Many microbial pathogen populations consist of multiple strains that induce strain-specific antibody responses in their vertebrate hosts. Females can transmit these antibodies to their offspring, thereby providing them with short-term strain-specific protection against microbial pathogens. We investigated this phenomenon using multiple strains of the tick-borne microbial pathogen Borrelia afzelii and its natural rodent reservoir host, the bank vole, as a model system. We found that female bank voles infected with B. afzelii transmitted to their offspring maternal antibodies that provided highly efficient but strain-specific protection against a natural tick bite challenge. The transgenerational transfer of antibodies could be a mechanism that maintains the high strain diversity of this tick-borne pathogen in nature.
KEYWORDS: Borrelia afzelii, Lyme disease, ecology of infectious disease, maternal antibodies, maternal effects, outer surface protein C, strain-specific immunity, tick-borne disease, Borrelia burgdorferi, vector-borne pathogen
ABSTRACT
Multistrain microbial pathogens often induce strain-specific antibody responses in their vertebrate hosts. Mothers can transmit antibodies to their offspring, which can provide short-term, strain-specific protection against infection. Few experimental studies have investigated this phenomenon for multiple strains of zoonotic pathogens occurring in wildlife reservoir hosts. The tick-borne bacterium Borrelia afzelii causes Lyme disease in Europe and consists of multiple strains that cycle between the tick vector (Ixodes ricinus) and vertebrate hosts, such as the bank vole (Myodes glareolus). We used a controlled experiment to show that female bank voles infected with B. afzelii via tick bite transmit protective antibodies to their offspring. To test the specificity of protection, the offspring were challenged using a natural tick bite challenge with either the maternal strain to which the mothers had been exposed or a different strain. The maternal antibodies protected the offspring against a homologous infectious challenge but not against a heterologous infectious challenge. The offspring from the uninfected control mothers were equally susceptible to both strains. Borrelia outer surface protein C (OspC) is an antigen that is known to induce strain-specific immunity. Maternal antibodies in the offspring reacted more strongly with homologous than with heterologous recombinant OspC, but other antigens may also mediate strain-specific immunity. Our study shows that maternal antibodies provide strain-specific protection against B. afzelii in an ecologically important rodent reservoir host. The transmission of maternal antibodies may have important consequences for the epidemiology of multistrain pathogens in nature.
IMPORTANCE Many microbial pathogen populations consist of multiple strains that induce strain-specific antibody responses in their vertebrate hosts. Females can transmit these antibodies to their offspring, thereby providing them with short-term strain-specific protection against microbial pathogens. We investigated this phenomenon using multiple strains of the tick-borne microbial pathogen Borrelia afzelii and its natural rodent reservoir host, the bank vole, as a model system. We found that female bank voles infected with B. afzelii transmitted to their offspring maternal antibodies that provided highly efficient but strain-specific protection against a natural tick bite challenge. The transgenerational transfer of antibodies could be a mechanism that maintains the high strain diversity of this tick-borne pathogen in nature.
INTRODUCTION
The maternal environment and maternal phenotype can have important consequences for the offspring phenotype and offspring fitness (1). In vertebrate hosts, an important maternal effect is the transmission of antibodies from mothers to their offspring (2, 3). Young vertebrates are susceptible to infectious diseases while their immune systems are developing (3). Maternally transmitted antibodies protect the offspring against pathogens until they can produce their own antibodies (3). Theoretical models have shown that the evolution of the maternal transfer of immunity depends on a number of factors, including host life span, the rate of loss of maternal protection, pathogen virulence, and host recovery rate (4). The transgenerational transmission of acquired immunity can have important consequences for the epidemiology of the pathogen (2, 5). Despite its potential importance in nature, the transgenerational transfer of acquired immunity has not received much attention in the ecology of zoonotic diseases (2, 5).
Maternal antibodies may be particularly important for the epidemiology of pathogens that consist of multiple genetically distinct strains that circulate in the host population at the same time. These strains can be distinguished by the acquired immune system of the vertebrate host, resulting in the development of strain-specific antibody responses. Theoretical models of acquired immunity (without maternal transmission) have shown that strain-specific antibodies play a critical role in shaping the epidemiology and population structure of pathogen strains (6–8). Theoretical models of host populations infected with multiple pathogen strains have shown that the evolution of maternal transfer of immunity depends on a number of factors, including the force of infection, the level of cross-immunity between strains, and the probability that the offspring will encounter the same strain as their mother (4). In such systems, a pathogen strain that is common in the maternal generation would be at a selective disadvantage in the offspring generation due to the maternal transmission of strain-specific antibodies against this common strain (4).
Tick-borne spirochete bacteria belonging to the Borrelia burgdorferi sensu lato genospecies complex are the etiological agents of Lyme borreliosis (9, 10). B. burgdorferi sensu lato is a good model system for studying whether maternally transmitted antibodies can influence strain-specific infection success. The populations of B. burgdorferi sensu lato consist of multiple strains that circulate between Ixodes ticks and vertebrate hosts, such as rodents and birds (11–15). Immature Ixodes ticks search for a blood meal from spring until early autumn, and the transmission of B. burgdorferi sensu lato therefore coincides with the reproduction and population expansion of its vertebrate hosts (10, 16). There is no vertical transmission of B. burgdorferi sensu lato in either the tick (17) or the vertebrate host (18–20). In nature, vertebrate hosts develop a strong antibody response against B. burgdorferi sensu lato (18, 19), and infection studies in rodents have shown that this antibody response is strain specific (21–24). This antibody response is not effective at clearing the pathogen, which is why rodent hosts remain infected for months or even years (25–28). However, this antibody response is effective at preventing reinfection with the same strain (29, 30), and the transfer of antisera from infected donors to naive recipients (i.e., passive immunization) prevents infection in the latter (31–33). Studies in various vertebrate species have shown that infected neonates develop much more disease than infected adults (34, 35), suggesting that it is important for mothers to protect their young offspring. Previous field studies on seabirds (36, 37) and one dog (38) found that B. burgdorferi-infected mothers transmit antibodies to their offspring. However, to date no one has used an experimental approach to test whether maternal antibodies protect offspring against infection with B. burgdorferi sensu lato and whether this protection is strain specific.
In this study, we used Borrelia afzelii, which is the most common cause of Lyme borreliosis in Europe (39); its tick vector, Ixodes ricinus; and the bank vole (Myodes glareolus), which is an important reservoir host for both B. afzelii and I. ricinus (39). The purpose of this study was to test (i) whether female bank voles that were experimentally infected with B. afzelii transmit maternal antibodies to their offspring, (ii) whether maternal antibodies can protect bank vole offspring against infection from B. afzelii-infected I. ricinus ticks, and (iii) whether this maternal antibody protection is specific for the strain of B. afzelii.
RESULTS
Maternal infection status and maternal antibody transmission.
The maternal infection status was unambiguous: control mothers tested negative for 4 of 4 maternal infection criteria, whereas infected mothers tested positive for at least 3 of 4 maternal infection criteria (see Table S1 in the supplemental material). The mean B. afzelii-specific IgG antibody response of the infected mothers (mean = 3,811 absorbance units, 95% confidence interval [CI] = 2,692 to 5,395 absorbance units) was 7.4 times higher than that of the uninfected mothers (mean = 512 absorbance units, 95% CI = 371 to 706 absorbance units), and this was significant (Fig. S1; t = −9.335, degrees of freedom [df] = 11, P < 0.001). This result shows that the infected mothers developed a strong IgG antibody response against the B. afzelii infection.
