Abstract
We have derivatized Momordica saponins (MS) I and II through their coupling at C3 glucuronic acid site with dodecylamine. The derivatives show significantly different immunostimulant activity profiles from their respective natural parent saponins. In particular, adjuvant VSA-1 (5), the derivative of MS I, potentiates a significantly higher IgG2a responose than the corresponding natural product. Its IgG1 and IgG2a production is similar to that of GPI-0100, indicating a potential mixed and antigen-specific Th1/Th2 immune response, which is different from the Th2 immunity induced by the natural saponin MS I. In addition, toxicity evaluations show that adjuvant VSA-1 (5) is much less toxic than the widely used natural saponin mixture Quil A. These results prove that derivatizing Momordica saponins can be a viable way for easy access to structurally defined saponin immunostimulants with favorable adjvuant activity and low toxicity.
Graphical Abstract
INTRODUCTION
A vaccine adjuvant is part of a vaccine formulation for boosting and/or modulating the immune responses induced by the vaccine. Modern vaccines frequently utilize refined and homogeneous antigens to minimize undesirable side effects. However, subunit antigens are often less immunogenic and require use of adjuvants/immunostimulants for a strong, long-lasting, and antigen-specific immune response.1–11 Despite the important role of adjuvants in vaccination, only a few are approved for human vaccines.10,12,13 Discovery and development of new vaccine adjuvants become a prioritized endeavor in the vaccine field.3,14–16
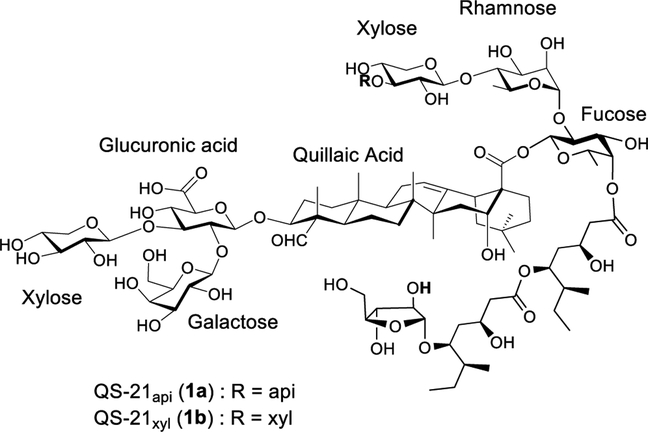
FDA recently approved the combination adjuvant AS01b for human use.17,18 One of its components, the natural saponin QS-21 isolated from the tree bark of Quillaja saponaria Molina (QS) (Figure 1), can potentiate a balanced antigen-specific Th1/Th2 response with CTL production.19–21. QS-21 has shown significant advantages over a broad range of clinical and experimental adjuvants20,22 and has been evaluated in numerous anticancer and anti-infective vaccines in preclinical and clinical trials.22 However, QS-21 has its own limitations. For example, abundance of QS-21 in QS tree bark extracts is low.23 Overexploitation of QS bark has already led to ecological destruction and supply shortage and resulted in more strict environmental regulations and increased price.24 The current natural supply of QS-21 was estimated to be only sufficient for about 6 million doses (calculated based on 100 μg/dose for human use), not sufficient for widespread clinical uses.22 The limited supply of QS-21, along with its hydrolytical instability, dose-limiting toxicity, and laborious and inefficient purification, hinders its wider use.22,24
Figure 1.
Structure of QS-21.
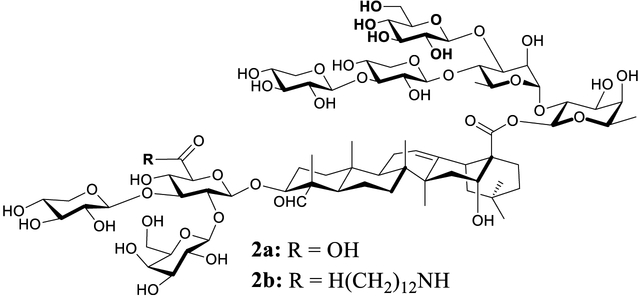
The favorable adjuvant activity of QS-21 coincides with its unique molecular structure. It is one of the rare saponins bearing an acyl side chain that is responsible for its capability in stimulating a cell-mediated immune response. Earlier structure–activity-relationship (SAR) results indicated that elimination of the acyl side chain resulted in loss of QS-21’s Th1 and antigen-specific CTL capacity.25,26 Our own research in synthesizing and evaluating QS analogs demonstrated the dramatic difference in adjuvant activity between analogs with or without a side chain.27–30 We recently demonstrated that the deacylated QS-17/18 (2a) mainly potentiated IgG1 immunity, and incorporation of a dodecylamide side chain at the C3 glucuronic acid moiety led to new adjuvant 2b with different adjuvant activity (Figure 2). It not only potentiates IgG1 response but also boosts IgG2a response significantly. For 2b, the ratio of IgG2a/IgG1 is comparable to that of GPI-0100 which is a complex mixture of semisynthetic sapoinins prepared from the QS tree bark extracts.31,32 GPI-0100 retains the capacity of natural QS saponins in potentiating humoral and T-cell immunity and has been used in preclinical and clinical studies.33 The similar IgG2a/IgG1 profiles between 2b and GPI-0100 suggest that saponin 2b might have the favorable adjuvant property of GPI-0100.29,30
Figure 2.
Synthesized QS analogs with different adjuvant activities.
