Abstract
Social processes are associated with depression, particularly understanding and responding to others, deficits in which can manifest as callousness/unemotionality (CU). Thus, CU may reflect some of the genetic risk to depression. Further, this vulnerability likely reflects the neurological substrates of depression, presenting biomarkers to capture genetic vulnerability of depression severity. However, heritability varies within brain regions, so a high-resolution genetic perspective is needed. We developed a toolbox that maps genetic and environmental associations between brain and behavior at high resolution. We used this toolbox to estimate brain areas that are genetically associated with both depressive symptoms and CU in a sample of 258 same-sex twin pairs from the Colorado Longitudinal Twin Study (LTS). We then overlapped the two maps to generate coordinates that allow for tests of downstream effects of genes influencing our clusters. Genetic variance influencing cortical thickness in the right dorsal lateral prefrontal cortex (DLFPC) sulci and gyri, ventral posterior cingulate cortex (PCC), pre-somatic motor cortex (PreSMA), medial precuneus, left occipital-temporal junction (OTJ), parietal–temporal junction (PTJ), ventral somatosensory cortex (vSMA), and medial and lateral precuneus were genetically associated with both depression and CU. Split-half replication found support for both DLPFC clusters. Meta-analytic term search identified “theory of mind”, “inhibit”, and “pain” as likely functions. Gene and transcript mapping/enrichment analyses implicated calcium channels. CU reflects genetic vulnerability to depression that likely involves executive and social functioning in a distributed process across the cortex. This approach works to unify neuroimaging, neuroinformatics, and genetics to discover pathways to psychiatric vulnerability.
Subject terms: Clinical genetics, Depression
Introduction
As depression follows a normal distribution of risk across the population1, relating depression to psychological features will better define pathways for addressing disorder vulnerability2. In particular, disruption in the ability to process social cues is associated with depressive symptomology, and can lead to increased deficits in daily functioning in both patient and subclinical groups3. Depressed individuals’ symptoms relate to specific facets of social behavior, namely, reasoning through others emotions4–6, fitting under the “understanding mental states” subcategory of the “social dimensions” construct in the US National Institute of Mental Health Research Domain Criteria (RDoC) matrix. Many forms of social response have been associated with depression7, and it is thought that broad deficits in social functioning may be influential in categories of severe depression. In particular, social deficits in theory of mind, the ability to understand others’ thoughts, are related to poor mentalizing/metacognition, or inability to understand the self. Further, theory of mind predicts depression diagnosis above and beyond metacognition in behavioral studies8.
An inability to understand and respond to others’ emotions due to multiple cognitive deficits may manifest as callousness/unemotionality (CU8), which has been suggested as a marker of internalizing disorder symptom severity9,10. Although typically examined in the context of externalizing disorders9–11, CU has also been consistently associated with depression12,13. This association may arise because, while CU reflects a disregard for others, CU has been related directly to theory of mind, empathy skills, and social processing14, including emotional understanding in social contexts12. Thus, poor social processing/CU may be a mechanism that indexes depression severity15 or sustains depression3. Finally, as CU is related to multiple forms of empathetic behaviors, including cognitive and social-emotional responses, it forms an important first step in exploring the biological overlap between social processes and depression.
Multiple biological perspectives could advance our understanding of CU in depression. Family and genetic studies can estimate the relative importance of genes and environments across traits. Coinheritance between depression and CU is likely, as behavior genetics has established that depression is partially genetic in origin16. Further, a recent genome-wide association analysis implicated over 150 genes in depression etiology1, any of which could relate more specifically to social processing. However, genes/variants and their downstream mechanisms are difficult to scrutinize17.
In contrast to this lack of contextualization and deep phenotyping in genetic research, brain mapping integrates onto other areas of biology/psychology (like transcriptomics18 and potential cognitive functions19), thanks to the specificity gained when using high-resolution brain maps. Here, we implement an integrative framework in which we directly map brain areas in which genetic influences on CU and depression overlap. Specifically, the goal of the current study is test whether CU captures some of the genetic vulnerability to depression; and localize the brain areas contributing to this vulnerability. These genetically associated brain areas can then be used with biological and neuro-informatic tools for mapping across different levels within the RDoC matrix, such as RNA expression and biological pathway analyses, to expand understanding of the coinheritance of CU and depression.
Depression and CU in the brain
Spatial brain mapping studies can localize where behavioral measures are associated with brain morphology. By overlaying neural correlates of depression with neural correlates from other measured behaviors, we gain specificity with respect to areas associated with particular aspects of depression. While the neuroanatomical correlates of depression and CU have been studied extensively, this will be the first study examining their anatomical overlap.
The largest meta-analysis of neuroanatomical differences in depression to date used region of interest (ROI) measures of cortical thickness. It found that major depressive disorder (MDD) was associated with cortical thinning in the insula, anterior and posterior cingulate, and temporal gyri:20 areas key in salience21, internal mentation22, and switching between internal thought and executive control23. However, this ROI approach does not consider how subcomponents of large ROIs may differentially relate to more specific facets of psychological phenomena. A meta-analysis of voxel-based morphometry (VBM) studies, which employed a voxel-wise spatial resolution, found that MDD was associated with lower brain volume in specific subregions of the rostral anterior cingulate cortex and the dorsolateral and dorsomedial frontal cortex24. And the anterior cingulate cortex shows differential gene expression and differential task activation across the ROI25. Thus, whole-brain approaches that employ a finer degree of spatial resolution are warranted.
