Abstract
Neuromyelitis optica spectrum disorder (NMOSD) causes disabling and persistent central neuropathic pain (NP). Because the pain syndrome in NMOSD is severe and often intractable to analgesic treatment, it interferes with quality of life in patients. No interventional trials have been published looking at response to interventions for pain in NMOSD. This is a synthesis of the literature surveying the impact on quality of life of interventions in all mechanisms of central spinal NP. This review has important implications for management of pain in NMOSD. A systematic database search was conducted using Pubmed, Embase and CINAHL Plus with keywords including “spinal cord,” “quality of life” and “neuropathic pain” in an attempt to identify original research that targeted spinal NP treatment and used quality of life as an outcome measure. Both pharmacologic and non-pharmacologic treatments were sought out. Twenty-one studies meeting our eligibility criteria were identified and evaluated, 13 using pharmacologic treatments and 8 using nonpharmacologic interventions. Overall, sample sizes were modest, and effects on decreasing pain and/or improving quality of life were sub-optimal. This review provides researchers with a foundation from which to start a more thorough and thoughtful investigation into the management of NP in NMOSD and underscores the importance of including quality of life as a clinically meaningful outcome measure.
Keywords: neuromyelitis optica, neuropathic pain, quality of life, multiple sclerosis, spinal cord injury, chronic pain
Neuromyelitis optica spectrum disorder (NMOSD) is a relapsing autoimmune disorder of the central nervous system (CNS) that preferentially targets the spinal cord and optic nerves, leading to blindness, paralysis and death. NMOSD disproportionately affects non-Caucasians and females (Mealy, Wingerchuk, Greenberg & Levy, 2012; Jarius et al, 2012), and has a worldwide prevalence estimated to be 0.52 to 4.4/100,000 (Marrie & Gryba, 2013), affects approximately 4,000–8,000 people in the US, with a wide range of disease age at onset from infancy through old age (Mealy, et al., 2012; Jarius et al, 2012). In contrast to multiple sclerosis (MS) which primarily targets myelin, NMOSD causes irreparable neuronal cell death which leads to more severe disability and a poorer prognosis (Popescu & Lucchinetti, 2016). Historically, within five years of symptom onset, 60% of NMOSD patients were blind in at least one eye, 52% were weak in at least one limb requiring gait assistance and mortality was as high as 30% (Wingerchuk, Hogancamp, O’Brien & Weinshenker, 1999), though those data have improved with the identification of the highly specific AQP4 antibody (Wingerchuk, Lennon, Pittock, Lucchinetti & Weinshenker, 2006; Mealy et al., 2018).
NMOSD causes severe, persistent pain which is more prevalent (83.8%−91%) than in MS (~47%) (Kanamori et al.,2011; Qian et al., 2012;Pellkofer et al., 2013) and most other neurological diseases (Borsook, 2012). The most common type of pain in NMOSD is central neuropathic pain (CNP), which is characterized as agonizing burning, shooting, tingling, lancinating, and squeezing sensations that are distressing, persistent and debilitating. Half of patients with NMOSD characterize their CNP as severe and two thirds report constant pain (Zhao, S., Mutch, K., Elsone, L., Nurmikko, T., & Jacob, A., 2014; Pellkofer et al., 2013). CNP typically presents weeks to months after the cord damage has occurred long after the acute injury, and may be a result of secondary changes due to reorganization of the damaged circuits of the somatosensory system (Sjolund, 2002). The pain can persist for years, decades or throughout the patient’s life. The presence and severity of pain in NMOSD may be influenced by lesion span and location: NMOSD lesions are generally transverse, involving both the central gray matter and dorsal horns. The dorsal horns are innervated by primary afferent fibers and contain a large number of excitatory (glutamatergic) and inhibitory (GABA (γ-aminobutyric acid)-ergic or glycinergic) interneurons, as well as projection neurons that convey sensory information to the brain (Todd, 2010; Bradl et al., 2014). Damage to the central gray matter in NMOSD leads to astrocytic damage and tissue necrosis, thus disrupting sensory pain tracts going to and from the brain (Qian et al.,2012; Kanamori et al.,2011). As a consequence of ongoing spontaneous activity arising in the periphery, surviving neurons develop increased background activity and increased responses to ascending nerve impulses, including normally harmless tactile stimulation (Centonze, 2014).
Because many provider visits are dedicated to assessment and treatment of the underlying neurological disease, treatment of CNP is lacking despite increasing awareness of its impact on quality of life (QoL). Table 1 shows a comprehensive overview that includes seven studies assessing pain and QoL in NMOSD to date, none of which focus on an intervention or treatment. However, three studies have found promising results when examining pain as a secondary outcome, two in patients receiving a humanized monoclonal antibody that targets interleukin(IL)-6 for disease suppression, (Araki et al., 2014; Ringelstein et al., 2015) and one in patients receiving low-dose mycophenolate mofetil (Huang et al., 2018), though none of these studies investigated QoL outcomes.
Table 1:
Studies evaluating pain in NMOSD patients (listed chronologically)
| Author (Year); Country | Patient Sample | Study Design |
Pain Measure |
QoL Measure |
Findings & Comments* |
|---|---|---|---|---|---|
| Anti-Epileptics | |||||
| Kanamori et al. (2011); Japan | 37 NMOSD patients (35 AQP4+; 36 female); 51 MS patients as comparator (37 female) | Descriptive comparative | BPI | SF-36 | This was the first study that explored pain in NMO. Pain in NMOSD (83.8%) was more common than in MS (47.1%), and also more severe. QoL was also significantly poorer in the NMOSD group in the areas of bodily pain, physical functioning and general health. Consecutive sampling was used for participant recruitment. While this study did not investigate or account for medication use and was limited in the number of variables assessed, this pivotal study shed light on the issue of pain in NMO. |
| Qian et al. (2012); US | 29 NMOSD patients (24 AQP4+; 24 female; 14 White/Caucasian, 14 Black/African descent, 1 Asian descent); 66 MS patients as comparator (52 female; 56 White/Caucasian, 10 Black/African descent) | Descriptive comparative | MPQ; 10-point NRS | SF-36 | This study assessed pain and other symptoms, neurological function, spinal cord damage, and QoL. Pain in NMOSD (86.2%) was more common than in MS (40.9%), and also more severe, even after controlling for disability and spinal cord damage. Pain correlated with worse physical and mental scores on SF-36. This was the first study to examine medication use: prescription pain medications were used significantly more frequently in NMOSD participants, more often required multiple medications and all treated patients experienced pain despite treatment. |
| Pellkofer et al. (2013); Germany | 11 NMOSD patients (11 AQP4+; 9 female; all White/Caucasian) | Descriptive | 10-point NRS | SF-36 | This study aimed to investigate presence and clinical characteristics of pain, stress and depression, and evaluated endocannabinoid levels and abnormalities in somatosensory functioning. Recruitment was through consecutive sampling. 91% endorsed NP within previous 3 months and 72% reported ongoing NP; decreased QoL in 3 of 8 measures: physical functioning, general health and bodily pain. This study also aimed to explore the role of endogenous cannabinoids and found plasma levels of 2-AG to be significantly higher in NMOSD patients, suggesting that central sensitization is controlled by it. |
