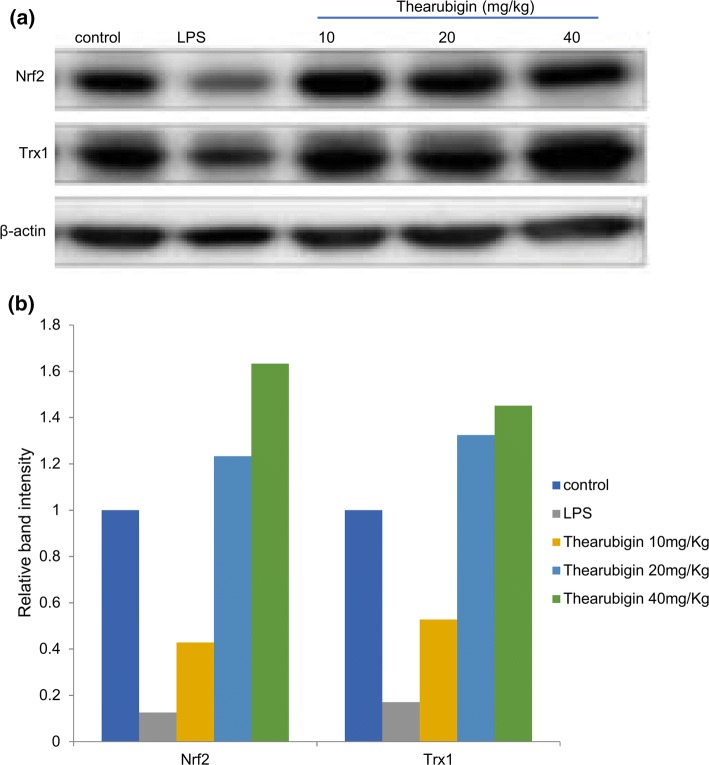
The antioxidant activity of thearubigin was estimated by determining the levels of Trx1 and Nrf2 using western blot and real-time PCR. The results of real-time PCR indicated an upregulation in the Trx1 and Nrf2 expression upon treatment with thearubigin in comparison with the control experiment (Fig. 5). Additionally, the result of qRT-PCR was confirmed by densitometric analysis of western blot (Fig. 6). The levels of Trx1 and Nrf2 were found to be significantly upregulated in comparison with the control experiment in the western blot analysis. However, the LPS-induced ALI group had a significantly downregulated expression of Trx1 and Nrf2 as compared to the control-treated experimental group.
Fig. 5.
Effect of thearubigin treatment on Nrf2 and Trx1 expressions in lung tissues. The mRNA expression levels of a Nrf2 and b Trx1 are shown. Data are presented as the mean ± standard error (n = 10). *P < 0.05 compared with the LPS-induced ALI group. Nrf2 nuclear factor erythroid-2-related factor 2, Trx1 thioredoxin isoform 1
Fig. 6.
Effect of thearubigin treatment on Nrf2 and Trx1 expressions in lung tissues. The protein expression levels were determined as a western blot analysis, b densitometry of western blot was performed to estimate the relative protein concentration. Data are presented as the mean ± standard error (n = 10). Nrf2 nuclear factor erythroid-2-related factor 2, Trx1 thioredoxin isoform 1
Discussion
This study was carried out to identify the antioxidative and anti-inflammatory effects of bioactive compound thearubigin on LPS-induced ALI in neonatal rats. We also attempted to decipher the mechanism of thearubigin action on inflammation in neonatal lungs. The results indicated that treatment with thearubigin caused a reduction in the wet-to-dry ratio of lungs, BALF protein concentration, MPO activity, inflammatory cell migration, cytokine production. Thearubigin caused significant antioxidant action as indicated by a significant reduction in the levels of MDA, H2O2 and the OH ion as well as a significant upregulation of Trx1 and Nrf2.
LPS induction is known to significantly increase the levels of proteins, serum and BALF neutrophils and inflammatory cytokines (Jerala 2007; Lu et al. 2008). ALI is also marked by damage to alveolar cavities, infiltration of neutrophils in lungs and edema (Matthay and Zemans 2011). Pulmonary inflammation is marked by elevated lung wet-to-dry weight ratio (Jacob et al. 2008), an increase in protein concentration of BALF resulting from extravasation (Muller-Redetzky et al. 2014). These indicators of inflammation are commonly increased upon induction with LPS. Treatment with thearubigin caused a significant reduction of these inflammatory parameters indicating a reduction in lung edema and vascular protein leakage. These results indicate an anti-inflammatory activity in thearubigin. The inflammation and edema in lungs were also observed in the histopathological analysis. The observation of edema, inflammatory cell migration and epithelium thickening was observed in the histopathological analysis. Thearubigin treatment vastly attenuated these inflammatory indicators. This observation validated the therapeutic effect of thearubigin in the neonatal ALI.
