Abstract
Background:
Solithromycin is a fourth-generation macrolide antibiotic with potential efficacy in pediatric community-acquired bacterial pneumonia. Pharmacokinetic (PK) studies of solithromycin in pediatric subjects are limited, therefore application of minimally invasive drug sampling techniques, such as dried blood spots (DBS), may enhance PK study enrollment in children. The objectives of this study were to compare solithromycin concentrations in DBS with those in liquid plasma samples (LPS) and to quantify the effects of modeling DBS concentrations on the results of a population PK model.
Methods:
Comparability analysis was performed on matched DBS and LPS solithromycin concentrations collected from two different phase 1 clinical trials of solithromycin treatment in children (clinicaltrials.gov # and #). Comparability of solithromycin concentrations was evaluated based on DBS-LPS ratio, median percentage prediction error (MPPE), and median absolute percentage prediction error (MAPE). The effect of correcting DBS concentrations for both hematocrit and protein binding was investigated. Additionally, a previously published population PK model (NONMEM®) was leveraged to compare parameter estimates resulting from either DBS or LPS concentrations.
Results:
A total of 672 paired DBS-LPS concentrations were available from 95 subjects (age: 0–17 years of age. The median (range) LPS and DBS solithromycin concentrations were 0.3 (0.01–12) μg/mL and 0.32 (0.01–14) μg/mL, respectively. MPPE and MAPE of raw DBS to LPS solithromycin concentrations were 5.26% and 22.95%, respectively. Additionally, the majority of population PK parameter estimates resulting from modeling DBS concentrations were within 15% of those obtained from modeling LPS concentrations.
Conclusion:
Solithromycin concentrations in DBS were similar to those measured in LPS and did not require correction for hematocrit or protein binding.
Keywords: solithromycin, dried blood spots, pharmacokinetics, NONMEM, pediatric drug development, clinical pharmacology
1. INTRODUCTION
The use of dried blood spot (DBS) sampling to quantify drug concentrations is an innovative technique that offers several practical advantages over traditional plasma sampling. These advantages include lower sample volume requirements, simplified collection techniques, and less restrictive storage conditions.1,2 DBS sampling is particularly useful in neonatal and pediatric populations, in which obtaining liquid plasma samples (LPS) is challenging owing to blood volume restrictions.3,4 Furthermore, because of both ethical and logistical constraints, robust pharmacokinetic (PK) studies in infants and children are infrequently performed. Therefore, the use of DBS sampling may enable the execution of these PK investigations, which can help improve dosing recommendations in this vulnerable population.4,5 However, before DBS sampling can be utilized instead of LPS, there are several bioanalytical issues that require assessment, including development of analytical techniques to quantify DBS drug concentrations, characterization of the relationship between DBS and LPS drug concentrations, and evaluation of the impact of hematocrit on DBS drug concentrations.6,7
Solithromycin is a novel fourth generation macrolide antibiotic active against common community-acquired bacterial pneumonia (CABP) pathogens, including Streptococcus pneumoniae, Haemophilus influenzae, and atypical pathogens.8,9 In adults, two large phase 3 clinical trials showed that the efficacy of solithromycin was non-inferior to that of moxifloxacin for the treatment of CABP.10,11 PK data from adult studies have shown that solithromycin has a bioavailability of approximately 67% and a large volume of distribution (Vd) of > 500 L, it is predominantly metabolized by cytochrome P450 3A4, and its pharmacodynamic activity is best characterized by the area under the free drug concentration-time curve/minimum inhibitory concentration (fAUC0-24/MIC).12 Given its potential for the treatment of CABP, the application of DBS sampling to characterize the PK of solithromycin may improve drug dosing recommendations in pediatric patients. Our group previously published both an interim PK analysis of solithromycin LPS and DBS concentrations as well as a population PK model of solithromycin in infants, children, and adolescents.13,14 In this study, we report the final DBS and LPS analysis for the complete dataset and compare the results of population PK model between the two matrices.