| Zhao et al. (2014); UK | 50 NMOSD patients (41 AQP4+; 39 female; no race/ethnicity data made available) | Descriptive | BPI | SF-36 | This study specifically explored NP more in-depth. Patients were assessed for pain and QoL through use of structured interviews and measurement tools, and a retrospective record review was conducted to examine MRI data, medication use and neurologic functioning. 62% of patients experienced NP, with 68% of those having constant pain affecting ADLs. Interestingly, 72% of females versus 27% of males reported pain. 25% of patients reported pain as their worst symptom, despite mobility and/or vision issues. Physical component of QoL was equally low in those patients with and without pain, specifically in the areas of physical functioning, general health and bodily pain. Mental component was significantly lower in those with pain. |
| Mutch et al. (2014); UK | 15 NMOSD patients (9 AQP4+; 11 female; no race/ethnicity data made available) | Qualitative descriptive | Semi-structured interviews | Semi-structured interviews | First qualitative study to explore QoL, including pain. Poor vision, reduced mobility, bladder dysfunction and pain affected participants’ independence and experience of living with NMO. Expressed anxiety regarding unpredictability of disease and desire for normalcy. Patients reported anxiety and low mood, particularly following diagnosis and after relapses. Twelve patients reported pain, and indicated that it considerably affected their daily activities, mood, walking ability, enjoyment of life and relationships. |
| Asseyer et al. (2018);Germany | 49 NMOSD patients (29 AQP4+, 14 MOG+; 41 female; no race/ethnicity data made available) | Descriptive | painDETECT | SF-36 | Eighty-six percent of patients reported pain, regardless of antibody status. Pain correlated with QoL, but treatment of pain was not effective at improving QoL. |
| Eaneff et al. (2017); US, UK, Sweden | 522 NMOSD patients (selfreported; AQP4 status unknown; 283 female; race data available for 142 patients: 99 White/Caucasian, 22 Black/African descent, 15 Asian; 6 other) | Descriptive | Self-report | PLM quality of life survey | This study reports the patient perspective of those with NMOSD via an online community called PatientsLikeMe and found that 53% of NMOSD patients report moderate to severe pain. Fifty-nine percent reported that their health limited their work and activities all or most of the time. Physical and emotional health interfered with social activities. |
values were considered significant at p<0.05, unless otherwise noted
NMOSD=neuromyelitis optica spectrum disorder; MS=multiple sclerosis; NP=neuropathic pain; BPI=Brief Pain Inventory; SF-36=Short Form 36 health survey; AQP4+=aquaporin 4+; MPQ=McGill Pain Questionnaire; NRS=numeric rating scale; QoL=quality of life; 2-AG=2-Arachidonoylglycerol; ADLs=activities of daily living; PLM=PatientsLikeMe
Research on the impact of persistent pain on QoL in NMOSD has found that those patients with CNP experience more depression, less enjoyment of life, and more difficulty with ambulation (Mutch et al., 2014; Pellkofer et al., 2013; Zhao et al., 2014). CNP is particularly resistant to most currently available treatments (Qian et al., 2012; Zhao et al., 2014). The most common medication classes for the treatment of CNP, used off-label, are anti-epileptics, anti-depressants and non-steroidal anti-inflammatories, but many patients still require frequent opioid use (Qian et al., 2012; Zhao et al., 2014). Cannabinoids have been recently considered for CNP as they become more available for use, though no data specific to this population have been analyzed. Despite this analgesic armament, NMOSD patients continue to have pain, in contrast to nearly half of MS patients treated for their CNP who report being pain-free (Qian et al., 2012).
Another factor to consider is that the medications used for treatment of CNP have side effects, particularly at higher doses, and are independently associated with slower reaction times and fatigue (Qian et al., 2012). Acknowledging that randomized control trials have demonstrated efficacy of non-pharmacologic interventions for other pain conditions, researchers have sought to extend this work to SCI and MS populations for the treatment of CNP, with interventions such as nerve stimulation, acupuncture, exercise and massage therapy(Widerstrom-Noga and Turk, 2003; Boldt et al., 2014;Namjooyan et al., 2014). These studies are limited by small sample size and the potential bias of symptom self-report, and they often include therapies not generally covered by insurance.
Despite research of both pharmacologic and non-pharmacologic interventions in CNP, as well as research suggesting that CNP impacts QoL, few studies have specifically examined whether a given intervention that targets pain has any side benefit on QoL. This review sought to extract, evaluate, and synthesize the literature regarding the impact of CNP interventions on QoL.This review is subdivided by pharmacologic and nonpharmacologic interventions. As no such literature exists specifically in NMOSD, central spinal pain was sought out broadly and included related conditions including MS and spinal cord injury of multiple etiologies.
Methods
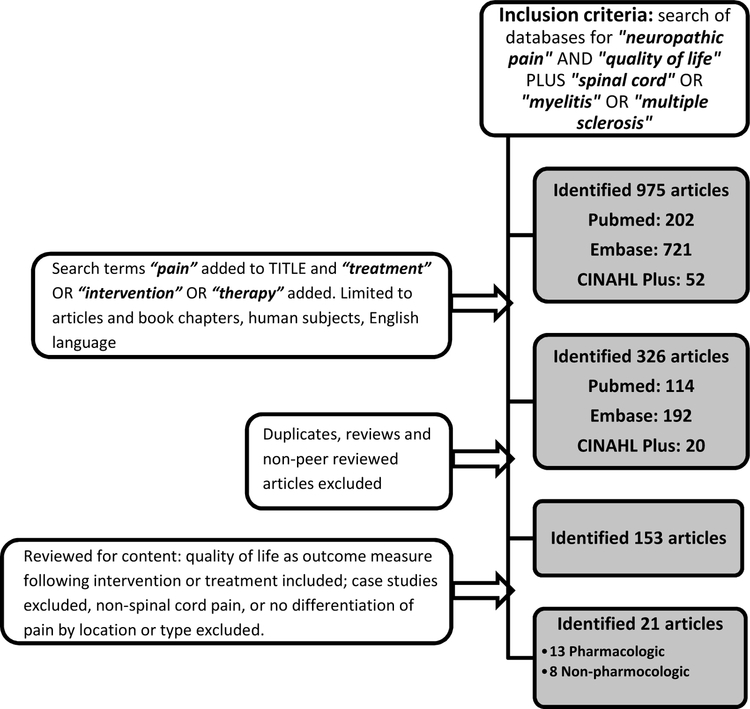
A research review was conducted by performing database searches of Pubmed, Embase, and CINAHL Plus. Search terms included “neuropathic pain” and “quality of life” for each search, and the terms “spinal cord,” “myelitis” and “multiple sclerosis” were each independently added to these terms in an effort to capture a comprehensive look at all causes for central NP of the spinal cord; this generated 975 citations (202 Pubmed, 721 Embase, 52 CINAHL). Results were further limited to articles and book chapters, human subjects, English language, by adding to the search “treatment” OR “intervention” OR “therapy” and by searching for “pain” specifically in article titles, which narrowed the search to 326 (114 Pubmed, 192 Embase, 20 CINAHL). Articles were not limited by date. Literature reviews that were not systematic, publications that were not peer-reviewed, and descriptive case reports were excluded. Duplicate articles were removed. Treatments directed to non-spinal cord etiologies or those that did not differentiate among location (spinal cord vs. supraspinal vs. peripheral) and/or types (neuropathic versus nociceptive) of pain were also excluded, and clinical judgment was exercised when interpretable based on description and location of the pain, in an effort to be inclusive when appropriate. Articles were included only if QoL was considered as an outcome (primary or secondary) following an intervention for CNP treatment, leaving 153 abstracts/articles for analysis in this review.
Results
Twenty-one interventional studies met inclusion criteria and were reviewed (see Figure 1). Across all studies, 910 patients were analyzed (438 SCI, 290 MS, 182 other). There were no studies focused on patients with NMOSD.
Figure 1:
Flowchart depicting study selection procedure
Sex was described in all but one study (n=24). Of the remaining aggregate sample of 886, 53% of participants were female. Demographic characteristics of race/ethnicity were described only in the three U.S. studies (aggregate n=263). Among these, 236 were White/Caucasian descent (90%), 22 were Black/African descent (8%), three were Hispanic/Latino descent (1%), one was Native American and one was classified as “other.”