For the offspring blood samples that were taken the day before the infectious challenge (at 34 days postbirth [PB]), the mean level of B. afzelii-specific IgG antibodies was 1.6 times higher for the maternal antibody-positive (MatAb+) offspring (mean = 815 absorbance units, 95% CI = 731 to 906 absorbance units) than for the maternal antibody-negative (MatAb−) offspring (mean = 511 absorbance units, 95% CI = 459 to 566 absorbance units), and this difference was significant (Fig. 1; t = −5.589, df = 39, P < 0.001). This result shows that B. afzelii-infected mothers transmitted maternal antibodies to the MatAb+ offspring, whereas uninfected mothers did not transmit such antibodies to the MatAb− offspring.
FIG 1.
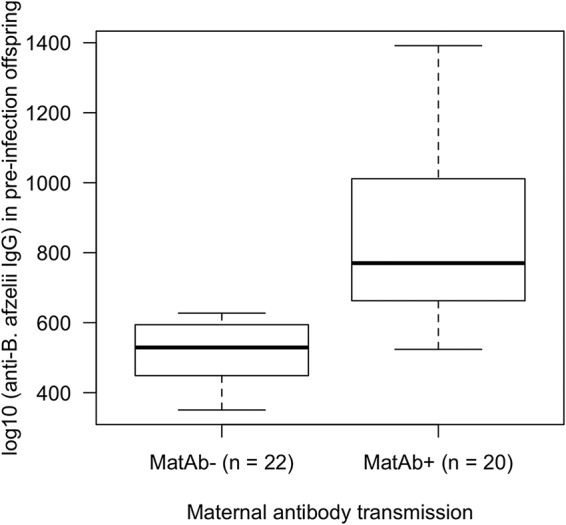
The level of maternally transmitted B. afzelii-specific IgG antibodies was significantly higher in the MatAb+ offspring (n = 20) than in the MatAb− offspring (n = 22) at 34 days postbirth (PB). The MatAb− and the MatAb+ offspring are the offspring of 7 uninfected control mothers and 6 B. afzelii-infected mothers, respectively. The level of the maternally transmitted B. afzelii-specific IgG antibody response was measured in the blood of the offspring at 34 days PB using a commercial Lyme borreliosis ELISA. Shown are the medians (black lines), the 25th and 75th percentiles (edges of the box), and the minimum and maximum values (whiskers).
The OspC A10 protein specificity ratio of the maternally transmitted IgG antibodies was 3.07 times higher in the MatAb+ offspring than in the MatAb− offspring, and this difference was significant (Fig. S3; t = −10.015, df = 39, P < 0.001). This result shows that the maternal IgG antibodies in the MatAb+ offspring reacted more strongly with the recombinant OspC (rOspC) A10 protein than with the rOspC A3 protein compared to the maternal IgG antibodies in the MatAb− offspring.
Offspring infection status following infectious challenge.
As expected, there was no mother-offspring transmission of the pathogen; the ear tissue biopsy specimens of all offspring before the infectious challenge (34 days PB) tested negative for B. afzelii. The infectious challenge was successful: we collected at least one engorged B. afzelii-infected nymph from 38 of the 40 offspring that were challenged with infected ticks. The 2 offspring from which no engorged infected ticks were collected were excluded from the analysis (Tables S2 and S3). The final sample sizes were therefore 8, 11, 9, and 10 offspring for the MatAb− group challenged with strain NE4049, the MatAb− group challenged with strain Fin-Jyv-A3, the MatAb+ group challenged with strain NE4049, and the MatAb+ group challenged with strain Fin-Jyv-A3, respectively. The infection status of the offspring was unambiguous: uninfected offspring tested negative for at least 5 of the 6 offspring infection criteria, whereas infected offspring tested positive for at least 4 of the 6 offspring infection criteria (Tables S2 and S3).
Maternal antibody protection and strain specificity.
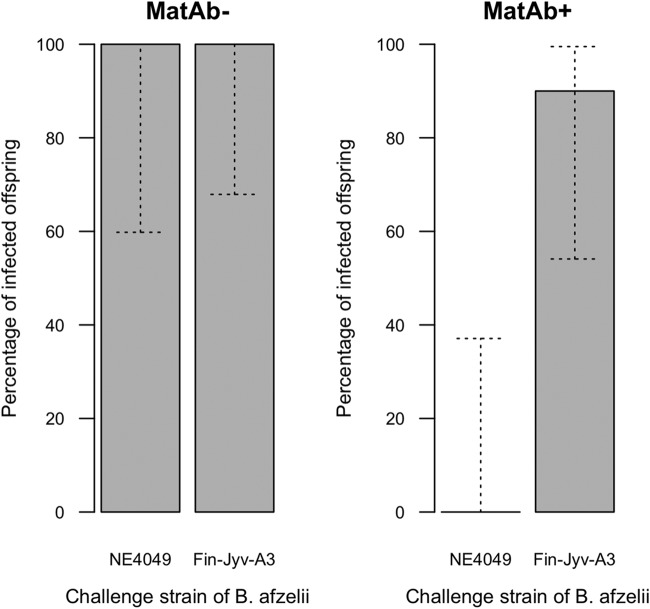
The analysis of offspring infection status found a highly significant interaction between maternal antibody status and the challenge strain (Fig. 2; the results of the generalized linear model [GLM] were as follows: Δdf = 1, Δχ2 = 71.659, and P < 0.001). All of the MatAb− offspring became infected regardless of whether they were challenged with strain NE4049 (100.0% = 8 infected/8 total) or strain Fin-Jyv-A3 (100.0% = 11 infected/11 total). This result shows that naive offspring were highly susceptible to both strains. The MatAb+ offspring were perfectly protected against strain NE4049 (0.0% = 0 infected/9 total) but almost completely susceptible to strain Fin-Jyv-A3 (90.0% = 9 infected/10 total), and this difference was significant (χ2 = 11.992, df = 1, P < 0.001). This result shows that maternal antibodies developed against the maternal strain (strain NE4049) protected offspring against this strain but not against a different strain (Fin-Jyv-A3) (Fig. 2).
FIG 2.
The percentage of infected offspring depends on the maternal antibody status of the offspring and on the strain with which they were challenged. MatAb− (left) and MatAb+ (right) refer to the offspring from the uninfected control mothers and the mothers infected with B. afzelii strain NE4049, respectively. The offspring were challenged via tick bite with either B. afzelii strain NE4049 or B. afzelii strain Fin-Jyv-A3 at 35 days postbirth (PB). The infection status of the offspring was determined using 6 different offspring infection criteria at 35 days postinfection (p.i.) and at 70 days p.i., which correspond to 70 days PB and 105 days PB, respectively. The MatAb− offspring were equally susceptible to both strains. The MatAb+ offspring were protected against the maternal strain (NE4049) but not the new strain (Fin-Jyv-A3). The gray solid bars show the means, and the dashed bars show the 95% confidence intervals.
B. afzelii-specific IgG antibody response in the offspring postinfection.
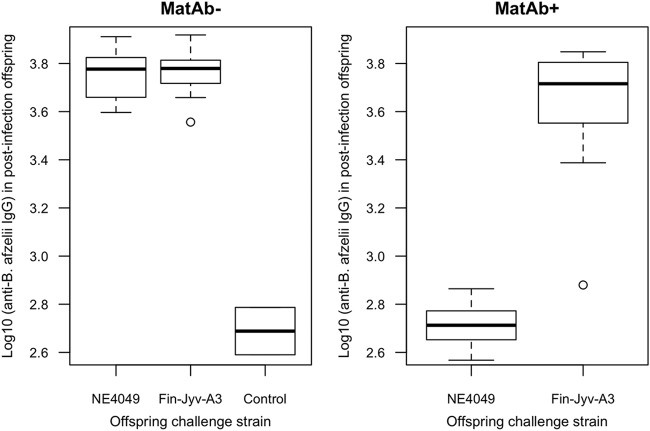
In the offspring, the B. afzelii-specific IgG antibody response at 35 days postinfection (p.i.) followed the expectation for strain-specific protection (Fig. 3). The interaction between maternal antibody status and the challenge strain was significant (F1, 33 = 54.664, P < 0.001). After splitting the analysis by maternal antibody status, the challenge strain had a significant effect on the B. afzelii-specific IgG antibody response at 35 days p.i. of the MatAb+ offspring (F1, 17 = 75.174, P < 0.001) but not the MatAb− offspring (F1, 16 = 0.088; P = 0.771). As expected, the two negative-control offspring showed no sign of infection.