2. MATERIALS AND METHODS
2.1. Patient Data
Data in this study were obtained by combining data from two different phase 1 clinical trials of solithromycin treatment in children/adolescents (CE01-119 and CE01-120; clinicaltrials.gov # and #, respectively). Study CE01-120 included 83 subjects with an age range of 0–17 years of age.13 Study CE01-119 included 13 adolescents ranging from 12 to 17 years of age.14 The baseline characteristics of the combined study subjects calculated at the time of first dose were as follows: a median (range) age, 7 years (0–17); total body weight, 23 kg (3.8–105); serum creatinine level, 0.4 mg/dL (0.1–1.2); blood urea nitrogen, 9 mg/dL (2–30); aspartate aminotransferase level, 28 U/L (11–97); alanine aminotransferase level, 23 U/L (5–205); alkaline phosphatase level, 161 IU/L (51–2701); total bilirubin level, 0.3 mg/dL (0.1–10.5); albumin level, 3.6 g/dL (1.5–4.8); and hematocrit value, 34.6% (22.9–47.3). All subjects enrolled in these studies provided informed consents, obtained either from the patient (if of appropriate age according to local requirements), parent, or legally authorized representative. Each enrolling study site had the protocol reviewed and approved by their respective institutional review board.13,14
2.2. Drug Administration and PK Sampling
In study CE01-120, solithromycin was administered intravenously (IV) and orally (suspension and capsules) (IV, 6 to 8 mg/kg [400 mg adult maximum]; capsules/suspension, 14 to 16 mg/kg [800 mg adult maximum] on day 1, and 7 to 15 mg/kg [400 mg adult maximum] on days 2 to 5).13 In study CE01-119, adolescents were administered solithromycin orally (capsules) at doses of 12 mg/kg on day 1 [800 mg adult maximum] and 6 mg/kg daily on days 2 to 5 [400 mg adult maximum].14 Further details regarding solithromycin dosing are available in published clinical trials.13,14 Following oral administration, PK samples were collected at 0.5–1.5, 2–4, 8–10, and 23-< 24 h after administration. After IV administration, PK samples were generally obtained within 10 min after completion of a 60-minute infusion, and at 2–4, 8–10, and 23-< 24 h after the start of infusion for the first and multiple doses.13,14 PK analysis dataset was generated and formatted by Duke Clinical Research Institute by merging clinical database data (dosing, demographics, and laboratory data) with raw concentration values received from the central laboratory. More information on the bioanalysis of solithromycin is available in Supplementary Information 1. All data manipulation and visualization was performed using R (version 3.0.2, R Foundation for Statistical Computing, Vienna, Austria) and RStudio (version 1.0.143, RStudio, Boston, MA, USA) with the packages lattice, latticeExtra, and ggplot2.15–17
2.3. Comparability Analyses of DBS-LPS Concentrations
Comparability analyses were performed using a merged dataset, combining solithromycin DBS and LPS concentrations from CE01-119 and CE01-120 trials. Besides the raw concentrations, DBS concentrations were also corrected for hematocrit [(DBS)/(1-(hematrocrit/100))] and protein binding [(DBS)/(1-(hematocrit/100)*(0.3)].6,18 Bias and imprecision of LPS and DBS concentration comparisons were assessed via calculation of DBS-LPS concentration ratios, median percentage prediction error (MPPE), and median absolute percentage prediction error (MAPE), as follows:
where, CONCDBS is the concentration of solithromycin in DBS samples and CONCplasma is the concentration of solithromycin in LPS samples. MPPE and MAPE values < 20% were considered acceptable.19
2.4. Comparability Analyses of DBS-LPS Population PK Model Results