With respect to neuroanatomical correlates of CU, decreases in volume of the rostral and dorsal cingulate cortex have been observed, overlapping spatially with regions that have been identified for depression26. Additionally, the rostral and dorsal anterior cingulate cortex areas that overlap between CU and depression were also found to distinguish suicidal cases from controls in another VBM study27, giving some evidence to support our hypothesis that CU represents a social severity dimension of depression and that depression and CU symptoms should be jointly studied.
This study
The goal of this study is to capture the dimension of depression that overlaps with CU and validate this approach by integrating results from across the imaging genetics literature through one analytical pipeline. Imaging can act as a conduit to understand the mechanisms of genetic effects but also transcriptomics, cognition, and behavior. Mapping genetic association with behavior across the brain enables us to explore multiple levels of biological analysis simultaneously and look for convergence across them to validate theories of behavior.
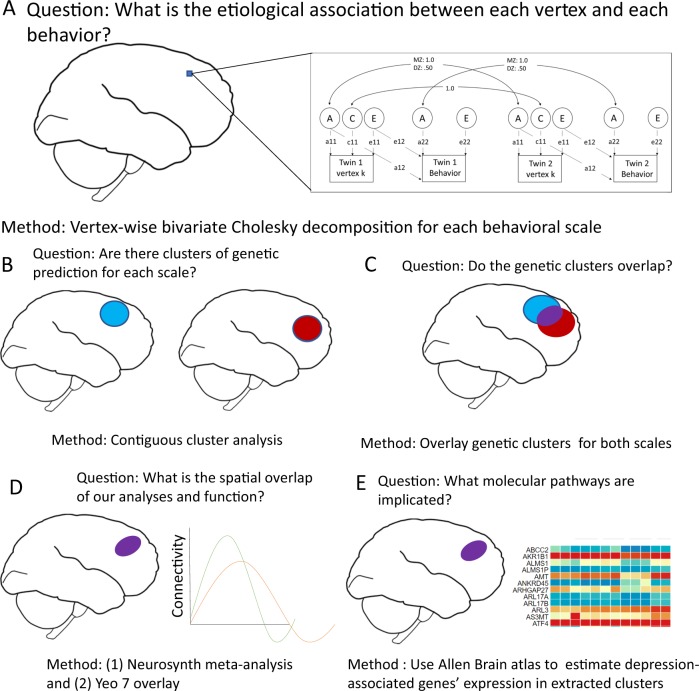
Using structural magnetic resonance imaging (MRI) data from 258 young adult twin pairs, we asked, where are the genes influencing the vulnerability to social deficits and depression influencing brain morphology? Do these morphological differences overlap? And, can we map a specific pattern and use this pattern to speculate further on mechanisms? To answer these questions, we used the methodology pictured in Fig. 1 (a tutorial for this approach can be found on our github: https://github.com/AlexHatoum/Wild-Card-Toolbox). In step 1, we estimated the genetic and environmental association between depression and CU to evaluate the relative importance of shared inherited vulnerability. In step 2, we developed a toolbox that creates genetic and environmental brain maps for each trait. Rather than map standard beta coefficients (i.e., clusters associated with phenotypic variability), our procedure maps effect sizes for genetic and environmental variances (i.e., clusters associated with our traits via a genetic or environmental etiology), creating brain maps of genetic association between cortical thickness and the two behavioral traits. We estimate areas that represent the genetic vulnerability to CU and depression by overlaying the clusters from the separate depression and CU genetic maps onto one map. Finally, by integrating these brain maps with neuroinformatic tools in step 3, we can begin to characterize likely functions and specific molecular mechanisms of the genes influencing CU and depression, which is impossible in a standard biometrical design. Specifically, in step 3, we used MNI coordinates to align our genetically associated clusters with a meta-analytic database of effects across multiple Functional Magnetic Resonance Imaging (fMRI) and transcriptomic studies. By using the spatial coordinates, we can further investigate our hypothesis about the overlap by comparing our coarser genetically derived phenotypes to results from studies using more specific cognitive functions. Thus, our main analysis is the generation of genetically influenced brain map for depression and CU, and our follow-up analyses explore likely effects of this genetic variance implicated by this map by using high-resolution brain coordinates.
Fig. 1. Five steps for whole-cortex mapping by genetic association and follow-up using informatic tools.
