Dickeya zeae is an emerging banana soft rot pathogen in China. We used genome sequencing and annotation to create an inventory of potential virulence factors and virulence gene regulators encoded in Dickeya zeae MS2, a particularly virulent strain. We created mutations in several genes and tested these mutants in a banana seedling infection model. A strain with a mutated proline iminopeptidase gene, homologs of which are important for disease in the Xanthomonas species phytopathogens, was attenuated for soft rot symptoms in our model. Understanding how the proline iminopeptidase functions as a virulence factor may lead to insights about how to control the disease, and it is of general importance as homologs of the proline iminopeptidase occur in dozens of plant-associated bacteria.
KEYWORDS: genome sequence, LuxR homolog, iminopeptidase, quorum sensing
ABSTRACT
The phytopathogen Dickeya zeae MS2 is a particularly virulent agent of banana soft rot disease. To begin to understand this banana disease and to understand the role of quorum sensing and quorum-sensing-related regulatory elements in D. zeae MS2, we sequenced its genome and queried the sequence for genes encoding LuxR homologs. We identified a canonical LuxR-LuxI homolog pair similar to those in other members of the genus Dickeya. The quorum-sensing signal for this pair was N-3-oxo-hexanoyl-homoserine lactone, and the circuit affected motility, cell clumping, and production of the pigment indigoidine, but it did not affect infections of banana seedlings in our experiments. We also identified a luxR homolog linked to a gene annotated as encoding a proline iminopeptidase. Similar linked pairs have been associated with virulence in other plant pathogens. We show that mutants with deletions in the proline iminopeptidase gene are attenuated for virulence. Surprisingly, a mutant with a deletion in the gene encoding the LuxR homolog shows normal virulence.
IMPORTANCE Dickeya zeae is an emerging banana soft rot pathogen in China. We used genome sequencing and annotation to create an inventory of potential virulence factors and virulence gene regulators encoded in Dickeya zeae MS2, a particularly virulent strain. We created mutations in several genes and tested these mutants in a banana seedling infection model. A strain with a mutated proline iminopeptidase gene, homologs of which are important for disease in the Xanthomonas species phytopathogens, was attenuated for soft rot symptoms in our model. Understanding how the proline iminopeptidase functions as a virulence factor may lead to insights about how to control the disease, and it is of general importance as homologs of the proline iminopeptidase occur in dozens of plant-associated bacteria.
INTRODUCTION
Members of the bacterial genus Dickeya cause serious soft rot diseases of crops, fruits, and ornamental plants. As a group, Dickeya species are considered one of the most important bacterial phytopathogens worldwide (1, 2). Most of the Dickeya species have a broad host range and can infect both dicotyledons and monocotyledons (1, 3). Characteristic Dickeya infections show invasion at the center or a portion of the rhizome, resulting in maceration and rotting (4). The plant growing point is destroyed, internal material decays, and vascular discoloration is obvious.
The past decade has seen the emergence of severe banana soft rot disease in Guangdong Province, China, and the causative agent has been identified as a variant of Dickeya zeae (1, 5), formerly known as Erwinia chrysanthemi pv. zeae (6). This bacterial species has been isolated from diseased pseudostem tissues of the banana Musa ABB. Little is known about the banana-disease-causing D. zeae, but the genomic sequence of one isolate (MS1) has been published previously (5). This isolate and a second isolate (MS2) have been surveyed for their ability to cause disease in a range of plants (1). Interestingly, D. zeae MS2, but not MS1, produces an antimicrobial agent (1).
In various Dickeya soft rot diseases, plant cell-wall-degrading enzymes (PCWDEs) (for a review, see reference 7), siderophores (for a review, see reference 8), type III secretion systems (9, 10), and the blue pigment indigoidine (11, 12) have been implicated as virulence factors. In D. zeae EC1, isolated from infected rice, a phytotoxin called zeamine has been identified, and this compound also has antibacterial and nematicidal activity (13, 14).
To better understand the banana pathogens and the potential involvement of quorum sensing or quorum-sensing-related transcription factors in virulence, we report here the sequence and annotation of the D. zeae MS2 genome. We further focus this report on LuxR homologs and genes known to be controlled by LuxR homologs in related bacteria. Like other members of the genus Dickeya (5, 15, 16), we found that strain MS2 possesses genes coding for an acyl-homoserine lactone (AHL) quorum-sensing circuit, that is, a gene coding for a LuxI homolog (an acyl-homoserine lactone synthase) linked to a gene coding for a LuxR homolog (a putative AHL signal receptor). By analogy to other members of the genus Dickeya, we call these genes expI and expR (15, 17, 18). We also identified a gene coding for a so-called orphan or solo LuxR homolog. This gene is linked to a gene coding for a putative proline iminopeptidase and, thus, falls into a group of luxR homologs with similar genomic contexts (19–21). These orphan LuxR homologs have been shown by others to activate virulence and proline iminopeptidase functions in several plant pathogens, and rather than responding to AHLs, they respond to plant-derived signals (22–24). We have studied one such system, the pipR-pipA system, in an endophyte found in the roots of cottonwood trees (25). By analogy to the endophyte system, we call the D. zeae MS2 genes pipR and pipA.
RESULTS AND DISCUSSION
Genome sequencing of D. zeae MS2 reveals potential virulence factors and virulence gene regulators, as well as a biosynthetic gene cluster required for antibacterial activity.
D. zeae MS2 was isolated from diseased banana soft rot tissue on a plant growing in a banana field in southern China. A 16S rRNA gene sequence analysis showed that this bacterium is very closely related to the previously sequenced banana pathogen D. zeae MS1 (1). Hu et al. (1) demonstrated that MS2 infects a variety of mono- and dicot plants, including young banana pseudostems, as illustrated in Fig. 1. Seven days after needle inoculation, banana stems showed obvious discoloration, and the disease had spread to the leaves of the seedling whereas a mock-infected seedling showed no sign of disease (Fig. 1; see also Fig. S1 in the supplemental material). To learn more about this potent pathogen, we sequenced the D. zeae MS2 genome, which is 4,740,052 bp with a G+C content of 53.44%. We closed the genome and found 4,275 open reading frames (ORFs) with seven copies of the rRNA operon. The D. zeae MS2 genome contains genes coding for virulence factors described for other members of the genus, including D. zeae MS1 (5), D. zeae EC1 (16), D. zeae Ech586 (26), and Dickeya dadantii 3937 (27) (summarized in Table S1 in the supplemental material). Consistent with previous findings that MS2 produces high levels of PCWDE and extracellular protease activity (1), its genome contains a large number (n = 14) of plant cell-wall-degrading enzyme (PCWDE) genes, including those required for pectin and cellulose degradation. These PCWDEs are primarily responsible for the soft rot symptoms in plant hosts (for a review, see reference 7). The type I, II, III, IV, and VI secretion systems are conserved in MS2. These secretion systems have secretory functions important for virulence in other bacteria. Type I secretion is important for export of proteases (16, 28, 29), type II is important for export of pectinases (16, 30, 31), and type III and type VI secretion systems deliver effector proteins to host cells (32–37).
FIG 1.