A previously published population PK model was used to compare parameter estimates obtained from modeling LPS and DBS concentrations.13 Briefly, this model was developed using 780 solithromycin plasma concentrations from 83 subjects, with age ranging from 4 days to 17 years of age. The final model was a two-compartment model with linear elimination, first-order absorption, an absorption lag time, an allometric scale of clearance (CL), and Vd parameters based on actual body weight. This model also applied a sigmoidal maximum-effect (Emax) maturation function to characterize the relationship between postmenstrual age (PMA) and CL.13
Population PK model comparability analysis was performed by re-estimating the final model parameter estimates using either LPS or DBS solithromycin concentrations. Modeling analysis was performed using the first-order conditional estimation method in NONMEM® version 7.4 (ICON Development Solutions; Ellicott City, MD, USA). For each matrix, both the final model parameter estimates and the empirical Bayesian estimates (EBEs) were computed and compared. Additionally, MPPE and MAPE values were calculated based on the EBEs of CL and Vd terms as follows:
where, θDBS refers to the PK parameter value derived using DBS solithromycin concentrations and θLPS refers to the PK parameter value derived using LPS solithromycin concentrations. MPPE and MAPE values < 20% were considered acceptable.19
RESULTS AND DISCUSSION
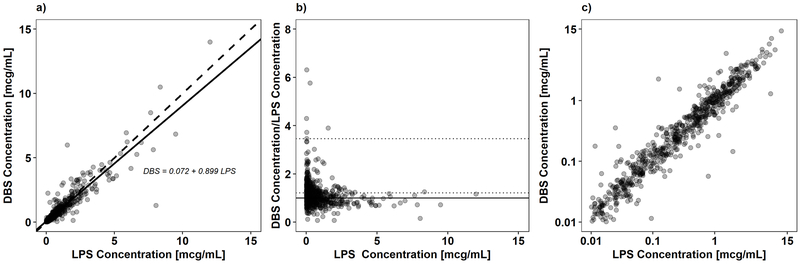
A total of 672 paired DBS-LPS solithromycin samples from 95 subjects were included in this analysis. Correction of DBS solithromycin concentrations for hematocrit and protein binding did not improve the measures of bias and precision; therefore, all further analyses were performed using raw DBS solithromycin concentrations. The median (range) LPS and DBS solithromycin concentrations were 0.3 (0.01–12) μg/mL and 0.32 (0.01–14) μg/mL, respectively. As shown in Figure 1a, an unweighted linear regression (R2 = 0.85) best characterized the correlation between DBS and LPS concentrations. The median (range) DBS to LPS ratio was 1.05 (0.07–18.37). A plot of DBS to LPS ratio vs. LPS concentrations is shown in Figure 1b. A log-log plot of DBS vs. LPS is shown in Figure 1c. The MPPE and MAPE of raw DBS to LPS solithromycin concentrations were 5.26% and 22.95%, respectively.
Figure 1.
Comparability analysis of matched dried blood spot (DBS) and liquid plasma samples (LPS) solithromycin concentrations. Panel a) shows the weighted linear regression of the paired LPS-DBS solithromycin concentrations. The solid and dashed lines represent the unity line and regression fit, respectively. Panel b) shows solithromycin DBS-LPS ratio versus LPS solithromycin concentration. The dashed lines denote the mean ratio and 1.96 × standard deviation. The solid black line denotes a ratio of 1. Three ratios > 7 were removed from the figure to allow for improved visualization of the data. Panel c) shows the paired LPS-DBS solithromycin concentrations plotted on a log scale.
The final population PK model parameter estimates resulting from modeling LPS and DBS solithromycin concentrations are shown in Table 1. The vast majority (83.3% [15/18]) of the estimates obtained from modeling DBS solithromycin concentrations were within 15% of those obtained from modeling LPS solithromycin concentrations. The three terms showing > 15% differences between the two matrices were the Hill coefficient for the sigmoidal maturation function (26.3% difference), the maturation half-life as a function of PMA (27.8% difference), and the off diagonal estimate for the covariance between central Vd and peripheral Vd (38.2% difference). Despite these differences, the DBS parameter estimates for CL terms, Vd terms, and the absorption rate constant were all within 10% of their respective LPS parameter estimates. Furthermore, the MPPE and MAPE estimates comparing the EBEs from each matrix for CL, central Vd, and peripheral Vd were −1.71% and 18.9%, 5.48% and 23%, and 6.15% and 18%, respectively.