a Additive genetic (A), Common environmental (C) and non-shared Environmental (E) Cholesky decomposition is used to find the etiological association of each vertex with each behavioral scale. Multiplication of standardized paths labeled 11 and 12 represents the phenotypic correlations predicted by additive genetic (bivariate heritability) and non-shared environmental (bivariate environmentality) influences, respectively. b Vertices whose associations with behavior are significant (p < 0.05) and are part of a contiguous cluster of larger than 20 mm (cluster-extent correction) are estimated across the cortex surface separately by each trait and separately for A and E components. This procedure recovered four categories of clusters: additive genetic clusters influencing CESD, additive genetic clusters influencing ICU, non-shared environmental clusters influencing CESD, and non-shared environmental clusters influencing ICU. c Areas that represent significant conjunction of genetic association are created by overlaying the genetic clusters from CESD and ICU after cluster-extent correction. d The coordinates for overlap were transformed in MNI space and were used to map onto the Yeo 7 functional connectivity patterns and conduct meta-analytic term searches of likely associated functions. e Genes associated with depression in a large genome-wide association study were extracted from Neurosynth-gene/Allen Brain Atlas dataset to examine the expression of each of those genes in our clusters
We conduct this analysis in a general population sample to include subsyndromal levels of depression and CU within a large enough sample to find patterns of coheritability between brain and phenotype. We chose high-resolution brain mapping because prior literature in neuroimaging genetics suggests vertex-wise approaches will more appropriately capture the individual differences patterns of genetic effects. In particular, common brain atlases used in anatomical research were derived agnostically to genes influencing individual differences and do not capture the specificity of the architecture of genetic effects on behavioral traits, as has been shown for language-related brain areas28. Further, past work has shown there are differences in the genetic variance structure within and between commonly utilized ROIs; thus, measuring genetic variability in ROIs vs. vertices leads to relative differences in genetic variance effects between regions being overestimated29 and more fine-grained metrics, such as voxel or vertex measures, are preferable to ROI approaches for making comparisons across the cortex for individual differences genetics29. Notably for this study, it is these genetic individual differences patterns that are implicated in the mechanisms of psychopathology, requiring high-resolution coordinates to specify accurately. Finally, using high-resolution analysis and Montreal Neurological Institute coordinates (MNI) coordinates allows for integration with functional and transcriptomics literature more broadly.
Methods and materials
Sample
Participants were 258 same-sex twin pairs (225 complete, 120 monozygotic (MZ), and 115 dizygotic (DZ), 132 female pairs and 93 male pairs; singletons were used in calculating the mean and variance), aged 28–31 years (M = 28.7, SD = 0.6), recruited from the Colorado Longitudinal Twin Study (LTS). Twin pairs who had completed an ongoing neuroimaging study of neural substrates of executive functions and psychopathology and whose imaging data passed quality control were included. More about the sample can be found in the online methods.
Structural MRI scan
Images were acquired on a Siemens Prisma and Trio 3 Tesla MRI scanner with 32-channel parallel imaging located at the University of Colorado Boulder. The total scanning session lasted 1 h 25 min; the current analyses focus on gray matter structure, obtained with a high-resolution T1-weighted Magnetization Prepared Gradient Echo sequence in 224 sagittal slices, with a repetition time (TR) = 2400 ms, echo time (TE) = 2.01 ms, flip angle = 8°, field of view (FoV) = 256 mm, and voxel size of 0.8 mm3.
Behavioral assessment
On the day of the scan, participants completed the Center for Epidemiological Studies-Depression (CESD) scale, a 20-item Likert scale assessing the frequency of past-week depression symptoms30. We chose this measure because tendencies toward an emotional vulnerability should manifest itself in higher frequency of depression, we wanted to include subsyndromal levels of depression, and this measure has shown reasonable stability across 10 years of longitudinal data31.
Prior to the scanning session, participants completed an online questionnaire battery that included the Inventory of Callous and Unemotional traits (ICU)13, a 24-item Likert questionnaire with three subscales: callousness (e.g., The feelings of others are unimportant to me), uncaring (e.g., I do things to make others feel good, reverse coded), and unemotional (e.g., I do not show my emotions to others). We used this scale as a measure of CU because it has been used to define clinical subtypes of conduct disorder in the past13, the ICU total score relates to social and emotional processing12, and, though the factor structure changes in adulthood, the scale retains a high internal consistency and predicts social, emotional, and depressive behaviors in individuals similar in age to our sample13. We conducted all analyses with the ICU total scale, which is more reliable and normally distributed than the subscales, which were all highly intercorrelated (see Supplemental Table S1).
For the CESD and ICU, the dependent variable was the average item rating provided that at least 80% of the items were answered, multiplied by the number of items. To improve normality, both scales were square-root transformed (see Supplemental Table S1).
Data analysis
All cortical thickness estimates were processed using a standard Freesurfer pipeline32 (full description in online methods). Each vertex and psychopathology measure was residualized on brain mean thickness and sex prior to model estimation.
Behavioral genetic ACE models decompose phenotypic variance into three sources: Additive genetic (A; the sum of a large number of genetic variants), Common environmental (C; environmental influences that lead siblings to correlate), and non-shared Environmental (E; environmental influences that lead siblings to not correlate). Because MZ twins share all their genes, their additive genetic influences correlate 1.0; DZ twins share on average half their genes identical by descent, so their additive genetic influences correlate 0.5. By definition, C effects correlate 1.0 and E effects correlate 0.0 for both types of twins.
To examine the genetic and environmental covariance between the psychopathology measures and brain measures, the standard ACE model for a single variable can be extended to multivariate analyses. To ensure that the estimated component covariance matrices are positive definite, they are expressed as the product of a lower triangular matrix and its transpose (Fig. 1a). This is the Cholesky decomposition33, which decomposes the phenotypic covariance between two measures into that explained by genes and environments. The genetic correlation (rG) of the two phenotypes equals (a11 × a12)/√(a112 × (a122 + a22)).
Depression and CU coinheritance
To examine the etiological overlap between depression and CU, we started by estimating their phenotypic overlap through a partial correlation analyses (accounting for sex and mean cortical thickness). We used a series of structural models to show that our association is specific to our measure of depressive symptom frequency and CU, rather than a broad psychiatric vulnerability (Supplemental Fig. S1). Finally, we used a standard bivariate Cholesky decomposition to estimate the relative contribution of genes and environment to the overlap between the measures.