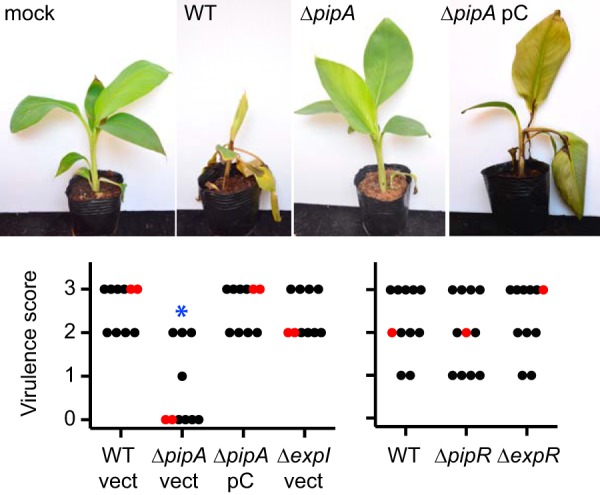
Virulence of D. zeae MS2 wild-type and mutant strains. (Top) Photographs of representative infected plants. (Bottom) Virulence scores. For each strain, 10 or 11 banana seedlings with 3 or 4 leaves were infected, and disease was scored 7 days later (see Materials and Methods and Fig. S1 in the supplemental material for details). The red dots indicate the median virulence scores. The blue asterisk indicates that the virulence score distributions between the wild type (WT) and the pipA mutant (ΔpipA) differed significantly (Mann-Whitney U = 6; n1 = n2 = 10; P < 0.01, two-tailed). For ΔpipA pC, the pC indicates the infecting bacteria carried the pipA-complementing plasmid pCpipA. Bacteria carrying the plasmid control vector pBBR1MCS-5 (vect).
It is often the case that secondary metabolites serve as virulence factors (38, 39). Therefore, we performed an antiSMASH 5.0 analysis (https://antismash.secondarymetabolites.org) (40) to identify secondary metabolite biosynthetic gene clusters (BGCs) in the MS2 genome. This program identified genes predicted to encode achromobactin and chrysobactin siderophores (8) as well as the blue pigment indigoidine, which is hypothesized to help phytopathogens overcome plant-generated oxidative stress during the host defense response (11, 12). The analysis also confirmed that MS2 lacks biosynthesis genes for the antibacterial and phytotoxic zeamines produced by D. zeae EC1 (1, 14) and the antibiotic carbapenem produced by some strains of the related Pectinobacterium sp. (41), even though MS2 possesses antibiotic activity against Escherichia coli and several fungal strains (1). Interestingly, antiSMASH identified a biosynthetic gene cluster (C1030_04995 to C1030_05185; see Table S1) with sequence similarity to nonribosomal peptide synthetase and bacteriocin clusters. This gene cluster has been found only in the D. zeae MS2 genome and in the genomes of two other Dickeya sp. (Dickeya paradisiaca NCPPB2511 and D. dadantii Ech703). We asked whether this cluster was responsible for the previously reported antimicrobial activity (1) by constructing a mutation in the nonribosomal peptide synthase gene. The mutated gene (C1030_05075) codes for a polypeptide missing the putative amino acid adenylation domain (amino acids 674 to 1051). While supernatant fluid from wild-type cultures inhibited growth of E. coli, culture fluid from the mutant did not (Fig. 2). Inhibition of E. coli by culture fluid was restored in the mutant by complementation with a wild-type peptide synthase gene (Fig. 2).
FIG 2.
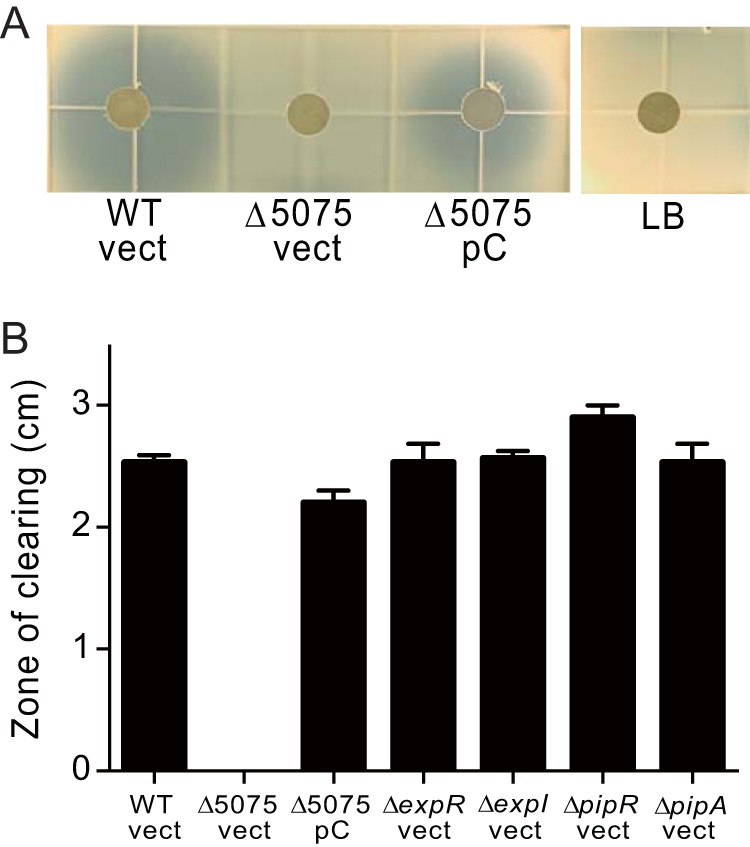
Influence of D. zeae MS2 and MS2 mutant culture fluid on growth of E. coli. (A) E. coli agarose overlay assays (Materials and Methods) with culture fluid from wild-type strain MS2 with the plasmid backbone pBBR1MCS-5 (vect), a strain with a mutation in the nonribosomal peptide synthase gene C1030_05075 (Δ5075) containing either the pBBR1MCS-5 plasmid (vect) or a plasmid containing a copy of C1030_05075 (pC), or fresh LB plus gentamicin (LB). Confluent E. coli growth in the agarose appears as a whitish color, while the zones of growth inhibition are a gray-blue color. (B) Sizes of the clearing zones around paper disks saturated with cell-free culture fluid from the indicated strains harboring either pBBR1MCS-5 vector (vect) or pC5075 complementation plasmid (pC). Results are means of three experiments, and error bars are standard deviations.
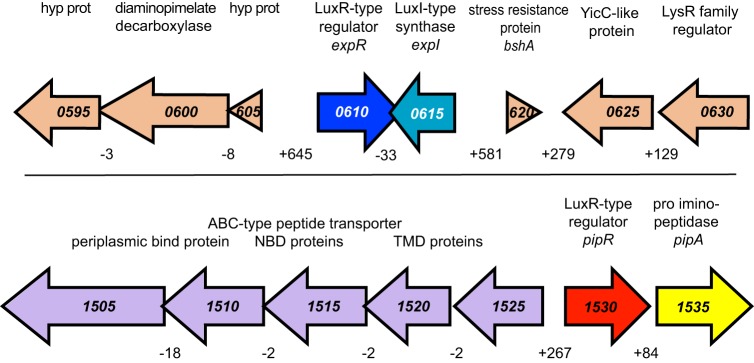
One might anticipate that coordinating expression of genes coding for the large arsenal of potential virulence factors could involve a number of regulatory proteins. Furthermore, many of the factors that we described are excreted or secreted, and it is common for such factors to be controlled by quorum sensing (42). As mentioned previously, we identified genes coding for homologs of the LuxR quorum-sensing transcriptional activator: expR, which is linked to the quorum-sensing signal synthase gene expI, and pipR, which is linked to pipA, a gene coding for a putative proline aminopeptidase (Fig. 3).
FIG 3.
D. zeae MS2 genomic regions surrounding the luxR homologs expR and pipR. (Top) The region surrounding expR (blue) and expI (teal). (Bottom) The region surrounding pipR (red). The context of genes surrounding pipR is similar to that of other bacteria (25). This includes a flanking gene annotated as a proline iminopeptidase gene (yellow, pipA) and genes coding for a transport system (purple). Positive numbers below intergenic regions indicate the number of bases between open reading frames. Negative numbers indicate that the open reading frames overlap. All gene numbers have the prefix “C1030_0.”
The influence of D. zeae MS2 expR and expI mutations on phenotypes regulated by quorum sensing in related bacteria.