Effect of pharmacologic interventions on QoL
Thirteen studies examined the effect of a pharmacologic intervention or treatment on QoL as a primary or secondary outcome (Table 2). Five studies evaluated the benefit of anti-epileptic medications (AEDs) for first-line treatment for CNP (Finnerup et al., 2015) in MS (2 studies), SCI (2 studies) and one was in a heterogeneous group of patients with CNP, a subset of whom had an undifferentiated spinal etiology. The rationale for using this class of medication for treatment of CNP was similar to the rationale for use in seizures: the drugs decrease hyperexcitability in damaged areas of the CNS caused by a sustained depolarization and inhibit voltage-dependent sodium channels, reducing the capacity of neurons to generate high-frequency action potentials (Salinas et al., 2012). A randomized, double-blind, placebo-controlled clinical trial using early AED intervention with carbamazepine to prevent the onset of CNP in SCI did not show a significant difference between the intervention and control groups (Salinas et al., 2012) suggesting that preemptive analgesia does not prevent the subsequent onset of CNP. The other four studies were conducted using patients after CNP onset, three of which found that reducing pain positively impacted QoL. Gabapentin, pregabalin and levetiracetam reduced pain by 50–100% on average and improved self-reported Visual Acuity Scale ratings through the course of treatment (Levendoglu, Ogun, Ozerbil, Ogun & Ugurlu, 2004; Vranken et al., 2008; Rossi et al., 2009) (Table 2). Overall, these findings support that use of AEDs for CNP treatment and improvement of QoL.
Table 2:
Trials examining the effect of pharmacologic interventions on QoL (grouped by mechanism)
| Author (Year); Country | Patient Sample | Study Design | Intervention | QoL Measure | Findings & Comments* |
|---|---|---|---|---|---|
| Anti-Epileptics | |||||
| Levendoglu et al. (2004); Turkey | SCI patients c/ NP; n=20 (7 female; no race data made available) |
RCT c/ cross-over | Gabapentin, titrated up to 3600 mg/day | Modified Lattinen Test | Significant decrease in intensity and frequency of most descriptors of pain (sharp, hot, unpleasant, deep, and surface) and disability and sleep were significantly better in the GBP treatment group. This was the 1st RCT to include QoL measures, in one of the most commonly used medications for NP treatment, however another measure of QoL would be helpful to capture a fuller picture of effects on health status. |
| Breuer et al. (2007); US | MS patients c/ spinal NP; n=12 (10 female; 8 white, 8 African descent) |
RCT c/ cross-over | Lamotrigine in addition to stable regimen, titrated up to 400 mg | MSQoL-54 | Pilot study showed no significant difference in pain or QoL; did not support need for larger trial. |
| Vranken et al. (2008); Netherlands | Patients c/ NP; subset was spinal; n=40 (19 female; 20 in treatment group; no race data made available); 21 patients with spinal cause |
RCT | Pregabalin in addition to stable regimen, titrated up to 600 mg/day | EQ-5D; SF-36; PDI | There was significant decrease in mean pain score for pregabalin treatment group, compared with placebo. No difference in PDI between groups but treatment group showed significant improvement for the EQ-5D utility score and EQ-5D VAS score compared with the placebo group and SF-36 indicated that treatment led to a significant improvement in the bodily pain domain only. |
| Rossi et al. (2009); Italy | MS patients c/ spinal NP; n=20 (15 female; 12 in treatment group; no race data made available) |
RCT | Levetiracetam, 500 mg/day | MSQoL-54 | Significant reduction in pain for treatment group; no difference in reported QoL, except for the item, ‘overall rating of quality of life.’ |
| Salinas et al. (2012); Colombia | SCI patients s/ NP; n=46 (4 female; 24 in treatment group; no race data made available) |
RCT | Carbamazepine, titrated up to 600 mg/day | SF-36 | This novel study looked at prevention of NP with early treatment, rather than treatment in those who already experience it. Early intervention did not decrease incidence of NP over time and there was no difference in QoL between groups, despite appropriate power. |
| Anti-Depressan ts | |||||
| Vranken et al. (2011); Netherlands | SCI and stroke patients c/ NP; n=48 (24 in treatment group; Table 1, demographics, is missing from manuscript) |
RCT | Duloxetine in addition to stable regimen, titrated up to 60 mg/day | EQ-5D; SF- 36; PDI | No difference in pain intensity with treatment. Treatment group showed a significant improvement for the bodily pain domain only of the SF-36. No significant differences were observed in other QoL indices. The distribution of spinal versus supraspinal NP is said to be in Table 1 (Demographics), but no such table was included in the manuscript and spinal NP could not be separately assessed. |
| Vollmer et al. (2013); US | MS patients c/ spinal NP; n=239 (189 female; 221 white, 15 African descent, 2 Hispanic, 1 Native American; 118 in treatment group); 209 in OLE |
Multi-center RCT, c/ OLE | Duloxetine in addition to stable regimen, titrated up to 60 mg/day in RCT and up to 120 mg/day in OLE | MSQoL-54 | This well-conceived, well-executed, international study showed significant pain reduction in treatment group; QoL was not impacted. In OLE, pain reduction was reported in patients in both groups, with greater improvement reported by patients who had received placebo during the acute phase. |
| Cannabinoids | |||||
| Svendsen et al. (2004); Denmark | MS patients c/ spinal NP; n=24 (14 female; no race data made available) |
RCT c/ crossover | Dronabinol, titrated up to 10 mg | SF-36 | Pain intensity and reduction significantly improved on treatment; the only improvements to QoL were seen in bodily pain and mental health. |
| Turcotte et al. (2015); Canada | MS patients c/ NP refractory to GPB; n=15 (13 female; 8 in treatment group; no race data made available) | RCT | Nabilone, titrated up to 1 mg, in addition to stable GBP dose | VASimpact | This small but well-designed study explores the important need for combining medication therapies, and shows that combination of GBP with nabilone significantly reduces pain. No change in pain impact was noted, however, the use of a VAS to capture impact of pain may have been inadequate and a more comprehensive measure of QoL would have been prudent. |
| Opioids | |||||
| Norrbrink & Lundeberg (2009); Sweden | SCI patients c/ NP; n=35 (7 female; 23 in treatment group; no race data made available) |
Multi-center RCT | Tramadol in addition to stable regimen, titrated up to 400 mg/day | LiSat-9 | Decrease in pain intensity in treatment group compared with those on placebo. Global life satisfaction improved in placebo group only. |
| Barrera-Chacon et al. (2011); Spain | SCI patients c/ NP refractory to AED treatment; n=54 (10 female; no race data made available) |
Multi-center, observational descriptive | Oxycodone, usually in conjunction with AED use | Modified EQ-5D | Significant decrease in pain intensity, improved health-related QoL and diminished impact of pain on physical activity and sleep. As doses of oxycodone were not investigated, further research in controlled trials assessing appropriate dosing for NP treatment is warranted. |
| Other | |||||
| Han et al. (2016); Korea | SCI patients c/ NP; n=40 (14 female; 20 in treatment group; no race data made available) | Multi-center RCT | BTX-A, 200 U subcutaneous injection, in addition to stable regimen | WHOQOL-BREF | The BTX-A group showed significant reductions in pain score at 4 and 8 weeks following injection, compared to placebo. Trend towards significant impact in physical health domain of QoL only. |
| Gonzalez et al. (2006); Sweden | Post-polio syndrome patients; subset had NP; n=142; 75 patients with NP (33 of whom are in treatment group; 92 females in total group, not differentiated by pain status; no race data made available) |
RCT | IVIG, 90 g over 3 days c/ 2nd equal dose at 3 months | SF-36 | Pain assessment was a secondary end-point of this study that primarily assessed strength, and not all patients included had NP. In the subcohort of patients with significant pain, those receiving IVIG had a greater pain reduction. QoL did not differ between groups. |
values were considered significant at p<0.05, unless otherwise noted
SCI=spinal cord injury; MS=multiple sclerosis; NP=neuropathic pain; RCT=Randomized Controlled Trial; GBP=gabapentin; MSQoL=Multiple Sclerosis Quality of Life instrument; EQ-5D=EuroQoL-5 Dimensions instrument; VAS=Visual Analog Scale; WHOQOL-BREF=World Health Organization Quality of Life-abbreviated; PDI=Pain Disability Index; SF-36=Short Form 36 health survey; OLE=open-label extension; BTX-A= botulinum toxin type A; IVIG= Intravenous immunoglobulin
Two RCTs studied the effects of the serotonin noradrenaline reuptake inhibitor (SNRI) duloxetine use on pain (Vranken et al., 2011; Vollmer, Robinson, Risser & Malcolm, 2014), although SNRIs are not as commonly used for pain worldwide compared to tricyclic anti-depressants (Finnerup et al., 2015). The stated rationale for using duloxetine was to inhibit the reuptake of serotonin and noradrenaline to potentiate monoamine neurotransmission in the descending inhibitory spinal pathways. This results in reduced nociceptive afferent transmission in the ascending spinal pain pathways to decrease the sensation of pain. Potentiation of both serotonin and noradrenaline is required to produce effective analgesia (Lunn, Hughes & Wiffen, 2014). The smaller of these studies (n=40) examined the effects of duloxetine in SCI-induced pain showed improvements in QoL including in the area of pain, but no improvement in independent pain scales (Vranken et al., 2011). In the larger study (n=239) duloxetine in MS patients with CNP resulted in significant reductions in pain throughout the end of the extension phase, without effecting any change in QoL (Vollmer et al, 2014). These seemingly conflicting results may be because the studies used different survey tools, highlighting the importance of using standardized, validated measures of pain and quality of life.