Discovery procedure for brain maps
The analysis plan is shown in Fig. 1. For each vertex, we estimated a separate Cholesky decomposition with the first variable being the vertex and the second being the CESD or ICU scale. We noticed substantial C variance across some areas of the cortex (Supplemental Fig. S2), so we specified our Cholesky decompositions with a freed C path loading on the vertex but set the C cross path and specific C loading on the psychopathology variable to be zero, as there were no C effects on the CESD or ICU measures. We then computed the parameter representing the bivariate heritability, the phenotypic correlation predicted by the overlap in genetic influences (standardized a11 × a12), at each vertex and projected it to a surface map in Freeview34 to create whole-cortex heat maps of genetic effects on the brain-behavior association. From the generated whole-cortex map, we estimated clusters above significance for CESD and ICU, respectively, and then overlaid the CESD and ICU clusters.
To determine significant clusters for each disorder, we (1) estimated a chi-square difference test p-value for each Cholesky bivariate cross path, and (2) used vertex-wise cluster extent p-value correction of values below (0.05) significance at a window of twice the original smoothing kernel (i.e. cluster extent threshold = 20 mm). We chose this procedure partially due to its practicality in integration with genetic estimates and to estimate clusters that were contiguous for follow-up analyses.
Split-half replication
To explore the replicability of our approach, we split our sample into halves by families (so that twin pairs would be kept together) by random draw (sample 1, n = 132 pairs; sample 2, n = 126 pairs) and ran the full analyses separately in each sample. In each half of the sample we used a conjunction minimum alpha of 0.05 (ref. 35) and cluster-extent correction of 20 mm to define significant clusters. We then overlaid the clusters from (1) the full sample analysis, (2) the analysis in sample 1, and (3) analysis in sample 2. Because the full sample was more conservative than either half, we used the criterion of significant overlap in all three analyses as our standard, i.e., a cluster must have been independently associated below the split-half criteria in both half-samples and by a more conservative threshold with the full sample for us to have high confidence in its effect.
Transcripts, cell types, and functions associated with our genetic clusters
Using MNI coordinates, we examined the overlap of our clusters with other sources of data: (1) the Allen brain atlas transcriptomic atlas and genome-wide association study (GWAS) results from the Psychiatric Genomics Consortium depression mega-analyses of 480,359 individuals1, (2) Neurosynth meta-analytical database of functional activation across over 10,000 fMRI studies19, and the (3) Yeo 7-network parcellation36.
With the Allen brain atlas, we took the list of associated genes from the psychiatric genomics consortium MDD GWAS gene-burden results1 and used Allen brain atlas through Neurosynth gene37 by downloading each gene image, renormalizing it across the cortex with FMRIB Software Library (FSL38) and visualizing its expression. We excluded genes from the major histocompatibility complex, as these associations may be spurious due to long-range linkage disequilibrium, and any genes not obtained through RNA arrays in the Allen brain atlas, leaving us with 100 genes. We put the expression values by each region in one matrix with k-means clustering. We used the elbow method (Supplemental Fig. S3) to see how many genetic clusters were recovered from our analyses. We then put the gene list of each cluster through the Reactome39 pathway analysis database, using False Discovery Rate (FDR) to account for multiple comparisons.
With Neurosynth, we entered our clusters from the discovery sample into Neurosynth decoder to obtain “terms” that were most associated with functional activation across studies, as determined by a meta-analytic naïve Bayes classifier across over 10 000 fMRI studies19. This analysis finds which of our coordinates most overlap with those found in the literature and which terms (fMRI patterns, tasks, or studied behaviors) are associated with those studies. We then identified which of these terms most commonly appeared across clusters (after filtering out non-specific brain terms). Finally, we overlaid the coordinate of our clusters on the seven resting-state networks from the Yeo parcellation36 to identify to which networks the clusters belonged.
Results
Is CU genetically correlated with depressive symptoms?
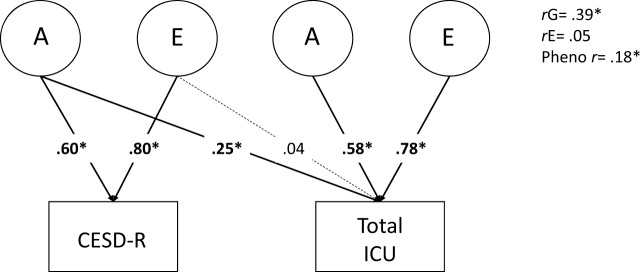
We began by estimating the phenotypic, genetic, and environmental overlap between depressive symptom frequency, measured by the CESD, and CU, measured by the ICU. Figure 2 shows the AE Cholesky decomposition. Based on the best fitting models for each univariate trait, C paths were not estimated (see Supplemental Table S2 for full model comparisons for each trait). Their genetic correlation (rG) was 0.40 (p < 0.001, see Supplemental Table S3 for genetic correlations between CESD and the ICU callous, uncaring, and unemotional subscales). The environmental correlation (rE) was not significantly greater than zero (rE = 0.04, p = 0.500). We concluded that the correlation between CU and depressive symptoms was due almost entirely to genetic covariance.