The D. zeae MS2 ExpR and ExpI show 99% amino acid sequence identity to ExpR and ExpI in D. zeae EC1. The EC1 AHL is 3OC6-HSL, and in this isolate, ExpR and ExpI control motility, cell aggregation, and, to a minor degree, starch degradation in experiments with potato slices (3). A quantitative radioactivity assay (43) showed a major high-pressure liquid chromatography (HPLC) peak with a retention time matching that of 3OC6-HSL (Fig. 4A). This peak (fractions 19 to 21) contained greater than 50% of the total radioactive carbon in the Fig. 4A peaks. There were also two minor peaks of radioactivity. The radioactivity in fractions 23 and 24 constituted about 24% of the total, and the identity of the compound in this peak remains unidentified. The radioactivity in fractions 33 and 34 constituted about 20% of the total, and this peak matches the retention time of 3OC8-HSL. These results indicate that like other Dickeya spp., MS2 produces 3OC6-HSL and lesser amounts of 3OC8-HSL (3, 18).
FIG 4.
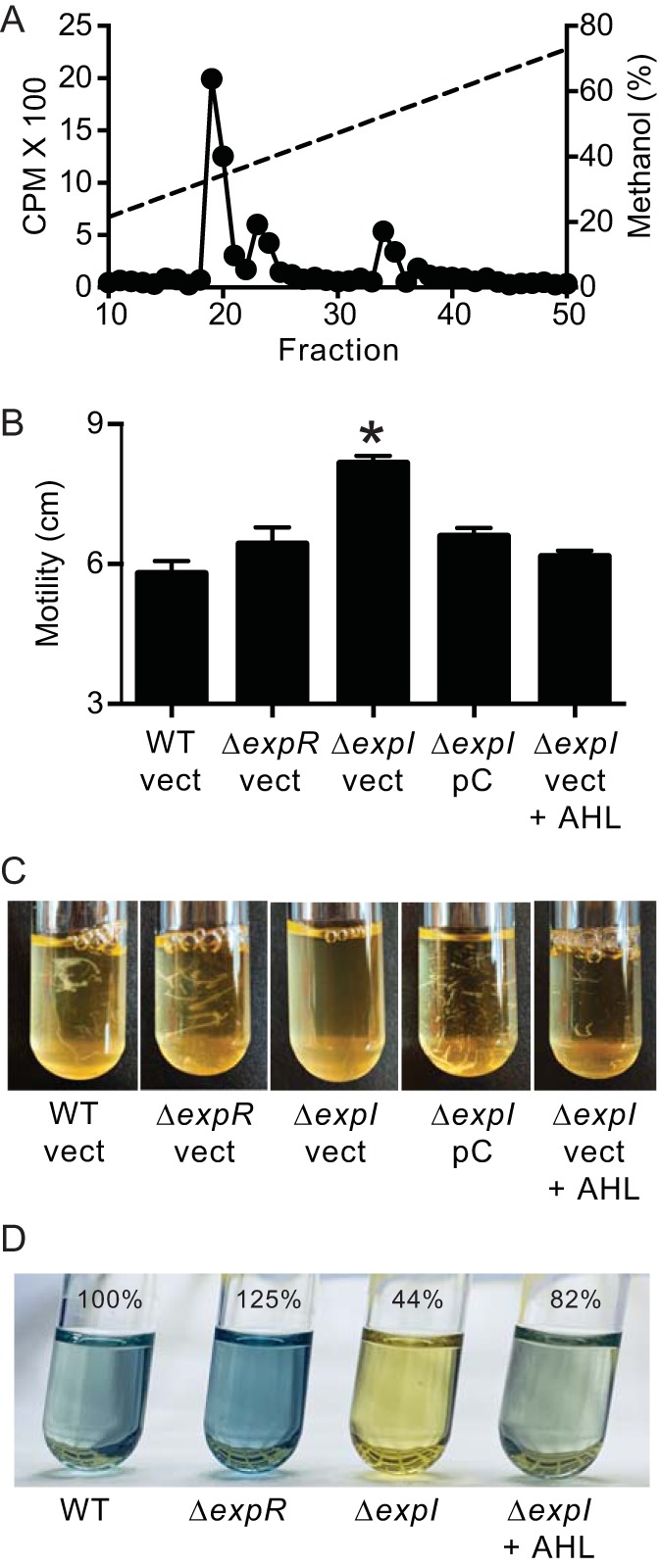
The major AHL produced by D. zeae MS2 and effects of expR and expI mutations on motility, aggregation, and indigoidine production. (A) HPLC profiles of 14C-AHLs synthesized by D. zeae MS2. The x axis indicates the fraction numbers collected over a 10 to 100% methanol-in-water gradient. The left y axis denotes the counts per minute (CPM) of radiolabel in each fraction (black circles), and the right y axis indicates the methanol concentration of the HPLC run (dashed line). (B) Motility on 0.3% agar plates. Data are means of three replicates, and the error bars are the standard deviations. The black asterisk indicates that the difference between the wild type (WT) and ΔexpI mutant is statistically significant (P < 0.01) using a two-tailed t test. (C) Cellular aggregation. (D) Indigoidine production. The amount of indigoidine produced relative to the wild type is given as a percent (means of three replicates). Strains are D. zeae MS2 wild type (WT) and ΔexpR and ΔexpI mutants; where indicated, strains harbored the pBBR1MCS-5 vector control (vect) or pCexpI (pC) or were grown with 2 μM 3OC6-HSL (AHL).
We then constructed ExpR and ExpI mutants. Growth of the mutants in lysogeny broth (LB) was indistinguishable from that of the wild type (see Fig. S2 in the supplemental material). By using a 3OC6-HSL bioassay, we estimate that wild-type MS2 produced the equivalent of about 1.5 μM 3OC6-HSL. We did not detect 3OC6-HSL in cultures of the ExpI mutant (limit of detection, 5 nM), and the ExpR mutant produced about 350 nM 3OC6-HSL. Similar to strain EC1, the MS2 ExpI mutant exhibited a small increase in motility (Fig. 4B) and a decrease in cell aggregation in the early logarithmic phase (Fig. 4C). Both phenotypes were recovered by complementing the mutation with an expI plasmid or by addition of 3OC6-HSL to the culture medium (Fig. 4). We also noticed that the indigoidine biosynthetic cluster and the expRI genes were located within a 35-kb region of the chromosome. Often luxR homologs are located near biosynthetic gene clusters under their regulatory control (44); thus, we extracted indigoidine from the quorum sensing mutants and found that the AHL synthase mutant, but not the receptor mutant, produced lower levels of blue pigment. Pigment production was restored by exogenous addition of 3OC6-HSL (Fig. 4D). This AHL effect is modest (about 2-fold), perhaps because there are multiple regulatory inputs into indigoidine production. In other Dickeya species, indigoidine production is influenced by carbon source, oxidative stress, and the PecS global repressor (11).
Our results are consistent with the notion that ExpR functions as a repressor of motility, aggregation, and pigment production as is the case for D. dadantii 3937 (45, 46), where, in the absence of AHL, the LuxR homolog binds to operator DNA and blocks transcription. When bound to the cognate AHL, the transcription factor is released from the operator and transcription commences.
We found that both the ExpR and ExpI mutants remained fully virulent in syringe-needle inoculations of banana pseudostems (Fig. 1). Also, neither mutant displayed any reduction in PCWDE production (see Fig. S3 in the supplemental material) or E. coli killing (Fig. 2). The ExpR-ExpI quorum sensing system is conserved in nearly all sequenced Dickeya (46 of 49) and Pectobacterium (57 of 58) strains and has been found to be important in infection and/or plant macerating enzyme production in nearly all strains tested (18), with the exception of D. dadantii 3937 and D. zeae MS2. Whether the lack of AHL control in banana virulence is a general feature of the Dickeya banana pathogens unique to MS2 or perhaps an experimental limitation (needle inoculation of the plant, which may not require normal motility) requires further experiments. As AHL-quorum-sensing systems have been targeted for infection control in several pectinolytic phytopathogens (47, 48), it seems important to investigate such questions.
