Abstract
Background:
Sevoflurane with its anti-inflammatory properties has shown to decrease mortality in animal models of sepsis. However, the underlying mechanism of its beneficial effect in this inflammatory scenario remains poorly understood. Macrophages play an important role in the early stage of sepsis as they are tasked with eliminating invading microbes and also attracting other immune cells by the release of pro-inflammatory cytokines such as interleukin-1β (IL-1 β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). Thus, we hypothesized that sevoflurane mitigates the pro-inflammatory response of macrophages, while maintaining their bactericidal properties.
Methods:
Murine bone marrow-derived macrophages (BMDM) were stimulated in vitro with lipopolysaccharide (LPS) in the presence and absence of 2% sevoflurane. Expression of cytokines and inducible NO synthase (iNOS) as well as uptake of fluorescently labeled E. coli were measured. Our in vivo endotoxemia model consisted of an intraperitoneal LPS injection after anesthesia with either ketamine/xylazine or 4% sevoflurane. Male mice (n=6 per group) were observed for a total of 20 hours. During the last 30 minutes fluorescently labeled E. coli were intraperitoneally injected. Peritoneal cells were extracted by peritoneal lavage and iNOS expression as well as E. coli uptake by peritoneal macrophages was determined using flow cytometry.
Results:
In vitro, sevoflurane enhanced LPS-induced iNOS expression after 8 hours by 466%, and increased macrophage uptake of fluorescently labeled E. coli by 70% compared to vehicle-treated controls. Inhibiting iNOS expression pharmacologically abolished this increase in bacteria uptake. In vivo, iNOS expression was increased by 669% and phagocytosis of E. coli by 49% compared to the control group.
Conclusion:
Sevoflurane enhances phagocytosis of bacteria by LPS-challenged macrophages in vitro and in vivo via an iNOS-dependent mechanism. Thus, sevoflurane potentiates bactericidal and anti-inflammatory host-defense mechanisms in endotoxemia.
Introduction
Volatile anesthetic gases, such as sevoflurane, are widely used to induce and maintain general anesthesia in patients with various comorbidities such as sepsis. Modern volatile anesthetics such as sevoflurane have been shown to be protective in scenarios of ischemia-reperfusion injury in various organs including the heart1, kidney2, and liver3. Furthermore, sevoflurane is known to have anti-inflammatory properties as it reduces the inflammatory response associated with alveolar epithelial cells4,5 and decreases the activation of nuclear factor-kappa B (NF-κB)6–12. NF-κB, a well-known nuclear transcription factor, is seen as one of the key regulators for initiating an immune response towards inflammation. Inhalation of sevoflurane also appears to be beneficial during acute lung injury (ALI)13,14. Less is known about the effects of sevoflurane on infectious inflammation. Animal studies suggest favorable influences as sevoflurane improved survival in bacterial sepsis15,16. This finding is particularly interesting because propofol, another commonly used anesthetic agent, showed adverse effects in infectious conditions, as exposure to propofol increased bacterial burden of infected animals17 and decreased survival in sepsis16.
Sevoflurane interacts with immune cells like neutrophils and decreases their adhesion to the endothelium18,19 as well as transmigration20, and reduces apoptosis21. Only a few studies investigated the effects of sevoflurane on other immune cells. Macrophages, as part of the innate immune system, are among the first to interact with microbial invaders and defend the host against pathogens. They are recruited to the site of infection and attract other immune cells by releasing pro-inflammatory cytokines such as interleukin-1β (IL-1 β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α)22. Invading microorganisms are eradicated when macrophages engulf them via phagocytosis, while bactericidal proteins such as inducible nitric oxide synthase (iNOS) are upregulated, leading to increased expression of nitric oxide (NO)22,23. Antigens from eradicated pathogens are then presented to the adaptive immune system and a specific immune response can be initiated24,25. Thus, macrophages play a pivotal role in restoring tissue homeostasis and overcoming inflammation23.
We hypothesized that sevoflurane prevents macrophages from eliciting an exaggerated immune response by attenuating the expression of NFκB-dependent gene products, thereby contributing to sevoflurane’s overall beneficial effect in severe inflammation. The first aim was thus to assess the inflammatory response of murine macrophages upon stimulation with bacterial lipopolysaccharide (LPS) in the presence and absence of sevoflurane. The second aim was to evaluate macrophage function under the influence of sevoflurane in vitro as well as in vivo in a LPS-induced endotoxemia model in mice.
Material and Methods
Cell culture
All cell culture procedures were conducted under sterile conditions in a laminar flow cabinet, with reagents warmed to 37°C before use, if not stated otherwise. Abelson murine leukemia virus-transformed macrophages, RAW 264.7 cells (ATCC® TIB-71™), were cultured under standard cell culture conditions (37°C, 80% relative humidity and 5% carbon dioxide (CO2) in DMEM with 10% heat-inactivated FBS (Gemini Bio-Products, West Sacramento, CA, USA), 1 U/ml penicillin, 100 μM/ml streptomycin, and 1 mM sodium pyruvate (all from Thermo Fisher Scientific, Waltham, MA, USA). RAW 264.7 macrophages were used for nitrate measurement and degradation of NF-κB inhibitor, alpha (IκBα). All other in vitro experiments were conducted with murine bone marrow-derived macrophages (BMDM).
Animals
After approval by the institutional ethical board all animal procedures and experiments were conducted in accordance with the guidelines of the University of Illinois at Chicago Institutional Animal Care and Use Committee and Office of Laboratory Animal Welfare (OLAW). C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and used in all experiments. Male C57BL/6 mice, 8-12 weeks old with a target weight of 25-30g were used. We only used male animals as hormonal changes occurring during the menstrual cycle could possibly have an impact on the experimental setup. Four animals per cage were housed. Animals had free access to food and water and were subjected to a 12-hour day and night cycle. Experiments were performed in the animal facility at the University of Illinos at Chicago. Anesthesia was either induced with an intraperitoneal injection of ketamine (100mg/kg bodyweight, Hospira Inc. Lake Forest, IL, USA) and xylazine (10mg/kg bodyweight, AKORN Animal Health, Lake Forest, IL, USA) or with 4% sevoflurane (Baxter, Deerfield, IL, USA), corresponding to a 1.2 MAC in mice15.
Isolation of murine bone marrow-derived macrophages
BMDMs were isolated and differentiated as previously described26. After induction of anesthesia with ketamine/xylazine, mice were euthanized via exsanguination and removal of the heart. Thereafter, bone marrow cells from the femur and tibia were isolated and differentiated for 5-7 days in RPMI containing 10% heat-inactivated FBS (Gemini Bio-Products, West Sacramento, CA, USA), 1% (vol/vol) Antibiotic-Antimycotic, 1 mM sodium pyruvate (both from Thermo Fisher Scientific, Waltham, MA, USA) and 10-15% L929 conditioned cell culture medium. For experimental procedures in 6 well plates, macrophages were differentiated in the same plate. For experiments in other plates, BMDMs were removed after 5-7 days with 5 mM EDTA (Sigma-Aldrich, St. Louis, MO, USA) in DPBS and reseeded into the appropriate cell culture dish.