FIG 3.
The B. afzelii-specific IgG antibody response of the offspring at 35 days postinfection (p.i.) depends on the maternal antibody status and the challenge strain. MatAb− (left) and MatAb+ (right) refer to the offspring from the uninfected control mothers and the mothers infected with B. afzelii strain NE4049, respectively. The offspring were challenged via tick bite with either strain NE4049 or strain Fin-Jyv-A3 at 35 days postbirth (PB). The MatAb− offspring were equally susceptible to both strains. The MatAb+ offspring were protected against the maternal strain (NE4049) but not the new strain (Fin-Jyv-A3). The control group refers to 2 MatAb− offspring that were each infested with 4 uninfected I. ricinus nymphs. These two individuals show the baseline B. afzelii-specific IgG antibody response for bank vole offspring that were bitten by ticks but not infected with B. afzelii. Shown are the medians (black lines), the 25th and 75th percentiles (edges of the box), the minimum and maximum values (whiskers), and the outliers (circles).
B. afzelii spirochete load in the ear tissue biopsy specimen of the offspring postinfection.
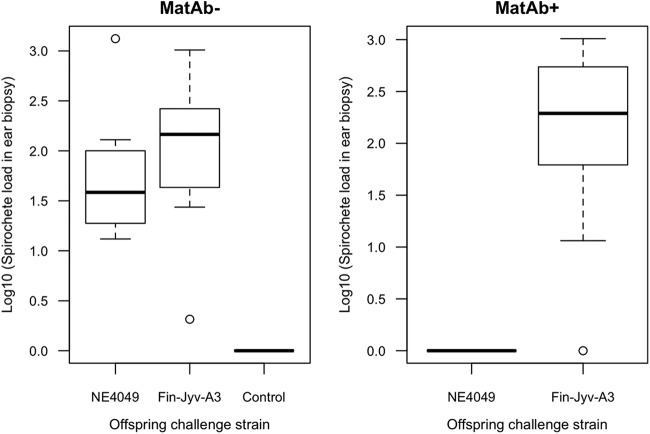
In the offspring, the B. afzelii spirochete load in the ear tissue biopsy specimens obtained at 35 days p.i. followed the expectation for strain-specific protection (Fig. 4). The interaction between maternal antibody status and the challenge strain was significant (F1, 34 = 16.101, P < 0.001). After splitting the analysis by maternal antibody status, the challenge strain had a significant effect on the ear tissue spirochete load at 35 days p.i. in the MatAb+ offspring (F1, 17 = 44.082, P < 0.001) but not the MatAb− offspring (F1, 17 = 0.609; P = 0.446). As expected, the two negative-control offspring showed no sign of infection.
FIG 4.
The B. afzelii spirochete load in the ear tissue biopsy specimens of the bank vole offspring at 35 days postinfection (p.i.) depends on the maternal antibody status and the challenge strain. MatAb− (left) and MatAb+ (right) refer to the offspring from the uninfected control mothers and the mothers infected with B. afzelii strain NE4049, respectively. The offspring were challenged via tick bite with either strain NE4049 or strain Fin-Jyv-A3 at 35 days postbirth (PB). The MatAb− offspring were equally susceptible to both strains. The MatAb+ offspring were protected against the maternal strain (NE4049) but not the new strain (Fin-Jyv-A3). The control group refers to 2 MatAb− offspring that were each infested with 4 uninfected I. ricinus nymphs. These two individuals showed the baseline B. afzelii spirochete load in the ear tissue biopsy specimens for bank vole offspring that were bitten by ticks but not infected with B. afzelii. Shown are the medians (black lines), the 25th and 75th percentiles (edges of the box), the minimum and maximum values (whiskers), and the outliers (circles).
DISCUSSION
Maternal antibodies are protective and strain specific.
Our study provides experimental evidence that maternally transmitted antibodies protect offspring against infection with B. afzelii in an important reservoir host. The maternal antibodies were highly protective for the MatAb+ offspring, despite the fact that the enzyme-linked immunosorbent assay (ELISA) absorbance values in the MatAb+ offspring (815 absorbance units) were not much higher than those in the MatAb− offspring (511 absorbance units) and were much lower than those in the mothers (3,811 absorbance units). One explanation is that not many antibodies are needed for offspring protection because, during their blood meal, nymphs inoculate only a few hundred spirochetes into the vertebrate host (40). Earlier studies on a marine Lyme borreliosis system that consists of B. garinii and seabirds showed a correlation in antibody concentrations between mothers and their chicks, but there was no proof of protection (36). Our results are similar to those of a study on the relapsing fever spirochete (Borrelia duttonii) in laboratory mice (Mus musculus strain ddY), which found that maternal antibodies protected offspring against infection (41). A strength of our study is that we used a natural rodent reservoir host rather than lab mice and that we used ticks rather than needles to deliver the infectious challenge. The mode of delivery (needle versus tick bite) influences the antibody response and its protective efficacy (42–44).
Our study also suggests that the protection afforded by the maternal antibodies is strain specific. Offspring from mothers infected with B. afzelii strain NE4049 were 100% protected against this strain, but they were highly susceptible to B. afzelii strain Fin-Jyv-A3, to which their mothers had not been exposed. Our results are consistent with those of numerous studies that have shown that strains of B. burgdorferi sensu lato (or their OspC antigens) induce strain-specific antibody responses in their rodent hosts (21, 22, 28, 45, 46). Again, it is critical to use the natural route of infection (i.e., ticks), as needle inoculation of B. burgdorferi sensu lato can produce strong patterns of cross-immunity between strains (47). Field studies at small spatial scales have shown that local populations of B. burgdorferi sensu lato contain a community of a dozen strains that circulate in the same reservoir host and tick populations (11–15, 48–52). Theoretical models have shown that strain-specific antibody responses are important for structuring pathogen populations into communities of antigenically distinct strains (6–8). Numerous Lyme disease researchers have suggested that the host immune response against the immunodominant OspC antigen could drive the population structure of B. burgdorferi sensu lato pathogens (10, 11, 13, 14, 53, 54). The results from our study suggest that the transgenerational transfer of antibodies in vertebrate reservoir hosts could play a critical role in structuring the local community of pathogen strains.
Duration of protection of maternal antibodies.
In rodents, maternally transmitted antibodies can protect the offspring for 6 to 10 weeks (3, 55). In the present study, maternally transmitted antibodies protected the bank vole offspring at 5 weeks postbirth. The study on B. duttonii in ddY mice also found that the maternally transmitted antibodies protected the offspring at 5 weeks postbirth (41). A study on Mongolian gerbils (Meriones unguiculatus), where the mothers were vaccinated against B. burgdorferi, found that antibody levels remained stable between 8 and 19 days of age (when the offspring nursed on their mother's milk) but then decreased rapidly and reached undetectable levels at 40 days of age (56). A study on bank voles found that maternally transmitted antibodies against Puumala hantavirus could protect the offspring for a period of 2.5 months postbirth (55). Our study suggests that maternal antibodies may protect subadult rodents against B. burgdorferi sensu lato and help to reduce the prevalence of infection in this age group (18, 19, 57).