Dickeya zeae MS2 possesses a predicted plant-responsive luxR homolog (pipR) adjacent to a pipA homolog, but this regulator is not required for plant virulence.
As described above, D. zeae MS2 also encodes a second LuxR homolog with 49% amino acid identity to the PipR regulator from Pseudomonas sp. GM79 (25). We have called the D. zeae MS2 genes pipR and pipA. The MS2 pipR possesses the two conserved amino acid substitutions (M65 and W69) distinguishing the plant-responsive LuxR homologs from AHL-responsive LuxR homologs (19–21). We created a PipR mutant and found it had no defect in E. coli killing (Fig. 2), indigoidine production (data not shown), motility, or PCWDE production (see Fig. S4 in the supplemental material). However, as PipR homologs of other bacteria require a plant-derived signal (22, 49), it is possible that the MS2 PipR is not active in laboratory cultures. However, the MS2 PipR mutant was fully virulent in syringe needle inoculations of banana pseudostems (Fig. 1), suggesting that the MS2 PipR is not required to activate any banana virulence genes.
The D. zeae MS2 pipA product has proline iminopeptidase activity and is required for banana plant disease.
Although we found that the MS2 PipR mutant has no apparent phenotype (see discussion above), we wondered if the gene adjacent to pipR, pipA, played any role in MS2 pathogenesis because in two Xanthomonas sp. pathogens, mutants in the pipA homolog are impaired in plant virulence (22, 24). The pipA gene of Pseudomonas GM79 codes for an aminopeptidase that shows a preference for cleavage of N-terminal prolines but can also cleave N-terminal alanines (25). This gene product shares 64% amino acid identity with the D. zeae MS2 pipA gene product. We first asked if the MS2 pipA product is a proline iminopeptidase. We purified the MS2 PipA enzyme as a hexahistidine-tagged fusion protein and assayed its ability to cleave N-terminal amino acids coupled to a fluorescent substrate (β-naphthylamide). The purified protein was most active against the proline substrate but also showed some activity with the hydroxyproline and alanine substrates. There was relatively little activity with any of the other substrates tested (Table 1). These results are similar to those reported for the Pseudomonas GM79 pipA product (25).
TABLE 1.
Substrate specificities of purified MS2 PipA-His6 enzyme
| Substrate | Relative PipA-His6 activitya |
|---|---|
| l-Proline-β-naphthylamide | 100.0 ± 0.7 |
| l-Alanine-β-naphthylamide | 34.5 ± 2.6 |
| l-Hydroxy-proline-β-naphthylamide | 5.8 ± 0.3 |
| l-Serine-β-naphthylamide | 1.4 ± 0.1 |
| l-Histidine-β-naphthylamide | 0.3 ± 0.1 |
| l-Leucine-β-naphthylamide | 0.7 ± 0.0 |
| l-Glutamic acid-β-naphthylamide | NDb |
| l-Lysine-β-naphthylamide | ND |
Enzyme purification and assay conditions were as described in Materials and Methods. Naphthylamide substrate results were measured as relative fluorescence units per minute per milligram of protein and normalized to activity exhibited by His6-PipA with l-proline-β-naphthylamide as substrate ± the standard deviation. Results are the averages of 4 to 8 assays ± the standard deviation.
ND, not detected; not above background of the no enzyme addition control.
We next screened the MS2 ΔpipA strain for a variety of phenotypes. Relative to the parent, there was no effect on growth in LB (Fig. S2) nor did the mutation affect E. coli killing (Fig. 2), cellular aggregation (data not shown), motility, or maceration in the potato slice assay (Fig. S4). However, the MS2 pipA mutant was greatly impaired in its ability to cause banana leaf rot (Fig. 1). Full virulence of the PipA mutant was restored by a pipA-complementing plasmid (Fig. 1). These data demonstrate that PipA is important for banana virulence as has been reported for Xanthomonas PipA homologs in cabbage black rot disease and bacterial pustule disease in soybeans (22, 24).
The MS2 PipR mutant has no defect in plant disease (Fig. 1), which suggests that it is not needed for sufficient in planta pipA expression as has been reported in other bacteria (22–24). We note that in the biocontrol strain Pseudomonas protegens Pf-5, the PipR homolog (PsoR) does not activate its downstream pipA homolog (21). We have not determined how the pipA product might be involved in D. zeae MS2 virulence. This also remains an open question for other phytopathogens where pipA homologs have been shown to be involved in virulence (22, 24), although recently it has been reported that the PipA homolog in Xanthomonas campestris pv. campestris 8004 is a type III effector that can elicit a hypersensitive response in nonhost plants and suppress plant immunity when expressed transgenically in Arabidopsis (50). Whether PipA is a type III effector protein in MS2 will be tested in future experiments; interestingly, many bacteria harboring PipR-PipA-type systems do not even encode type III secretion systems. Any understanding of how PipA functions at the molecular level to affect plant health could be important.
Unlike expR and expI, which are highly conserved in the genus Dickeya, pipR and pipA are found only in 5 of the 11 sequenced D. zeae genomes (strains MK19, NCPPB 2539, NCPPB 3532, MS1, and MS2). We note pipR and pipA are in the genomes of both banana isolates (MS1 and MS2). It will be interesting to learn how similar other D. zeae banana isolates are to strain MS2, whether or not pipA is present and plays a role in banana virulence, and how D. zeae has evolved to become an emerging pathogen of bananas in Southeast Asia.
MATERIALS AND METHODS
Bacterial strains, plasmids, growth conditions, and chemicals.
Bacterial strains and plasmids are described in Tables 2 and 3. D. zeae MS2 and its derived strains were grown in LB (51) or in M9 minimal medium (52) with 10 mM glucose. Bacteria were grown with shaking (200 rpm) at 30°C. Escherichia coli strains were grown in LB at 37°C with shaking. Antibiotics were used at the following final concentrations: kanamycin, 50 μg/ml; ampicillin, 100 μg/ml; streptomycin, 50 μg/ml; chloramphenicol, 35 μg/ml; and gentamicin, 20 μg/ml. Plating was on LB or minimal medium solidified with 1.5% agar. Chemically synthesized AHL compounds used in this work included 3OC6-HSL [N-(β-ketocaproyl)-l-homoserine lactone, item K3007; Sigma-Aldrich, St. Louis, MO] and 3OC8-HSL [N-(β-oxo-octanoyl)-l-homoserine lactone, item 10011206; Cayman Chemical, Ann Arbor, MI].