Experimental exposure in vitro
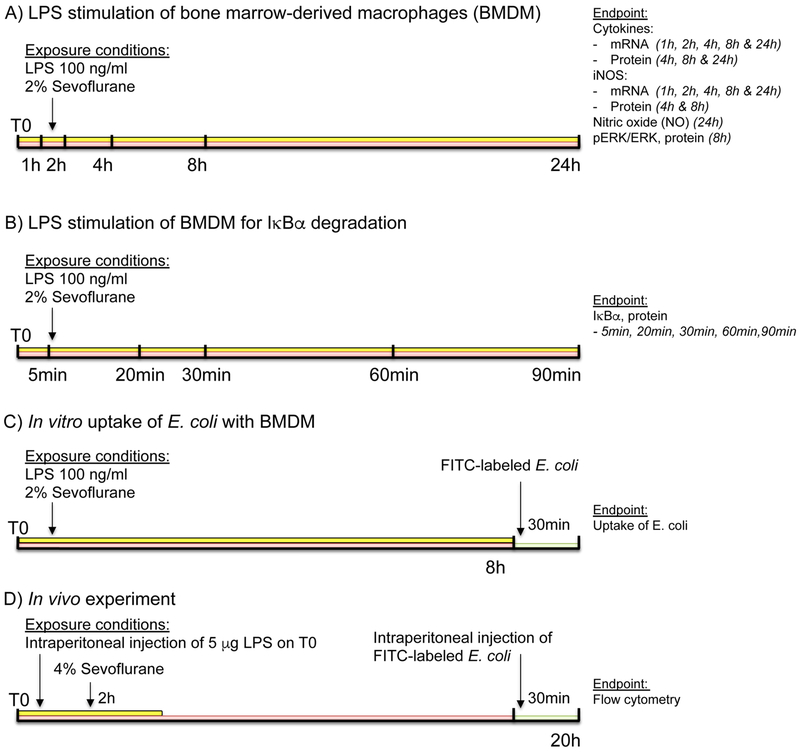
Cells were incubated with bacterial LPS from Escherichia coli (E. coli) serotype 055:B5 (Sigma-Aldrich, St. Louis, MO, USA) at a concentration of 100 ng/ml for up to 24 hours. Sevoflurane (Baxter, Deerfield, IL, USA) was vaporized with a Penlon (Minnetonka, MN, USA) Sigma Elite vaporizer, and the cells were incubated in an airtight Oxoid chamber, (Pratteln, Switzerland) for the entire time of LPS exposure. This time varied according to the endpoint (inflammatory mediators IL-1β, IL-6 and TNF-α: mRNA 0-24 hours, protein: 4-24 hours; iNOS mRNA 0-24 hours; iNOS protein 4 and 8 hours; nitrite 24 hours; IκBα 0-90 min; ERK 8 hours; BMDM E. coli uptake: in vitro 8 hours in vivo experiments 20 hours). A schematic illustration of the experimental setting is shown in Figure 1.
Figure 1.
Schematic illustration of the experimental setting. A) Stimulation of bone marrow-derived macrophages (BMDM) with lipopolysaccharide (LPS) in the presence and absence of 2% sevoflurane. B) Experimental setup for degradation of IκBα. C) In vitro update of FITC-labeled E. coli. D) In vivo inducible nitric oxide synthase (iNOS) expression and uptake of FITC-labeled E. coli by murine peritoneal macrophages.
For iNOS inhibition, the selective iNOS inhibitor 1400W (Calbiochem, Billerica, MA, USA) was used at a final concentration of 1 μM. Sevoflurane concentration in the outflowing air from the airtight chamber was measured with an E-CAiO gas analyzer and displayed on a CARESCAPE Monitor B650, both from GE Healthcare (Chicago, IL, USA). Sevoflurane was vaporized in synthetic air with 5% CO2 (21% O2, 74% N2, and 5% CO2).
Quantitative real-time polymerase chain reaction analysis
Total RNA was isolated from BMDMs with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. After RNA purification, 1 μg RNA was used to produce cDNA using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). Quantitative real-time polymerase chain reaction (qPCR) was performed with Fast SYBR green master mix (Applied Biosystems, Foster City, CA, USA) on a ViiA™ 7 real-time PCR system (Applied Biosystems, Foster City, CA, USA). The following primers were used: Interleukin-1β(IL-1β) forward primer 5’-ttc cca tta gac aac tgc act ac-3’ and reverse primer 5’-gtc gtt gct tgg ttc tcc tt-3’; interleukin-6 (IL-6) forward primer 5’-cac aag tcc gga gag gag ac-3’ and reverse primer 5’-ttc tgc aag tgc atc atc gt-3’; glyceraldehyde 3-phosphate dehydrogenase (GADPH) forward primer 5’-ggg tgt gaa cca cga gaa ata-3’ and reverse primer 5’-gtc atg agc cct tcc aca at-3’; inducible nitric oxide synthase (iNOS) forward primer 5’-cag ctg ggc tgt aca aac ctt-3’ and reverse primer 5’-cat tgg aag tga agc gtt tcg-3’; TNF-α forward primer 5’-agt tct atg gcc cag acc ct-3’ and reverse primer 5’-cac ttg gtg gtt tgc tac ga-3’. Cycle threshold (Ct) values were transformed (2-Ct) and normalized to corresponding GADPH values for each sample. Graphs show fold the change compared to controls at time point 0 minute.
Enzyme-linked immunosorbent assay
The concentrations of murine IL-1β and IL-6 were determined in the supernatant by ELISA, according to the manufacturer’s manual (ELISA DuoKits, R&D Systems, Minneapolis, MN, USA). Optical density (OD) at 450nm with a wavelength correction of 540nm was measured on the Tecan (Männedorf, Switzerland) GENios Pro microplate reader.
Nitrite measurement
NO release by macrophages was measured indirectly by determination of nitrite production. Nitrite level reflects the total NO production as it forms immediately upon oxidation of NO, and also is more stable than NO which has a half-life of a few seconds27. 9×105 RAW 264.7 cells/well were seeded into 6 well plates and stimulated for 24 hours. Nitrite concentrations in the supernatant were measured with the Griess reagent measurement kit (Cell Signaling, Danvers, MA, USA) according to the manufacturer’s protocol.