Maternally transmitted antibodies and offspring fitness.
Our study did not show whether protected and uninfected offspring had higher fitness than the unprotected and infected offspring. Lyme disease causes morbidity in human patients (58), but whether infection reduces the fitness of reservoir hosts is less clear (10, 53). Long-term mark-recapture studies of the white-footed mouse (Peromyscus leucopus) and the black-legged kittiwake (Rissa tridactyla) have found no effect of B. burgdorferi sensu lato infection on host survival (18, 57, 59). An enclosure study on bank voles found subtle effects of B. afzelii infection on host reproduction but not on host survival (60). Numerous studies have shown that infection with B. burgdorferi induces pathology (e.g., arthritis and carditis) in laboratory mice (Mus musculus) (61–63) but not in wild rodents (20, 35, 64, 65). Interestingly, young individuals are more likely to develop disease than old individuals (34, 35), suggesting that maternally transmitted antibodies increase offspring fitness by delaying pathogen acquisition to an older and more disease-resistant host age class.
Importance of maternal antibodies for ecology of Lyme borreliosis.
Previous studies on wild bank vole populations in Finland have shown that maternally transmitted antibodies are important for the epidemiology of the Puumala hantavirus (5). The ecology of Lyme borreliosis suggests that maternally transmitted antibodies could be important for controlling the epidemiology of B. burgdorferi sensu lato pathogens in nature. The search for a blood meal by the tick vector, the resultant transmission of B. burgdorferi sensu lato, and the reproduction of the rodent host all occur at the same time of the year (10, 16). Ixodes nymphs, which transmit B. burgdorferi sensu lato, search for reservoir hosts from the spring to the autumn (66). Field studies have shown that wild rodent populations can build up high levels of acquired immunity to B. burgdorferi sensu lato (18, 19). For example, >90% of white-footed mice (P. leucopus) in Connecticut were seropositive for B. burgdorferi by the end of August (19). This study suggests that the majority of infected female rodents transfer protective antibodies to their offspring during the summer. In summary, the Lyme disease system is characterized by strong seasonal interactions between pathogen transmission, offspring production, and maternal transmission of highly protective antibodies to offspring (67).
OspC and strain-specific immunity.
Our study also showed that the protection provided by the maternal antibodies was strain specific. ospC is the most polymorphic gene in the genome of B. burgdorferi sensu lato (11–13) and encodes outer surface protein C (OspC). Studies have shown that OspC induces a strain-specific antibody response that protects rodents from tick bite (21–23, 30). The two ospC alleles used in this study (the A3 and A10 alleles) have a genetic distance of 23.19% and an amino acid distance of 62.57%. We had previously shown in a vaccination trial that rOspC proteins A3 and A10 induce strain-specific protection against strains of B. afzelii carrying the corresponding ospC alleles and that cross-immunity was low (21). In the present study, we showed that maternal antibodies (sampled in the offspring) against B. afzelii strain NE4049, which carries ospC allele A10, reacted much more strongly with rOspC A10 than with rOspC A3. Numerous studies have shown that infection with a particular ospC strain induces a much stronger antibody response against the homologous OspC antigen than against the heterologous OspC antigen (21, 24, 45, 68). In summary, our study is consistent with the idea that OspC plays a role in inducing strain-specific immunity, but other antigens could also be important.
Importance of maternal antibodies for population structure of ospC type strains.
B. burgdorferi sensu lato pathogens often contain a high ospC diversity at small spatial scales (12–15, 48, 52, 69, 70). An important goal of Lyme disease ecology is to understand the factors that allow this diversity of ospC strains to coexist in nature (10, 12, 14, 15, 53, 54). Long-term field studies on B. afzelii in tick and rodent populations have shown that the community of strains carrying different ospC major groups (oMGs) was stable over more than a decade, with some strains being an order of magnitude more common than others (14, 15). Strains that were common in the field had higher rates of host-to-tick transmission in laboratory studies (14, 71), and an important question is why these high-transmission strains do not eliminate the low-transmission strains. The ospC polymorphism is maintained by balancing selection, and two alternative hypotheses are multiple-niche polymorphism (MNP) and negative frequency-dependent selection (NFDS) (11, 12, 54). Under MNP, the different oMG strains are adapted to different host species and the frequency of each oMG strain depends on the abundance of its host species (12, 54). Under NFDS, the immune system of the vertebrate host is more efficient at controlling the common oMG strains, and the rare oMG strains therefore have a selective advantage (11, 13). Our study suggests that balancing selection could result from the maternal transfer of OspC-specific antibodies. At the start of the transmission season, the naive mothers become infected with the common oMG strains. Later in the transmission season, the MatAb+ offspring are protected against the common oMG strains, which gives a selective advantage to the rare oMG strains. In summary, the seasonal transgenerational transmission of strain-specific antibodies could play a role in maintaining the high local diversity of ospC strains in nature.
Conclusions.
We used experimental infections with a common Lyme disease pathogen (B. afzelii) and its natural reservoir host (the bank vole) to show that females transmit maternal antibodies to their offspring. These maternal antibodies were completely protective against the strain that the mother had encountered but provided no protection against a different strain. The binding affinity for homologous and heterologous rOspC antigens was consistent with the expected pattern, suggesting that this immunodominant antigen was involved in the maternal transmission of strain-specific immunity. The intergenerational transfer of protective strain-specific antibodies could have important implications for the epidemiology of multistrain pathogen populations in the field.
Future studies should investigate whether maternally transmitted antibodies are important for protecting other important reservoir host species against B. burgdorferi sensu lato. They should investigate the duration of protection, whether the mechanism of antibody transfer involves the placenta, milk, or both (41, 56), and whether females infected with multiple strains transmit antibody responses that are protective against each of those strains. Studies are needed to determine which B. burgdorferi sensu lato antigens are responsible for strain-specific immunity. Finally, theoretical models should investigate how the maternal transfer of antibodies in the host population influences the epidemiology of this multistrain tick-borne pathogen.
MATERIALS AND METHODS
Bank voles, Ixodes ricinus ticks, and Borrelia afzelii.
In 2014, we used field-captured bank voles to establish a breeding colony at the University of Neuchâtel (72). The bank voles used in this study were from the third and fourth lab-born generations and were therefore free from tick-borne pathogens. The I. ricinus ticks came from a laboratory colony established in 1978 at the University of Neuchâtel. During the study, the bank voles were maintained in individual cages and were given food and water ad libitum. The bank voles were experimentally infected via tick bite with one of two isolates of B. afzelii: NE4049 and Fin-Jyv-A3. NE4049 was isolated from an I. ricinus tick in Switzerland and has multilocus sequence type (MLST) 679 and strain identification number 1887 in the Borrelia MLST database. Fin-Jyv-A3 was isolated from a bank vole in Finland and has MSLT 676 and strain identification number 1961 in the Borrelia MLST database. These two isolates (referred to here as “strains”) are highly infectious to both rodents and I. ricinus ticks (21, 73). Furthermore, these two strains carry two different ospC alleles, A10 and A3, which code for two different variants of outer surface protein C (OspC). Immunization with recombinant OspC (rOspC) A10 and rOspC A3 induces strain-specific protective antibody responses in laboratory mice (21).
Ethics statement and animal experimentation permits.
This study followed the Swiss legislation on animal experimentation. The commission that is part of the “Service de la Consommation et des Affaires Vétérinaires (SCAV)” of the canton of Vaud, Switzerland evaluated and approved the ethics of this study. The SCAV of the canton of Neuchâtel, Switzerland issued the animal experimentation permits for the study (NE02-2018) and for the maintenance of the I. ricinus tick colony on vertebrate hosts at the University of Neuchâtel (NE05-2014).
Creation of I. ricinus nymphs infected with B. afzelii.