Fig. 2. Additive genetic (A) and non-shared Environmental (E) Cholesky decomposition of the relationship between the Center for Epidemiological Studies Depression scale (CESD) and the Inventory of Callous and Unemotional traits (ICU).
Numbers on arrows are standardized path estimates. Each task was residualized on sex and mean thickness prior to analysis. Genetic (rG), environmental (rE), and phenotypic (pheno r) correlations are shown to the right of the path model. The model fit acceptably, χ2(20) = 30.264, p = 0.070, RMSEA = 0.059, TLI = 0.905, CFI = 0.842. *p < 0.05, determined by χ2 difference tests. Dotted line indicates p > 0.05
Where are CU/depressive symptom genetic influences related to brain morphology?
We created a map of areas where cortical thickness genetically correlated with CESD and ICU scores. We then overlaid the clusters from the two maps to discover regions that showed conjunction for genetic prediction.
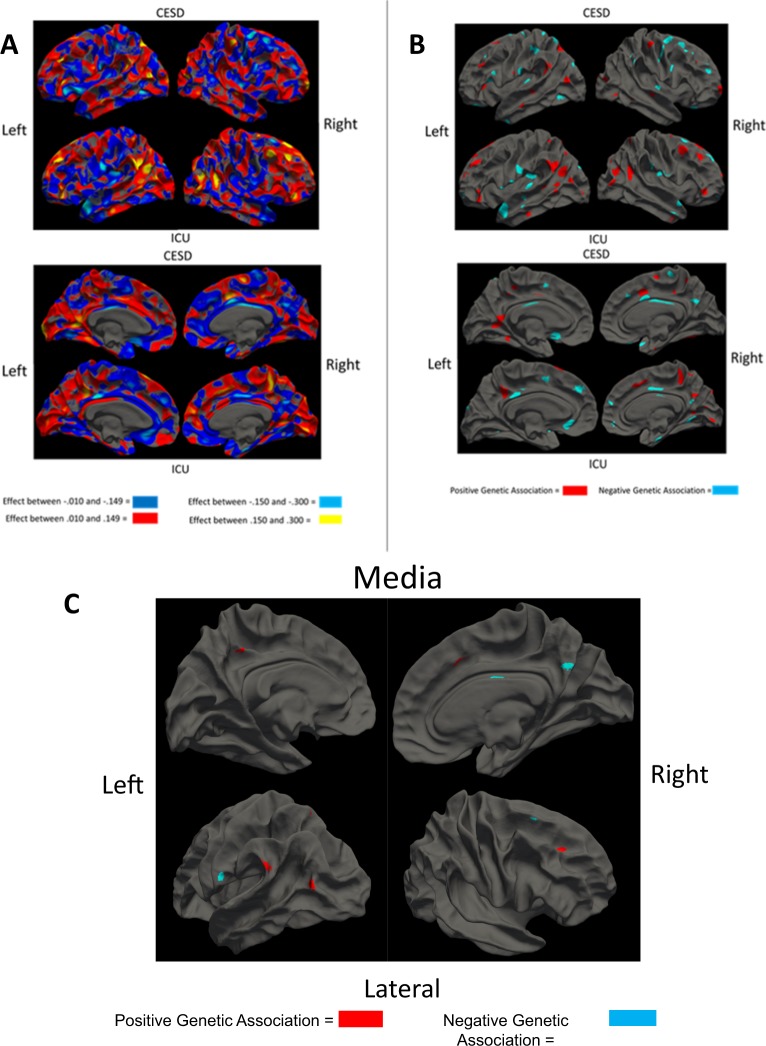
As shown in Fig. 3 and Table 1, we found that genetic influences on thicker cortex in the right dorsal lateral prefrontal cortex (DLFPC) sulci, the right pre-somatic motor cortex (PreSMA), left medial and lateral precuneus, occipital-temporal junction (OTJ), and temporoparietal junction (TPJ) were associated with both traits (i.e., these areas showed positive genetic associations above significance with both measures). We found genetic influences on thinner cortex in the right ventral posterior cingulate cortex (PCC), right medial precuneus, right DLPFC gyrus, and left ventral somatosensory cortex in the pathophysiology of both traits. Finally, split-half replication gave support for both right DLPFC areas in the same direction as discovered in the full sample (Supplemental Fig. S4). Comparison to phenotypic maps (Supplemental Fig. S5) showed that overlay regions discovered would have been qualitatively different without the genetic approach, as phenotypic areas did not overlap substantially with our genetic areas.
Fig. 3. Neural associations with the Center for Epidemiological Studies Depression scale (CESD) and Inventory of Callous and Unemotional traits (ICU).
a Depicts whole-cortex heat maps of the genetic association of each vertex with each behavioral measure as bivariate heritability. b Depicts p-values for genetic association between each vertex and each behavioral scale below cluster-corrected significance (p < 0.05). Lateral views are on top and medial views below. These analyses correspond to those outlined by Fig. 1b. c Depicts overlap areas for our genetic clusters. These genetic clusters coordinates were used in all future analyses
Table 1.