Western Blot
Cells were rinsed one time with ice-cold Dulbecco’s phosphate buffered saline (DPBS, Cellgro, Manassas, VA, USA) and then lysed with RIPA buffer (Boston Bioproducts, Ashland, MA, USA) containing phosphatase and proteinase inhibitors (both from Sigma-Aldrich, St. Louis, MO, USA). Thereafter, the lysate was scraped from the cell culture plate and left on ice for 30 minutes, followed by centrifugation at 4°C and 12,000 x g for 20 minutes. Total protein was measured using a DC protein assay (Bio-Rad, Hercules, CA, USA) using albumin as the protein standard (Thermo Fisher, Waltham, MA, USA). The supernatant was stored at −80°C until further analysis, at which time equal amounts of protein were diluted using Laemmli buffer (Bio-Rad, Hercules, CA, USA) plus beta-mercaptoethanol (Bio-Rad, Hercules, CA, USA) and boiled for 5 minutes at 95°C. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane. After blocking for 1 hour with either 5% milk or 5% bovine serum albumin (BSA) in tris-buffered saline with 0.1% Tween20 (TBST), membranes were washed three times and probed with primary antibody overnight at 4°C. After washing with TBST, membranes were probed with a secondary antibody (KPL, Gaithersburg, MD, USA) and chemiluminescent signal was detected using supersignal chemiluminescence substrate (Thermo Fisher, Waltham, MA, USA) and an Odyssey Fc imager (Li-cor, Lincoln, NE, USA). Digital images were analyzed with ImageJ 1.49v software (National Institute of Health, Bethesda, MD, USA). For protein phosphorylation, membranes were exposed to stripping buffer (100 mmol/l glycine, 1% SDS, 0.1% NP-40, pH 2 in H2O) and reprobed with antibodies as previously described. GAPDH or β-actin were used as loading controls. The following primary antibodies were used: β-actin from BD Bioscience (San Jose, CA, USA); total and phosphorylated (pThr202/pTyr204) extracellular signal-regulated kinases (ERK) from Cell Signaling (Danvers, MA, USA); GAPDH and IκBα from Santa Cruz Biotechnology (Dallas, TX, USA); iNOS from EMD Millipore (Billerica, MA, USA).
Measurement of uptake of fluorescently labeled E. coli
Macrophage-mediated uptake of heat inactivated E. coli was performed as previously described28. 105 BMDM per well were seeded in a black, clear-bottom 96 well plate (Corning, Corning, NY, USA) and pretreated for 8 hours with or without 100 ng/ml LPS in the presence or absence of 2% sevoflurane. Thereafter, the medium was removed and replaced with medium containing approximately 108 FITC-labeled heat-inactivated E. coli and incubated for 30 minutes. The fluorescent signal of extracellular E. coli was quenched with Trypan blue (Sigma-Aldrich, St. Louis, MO, USA), as previously described28–30, and the cells were then fixed in 4% paraformaldehyde (PFA) (Electron Microscopy Science, Hatfield, PA, USA) and stained with DAPI (Molecular Probes, Eugene, OR, USA). The fluorescent signal from FITC (λExcitation 494nm/ λEmission 518nm) and DAPI (λExcitation 364nm/ λEmission 454nm) were obtained using a Synergy H4 fluorescent microplate reader (BioTek, Winooski, VT, USA). Uptake of heat-inactivated FITC-labeled E. coli was calculated as: Uptake = (FITC signal-background)/(DAPI signal). Uptake was normalized to BMDM that were exposed to medium without additives.
LPS-induced endotoxemia model
Animals were randomly assigned to the following groups: 1. Sham, 2. Sevo/Sham, 3. LPS and 4. Sevo/LPS. Endotoxemia was induced by intraperitoneal injection of 5 μg LPS, Serotype O111:B4 (Sigma-Aldrich, St. Louis, MO, USA) in 200 μl of sterile pre-warmed saline31. Experiments were always initiated at the same day time (start at 2pm). To ensure solubilization the suspension was sonicated for 10 minutes prior to injection. Mice were anesthetized either with ketamine/xylazine (Sham and LPS) or with sevoflurane (Sevo/Sham and Sevo/LPS). Goal was to reach a group size of 6, resulting in a total of 24 animals based on previous experiments, respecting replacement, refinement, or reduction of animals in research (3R) without sample size calculation. Animals were randomly assigned to one of the groups without blinding. Sevoflurane-treated animals were exposed to 4% sevoflurane for 2 hours after injection of LPS. Sevoflurane concentration was used as previously described15,32. For the last 30 minutes (at 19.5 hours) 107 FITC-labeled heat-inactivated E. coli in 500 μl pre-warmed PBS were injected intraperitoneally. After a total of 20 hours, the anesthetized mice were sacrificed by cervical dislocation31. Subsequently, peritoneal lavage with 6 ml ice-cold PBS was performed33. The lavage was centrifuged for 5 minutes at 300 x g and the cell pellet resuspended in 1 ml ice-cold FACS buffer (PBS and 0.5% BSA). Before initiation of the experiments outcomes were defined. The primary outcome was uptake of E. coli by peritoneal macrophages; the secondary outcome was iNOS expression by peritoneal macrophages.
Flow cytometry
Prior to staining, the cells were treated with anti-CD16/CD32 mAb (BD Bioscience, San Jose, CA, USA) according to the manufacturer’s recommendation to block FcγRII/III receptors. If not stated otherwise, all stainings were conducted on ice and protected from light. First, surface staining with the following antibodies was performed for 30 minutes: PerCP Cy5-5 labeled Ly6C, Pacific Blue labeled Ly6G, Apc Cy 7 labeled CD11b, Brillian Violet 510 labeled CD11c and PE labeled F4/80 (all from Biolegend, San Diego, CA, USA). Thereafter, the cell suspension was washed in PBS and stained for dead cells with fixable viability dye eFluor 660 (eBioscience, San Diego, CA, USA) for 15 minutes. To prepare the cells for the intracellular iNOS staining, they were fixed and permeabilized with a kit (eBioscience, San Diego, CA, USA) according to the manufacturer’s protocol. This step was followed by intracellular staining with PE Cy7 labeled iNOS antibody, which was performed for 20 minutes at room temperature. For compensation Ultracomp eBeads (Invitrogen, Carlsbad, CA, USA) were stained with 0,5 μl of each dye for 20 minutes. The cells and beads were analyzed with a BD LSR Fortessa flow cytometer. For analysis, FlowJo v10 (FlowJo, LCC, Ashland, OR, USA) software was used; fluorescence minus one (FMO) controls were used to set the gates.
Statistical analysis
Values are shown as means ± standard deviation (SD). Data analysis and graphical presentations were performed using GraphPad Prism version 7.0 and 8.0 (GraphPad, La Jolla, CA, USA). Normal distribution was visually analyzed using Q-Q plots. Comparisons between two groups were performed by an unpaired two-tailed t-test, and for 3 or more groups using one-way analysis of variance (ANOVA) with Bonferroni’s post hoc comparison. In experiments with one single time point analysis, comparison of one-way ANOVA was used. To assess the effect of the treatment and time an ordinary two-way ANOVA (no repeated measures) with Bonferroni’s post hoc test was used with time and treatment as factors to be evaluated. No statistical power analysis was conducted prior to the study. The sample size was based on previous experience with the experimental design(s). There was no exclusion of outliers. A p-value of < 0.05 was considered statistically significant. All experimental procedures were performed at least three times.