Cannabinoid agents were investigated in two small RCTs, both in MS (Svendsen et al., 2004; Turcotte et al., 2015). Cannabinoids are ligands that bind on the presynaptic cannabinoid receptor, resulting in reduced calcium influx from voltage-gated calcium channels and hyperpolarization, thus decreasing cellular excitability (Turcotte et al., 2015). Both of the cannabinoid agents used in these studies uncovered a significant impact on pain with the larger of the two additionally impacting QoL. The smaller, more recent study evaluated the use of nabilone, a synthetic tetrahydrocannabinol, in patients who were already on stable doses of gabapentin (Turcotte et al., 2015). As used this study, multimodal approaches to CNP treatment that target dual mechanisms of CNP treatment may be more effective at improving both pain and QoL.
Two opioids were assessed for treatment of CNP: tramadol and oxycodone. Opioids bind to an opioid receptor, causing inhibition of adenylyl-cyclase and hyperpolarization of neurons, and decreased excitability (Ordonez Gallego, Gonzalez Baron & Espinosa Arranz, 2007). One of the medications, tramadol, has a second mechanism of action similar to SNRIs, as described above (Norrbrink and Lundeberg, 2009). Both studies showed improvement in pain, though a limitation of the oxycodone study (Ordonez Gallego et al., 2007) is that it was an observational study and oxycodone dosing was not standardized by the study protocol.
Another study examined the effect of botulinum toxin type A (BTX-A) for CNP treatment in patients with SCI (n=40). The mechanism of action proposed in CNP is based on its mechanism in nociceptive pain, which suggests that BTX-A may inhibit neurogenic inflammation and the peripheral sensitization of nerve fibers by inhibiting the release of local neuropeptides, thereby reducing pain. The study reported a significant reduction in pain, though only a marginal trend toward significance on QoL.
Intravenous immunoglobulin (IVIG) contains the pooled polyvalent IgG antibodies extracted from the plasma of thousands of blood donors and is generally used to decrease inflammation. The exact mechanism of action has not been well-elucidated, but it is theorized that a high load of exogenous antibodies leads to a robust antibody recycling process that turns over both exogenous and endogenous antibodies (Sapir & Shoenfeld, 2005). This therapy was examined in post-polio syndrome (n=142; 75 with CNP): polio causes acute inflammation of the spinal cord leading to weakness, fatigue and pain persisting long after the acute infection has resolved (Gonzalez et al., 2006). Ongoing denervation has been suggested to be the most important reason for progressive muscle weakness associated with poliomyelitis infection. Patients with post-polio syndrome have increased expression of messenger RNA for proinflammatory cytokines in cerebrospinal fluid, which may suggest an ongoing inflammatory process in the CNS (Gonzalez et al., 2006). As such, the researchers hypothesized that targeting inflammation with IVIG may improve weakness or stop its progression. Pain was a secondary study outcome, but notably it was reduced in this patient population. QoL was not significantly impacted.
Effect of non-pharmacologic interventions on QoL
Eight studies were identified that examined the effect of a non-pharmacologic intervention for CNP on QoL. Non-pharmacologic interventions included the use of physical therapy (PT) and exercise, transcutaneous electrical nerve stimulation (TENS), cognitive behavioral therapy (CBT) and complementary and alternative medicine (CAM) approaches (Table 3).
Table 3:
Trials examining the effect of non-pharmacologic interventions on QoL (grouped by mechanism)
| Author (Year); Country | Patient Sample | Study Design | Intervention | QoL Measure | Findings & Comments |
|---|---|---|---|---|---|
| Physical Medicine and Rehabilitation Medicine | |||||
| Norrbrink (2009); Sweden | SCI patients c/ NP; n=24 (4 female; no race data made available) |
RCT c/ cross-over | Hi- versus low- frequency TENS | LiSat-9 | In this study with high attrition and difficulty with enrollment, pain intensity was unchanged compared with baseline values on a group level and no differences were found between the two modes of stimulation, and no effect on secondary measures including life satisfaction was noted. |
| Norrbrink et al. (2012); Sweden | SCI patients c/musculoskeletal and/or NP; n=8; 7 patients with NP pain (1 of whom is female; no race data made available) |
Observational descriptive | 10-week exercise program | SCI QoL data set | Descriptive statistics were utilized alone since inferences could not be made in this small cohort. For those with neuropathic pain, median pain intensity ratings decreased from 5 on a 010 numerical rating scale at baseline to 3 at the end of study. All median ratings of QoL showed improvement. Results in this exploratory study are promising and need to be further explored in a larger controlled study. |
| Cognitive Behavioral Therapy Programs | |||||
| Norrbrink Budh et al. (2006); Sweden | SCI patients c/ NP; n=38 (24 female; 27 in treatment group; no race data made available) |
Quasi-experimental | CBT | NHP | In this non-randomized study, no difference from baseline in pain or QoL was found in treatment group, or between groups. Improvement in sleep quality and mood detected in treatment group, not affecting composite score. |
| Nicholson-Perry et al. (2009); Australia | SCI in patients c/ NP; n=36 (8 female; 19 in treatment group; no race data made available) | Quasi-experimental | CBT | Modified SF-36 with physical and mental domains | No changes in pain intensity between or within groups over time, though there was significant improvement in pain-catastrophizing for the treatment group. QoL improved for mental but not physical status. |
| Heutink et al. (2012); Netherlands | SCI patients c/ NP; n=61 (22 female; 31 in treatment group; no race data made available) |
Multi-center unblinded RCT | CBT | LiSat-9 | In the first RCT of CBT, short-term decrease in pain was detected, but this was not sustained at 3-month follow-up. No change in life satisfaction was identified, but anxiety and depression decreased in the intervention group. |
| Heutink et al. (2014); Netherlands | SCI patients c/ NP; n=29 (9 female; no race data made available) |
Multi-center extension study of Heutink, 2012 (treatment group only) | CBT | LiSat-9 | Pain intensity was significantly decreased at 12- month follow-up; no changes in life satisfaction or depression over time, though significant decrease in anxiety was noted. |
| Complementary and Alternative Medicine | |||||
| Wardell et al. (2006); US | SCI patients c/ NP; n=12 (0 female; 4 white, 3 African descent, 1 Hispanic, 1 other; 7 in HT group) |
Quasi-experimental; Convergent Mixed Methods | Healing Touch (energy-based program) vs. Guided Progressive Relaxation | SWLS; unstructured focus groups & interviews, written responses | This small pilot study of all-male veterans did not reveal significant changes in pain or life satisfaction. However, the HT group had variable responses and the qualitative component indicated that a subset of patients experienced benefit suggesting that while this pilot study was not powered appropriately to find differences, a larger study may. |
| Norrbrink & Lundeberg (2011); Sweden | SCI patients c/ NP; n=30 (6 female; 15 in treatment group; no race data made available) |
Quasi-experimental; Sequential Controlled-Trial | Acupuncture vs. Massage Therapy | LiSat-9 | This small exploratory study found a significant difference between the two groups at end of treatment in favor of acupuncture, but no within-group differences in pain at follow-up. No impact on life satisfaction. |