Table 1.
Comparison of final population PK model parameter estimates of liquid plasma sample (LPS) concentrations and dried blood spot (DBS) concentrations.
| Matrix | LPS Concentrations (780 samples from 95 subjects) | DBS Concentrations (793 samples from 95 subjects) | ||
|---|---|---|---|---|
| Structural Model | Estimate | RSE (%) | Estimate | RSE (%) |
| Ka (h−1) | 0.381 | 14 | 0.353 | 12 |
| CLPOP,70KG (L/h) | 55 | 22 | 59.5 | 10 |
| VcPOP,70KG (L) | 162 | 16 | 171 | 15 |
| QPOP,70KG (L/h) | 23.4 | 30 | 23 | 29 |
| VpPOP,70KG (L) | 119 | 12 | 123 | 12 |
| F capsule (%) | 69.1 | 18 | 70.2 | 21 |
| F suspension (%) | 52.9 | 19 | 55 | 14 |
| LAG capsule (h) | 0.494 | 2 | 0.491 | 3 |
| LAG suspension (h) | 0.365 | 24 | 0.325 | 20 |
| HILL | 1.09 | 77 | 0.81 | 17 |
| TM50 (weeks) | 52.6 | 40 | 67.2 | 30 |
| Inter-individual Variability (IIV) | ||||
| IIV CL (%) | 81.9 | 8 | 79.4 | 2 |
| Covariance CL-Vc | 0.569 | 25 | 0.585 | 22 |
| IIV Vc (%) | 89.6 | 13 | 93.1 | 20 |
| Covariance Vc-Vp | 0.262 | 38 | 0.362 | 31 |
| IIV Vp (%) | 53.7 | 28 | 47 | 51 |
| Covariance CL-Vp | 0.355 | 38 | 0.376 | 30 |
| Residual Variability | ||||
| Proportional error (%) | 53.9 | 9 | 55.5 | 9 |
Ka, absorption rate constant; CLPOP,70KG, population clearance estimate scaled to a 70-kg adult; VcPOP,70KG, population central volume of distribution estimate scaled to a 70-kg adult; QPOP,70KG, population inter-compartmental clearance estimate scaled to a 70-kg adult; VpPOP,70KG, population peripheral volume of distribution estimate scaled to a 70-kg adult; F, bioavailability; LAG, lag time (h) in drug absorption; HILL, Hill coefficient for the sigmoidal maturation function; TM50, maturation half-life calculated as a function of post-menstrual age (weeks); IIV CL, inter-individual variability in drug clearance reported as CV%; Covariance CL-Vc, covariance between CL and Vc; IIV Vc, inter-individual variability in central volume of distribution reported as CV%, Covariance Vc-Vp, covariance between CL and Vc; IIV Vp, inter-individual variability in peripheral volume of distribution reported as CV%; and Covariance CL-Vp, covariance between CL and Vp. Proportional error reported as CV%
Consistent with the results from CE01-119 study, solithromycin concentrations in DBS and LPS were comparable, albeit with some variability. This variability might be attributed to multiple factors, including red blood cell partitioning, nonhomogeneous distribution across the blood spot sample, and inherent physicochemical properties of the molecule.20 A slope of DBS to LPS concentration ratio near unity indicated that significant red blood cell partitioning occurred, which is in agreement with data from a previous study (~75% whole blood:plasma partitioning based on total radioactivity; sponsor data on file). This finding is in line with results obtained for other drugs with high red blood cell partitioning.21 Besides the comparability of solithromycin DBS and LPS concentrations, population PK modeling results did not differ significantly between the two matrices. Given the similarity in the final model estimates for CL, Vd, and absorption rate constant between the two matrices, it is likely that maintenance dosing recommendations made based on the results of population PK models would not be altered by the use of DBS concentrations.