Cluster coordinates for overlay clusters in millimeter space
| Cluster | COG X | COG Y | COG Z | Number of vertices |
|---|---|---|---|---|
| L-Lateral Precuneus | −14 | −67 | 57 | 6 |
| L-Medial Precuneus | −6.54 | −42.3 | 43.6 | 38 |
| L-Occipital Junction | −46.1 | −72.8 | 14.7 | 138 |
| L-Temporal Junction | −57.7 | −49.1 | 29.9 | 191 |
| L-ventral SMA | −60.8 | −16.7 | 23.9 | 73 |
| R-DLPFC* | 23.5 | 32.6 | 35.2 | 61 |
| R-Lateral Frontal* | 23 | 16.4 | 57.2 | 21 |
| R-PCC | 4.87 | −13 | 30.6 | 42 |
| R-Posterior Precuneus | 5.48 | −59 | 31.1 | 99 |
| R-PreSMA | 11.1 | 12.6 | 43.6 | 28 |
Note. Cluster coordinates for each of the overlay clusters discovered in our analysis. Coordinates for the Center Of Gravity (COG) of the peak activation are given in mm space for X, Y and Z coordinates and size was determined based on the number of vertices in each cluster. The name of each area was determined by entering the coordinates into Neurosynth and using the top gyri/sulci name
R right hemisphere and L left hemisphere, DLPFC dorsal lateral prefrontal cortex, PCC posterior cingulate cortex, SMA somatamotor area
*Clusters that replicated in the sample split-half replication
Our method also creates an environmental association map. If genetic and environmental association are in the same direction, it is consistent with an explanation of causality40, though not sufficient to establish a causal relationship. Environmental associations were not consistently in the same direction of effect as the genetic clusters (see Supplemental Fig. S6). Thus, from the environmental map analysis and the bivariate Cholesky decomposition of ICU and CESD, we concluded that the areas in Fig. 3 are likely biomarkers that reflect genetic vulnerability to CU and CESD and implicate a shared genetic liability.
What genetic pathways are implicated?
We used follow-up analyses to gain insight into potential mechanisms involved in this genetic vulnerability. Results of clustering of Psychiatric Genomics Consortium depression-related genes are shown in Supplemental Fig. S7. We found three clusters: overexpressed, mixed expression, and underexpressed (genes listed in axis of Supplemental Fig. S7). The overexpressed cluster showed significant enrichment for genes in “Depolarization of the Presynaptic Terminal Triggers the Opening of Calcium Channels_Homo sapiens_R-HSA-112308” pathway (FDR corrected p = .03). No other pathways were significant after FDR correction.
What likely cognitive/behavioral pathways are involved?
To identify likely cognitive/behavioral mechanisms reflecting this vulnerability, we conducted a meta-analytic term search using Neurosynth. Supplemental Tables S4 and S5 show the 25 most positively associated function terms from Neurosynth for each genetic overlap cluster from the full sample (in some cases, fewer than 25 terms were positively associated). The top repeated behavioral terms were “Theory of Mind”, “inhibit”, and “pain” across all regions (using a wildcard* for different forms of the same word and spelling out acronyms).
We projected our genetic derived clusters onto the Yeo 7-network parcellation, a popular, low-dimensionality parcellation derived from a clustering analysis of resting state data from 1000 participants36. Supplemental Table S6 reports the results of this analyses. The default network was the most common network (4 areas); all but one positively associated cluster from our genetic analysis fell in this network, in line with past research that implicated default network functions to depression41. All but two areas (8 of 10 positively and negatively associated areas) fell in networks with higher-level cognitive functions (i.e., default mode, ventral and dorsal attention, and frontal networks).
Discussion
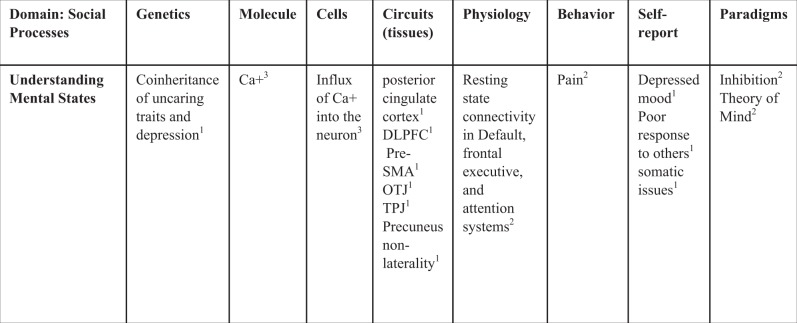
By directly estimating brain areas genetically associated with depression and CU, we found (1) the association between CU and depressive symptoms was entirely genetic in origin. (2) Genetic influences on thicker cortex in right DLFPC sulci, the right PreSMA, left medial and lateral precuneus, OTJ, and TPJ were associated with both traits, and genetic influences on thinner cortex in the right ventral PCC, right medial precuneus, right DLPFC gyrus, and left somatosensory cortex were associated with both traits. (3) Likely molecular pathways are influencing calcium channel depolarization. (4) Likely associated behaviors are “theory of mind”, “inhibit”, and “pain.” (5) Likely neural functions as determined by connectivity are associated with default-mode and higher-level cognitive systems. Figure 4 links our results across different methods to the RDoC social dimensions matrix. We discuss the implications of these findings below.
Fig. 4. We used the RDoC “Social Processing: Understanding Mental States” domain dimension matrix to organize our results across different levels of biology and literature.
