1. Introduction
Lumbar disc herniation (LDH) is a clinical syndrome in which localized displacement of disc material (nucleus pulposus or annulus fibrosus) is observed beyond the intervertebral disc, compressing and irritating nearby nerves, and may result in lower back pain and/or typical sciatica, manifested as pain, weakness and numbness in lower extremities.2,27 LDH is the most common etiology in low back pain. The overall prevalence of symptomatic lumbar disc herniation in the U.S. and European countries is reported approximately 1–3% of the population,23,50 and a provincial level epidemiological investigation in China showed the incidence rate was 7.62%.51 Due to the inefficiency of medical treatment and persisting pain after surgery, LDH is a major source of chronic pain, rendering patients to physical inactivity, increased psychological distress, reduced social functional capacity, and thus remarkably diminishing their quality of life.19,25 On account of its high prevalence and significant contribution to disability, understanding brain functional properties of chronic pain consequent to LDH is of great importance.
Emerging evidence now support the idea that chronic pain is not just associated with molecular or structural changes in peripheral afferents and spinal cord circuitry that elicit disturbed nociceptive processes,24,45,49,54 but also accompanied with brain morphological and functional reorganization.8,11,16 This reorganization is observed in both event-related5,34 and resting-state functional connectomes; for instance, increased associations between Default Mode Network (DMN) and mesolimbic regions across clinical pain populations,7,12,35,47 and synchronization changes within and across brain intrinsic networks in different chronic pain conditions.6,36 Over the years, functional connectivity analysis has become an important tool for the characterizing brain physiological mechanisms of chronic pain.
Functional connectivity based networks can be studied regarding the topology of derived graphs as global markers of network disruption, unraveling relationships between disease conditions and brain function.1,26,48 Chronic pain is related to altered brain functional network topological measures, affecting the segregation and integration properties of brain information exchange both in animals and humans.4,40,57 The unitary global measure degree rank order disruption extent is associated with clinical properties of chronic back pain (CBP), complex regional pain syndrome (CRPS), and knee osteoarthritis (OA).31 The association was replicated in two independent CBP populations with two other graph indices.30 These observations suggest that examining graph topological measures and the extent of their disruption provides a new window for understanding the physiology of the brain in chronic pain.
Despite the remarkably high prevalence of LDH with chronic pain (LDH-CP), underlying brain physiology, especially the extent of disruption of global topological measures and how similar to, or different from, those observed in chronic back pain patients without LDH, remains unknown. Therefore, here we studied global brain functional properties in a large group of LDH-CP in Chinese population. We collected 146 LDH-CP and 165 healthy control (HC) subjects’ resting-state functional MRI (RS-fMRI) brain images, along with demographic information and pain-related behavioral measures. We applied a graph-theory-based network analysis to compare brain properties between matched patients and healthy controls. The discovered results were then tested for corroboration in an out-of-sample validation group.
2. Methods
2.1. Participants
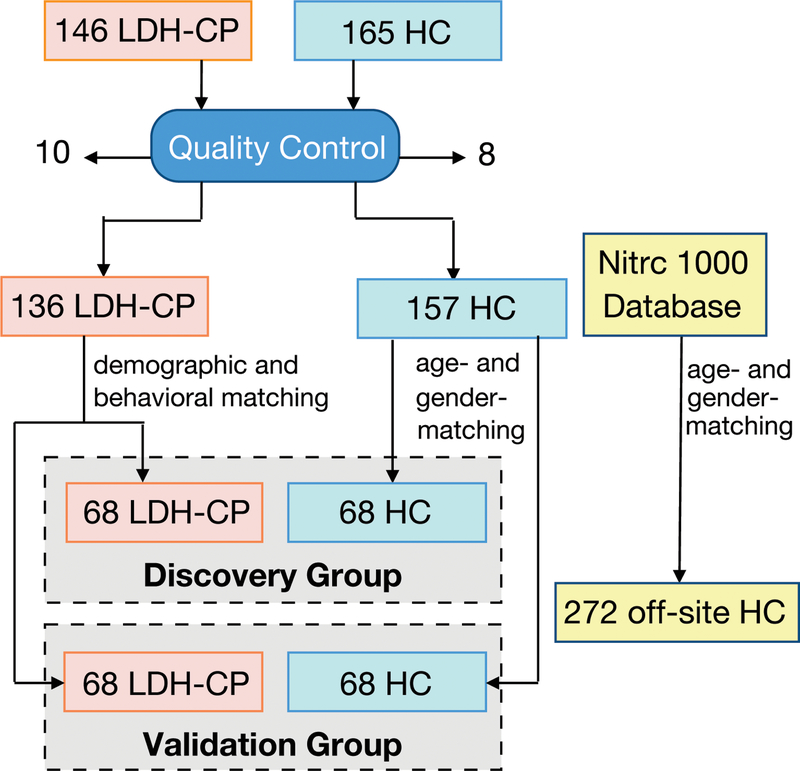
146 lumbar disc herniation (LDH) patients with chronic pain that persisted for at least 12 weeks were recruited, and LDH was diagnosed by medical history, physical examination, and consistent MRI assessment confirmed independently by two radiologists.27 These LDH patients were treated with physical therapy and/or took NSAIDs for pain relief. 165 healthy volunteers without chronic pain at least for the last 52 weeks (one year) were recruited as healthy controls (HC) (Fig. 1). Participants were excluded if they 1) were less than 18 or more than 75 years old; 2) reported history of head injury and/or cerebral disease (e.g. stroke or encephalopathy); 3) had diabetes and psychiatric disease; 4) reported history of brain neurosurgical procedures and/or epilepsy; 5) were unable to cooperate (e.g. psychogenic or cognitively impaired); 6) reported pregnancy, drug dependence or abuse; 7) were not suitable for MRI scan; 8) were enrolled in other clinical trial(s) involving investigational drug(s). All participants were scanned for structural and resting-state functional MRI (rs-fMRI). 10 LDH patients with chronic pain (LDH-CPs) and 8 HCs who didn’t pass our data quality control were removed from further data analyses (see “2.5. Quality control” for the details).
Figure 1. Chart of study flow.
146 lumbar disc herniation patients with chronic pain (LDH-CP) and 165 healthy controls (HC) participated in this study and their brain anatomical and resting-state functional MRI images were acquired, of which 10 LDH-CPs and 8 HCs were excluded based on quality control (QC) (expounded in 2.5). The 136 QC-passed LDH-CPs were allocated to either the discovery or the validation group with matched age, gender, education level, current smoking status, pain duration and three pain-related components (the details are in 2.7). For each group, 68 HCs were randomly chosen from the pool of 157 QC-passed HCs with matched age and gender to its corresponding LDH-CP group. As an external reference group, 272 age- and gender-matched HCs were chosen from NITRC 1000 Connectome project database.
This study was approved by the Institutional Review Board of the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, China (Approval number: Clinical Scientific Research Ethical Review No. 8–2017) and all participants signed a written informed consent. All experiments were performed in accordance with Helsinki declaration, International Conference on Harmonization-Good Clinical Practice (ICH-GCP), the China Food and Drug Administration-Good Clinical Practice (CFDA-GCP) guidelines, and relevant laws and regulations in China.
2.2. Behavioral measures
All participants completed a demographic survey regarding age, gender, marital status, education (illiteracy, primary school, middle school, high school, undergraduate, and post-graduate or higher), residence, smoking status (current smoker or not), alcohol drinking habits, exercise, and employment status. Educational levels were categorized to low education (middle school or below) and high education (high school or above). LDH-CPs reported their pain intensity and pain duration as well.