CONCLUSION
Solithromycin DBS concentrations were similar to LPS concentrations with comparable population PK model results, suggesting that DBS concentrations would result in similar dosing recommendations if used instead of LPS concentrations. These results support the continued use of DBS sampling techniques in clinical pharmacology research.
Supplementary Material
ACKNOWLEDGMENTS
Biomedical Advanced Research and Development Authority: James King, MD, Claiborne Hughes, MS, PMP, and Shar’Ron DeDreu, MS.
Melinta Therapeutics, Inc., Chapel Hill, NC, USA: Brian Jamieson, MD, Robert Hernandez, PhD, David Oldach, MD, and Melissa Allaband.
Duke Clinical Research Institute, Durham, NC, USA: Adam Silverstein, PhD (statistician), Felix Boakye-Agyeman, MD (pharmacokineticist), Danielle Sutton (data management), Elizabeth VanDyne (safety), Carrie Elliott (lead clinical research associate), Theresa Jasion, MS (project leader), and Christoph Hornik, MD, MPH.
Clinical trial sites: Laura P. James, MD (principal investigator [PI]), and Carol Pierce, BSN, CCRC (study coordinator [SC]), Arkansas Childrens Hospital Research Institute, Little Rock, AR, USA; Ram Yogev, MD (PI), and Laura Fearn, RN (SC), Lurie Children’s Hospital of Chicago, IL, USA; Amira Al-Uzri, MD, MCR (PI), and Kira Clark, MPH, CHES (SC), Oregon Health and Science University, Portland, OR, USA; Miroslava Boshevae, MD (PI), Medical University, Plovdiv, Bulgaria; Felice C. Adler-Shohet, MD, FAAP (PI), and Stephanie Osborne, BS, RN, CCRC (SC), CHOC Children’s, Orange, CA, USA; Susan R. Mendley, MD (PI), and Donna Cannonier, MS, LPN, BSW, MBA (SC), University of Maryland School of Medicine, Baltimore, MD, USA; Munib Daudjee, MD (PI), and Jessica Orsak, MA (SC), Mercury Clinical Research, Houston, TX, USA; John S. Bradley, MD (PI), and Sara Hingtgen, RN, MSN (SC), Rady Children’s Hospital San Diego and the University of California San Diego, San Diego, CA, USA; Claudia Espinosa, MD, MSc (PI), and Andrew Michael, RN (SC), Kosair Children’s Hospital, Louisville, KY, USA; Eva Tsonkovak, MD (PI), Multiprofile Hospital for Active Treatment, Ruse, Bulgaria; Kathryn Moffett, MD (PI), and Tammy Carrington (SC), West Virginia University Hospital, Morgantown, WV, USA; Lucila Marquez, MD, MPH (PI), and Farida Lalani, MPH (SC), Baylor College of Medicine and Texas Children’s Hospital, Houston, TX, USA; Kari A. Simonsen, MD (PI), and Kym Abraham (SC), University of Nebraska Medical Center, Omaha, NE, USA; Stefan Stoilov, MD (PI), University Multiprofile Hospital for Active Treatment and Emergency Medicine “N. I. Pirogov”, Sofia, Bulgaria; Barry Bloom, MD (PI), and Paula Delmore, MSN (SC), Wesley Medical Center, Wichita, KS, USA; John Vanchiere, MD, PhD (PI), and Lisa Latiolais, RN, BSN, CCRC (SC), Louisiana State University Medical Center, New Orleans, LA, USA; Joshua Wolf, MD (PI), and Kim Allison (SC), St. Jude Children’s Research Hospital, Memphis, TN, USA; Nathan Price, MD (PI), and Gretchen Cress, RN, BSN, MPH (SC), University of Iowa Hospitals and Clinics, Iowa City, IA, USA; Rachel Orscheln, MD (PI), and Susan Jones, RN, BSN, CRC (SC), Saint Louis Childrens Hospital, St. Louis, MO, USA; Water Dehority, MD, MSc (PI), and Christina Batson (SC), University of New Mexico, Department of Pediatrics, Albuquerque, NM, USA.