Nymphs infected with B. afzelii strain NE4049 or Fin-Jyv-A3 were created experimentally (see Section 1 in the supplemental material for details). The percentage of infected nymphs was 77.9% and 91.8% for strain NE4049 and strain Fin-Jyv-A3, respectively.
Infectious challenge of bank vole mothers.
Five-week-old female bank voles were randomly assigned to one of two experimental groups: a control group (n = 9) and a group infected with B. afzelii strain NE4049 (n = 11). Each female in the control group was infested with 4 uninfected nymphs; each female in the infected group was infested with 4 nymphs infected with strain NE4049. At 5 weeks p.i., a blood sample and an ear tissue biopsy specimen were taken from each female to confirm their infection status. Females were coupled with different males at 2 weeks and at 6 weeks p.i., and the offspring from the first successful coupling were used in the present study. Seven control mothers and 6 B. afzelii-infected mothers produced a total of 22 offspring and 20 offspring, respectively (Table S1). At 18 weeks p.i., the mothers were sacrificed using CO2 asphyxiation, and the following organs were aseptically dissected: bladder, left ear, right ear, left rear tibiotarsal joint, and right rear tibiotarsal joint. The tissue samples were stored at −80°C until further analysis. The infection status of the mothers was based on 4 infection criteria (Table S1).
Rearing of bank vole offspring.
At 21 days postbirth (PB), the offspring were separated from their mothers and moved to individual cages. At 34 days PB, a blood sample and an ear tissue biopsy specimen were taken from each of the 42 offspring. The blood samples collected at 34 days PB were tested for maternal IgG antibodies against B. afzelii using a Borrelia-specific ELISA (see below). To confirm that there was no mother-to-offspring transmission of B. afzelii, the ear tissue biopsy specimens collected at 34 days PB were tested for the presence of B. afzelii using quantitative PCR (qPCR) (see below). As the offspring from the uninfected mothers and the infected mothers are expected to test negative and positive for maternal antibodies (MatAb), they are referred to here as the MatAb− and MatAb+ offspring, respectively. At 35 days PB, the offspring were challenged with I. ricinus nymphs that were infected with either strain NE4049 or strain Fin-Jyv-A3 (see below).
Infectious challenge of bank vole offspring.
To test whether maternal antibodies provide strain-specific protection, the MatAb− offspring (n = 22) and the MatAb+ offspring (n = 20) were challenged via tick bite with strain NE4049 or strain Fin-Jyv-A3 at 35 days PB. Offspring were assigned to balance sample sizes and family effects among the four combinations of MatAb status and challenge strain, which were as follows: MatAb− offspring challenged with strain NE4049 (n = 9), MatAb− offspring challenged with strain Fin-Jyv-A3 (n = 11), MatAb+ offspring challenged with strain NE4049 (n = 10), and MatAb+ offspring challenged with strain Fin-Jyv-A3 (n = 10). The remaining 2 MatAb− offspring were challenged with uninfected nymphs as negative controls. The infectious tick bite challenge for the offspring was the same as that for the mothers. At 35 days PB, offspring were challenged with 4 nymphs infected with either strain NE4049 or strain Fin-Jyv-A3. The engorged nymphs were collected and tested for B. afzelii to confirm that each offspring had been infested with at least one infected nymph (Tables S2 and S3). At 35 days p.i. (70 days PB), a second blood sample and a second ear tissue biopsy specimen were taken from each of the 42 offspring to confirm their infection status. At 70 days p.i. (105 days PB), the offspring were sacrificed using CO2 asphyxiation and the following organs were aseptically dissected: bladder, left ear, right ear, left rear tibiotarsal joint, right rear tibiotarsal joint, ventral skin, and dorsal skin. Tissue samples (20 to 25 mg) from the bladder, left ear, and left rear tibiotarsal joint were tested for the presence of B. afzelii using qPCR (see below). Tissue samples from the right ear, right rear tibiotarsal joint, ventral skin, and dorsal skin were cultured in Barbour-Stoenner-Kelly II (BSK-II) medium (see below).
Infection status of bank vole offspring.
A bank vole offspring was considered to have been successfully challenged with B. afzelii if at least one engorged B. afzelii-infected nymph was collected and/or if it developed a systemic infection following the infectious tick challenge. A bank vole was defined as having a systemic infection with B. afzelii if it tested positive for more than one of the following six offspring infection criteria: (i) the presence of B. afzelii-specific IgG antibodies at 35 days p.i., (ii) the presence of B. afzelii in the ear tissue biopsy specimen at 35 days p.i., (iii) the presence of B. afzelii in the bladder at 70 days p.i., (iv) the presence of B. afzelii in the left ear at 70 days p.i., (v) the presence of B. afzelii in the left rear joint at 70 days p.i., and (vi) the presence in culture of live spirochetes from dissected organs at 70 days p.i. Note that 35 days p.i. and 70 days p.i. correspond to 70 days postbirth (PB) and 105 days PB, respectively.
Borrelia-specific qPCR and ospC-specific qPCR.
The B. afzelii infection status of the engorged nymphs and the bank vole tissue samples was tested using qPCR. The DNA was extracted from the engorged nymphs and the bank vole tissue samples as previously described (21, 72). The qPCR assay targets a 132-bp fragment of the flagellin gene of B. burgdorferi sensu lato and was performed as previously described (21, 72). The identity of the strains in the engorged nymphs and the offspring ear tissue biopsy specimens was confirmed using a strain-specific qPCR (73). This qPCR targets a 143-bp fragment of the ospC gene, uses two different probes that detect either ospC allele A3 or ospC allele A10, and was performed as previously described (73).
Borrelia-specific ELISA and OspC-specific ELISA.
The serum samples from the bank voles were tested for the presence of B. afzelii-specific IgG antibodies with an ELISA using Serion ELISA classic Borrelia burgdorferi IgG/IgM immunoassay plates (Ruwag, Germany), as previously described (21, 72). These ELISA plates use the conserved fragments of three recombinant antigens of B. burgdorferi sensu lato: OspC, Flagellin, and VlsE. The maternally transmitted OspC-specific IgG antibody levels in the offspring before the infectious challenge (34 days PB) were measured using a homemade ELISA with recombinant OspC (rOspC) proteins A3 and A10 (21) (see Section 4 in the supplemental material for details).
Culture of B. afzelii spirochetes from bank vole tissues.
To demonstrate that the bank vole offspring were infected with live B. afzelii spirochetes, tissue biopsy specimens were cultured in BSK-II medium. Tissue biopsy specimens from the skin (ventral skin and/or dorsal skin), right ear, and right rear tibiotarsal joint were placed in individual tubes for each of the 42 offspring. The culture tubes were kept in an incubator at 34°C and were screened for live spirochetes over a period of 4 weeks using a dark-field microscope.
Statistical analysis.
All statistical analyses were done using R software (version 1.0.143; R Development Core Team, 14 August 2015). Here, we use the word “response” to refer to the amount of IgG antibodies that developed in response to the B. afzelii infection in infected individuals (mothers or offspring). In contrast, we use the word “level” to refer to the amount of maternally transmitted IgG antibodies that were measured in the offspring the day before their infectious challenge. The IgG antibody response or level was measured in absorbance units and was log10 transformed to improve the normality of the residuals. All means are reported with their 95% confidence intervals (CI).
Maternal infection status and maternal antibody transmission.
To test whether the mother bank voles developed an IgG antibody response against B. afzelii at 35 days p.i., we compared this variable (log10 transformed) between infected mothers and uninfected mothers using an independent two-sample t test.
To test whether there was maternal transmission of B. afzelii-specific IgG antibodies from mothers to their offspring, we compared the level of this variable (log10 transformed) in the preinfection blood sample (at 34 days PB) between the MatAb− offspring and the MatAb+ offspring using an independent two-sample t test.