values were considered significant at p<0.05, unless otherwise noted
SCI=spinal cord injury; MS=multiple sclerosis; NP=neuropathic pain; RCT=Randomized Controlled Trial; GBP=gabapentin; SCI QoL=Spinal Cord Injury Quality of Life instrument; EQ-5D=EuroQoL-5 Dimensions instrument; HT=Healing Touch; SWLS=Satisfaction with Life Scale; LiSat-9=Life Satisfaction-9; CBT=Cognitive Behavioral Therapy: NHP=Nottingham Health Profile extension
In rats, regular moderate aerobic exercise reversed signs of CNP and increased endogenous opioid content in brainstem regions important in pain modulation (Stagg et al., 2011). This approach was translated to a small human study in SCI that showed significant improvements in both QoL and pain, suggesting that a larger study with PT and exercise is warranted (Norrbrink, Lindberg, Wahman, & Bjerkefors, 2012). Transcutaneous electrical nerve stimulation (TENS) uses electric current to stimulate denervated nerves through electrodes placed on the skin (Norrbrink, 2009). Effectiveness has been shown for use in peripheral neuropathy, but results in the treatment of central NP have been equivocal. Sites in the spinal cord and brainstem that utilize opioid, serotonin, and muscarinic receptors have been shown to be activated by peripheral nerve stimulation by TENS, but lack of standards on ideal frequency and stimulation amplitude needed to achieve pain reduction has hampered the use of TENS in clinical trials of CNP (Norrbrink, 2009; Sluka et al., 2013).
Psychological factors are believed to influence the maintenance and aggravation of CNP, suggesting that psychological interventions with traditional biomedical interventions may reduce the burden of CNP (Heutink et al., 2014). Four studies evaluated the effectiveness of Cognitive Behavioral Therapy (CBT) for CNP treatment, all in SCI (Norrbrink Budh, Kowalski, & Lundeberg, 2006; Nicholson Perry et al., 2009; Heutink et al., 2012; Heutink et al., 2014). CBT focuses on modifying an individual’s beliefs, expectations and coping abilities (Norrbrink Budh et al., 2006). One study reduced pain without improving QoL, two studies improved QoL only, and one had no impact on pain or QoL. CBT is aimed at modifying a patient’s response to pain rather than directed at the pain itself, explaining the finding that three of the studies did not impact pain significantly. Interestingly, all four studies had some impact on anxiety and/or depression, adding strength to the argument that this intervention improves pain responses rather than the physical experience of pain itself. Complementary and Alternative Medicine (CAM) refers to treatments that are outside of conventional medicine which have for the most part not been rigorously tested and often evolve from traditional Asian medicine. The American public’s use of CAM therapeutic modalities has grown exponentially in recent years, including for CNP (Namjooyan et al., 2014). This may be a product of the fact that CNP is often not affected in a clinically meaningful way, leading patients to look for other options. Common examples of these are acupuncture and massage therapy. Acupuncture is the stimulation of specific points through which the life-energy flows along the skin of the body using thin needles, in an attempt to achieve balance. Healing Touch (HT) similarly aims to achieve balance in life-energy through touch. Progressive muscle relaxation (PMR) is a technique focused on controlling the state of muscular tension and involves learning to monitor tension in each specific muscle group in the body by deliberately inducing and releasing tension in each group. The study examining HT versus PMR was the only mixed methods study reviewed; no significant impact was found on pain and QoL differences favoring HT were captured in the qualitative component alone (Wardell, Rintala, Duan, & Tan, 2006). In the study investigating the effects of acupuncture versus massage therapy, no differences in amount of pain were found but the data suggest that acupuncture may have prevented worsening of pain compared to therapeutic massage (Norrbrink and Lundeberg, 2011).
The concept of integrative medicine combines evidence-based treatments with alternative and non-pharmacologic options, in an attempt to approach treatment in a more holistic manner, and may be a promising next step in this arena. There are no trials or studies that systematically used a combination of pharmacologic and non-pharmacologic treatments for central spinal CNP using QoL as an outcome measure.
Discussion
This is a comprehensive literature review examining the state of the science in spinal CNP treatment utilizing QoL as a primary or secondary measure. Hundreds of articles were found that examined the impact of a given intervention on CNP or the impact of CNP on QoL, but only these 21 could be identified that included an examination of the impact of a pain intervention on QoL. However, impacting pain alone may not be clinically meaningful to a patient if QoL is not also enhanced. Given that pain affects QoL (Newland et al., 2009; Kanamori, 2011), it seems reasonable that an objective of pain treatment should be aimed at improving QoL in patients as well. It is striking that so few studies have applied QoL measures as an outcome. In order to meet patient-centered goals of improving QoL, future studies should include measures of both pain and QoL.
Also notable is the seeming mismatch between reported effects on pain levels and QoL, such that improvement in pain did not necessarily translate to improvement in QoL: of the 21 studies examined, 6 were shown to positively impact both pain and QoL, 4 failed to impact either, 7 impacted pain only and 4 impacted QoL only. There are several possible explanations as to why half of the studies had a mismatch between effects on pain and effects on QoL. First, many of the studies were relatively small, with a median sample size of 29.5 (range 8–239). Thus, some studies may have been adequately powered to address the primary outcome, but not sufficiently powered to identify significant differences in secondary outcomes, including QoL. Second, there was a wide array of both pain measures and QoL measures used, and while most have been validated in some populations, not all have been validated specifically in the populations that were investigated; see reviews of instruments for SCI, MS, and NP (Breivik et al., 2008; Stadhouder et al., 2010; Kuspinar and Mayo, 2014). Using validated measurement tools, or combining tools may draw out significant findings.
Another possible explanation for the mismatch between treatment of NP and its effect on QoL could be explained by recent research on symptom clusters. Symptom clusters consist of two or more related symptoms that co-occur and that may or may or may not share a common etiology (Kim, Abraham, & Malone, 2013). In chronic disorders, patients often present with multiple inter-related symptoms, which may explain why treating one symptom does not necessarily impact quality of life. While much of the research in symptom clusters has focused on cancer, the concept is applicable to a wide array of chronic conditions, including NMOSD, MS and SCI. The implication for NMOSD is that treating CNP in isolation may not impact QoL in a disease that causes other debilitating symptoms, including anxiety, depression, fatigue, sleep dysfunction and bladder dysfunction (Pan et al., 2015; Mutch et al., 2015; Hollinger et al., 2016; Shi et al., 2016; Mealy, Boscoe, Caro & Levy, M. 2018).