TABLE 2.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Source/reference |
|---|---|---|
| D. zeae strains | ||
| MS2 | Wild type; isolated from infected banana | 1 |
| Δ5075 | C1030_05075 deletion mutant (annotated as nonribosomal peptide synthetase gene) of MS2 | This work |
| ΔexpR | expR deletion mutant of MS2 | This work |
| ΔexpI | expI deletion mutant of MS2 | This work |
| ΔpipR | pipR deletion mutant of MS2 | This work |
| ΔpipA | pipA deletion mutant of MS2 | This work |
| E. coli strains | ||
| DH5α | fhuA2 Δ(argF-lacZ)U169 phoA glnV44 Φ80 Δ(lacZ)M15 gyrA96 recA1 relA1 endA1 thi-1 hsdR17 | Invitrogen |
| C118 λpir | Δ(ara-leu) araD ΔlacX74 galE galK phoA20 thi-1 rpsE rpoB argE (Am) recA1 λpir | 63 |
| SM10 λpir | thi-1 thr leu tonA lacY supE recA::RP4-2-Tc::Mu λpir Kanr | 56 |
| DH12S(pHV402, pLC42) | 3OC6-HSL bioassay reporter; Camr Kanr | 64 |
| Plasmids | ||
| pKNG101 | Suicide vector; strAB sacB oriR6K monRK2; Strr | 65 |
| pKNG5075 | Knockout plasmid for 5075-mutant strain construction; Strr | This work |
| pKNGexpR | Knockout plasmid for ExpR-mutant strain construction; Strr | This work |
| pKNGexpI | Knockout plasmid for ExpI-mutant strain construction; Strr | This work |
| pKNGpipR | Knockout plasmid for PipR-mutant strain construction; Strr | This work |
| pKNGpipA | Knockout plasmid for PipA-mutant strain construction; Strr | This work |
| pBBR1MCS-5 | Broad-host-range vector; Genr | 62 |
| pC5075 | pBBR1MCS-5 expressing Plac C1030_05075; Genr | This work |
| pCexpI | pBBR1MCS-5 expressing Plac expI; Genr | This work |
| pCpipA | pBBR1MCS-5 expressing Plac pipA; Genr | This work |
| pBBR1MCS-4 | Broad-host-range vector; Ampr | 62 |
| pPipAhis | C-terminal PipA-His6 expression plasmid; Ampr | This work |
TABLE 3.
Primers used in this study
| Primer | Sequence (5′–3′) | Description |
|---|---|---|
| pKNG-F | CGTATTCAGTGTCGCTGATTTGTA | Suicide vector backbone |
| pKNG-R | GGTACCATAAGTAGAAGCAGCAAC | Suicide vector backbone |
| expI-KO-UP-F | AACAACTTCAGACAATACAAATCAGCGACACTGAATACGTCACCCTGAACGGACGTGATC | Construction of ΔexpI mutant |
| expI-KO-UP-R | CGAAATGAACCTGATCAAGCTCCCCCTATCCTTAACTTCATGTC | Construction of ΔexpI mutant |
| expI-KO-DN-F | GAAGTTAAGGATAGGGGGAGCTTGATCAGGTTCATTTCGACC | Construction of ΔexpI mutant |
| expI-KO-DN-R | GGTAAAGCTACTTGGGTTGCTGCTTCTACTTATGGTACCGTGCCGTCATGATCAGATTGC | Construction of ΔexpI mutant |
| pipA-KO-UP-F | AAACAACTTCAGACAATACAAATCAGCGACACTGAATACGGATGCGGTGATTTACGACTACAC | Construction of ΔpipA mutant |
| pipA-KO-UP-R | GGTGTTAGCAACCATTAACAGGTGTACATGAGTGACGTTTACC | Construction of ΔpipA mutant |
| pipA-KO-DN-F | AAACGTCACTCATGTACACCTGTTAATGGTTGCTAACACCAGG | Construction of ΔpipA mutant |
| pipA-KO-DN-R | TGGTAAAGCTACTTGGGTTGCTGCTTCTACTTATGGTACCCAAGAGCGCATAGGTAGTCATTAG | Construction of ΔpipA mutant |
| 5075-KO-UP-F | AAACAACTTCAGACAATACAAATCAGCGACACTGAATACGGTGCTGCTGGATACCTTCG | Construction of Δ5075 mutant |
| 5075-KO-UP-R | CGCCATGCCAAAAGTTTTACCAGTCATCCACCACGTTGATC | Construction of Δ5075 mutant |
| 5075-KO-DN-F | ATCAACGTGGTGGATGACTGGTAAAACTTTTGGCATGGCGC | Construction of Δ5075 mutant |
| 5075-KO-DN-R | TGGTAAAGCTACTTGGGTTGCTGCTTCTACTTATGGTACCAAACGCGGACTGAAAGTTGG | Construction of Δ5075 mutant |
| pipA-F | CCCAAGCTTGTAACTAGCATTTTTGCTAGTG | Construction of pPipAhis |
| pipA-R | AACTGCAGAGCAACCATTAGTGATGATGATGATGATGACAATACTGCGTC | Construction of pPipAhis |
| pBBR-F | GCGCTTGGCGTAATCATGGTC | pBBR1MCS-5 backbone |
| pBBR-R | TCGTGACTGGGAAAACCCTGG | pBBR1MCS-5 backbone |
| C5075-F | AAACAGCTATGACCATGATTACGCCAAGCGCGCAAACGTTACCGCACAACCAGAG | Construction of pC5075 |
| C5075-R | TGTAGCCGCCTCGCCGCCCTATACCTTGTCTGCCTTAGCTGACTGGAGGCAATGC | Construction of pC5075 |
| CexpI-F | ACACAGGAAACAGCTATGACCATGATTACGCCAAGCGCTCCCAGCGGGAGTTATCTCAC | Construction of pCexpI |
| CexpI-R | GATTAAGTTGGGTAACGCCAGGGTTTTCCCAGTCACGACTAAACACGCCATTCGGCTTG | Construction of pCexpI |
| CpipA-F | CCCAAGCTTATAACTAGCATTTTTGCTAG | Construction of pCpipA |
| CpipA-R | AACTGCAGCCTACCTGGTGTTAGCAACC | Construction of pCpipA |
Genome sequencing.
DNA was isolated from D. zeae MS2 grown to mid-logarithmic phase in LB. Genomic sequencing, assembly, and electronic annotation were performed by Total Genetics Solution Company (Shenzhen, China). Genome sequencing was performed using PacBio RSII (250-fold coverage) and Illumina HiSeq 2500 (400-fold coverage). The whole-genome sequencing approach resulted in 3,495,253 high-quality filtered reads with an average paired-end read length of 150 bp and 110-fold sequencing coverage on average. De novo assembly was with SMRT Analysis version 2.3.0 (Pacific Biosciences, Menlo Park, CA) and SOAPaligner version 2.21 (https://github.com/aquaskyline/SOAPdenovo2). Functional annotation was done by RefSeq Prokaryotic Genome Annotation Project (RGAP) and BLAST. The genome was closed and consists of 4,740,052 bp. The genome was annotated by using the NCBI Prokaryotic Genome Annotation Pipeline (PGAP) (53), and putative biosynthetic gene clusters were identified by using antiSMASH 5.0 (40) with default settings. LuxI and LuxR homologs were identified by searching for their associated PFAM domains (pfam00765 and pfam03472/pfam00196, respectively) (54). To identify the genes for secretion systems and PCWDEs, we performed tBLASTn analyses of those previously described systems in the related Dickeya genomes, including D. zeae MS1 (5), D. zeae EC1 (16), D. zeae Ech586 (26), and D. dadantii 3937 (27).
Mutant and plasmid construction.
All plasmids and primer sequences are described in Tables 2 and 3, respectively. For knockout plasmids used to construct the ExpR and PipR mutants (ΔexpR and ΔpipR), about 500 bp of upstream and downstream flanking DNA was synthesized (IGE Biotechnology Ltd, Guangzhou, China) and then cloned into the suicide plasmid pKNG101 by standard restriction enzyme-based cloning. To construct the ΔC1030_05075, ΔexpI, and ΔpipA knockout plasmids, we used genomic DNA as a template to PCR amplify about 750 bp of upstream and downstream DNA of the intended deletion with overlapping homology, including that of the suicide plasmid pKNG101. We used E. coli DH5α for in vivo plasmid assembly as described elsewhere (55). All constructs were confirmed by DNA sequencing and introduced in the conjugal donor strain E. coli SM10 λpir by electroporation. Plasmids were moved to strain MS2 by conjugal transfer (56) and double-crossover transconjugants were selected on M9 minimal agar plates containing 10% sucrose for sacB counterselection. To create the complementing plasmids, expI (including 175 bp of DNA upstream of the translational start codon) or pipA (including 85 bp upstream of the start codon) was PCR amplified and cloned in pBBR1MCS-5 by using E. coli-mediated DNA assembly (55) or standard restriction enzyme-based techniques. All plasmid constructions were confirmed by DNA sequencing and introduced into MS2 via electroporation. Growth of mutants in LB was indistinguishable from that of the wild type (see Fig. S2 in the supplemental material).