The specificity of the maternal IgG antibodies in the preinfection blood samples (at 34 days PB) of the offspring was measured as their relative ability to bind the two strain-specific OspC antigens A3 and A10. We calculated an OspC A10 specificity ratio for each offspring by dividing the level of IgG antibodies that bound to rOspC A10 by the level of IgG antibodies that bound to rOspC A3. We compared the log10-transformed OspC A10 specificity ratio between the MatAb+ offspring and the MatAb− offspring using an independent two-sample t test.
Maternal antibody protection and strain specificity.
We tested whether the maternal antibodies protected the offspring against a tick bite challenge with B. afzelii and whether this protection was strain specific. Offspring were classified as being uninfected (which was given a value of 0) or infected (which was given a value of 1), depending on the 6 offspring infection criteria. Offspring infection status was modeled using generalized linear models (GLMs) with binomial errors. The two explanatory factors included offspring maternal antibody status (2 levels, MatAb+ and MatAb−), offspring challenge strain (2 levels, NE4049 and Fin-Jyv-A3), and their interaction. The statistical significance of the explanatory factors was determined using log-likelihood ratio tests that compared the change in deviance between nested models to a chi-square distribution.
B. afzelii-specific IgG antibody response and B. afzelii ear tissue spirochete load in offspring postinfection.
In the previous analysis, the comprehensive infection status of the offspring was based on 6 offspring infection criteria. In our experience, scientists differ with respect to their preference for these infection criteria (e.g., some prefer antibody data, whereas others prefer direct detection of microbes via qPCR or culture). We therefore present 2 of the 6 offspring infection criteria for the offspring to show that they have the same pattern as the comprehensive infection status. To avoid redundancy, the other 4 offspring infection criteria are not shown, but they all show the same pattern.
The two offspring infection criteria analyzed here were (i) the B. afzelii-specific IgG antibody response in the offspring postinfection and (ii) the B. afzelii ear tissue spirochete load in the offspring postinfection. Both response variables were log10 transformed to normalize the residuals. We used linear models with normal errors to model both response variables as a function of two explanatory factors: offspring maternal antibody status (2 levels, MatAb+ and MatAb−), offspring challenge strain (2 levels, NE4049 and Fin-Jyv-A3), and their interaction. The statistical significance of each explanatory factor was based on the type II sums of squares, which were calculated using the analysis of variance function Anova() in the R package car.
Data availability.
The raw data for this study are stored on Zenodo (https://doi.org/10.5281/zenodo.3445357) in an Excel file titled “Raw data for maternal antibodies ms_v03.xlsx.”
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the Swiss National Science Foundation to Maarten J. Voordouw (grant FN 31003A_141153) and by a grant from the Natural Sciences and Engineering Research Council of Canada to Maarten J. Voordouw (RGPIN-2019-04483).
We thank Jacob Koella for advice on the experimental design. We thank Steve Perlman and three anonymous reviewers for their comments on the manuscript.
We declare that we have no competing interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01887-19.
REFERENCES
- 1.Mousseau TA, Fox CW. 1998. Maternal effects as adaptations. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 2.Boulinier T, Staszewski V. 2008. Maternal transfer of antibodies: raising immuno-ecology issues. Trends Ecol Evol 23:282–288. doi: 10.1016/j.tree.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Grindstaff JL, Brodie ED, Ketterson ED. 2003. Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc Biol Sci 270:2309–2319. doi: 10.1098/rspb.2003.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garnier R, Boulinier T, Gandon S. 2012. Coevolution between maternal transfer of immunity and other resistance strategies against pathogens. Evolution 66:3067–3078. doi: 10.1111/j.1558-5646.2012.01665.x. [DOI] [PubMed] [Google Scholar]
- 5.Kallio ER, Begon M, Henttonen H, Koskela E, Mappes T, Vaheri A, Vapalahti O. 2010. Hantavirus infections in fluctuating host populations: the role of maternal antibodies. Proc Biol Sci 277:3783–3791. doi: 10.1098/rspb.2010.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S, Maiden MCJ, Feavers IM, Nee S, May RM, Anderson RM. 1996. The maintenance of strain structure in populations of recombining infectious agents. Nat Med 2:437–442. doi: 10.1038/nm0496-437. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, Anderson RM. 1999. Population structure of pathogens: the role of immune selection. Parasitol Today 15:497–501. doi: 10.1016/S0169-4758(99)01559-8. [DOI] [PubMed] [Google Scholar]
- 8.Cobey S, Lipsitch M. 2012. Niche and neutral effects of acquired immunity permit coexistence of pneumococcal serotypes. Science 335:1376–1380. doi: 10.1126/science.1215947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanek G, Reiter M. 2011. The expanding Lyme Borrelia complex—clinical significance of genomic species? Clin Microbiol Infect 17:487–493. doi: 10.1111/j.1469-0691.2011.03492.x. [DOI] [PubMed] [Google Scholar]
- 10.Kurtenbach K, Hanincova K, Tsao JI, Margos G, Fish D, Ogden NH. 2006. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat Rev Microbiol 4:660–669. doi: 10.1038/nrmicro1475. [DOI] [PubMed] [Google Scholar]
- 11.Barbour AG, Travinsky B. 2010. Evolution and distribution of the ospC gene, a transferable serotype determinant of Borrelia burgdorferi. mBio 1:e00153-10. doi: 10.1128/mBio.00153-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brisson D, Dykhuizen DE. 2004. ospC diversity in Borrelia burgdorferi: different hosts are different niches. Genetics 168:713–722. doi: 10.1534/genetics.104.028738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang IN, Dykhuizen DE, Qiu W, Dunn JJ, Bosler EM, Luft BJ. 1999. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics 151:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durand J, Jacquet M, Rais O, Gern L, Voordouw MJ. 2017. Fitness estimates from experimental infections predict the long-term strain structure of a vector-borne pathogen in the field. Sci Rep 7:1851. doi: 10.1038/s41598-017-01821-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raberg L, Hagstrom A, Andersson M, Bartkova S, Scherman K, Strandh M, Tschirren B. 2017. Evolution of antigenic diversity in the tick-transmitted bacterium Borrelia afzelii: a role for host specialization? J Evol Biol 30:1034–1041. doi: 10.1111/jeb.13075. [DOI] [PubMed] [Google Scholar]
- 16.Tersago K, Schreurs A, Linard C, Verhagen R, Van Dongen S, Leirs H. 2008. Population, environmental, and community effects on local bank vole (Myodes glareolus) Puumala virus infection in an area with low human incidence. Vector-Borne Zoonot 8:235–244. doi: 10.1089/vbz.2007.0160. [DOI] [PubMed] [Google Scholar]
- 17.Richter D, Debski A, Hubalek Z, Matuschka FR. 2012. Absence of Lyme disease spirochetes in larval Ixodes ricinus ticks. Vector-Borne Zoonot 12:21–27. doi: 10.1089/vbz.2011.0668. [DOI] [PubMed] [Google Scholar]