There was relative balance in the number of females to males represented overall (54%). SCI made up 39% of the total number of participants among trials, which has a high male-to-female ratio of 2.6–7:1 internationally (Singh, Tetreault, Kalsi-Ryan, Nouri & Fehlings, 2014). Counterbalancing this were the MS patients (42% of the total sample) where the female-to-male ratio is 2–3:1 (Koch-Henriksen & Sørensen, 2010). Only three studies reported on race/ethnicity, and all of these were from the United States and all predominantly white. Although many of the countries represented are fairly homogenous groups, demographics continue to change worldwide and it is problematic to fail to report on social determinants of health and inequities between groups, especially among groups where health inequities are known to be present and where perceptions of pain differ (Bernardes, Keogh & Lima, 2008; Rahim-Williams et al., 2012). Some research suggests that biopsychosocial mechanisms may underlie these differences (Paller, Campbell, Edwards, & Dobs, 2009). Of interest would be a comparison of responses to non-pharmacologic interventions, and particularly to CBT which is guided by the biopsychosocial model (Nicholson Perry et al., 2009; Heutink et al., 2012; Heutink et al., 2014).
Conclusion
NMOSD causes damage to CNS pathways in the spinal cord as occurs in MS and SCI, suggesting that it is reasonable to extrapolate data from MS and SCI to guide therapy in NMOSD while also recognizing that different outcomes may result in part due to disparities in sex and race in NMOSD. CNP is difficult to treat and is pervasive in NMOSD. Treatments are ineffective and individual pain interventions are not sufficient to impact QoL. These factors only further underscore the need for broadening treatment options and using a multimodal approach in this population. It is important to focus attention on what symptoms form clusters and on comprehensive treatment regimens that address these clusters. A practical and potentially clinically meaningful trial for future research may examine a combination of an AED, anti-depressant, CBT and exercise program. This may highlight how the cluster of symptoms is impacted differently between the two groups, with emphasis on how this translates to improved quality of life.
Highlights:
Pain in neuromyelitis optica spectrum disorder is debilitating and chronic
Effect of spinal neuropathic pain have been modestly addressed in treatment trials
Quality of life needs to be incorporated as an outcome measure in trials
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Maureen A. Mealy has no disclosures to report.
Sharon L. Kozachik has no disclosures to report.
Michael Levy receives research support from NIH, Guthy Jackson Charitable Foundation, Viropharma, Acorda, Sanofi, NeuralStem and Genentech, and serves as a consultant for Chugai Pharmaceuticals, GlaxoSmithKline and Medimmune.
Contributor Information
Maureen A. Mealy, Johns Hopkins University School of Medicine, Department of Neurology, Baltimore, MD, USA, Johns Hopkins University School of Nursing, Baltimore, MD, USA.
Sharon L. Kozachik, Johns Hopkins University School of Nursing, Baltimore, MD, USA.
Michael Levy, Johns Hopkins University School of Medicine, Department of Neurology, Baltimore, MD, USA.
References
- Araki M, Matsuoka T, Miyamoto K, Kusunoki S, Okamoto T, Murata M,Yamamura T (2014). Efficacy of the anti-IL-6 receptor antibody tocilizumab in neuromyelitis optica: A pilot study.Neurology, 82(15), 1302–1306. doi: 10.1212/WNL.0000000000000317 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseyer S, Schmidt F, Chien C, Scheel M, Ruprecht K, Bellmann-Strobl J, Paul F (2018). Pain in AQP4-IgG-positive and MOG-IgG-positive neuromyelitis optica spectrum disorders. Multiple Sclerosis Journal - Experimental, Translational and Clinical, 4(3), 2055217318796684. doi: 10.1177/2055217318796684 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardes SF, Keogh E, & Lima ML (2008). Bridging the gap between pain and gender research: A selective literature review. European Journal of Pain (London,England), 12(4), 427–440. doi:S1090-3801(07)00646-5 [pii] [DOI] [PubMed] [Google Scholar]
- Boldt I, Eriks-Hoogland I, Brinkhof MW, de Bie R, Joggi D, & von Elm E (2014). Nonpharmacological interventions for chronic pain in people with spinal cord injury. The Cochrane Database of Systematic Reviews, 11, CD009177. doi: 10.1002/14651858.CD009177.pub2 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D (2012). Neurological diseases and pain. Brain : A Journal of Neurology, 135(Pt 2), 320–344. doi: 10.1093/brain/awr271 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradl M, Kanamori Y, Nakashima I, Misu T, Fujihara K, Lassmann H, & Sandkuhler J (2014). Pain in neuromyelitis optica--prevalence, pathogenesis and therapy. Nature Reviews.Neurology, 10(9), 529–536. doi: 10.1038/nrneurol.2014.129 [doi] [DOI] [PubMed] [Google Scholar]
- Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Hals EK, Stubhaug A (2008). Assessment of pain. British Journal of Anaesthesia, 101(1), 17–24. doi: 10.1093/bja/aen103 [doi] [DOI] [PubMed] [Google Scholar]
- Breuer B, Pappagallo M, Knotkova H, Guleyupoglu N, Wallenstein S, & Portenoy RK (2007). A randomized, double-blind, placebo-controlled, two-period, crossover, pilot trial of lamotrigine in patients with central pain due to multiple sclerosis. Clinical Therapeutics, 29(9), 2022–2030. doi:S0149-2918(07)00304-9 [pii] [DOI] [PubMed] [Google Scholar]
- Centonze D (2014). Advances in the management of multiple sclerosis spasticity: Multiple sclerosis spasticity nervous pathways. European Neurology, 72 Suppl 1, 6–8. doi: 10.1159/000367615 [doi] [DOI] [PubMed] [Google Scholar]
- Eaneff S, Wang V, Hanger M, Levy M, Mealy MA, Brandt AU, Wicks P (2017). Patient perspectives on neuromyelitis optica spectrum disorders: Data from the PatientsLikeMe online community. Multiple Sclerosis and Related Disorders, 17, 116–122. doi:S2211-0348(17)30164-5 [pii] [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Wallace M (2015). Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. The Lancet.Neurology, 14(2), 162–173. doi: 10.1016/S1474-4422(14)70251-0 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez H, Sunnerhagen KS, Sjoberg I, Kaponides G, Olsson T, & Borg K (2006). Intravenous immunoglobulin for post-polio syndrome: A randomised controlled trial. The Lancet.Neurology, 5(6), 493–500. doi:S1474-4422(06)70447-1 [pii] [DOI] [PubMed] [Google Scholar]
- Han ZA, Song DH, Oh HM, & Chung ME (2016). Botulinum toxin type A for neuropathic pain in patients with spinal cord injury. Annals of Neurology, 79(4), 569–578. doi: 10.1002/ana.24605 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heutink M, Post MW, Bongers-Janssen HM, Dijkstra CA, Snoek GJ, Spijkerman DC, & Lindeman E (2012). The CONECSI trial: Results of a randomized controlled trial of a multidisciplinary cognitive behavioral program for coping with chronic neuropathic pain after spinal cord injury. Pain, 153(1), 120–128. doi: 10.1016/j.pain.2011.09.029 [doi] [DOI] [PubMed] [Google Scholar]