Banana virulence assays.
Young banana plants (Musa ABB) were obtained from the Fruit Research Institute, Guangdong Academy of Agricultural Sciences (Guangzhou, China). Efforts were made to obtain plants of similar size and characteristics. Banana plants were grown in plastic pots with garden soil and acclimated in a greenhouse at about 25°C with 12-h alternating light-dark cycles for 2 weeks prior to infection. For infections, bacteria were grown overnight in LB with shaking at 28°C and then diluted 1:100 into 5 ml fresh LB and grown to mid-log phase (optical density at 600 nm [OD600] of 1.0). When appropriate, antibiotics were added for plasmid maintenance. Plants were infected via needle injection (0.1 ml of culture in 1-ml syringe with 26-gauge needle) into the central pseudostem. Plants were incubated at 30°C with 12-h alternating light-dark cycles for 7 days, and then disease was assessed by using a modified virulence score (57) as described in Fig. S1 in the supplemental material.
E. coli inhibition assay.
To survey D. zeae strains for the ability to inhibit growth of E. coli, we used a method similar to one described previously (14, 58). Briefly, 20 ml of LB agar (1.5% agar) plus gentamicin was solidified in square plastic plates (10 cm by 10 cm). The plates were overlaid with 15-ml of 1% agarose cooled to 50°C and inoculated with E. coli pBBR1MCS-5 (108 CFU). Cell-free culture fluid from the indicated D. zeae strains was prepared from overnight LB cultures filtered through a 0.2-μm filter. A paper disk (6 mm from Becton, Dickinson and Company, NJ, USA) was saturated with 40 μl of culture fluid and placed on top of the E. coli-impregnated agarose using sterilized tweezers. Plates were incubated at 37°C for 24 h. When the plates were imaged using transmitted light from a “bucket of light” (59), confluent E. coli growth in the agarose appeared as a whitish color while zones of growth inhibition appeared as a gray/blue color emanating from the paper disk (Fig. 2A). The diameters of these gray/blue-colored zones of clearing were measured using a ruler.
Detection of AHL signals.
To detect and identify AHL signals, we utilized a 14C-radiolabel assay (43) as follows. Bacteria were grown in 4 ml of minimal medium to log phase and then incubated for 16 h in the presence of 5 μCi (about 90 nM) of [1-14C]-methionine (American Radiolabeled Chemicals, Inc., St. Louis, MO). The 14C-labeled AHLs were extracted with acidified ethyl acetate (0.1 ml of glacial acetic acid per liter of solvent) and fractionated by C18 reverse-phase HPLC. Radioactivity in each fraction was determined by scintillation counting. To determine the retention times of synthetic AHL standards, we used the same HPLC protocol and monitored AHL elution by UV absorbance at 210 nm. We utilized the bioassay with the reporter strain E. coli DH12S(pHV402, pLC42) to measure 3OC6-HSL in culture extracts with a standard curve generated using synthetic 3OC6-HSL [N-(β-ketocaproyl)-l-homoserine lactone, item K3007; Sigma-Aldrich, St. Louis, MO] according to a protocol described previously (60).
Motility assays, cell aggregation assays, and measurement of indigoidine.
Bacterial motility was assessed by using soft-agar motility plates (0.3% agar) as described previously (3), except that 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) was omitted from the medium. Cell aggregation was assessed by eye at 3 to 4 h postinoculation (early logarithmic phase) into 4 ml of LB broth incubated at 30°C with shaking. Indigoidine was extracted with dimethyl sulfoxide (DMSO) from agar-grown cells as described previously (61). Blue pigment was measured by absorption at A615, and values were normalized relative to the wild-type D. zeae MS2.
Purification and enzyme activity of PipA-His6.
To obtain purified PipA, the pipA gene was amplified from genomic DNA and cloned into pBBR1MCS-4 (62) to create pPipAhis. The reverse primer contained a C-terminal hexahistidine tag to aid in protein purification (Table 3). PipA-His6 overexpression, purification, and enzyme activity assays were performed as described previously (25) except that nickel resin was used in place of cobalt resin.
Data availability.
The genome sequence has been deposited at the National Center for Biotechnology Information (NCBI accession number CP025799 and BioProject number PRJNA429264).
Supplementary Material
ACKNOWLEDGMENTS
We thank Ajai Dandekar for helpful statistical analysis discussions.
This research was sponsored by grants from the National Natural Science Foundation of China (31972230), National Key Project for Basic Research of China (2015CB150600), Team R&D Project of Guangdong Province (key areas of research and development program 2018B020205003), and the China Scholarship Council fellowship program grant (201808440406) to L.F.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01611-19.
REFERENCES
- 1.Hu M, Li J, Chen R, Li W, Feng L, Shi L, Xue Y, Feng X, Zhang L, Zhou J. 2018. Dickeya zeae strains isolated from rice, banana and clivia rot plants show great virulence differentials. BMC Microbiol 18:136. doi: 10.1186/s12866-018-1300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald P, Dow M, Verdier V, Beer SV, Machado MA, Toth I, Salmond G, Foster GD. 2012. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol 13:614–629. doi: 10.1111/j.1364-3703.2012.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussain BM, Zhang HB, Xu JL, Liu Q, Jiang Z, Zhang LH. 2008. The acyl-homoserine lactone-type quorum-sensing system modulates cell motility and virulence of Erwinia chrysanthemi pv. zeae. J Bacteriol 190:1045–1053. doi: 10.1128/JB.01472-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perombelon MCM, Kelman A. 1980. Ecology of the soft rot erwinias. Annu Rev Phytopathol 18:361–387. doi: 10.1146/annurev.py.18.090180.002045. [DOI] [Google Scholar]
- 5.Zhang JX, Lin BR, Shen HF, Pu XM. 2013. Genome sequence of the banana pathogen Dickeya zeae strain MS1, which causes bacterial soft rot. Genome Announc 13:e00317-13. doi: 10.1128/genomeA.00317-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samson R, Legendre JB, Christen R, Fischer-Le Saux M, Achouak W, Gardan L. 2005. Transfer of Pectobacterium chrysanthemi (Burkholder et al. 1953) Brenner et al. 1973 and Brenneria paradisiaca to the genus Dickeya gen. nov. as Dickeya chrysanthemi comb. nov. and Dickeya paradisiaca comb. nov. and delineation of four novel species, Dickeya dadantii sp. nov., Dickeya dianthicola sp. nov., Dickeya dieffenbachiae sp. nov. and Dickeya zeae sp. nov. Int J Syst Evol Microbiol 55:1415–1427. doi: 10.1099/ijs.0.02791-0. [DOI] [PubMed] [Google Scholar]
- 7.Hugouvieux-Cotte-Pattat N, Condemine G, Nasser W, Reverchon S. 1996. Regulation of pectinolysis in Erwinia chrysanthemi. Annu Rev Microbiol 50:213–257. doi: 10.1146/annurev.micro.50.1.213. [DOI] [PubMed] [Google Scholar]
- 8.Expert D. 1999. Withholding and exchanging iron: interactions between Erwinia spp. and their plant hosts. Annu Rev Phytopathol 37:307–334. doi: 10.1146/annurev.phyto.37.1.307. [DOI] [PubMed] [Google Scholar]