Persistent inflammation of lungs plays a key role in the pathogenesis of lung injury (Goodman et al. 2003; Manicone 2009). Therefore, immune system suppression should be a preferable strategy for the management of ALI. In this study, the attenuation of inflammation by thearubigin treatment involved a reduction in the levels of inflammatory cytokines. These results indicated that the anti-inflammatory action of thearubigin is mediated by attenuation of inflammatory cytokines.
ALI is well known to be marked by infiltration of neutrophils in lungs (Matthay and Zemans 2011). In ALI, the migration of neutrophils from lung epithelia and endothelia leads to accumulation their accumulation in the alveolar cavities. The activated neutrophils lead to an increase in the levels of reactive oxygen species (ROS), release of proinflammatory cytokines and MPO and an elevated permeability of microvesicles. (Grommes and Soehnlein 2011). Thus, long-term presence of activated neutrophils in alveolar cavities is a hallmark of ALI. In the present study, treatment with thearubigin caused a significant reduction of macrophages, neutrophils as well as total cell count. MPO is known to be released by macrophages and neutrophils during ALI. (Chagnon et al. 2015). Conversely, the elevated activity of MPO is a reliable indicator of the presence of neutrophils in the pulmonary cavities and parenchyma (Reumaux et al. 2003). Several studies have reported the use of MPO levels as a reliable indicator of ALI such as Rittirsch et al. (2008), Tsai et al. (2014). Our study also showed that treatment with thearubigin caused a significant reduction in MPO activity. This was corroborated by our observation of low neutrophil levels in pulmonary cavities as well as reduced inflammation as seen in the histopathological examination.
Activated cytokines produce reactive oxygen species (Tasoulis et al. 2009). Elevated presence of reactive oxygen species leads to lipid peroxidation, oxidation of proteins and DNA damage. Thearubigin possesses antioxidant properties in addition to being a strong anti-inflammatory (Maity et al. 2003). LPS-mediated induction of ALI caused elevation of H2O2, MDS and -OH ion. In the present study, the levels of Nrf2 and Trx1 were found to be significantly downregulated in LPS-induced ALI animals. Nrf2 is known to be a master regulator of antioxidant process which is regulated by Nrf2 activator such as CDDO-Me (Vomund et al. 2017). Trx1 is another protein that mediates redox reactions under oxidative stress conditions (Furukawa et al. 2011). Both Nrf2 and Trx1 have been recommended as target molecules by activators for treatment of acute lung injury such as COPD (Rahman and MacNee 2012). Thearubigin treatment led to elevated levels of these two redox proteins indicated that thearubigin can potentially act as a natural activator of Nrf2 and Trx1.
The plant bioactive thearubigin showed protective action against LPS-induced neonatal ALI model rats. Thearubigin caused attenuation of all the inflammatory parameters studied here. Thearubigin provided protection against inflammation by preventing the production and accumulation of inflammatory cytokines. Thearubigin also showed strong antioxidant properties by activating redox proteins such as Nrf2 and Trx1. Therefore, we propose that thearubigin can be a potential bioactive compound for the development of therapy for neonatal ALI. However, further studies are required to decipher the mechanism by which thearubigin activates the observed redox proteins.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The project is funded by the National Natural Science Foundation of China (No: 81660013).
Authors’ contributions
XW designed the study and carried out experiments. PH, SY, CW carried out experiments and interpreted the data. XW wrote the paper.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All the animals’ upkeeping and experimental procedures were carried out in accordance with the ethical standards given in the Declaration of Helsinki. The animal experiments were conducted with prior approval from the institutional review board and institutional animal care and use committee (IACUC). All efforts were made to minimize the number of animals and their suffering.