Results
Sevoflurane suppresses LPS-induced expression and production of pro-inflammatory cytokines by bone marrow-derived macrophages
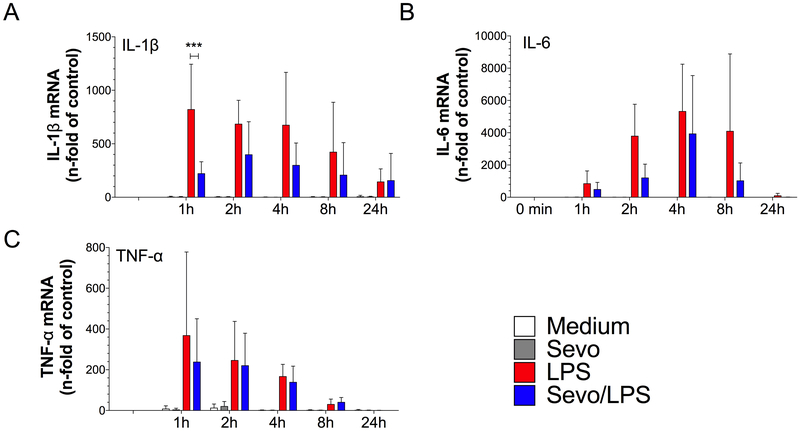
Expression of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α in BMDMs following LPS stimulation was determined using qPCR. As expected, we observed a time-dependent increase in the expression of pro-inflammatory cytokines after stimulation with LPS as compared to control (t0min) (Figure 2A–C). LPS-induced IL-1β expression in BMDMs was reduced by sevoflurane at 1 hour (p=0.0006) (Figure 2A), whereas no difference was observed in the expression of TNF-α at any of the observed time points (Figure 2C).
Figure 2.
mRNA cytokine expression in bone marrow-derived macrophages. Cells were exposed to lipopolysaccharide (LPS) in the presence of absence of 2% sevoflurane for 0, 1, 2, 4, 8 and 24 hours. RNA was extracted and quantitative real-time PCR (qPCR) was performed. A) mRNA expression of the proinflammatory mediators interleukin-1β (IL-1β), B) interleukin-6 (IL-6) and C) tumor necrosis factor-α (TNF-α). Values represent means ± standard deviation; n≥3<6, two-way ANOVA (group and time interaction); *** p=0.0006
To evaluate whether reduced IL-1β and IL-6 mRNA expression was associated with a decrease in protein level, the concentration of these cytokines was measured in the supernatant from LPS-treated BMDMs using ELISA. Stimulation with LPS increased the release of both IL-1β and IL-6 at 4 hours, 8 hours, and 24 hours (Figure 3A and 3B). The release of IL-1β upon LPS stimulation at 24 hours (546±346 ng/ml) [mean±SD]) was reduced in the presence of 2% sevoflurane to a value of 145±155 ng/ml (p<0.0001) (Figure 3A). Similarly, after LPS stimulation there was a time-dependent increase in IL-6 protein concentration over time to a value of (9006±5817 ng/ml) at 24 hours, while sevoflurane reduced LPS-induced IL-6 release to 4183±2797 ng/ml; p<0.0001) (Figure 3B).
Figure 3.
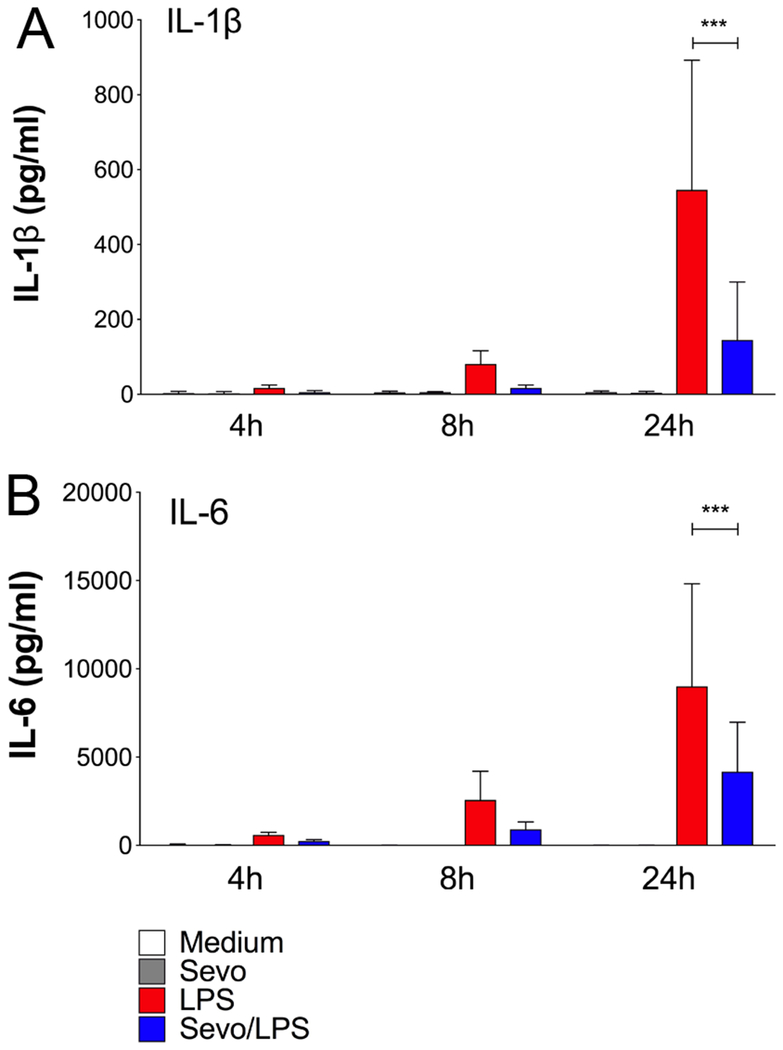
Cytokine protein production in bone marrow-derived macrophages (BMDM). Cells were stimulation with lipopolysaccharide (LPS) in the presence or absence of 2% sevoflurane for 4, 8 and 24 hours. Interleukin-1β (IL-1β) and interleukin-6 (IL-6) were measured in the supernatant of cultured BMDM by ELISA. A) IL-1β and B) IL-6. Values represent means ± standard deviation; n=9 (treatment groups) and n=6 (controls); two-way ANOVA (group and time interaction); *** p<0.0001
Sevoflurane differentially modulates iNOS expression upon LPS stimulation
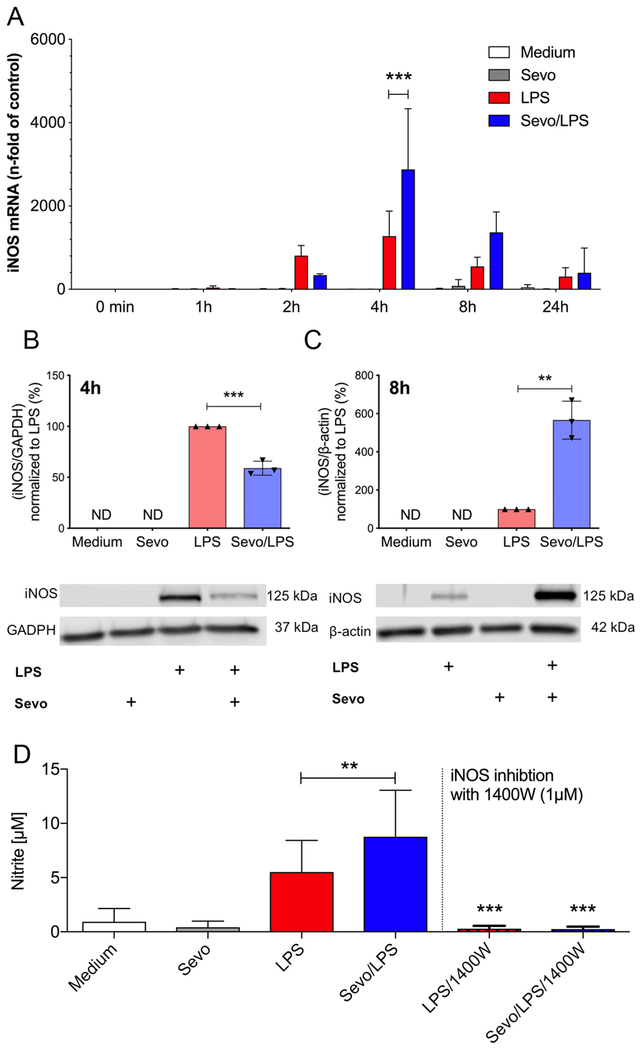
We also assessed the effect of sevoflurane on another classic NFκB-dependent inflammatory mediator, namely iNOS. LPS stimulation promoted a time-dependent increase in iNOS mRNA expression, which peaked at 4 hours (Figure 4). The effect of sevoflurane on LPS stimulated iNOS expression level, however, differed over the 24-hour time course as compared to other pro-inflammatory cytokines. Sevoflurane increased iNOS gene expression at 4 hours (p<0.0001). At 8 hours iNOS expression did not differ (p=0.099) in sevoflurane-treated cells compared to cells exposed to air only (Figure 4A). Effects of sevoflurane on iNOS protein levels mirrored the changes observed by qPCR. At 4 hours of LPS stimulation, sevoflurane reduced iNOS expression by 41% compared to BMDM treated with LPS alone (p=0.0005) (Figure 4B) and sevoflurane increased iNOS expression by 466% at 8h compared to LPS alone (p=0.0012) (Figure 4C).