In addition, a battery of self-reported questionnaires related to pain were filled on the day when LDH-CPs were scanned, items included: Numerical Rating Scale (NRS)17, Oswestry Disability Index (ODI),15,29 McGill Pain Questionnaire - Short Form (sf-MPQ),13,21 Pain Catastrophizing Scale (PCS),44,46 Pain Anxiety Symptoms Scale (PASS),33,59 Positive and Negative Affect Schedule (PANAS),22,52 Beck Depression Inventory (BDI),10,58 and Pain Sensitivity Questionnaire (PSQ).41,43 NRS is an 11-point numerical rating scale used to measure pain intensity, where 0 corresponds to no pain and 10 indicates worst possible pain. ODI assesses physical impairment in relation to pain; sf-MPQ is a well-validated pain measure with sensory and affective components of pain (MPQ/s and MPQ/a), which also includes a visual analog pain scale (VAS) of pain. PCS is a 5-point instrument to assess 13 thoughts or feelings on past pain experience. PCS yields three sub-scale scores assessing rumination (PCS/r), magnification (PCS/m), and helplessness (PCS/h) aspects. PASS measures fear and anxiety responses specific to pain. The PASS consists of four aspects of pain-related anxiety: cognitive suffering (PASS/c), escape-avoidance behaviors (PASS/e), fear of pain (PASS/f), and physiological symptoms of anxiety (PASS/p). PANAS has two mood scales, one measuring positive affect (PANAS/p) and the other measuring negative affect (PANAS/n). Each scale is rated on a 5-point, 10-item scale. BDI is a 21-item instrument for measuring the severity of depression. PSQ is a 11-point, 17-item instrument used to assess individual pain sensitivity. PSQ is based on pain intensity ratings of hypothetical situations, which include various modalities (heat, cold, pressure, pinprick) and measures (pain threshold, intensity ratings). PSQ-minor (PSQ/min) and PSQ-moderate (PSQ/mod) were two subscales corresponding to mildly painful and moderately painful situation respectively. HCs were only required to fill PANAS, BDI, and PSQ.
All questionnaires were administered in Chinese and collected on an electronic tablet device using Research Electronic Data Capture (REDCap).20 REDCap is a secure, convenient, and efficient web application for capturing electronic survey data.
2.3. MRI scanning parameters
Subjects were scanned on a 3 Tesla GE-Discovery 750 scanner at Wenzhou Medical University (WMU), Zhejiang, China. T1-anatomical brain images were acquired with following parameters: voxel size 1 × 1 × 1 mm3; TR/TE = 7.7/3.4 ms; flip angle = 12°; in-plane resolution = 256 × 256; slices per volume = 176; field of view = 256 mm. Rs-fMRI images were acquired on the same day with the following parameters: TR/TE = 2500/30 ms; flip angle = 90°; voxel size = 3.4375 × 3.4375 × 3.5 mm3; in-plane resolution = 64×64; number of volumes = 230; number of slices = 42, which covers the whole brain from the cerebellum to the vertex.
2.4. Rs-fMRI data preprocessing
A preprocessing pipeline was applied to all collected rs-fMRI data and NITRC open data from NITRC 1000 functional connectomes project (http://fcon_1000.projects.nitrc.org/), which were used as an off-site control. We used the FMRIB Expert Analysis Tool (www.fmrib.ox.ac.uk/fsl), MATLB2016a, and Bash Shell Scripting, performing the following steps: discard the first 4 volumes (10 seconds) for magnetic field stabilization; motion correction; slice-time correction; intensity normalization; high-pass temporal filtering (0.008 Hz) for correcting low-frequency signal drift; regression of cerebrospinal fluid (CSF) signal averaged overall voxels of eroded ventricle region, averaged white matter (WM) signal, and averaged global signal of whole brain; motion-volume censoring by detecting volumes with frame-wise displacement (FD) larger than 0.5 millimeter, Derivative Variance Root mean Square (DVARS) after Z normalization larger than 2.3, and standard deviation (SD) after Z normalization larger than 2.3, and scrubbing above detected (number of volume = i) and adjacent four volumes (i-2, i-1, i, i+1, i+2).37,39 FD is a measure of head motion from one volume to the next, and is calculated as the sum of absolute value of three translational displacements in x, y, z axis and three rotational displacements in pitch, yaw, and roll (units of radians), which were multiplied 50 to convert to similar units to translational displacements.39 DVARS is a measure of the change in volume intensity within a pre-defined gray matter (GM) mask from one volume to the next, calculated as the root mean square of the backward differentiated volumes; SD is a measure of deviation of volume intensity within the pre-defined GM mask. Because we were interested in low-frequency fluctuations of resting-state fMRI signal, the above scrubbed time series were band-pass filtered (0.008–0.1 Hz) by applying a Butterworth filter.
All pre-processed rs-fMRI data were registered to our in-house MNI_152_2mm template using FNIRT [ref. https://www.fmrib.ox.ac.uk/datasets/techrep/tr07ja2/tr07ja2.pdf], which were derived from NITRC 1000 functional connectomes project data sets.
2.5. Quality control
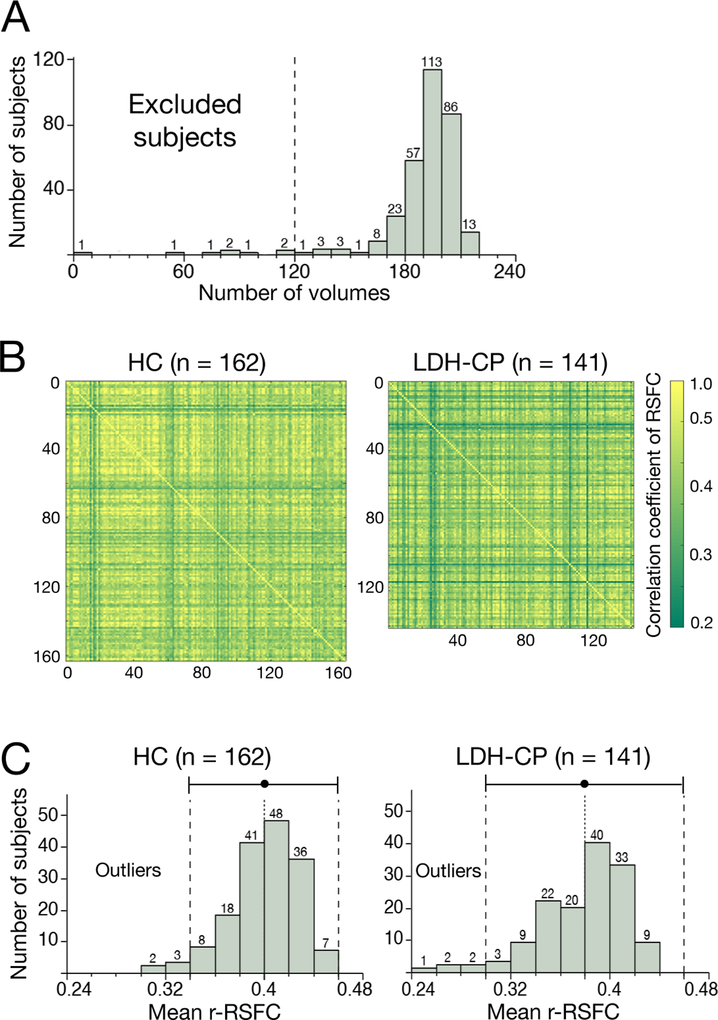
For rs-fMRI images, a robust quality control pipeline was used, and each image was assessed for excessive motion or bad signal to noise ratio. The number of censored motion-volumes after preprocessing reflects the extent of a subject’s motion during scanning, so the number of remaining-volume is evaluated for each subject. Any subject with less than 120 remaining volumes was excluded, which guaranteed a minimum of 5-minute-scanning images for functional connectivity analysis (Fig. 2A).
Figure 2. Quality control of functional MRI data.
A) Histogram of remaining volumes after preprocessing (scrubbing). At this stage, 8 subjects (3 HCs and 5 LDH-CPs) were excluded with less than 120 volumes (equivalent to 5-minute scanning). B) Correlation matrixes across subjects of correlation matrix across ROIs of resting-state functional connectivity (RSFC) on regions of interest (ROIs) between subjects. Darker green represents less similarity of RSFC on the ROIs. C) Histograms of mean correlation coefficient (between subjects) of correlation matrix of RSFC (r-RSFC) on the ROIs. Dot and error bars show an average and double values of standard deviation (SD). Outliers were these subjects whose mean correlation coefficient is less than 2*SD from the average. At this stage, 10 subjects (5 HCs and 5 LDH-CPs) were excluded.
In addition, to further increase the consistency of functional connectivity within LDH-CPs or HCs, an outlier detection procedure was performed and the details were as follows: firstly for each subject, correlation coefficients of resting-state functional connectivity (RSFC) between 256 ROIs were generated; then, the upper (or lower) triangular correlation matrix of each subject was transformed into a vector; and correlation coefficients of all subjects in each group (146 LDH-PCs or 165 HCs) were separately calculated (Fig. 2B); finally, average of each column was calculated, representing the mean correlation coefficient across subjects of correlation coefficient across the 256 ROIs. The subjects with low average within group correlation (reflecting low similarity with the rest of the group; > two standard deviations from the average in each group), were identified as outliers and excluded from further analyses (Fig. 2C).