- 18.Hofmeister EK, Ellis BA, Glass GE, Childs JE. 1999. Longitudinal study of infection with Borrelia burgdorferi in a population of Peromyscus leucopus at a Lyme disease-enzootic site in Maryland. Am J Trop Med Hyg 60:589–609. [DOI] [PubMed] [Google Scholar]
- 19.Bunikis J, Tsao J, Luke CJ, Luna MG, Fish D, Barbour AG. 2004. Borrelia burgdorferi infection in a natural population of Peromyscus leucopus mice: a longitudinal study in an area where Lyme borreliosis is highly endemic. J Infect Dis 189:1515–1523. doi: 10.1086/382594. [DOI] [PubMed] [Google Scholar]
- 20.Wright SD, Nielsen SW. 1990. Experimental infection of the white-footed mouse with Borrelia burgdorferi. Am J Vet Res 51:1980–1987. [PubMed] [Google Scholar]
- 21.Jacquet M, Durand J, Rais O, Voordouw MJ. 2015. Cross-reactive acquired immunity influences transmission success of the Lyme disease pathogen, Borrelia afzelii. Infect Genet Evol 36:131–140. doi: 10.1016/j.meegid.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Probert WS, Crawford M, Cadiz RB, LeFebvre RB. 1997. Immunization with outer surface protein (Osp) A, but not OspC, provides cross-protection of mice challenged with North American isolates of Borrelia burgdorferi. J Infect Dis 175:400–405. doi: 10.1093/infdis/175.2.400. [DOI] [PubMed] [Google Scholar]
- 23.Bhatia B, Hillman C, Carracoi V, Cheff BN, Tilly K, Rosa PA. 2018. Infection history of the blood-meal host dictates pathogenic potential of the Lyme disease spirochete within the feeding tick vector. PLoS Pathog 14:e1006959. doi: 10.1371/journal.ppat.1006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baum E, Hue F, Barbour AG. 2012. Experimental infections of the reservoir species Peromyscus leucopus with diverse strains of Borrelia burgdorferi, a Lyme disease agent. mBio 3:e00434-12. doi: 10.1128/mBio.00434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gern L, Siegenthaler M, Hu CM, Leuba-Garcia S, Humair PF, Moret J. 1994. Borrelia burgdorferi in rodents (Apodemus flavicollis and A. sylvaticus): duration and enhancement of infectivity for Ixodes ricinus ticks. Eur J Epidemiol 10:75–80. doi: 10.1007/BF01717456. [DOI] [PubMed] [Google Scholar]
- 26.Richter D, Klug B, Spielman A, Matuschka FR. 2004. Adaptation of diverse Lyme disease spirochetes in a natural rodent reservoir host. Infect Immun 72:2442–2444. doi: 10.1128/IAI.72.4.2442-2444.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacquet M, Margos G, Fingerle V, Voordouw MJ. 2016. Comparison of the lifetime host-to-tick transmission between two strains of the Lyme disease pathogen Borrelia afzelii. Parasit Vectors 9:645. doi: 10.1186/s13071-016-1929-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rynkiewicz EC, Brown J, Tufts DM, Huang C-I, Kampen H, Bent SJ, Fish D, Diuk-Wasser MA. 2017. Closely-related Borrelia burgdorferi (sensu stricto) strains exhibit similar fitness in single infections and asymmetric competition in multiple infections. Parasit Vectors 10:64. doi: 10.1186/s13071-016-1964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piesman J, Dolan MC, Happ CM, Luft BJ, Rooney SE, Mather TN, Golde WT. 1997. Duration of immunity to reinfection with tick-transmitted Borrelia burgdorferi in naturally infected mice. Infect Immun 65:4043–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogovskyy AS, Bankhead T. 2014. Bacterial heterogeneity is a requirement for host superinfection by the Lyme disease spirochete. Infect Immun 82:4542–4552. doi: 10.1128/IAI.01817-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson RC, Kodner C, Russell M. 1986. Passive immunization of hamsters against experimental infection with the Lyme disease spirochete. Infect Immun 53:713–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mbow ML, Gilmore RD, Titus RG. 1999. An OspC-specific monoclonal antibody passively protects mice from tick-transmitted infection by Borrelia burgdorferi B31. Infect Immun 67:5470–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barthold SW, Bockenstedt LK. 1993. Passive immunizing activity of sera from mice infected with Borrelia burgdorferi. Infect Immun 61:4696–4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moody KD, Barthold SW, Terwilliger GA. 1990. Lyme borreliosis in laboratory animals: effect of host species and in vitro passage of Borrelia burgdorferi. Am J Trop Med Hyg 43:87–92. doi: 10.4269/ajtmh.1990.43.87. [DOI] [PubMed] [Google Scholar]
- 35.Moody KD, Terwilliger GA, Hansen GM, Barthold SW. 1994. Experimental Borrelia burgdorferi infection in Peromyscus leucopus. J Wildl Dis 30:155–161. doi: 10.7589/0090-3558-30.2.155. [DOI] [PubMed] [Google Scholar]
- 36.Gasparini J, McCoy KD, Tveraa T, Boulinier T. 2002. Related concentrations of specific immunoglobulins against the Lyme disease agent Borrelia burgdorferi sensu lato in eggs, young and adults of the kittiwake (Rissa tridactyla). Ecol Lett 5:519–524. doi: 10.1046/j.1461-0248.2002.00345.x. [DOI] [Google Scholar]
- 37.Gasparini J, McCoy KD, Haussy C, Tveraa T, Boulinier T. 2001. Induced maternal response to the Lyme disease spirochaete Borrelia burgdorferi sensu lato in a colonial seabird, the kittiwake Rissa tridactyla. Proc Biol Sci 268:647–650. doi: 10.1098/rspb.2000.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eschner A. 2008. Effect of passive immunoglobulin transfer on results of diagnostic tests for antibodies against Borrelia burgdorferi in pups born to a seropositive dam. Vet Ther 9:184–191. [PubMed] [Google Scholar]
- 39.van Duijvendijk G, Sprong H, Takken W. 2015. Multi-trophic interactions driving the transmission cycle of Borrelia afzelii between Ixodes ricinus and rodents: a review. Parasit Vectors 8:643. doi: 10.1186/s13071-015-1257-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kern A, Collin E, Barthel C, Michel C, Jaulhac B, Boulanger N. 2011. Tick saliva represses innate immunity and cutaneous inflammation in a murine model of Lyme disease. Vector-Borne Zoonot 11:1343–1350. doi: 10.1089/vbz.2010.0197. [DOI] [PubMed] [Google Scholar]
- 41.Morshed MG, Yokota M, Nakazawa T, Konishi H. 1993. Transfer of antibody against Borrelia duttonii from mother to young in ddY mice. Infect Immun 61:4147–4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gern L, Schaible UE, Simon MM. 1993. Mode of inoculation of the Lyme disease agent Borrelia burgdorferi influences infection and immune responses in inbred strains of mice. J Infect Dis 167:971–975. doi: 10.1093/infdis/167.4.971. [DOI] [PubMed] [Google Scholar]
- 43.Roehrig JT, Piesman J, Hunt AR, Keen MG, Happ CM, Johnson BJB. 1992. The hamster immune response to tick-transmitted Borrelia burgdorferi differs from the response to needle-inoculated, cultured organisms. J Immunol 149:3648–3653. [PubMed] [Google Scholar]