- Heutink M, Post MW, Luthart P, Schuitemaker M, Slangen S, Sweers J, Lindeman E (2014). Long-term outcomes of a multidisciplinary cognitive behavioural programme for coping with chronic neuropathic spinal cord injury pain. Journal of Rehabilitation Medicine, 46(6), 540–545. doi: 10.2340/16501977-1798 [doi] [DOI] [PubMed] [Google Scholar]
- Hollinger KR, Franke C, Arenivas A, Woods SR, Mealy MA, Levy M, & Kaplin AI (2016). Cognition, mood, and purpose in life in neuromyelitis optica spectrum disorder. Journal of the Neurological Sciences, 362, 85–90. doi: 10.1016/j.jns.2016.01.010 [doi] [DOI] [PubMed] [Google Scholar]
- Huang Q, Wang J, Zhou Y, Yang H, Wang Z, Yan Z, Qiu W (2018). Low-dose mycophenolate mofetil for treatment of neuromyelitis optica spectrum disorders: A prospective multicenter study in south china. Frontiers in Immunology, 9, 2066. doi: 10.3389/fimmu.2018.02066 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarius S, Ruprecht K, Wildemann B, Kuempfel T, Ringelstein M, Geis C, Paul F (2012). Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: A multicentre study of 175 patients. Journal of Neuroinflammation, 9, 14-2094-9-14. doi: 10.1186/1742-2094-9-14 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori Y, Nakashima I, Takai Y, Nishiyama S, Kuroda H, Takahashi T, Itoyama Y (2011). Pain in neuromyelitis optica and its effect on quality of life: A cross-sectional study. Neurology, 77(7), 652–658. doi: 10.1212/WNL.0b013e318229e694 [doi] [DOI] [PubMed] [Google Scholar]
- Kim HJ, Abraham I, & Malone PS (2013). Analytical methods and issues for symptom cluster research in oncology. Current Opinion in Supportive and Palliative Care, 7(1), 45–53. doi: 10.1097/SPC.0b013e32835bf28b [doi] [DOI] [PubMed] [Google Scholar]
- Koch-Henriksen N, & Sørensen PS (2010). The changing demographic pattern of multiple sclerosis epidemiology. The Lancet Neurology, 9(5), 520–532. doi: 10.1016/S1474-4422(10)70064-8 [DOI] [PubMed] [Google Scholar]
- Kuspinar A, & Mayo NE (2014). A review of the psychometric properties of generic utility measures in multiple sclerosis. Pharmacoeconomics, 32(8), 759–773. doi: 10.1007/s40273-014-0167-5 [doi] [DOI] [PubMed] [Google Scholar]
- Levendoglu F, Ogun CO, Ozerbil O, Ogun TC, & Ugurlu H (2004). Gabapentin is a first line drug for the treatment of neuropathic pain in spinal cord injury. Spine, 29(7), 743–751. doi:00007632-200404010-00007 [pii] [DOI] [PubMed] [Google Scholar]
- Lunn MP, Hughes RA, & Wiffen PJ (2014). Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. The Cochrane Database of Systematic Reviews, 1, CD007115. doi: 10.1002/14651858.CD007115.pub3 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie RA, & Gryba C (2013). The incidence and prevalence of neuromyelitis optica: A systematic review. International Journal of MS Care, 15(3), 113–118. doi: 10.7224/1537-2073.2012-048 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mealy MA, Kessler RA, Rimler Z, Reid A, Totonis L, Cutter G,.Levy M. (2018). Mortality in neuromyelitis optica is strongly associated with african ancestry. Neurology(R) Neuroimmunology & Neuroinflammation, 5(4), e468. doi: 10.1212/NXI.0000000000000468 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mealy MA, Boscoe A, Caro J, Levy M (2018). Assessment of Patients with Neuromyelitis Optica Spectrum Disorder Using EQ-5D. Int J MS Care. Online ahead of print: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mealy MA, Wingerchuk DM, Greenberg BM, & Levy M (2012). Epidemiology of neuromyelitis optica in the united states: A multicenter analysis. Archives of Neurology, 69(9), 1176–1180. doi: 10.1001/archneurol.2012.314 [doi] [DOI] [PubMed] [Google Scholar]
- Mutch K, Methley A, Moore P, & Jacob A (2014). Life on hold: The experience of living with neuromyelitis optica. Disability and Rehabilitation, 36(13), 1100–1107. doi: 10.3109/09638288.2013.833301 [doi] [DOI] [PubMed] [Google Scholar]
- Mutch K, Zhao S, Hamid S, Methley A, Elsone L, Singh G, Jacob A (2015). Bladder and bowel dysfunction affect quality of life. A cross sectional study of 60 patients with aquaporin-4 antibody positive neuromyelitis optica spectrum disorder. Multiple Sclerosis and Related Disorders, 4(6), 614–618. doi: 10.1016/j.msard.2015.07.015 [doi] [DOI] [PubMed] [Google Scholar]
- Namjooyan F, Ghanavati R, Majdinasab N, Jokari S, & Janbozorgi M (2014). Uses of complementary and alternative medicine in multiple sclerosis. Journal of Traditional and Complementary Medicine, 4(3), 145–152. doi: 10.4103/2225-4110.136543 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland PK, Naismith RT, & Ullione M (2009). The impact of pain and other symptoms on quality of life in women with relapsing-remitting multiple sclerosis. The Journal of Neuroscience Nursing : Journal of the American Association of NeuroscienceNurses, 41(6), 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson Perry K, Nicholas MK, Middleton J, & Siddall P (2009). Psychological characteristics of people with spinal cord injury-related persisting pain referred to a tertiary pain management center.Journal of Rehabilitation Research and Development, 46(1), 57–67. [PubMed] [Google Scholar]
- Norrbrink Budh C, Kowalski J, & Lundeberg T (2006). A comprehensive pain management programme comprising educational, cognitive and behavioural interventions for neuropathic pain following spinal cord injury. Journal of Rehabilitation Medicine, 38(3), 172–180. doi:M1N46588657H1718 [pii] [DOI] [PubMed] [Google Scholar]
- Norrbrink C (2009). Transcutaneous electrical nerve stimulation for treatment of spinal cord injury neuropathic pain. Journal of Rehabilitation Research and Development, 46(1), 85–93. [PubMed] [Google Scholar]
- Norrbrink C, Lindberg T, Wahman K, & Bjerkefors A (2012). Effects of an exercise programme on musculoskeletal and neuropathic pain after spinal cord injury--results from a seated double-poling ergometer study. Spinal Cord, 50(6), 457–461. doi: 10.1038/sc.2011.160 [doi] [DOI] [PubMed] [Google Scholar]
- Norrbrink C, & Lundeberg T (2009). Tramadol in neuropathic pain after spinal cord injury: A randomized, double-blind, placebo-controlled trial. The Clinical Journal of Pain, 25(3), 177–184. doi: 10.1097/AJP.0b013e31818a744d [doi] [DOI] [PubMed] [Google Scholar]
- Norrbrink C, & Lundeberg T (2011). Acupuncture and massage therapy for neuropathic pain following spinal cord injury: An exploratory study. Acupuncture in Medicine : Journal of the British Medical Acupuncture Society, 29(2), 108–115. doi: 10.1136/aim.2010.003269 [doi] [DOI] [PubMed] [Google Scholar]
- Ordonez Gallego A, Gonzalez Baron M, & Espinosa Arranz E (2007). Oxycodone: A pharmacological and clinical review. Clinical & Translational Oncology : Official Publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico, 9(5), 298–307. doi:946 [pii] [DOI] [PubMed] [Google Scholar]