2.6. Study design
136 QC-passed LDH-CP (87 males and 49 females, mean age = 44 years, age range = 18–69 years) were evenly allocated to either an exploratory group (Discovery) or a validation group (Validation) with matched demographics and pain-related behavioral data (Table 1). For each group, 68 HCs were randomly chosen from the pool of 157 QC-passed HCs with matched age and gender to its corresponding LDH-CP group (Table 2, 3). In addition, 272 off-site healthy control subjects (174 males and 98 females, mean age = 42.4 years, age range = 18–85 years) were chosen from NITRC 1000 functional connectomes project (http://fcon_1000.projects.nitrc.org/) as an external reference group. Data explorations were first performed only in the Discovery group (68 LDH-PCs and 68 HCs) and the results acquired in the Discovery were tested for corroboration in the Validation group (68 LDH-PCs and 68 HCs).
Table 1.
No significant difference of demographic and pain-related characteristics between Discovery LDH-PC and Validation LDH-PC. Pain duration was log-transformed for the normal distribution assumption of statistical analysis. Two-tailed t-test was performed for parametric data and chi-square test was performed for categorical data.
| Discovery (n = 68) | Validation (n = 68) | p value | |||
|---|---|---|---|---|---|
| Mean or n | SEM or % | Mean or n | SEM or % | ||
| Age | 44.0 | 1.5 | 44.0 | 1.4 | 0.99 |
| Male | 43 | 63.2 | 44 | 64.7 | 0.86 |
| Low education | 44 | 64.7 | 43 | 63.2 | 0.86 |
| Current smoking | 20 | 29.4 | 19 | 27.9 | 0.85 |
| Pain duration (log) | 1.97 | 0.07 | 1.97 | 0.06 | 0.98 |
| Pain intensity | 0.06 | 0.12 | 0.03 | 0.11 | 0.81 |
| Pain emotion | 0.01 | 0.11 | 0.02 | 0.12 | 0.93 |
| Pain sensitivity | −0.04 | 0.11 | −0.01 | 0.11 | 0.84 |
Table 2.
Demographic characteristics in Discovery group. HC and LDH-CP was age- and gender-matched, but significant difference in education level (p < 0.01) and current smoking status (p = 0.02). Two-tailed t-test was performed for parametric data and chi-square test was performed for categorical data.
| HC (n = 68) | LDH-CP (n = 68) | p value | |||
|---|---|---|---|---|---|
| Mean or n | SEM or % | Mean or n | SEM or % | ||
| Age | 43.7 | 1.7 | 44.0 | 1.5 | 0.87 |
| Male | 41 | 60.3 | 43 | 63.2 | 0.72 |
| Low education | 24 | 35.29 | 44 | 64.71 | < 0.01 |
| Current smoking | 9 | 13.2 | 20 | 29.4 | 0.02 |
Table 3.
Demographic characteristics in Validation. HC and LDH-CP was age- and gender-matched; had significant difference in education level (p < 0.01) but no difference in current smoking status (p = 0.10). Two-tailed t-test was performed for parametric data and chi-square test was performed for categorical data
| HC (n = 68) | LDH-CP (n = 68) | p value | |||
|---|---|---|---|---|---|
| Mean or n | SEM or % | Mean or n | SEM or % | ||
| Age | 43.6 | 1.7 | 44.0 | 1.4 | 0.83 |
| Male | 41 | 60.3 | 44 | 64.7 | 0.60 |
| Low education | 28 | 41.2 | 43 | 63.2 | < 0.01 |
| Current smoking | 11 | 16.2 | 19 | 27.9 | 0.10 |
2.7. Principal component analysis for reducing dimensionality of behavioral measures
Principal component analysis (PCA) with the varimax rotation method by Kaiser normalization was used to convert 16 correlated behavioral measures (NRS, MPQ/vas, ODI, MPQ/s, MPQ/a, PCS/r, PCS/m, PCS/h, PASS/c, PASS/e, PASS/f, PASS/p, PANAS/n, BDI, PSQ/min, and PSQ/mod) into linearly uncorrelated, orthogonal, principal pain-related components. Considering that pain characteristics are more related to negative psychological factors, the only positive measure, PANAS/p was removed from this dimensionality reduction analysis. Two criteria for component selection were employed: (1) eigenvalue more than 1, and (2) percentage of explained variance above 10%. After the principal components were selected, component scores corresponding to each LDH-CP subject were created on each principal component and standardized along subject column to reflect a z-score.
2.8. Global graph properties
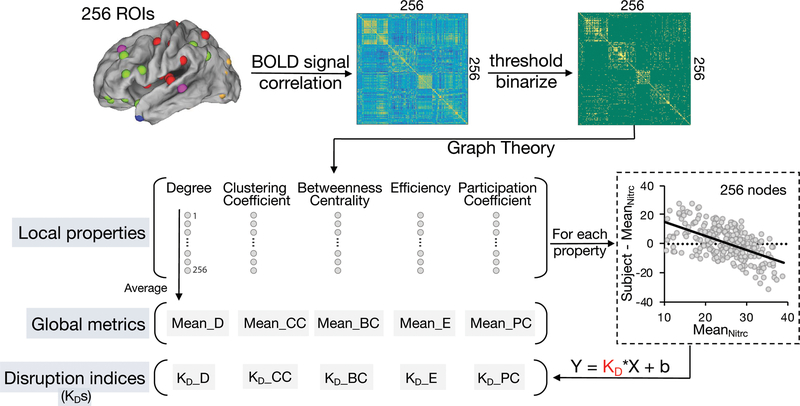
As shown in Fig. 3, 256 regions of interest (ROIs) originated from 264 ROIs38 (excluded 8 ROIs located in cerebellum) were used to construct ROI-based functional networks. Blood oxygenation level dependent (BOLD) signal of each ROI was extracted as an average over voxels within 10 mm diameter spheres centered at peak coordinates. Following this, a 256 × 256 correlation matrix was generated, using Pearson correlation coefficients between BOLD signals for pairs of ROIs, representing resting-state functional connectivity (RSFC) strengths before thresholding. Fisher’s z transformation was applied to convert the Pearson correlation coefficient to normalized distributions. As described previously,1,31 to compare extracted functional networks and calculate global graph metrics, it is necessary to predetermine a link density, which is defined as the percentage of links (edges) with respect to the maximum number of possible links. In this study, 9 link densities were applied, from 2% to 10%.38 Any pair of nodes of the brain functional network was set as “undirectly connected” if their correlation coefficient was above a subject-dependent threshold, thus generating a binarized network.
Figure 3. Calculation of graph local and global metrics and disruption indices.
For each subject in LDH-CP, LDH-HC, and off-site HC, 256 ROIs originated from 264 ROIs38 (excluded 8 ROIs located in cerebellum) were used to construct ROI-based functional networks. BOLD signal of each ROI was extracted as an average over voxels within 10 mm diameter spheres centered at peak coordinates. Following this, a 256 × 256 correlation matrix was generated, showing Pearson correlation coefficient between BOLD signals. Then under each of 9 given link density (from 2 to 10%), nodal-level (local) graph properties were respectively computed using the brain connectivity toolbox (BCT)42. Finally, the average of each property across 256 nodes produced their corresponding global graph metrics. Meanwhile, using the mean nodal-level graph properties across 272 subjects chosen in NITRC 1000 functional connectomes project as normative topological properties, for each subject in LDH-CP and LDH-HC, firstly degree (or other graph metrics) of each node was subtracted from the mean degree (or other graph metrics) of off-site HC (n = 272) of its corresponding node, at a pre-defined link density. Following this, the difference of degree (y axis) and its corresponding mean degree of off-site HC (x axis) was plotted across all 256 ROIs. Then KD was estimated as the slope of the fitted line. The details about calculating graph topological disruption index are expounded in 2.9.
Under each given link density, 6 nodal-level (local) graph properties were respectively computed for each subject using the brain connectivity toolbox (BCT):42 ‘degree’ - a measure of network hubness, ‘clustering coefficient’ - a measure of network segregation, ‘betweenness centrality’ - a measure of within-network communication, ‘efficiency’ - a measure of network integration, ‘small-worldness’ - a measure of network randomness’, and ‘participation coefficient’ - a measure of diversity within a network. The average for each of the six metrics across 256 nodes produced their corresponding global graph metrics, for each subject. Note that global degree of each subject is the same for a given link density. Differences in global graph metrics between groups (LDH-CP, HC, and off-site HC) were computed using a repeated measure ANCOVA with age, gender, educational level, and current smoking status as covariates of no interest.