Figure 4.
Inducible nitric oxide synthase (iNOS) gene expression and nitric oxide (NO) production in bone marrow-derived macrophages (BMDM). A) Cells were exposed to lipopolysaccharide (LPS) in the presence and absence of 2% sevoflurane for 0, 1, 2, 4, 8 and 24 hours. RNA was extracted and quantitative real-time PCR (qPCR) was performed to determine iNOS mRNA. Values represent means ± standard deviation; n=4, two-way ANOVA (group and time interaction); *** p<0.0001 B) Protein levels of iNOS were determined after 4 hours (A) or 8 hours (B) of LPS stimulation in the presence or absence of 2% sevoflurane. Whole cell lysates were used for Western blot analysis. Representative Western blots are shown for B) and C). For B) and C) values represent means ± standard deviation; n=3; unpaired two-tailed t-test; ** p=0.0012, *** p=0.0005 D) NO production in BMDM after 24 hours stimulation with LPS in the presence or absence of 2% sevoflurane was measured indirectly by determination of nitrite production using Griess reagent. To inhibit iNOS production 1400W was added for the entire incubation. Values represent means ± standard deviation; n=15 samples without 1400W, n=6 samples with 1400W; one-way ANOVA; LPS vs Sevo/LPS ** p=0.009, LPS vs LPS1400W ** p=0.0007, Sevo/LPS vs Sevo/LPS/1400W *** p=0.0005
Sevoflurane enhances nitrite production during LPS stimulation
To assess, whether the increase in iNOS protein resulted correspondingly in enhanced NO production, we measured nitrite concentration in the culture media. As expected, LPS stimulation led to an increase in nitrite production by macrophages compared to unstimulated controls (p<0.0001) (Figure 4D). The nitrite production after 24 hours of LPS stimulation (5.5±2.9 μM [mean±SD]) was enhanced when cells were co-exposed to sevoflurane (8.8±4.3 μM; p=0.009). Furthermore, LPS-induced increase in nitrite concentration was completely abolished in cells treated with the iNOS specific inhibitor 1400W (1μM; LPS vs LPS1400W, p=0.0007; Sevo/LPS vs Sevo/LPS/1400W, p=0.0005), confirming increased nitrate level is promoted by iNOS activation.
Sevoflurane decreased LPS-induced ERK phosphorylation whereas IκBα degradation was not altered
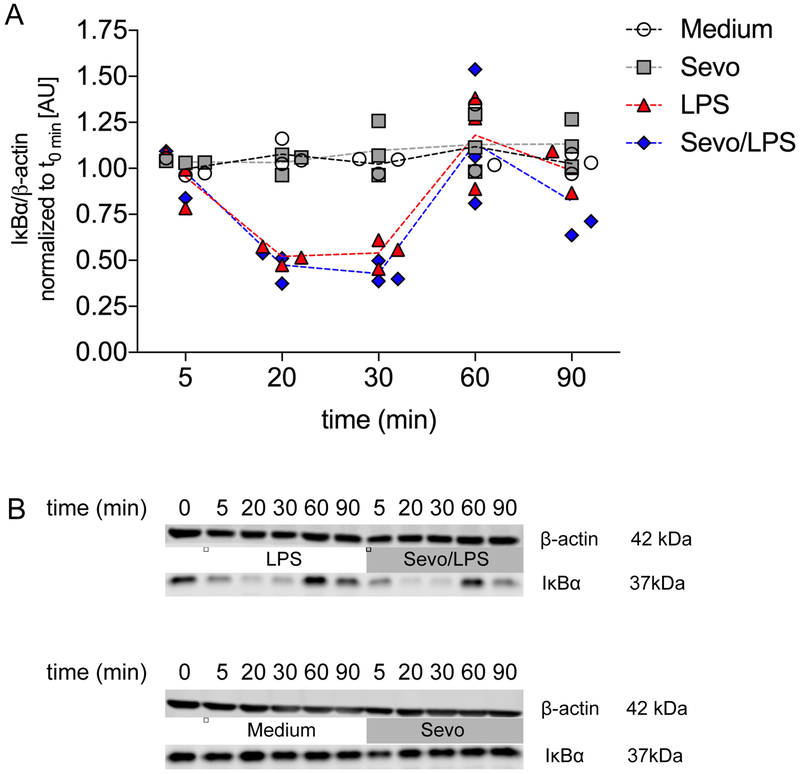
As pro-inflammatory genes in macrophages were observed to be differentially modulated by sevoflurane, we next assessed potential molecular mechanisms by interrogating signaling proteins associated with the various inflammatory pathways. As the data indicated sevoflurane promotes a decrease in the expression of NF-κB dependent genes, such as pro-inflammatory cytokines IL-1β and IL-6, we next assessed whether degradation of IκBα, which suppresses NF-κB activation, would play a role in the reduced expression of IL-1β and IL-6. As this step is found at the very beginning of the inflammatory cascade, early time points (0, 5, 20, 30, 60 and 90 minutes) were chosen34. LPS led to a time-dependent degradation of IκBα, which peaked at 20 minutes (p=0.004 compared to medium), which was still reduced at 30 minutes (p=0.002 compared to medium), and finally returned to baseline at 60 minutes (p>0.999 compared to controls). In the presence of sevoflurane, LPS-induced degradation of IκBα was not affected (p>0.999) (Figure 5A, B).