- 44.Barthold SW, Fikrig E, Bockenstedt LK, Persing DH. 1995. Circumvention of outer surface protein A immunity by host-adapted Borrelia burgdorferi. Infect Immun 63:2255–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Earnhart CG, Buckles EL, Dumler JS, Marconi RT. 2005. Demonstration of OspC type diversity in invasive human Lyme disease isolates and identification of previously uncharacterized epitopes that define the specificity of the OspC murine antibody response. Infect Immun 73:7869–7877. doi: 10.1128/IAI.73.12.7869-7877.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bockenstedt LK, Hodzic E, Feng S, Bourrel KW, de Silva A, Montgomery RR, Fikrig E, Radolf JD, Barthold SW. 1997. Borrelia burgdorferi strain-specific Osp C-mediated immunity in mice. Infect Immun 65:4661–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Devevey G, Dang T, Graves CJ, Murray S, Brisson D. 2015. First arrived takes all: inhibitory priority effects dominate competition between co-infecting Borrelia burgdorferi strains. BMC Microbiol 15:61. doi: 10.1186/s12866-015-0381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Durand J, Jacquet M, Paillard L, Rais O, Gern L, Voordouw MJ. 2015. Cross-immunity and community structure of a multiple-strain pathogen in the tick vector. Appl Environ Microbiol 81:7740–7752. doi: 10.1128/AEM.02296-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hellgren O, Andersson M, Raberg L. 2011. The genetic structure of Borrelia afzelii varies with geographic but not ecological sampling scale. J Evol Biol 24:159–167. doi: 10.1111/j.1420-9101.2010.02148.x. [DOI] [PubMed] [Google Scholar]
- 50.Andersson M, Scherman K, Raberg L. 2013. Multiple-strain infections of Borrelia afzelii: a role for within-host interactions in the maintenance of antigenic diversity? Am Nat 181:545–554. doi: 10.1086/669905. [DOI] [PubMed] [Google Scholar]
- 51.Durand J, Herrmann C, Genné D, Sarr A, Gern L, Voordouw MJ. 2017. Multistrain infections with Lyme borreliosis pathogens in the tick vector. Appl Environ Microbiol 83:e02552-16. doi: 10.1128/AEM.02552-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qiu WG, Dykhuizen DE, Acosta MS, Luft BJ. 2002. Geographic uniformity of the Lyme disease spirochete (Borrelia burgdorferi) and its shared history with tick vector (Ixodes scapularis) in the northeastern United States. Genetics 160:833–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsao J. 2009. Reviewing molecular adaptations of Lyme borreliosis spirochetes in the context of reproductive fitness in natural transmission cycles. Vet Res 40:36. doi: 10.1051/vetres/2009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brisson D, Drecktrah D, Eggers C, Samuels DS. 2012. Genetics of Borrelia burgdorferi. Annu Rev Genet 46:515–536. doi: 10.1146/annurev-genet-011112-112140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kallio ER, Poikonen A, Vaheri A, Vapalahti O, Henttonen H, Koskela E, Mappes T. 2006. Maternal antibodies postpone hantavirus infection and enhance individual breeding success. Proc Biol Sci 273:2771–2776. doi: 10.1098/rspb.2006.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garnier R, Gandon S, Chaval Y, Charbonnel N, Boulinier T. 2013. Evidence of cross-transfer of maternal antibodies through allosuckling in a mammal: potential importance for behavioral ecology. Mamm Biol 78:361–364. doi: 10.1016/j.mambio.2012.11.004. [DOI] [Google Scholar]
- 57.Voordouw MJ, Lachish S, Dolan MC. 2015. The Lyme disease pathogen has no effect on the survival of its rodent reservoir host. PLoS One 10:e0118265. doi: 10.1371/journal.pone.0118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stanek G, Wormser GP, Gray J, Strle F. 2012. Lyme borreliosis. Lancet 379:461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 59.Chambert T, Staszewski V, Lobato E, Choquet R, Carrie C, McCoy KD, Tveraa T, Boulinier T. 2012. Exposure of black-legged kittiwakes to Lyme disease spirochetes: dynamics of the immune status of adult hosts and effects on their survival. J Evol Biol 81:986–995. doi: 10.1111/j.1365-2656.2012.01979.x. [DOI] [PubMed] [Google Scholar]
- 60.Cayol C, Giermek A, Gomez-Chamorro A, Hytönen J, Kallio ER, Mappes T, Salo J, Voordouw MJ, Koskela E. 2018. Borrelia afzelii alters reproductive success in a rodent host. Proc Biol Sci 285:20181056. doi: 10.1098/rspb.2018.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang G, Ojaimi C, Iyer R, Saksenberg V, McClain SA, Wormser GP, Schwartz I. 2001. Impact of genotypic variation of Borrelia burgdorferi sensu stricto on kinetics of dissemination and severity of disease in C3H/HeJ mice. Infect Immun 69:4303–4312. doi: 10.1128/IAI.69.7.4303-4312.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang GQ, Ojaimi C, Wu HY, Saksenberg V, Iyer R, Liveris D, McClain SA, Wormser GP, Schwartz I. 2002. Disease severity in a murine model of Lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J Infect Dis 186:782–791. doi: 10.1086/343043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wooten RM, Weis JJ. 2001. Host-pathogen interactions promoting inflammatory Lyme arthritis: use of mouse models for dissection of disease processes. Curr Opin Microbiol 4:274–279. doi: 10.1016/S1369-5274(00)00202-2. [DOI] [PubMed] [Google Scholar]
- 64.Zhong X, Nouri M, Råberg L. 2019. Colonization and pathology of Borrelia afzelii in its natural hosts. Ticks Tick Borne Dis 10:822–827. doi: 10.1016/j.ttbdis.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 65.Schwanz LE, Voordouw MJ, Brisson D, Ostfeld RS. 2011. Borrelia burgdorferi has minimal impact on the Lyme disease reservoir host Peromyscus leucopus. Vector-Borne Zoonot 11:117–124. doi: 10.1089/vbz.2009.0215. [DOI] [PubMed] [Google Scholar]
- 66.Randolph SE, Miklisova D, Lysy J, Rogers DJ, Labuda M. 1999. Incidence from coincidence: patterns of tick infestations on rodents facilitate transmission of tick-borne encephalitis virus. Parasitology 118:177–186. doi: 10.1017/S0031182098003643. [DOI] [PubMed] [Google Scholar]
- 67.Peel AJ, Pulliam JRC, Luis AD, Plowright RK, O’Shea TJ, Hayman DTS, Wood JLN, Webb CT, Restif O. 2014. The effect of seasonal birth pulses on pathogen persistence in wild mammal populations. Proc Biol Sci 281:20132962. doi: 10.1098/rspb.2013.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Melo R, Richer L, Johnson DL, Gomes-Solecki M. 2016. Oral immunization with OspC does not prevent tick-borne Borrelia burgdorferi infection. PLoS One 11:e0151850. doi: 10.1371/journal.pone.0151850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jacquot M, Bisseux M, Abrial D, Marsot M, Ferquel E, Chapuis J-L, Vourc'h G, Bailly X. 2014. High-throughput sequence typing reveals genetic differentiation and host specialization among populations of the Borrelia burgdorferi species complex that infect rodents. PLoS One 9:e88581. doi: 10.1371/journal.pone.0088581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pérez D, Kneubühler Y, Rais O, Jouda F, Gern L. 2011. Borrelia afzelii ospC genotype diversity in Ixodes ricinus questing ticks and ticks from rodents in two Lyme borreliosis endemic areas: contribution of co-feeding ticks. Ticks Tick Borne Dis 2:137–142. doi: 10.1016/j.ttbdis.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 71.Tonetti N, Voordouw MJ, Durand J, Monnier S, Gern L. 2015. Genetic variation in transmission success of the Lyme borreliosis pathogen Borrelia afzelii. Ticks Tick Borne Dis 6:334–343. doi: 10.1016/j.ttbdis.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 72.Gomez-Chamorro A, Battilotti F, Cayol C, Mappes T, Koskela E, Boulanger N, Genné D, Sarr A, Voordouw MJ. 2019. Susceptibility to infection with Borrelia afzelii and TLR2 polymorphism in a wild reservoir host. Sci Rep 9:6711. doi: 10.1038/s41598-019-43160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Genné D, Sarr A, Gomez-Chamorro A, Durand J, Cayol C, Rais O, Voordouw MJ. 2018. Competition between strains of Borrelia afzelii inside the rodent host and the tick vector. Proc Biol Sci 285:20181804. doi: 10.1098/rspb.2018.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data for this study are stored on Zenodo (https://doi.org/10.5281/zenodo.3445357) in an Excel file titled “Raw data for maternal antibodies ms_v03.xlsx.”