2.9. Graph topological disruption index (KD)
Graph topological disruption index (KD) is a well-recognized network-based assessment for brain functional reorganization, indicating a network property change in some regions while the opposite trend in other regions of the brain.30,31,48 By definition, KD is relative to a normative topological network. In this study, the topological network derived from 272 subjects chosen in NITRC 1000 functional connectomes project was used as the off-site control and 5 graph topological disruption indices were computed in terms of their nodal metrics: degree (D), betweenness centrality (BC), clustering coefficient (CC), efficiency (E), and participation coefficient (PC) and referred as KD_D, KD_BC, KD_CC, KD_E, and KD_PC, respectively.
To construct KD_D for any given subject, as shown in (Fig. 3), degree of each node was subtracted from the mean degree of off-site control of its corresponding node at a pre-defined link density. Following this, the difference of nodal degree (y axis) was plotted against the mean degree of off-site control (x axis) for all 256 nodes. Then KD_D is defined as the gradient of a straight line fitted to a scatter plot following the linear regression (y = KD_D *x + b), where y = nodal degree of the subject – mean nodal degree of off-site controls, x = mean nodal degree of off-site controls, and b = residual or intercept of the regression. Similarly, the above method for deriving KD_D was used to construct the other 4 KDs.
For each KD, difference between groups (LDH-CP and HC) were computed using a repeated measure ANCOVA with age, gender, educational level, and current smoking status as covariates of no interest. Pearson’s correlation analyses were performed to calculate correlation coefficient between KDs within all HCs (n = 157) and all LDH-CPs (n = 136), respectively.
2.10. Correlation between graph disruption indices and pain-related components
Pearson’s correlation coefficients were computed between KDs at 5% link density and 3 pain-related principal components derived from behavioral measures: pain intensity, pain emotion, and pain sensitivity. As LDH-CP patients were stratified to males (n = 43), females (n = 25), males with low education (Male-LE, n = 23), and males with high education (Male-HE, n = 20), correlation analyses were repeated within each stratified subgroup. Females were not educationally stratified because most (21 out of 25) were low-educated. Bonferroni’s corrected p value, 0.017 (0.05/3) was used as a significant threshold considering that the tests were repeated 3 times for the 3 pain-related components in each subgroup for each KD. At the end, a repeated measure ANOVA was performed to confirm a significant association not only at 5% link density but also across all densities.
2.11. Software
Statistical analyses were performed using MATLAB 2016a and JMP Pro version 13.2 (SAS Institute, Cary, NC). Visualized network schemas were generated by Cytoscape, an open source software.14 Brain schemas of ROI and functional connectivity network were visualized on a surface rendering of a human brain atlas with the BrainNet Viewer (http://www.nitrc.org/projects/bnv/).55
3. Results
3.1. Demographics and pain characteristics of LDH-CP patients
Table 4 summarized demographics and pain characteristics of the LDH-CP patients. Mean NRS (0–10; 0 = no pain, 10 = worst pain imaginable) was 4.99/10 with a standard error (SE) of 0.18; median pain duration was 104 weeks and the range of pain duration was between 12 and 1040 weeks. 58.8% of the patients were males and 64.0% were with middle school or below education, respectively.
Table 4. Demographic characteristics of healthy controls (HC) and demographic and pain-related characteristics of lumbar disc herniation patients with chronic pain (LDH-CP).
Differences were observed in age (p = 0.01), gender (p = 0.01), education level (p < 0.01), current smoking status (p < 0.01), PANAS/p (p < 0.01), PANAS/n (p < 0.01), and BDI (p < 0.01). (SEM: Standard Error of Mean; y/o: years old; wks: weeks; -: Not Available; NRS: Numerical Rating Scale; MPQ: McGill Pain Questionnaire; MPQ/vas: MPQ/visual analog scale; MPQ/s: MPQ/sensory; MPQ/a: MPQ/affective components of pain; ODI: Oswestry Disability Index; PASS: Pain Anxiety Symptoms Scale; PASS/e: PASS/escape-avoidance behaviors; PASS/c: PASS/cognitive suffering; PASS/f: PASS/fear of pain; PASS/p: PASS/physiological symptoms of anxiety; PCS: Pain Catastrophizing Scale; PCS/r: PCS/rumination; PCS/m: PCS/magnification; PCS/h: PCS/helplessness; PANAS: Positive and Negative Affect Scale; PANAS/n: PANAS/negative; BDI: Beck Depression Inventory; PSQ: Pain Sensitivity Questionnaire; PSQ/min: PSQ/minor; PSQ/mod: PSQ/moderate)
| HC (n = 157) | LDH-CP (n = 136) | p value | |||
| Age, mean, SEM (y/o) | 40.1 | 1.0 | 44.0 | 1.1 | 0.01 |
| Male, n, % | 77 | 49.0 | 80 | 58.8 | 0.01 |
| Low education, n, % | 53 | 33.8 | 87 | 64.0 | < 0.01 |
| Current smoking, n, % | 13 | 8.3 | 39 | 28.7 | < 0.01 |
| Pain duration, median, min, max (wks) | - | - | 104 | 12, 1040 | - |
| Pain measures | Mean | SEM | Mean | SEM | p value |
| NRS | - | - | 4.99 | 0.18 | - |
| MPQ/vas | - | - | 4.88 | 0.18 | - |
| ODI | - | - | 19.8 | 0.9 | - |
| MPQ/s | - | - | 16.5 | 0.4 | - |
| MPQ/a | - | - | 7.2 | 0.2 | - |
| PASS/e | - | - | 13.1 | 0.6 | - |
| PASS/c | - | - | 7.4 | 0.5 | - |
| PASS/f | - | - | 7.9 | 0.6 | - |
| PASS/p | - | - | 3.4 | 0.4 | - |
| PCS/r | - | - | 8.2 | 0.3 | - |
| PCS/m | - | - | 4.0 | 0.2 | - |
| PCS/h | - | - | 7.1 | 0.5 | - |
| PANAS/p | 24.4 | 0.6 | 21.4 | 0.6 | < 0.01 |
| PANAS/n | 15.5 | 0.4 | 17.4 | 0.5 | < 0.01 |
| BDI | 3.8 | 0.4 | 8.9 | 0.6 | < 0.01 |
| PSQ/min | 3.6 | 0.1 | 3.7 | 0.1 | 0.60 |
| PSQ/mod | 5.2 | 0.2 | 5.2 | 0.2 | 0.83 |
Compared to HCs in this study, there was a significant difference in current smoking status (p < 0.01), PANAS/p (p < 0.01), PANAS/n (p < 0.01), and BDI (p < 0.01), indicating LDH-CP patients suffered from more negative mood and depression. Other relationships between demographic variables and pain-related outcomes were not significant.
3.2. Three pain-related principal components derived from behavioral measures in LDH-PC patients
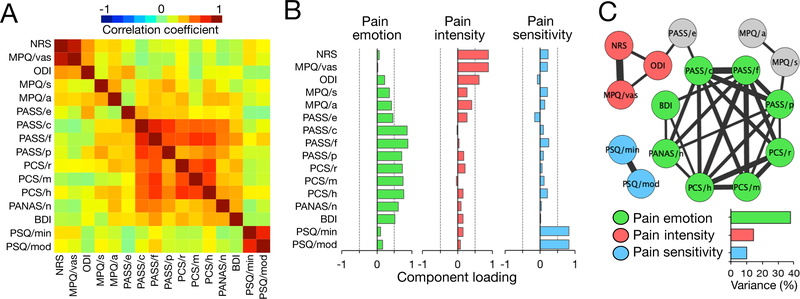
PCA was used to convert 16 correlated behavioral measures in LDH-PC patients (Fig. 4A)into linearly uncorrelated, orthogonal, principal components, which correspond to the latent dimensions of the data. In our study, only 3 components with an eigenvalue greater than one and explained variance > 10% were determined as principal components: pain emotion (explained variance 37.9%, eigenvalue 6.1), pain intensity (explained variance 14.4%, eigenvalue 2.3), and pain sensitivity (explained variance 10.1%, eigenvalue 1.6) (Fig. 4B). As shown in Fig. 4B and Table 5, using a threshold of 0.5 loading, components of pain emotion consisted of PASS/e, PASS/c, PASS/f, PCS/r, PCS/m, and PCS/h; pain intensity of NRS, MPQ/vas, and ODI; and pain sensitivity of PSQ/min and PSQ/mod. The three pain-related principal components explained 62.4% variance of the data and the relationship between the three components and the reorganized network of behavioral measures after thresholding (top 25% linkage density) are shown in Fig. 4C.