Source of Funding: U.S. Biomedical Advanced Research and Development Authority provided funding to Daniel Gonzalez, Christoph P. Hornik, Jason E. Lang, Theresa Jasion, and Michael Cohen-Wolkowiez under grant number HHSO100201300009C. This research was sponsored by the U.S. Biomedical Advanced Research and Development Authority (HHSO100201300009C), which had a contract with Cempra Pharmaceuticals, Inc., a wholly-owned-subsidiary of Melinta Therapeutics, Inc., to perform the study.
Conflicts of Interest: R.J.B. is supported by the National Institute of General Medical Sciences (NIGMS) of the NIH under award T32GM086330. C.P.H. receives salary support for research from the Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD) (K23HD090239), the U.S. government for his work in pediatric and neonatal clinical pharmacology (Government Contract HHSN267200700051C, PI: Benjamin under the Best Pharmaceuticals for Children Act), and the industry for drug development in children. M.C-W. receives support for research from the NIH (1R01-HD076676-01A1), the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the National Institute of Allergy and Infectious Disease (NIAID) (HHSN272201500006I and HHSN272201300017I), the NICHD (HHSN275201000003I), the Biomedical Advanced Research and Development Authority (BARDA) (HHSO100201300009C), the nonprofit organization, Thrasher Research Fund (www.thrasherresearch.org), and from the industry for drug development in adults and children (www.dcri.duke.edu/research/coi.jsp). R.H. was an employee of Melinta Therapeutics, Inc. D.G. receives support for research from the NICHD (K23HD083465) and BARDA (HHSO100201300009C). The rest of the authors have no conflicts to disclose. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Compliance with Ethical Standards: All clinical studies, from which data were obtained, were conducted in accordance with the ethical standards of the institutional and/or national research committee and the Helsinki Declaration.
REFERENCES
- 1.Evans C, Arnold M, Bryan P, et al. Implementing dried blood spot sampling for clinical pharmacokinetic determinations: considerations from the IQ consortium microsampling working group. AAPS J. 2015;17(2):292–300. doi: 10.1208/s12248-014-9695-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enderle Y, Foerster K, Burhenne J. Clinical feasibility of dried blood spots: Analytics, validation, and applications. J Pharm Biomed Anal. 2016;130:231–243. doi: 10.1016/j.jpba.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 3.Gupta K, Mahajan R. Applications and diagnostic potential of dried blood spots. Int J App Basic Med Res. 2018;8(1):1. doi: 10.4103/ijabmr.IJABMR_7_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kothare PA, Bateman KP, Dockendorf M, et al. An integrated strategy for implementation of dried blood spots in clinical development programs. AAPS J. 2016;18(2):519–527. doi: 10.1208/s12248-015-9860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laughon MM, Benjamin DK, Capparelli EV., et al. Innovative clinical trial design for pediatric therapeutics. Expert Rev Clin Pharmacol. 2011;4(5):643–652. doi: 10.1586/ecp.11.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilhelm AJ, den Burger JCG, Swart EL. Therapeutic drug monitoring by dried blood spot: progress to date and future directions. Clin Pharmacokinet. 2014;53(11):961–973. doi: 10.1007/s40262-014-0177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Kesel PM, Sadones N, Capiau S, Lambert WE, Stove CP. Hemato-critical issues in quantitative analysis of dried blood spots: challenges and solutions. Bioanalysis. 2013;5(16):2023–2041. doi: 10.4155/bio.13.156. [DOI] [PubMed] [Google Scholar]