Figure 5.
Degradation of the NF-κB inhibitor IκBα in bone marrow-derived macrophages. A) Cells were exposed to lipopolysaccharide (LPS) in the presence and absence of 2% sevoflurane for 0, 5, 20, 30, 60 and 90 min, followed by Western blot analysis. Scatter plot with actual values and the connecting line representing the mean; n=3, two-way ANOVA (group and time interaction) B) Representative Western blots.
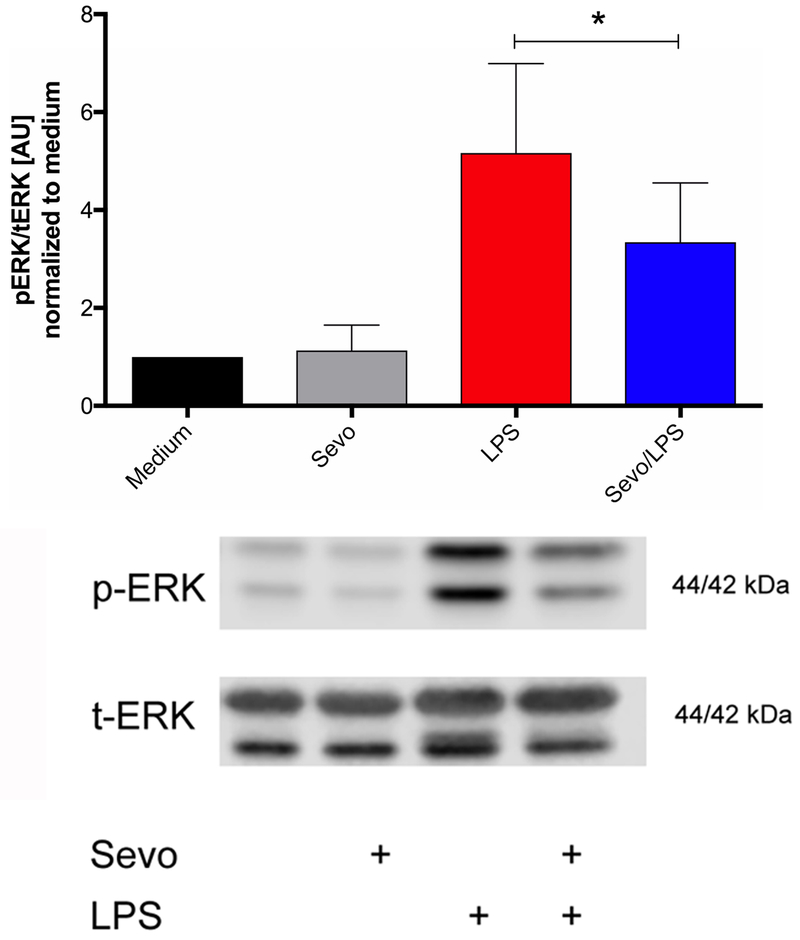
Next, mitogen-activated protein kinases (MAPK) pathways were explored. ERK, which is part of the MAPK pathway, is activated by phosphorylation at Thr202/Tyr204 by MEK1. Phosphorylated ERK then influences the activity of several transcription factors. After 8 hours of LPS stimulation, ERK phosphorylation increased (control vs LPS, p>0.0001; control vs Sevo/LPS p=0.0004) and sevoflurane decreased LPS-induced ERK phosphorylation (p=0.036) compared to LPS alone (Figure 6).
Figure 6.
Expression of extracellular signal-regulated kinase (ERK) phosphorylation (p-ERK) in bone marrow-derived macrophages normalized by total ERK (t-ERK). Cells were stimulated for 8 hours with lipopolysaccharide (LPS) in the presence or absence of 2% sevoflurane, followed by Western blot analysis. Representative Western blots are shown below. Values represent means ± standard deviation; n=7, one-way ANOVA); * p=0.036
Sevoflurane increased uptake of fluorescently labeled heat-inactivated E. coli by macrophages in vitro and in vivo
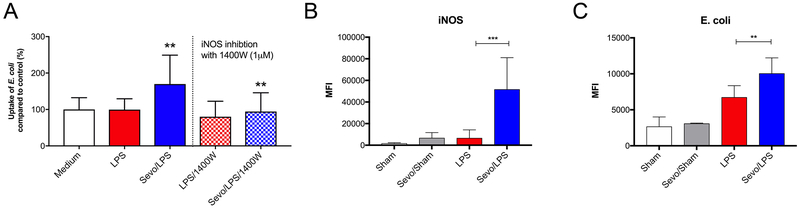
To test whether enhanced iNOS expression affects phagocytosis by macrophages, following exposure to medium alone, LPS or LPS and sevoflurane for 8 hours, BMDMs were incubated with FITC-labeled heat-inactivated E. coli for 30 minutes. All samples were normalized to BMDM pretreated with medium without additional additives. There was no difference between BMDMs pretreated with medium (100% ± 32.4% [mean±SD]) and LPS (99.8% ± 29.6%; p>0.999). However, in LPS/Sevo treated BMDMs, we observed a statistically significant increase in the uptake of FITC-labeled E. coli (169.9% ± 79.2%, p=0.004). In addition, in presence of the iNOS inhibitor 1400W, the increase in E. coli uptake induced by sevoflurane was abolished (Figure 7A), indicating E. coli uptake in the presence of sevoflurane was mediated by iNOS.
Figure 7.