Figure 4. Three pain-related components of behavioral measures in all lumber disc herniation patients with chronic pain (n = 136).
A) Correlation matrix of behavioral measures. Principal component analysis (PCA) was applied to reduce behavioral dimensions. B) Loadings of each original measure on three components. Three pain-related components were selected with larger than 1.0 eigenvalue and more than 10% explained variance: pain emotion, pain intensity, and pain sensitivity. Note that gray dot lines represent ± 0.5 component loadings. C) The relationship between reorganized network of behavioral measures and the three pain-related components. The three components, pain emotion (green), pain intensity (red), and pain sensitivity (blue) accounted for %37, %20, and 10% of total variance. The network shows only top 25% linkage density and the width of a link represents correlation strength (0.40–0.99). Each original measure was projected onto 3 principal components using a threshold of 0.5 loading and circle-colored correspondingly. Three measures (MPQ/s, MPQ/a, and PASS/e) did not make significant contribution to the 3 principle components. (NRS: Numerical Rating Scale; MPQ: McGill Pain Questionnaire; MPQ/vas: MPQ/visual analog scale; MPQ/s: MPQ/sensory; MPQ/a: MPQ/affective components of pain; ODI: Oswestry Disability Index; PASS: Pain Anxiety Symptoms Scale; PASS/e: PASS/escape-avoidance behaviors; PASS/c: PASS/cognitive suffering; PASS/f: PASS/fear of pain; PASS/p: PASS/physiological symptoms of anxiety; PCS: Pain Catastrophizing Scale; PCS/r: PCS/rumination; PCS/m: PCS/magnification; PCS/h: PCS/helplessness; PANAS: Positive and Negative Affect Scale; PANAS/n: PANAS/negative; BDI: Beck Depression Inventory; PSQ: Pain Sensitivity Questionnaire; PSQ/min: PSQ/minor; PSQ/mod: PSQ/moderate)
Table 5.
Component loadings of 16 measures corresponding to 3 pain-related components. Each black-highlight item in the table indicates at least 25% of the variance in its original assessment was explained by its column-corresponding component
| Measures | Pain emotion | Pain intensity | Pain sensitivity |
|---|---|---|---|
| NRS | 0.07 | 0.89 | 0.22 |
| MPQ-vas | 0.00 | 0.88 | 0.21 |
| ODI | 0.22 | 0.62 | −0.09 |
| MPQ/s | 0.37 | 0.28 | 0.21 |
| MPQ/a | 0.41 | 0.40 | 0.13 |
| PASS/e | 0.45 | 0.28 | −0.16 |
| PASS/c | 0.86 | −0.01 | 0.08 |
| PASS/f | 0.87 | 0.05 | 0.24 |
| PASS/p | 0.70 | 0.17 | 0.09 |
| PCS/r | 0.72 | 0.22 | 0.06 |
| PCS/m | 0.74 | −0.03 | 0.11 |
| PCS/h | 0.77 | 0.16 | 0.20 |
| PANAS/n | 0.62 | 0.10 | 0.03 |
| BDI | 0.52 | 0.16 | 0.01 |
| PSQ/min | 0.10 | 0.17 | 0.82 |
| PSQ/mod | 0.17 | 0.09 | 0.83 |
| Eigenvalue | 6.06 | 2.31 | 1.62 |
| Variance (%) | 37.9 | 14.4 | 10.1 |
3.3. Global graph metrics show significant difference in Discovery and a trend in Validation
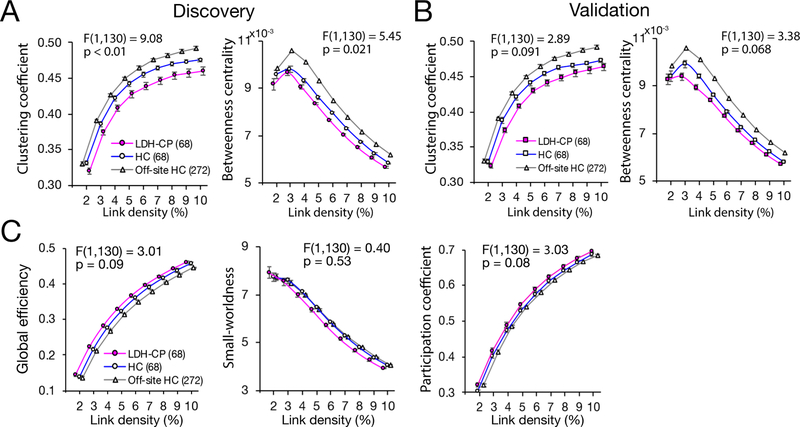
Significant difference in global graph metrics between LDH-PC and HC was observed in the Discovery using a repeated measure ANCOVA with age, gender, educational level, and current smoking status as covariates of no interest. Compared to HC, clustering coefficient (F(1,130) = 9.08, p < 0.01) and betweenness centrality (F(1,130) = 5.45, p = 0.021) of LDH-PC decreased across all link densities (Fig. 5A). The two metrics in HC were closer to those in the off-site HC than LDH-CP (Fig. 5A). However, when tested in the Validation group, the two global graph metrics only showed a trend of decrease in clustering coefficient (F(1,130) = 2.89, p = 0.091) and betweenness centrality (F(1,130) = 3.38, p = 0.068) (Fig. 5B). No statistical differences were shown in the global efficiency, the small-worldness, and the participation coefficient between HC and LDH-CP in the Discovery group (Fig. 5C).
Figure 5. Significant difference in global graph metrics between LDH-PC and HC in the Discovery and a trend in the Validation.
A) In the Discovery, a repeated measure ANCOVA with age, gender, educational level, and current smoking status as covariates of no interest determined that global graph metrics of brain networks differed statistically significantly between LDH-PC and HC. Clustering coefficient (F(1,130) = 9.08, p < 0.01) and betweenness centrality (F(1,130) = 5.45, p = 0.021) significantly decreased across all link densities. The two metrics of HC were closer to the off-site HC than LDH-CP. Data plotted as mean+/−SEM. B) In the Validation, global graph metrics showed a similar trend of difference. Clustering coefficient (F(1,130) = 2.89, p = 0.091) and betweenness centrality (F(1,130) = 3.38, p = 0.068). The significant differences were not replicated in the Validation. C) No significant difference between LDH-CP and HC in Discovery group for global efficiency (F(1,130) = 3.01, p = 0.09), small-worldness (F(1,130) = 0.40, p = 0.53), and participation coefficient (F(1,130) = 3.03, p = 0.08).
3.4. LDH-CP patients’ brain network is significantly disrupted
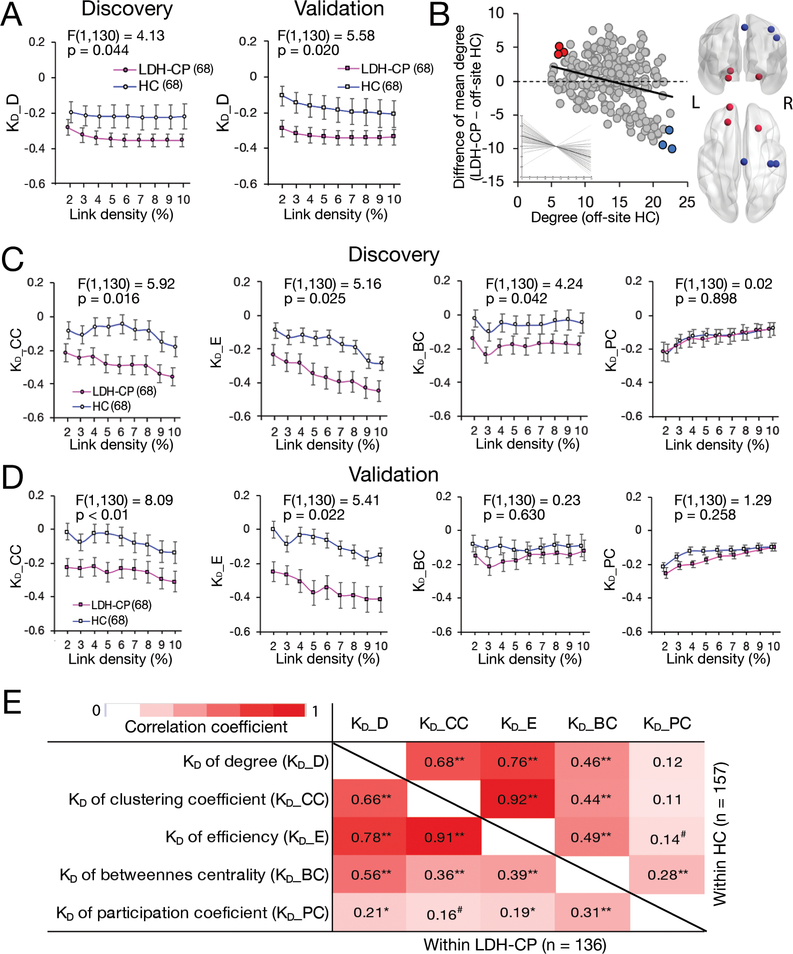
In the Discovery group, a repeated measure ANCOVA, with age, gender, educational level, and current smoking status as covariates of no interest, determined that graph disruption index of degree, KD_D, of LDH-CP was statistically significantly decreased compared to HC across all link densities (F(1,130) = 4.13, p = 0.044), which was replicated in the Validation group (F(1,130) = 5.58, p = 0.020) (Fig. 6A), and the most disrupted ROIs in LDH-CP were shown in Fig. 6B, which are located in medial ventral prefrontal cortex (red circles) and sensory motor cortex (blue circle). LDH-CP patients also showed decreased KD_CC (F(1,130) = 5.92 , p = 0.016) and KD_E (F(1,130) = 5.16, p = 0.025), with replication in the Validation group (KD_CC: (F(1,130) = 8.09, p < 0.01; KD_E: F(1,130) = 5.41, p = 0.022) (Fig. 6C, 6D).