- 8.Donald BJ, Surani S, Deol HS, Mbadugha UJ, Udeani G. Spotlight on solithromycin in the treatment of community-acquired bacterial pneumonia: Design, development, and potential place in therapy. Drug Des Devel Ther. 2017;11:3559–3566. doi: 10.2147/DDDT.S119545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buege MJ, Brown JE, Aitken SL. Solithromycin: A novel ketolide antibiotic. Am J Health Syst Pharm. 2017;74(12):875–887. doi: 10.2146/ajhp160934. [DOI] [PubMed] [Google Scholar]
- 10.Barrera CM, Mykietiuk A, Metev H, et al. Efficacy and safety of oral solithromycin versus oral moxifloxacin for treatment of community-acquired bacterial pneumonia: A global, double-blind, multicentre, randomised, active-controlled, non-inferiority trial (SOLITAIRE-ORAL). Lancet Infect Dis. 2016;16(4):421–430. doi: 10.1016/S1473-3099(16)00017-7. [DOI] [PubMed] [Google Scholar]
- 11.File TM, Rewerska B, Vucinić-Mihailović V, et al. SOLITAIRE-IV: A randomized, double-blind, multicenter study comparing the efficacy and safety of intravenous-to-oral solithromycin to intravenous-to-oral moxifloxacin for treatment of community-acquired bacterial pneumonia. Clin Infect Dis. 2016;63(8):1007–1016. doi: 10.1093/cid/ciw490. [DOI] [PubMed] [Google Scholar]
- 12.Zhanel GG, Hartel E, Adam H, et al. Solithromycin: A novel fluoroketolide for the treatment of community-acquired bacterial pneumonia. Drugs. 2016;76(18):1737–1757. doi: 10.1007/s40265-016-0667-z. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez D, James LP, Al-Uzri A, et al. Population pharmacokinetics and safety of solithromycin following intravenous and oral administration in infants, children, and adolescents. Antimicrob Agents Chemother. 2018;62:1–14. doi: 10.1128/AAC.00692-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez D, Palazzi DL, Bhattacharya-Mithal L, et al. Solithromycin pharmacokinetics in plasma and dried blood spots and safety in adolescents. Antimicrob Agents Chemother. 2016;60(4):2572–2576. doi: 10.1128/AAC.02561-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarkar D, Andrews F. latticeExtra: extra graphical utilities based on lattice. https://cran.r-project.org/package=latticeExtra Published 2016. Accessed November 11, 2017.
- 16.Sarkar D Lattice: Multivariate Data Visualization with R. New York: Springer; 2008. [Google Scholar]
- 17.Wickham H Ggplot2: Elegant Graphics for Data Analysis. New York: Springer; 2009. [Google Scholar]
- 18.Jamieson BD, Ciric S, Fernandes P. Safety and pharmacokinetics of solithromycin in subjects with hepatic impairment. Antimicrob Agents Chemother. 2015;59(8):4379–4386. doi: 10.1128/AAC.04652-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9(4):503–512. http://www.ncbi.nlm.nih.gov/pubmed/7310648. [DOI] [PubMed] [Google Scholar]
- 20.Edelbroek PM, van der Heijden J, Stolk LML. Dried blood spot methods in therapeutic drug monitoring: methods, assays, and pitfalls. Ther Drug Monit. 2009;31(3):327–336. doi: 10.1097/FTD.0b013e31819e91ce. [DOI] [PubMed] [Google Scholar]
- 21.Cohen-Wolkowiez M, Sampson M, Bloom BT, et al. Determining population and developmental pharmacokinetics of metronidazole using plasma and dried blood spot samples from premature infants. Pediatr Infect Dis J. 2013;32(9):956–961. doi: 10.1097/INF0b013e3182947cf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.