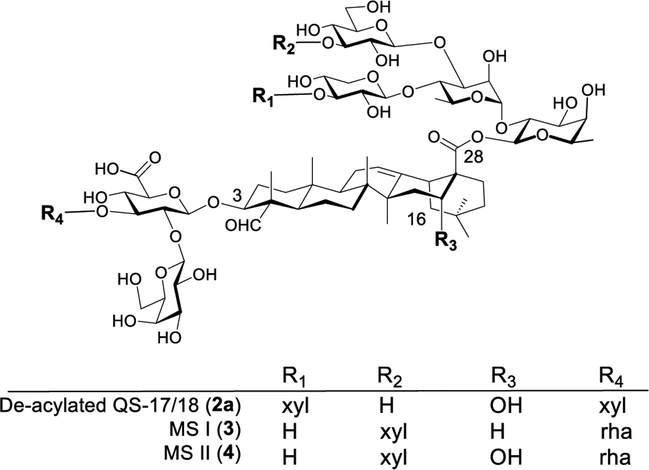
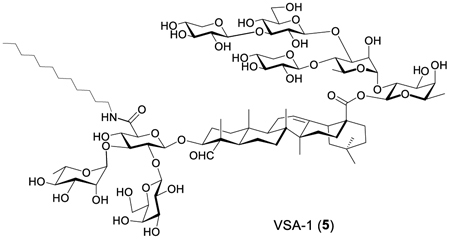
Herein we report a simple strategy to obtain saponin adjuvants with activity similar to that of QS analog 2b. It involves only one-step derivatization of Momordica saponins (MS) I and II.34 These two saponins were isolated from widely available and inexpensive seeds of Momordica cochinchinensis Spreng (MC). Their structures only differ in the triterpenoid core; i.e., MS saponin I (3) has a gypsogenin core (R3 = H), while MS II (4) has a quillaic acid core (R3 = OH) identical to the core of QS-21 and QS-17/18 (Figure 3). The structures of saponins 3 and 4 strikingly resemble that of the deacylated QS-17/18 (2a); therefore, we infer that by incorporating a side chain to saponins 3 and 4, we can obtain two new adjuvants with an immunostimulation profile similar to that of 2b, given their structural similarity and consequently their similar hydrophile–lipophile balance (HLB) values (Figure 3). Oda and co-workers correlated HLB values to adjuvant activity in their SAR studies of soyasaponins.35
Figure 3.
Structural similarity between MS and deacylated QS-17/18.
The advantage of having structurally defined new saponin adjuvants from natural sources other than QS tree bark is multifold. For example, new natural saponin sources can circumvent the “limited supply” issue of QS saponins. Momordica cochinchinensis Spreng is a perennial plant and grows mainly in China and Southeast Asia. It is easy to grow; the seeds (Mubiezi) are readily available and have been utilized in traditional Chinese medicine for more than 1000 years.36 The abundance of 3 and 4 in the MC seeds is high, and their isolation is more efficient and thus cost-effective than QS saponins. Recently, a MC seed extract was evaluated for adjuvant activity in an experimental swine vaccine against foot-and-mouth disease. The MC saponins showed synergistic effect with oil emulsion in boosting antigen-specific IgG in guinea pigs.37 However, in comparison of adjuvant activities against other adjuvants, i.e., Freund’s adjuvant, Quil A (QA), and propolis, in chickens immunized with the antigen F4 fimbriae, MS 3 and 4 showed lower capacity than Freund’s adjuvant and QA in boosting IgG response in both serum and egg yolk.38 These results are in agreement with our observations that without a fatty side chain, the deacylated QS-17/18 (2a) only showed modest humoral immunity.29,30
RESULTS AND DISCUSSION
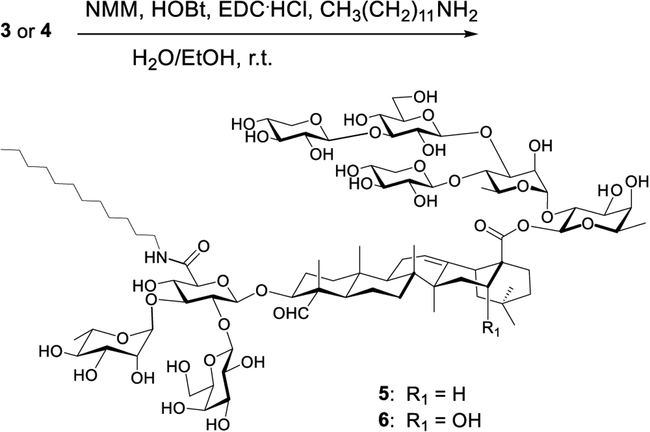
We isolated the two natural MC saponins from MC seeds by using a known procedure.34 We then synthesized the two MS derivatives, i.e., 5 and 6, from natural saponins 3 and 4, respectively, by using a routine one-step amide-formation reaction (Scheme 1).39
Scheme 1.
Derivatizing Momordica Saponins
With pure natural saponins 3 and 4 and their derivatives 5 and 6 in hand, we evaluated their ability to potentiate antibody responses to chicken egg ovalbumin (OVA). We immunized groups of BALB/c mice (female, 8–10 weeks of age, six per group) via the subcutaneous route (sc) with OVA (20 μg) alone or with GPI-0100 (100 μg) or saponins 3–6 (at 100 μg dose) on days 0, 14, and 28. Serum samples were collected, and mice were weighed prior to each immunization and at 2 weeks after the last immunization. The serum IgG, IgG1, and IgG2a antibody activities to OVA antigen were determined with enzyme-linked immunosorbent assay (ELISA).