Figure 6. Disruptions of degree (KD_D), clustering coefficient (KD_CC) and efficiency (KD_E) in LDH-PC patients reflect globally altered connectivity.
A) A repeated measure ANCOVA with age, gender, educational level, and current smoking status as covariates of no interest determined that KD_D of LDH-CP statistically significantly decreased compared to HC across all link densities in the Discovery (F(1,130) = 4.13, p = 0.044) and the result was replicated in the Validation (F(1,130) = 5.58, p = 0.020) . Data plotted as mean+/−SEM. B) The left scatter plot showed the average KD_D and the most disrupted ROIs (colored circles) in LDH-CP. Insert shows individual KD_D value. The right brain schema indicated that the ROIs are located in medial ventral prefrontal cortex (red) and sensory motor cortex (blue). C) Other than KD_D, in the Discovery, a repeated measure ANCOVA with age, gender, educational level, and current smoking status as covariates of no interest determined that KD_CC, KD_E, and KD_BC of LDH-CP statistically significantly decreased compared to HC across all link densities (F(1,130) = 5.92, p = 0.016, (F(1,130) = 5.16, p = 0.025, and (F(1,130) = 4.24, p = 0.024). D) The decreases of KD_CC and KD_E were replicated in the Validation except of KD_BC (F(1,130) = 8.09, p < 0.01, (F(1,130) = 5.41, p = 0.022, and (F(1,130) = 0.23, p = 0.630). E) Four of graph disruptions, KDs of degree, clustering coefficient (CC), efficiency (E) and betweenness centrality (BC) were highly correlated with each other within both HC (upper triangular) and LDH-CP (lower triangular). Graph disruption of participation coefficient (PC) showed weak correlations to the other KDs except for that of BC. Pearson’s correlation analyses were performed; p < 0.1, p < 0.05, and p < 0.01.
3.5. LDH-CP patients’ graph degree disruption index is associated with pain intensity in males with high educational level
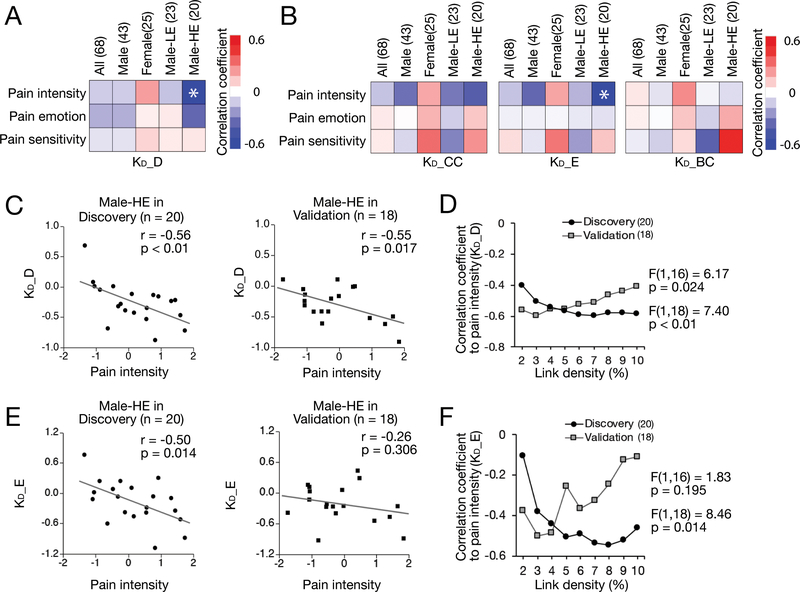
Since the graph degree disruption index, KD_D, was not significantly associated with any pain-related component derived from behavioral measures, we stratified the patients by gender and educational level to take these effects away from the analyses. Only in males with high educational level (Male-HE), the component of pain intensity and KD_D was significantly inversely correlated after Bonferroni’s correction (Fig. 7A, 7B). Fig. 7C depicted this correlation in the discovery (r = −0.56, p < 0.01) and validation results (r = −0.55, p = 0.017) at 5% link density. This correlation was further confirmed across all densities by a repeated measure ANOVA performed in both the Discovery (F(1,18) = 7.40, p < 0.01) and the Validation group (F(1,16) = 6.17, p = 0.024) (Fig. 7D).
Figure 7. The extent of disruption of degree (KD_D) in LDH-CP patients is associated with pain intensity in males with higher education.
A) In the Discovery, Pearson correlation analyses were performed between 3 pain-related components (pain intensity, pain emotion, and pain sensitivity) and KD across all link densities for All group (n = 68), Male subgroup (n = 43), Female subgroup (n = 25), Male with Lower Education level subgroup (Male-LE, n = 23), and Male with Higher Education level subgroup (Male-HE, n = 20). KD_D in Male-HE subgroup showed a statistically significantly relationship to the component of pain intensity at 5% link density (p < 0.017 = 0.05/3; Bonferroni’s correction). No statistically significantly association was found in the All group and the other three subgroups. B) KD_E in Male-HE subgroup showed a statistically significantly relationship to the pain intensity component at 5% link density (p = 0.014). C) Significant association between KD_D and the pain intensity component in males with high education level was found and validated in the Discovery (p < 0.01, r = −0.55) and the Validation (p = 0.017, r = −0.55) at 5% link density. The decreased KD_D was associated with increased pain intensity. D) The significant association between degree disruption KD_D and the component of pain intensity in Male-HE were observed across all link densities in both the Discovery (F(1,18) = 7.40, p < 0.01) and the Validation (F(1,16) = 6.17, p = 0.024). E) KD_E was significantly associated with the pain intensity component in males with higher education level (p = 0.014, r = −0.50) in the Discovery but was not replicated in the Validation (p = 0.306, r = −0.26) at 5% link density. F) Correlation coefficients of KD_E to pain intensity across all link densities in male-HE. The significant association in Discovery (F(1,18) = 8.46, p = 0.014) was not replicated in Validation (F(1,16) = 1.83, p = 0.195; repeated measure ANOVA).
3.6. Correlations between graph disruption indices and their associations with pain-related components
The correlations between graph disruption indices were evaluated and shown in Fig. 6E. KD_D, KD_CC, KD_E, and KD_BC were significantly correlated with each other within either HC or LDH-CP. In particular, KD_D, KD_CC, and KD_E showed quite high correlation coefficients (more than 0.6, p < 0.01). Except for the significant association between KD_D and pain intensity in Male-HE, a statistically significant correlation between KD_E and pain intensity at 5% link density was also found in Male-HE in the Discovery (r = −0.50, p = 0.014) (Fig. 7E), although not replicated in the Validation (r = −0.26, p = 0.306) (Fig. 7E). Similar results can be observed when link densities were at 6%, 7%, 8%, 9%, and 10% respectively (F(1,16) = 1.83, p = 0.195) (Fig. 7F)
Discussion
Even though a substantial population suffers from LDH, usually accompanied with chronic pain, and the condition is associated with a staggering health care cost, there is a lack of knowledge on how the brain adapts to this condition and how such brain adaptions relate to pain characteristics of LDH. To unravel these relationships, we collected 146 LDH patients’ resting-state functional MRI images along with their demographical and behavioral measures and approximately same number of age- and gender-comparative healthy controls. We constructed brain connectome networks, measured their global topological properties and graph disruption indices together with their interactions with pain-related questionnaire outcomes, trying to identify how brain functional connectivity network under LDH chronic pain condition is reorganized.