- Paller CJ, Campbell CM, Edwards RR, & Dobs AS (2009). Sex-based differences in pain perception and treatment. Pain Medicine (Malden, Mass.), 10(2), 289–299. doi: 10.1111/j.1526-4637.2008.00558.x [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Zhao P, Cai H, Su L, Wood K, Shi FD, & Fu Y (2015). Hypoxemia, sleep disturbances, and depression correlated with fatigue in neuromyelitis optica spectrum disorder. CNS Neuroscience & Therapeutics, 21(7), 599–606. doi: 10.1111/cns.12411 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellkofer HL, Havla J, Hauer D, Schelling G, Azad SC, Kuempfel T, Huge V (2013). The major brain endocannabinoid 2-AG controls neuropathic pain and mechanical hyperalgesia in patients with neuromyelitis optica. PloS One, 8(8), e71500. doi: 10.1371/journal.pone.0071500 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu BF, & Lucchinetti CF (2016). Immunopathology: Autoimmune glial diseases and differentiation from multiple sclerosis. Handbook of Clinical Neurology, 133, 95–106. doi: 10.1016/B978-0-444-63432-0.00006-2 [doi] [DOI] [PubMed] [Google Scholar]
- Qian P, Lancia S, Alvarez E, Klawiter EC, Cross AH, & Naismith RT (2012). Association of neuromyelitis optica with severe and intractable pain. Archives of Neurology, 69(11), 1482–1487. doi:1355367 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahim-Williams B, Riley JL 3rd, Williams AK, & Fillingim RB (2012). A quantitative review of ethnic group differences in experimental pain response: Do biology, psychology, and culture matter? Pain Medicine (Malden, Mass.), 13(4), 522–540. doi: 10.1111/j.1526-4637.2012.01336.x [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringelstein M, Ayzenberg I, Harmel J, Lauenstein AS, Lensch E, Stogbauer F,Kleiter I (2015). Long-term therapy with interleukin 6 receptor blockade in highly active neuromyelitis optica spectrum disorder. JAMA Neurology, 72(7), 756–763. doi: 10.1001/jamaneurol.2015.0533 [doi] [DOI] [PubMed] [Google Scholar]
- Rossi S, Mataluni G, Codeca C, Fiore S, Buttari F, Musella A, Centonze D (2009). Effects of levetiracetam on chronic pain in multiple sclerosis: Results of a pilot, randomized, placebo-controlled study. European Journal of Neurology, 16(3), 360–366. doi: 10.1111/j.1468-1331.2008.02496.x [doi] [DOI] [PubMed] [Google Scholar]
- Salinas FA, Lugo LH, & Garcia HI (2012). Efficacy of early treatment with carbamazepine in prevention of neuropathic pain in patients with spinal cordinjury. American Journal of Physical Medicine & Rehabilitation / Association of Academic Physiatrists, 91(12), 1020–1027. doi: 10.1097/PHM.0b013e3182643c85 [doi] [DOI] [PubMed] [Google Scholar]
- Sapir T, & Shoenfeld Y (2005). Facing the enigma of immunomodulatory effects of intravenous immunoglobulin. Clinical Reviews in Allergy & Immunology, 29(3), 185–199. doi:CRIAI:29:3:185 [pii] [DOI] [PubMed] [Google Scholar]
- Shi Z, Chen H, Lian Z, Liu J, Feng H, & Zhou H (2016). Factors that impact health-related quality of life in neuromyelitis optica spectrum disorder: Anxiety, disability, fatigue and depression. Journal of Neuroimmunology, 293, 54–58. doi: 10.1016/j.jneuroim.2016.02.011 [doi] [DOI] [PubMed] [Google Scholar]
- Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, & Fehlings MG (2014). Global prevalence and incidence of traumatic spinal cord injury. Clinical Epidemiology, 6, 309–331. doi: 10.2147/CLEP.S68889 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjolund BH (2002). Pain and rehabilitation after spinal cord injury: The case of sensory spasticity? Brain Research.Brain Research Reviews, 40(1–3), 250–256. doi:S0165017302002072 [pii] [DOI] [PubMed] [Google Scholar]
- Sluka KA, Bjordal JM, Marchand S, & Rakel BA (2013). What makes transcutaneous electrical nerve stimulation work? making sense of the mixed results in the clinical literature. Physical Therapy, 93(10), 1397–1402. doi: 10.2522/ptj.20120281 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadhouder A, Buckens CF, Holtslag HR, & Oner FC (2010). Are existing outcome instruments suitable for assessment of spinal trauma patients? Journal of Neurosurgery.Spine, 13(5), 638–647. doi: 10.3171/2010.5.SPINE09128 [doi] [DOI] [PubMed] [Google Scholar]
- Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, & Philip Malan T Jr. (2011). Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: Role of endogenous opioids. Anesthesiology, 114(4), 940–948. doi: 10.1097/ALN.0b013e318210f880 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen KB, Jensen TS, & Bach FW (2004). Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? randomised double blind placebo controlled crossover trial. BMJ (Clinical Research Ed.), 329(7460), 253. doi: 10.1136/bmj.38149.566979.AE [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ (2010). Neuronal circuitry for pain processing in the dorsal horn. Nature Reviews.Neuroscience, 11(12), 823–836. doi: 10.1038/nrn2947 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte D, Doupe M, Torabi M, Gomori A, Ethans K, Esfahani F, … Namaka M. (2015). Nabilone as an adjunctive to gabapentin for multiple sclerosis-induced neuropathic pain: A randomized controlled trial. Pain Medicine (Malden, Mass.), 16(1), 149–159. doi: 10.1111/pme.12569 [doi] [DOI] [PubMed] [Google Scholar]
- Vollmer TL, Robinson MJ, Risser RC, & Malcolm SK (2014). A randomized, double-blind, placebo-controlled trial of duloxetine for the treatment of pain in patients with multiple sclerosis. Pain Practice : The Official Journal of World Institute of Pain, 14(8), 732–744. doi: 10.1111/papr.12127 [doi] [DOI] [PubMed] [Google Scholar]
- Vranken JH, Dijkgraaf MG, Kruis MR, van der Vegt MH, Hollmann MW, & Heesen M (2008). Pregabalin in patients with central neuropathic pain: A randomized, double-blind, placebo-controlled trial of a flexible-dose regimen. Pain, 136(1–2), 150–157. doi:S0304-3959(07)00369-7 [pii] [DOI] [PubMed] [Google Scholar]
- Vranken JH, Hollmann MW, van der Vegt MH, Kruis MR, Heesen M, Vos K, … Dijkgraaf MG. (2011). Duloxetine in patients with central neuropathic pain caused by spinal cord injury or stroke: A randomized, double-blind, placebo-controlledtrial. Pain, 152(2), 267–273. doi: 10.1016/j.pain.2010.09.005 [doi] [DOI] [PubMed] [Google Scholar]
- Wardell DW, Rintala DH, Duan Z, & Tan G (2006). A pilot study of healing touch and progressive relaxation for chronic neuropathic pain in persons with spinal cord injury. Journal of Holistic Nursing : Official Journal of the American Holistic Nurses’ Association, 24(4), 231–40; discussion 241–4. doi:24/4/231 [pii] [DOI] [PubMed] [Google Scholar]
- Widerstrom-Noga EG, & Turk DC (2003). Types and effectiveness of treatments used by people with chronic pain associated with spinal cord injuries: Influence of pain and psychosocial characteristics.Spinal Cord, 41(11), 600–609. doi: 10.1038/sj.sc.3101511 [doi] [DOI] [PubMed] [Google Scholar]
- Wingerchuk DM, Hogancamp WF, O’Brien PC, & Weinshenker BG (1999). The clinical course of neuromyelitis optica (devic’s syndrome). Neurology, 53(5), 1107–1114. [DOI] [PubMed] [Google Scholar]
- Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, & Weinshenker BG (2006). Revised diagnostic criteria for neuromyelitis optica. Neurology, 66(10), 1485–1489. doi:66/10/1485 [pii] [DOI] [PubMed] [Google Scholar]
- Zhao S, Mutch K, Elsone L, Nurmikko T, & Jacob A (2014). Neuropathic pain in neuromyelitis optica affects activities of daily living and quality of life. Multiple Sclerosis (Houndmills, Basingstoke, England), 20(12), 1658–1661. doi: 10.1177/1352458514522103 [doi] [DOI] [PubMed] [Google Scholar]