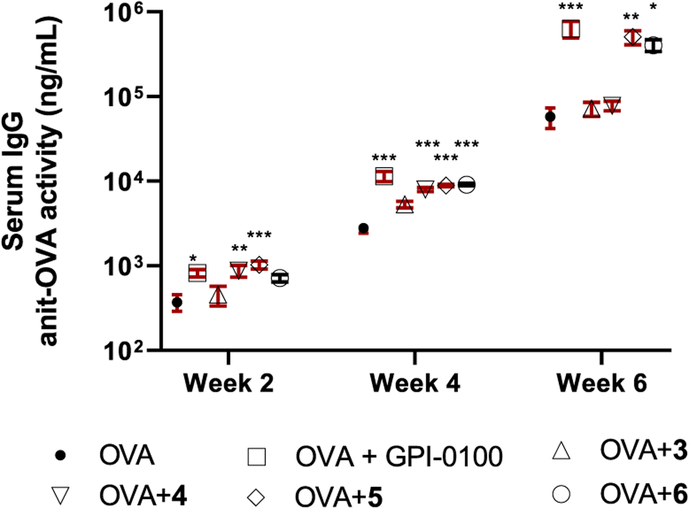
All groups of mice showed a serum anti-OVA IgG response at week 2, and the magnitude of the response continued to grow at weeks 4 and 6 (Figure 4). Similar to the positive control GPI-0100, VSA-1 (5) enhanced IgG responses to OVA antigen significantly at weeks 2, 4, and 6, compared with the group without an adjuvant. The OVA + 5 group also had significantly higher anti-OVA IgG responses than the OVA + 3 group at weeks 2 (P < 0.001), 4 (P < 0.001), and 6 (P < 0.01). The OVA group and the OVA + 3 group did not have significant difference in their IgG activity. The OVA + 4 group showed significant difference in IgG responses from the OVA group at weeks 2 and 4 but not at week 6. The OVA + 6 (derivative of natural MS II (4)) showed significantly higher anti-OVA IgG responses than the OVA group at weeks 4 and 6 but did not show significant difference from its parent compound 4 until week 6 (P < 0.05). Weight monitoring of mice indicated no sign of toxicity.
Figure 4.
Serum IgG anti-OVA response in mice immunized by the sc route with OVA alone or with GPI-0100 or saponins 3–6. Mice were immunized on days 0, 14, and 28. Serum samples were collected prior to each immunization and at 6 weeks after the initial immunization. Values are expressed as the mean ± SEM: *P < 0.05, **P < 0.01, and ***P < 0.001 compared with mice immunized with OVA alone.
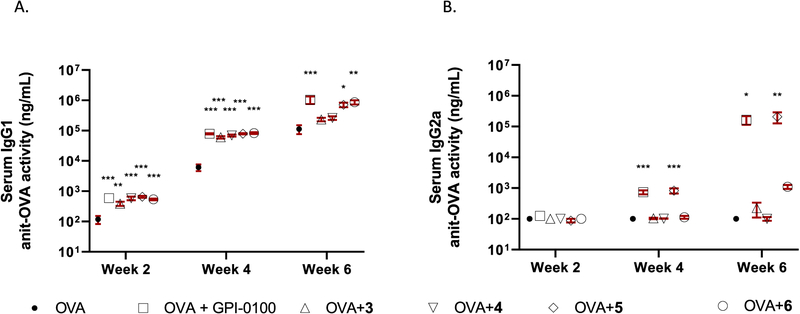
We then analyzed the IgG subclass distribution of different adjuvants. All adjuvants enhanced IgG1 responses significantly at weeks 2 and 4 (Figure 5A). However, only GPI-0100, VSA-1 (5), and 6 showed significantly higher IgG1 titers than that of the OVA group at week 6. In IgG2a assessment (Figure 5B), there was no significant difference among the groups at week 2, but GPI-0100 and VSA-1 (5) induced significantly higher IgG2a than seen in mice with OVA alone or with adjuvants 3, 4, or 6 at weeks 4 and 6.
Figure 5.
Serum IgG1 (A) and IgG2a (B) anti-OVA response in mice immunized by the sc route with OVA alone or with GPI-0100 or saponins 3–6. Mice were immunized on days 0, 14, and 28. Serum samples were collected prior to each immunization and at 6 weeks following the initial immunization. Values are expressed as the mean ± SEM: *P < 0.05, **P < 0.01, and ***P < 0.001 compared with mice immunized with OVA alone.
Analysis of the anti-OVA IgG2a and IgG1 activities at week 6 revealed that OVA + 5 had a significantly higher IgG2a/IgG1 ratio than OVA alone or OVA with 3, 4, or 6 but no significant difference from GPI-0100 (Table 1). The negligible IgG2a responses from the groups without an adjuvant or with adjuvant 3, 4, or 6 suggest that Th2-biased immune responses were selectively induced in these groups. Since GPI-0100 is known for its capability in potentiating a mixed Th1/Th2 response with CTL production, similar IgG2a/IgG1 distributions between GPI-0100 (0.194, Table 1, entry 2) and adjuvant 5 (0.312, Table 1, entry 5) groups suggest that these two adjuvants could have a similar activity profile, which warrants further evaluations.
Table 1.
Serum IgG1 and IgG2a Anti-OVA Activity at Week 6a
| entry | adjuvant | IgG1 (μg/mL) | IgG2a (μg/mL) | IgG2a/IgG1 |
|---|---|---|---|---|
| 1 | none | 113 ± 37 | 0.1 | 0.001** |
| 2 | GPI-0100 | 1,047 ± 292 | 166 ± 50 | 0.194 ± 0.060 (ns) |
| 3 | 3 | 233 ± 74 | 0.2 | <0.001** |
| 4 | 4 | 262 ± 37 | 0.1 | <0.001** |
| 5 | 5 | 713 ± 293 | 208 ± 81 | 0.312 ± 0.126 |
| 6 | 6 | 869 ± 334 | 1 | <0.001** |
Values are expressed as the mean ± SEM. Statistical significance compared with OVA + 5:
P < 0.05.