The most important and robust finding was that LDH-CP patients’ whole-brain network structure was altered. Graph disruption indices derived from three network topological measurements, degree, clustering coefficient, and efficiency, which respectively represents network hubness, segregation, and integration, were significantly decreased compared to HC across all predefined link densities, in both Discovery and Validation group. This graph-theory-based functional connectivity analysis with a rigorous validation strategy32 is a reliable and reproducible approach53 and provides robust conclusions and generalization to the population at large. Similar to discoveries in patients with chronic back pain (CBP), complex regional pain syndrome (CRPS), knee osteoarthritis (OA),31 the observation of alterations in whole-brain network of LDH-CP patients demonstrates that chronic pain is a disorder where the brain is globally reorganized in such a way that a network property changes in some regions while the opposite trend of change occur in other regions of the brain.
LDH-CP has its unique way of change in global network properties. Two of its global graph metrics, clustering coefficient and betweenness centrality, significantly differentiate patient and healthy control in the Discovery group and showed a similar trend in the Validation group, opposite to those in CBP, CRPS, and OA.31 A network with low clustering coefficient is characterized by weak connectivity in the local clusters, indicating the brain network of LDH-CP has highly connected inter-clusters and less connected intra-clusters; while betweenness centrality is a measure of the centrality of a node in a network, implying brain of LDH-CP has less nodes with stronger influences within its own network. Biologically these results putatively suggest that in LDH-CP within region synaptic contacts are weakened and at the same time synapses of long-distance axonal tracks are strengthened. These global network properties may attribute to the complexity of LDH-CP since pain location (lower back and/or lower extremities), dominating sensations (pain and/or hypo- or hypersensitivity, numbness and/or tingling), and limitation of mobility may coexist and vary in LDH-CP. As a result, the chronic pain accompanied by LDH may have specific nociceptive (peripheral and spinal cord) and non-nociceptive (central limbic) characteristics,3 manifesting different global network properties from other musculoskeletal chronic pain conditions.
The stratified approach of LDH-CP revealed that graph degree disruption, KD_D, is associated with the pain intensity only in the subgroup of males with high education (Fig. 7C, 7D), higher pain intensity always corresponding to a larger disruption. This phenomenon deviates from previous studies in the USA,31 where the association is not dependent on gender and education level. The deviation may be mainly caused by education level in the LDH-CP patients (64% finished middle-school or below education), which plays a major role in the development of pain perception for chronic pain patients18. Unlike the study in the US where most of participants completed at least high-school education, the participants in China with low education may degrade responses to the pain-related questionnaires or pain ratings, and/or directly diminish the linkage between graph degree disruption and perceived/experienced pain. Because we did not enroll enough females with high-education level, we cannot reach a conclusion regarding the gender effect on graph degree disruption. Therefore, future studies need to examine a more varied Chinese patient group regarding socioeconomic status and cultural background.
Five graph topological disruption indexes (KDs) in terms of their nodal metrics: degree (D), betweenness centrality (BC), clustering coefficient (CC), efficiency (E), and participation coefficient (PC) and referred as KD_D, KD_BC, KD_CC, KD_E, and KD_PC were used to study the topology of the brain functional network for LDH-CP. The correlation analyses across five KDs showed that, except KD_PC, the other 4 KDs are highly correlated with each other (Fig. 6E), among which KD_D, KD_CC, and KD_E significantly decreased in both the Discovery and the Validation groups when compared LDH-PC with HC (Fig. 6A, 6C, 6D). These observations imply that although local network properties are independent measurements, the graph topological indices derived from these local properties similarly reflect brain disorder and the extent of correlation may depend on clinical conditions.
An important limitation in this study is demographically imbalanced subjects present both between LDH-CP and HC and within LDH-CPs. It was difficult to recruit HCs with similar demographic background to LDH-CP because most of LDH-CP patients were older, and lived in rural areas with low education level in China. Due to a large gender gap of educational level in China,9,56 we do not have enough statistical power to make conclusions regarding the effect of gender on brain reorganization of LDH-CP. Furthermore, a national survey of prevalence and patterns of smoking among Chinese adults demonstrate that only 3.4% of women are smokers compared to more than 50% in men.28 Thus these demographic differences also bias our results.
Another limitation is a lack of details of perceptional abnormalities accompanying LDH. Depending on various forms of herniation, each individual may have different laterality and location of pain on the back or leg(s). In addition, LDH phenotypes such as existence of back pain or not, hypo- and/or hypersensitivity, numbness, and defects of motor functions should have been assessed and might bias our results.
In conclusion, in this paper we looked at the brain network changes of lumbar disc herniation patients with chronic pain and discovered that LDH-CP patients showed whole-brain functional network disruption. The relationship between pain and graph disruption indices was limited to males with high education. These results deviate somewhat from recent similar analysis for other musculoskeletal chronic pain conditions, yet we cannot determine whether the differences are due to types of pain or also to cultural differences between patients studied in China and the US.
Acknowledgements
We would like to thank Feng Xu at the Department of Pain Management for helping recruitment and Lili Yang at the Department of Radiology in the Second Affiliated Hospital of Wenzhou Medical University for maintaining MRI scanner. We also thank NIH (grant 1P50DA044121–01A1) for funding for data analysis.
Footnotes
Competing interests
The authors declare that they have no competing interests.
References
- [1].Achard S, Delon-Martin C, Vertes PE, Renard F, Schenck M, Schneider F, Heinrich C, Kremer S, Bullmore ET. Hubs of brain functional networks are radically reorganized in comatose patients. Proc Natl Acad Sci U S A 2012;109(50):20608–20613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Amin RM, Andrade NS, Neuman BJ. Lumbar Disc Herniation. Curr Rev Musculoskelet Med 2017;10(4):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Apkarian AV, Reckziegel D. Peripheral and central viewpoints of chronic pain, and translational implications. Neurosci Lett 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Baliki MN, Chang PC, Baria AT, Centeno MV, Apkarian AV. Resting-sate functional reorganization of the rat limbic system following neuropathic injury. Sci Rep 2014;4:6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci 2008;28(6):1398–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Baliki MN, Mansour AR, Baria AT, Apkarian AV. Functional reorganization of the default mode network across chronic pain conditions. PLoS One 2014;9(9):e106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 2012;15(8):1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV. Brain morphological signatures for chronic pain. PLoS One 2011;6(10):e26010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bauer J, Wang F, Nancy R, Zhao X. Gender Inequality in Urban China: Education and Employment. Modern China 1992;18(3):333–370. [Google Scholar]
- [10].Beck AT, Steer RA, Garbin MG. Psychometric Properties of the Beck Depression Inventory - 25 Years of Evaluation. Clin Psychol Rev 1988;8(1):77–100. [Google Scholar]
- [11].Cauda F, Palermo S, Costa T, Torta R, Duca S, Vercelli U, Geminiani G, Torta DM. Gray matter alterations in chronic pain: A network-oriented meta-analytic approach. Neuroimage Clin 2014;4:676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cauda F, Sacco K, Duca S, Cocito D, D’Agata F, Geminiani GC, Canavero S. Altered resting state in diabetic neuropathic pain. PLoS One 2009;4(2):e4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cohen SR, Mount BM, Strobel MG, Bui F. The Mcgill Quality-of-Life Questionnaire - a Measure of Quality-of-Life Appropriate for People with Advanced Disease - a Preliminary-Study of Validity and Acceptability. Palliative Med 1995;9(3):207–219. [DOI] [PubMed] [Google Scholar]