- 9.Wu X, Zeng Q, Koestler BJ, Waters CM, Sundin GW, Hutchins W, Yang CH. 2014. Deciphering the components that coordinately regulate virulence factors of the soft rot pathogen Dickeya dadantii. Mol Plant Microbe Interact 27:1119–1131. doi: 10.1094/MPMI-01-14-0026-R. [DOI] [PubMed] [Google Scholar]
- 10.Yap MN, Yang CH, Barak JD, Jahn CE, Charkowski AO. 2005. The Erwinia chrysanthemi type III secretion system is required for multicellular behavior. J Bacteriol 187:639–648. doi: 10.1128/JB.187.2.639-648.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reverchon S, Rouanet C, Expert D, Nasser W. 2002. Characterization of indigoidine biosynthetic genes in Erwinia chrysanthemi and role of this blue pigment in pathogenicity. J Bacteriol 184:654–665. doi: 10.1128/JB.184.3.654-665.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pait IGU, Kitani S, Kurniawan YN, Asa M, Iwai T, Ikeda H, Nihira T. 2017. Identification and characterization of lbpA, an indigoidine biosynthetic gene γ-butyrolactone signaling system of Streptomyces lavendulae FRI-5. J Biosci Bioeng 124:369–375. doi: 10.1016/j.jbiosc.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 13.Hellberg JE, Matilla MA, Salmond GP. 2015. The broad-spectrum antibiotic, zeamine, kills the nematode worm Caenorhabditis elegans. Front Microbiol 6:137. doi: 10.3389/fmicb.2015.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, Zhang H, Wu J, Liu Q, Xi P, Lee J, Liao J, Jiang Z, Zhang L-H. 2011. A novel multidomain polyketide synthase is essential for zeamine production and the virulence of Dickeya zeae. Mol Plant Microbe Interact 24:1156–1164. doi: 10.1094/MPMI-04-11-0087. [DOI] [PubMed] [Google Scholar]
- 15.Barnard AM, Salmond GP. 2007. Quorum sensing in Erwinia species. Anal Bioanal Chem 387:415–423. doi: 10.1007/s00216-006-0701-1. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Cheng Y, Lv M, Liao L, Chen Y, Gu Y, Liu S, Jiang Z, Xiong Y, Zhang L. 2015. The complete genome sequence of Dickeya zeae EC1 reveals substantial divergence from other Dickeya strains and species. BMC Genomics 16:571. doi: 10.1186/s12864-015-1545-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson RA, Eriksson AR, Heikinheimo R, Mae A, Pirhonen M, Koiv V, Hyytiainen H, Tuikkala A, Palva ET. 2000. Quorum sensing in the plant pathogen Erwinia carotovora subsp. carotovora: the role of expREcc. Mol Plant Microbe Interact 13:384–393. doi: 10.1094/MPMI.2000.13.4.384. [DOI] [PubMed] [Google Scholar]
- 18.Crepin A, Beury-Cirou A, Barbey C, Farmer C, Helias V, Burini JF, Faure D, Latour X. 2012. N-acyl homoserine lactones in diverse Pectobacterium and Dickeya plant pathogens: diversity, abundance, and involvement in virulence. Sensors 12:3484–3497. doi: 10.3390/s120303484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez JF, Venturi V. 2012. A novel widespread interkingdom signaling circuit. Trends Plant Sci 18:167–174. doi: 10.1016/j.tplants.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Patankar AV, Gonzalez JE. 2009. Orphan LuxR regulators of quorum sensing. FEMS Microbiol Rev 33:739–756. doi: 10.1111/j.1574-6976.2009.00163.x. [DOI] [PubMed] [Google Scholar]
- 21.Subramoni S, Gonzalez JF, Johnson A, Pechy-Tarr M, Rochat L, Paulsen I, Loper JE, Keel C, Venturi V. 2011. Bacterial subfamily of LuxR regulators that respond to plant compounds. Appl Environ Microbiol 77:4579–4588. doi: 10.1128/AEM.00183-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chatnaparat T, Prathuangwong S, Ionescu M, Lindow SE. 2012. XagR, a LuxR homolog, contributes to the virulence of Xanthomonas axonopodis pv. glycines to soybean. Mol Plant Microbe Interact 25:1104–1117. doi: 10.1094/MPMI-01-12-0008-R. [DOI] [PubMed] [Google Scholar]
- 23.Ferluga S, Venturi V. 2009. OryR is a LuxR-family protein involved in interkingdom signaling between pathogenic Xanthomonas oryzae pv. oryzae and rice. J Bacteriol 191:890–897. doi: 10.1128/JB.01507-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Jia Y, Wang L, Fang R. 2007. A proline iminopeptidase gene upregulated in planta by a LuxR homologue is essential for pathogenicity of Xanthomonas campestris pv. campestris. Mol Microbiol 65:121–136. doi: 10.1111/j.1365-2958.2007.05775.x. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer AL, Oda Y, Coutinho BG, Pelletier DA, Weiburg J, Venturi V, Greenberg EP, Harwood CS. 2016. A LuxR homolog in a cottonwood tree endophyte that activates gene expression in response to a plant signal or specific peptides. mBio 7:e01101-16. doi: 10.1128/mBio.01101-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pritchard L, Humphris S, Saddler GS, Parkinson NM, Bertrand V, Elphinstone JG, Toth IK. 2012. Detection of phytopathogens of the genus Dickeya using a PCR primer prediction pipeline for draft bacterial genome sequences. Plant Pathol 62:587–596. doi: 10.1111/j.1365-3059.2012.02678.x. [DOI] [Google Scholar]
- 27.Glasner JD, Yang C-H, Reverchon S, Hugouvieux-Cotte-Pattat N, Condemine G, Bohin J-P, Van Gijsegem F, Yang S, Franza T, Expert D, Plunkett G, San Francisco MJ, Charkowski AO, Py B, Bell K, Rauscher L, Rodriguez-Palenzuela P, Toussaint A, Holeva MC, He SY, Douet V, Boccara M, Blanco C, Toth I, Anderson BD, Biehl BS, Mau B, Flynn SM, Barras F, Lindeberg M, Birch PRJ, Tsuyumu S, Shi X, Hibbing M, Yap M-N, Carpentier M, Dassa E, Umehara M, Kim JF, Rusch M, Soni P, Mayhew GF, Fouts DE, Gill SR, Blattner FR, Keen NT, Perna NT. 2011. Genome sequence of the plant-pathogenic bacterium Dickeya dadantii 3937. J Bacteriol 193:2076–2077. doi: 10.1128/JB.01513-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Binet R, Letoffe S, Ghigo JM, Delepelaire P, Wandersman C. 1997. Protein secretion by Gram-negative bacterial ABC exporters–a review. Gene 192:7–11. doi: 10.1016/S0378-1119(96)00829-3. [DOI] [PubMed] [Google Scholar]
- 29.Delepelaire P, Wandersman C. 1991. Characterization, localization and transmembrane organization of the three proteins PrtD, PrtE and PrtF necessary for protease secretion by the Gram-negative bacterium Erwinia chrysanthemi. Mol Microbiol 5:2427–2434. doi: 10.1111/j.1365-2958.1991.tb02088.x. [DOI] [PubMed] [Google Scholar]
- 30.Ferrandez Y, Condemine G. 2008. Novel mechanism of outer membrane targeting of proteins in Gram-negative bacteria. Mol Microbiol 69:1349–1357. doi: 10.1111/j.1365-2958.2008.06366.x. [DOI] [PubMed] [Google Scholar]
- 31.Lindeberg M, Boyd CM, Keen NT, Collmer A. 1998. External loops at the C terminus of Erwinia chrysanthemi pectate lyase C are required for species-specific secretion through the out type II pathway. J Bacteriol 180:1431–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alfano JR, Collmer A. 1997. The type III (Hrp) secretion pathway of plant pathogenic bacteria: trafficking harpins, Avr proteins, and death. J Bacteriol 179:5655–5662. doi: 10.1128/jb.179.18.5655-5662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauer DW, Bogdanove AJ, Beer SV, Collmer A. 1994. Erwinia chrysanthemi hrp genes and their involvement in soft rot pathogenesis and elicitation of the hypersensitive response. Mol Plant Microbe Interact 7:573–581. doi: 10.1094/MPMI-7-0573. [DOI] [PubMed] [Google Scholar]