P < 0.01.
P < 0.001.
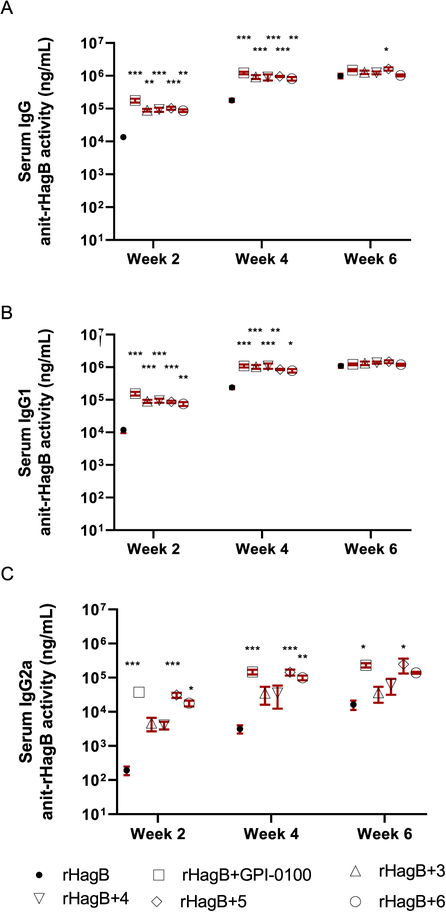
As a comparison, we also evaluated adjuvants 3–6 in augmenting immune responses to rHagB antigen, a recombinant and nonfimbrial adhesion hemagglutinin B from Porphyromonas gingivalis.33,40,41 In an experimental animal model, it was effective in inducing protective immunity against P. gingivalis-induced alveolar bone loss.42 By using the same procedure with rHagB antigen (at 35 μg dose), we observed the results similar to those with OVA antigen, and the IgG, IgG1 and IgG2a data are summarized in Figure 6A–C.
Figure 6.
Serum IgG (A), IgG1 (B), and IgG2a (C) anti-rHagB response in mice immunized by the sc route with rHagB alone or with GPI-0100 or saponins 3–6. Mice were immunized on days 0, 14, and 28. Serum samples were collected prior to each immunization and at 6 weeks after the initial immunization. Values are expressed as the mean ± SEM: *P < 0.05, **P < 0.01, and ***P < 0.001 compared with mice immunized with rHagB alone.
The antigen rHagB stimulated a strong humoral immune response. Although the group without adjuvant was lower in IgG and IgG1 activities than the groups with an adjuvant at weeks 2 and 4, the difference became insignificant at week 6 (except for IgG of the group with 5). However, the rHagB + GPI-0100 or rHagB + VSA-1 (5) groups showed significantly higher IgG2a activity than the group with rHagB alone at weeks 2, 4, and 6, which is consistent with our observation with the OVA antigen. The rHagB + 6 also had significantly higher IgG2a titers than the rHagB group at weeks 2 and 4 but not at week 6.
These results clearly showed that by incorporating a simple and chemically stable fatty side chain to natural MS I (3), the new derivative (5) not only retains and enhances the IgG1 immunity of 3 but also modulates its adjuvant activity profile by inducing a significant IgG2a immune response. However, when the same strategy was applied to MS II (4), no significant increase of IgG2a was observed. Given that the only structural difference between the two MS derivatives is in the triterpenoid core, i.e., gypsogenin (R1 = H) versus quillaic acid (R1 = OH) (Scheme 1), it appears that the structure of the triterpenoid core instead of the hydrophile–lipophile balance of the saponin structure35 plays an important role in affecting adjuvant activity of derivatives 5 and 6. On the other hand, similar adjuvant activity between MS derivative 5 (with gypsogenin core) and QS derivative 2 (with quillaic acid core) suggests that the SAR is not straightforward, and the overall adjuvant activity is a result of complicated coordination of different structural moieties, i.e., the triterpene core and C3 and C28 sugar domains. Nevertheless, identification of derivative 5 demonstrates a viable way for easy access to structurally defined and potent saponin immunostimulants through simple chemical derivatization of inexpensive natural products.
We then went on to evaluate acute toxicity of adjuvant VSA-1 (5) by using the same procedure for GPI-0100.31 Thus, BALB/c mice (female, 10 weeks of age) were given an injection of an adjuvant in 0.1 mL of PBS on the neck via the subcutaneous route with the doses indicated in Table 2. All of the mice in the groups treated with VSA-1 (5) (5000 μg) or Quil-A (100 or 200 μg) died within 5 days after injection. The survival mice all had healthy looking fur and appeared to be behaving normally. None of the survival mice seemed lethargic in any way by day 7, and no lesion formation was observed on any of the mice. The data in Table 2 showed that the acute toxicity of VSA-1 (5) was similar to that of GPI-0100 but much lower than that of Quil A.
Table 2.
Acute Toxicity Comparison of 5, Quil A, and GPI-0100a
| dose (μg) | 5 | GPI-0100b | Quil-A |
|---|---|---|---|
| controls | 5/5 | 5/5 | 5/5 |
| 100 | 5/5 | S/S | 0/5 |
| 200 | 5/5 | 5/5 | 0/5 |
| 500 | 5/5 | 5/5 | |
| 1000 | 5/5 | 5/5 | |
| 2000 | 5/5 | 4/5 | |
| 5000 | 0/5 | 0/5 |
Results are expressed as the number of surviving mice per group of 5 mice 5 days after injection.