DLPFC dorsal lateral prefrontal cortex, Pre-SMA pre-somatosensory area, OTJ occipital temporal junction, TPJ temporoparietal junction, Ca+ calcium, positive charge. 1Results were estimated directly in this study. 2Results were found using MNI coordinates that overlap spatially with those found in the fMRI literature, including the Yeo 7 networks and Neurosynth meta-analytic database. 3Results use the Allen Brain Atlas to visualize expression of PGC MDD-associated genes
Advantages of brain mapping approach
We are the first to directly estimate the cortical pattern that represents genetic vulnerability to a psychiatric disorder. Importantly, this approach is not limited to known associations42. Further, our approach allows for expansion of hypotheses in genetic association studies by integrating MRI atlas-based approaches to contextualize the genetic association patterns and implicate molecular pathways and brain functions.
In this case, we focused on the vulnerability for CU in depression, chosen due to its importance in depression severity43 and integration with RDoC domains2. Reassuringly, this approach converges on several areas previously associated with depression and social processing literature20,24, which means past neuroimaging studies of these behaviors may be driven by genetics. However, cortical thickness associations with depression in the temporoparietal and temporo-occipital junctions, key social processing areas, were novel. Finally, we identified likely mechanisms for follow-up analyses using Bayesian meta-analysis, such as theory of mind and inhibition, that are likely targets for behavioral intervention.
This vulnerability reflects an expanded cognitive network. The association could be explained by both theory of mind (in line with our hypothesis) and inhibition, a trait that we did not hypothesize initially, but that is thought to be related to theory of mind44. The association with theory of mind helps validate these results in line with our hypothesis as it implicates a cognitive social process, while the association with inhibition leads us to believe this may be a more top-down mechanism. Spatially, we found clusters specific to the posterior ventral cingulate cortex and DLFPC, which show broad connectivity patterns (functional and anatomical) between limbic/emotional systems and the association cortex44–46. Further, almost all clusters were in higher-order cognitive systems.
Limitations
There are limitations to our approach. First, the sample is matched on age (at around age 28). While this protects against both linear and non-linear confounding by age, results may not generalize to younger or older ages. Second, we did not examine moderation by sex. However, although sex interaction may be a factor in genetic depressive symptomology, there is still a genetic correlation between males and females16. Finally, informatic analyses focused on overlap based on spatial coordinates. We did not directly measure the overlap between measures of expression, function, and neuro-anatomy, so we do not know whether these analyses are directly tapping the same underlying variability. Of course, this would be impossible for transcription (in humans) and would require complex multi-mediation patterns for relating functional coordinates and anatomy.
Conclusion
We directly mapped genetic vulnerability to CU and depressive symptoms on the brain. We found common genetic variance in CU and depressive symptoms was associated with higher-order cognitive areas and functions. As the genetic vulnerability to psychiatric disorders is discovered, the use of high-resolution cortical methods will be invaluable in contextualizing the patterns of genetic effects.
Supplementary information
Acknowledgements
This research was supported by NIH grants MH063207, AG046938, and MH016880. Cold Spring Harbor Laboratory hosted the preprint for this manuscript on Bioarchive.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41398-019-0611-6).
References
- 1.Wray NR, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 2018;50:668–681. doi: 10.1038/s41588-018-0090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Insel T, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 3.Fekadu A, et al. What happens to patients with treatment-resistant depression? A systematic review of medium to long term outcome studies. J. Affect. Disord. 2009;116:4–11. doi: 10.1016/j.jad.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Schreiter S, Pijnenborg GHM, aan het Rot M. Empathy in adults with clinical or subclinical depressive symptoms. J. Affect. Disord. 2013;150:1–16. doi: 10.1016/j.jad.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Wolkenstein L, Schönenberg M, Schirm E, Hautzinger M. I can see what you feel, but I can’t deal with it: Impaired theory of mind in depression. J. Affect. Disord. 2011;132:104–11.. doi: 10.1016/j.jad.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Cusi AM, MacQueen GM, Spreng RN, McKinnon MC. Altered empathic responding in major depressive disorder: Relation to symptom severity, illness burden, and psychosocial outcome. Psychiatry Res. 2011;188:231–236. doi: 10.1016/j.psychres.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Weightman MJ, Air TM, Baune BT. A review of the role of social cognition in major depressive disorder. Front. Psychiatry. 2014;5:179. doi: 10.3389/fpsyt.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centifanti LCM, Meins E, Fernyhough C. Callous-unemotional traits and impulsivity: distinct longitudinal relations with mind-mindedness and understanding of others. J. Child Psychol. Psychiatry. 2016;57:84–92. doi: 10.1111/jcpp.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenbarth H, Demetriou CA, Kyranides MN, Fanti KA. Stability subtypes of callous-unemotional traits and conduct disorder symptoms and their correlates. J. Youth Adolesc. 2016;45:1889–1901. doi: 10.1007/s10964-016-0520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cecil CAM, McCrory EJ, Barker ED, Guiney J, Viding E. Characterising youth with callous–unemotional traits and concurrent anxiety: evidence for a high-risk clinical group. Eur. Child Adolesc. Psychiatry. 2018;27:885–898. doi: 10.1007/s00787-017-1086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frick PJ, Ellis M. Callous-unemotional traits and subtypes of conduct disorder. Clin. Child Fam. Psychol. Rev. 1999;2:149–168. doi: 10.1023/A:1021803005547. [DOI] [PubMed] [Google Scholar]