References
- Ago T, Yeh I, Yamamoto M, Schinke-Braun M, Brown JA, Tian B, Sadoshima J. Thioredoxin1 upregulates mitochondrial proteins related to oxidative phosphorylation and TCA cycle in the heart. Antioxid Redox Signal. 2006;8(9–10):1635–1650. doi: 10.1089/ars.2006.8.1635. [DOI] [PubMed] [Google Scholar]
- Cachofeiro V, Goicochea M, de Vinuesa SG, Oubina P, Lahera V, Luno J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl. 2008;111:S4–S9. doi: 10.1038/ki.2008.516. [DOI] [PubMed] [Google Scholar]
- Chagnon F, Bourgouin A, Lebel R, Bonin MA, Marsault E, Lepage M, Lesur O. Smart imaging of acute lung injury: exploration of myeloperoxidase activity using in vivo endoscopic confocal fluorescence microscopy. Am J Physiol Lung Cell Mol Physiol. 2015;309(6):L543–L551. doi: 10.1152/ajplung.00289.2014. [DOI] [PubMed] [Google Scholar]
- Chakraborty M, McGreal EP, Kotecha S. Acute lung injury in preterm newborn infants: mechanisms and management. Paediatr Respir Rev. 2010;11(3):162–170. doi: 10.1016/j.prrv.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Cheifetz IM. Year in review 2015: pediatric ARDS. Respir Care. 2016;61(7):980–985. doi: 10.4187/respcare.05017. [DOI] [PubMed] [Google Scholar]
- Deramaudt TB, Dill C, Bonay M. Regulation of oxidative stress by Nrf2 in the pathophysiology of infectious diseases. Medecine et maladies infectieuses. 2013;43(3):100–107. doi: 10.1016/j.medmal.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Ding Q, Liu GQ, Zeng YY, Zhu JJ, Liu ZY, Zhang X, Huang JA. Role of IL-17 in LPS-induced acute lung injury: an in vivo study. Oncotarget. 2017;8(55):93704–93711. doi: 10.18632/oncotarget.21474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa M, Tanaka R, Chuang VT, Ishima Y, Taguchi K, Watanabe H, Maruyama T, Otagiri M. Human serum albumin-thioredoxin fusion protein with long blood retention property is effective in suppressing lung injury. J Control Release. 2011;154(2):189–195. doi: 10.1016/j.jconrel.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Goodman RB, Pugin J, Lee JS, Matthay MA. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 2003;14(6):523–535. doi: 10.1016/S1359-6101(03)00059-5. [DOI] [PubMed] [Google Scholar]
- Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17(3–4):293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S, Deyo DJ, Cox RA, Traber DL, Hawkins HK. Assessment of lung inflammation in a mouse model of smoke inhalation and burn injury: strain-specific differences. Toxicol Mech Methods. 2008;18(7):551–559. doi: 10.1080/15376510802251993. [DOI] [PubMed] [Google Scholar]
- Jerala R. Structural biology of the LPS recognition. Int J Med Microbiol. 2007;297(5):353–363. doi: 10.1016/j.ijmm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Karmpaliotis D, Kosmidou I, Ingenito EP, Hong K, Malhotra A, Sunday ME, Haley KJ. Angiogenic growth factors in the pathophysiology of a murine model of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2002;283(3):585–595. doi: 10.1152/ajplung.00048.2002. [DOI] [PubMed] [Google Scholar]
- Kim K, Li Y, Jin G, Chong W, Liu B, Lu J, Lee K, Demoya M, Velmahos GC, Alam HB. Effect of valproic acid on acute lung injury in a rodent model of intestinal ischemia reperfusion. Resuscitation. 2012;83(2):243–248. doi: 10.1016/j.resuscitation.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JP, Li YC, Chen HY, Lin RH, Huang SS, Chen HL, Kuan PC, Liao MF, Chen CJ, Kuan YH. Protective effects of luteolin against lipopolysaccharide-induced acute lung injury involves inhibition of MEK/ERK and PI3K/Akt pathways in neutrophils. Acta Pharmacol Sin. 2010;31(7):831–838. doi: 10.1038/aps.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Kang P, Kim KY, Seol GH. Foeniculum vulgare mill. Protects against lipopolysaccharide-induced acute lung injury in mice through ERK-dependent NF-kappaB activation. Korean J Physiol Pharmacol. 2015;19(2):183–189. doi: 10.4196/kjpp.2015.19.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2(10):725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Li G, Zhou CL, Zhou QS, Zou HD. Galantamine protects against lipopolysaccharide-induced acute lung injury in rats. Braz J Med Biol Res. 2016;49(2):e5008. doi: 10.1590/1414-431x20155008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lin A. Wiring the cell signaling circuitry by the NF-kappa B and JNK1 crosstalk and its applications in human diseases. Oncogene. 