A) Bone marrow-derived macrophages (BMDM): uptake of FITC labeled heat-inactivated Escherichia coli (E. coli). Cells were exposed to lipopolysaccharide (LPS) in the presence or absence of 2% sevoflurane for 8 hours. Thereafter, the cells were exposed to E. coli for an additional 30 minutes. Fluorescence was determined using a fluorescence microplate reader. The same experiments were performed in the presence or absence of 1400W, an inhibitor of the inducible nitric oxide synthase (iNOS), which was added for the entire incubation. Values represent means ± standard deviation; n=15 samples without 1400W and n=9 samples with 1400W; one-way ANOVA; LPS vs Sevo/LPS ** p=0.004, Sevo/LPS vs Sevo/LPS/1400W ** p=0.009. B) Mice were exposed to intraperitoneally applied lipopolysaccharide (LPS) for a total of 20 hours. Mice in the sevoflurane group received 2-hour anesthesia with 4% sevoflurane, while control animals were anesthetized with ketamine/xylazine. Thirty minutes prior to collection of peritoneal macrophages for analysis, FITC-labeled E. coli were injected intraperitoneally. Mean fluorescence intensity (MFI) of inducible nitric oxide synthase (iNOS), after staining with fluorescently-labeled antibody, was measured using flow cytometry. C) MFI of FITC-labeled E. coli using flow cytometry. Exposition as just described. For B) and C) values represent means ± standard deviation; n=6, one-way ANOVA; ** p=0.006 LPS vs Sevo/LPS, *** p=0.0003 LPS vs Sevo/LPS
Finally, we assessed whether results obtained in vitro can be observed in vivo. Six animals per group were evaluated of a total number of 31 animals included. In each group, cell count and/or assessment was not possible in 2 animals (in the sham group only one animal) due to severe blood contamination of the lavage or technical difficulties when performing cell harvest and analysis. The average weight was 27.4g. Using the LPS model of endotoxemia, macrophages iNOS expression and E. coli uptake in the peritoneal cavity was determined. Macrophages were identified as CD11c- CD11b+ F4/80+ cells31,35. Data showed that iNOS expression was very low in all 3 control groups (Sham MFI=1813,83, LPS MFI=6736, Sevo/Sham MFI=6757). In the Sevo/LPS group, iNOS expression was markedly increased by 669% (MFI=51768) compared to the LPS group (p=0.0003) (Figure 7B). To quantify the number of bacteria internalized by macrophages, we analyzed the mean fluorescence intensity (MFI) of FITC-labeled E. coli. Consistent with results of our in vitro study, we detected an increase in E. coli uptake by 49% in the Sevo/LPS treated group compared to LPS alone (LPS MFI=6756, Sevo/LPS MFI=10068; p=0.006) (Figure 7C); demonstrating that the increased uptake of E. coli by macrophages after exposure to sevoflurane is also occurring in vivo.
Discussion
The present findings demonstrate that sevoflurane differentially modulates pro-inflammatory genes in murine macrophages. While sevoflurane suppressed the expression of pro-inflammatory cytokines, it enhanced the expression of iNOS. Furthermore, sevoflurane increased the uptake of E. coli in an iNOS-dependent manner in vitro and in vivo.
This study demonstrates that endotoxin-induced release of pro-inflammatory cytokines in murine macrophage can be reduced by sevoflurane, as shown previously in other cell types4,5,10,36,37. As NF-κB is the primary mechanism associated with the regulation of pro-inflammatory genes, we assumed that sevoflurane would reduce the activation of NF-κB in BMDM, as described in other cells6–12. As we did not observe a difference in the degradation of IκBα in presence of sevoflurane, we concluded that sevoflurane might be interacting directly with NF-κB. Boost et al. postulated that sevoflurane decreases IκBα degradation upon TNF-α stimulation6. But since LPS stimulates Toll-like receptor 4 (TLR4) we concluded that it affects the TLR4 pathway differently than the TNF-α pathway, the latter with involvement of TNF receptors, TNF receptor type 1-associated death domain (TRADD) and TNF receptor-associated factor 2 (TRAF2).
Interestingly, we observed that sevoflurane affected LPS-induced expression of NF-κB-dependent genes differentially. Sevoflurane promoted the expression of iNOS, which is in agreement with previous findings3,5. As sevoflurane differentially modulates pro-inflammatory genes in murine macrophages, we concluded that different inflammatory pathways may be involved. Our data showed that sevoflurane affected the MAPK pathway by decreasing ERK phosphorylation.
This may be a potential mechanism by which sevoflurane promotes iNOS expression in murine macrophages. Further, other groups reported decreased levels of ERK phosphorylation in the presence of sevoflurane38–40 and that inhibition of ERK phosphorylation was associated with enhanced iNOS expression41. As reduced ERK phosphorylation was observed only at later time points, and decreased expression of NFκB-dependent genes was observed after only 1 hour of stimulation, we presume that the decrease in iNOS expression at the early time point may be due to a decreased in NF-κB activation during the initial phase. The question remains. Which transcription factor is involved in the increase in iNOS expression? A possible explanation is that reduced ERK phosphorylation leads to increased NF-κB activation. Bhatt et al. showed reduced ERK phosphorylation upon stimulation of the TLR2 pathway enhances NF-κB activation41. However, it remains unclear whether the same mechanism occurs with the TLR4 pathway or if a different transcription factor is involved.
This is the first study to investigate the effect of sevoflurane on uptake of heat-inactivated E. coli by murine macrophages. We observed increased uptake of E. coli after exposure to sevoflurane, and the mechanism appears to be linked to the activity of iNOS as enhanced uptake was abolished when iNOS was pharmacologically inhibited. It has been shown that NO donors increase phagocytosis42 and that inhibiting NO release with L-NAME reduces bacterial uptake and bacterial killing43. However, the role of iNOS in bacterial clearance remains controversial, as there is a report showing higher bacterial clearance and resistance to bacterial meningitis in iNOS-deficient newborn mice44. These findings are in contrast with the current findings. Further, various groups observed that iNOS knockout animals exhibit an increase in mortality induced by different sepsis models26,45–48 consistent with the idea that reactive nitrogen species play a significant role in host defense against microbes49,50.
Inducible nitric oxide synthase is an important mediator in the inflammatory response to LPS stimulation. While iNOS protein levels are decreased in the Sevo/LPS group compared to LPS alone shortly after initiating endotoxin stimulation, iNOS expression in the Sevo/LPS group exceeds the LPS values at later time points. With regard to iNOS expression sevoflurane appears to be most likely interacting with at least two different inflammatory pathways, with opposed effects on iNOS expression. This is consistent with the findings that exposure to sevoflurane leads to decreased proinflammatory cytokine expression while enhancing bactericidal properties.
Obviously, the effect of sevoflurane upregulating iNOS can be evoked by using varying exposure times to sevoflurane. This becomes evident when comparing in vitro with in vivo results (in vitro: 8 hours, in vivo: 2 hours). However, in vitro scenarios are for sure not comparable with in vivo experimental setups, the latter being more complex with many different cell types over time involved. Moreover, it is known from previous studies that sevoflurane preconditioning increases nitric oxide release51, and that conditioning with a volatile anesthetic for a short time provides long-term protection3,15,52–54.
We observed markedly increased iNOS expression upon LPS stimulation when animals were exposed to sevoflurane. Additionally, we demonstrated that exposure to sevoflurane increased the uptake of E. coli by peritoneal macrophages in vivo in mice. However, as the impact of sex on iNOS expression and uptake of E. coli was not evaluated, conclusions have to be drawn in a careful way.
Erol et al. reported that the phagocytic function of human polymorphonuclear leukocytes was not altered by sevoflurane when compared with desflurane and propofol55, whereas another study showed reduced phagocytic function of human granulocytes after exposure to sevoflurane56. Interestingly, this reduction was observed for granulocytes but not in monocytes56, implying that the effects of sevoflurane on phagocytic function may be different between granulocytes and cells of monocyte-macrophage lineage.