- 12.Kimonis ER, et al. Assessing callous–unemotional traits in adolescent offenders: validation of the Inventory of Callous–Unemotional Traits. Int. J. Law Psychiatry. 2008;31:241–252. doi: 10.1016/j.ijlp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Byrd AL, Kahn RE, Pardini DA. A validation of the inventory of callous-unemotional traits in a community sample of young adult males. J. Psychopathol. Behav. Assess. 2013;35:20–34. doi: 10.1007/s10862-012-9315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ladegaard N, Larsen ER, Videbech P, Lysaker PH. Higher-order social cognition in first-episode major depression. Psychiatry Res. 2014;216:37–43. doi: 10.1016/j.psychres.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Lamers F, et al. Identifying depressive subtypes in a large cohort study. J. Clin. Psychiatry. 2010;71:1582–1589. doi: 10.4088/JCP.09m05398blu. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am. J. Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 17.Levinson DF, et al. Genetic studies of major depressive disorder: why are there no genome-wide association study findings and what can we do about it? Biol. Psychiatry. 2014;76:510–512. doi: 10.1016/j.biopsych.2014.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sunkin SM, et al. Allen Brain Atlas: an integrated spatio-temporal portal for exploring the central nervous system. Nucleic Acids Res. 2013;41:D996–D1008. doi: 10.1093/nar/gks1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmaal L, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry. 2017;22:900–909. doi: 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- 22.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goulden N, et al. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage. 2014;99:180–190. doi: 10.1016/j.neuroimage.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 24.Bora E, Fornito A, Pantelis C, Yücel M. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J. Affect. Disord. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 25.Fischl B, Sereno MI, Tootell RBH, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Brito SA, et al. Size matters: Increased grey matter in boys with conduct problems and callous–unemotional traits. Brain. 2009;132:843–852. doi: 10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- 27.Wagner G, et al. Structural brain alterations in patients with major depressive disorder and high risk for suicide: Evidence for a distinct neurobiological entity? Neuroimage. 2011;54:1607–1614. doi: 10.1016/j.neuroimage.2010.08.082. [DOI] [PubMed] [Google Scholar]
- 28.Amunts K, et al. Broca’s region revisited: cytoarchitecture and intersubject variability. J. Comp. Neurol. 1999;412:319–341. doi: 10.1002/(SICI)1096-9861(19990920)412:2<319::AID-CNE10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Eyler LT, et al. A comparison of heritability maps of cortical surface area and thickness and the influence of adjustment for whole brain measures: a magnetic resonance imaging twin study. Twin Res. Hum. Genet. 2012;15:304–314. doi: 10.1017/thg.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eaton, W. W., Smith, C., Ybarra, M., Muntaner, C., & Tien, A. Center for Epidemiologic Studies Depression Scale: review and revision (CESD and CESD-R). in The Use Of Psychological Testing For Treatment Planning And Outcomes Assessment: Instruments For Adults (ed Maruish, M. E.) 363–377 (Lawrence Erlbaum Associates, Mahwah, NJ, 2004).
- 31.Friedman NP, du Pont A, Corley RP, Hewitt JK. Longitudinal relations between depressive symptoms and executive functions from adolescence to early adulthood: a twin study. Clin. Psychol. Sci. 2018;6:543–560. doi: 10.1177/2167702618766360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagler DJ, Saygin AP, Sereno MI, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33:1093–103.. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Dordrecht: Springer Netherlands; 1992. [Google Scholar]
- 34.Fischl B. FreeSurfer. Neuroimage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friston KJ, Penny WD, Glaser DE. Conjunction revisited. Neuroimage. 2005;25:661–667. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 36.Yeo BTT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox, A. S., Chang, L. J., Gorgolewski, K. J. & Yarkoni, T. Bridging psychology and genetics using large-scale spatial analysis of neuroimaging and neurogenetic data. Preprint at https://www.biorxiv.org/content/biorxiv/early/2014/12/09/012310.full.pdf (2014).
- 38.Crum WR. Magnetic resonance brain image processing and arithmetic with FSL. Methods Mol. Biol. 2011;711:109–126. doi: 10.1007/978-1-61737-992-5_5. [DOI] [PubMed] [Google Scholar]
- 39.Joshi-Tope G, et al. Reactome: a knowledgebase of biological pathways. Nucleic Acids Res. 2004;33:D428–D432. doi: 10.1093/nar/gki072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heath AC, et al. Testing hypotheses about direction of causation using cross-sectional family data. Behav. Genet. 1993;23:29–50. doi: 10.1007/BF01067552. [DOI] [PubMed] [Google Scholar]
- 41.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder. JAMA Psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rimol LM, et al. Cortical thickness is influenced by regionally specific genetic factors. Biol. Psychiatry. 2010;67:493–499. doi: 10.1016/j.biopsych.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szanto K, et al. Social emotion recognition, social functioning, and attempted suicide in late-life depression. Am. J. Geriatr. Psychiatry. 2012;20:257–265. doi: 10.1097/JGP.0b013e31820eea0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wade M, et al. On the relation between theory of mind and executive functioning: a developmental cognitive neuroscience perspective. Psychon. Bull. Rev. 2018;25:2119–2140. doi: 10.3758/s13423-018-1459-0. [DOI] [PubMed] [Google Scholar]
- 45.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2010;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.