2007;26(22):3267–3278. doi: 10.1038/sj.onc.1210417. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔ CT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Luh SP, Chiang CH. Acute lung injury/acute respiratory distress syndrome (ALI/ARDS): the mechanism, present strategies and future perspectives of therapies. J Zhejiang Univ Sci B. 2007;8(1):60–69. doi: 10.1631/jzus.2007.B0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity S, Ukil A, Karmakar S, Datta N, Chaudhuri T, Vedasiromoni JR, Ganguly DK, Das PK. Thearubigin, the major polyphenol of black tea, ameliorates mucosal injury in trinitrobenzene sulfonic acid-induced colitis. Eur J Pharmacol. 2003;470(1–2):103–112. doi: 10.1016/S0014-2999(03)01760-6. [DOI] [PubMed] [Google Scholar]
- Manicone AM. Role of the pulmonary epithelium and inflammatory signals in acute lung injury. Expert Rev Clin Immunol. 2009;5(1):63–75. doi: 10.1586/177666X.5.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Redetzky HC, Suttorp N, Witzenrath M. Dynamics of pulmonary endothelial barrier function in acute inflammation: mechanisms and therapeutic perspectives. Cell Tissue Res. 2014;355(3):657–673. doi: 10.1007/s00441-014-1821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedruzzi LM, Stockler-Pinto MB, Leite M, Jr, Mafra D. Nrf2-keap1 system versus NF-kappaB: the good and the evil in chronic kidney disease? Biochimie. 2012;94(12):2461–2466. doi: 10.1016/j.biochi.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Antioxidant pharmacological therapies for COPD. Curr Opin Pharmacol. 2012;12(3):256–265. doi: 10.1016/j.coph.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reumaux D, de Boer M, Meijer AB, Duthilleul P, Roos D. Expression of myeloperoxidase (MPO) by neutrophils is necessary for their activation by anti-neutrophil cytoplasm autoantibodies (ANCA) against MPO. J Leukoc Biol. 2003;73(6):841–849. doi: 10.1189/jlb.1102567. [DOI] [PubMed] [Google Scholar]
- Rittirsch D, Flierl MA, Day DE, Nadeau BA, McGuire SR, Hoesel LM, Ipaktchi K, Zetoune FS, Sarma JV, Leng L, Huber-Lang MS, Neff TA, Bucala R, Ward PA. Acute lung injury induced by lipopolysaccharide is independent of complement activation. J Immunol. 2008;180(11):7664–7672. doi: 10.4049/jimmunol.180.11.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stodt UW, Stark J, Engelhardt UH. Comparison of three strategies for the isolation of black tea thearubigins with a focus on countercurrent chromatography. J Food Compos Anal. 2015;43:160–168. doi: 10.1016/j.jfca.2015.07.002. [DOI] [Google Scholar]
- Suliman HB, Carraway MS, Piantadosi CA. Postlipopolysaccharide oxidative damage of mitochondrial DNA. Am J Respir Crit Care Med. 2003;167(4):570–579. doi: 10.1164/rccm.200206-518OC. [DOI] [PubMed] [Google Scholar]
- Tasoulis MK, Livaditi O, Stamatakos M, Stefanaki C, Paneris P, Prigouris P, Flevari A, Goutas N, Vlachodimitropoulos D, Villiotou V, Douzinas EE. High concentrations of reactive oxygen species in the BAL fluid are correlated with lung injury in rabbits after hemorrhagic shock and resuscitation. Tohoku J Exp Med. 2009;219(3):193–199. doi: 10.1620/tjem.219.193. [DOI] [PubMed] [Google Scholar]
- Trocha M, Merwid-Lad A, Chlebda E, Sozanski T, Piesniewska M, Gliniak H, Szelag A. Influence of ezetimibe on selected parameters of oxidative stress in rat liver subjected to ischemia/reperfusion. Arch Med Sci AMS. 2014;10(4):817–824. doi: 10.5114/aoms.2013.38087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CL, Lin YC, Wang HM, Chou TC. Baicalein, an active component of Scutellaria baicalensis, protects against lipopolysaccharide-induced acute lung injury in rats. J Ethnopharmacol. 2014;153(1):197–206. doi: 10.1016/j.jep.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Vomund S, Schafer A, Parnham MJ, Brune B, von Knethen A. Nrf2, the master regulator of anti-oxidative responses. Int J Mol Sci. 2017 doi: 10.3390/ijms18122772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li W, Duan M, Zhou Z, Lin N, Wang Z, Sun J, Xu J. Large dose ketamine inhibits lipopolysaccharide-induced acute lung injury in rats. Inflamm Res. 2005;54(3):133–137. doi: 10.1007/s00011-004-1334-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.