Literature results, female BALB/c mice of 16 weeks of age.31
CONCLUSIONS
We have derivatized MS I and II by coupling them with dodecylamine at C3 glucuronic acid site. The obtained derivatives show significantly different immunostimulant activity profiles from their respective natural parent saponins. In particular, adjuvant VSA-1 (5), the derivative of MS I (3), induces an IgG2a responose significantly higher than the corresponding natural product. Its IgG1 and IgG2a productions are similar to that of GPI-0100, suggesting a potential mixed Th1/Th2 immune response against the specific antigens, different from the Th2 immunity induced by the natural saponins. Toxicity evaluations show that VSA-1 (5) has a toxicity profile similar to that of GPI-0100 and is much less toxic than the widely used natural saponin mixture Quil A. These results prove that derivatizing Momordica saponins is a viable way for easy access to structurally defined and potent saponin immunostimulants that can potentiate a mixed Th1/Th2 immune response and have low toxicity. Given the fact that Momordica saponins are readily available and easy to isolate, it will be practical for large-scale preparation of MS derivatives for potential preclinical studies and clinical applications.
EXPERIMENTAL SECTION
Chemistry. General.
Organic solutions were concentrated by rotary evaporation at ~12 Torr. Flash column chromatography was performed employing 230–400 mesh silica gel. Thin-layer chromatography was performed using glass plates precoated to a depth of 0.25 mm with 230–400 mesh silica gel impregnated with a fluorescent indicator (254 nm). Infrared (IR) data are presented as frequency of absorption (cm−1). Proton and carbon-13 nuclear magnetic resonance (1H NMR or 13C NMR) spectra were recorded on 400, 700, and 850 MHz NMR spectrometers. Chemical shifts are expressed in parts per million (δ scale) downfield from tetramethylsilane and are referenced to residual protium in the NMR solvent (CHCl3: δ = 7.26). Data are presented as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet and/or multiple resonances, AB = AB quartet), coupling constant in hertz (Hz), integration. Anhydrous solvents were used without distillation. Solvents for workup and column chromatography were obtained from commercial vendors and used without further purification. The purity of the products was determined by a combination of HPLC, HRMS, and 1H NMR and found to be ≥95%. All in vivo studies were performed in accordance with local IACUC guidelines.
General Procedure of Derivatizing Momordica Saponins I and II.
Momordica saponins were isolated by using the published procedure.34 To the obtained saponin (3) (120 mg, 0.07 mmol) in ethanol (3.0 mL) and water (1.0 mL) were added dodecylamine (50 mg, 0.27 mmol), N-methylmorpholine (NMM) (91 mg, 0.90 mmol), hydroxybenzotriazole (HOBt) (83 mg, 0.54 mmol), and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl) (107 mg, 0.54 mmol) at room temperature.39 The reaction mixture was stirred for 1 day and then filtered. The filtrate was purified with RP HPLC by using a Prep-C18, 21.2 mm × 50 mm, 5 μm column and H2O/MeCN gradients (90–10% H2O over 9 min with a 25 mL/min flow rate). The desired product had a retention time of 7.5–9.5 min and the fraction was concentrated on a rotary evaporator at room temperature to remove MeCN, and the remaining water was then removed on a lyophilizer to provide final product 5 (93 mg, 70%) as a white solid.
For 5, 1H NMR (600 MHz, CD3OD) (characteristic protons) δ 9.48 (s, 1H), 5.35 (d, J = 1.6 Hz, 1H), 5.33 (d, J = 8.2 Hz, 1H), 5.27 (t, J = 3.5 Hz, 1H), 5.04 (d, J = 1.5 Hz, 1H), 4.66 (d, J = 7.9 Hz, 1H), 4.59 (d, J = 7.8 Hz, 1H), 4.51–4.49 (m, 2H), 4.47 (m, 1H), 4.27 (dd, J = 3.0, 1.8 Hz, 1H), 4.03 (dd, J = 3.2, 1.8 Hz, 1H), 4.00 (dd, J = 11.4, 5.3 Hz, 1H), 3.15 (t, J = 10.9 Hz, 1H), 3.06 (dd, J = 9.2, 7.9 Hz, 1H), 2.82 (dd, J = 13.8, 3.9 Hz, 1H), 1.20 (s, 3H), 1.19 (s, 3H), 1.02 (s, 3H), 0.82 (s, 3H); 13C NMR (150.9 MHz, CD3OD) δ 209.3, 176.5, 169.8, 143.6, 121.8, 104.6, 103.9, 103.7, 102.8, 102.5, 101.8, 100.0, 94.0, 87.3, 84.3, 84.1, 81.5, 77.7, 77.6, 76.6, 76.1, 76.0, 75.40, 75.38, 74.9, 74.5, 74.0, 73.6, 73.0, 72.8, 72.4, 72.2, 71.6, 71.3, 70.8, 70.7, 70.4, 70.1, 69.9, 69.6, 69.2, 69.1, 68.1, 67.4, 65.7, 65.6, 60.81, 60.77, 54.8, 46.6, 46.0, 41.8, 41.6, 39.6, 38.7, 38.0, 35.7, 33.5, 32.2, 32.1, 31.7, 31.5, 30.1, 29.54, 29.51, 29.5, 29.4, 29.2, 29.1, 28.9, 27.5, 26.5, 24.8, 24.4, 23.2, 22.7, 22.6, 22.4, 20.2, 17.0, 16.5, 16.4, 15.1, 15.0, 13.1, 9.5. HRMS (ESI-TOF) m/z: [M – H]− calcd for C88H144NO39 1838.9315; found 1838.9305.