- [14].Doerks T, Copley RR, Schultz J, Ponting CP, Bork P. Systematic identification of novel protein domain families associated with nuclear functions. Genome Res 2002;12(1):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66(8):271–273. [PubMed] [Google Scholar]
- [16].Farmer MA, Baliki MN, Apkarian AV. A dynamic network perspective of chronic pain. Neurosci Lett 2012;520(2):197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Farrar JT, Young JP Jr., LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94(2):149–158. [DOI] [PubMed] [Google Scholar]
- [18].Fitzcharles MA, Rampakakis E, Ste-Marie PA, Sampalis JS, Shir Y. The association of socioeconomic status and symptom severity in persons with fibromyalgia. J Rheumatol 2014;41(7):1398–1404. [DOI] [PubMed] [Google Scholar]
- [19].Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health 2011;11:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hu L, Li J, Wang X, Payne S, Chen Y, Mei Q. Prior Study of Cross-Cultural Validation of McGill Quality-of-Life Questionnaire in Mainland Mandarin Chinese Patients With Cancer. Am J Hosp Palliat Care 2015;32(7):709–714. [DOI] [PubMed] [Google Scholar]
- [22].Huang L, Yang T, Ji Z. Exploring the applicability of Positive and Negative Affect Schedule in Chinese population. Journal of Chinese Mental Health 2003;17(1):54–56. [Google Scholar]
- [23].Jordan J, Konstantinou K, O’Dowd J. Herniated lumbar disc. BMJ Clin Evid 2011;2011. [PMC free article] [PubMed] [Google Scholar]
- [24].Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 2001;413(6852):203–210. [DOI] [PubMed] [Google Scholar]
- [25].Kose G, Hatipoglu S. The effect of low back pain on the daily activities of patients with lumbar disc herniation: a Turkish military hospital experience. J Neurosci Nurs 2012;44(2):98–104. [DOI] [PubMed] [Google Scholar]
- [26].Koubiyr I, Deloire M, Besson P, Coupe P, Dulau C, Pelletier J, Tourdias T, Audoin B, Brochet B, Ranjeva JP, Ruet A. Longitudinal study of functional brain network reorganization in clinically isolated syndrome. Mult Scler 2018:1352458518813108. [DOI] [PubMed] [Google Scholar]
- [27].Kreiner DS, Hwang SW, Easa JE, Resnick DK, Baisden JL, Bess S, Cho CH, DePalma MJ, Dougherty P 2nd, Fernand R, Ghiselli G, Hanna AS, Lamer T, Lisi AJ, Mazanec DJ, Meagher RJ, Nucci RC, Patel RD, Sembrano JN, Sharma AK, Summers JT, Taleghani CK, Tontz WL Jr., Toton JF, North American Spine S. An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J 2014;14(1):180–191. [DOI] [PubMed] [Google Scholar]
- [28].Liu S, Zhang M, Yang L, Li Y, Wang L, Huang Z, Wang L, Chen Z, Zhou M. Prevalence and patterns of tobacco smoking among Chinese adult men and women: findings of the 2010 national smoking survey. J Epidemiol Community Health 2017;71(2):154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lue YJ, Hsieh CL, Huang MH, Lin GT, Lu YM. Development of a Chinese version of the Oswestry Disability Index version 2.1. Spine (Phila Pa 1976) 2008;33(21):2354–2360. [DOI] [PubMed] [Google Scholar]
- [30].Mano H, Kotecha G, Leibnitz K, Matsubara T, Sprenger C, Nakae A, Shenker N, Shibata M, Voon V, Yoshida W, Lee M, Yanagida T, Kawato M, Rosa MJ, Seymour B. Classification and characterisation of brain network changes in chronic back pain: A multicenter study. Wellcome Open Res 2018;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mansour A, Baria AT, Tetreault P, Vachon-Presseau E, Chang PC, Huang L, Apkarian AV, Baliki MN. Global disruption of degree rank order: a hallmark of chronic pain. Sci Rep 2016;6:34853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mansour AR, Baliki MN, Huang L, Torbey S, Herrmann KM, Schnitzer TJ, Apkarian AV. Brain white matter structural properties predict transition to chronic pain. Pain 2013;154(10):2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mccracken LM, Zayfert C, Gross RT. The Pain Anxiety Symptoms Scale - Development and Validation of a Scale to Measure Fear of Pain. Pain 1992;50(1):67–73. [DOI] [PubMed] [Google Scholar]
- [34].Mutso AA, Petre B, Huang L, Baliki MN, Torbey S, Herrmann KM, Schnitzer TJ, Apkarian AV. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J Neurophysiol 2014;111(5):1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum 2010;62(8):2545–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ploner M, Sorg C, Gross J. Brain Rhythms of Pain. Trends Cogn Sci 2017;21(2):100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012;59(3):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. Functional network organization of the human brain. Neuron 2011;72(4):665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 2014;84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Qi R, Ke J, Schoepf UJ, Varga-Szemes A, Milliken CM, Liu C, Xu Q, Wang F, Zhang LJ, Lu GM. Topological Reorganization of the Default Mode Network in Irritable Bowel Syndrome. Mol Neurobiol 2016;53(10):6585–6593. [DOI] [PubMed] [Google Scholar]
- [41].Quan X, Fong DYT, Leung AYM, Liao Q, Ruscheweyh R, Chau PH. Validation of the Mandarin Chinese Version of the Pain Sensitivity Questionnaire. Pain Pract 2018;18(2):180–193. [DOI] [PubMed] [Google Scholar]
- [42].Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010;52(3):1059–1069. [DOI] [PubMed] [Google Scholar]
- [43].Ruscheweyh R, Marziniak M, Stumpenhorst F, Reinholz J, Knecht S. Pain sensitivity can be assessed by self-rating: Development and validation of the Pain Sensitivity Questionnaire. Pain 2009;146(1–2):65–74. [DOI] [PubMed] [Google Scholar]
- [44].Shen B, Wu B, Abdullah TB, Zhan G, Lian Q, Vania Apkarian A, Huang L. Translation and validation of Simplified Chinese version of the Pain Catastrophizing Scale in chronic pain patients: Education may matter. Mol Pain 2018;14:1744806918755283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].St John Smith E Advances in understanding nociception and neuropathic pain. J Neurol 2018;265(2):231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assessment 1995;7(4):524–532. [Google Scholar]
- [47].Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR. Brain resting state is disrupted in chronic back pain patients. Neurosci Lett 2010;485(1):26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Termenon M, Achard S, Jaillard A, Delon-Martin C. The “Hub Disruption Index,” a Reliable Index Sensitive to the Brain Networks Reorganization. A Study of the Contralesional Hemisphere in Stroke. Front Comput Neurosci 2016;10:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tsuda M, Tozaki-Saitoh H, Inoue K. Pain and purinergic signaling. Brain Res Rev 2010;63(1–2):222–232. [DOI] [PubMed] [Google Scholar]
- [50].Vialle LR, Vialle EN, Suárez Henao JE, Giraldo G. Lumbar Disc Herniation. Revista Brasileira de Ortopedia (English Edition) 2010;45(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wang GJ, Wang GJ, Peng JM, Cai KM, Zhang JS, Song WH, Xiao J. EPIDEMIOLOGY STUDY ON PATHOGENIC FACTORS OF LUMBAR DISC HERNIATION [Chinese]. Modern Preventive Medicine 2009;36(13):2401–2403. [Google Scholar]
- [52].Watson D, Clark LA, Tellegen A. Development and Validation of Brief Measures of Positive and Negative Affect - the Panas Scales. J Pers Soc Psychol 1988;54(6):1063–1070. [DOI] [PubMed] [Google Scholar]
- [53].Welton T, Kent DA, Auer DP, Dineen RA. Reproducibility of graph-theoretic brain network metrics: a systematic review. Brain Connect 2015;5(4):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science 2000;288(5472):1765–1769. [DOI] [PubMed] [Google Scholar]
- [55].Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One 2013;8(7):e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zeng J, Pang X, Zhang L, Medina A, Rozelle S. GENDER INEQUALITY IN EDUCATION IN CHINA: A META-REGRESSION ANALYSIS. Contemporary Economic Policy 2013;32 (2):474–491. [Google Scholar]
- [57].Zhang Y, Liu J, Li L, Du M, Fang W, Wang D, Jiang X, Hu X, Zhang J, Wang X, Fang J. A study on small-world brain functional networks altered by postherpetic neuralgia. Magn Reson Imaging 2014;32(4):359–365. [DOI] [PubMed] [Google Scholar]
- [58].Zheng YP, Wei LA, Goa LG, Zhang GC, Wong CG. Applicability of the Chinese Beck Depression Inventory. Compr Psychiatry 1988;29(5):484–489. [DOI] [PubMed] [Google Scholar]
- [59].Zhou XY, Xu XM, Wang F, Wu SY, Yang YL, Li M, Huang JM, Wei XZ. Validations and psychological properties of a simplified Chinese version of pain anxiety symptoms scale (SC-PASS). Medicine (Baltimore) 2017;96(10):e5626. [DOI] [PMC free article] [PubMed] [Google Scholar]