- 34.Bingle LE, Bailey CM, Pallen MJ. 2008. Type VI secretion: a beginner’s guide. Curr Opin Microbiol 11:3–8. doi: 10.1016/j.mib.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Pedron J, Mondy S, Des Essarts YR, Van Gijsegem F, Faure D. 2014. Genomic and metabolic comparison with Dickeya dadantii 3937 reveals the emerging Dickeya solani potato pathogen to display distinctive metabolic activities and T5SS/T6SS-related toxin repertoire. BMC Genomics 15:283. doi: 10.1186/1471-2164-15-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silverman JM, Brunet YR, Cascales E, Mougous JD. 2012. Structure and regulation of the type VI secretion system. Annu Rev Microbiol 66:453–472. doi: 10.1146/annurev-micro-121809-151619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang CH, Gavilanes-Ruiz M, Okinaka Y, Vedel R, Berthuy I, Boccara M, Chen JW, Perna NT, Keen NT. 2002. hrp genes of Erwinia chrysanthemi 3937 are important virulence factors. Mol Plant Microbe Interact 15:472–480. doi: 10.1094/MPMI.2002.15.5.472. [DOI] [PubMed] [Google Scholar]
- 38.Bender CL, Alarcon-Chaidez F, Gross DC. 1999. Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol Mol Biol Rev 63:266–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pierson LS III, Pierson EA. 2010. Metabolism and function of phenazines in bacteria: impacts on the behavior of bacteria in the environment and biotechnological processes. Appl Microbiol Biotechnol 86:1659–1670. doi: 10.1007/s00253-010-2509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blin K, Medema MH, Kottmann R, Lee SY, Weber T. 2017. The antiSMASH database, a comprehensive database of microbial secondary metabolite biosynthetic gene clusters. Nucleic Acids Res 45:D555–D559. doi: 10.1093/nar/gkw960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGowan SJ, Sebaihia M, Porter LE, Stewart G, Williams P, Bycroft BW, Salmond GPC. 1996. Analysis of bacterial carbapenem antibiotic production genes reveals a novel beta-lactam biosynthesis pathway. Mol Microbiol 22:415–426. doi: 10.1046/j.1365-2958.1996.00125.x. [DOI] [PubMed] [Google Scholar]
- 42.Schuster M, Lostroh CP, Ogi T, Greenberg EP. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol 185:2066–2079. doi: 10.1128/JB.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaefer AL, Harwood CS, Greenberg EP. 2018. “Hot stuff”: the many uses of a radiolabel assay in detecting acyl-homoserine lactone quorum-sensing signals. Methods Mol Biol 1673:35–47. doi: 10.1007/978-1-4939-7309-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brotherton CA, Medema MH, Greenberg EP. 2018. luxR homolog-linked biosynthetic gene clusters in Proteobacteria. mSystems 3:e00208-17. doi: 10.1128/mSystems.00208-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castang S, Reverchon S, Gouet P, Nasser W. 2006. Direct evidence for the modulation of the activity of the Erwinia chrysanthemi quorum-sensing regulator ExpR by acylhomoserine lactone pheromone. J Biol Chem 281:29972–29987. doi: 10.1074/jbc.M601666200. [DOI] [PubMed] [Google Scholar]
- 46.Reverchon S, Bouillant ML, Salmond G, Nasser W. 1998. Integration of the quorum-sensing system in the regulatory networks controlling virulence factor synthesis in Erwinia chrysanthemi. Mol Microbiol 29:1407–1418. doi: 10.1046/j.1365-2958.1998.01023.x. [DOI] [PubMed] [Google Scholar]
- 47.Dong YH, Wang LH, Xu JL, Zhang HB, Zhang XF, Zhang LH. 2001. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 411:813–817. doi: 10.1038/35081101. [DOI] [PubMed] [Google Scholar]
- 48.Faure D, Dessaux Y. 2007. Quorum sensing as a target for developing control strategies for the plan pathogen Pectobacterium. Eur J Plant Pathol 119:353–365. doi: 10.1007/s10658-007-9149-1. [DOI] [Google Scholar]
- 49.Wang L, Zhang L, Geng Y, Xi W, Fang R, Jia Y. 2011. XerR, a negative regulator of XccR in Xanthomonas campestris pv. campestris, relieves its repressor function in planta. Cell Res 21:1131–1142. doi: 10.1038/cr.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kan J, An L, Wu Y, Long J, Song L, Fang R, Jia Y. 2018. A dual role for proline iminopeptidase in the regulation of bacterial motility and host immunity. Mol Plant Pathol 19:2011–2024. doi: 10.1111/mpp.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 52.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 53.Tatusove T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, Zaslavsky L, Lomsadze A, Pruitt KD, Borodovsky M, Ostell J. 2016. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res 44:6614–6624. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schaefer AL, Lappala CR, Morlen RP, Pelletier DA, Lu TY, Lankford PK, Harwood CS, Greenberg EP. 2013. LuxR- and LuxI-type quorum-sensing circuits are prevalent in members of the Populus deltoides microbiome. Appl Environ Microbiol 79:5745–5752. doi: 10.1128/AEM.01417-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kostylev M, Otwell AE, Richardson RE, Suzuki Y. 2015. Cloning should be simple: Escherichia coli DH5a-mediated assembly of multiple DNA fragments with short end homologies. PLoS One 10:e0137466. doi: 10.1371/journal.pone.0137466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simon R, Priefer U, Puhler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Nat Biotechnol 1:784–791. doi: 10.1038/nbt1183-784. [DOI] [Google Scholar]
- 57.Down JM, Clarke BR, Milligan DE, Tang JL, Daniels MJ. 1990. Extracellular proteases from Xanthomonas campestris pv. campestris, the black rot pathogen. Appl Environ Microbiol 56:2994–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liao L, Cheng Y, Liu S, Zhou J, An S, Lv M, Chen Y, Gu Y, Chen S, Zhang LH. 2014. Production of novel antibiotics zeamines through optimizing Dickeya zeae fermentation conditions. PLoS One 9:e116047. doi: 10.1371/journal.pone.0116047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parkinson JS. 2007. A “bucket of light” for viewing bacterial colonies in soft agar. Methods Enzymol 423:432–435. doi: 10.1016/S0076-6879(07)23020-4. [DOI] [PubMed] [Google Scholar]
- 60.Puri AW, Schaefer AL, Fu Y, Beck DA, Greenberg EP, Lidstrom K. 2017. Quorum sensing in a methane-oxidizing bacterium. J Bacteriol 14:e00773-16. doi: 10.1128/JB.00773-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee YA, Yu CP. 2006. A differential medium for the isolation and rapid identification of a plant soft rot pathogen, Erwinia chrysanthemi. J Microbiol Methods 64:200–206. doi: 10.1016/j.mimet.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 62.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM II, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 63.Herrero M, de Lorenzo V, Timmis KN. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in Gram-negative bacteria. J Bacteriol 172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antunes LC, Schaefer AL, Ferreira RB, Qin N, Stevens AM, Ruby EG, Greenberg EP. 2007. Transcriptome analysis of the Vibrio fischeri LuxR-LuxI regulon. J Bacteriol 189:8387–8391. doi: 10.1128/JB.00736-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaniga K, Delor I, Cornelis GR. 1991. A wide-host-range suicide vector for improving reverse genetics in Gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109:137–141. doi: 10.1016/0378-1119(91)90599-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genome sequence has been deposited at the National Center for Biotechnology Information (NCBI accession number CP025799 and BioProject number PRJNA429264).