For 6 (9.8 mg, 50%), 1H NMR (600 MHz, CD3OD) (characteristic protons) δ 9.50 (s, 1H), 5.43 (d, J = 1.5 Hz, 1H), 5.33 (t, J = 3.6 Hz, 1H), 5.23 (d, J = 8.3 Hz, 1H), 5.04 (d, J = 1.4 Hz, 1H), 4.74 (d, J = 7.9 Hz, 1H), 4.56 (d, J = 7.8 Hz, 1H), 4.53 (s, 1H), 4.50–4.47 (m, 2H), 4.45 (d, J = 7.6 Hz, 1H); 4.24 (dd, J = 3.0, 1.9 Hz, 1H), 3.38 (t, J = 8.9 Hz, 1H), 3.18 (t, J = 10.9 Hz, 1H), 3.14 (dd, J = 9.3, 8.3 Hz, 1H), 2.91 (dd, J = 13.9, 4.3 Hz, 1H), 2.31 (t, J = 13.4 Hz, 1H), 1.43 (s, 3H), 1.20 (s, 3H), 1.04 (s, 3H), 0.96 (s, 3H), 0.89 (s, 3H), 0.81 (s, 3H); 13C NMR (150.9 MHz, CD3OD) δ 209.5, 175.6, 169.8, 143.5, 121.5, 104.7, 103.8, 103.5, 102.8, 102.7, 101.9, 99.3, 94.0, 87.4, 84.6, 84.5, 82.1, 77.2, 76.8, 76.4, 76.0, 75.43, 75.38, 75.1, 74.2, 74.0, 73.6, 73.4, 73.0, 72.4, 72.3, 71.6, 71.52, 71.47, 70.8, 70.7, 70.5, 70.04, 69.98, 69.6, 69.2, 69.1, 68.0, 67.4, 65.7, 60.8, 60.7, 54.8, 41.5, 41.0, 39.7, 38.7, 38.0, 35.7, 35.2, 32.7, 31.9, 31.7, 30.5, 29.9, 29.6, 29.52, 29.46, 29.42, 29.21, 29.15, 28.9, 26.5, 25.9, 24.6, 23.3, 23.1, 22.4, 20.0, 17.0, 16.44, 16.36, 15.1, 13.1, 9.6. HRMS (ESI-TOF) m/z: [M – H]− calcd for C88H144NO40 1854.9265; found 1854.9240.
Antigens.
The chicken egg albumin for in vivo use (Vac-pova) was purchased from InvivoGen. Recombinant Porphyromonas gingivalis HagB was prepared as previously described.33,40,41 Briefly, the HagB gene was cloned from P. gingivalis 381 into a pET vector with a lac promoter and histidine tag and expressed in Escherichia coli JM109 (kindly provided by Ann Progulske-Fox and Thomas Brown, University of Florida, Gainesville). Protein expression was induced following isopropyl-β-d-thiogalactopyranoside (IPTG) induction. rHagB was purified from the soluble fraction of the bacterial lysates by using a His-bind resin column, according to the manufacturer’s instruction (Novagen, Madison, WI). The purity of rHagB was confirmed by silver staining and Western blot analysis using a rabbit anti-rHagB antibody. The concentration of rHagB was estimated by the bicinchoninic acid protein determination assay (Pierce, Rockford, IL), using bovine serum albumin (BSA) as the standard.
Mice and Immunization.
BALB/c mice used in this study were purchased from Frederick Cancer Research (Frederick, MD) and maintained within an environmentally controlled, pathogen-free animal facility at the University of Alabama at Birmingham (UAB). To assess the adjuvant activity of the MS saponin-based immune adjuvants, groups of female mice (8–10 weeks of age; 6 mice per group) were immunized by the subcutaneous (sc) route with OVA (20 μg) or rHagB (35 μg) alone or with antigen plus proper adjuvant such as GPI-0100 (100 μg) or a MS adjuvant (100 μg) on days 0, 14, and 28. Prior to each immunization and at 2 weeks after last immunization, mice were weighed and blood samples were collected from the lateral tail vein by using heparinized capillary pipettes. The serum was obtained after centrifugation and stored at −20 °C until assayed. All studies were performed according to National Institutes of Health guidelines, and protocols were approved by the UAB Institutional Animal Care and Use Committee.
Evaluation of Antibody Responses.
The levels of specific serum IgG and IgG subclasses against OVA or rHagB in each group were determined by an enzyme-linked immunosorbent assay (ELISA). Maxisorpmicrotiter plates (NUNC International, Roskilde, Denmark) were coated with rHagB (1 μg/mL), OVA (0.1 μg/mL) or with optimal amounts of goat anti-mouse IgG, IgG1 or IgG2a (Southern Biotechnology Associates, Inc., Birmingham, AL) in borate buffer saline (BBS; 100 mM NaCl, 50 mM boric acid, 1.2 mM Na2B4O7 pH 8.2) at 4 °C overnight. Plates were blocked with 1% bovine serum albumin (BSA) and 0.02% sodium azide in BBS for 2 h at room temperature. Serial 2-fold dilutions of serum samples were added in duplicate to the plates. To generate standard curves, serial dilutions of a mouse immunoglobulin reference serum (MP Biomedicals, Solon, OH) were added to two rows of wells in each plate that had been coated with the appropriate anti-mouse IgG or IgG subclass reagent. After incubation (overnight at 4 °C) and washing of the plates, horseradish peroxidase-conjugated goat antimouse IgG or IgG subclass antibody (Southern Biotechnology Associates, Inc.) was added to appropriate wells. After 4 h of incubation at room temperature, plates were washed and developed by o-phenylenediamine substrate with hydrogen peroxide. Color development was recorded at 490 nm. The concentrations of antibodies were determined by interpolation on standard curves generated by using the mouse immunoglobulin reference serum and constructed by a computer program based on four-parameter logistic algorithms (Softmax/Molecular Devices Corp., Menlo Park, CA).
Statistical Analysis.
Statistical significance in antibody responses was evaluated by ordinary one-way ANOVA and the Dunnett test or Tukey multiple-comparisons test using GraphPad Prism 8.0.1. Differences were considered significant at a p value of <0.05.
ACKNOWLEDGMENTS
This work was supported by NIH Grant R01 GM120159 to P.W.
ABBREVIATIONS USED
- IgG
immunoglobulin G
- Th
T helper cells
- CTL
cytotoxic T cell
- rha
rhamnose
- xyl
xylose
- OVA
ovalbumin
- NMM
N-methylmorpholine
- HOBt
hydroxybenzotriazole
- EDC·HCl
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
- DCM
dichloromethane
- MeCN
acetonitrile
- THF
tetrahydrofuran
- rHagB
recombinant hemagglutinin B
- sc
subcutaneous
- ESI-TOF
electrospray ionization time-of-flight mass spectrometry
- ELISA
enzyme-linked immunosorbent assay
- IACUC
International Animal Care and Use Committee
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmedchem.9b01511.
1H NMR spectra of the known compounds 3 and 4 and 1H and 13C NMR spectra of the new compounds 5 and 6 (PDF)
The authors declare the following competing financial interest(s): P.W. is inventor on patent applications based on this work.
REFERENCES
- (1).Brunner R; Jensen-Jarolim E; Pali-Scholl I The ABC of clinical and experimental adjuvants-A brief overview. Immunol. Lett 2010, 128, 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Kensil CR; Mo AX; Truneh A Current vaccine adjuvants: an overview of a diverse class Front. Biosci, Landmark Ed. 2004, 9, 2972–2988. [DOI] [PubMed] [Google Scholar]
- (3).Leroux-Roels G Unmet needs in modern vaccinology: Adjuvants to improve the immune response. Vaccine 2010, 28 (3), C25–36. [DOI] [PubMed] [Google Scholar]
- (4).Sharp FA; Lavelle EC Discovery of Vaccine Adjuvants In Development of Therapeutic Agents Handbook, 1st ed.; Gad SC, Ed.; John Wiley & Sons, Inc,: Hoboken, NJ, 2012; pp 533–546. [Google Scholar]
- (5).Wang W; Singh M Selection of adjuvants for enhanced vaccine potency. World J. Vaccines 2011, 01, 33–78. [Google Scholar]
- (6).Weeratna RD; McCluskie MJ Recent Advances in Vaccine Adjuvants In Emerging Trends in Antibacterial Discovery: Answering the Call to Arms; Miller AA, Miller PF, Eds.; Caister Academic Press: Great Britain, 2011; pp 303–322. [Google Scholar]
- (7).Coffman RL; Sher A; Seder RA Vaccine adjuvants: putting innate immunity to work. Immunity 2010, 33, 492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Banday AH; Jeelani S; Hruby VJ Cancer vaccine adjuvants - Recent clinical progress and future perspectives. Immunopharmacol. Immunotoxicol 2015, 37, 1–11. [DOI] [PubMed] [Google Scholar]
- (9).Bastola R; Noh G; Keum T; Bashyal S; Seo JE; Choi J; Oh Y; Cho Y; Lee S Vaccine adjuvants: smart components to boost the immune system. Arch. Pharmacal Res 2017, 40, 1238–1248. [DOI] [PubMed] [Google Scholar]
- (10).Di Pasquale A; Preiss S; Tavares Da Silva F; Garçon N Vaccine Adjuvants: From 1920 to 2015 and Beyond. Vaccines (Basel, Switz.) 2015, 3, 320–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Temizoz B; Kuroda E; Ishii KJ Vaccine adjuvants as potential cancer immunotherapeutics. Int. Immunol 2016, 28, 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).O’Hagan DT; Friedland LR; Hanon E; Didierlaurent AM Towards an evidence based approach for the development of adjuvanted vaccines. Curr. Opin. Immunol 2017, 47, 93–102. [DOI] [PubMed] [Google Scholar]
- (13).Garçon N; Leroux-Roels G; Cheng W-F Vaccine Adjuvants In Understanding Modern Vaccines Perspectives in Vaccinology; Garçon N, Stern PL, Cunningham AL, Eds.; Elsevier: Amsterdam, 2011; Vol. 1; pp 89–113. [Google Scholar]
- (14).Harandi AM; Medaglini D; Shattock RJ Working Group convened by EUROPRISE. Vaccine adjuvants: a priority for vaccine research. Vaccine 2010, 28, 2363–2366. [DOI] [PubMed] [Google Scholar]
- (15).Bowen WS; Svrivastava AK; Batra L; Barsoumian H; Shirwan H Current challenges for cancer vaccine adjuvant development. Expert Rev. Vaccines 2018, 17, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Brito LA; O’Hagan DT Designing and building the next generation of improved vaccine adjuvants. J. Controlled Release 2014, 190, 563–579. [DOI] [PubMed] [Google Scholar]
- (17).Didierlaurent AM; Laupeze B; Di Pasquale A; Hergli N; Collignon C; Garçon N Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [DOI] [PubMed] [Google Scholar]
- (18).James SF; Chahine EB; Sucher AJ; Hanna C Shingrix: The New Adjuvanted Recombinant Herpes Zoster Vaccine. Ann. Pharmacother 2018, 52, 673–680. [DOI] [PubMed] [Google Scholar]
- (19).Kensil CR Saponins as vaccine adjuvants. Crit. Rev. Ther. Drug Carrier Syst 1996, 13, 1–55. [PubMed] [Google Scholar]
- (20).Kensil CR; Liu G; Anderson C; Storey J Effects of QS-21 on Innate and Adaptive Immune Responses In Vaccine Adjuvants: Immunological and Clinical Principles; Hackett CJ, Harn DAJ, Eds.; Humana Press Inc: Totowa, NJ, 2005; pp 221–234. [Google Scholar]
- (21).Kensil CR; Patel U; Lennick M; Marciani D Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol 1991, 146, 431–437. [PubMed] [Google Scholar]
- (22).Ragupathi G; Gardner JR; Livingston PO; Gin DY Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer. Expert Rev. Vaccines 2011, 10, 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Kamstrup S; San Martin R; Doberti A; Grande H; Dalsgaard K Preparation and characterisation of Quillaja saponin with less heterogeneity than Quil-A. Vaccine 2000, 18, 2244–2249. [DOI] [PubMed] [Google Scholar]
- (24).Martin RS; Briones R Industrial uses and sustainable supply of Quillaja Saponaria (Rosaceae) Saponins. Econ. Bot 1999, 53, 302–311. [Google Scholar]
- (25).Liu G; Anderson C; Scaltreto H; Barbon J; Kensil CR QS-21 structure/function studies: effect of acylation on adjuvant activity. Vaccine 2002, 20, 2808–2815. [DOI] [PubMed] [Google Scholar]
- (26).Pillion DJ; Amsden JA; Kensil CR; Recchia J Structure-function relationship among Quillaja saponins serving as excipients for nasal and ocular delivery of insulin. J. Pharm. Sci 1996, 85, 518–524. [DOI] [PubMed] [Google Scholar]
- (27).Wang P; Dai Q; Thogaripally P; Zhang P; Michalek SM Synthesis of QS-21-based immunoadjuvants. J. Org. Chem 2013, 78, 11525–11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Wang P; Devalankar DA; Dai Q; Zhang P; Michalek SM Synthesis and evaluation of QS-21-based immunoadjuvants with a terminal-functionalized side chain incorporated in the west wing trisaccharide. J. Org. Chem 2016, 81, 9560–9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Wang P; Škalamera Đ; Sui X; Zhang P; Michalek SM Synthesis and evaluation of a QS-17/18-based vaccine adjuvant. J. Med. Chem 2019, 62, 1669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Wang P; Škalamera Đ; Sui X; Zhang P; Michalek SM Synthesis and evaluation of QS-7-based vaccine adjuvants. ACS Infect. Dis 2019, 5, 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Marciani D; Press JB; Reynolds RC; Pathak AK; Pathak V; Gundy LE; Farmer JT; Koratich MS; May RD Development of semisynthetic triterpenoid saponin derivatives with immune stimulating activity. Vaccine 2000, 18, 3141–3151. [DOI] [PubMed] [Google Scholar]
- (32).Marciani DJ; Reynolds RC; Pathak AK; Finley-Woodman K; May RD Fractionation, structural studies, and immunological characterization of the semi-synthetic Quillaja saponins derivative GPI-0100. Vaccine 2003, 21, 3961–3971. [DOI] [PubMed] [Google Scholar]
- (33).Zhang P; Yang Q-B; Marciani DJ; Martin M; Clements JD; Michalek SM; Katz J Effectiveness of the quillaja saponin semisynthetic analog GPI-0100 in potentiating mucosal and systemic responses to recombinant HagB from Porphyromonas gingivalis. Vaccine 2003, 21, 4459–4471. [DOI] [PubMed] [Google Scholar]
- (34).Iwamoto M; Okabe H; Yamauchi T; Tanaka M; Rokutani Y; Hara S; Mihashi K; Higuchi R Studies on the constituents of Momordica cochinchinensis SPRENG. I. Isolation and characterization of the seed saponins, momordica saponins I and II. Chem. Pharm. Bull 1985, 33, 464–478. [Google Scholar]
- (35).Oda K Relationship between adjuvant activity and amphipathic structure of soyasaponins. Vaccine 2003, 21, 2145–2151. [DOI] [PubMed] [Google Scholar]
- (36).Tang W; Eisenbrand G Handbook of Chinese Medicinal Plants; Wiley-VCH: Weinheim, Germany, 2011; Vol. 2, p 768. [Google Scholar]
- (37).Xiao C; Rajput ZI; Liu D; Hu S Enhancement of serological immune responses to foot-and-mouth disease vaccine by a supplement made of extract of cochinchina momordica seeds. Clinical and Vaccine Immunology: CVI 2007, 14, 1634–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Sun JH; Jiang ZQ; Hu SH Effect of four adjuvants on immune response to F4 fimbriae in chickens. Vet. Immunol. Immunopathol 2008, 121, 107–112. [DOI] [PubMed] [Google Scholar]
- (39).Pu YJ; Vaid RK; Boini SK; Towsley RW; Doecke CW; Mitchell D A practical method for functionalized peptide or amide bond formation in aqueous-ethanol media with EDC as Activator. Org. Process Res. Dev 2009, 13, 310–314. [Google Scholar]
- (40).Zhang P; Martin M; Yang Q-B; Michalek SM; Katz J Role of B7 costimulatory molecules in immune responses and T-helper cell differentiation to recombinant HagB from Prophyromonas gingivalis. Infect. Immun 2004, 72, 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Zhang P; Martin M; Michalek SM; Katz J Role of mitogen-activated protein kinases and NF-κB in the regulation of pro-and anti-inflammatory cytokines by Porphyromonas gingivalis Hemagglutinin B. Infect. Immun 2005, 73, 3990–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Katz J; Black KP; Michalek SM Host responses to recombinant hemagglutinin B of Porphyromonas gingivalis in an experimental rat model. Infect. Immun 1999, 67, 4352–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]