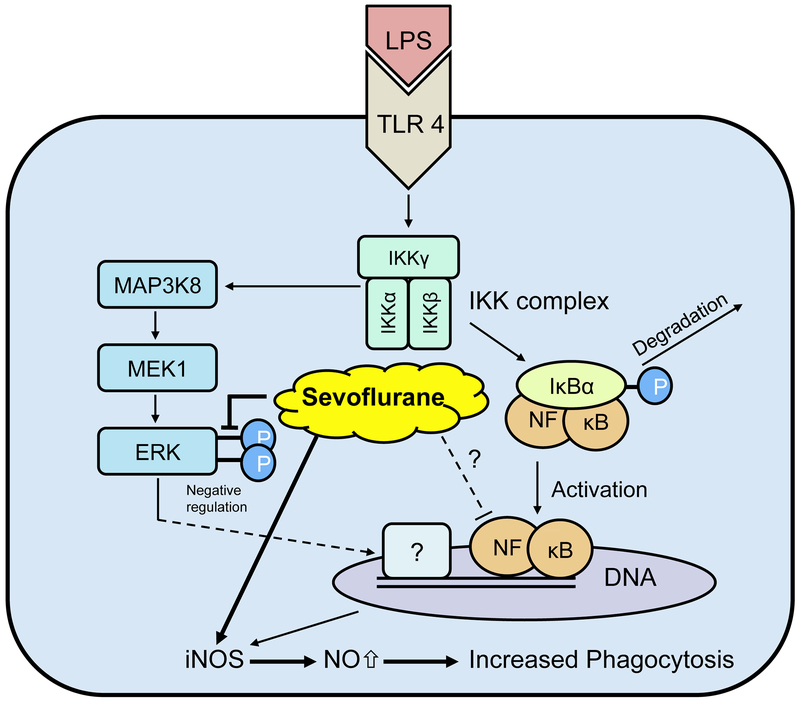
Considering these data, we provide a possible mechanism by which sevoflurane increases bacterial uptake and survival in animal models of endotoxemia/sepsis 15,16 (Figure 8). Stimulation of the TLR4 pathway with LPS leads to a well-understood inflammatory response. After stimulation of TLR4 in the plasma membrane, a signal transduction cascade is initiated which culminates in the phosphorylation of IκBα. Once IκBα is phosphorylated, it is degraded and NF-κB is no longer inhibited, enabling it to translocate to the nucleus to increase transcription of dependent genes. As sevoflurane did not affect LPS-induced IκBα degradation, we assume that sevoflurane may directly interact with the translocation of NF-κB into the nucleus or reduce transcription of NF-κB-dependent genes as highlighted in Figure 8. At later time points, sevoflurane reduced ERK phosphorylation, and this effect may be associated with an increase in iNOS expression through a mechanism that is not yet understood.
Figure 8.
Schematic illustration of proposed anti-inflammatory mechanism of action of sevoflurane. Exposure to lipopolysaccharide (LPS) leads to activation of Toll-like receptor 4 (TLR4) and initiates pro-inflammatory cascade. Degradation of IκBα was not altered by sevoflurane and thus sevoflurane may directly interact with NF-κB (highlighted with ‘?’). Sevoflurane reduces phosphorylation of extracellular signal-regulated kinases (ERK) that may cause enhanced expression of inducible nitric oxide synthase (iNOS) and increased macrophage-mediated bacterial clearance.
This study demonstrated that sevoflurane differentially modulates pro-inflammatory genes in murine BMDMs. While sevoflurane reduced LPS-induced release of pro-inflammatory cytokines, it enhanced the production of anti-microbial iNOS. Sevoflurane also increased macrophage uptake of heat-inactivated E. coli by a mechanism that seems to be linked to the activity of iNOS. These observations made in vitro were corroborated by in vivo studies that demonstrated sevoflurane promotes iNOS expression and bacterial uptake by peritoneal macrophages in the murine model of endotoxemia. These results provide an explanation, at least in part, for increased survival of septic rodents exposed to sevoflurane15,16. It remains unclear whether results observed in rodents will hold true in higher mammals such as humans.
Summary statement:
“In endotoxin-induced injury sevoflurane enhances the expression of iNOS leading to an increased uptake of E. coli by murine macrophages in vitro and in vivo.”
Acknowledgements
Funding statement: This study was supported by National Institutes of Health, Heart Lung and Blood Institute grants HL60678 and HL125356 (RDM) and from the Swiss National Science Foundation, project number 320030_160283 (BBS).
The authors kindly thank lab manager Maricela Castellon, M.S., Zhenlong Chen, Ph.D., Chenxia He, Ph.D., and André L. de Abreu, Ph.D. for technical support.
Conflicts of interests: BBS received a grant from Baxter AG, not related to this work. BBS was a participant of an Advisory Board Meeting of Baxter AG, not related to this topic. BBS chaired a session (Satellite Symposium on ‘General Anaesthesia and its effect on organ function – What do we know?) at Euroanaesthesia 2013, organized by Baxter AG. BBS is associate editor of ‘Anesthesiology’. BBS received a speaker’s fee from Abbvie, Switzerland (Pro/cons of volatile anaesthetics) for a Grand Round talk in a Swiss Hospital. BBS has a patent 04/10/14 – 20140100278: Injectable formulation for treatment and protection of patients having an inflammatory reaction or an ischemia-reperfusion event; M. Urner, L.K. Limbach, I.K. Herrmann, W.J. Stark, B. Beck Schimmer, applied as PCT (internationally), July 2009.
Footnotes
Clinical trial number and registry, not applicable
Prior Presentations: Annual Conference, Swiss Society of Anesthesiology and Resuscitation, November 4th 2016, Basel, Switzerland; College of Medicine Research Forum, University of Illinois at Chicago, November 18th 2016, Chicago, Illinois, USA; Annual Conference, International Anesthesia Research Society (IARS), May 21th 2019, Montreal, Quebec, Canada.
References
- 1.De Hert SG, Van der Linden PJ, Cromheecke S, Meeus R, Nelis A, Van Reeth V, ten Broecke PW, De Blier IG, Stockman BA, Rodrigus IE: Cardioprotective properties of sevoflurane in patients undergoing coronary surgery with cardiopulmonary bypass are related to the modalities of its administration. Anesthesiology 2004; 101: 299–310 [DOI] [PubMed] [Google Scholar]
- 2.Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW: Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology 2004; 101: 1313–24 [DOI] [PubMed] [Google Scholar]
- 3.Beck-Schimmer B, Breitenstein S, Urech S, De Conno E, Wittlinger M, Puhan M, Jochum W, Spahn DR, Graf R, Clavien PA: A randomized controlled trial on pharmacological preconditioning in liver surgery using a volatile anesthetic. Ann Surg 2008; 248: 909–18 [DOI] [PubMed] [Google Scholar]
- 4.Suter D, Spahn DR, Blumenthal S, Reyes L, Booy C, Z’Graggen B R, Beck-Schimmer B: The immunomodulatory effect of sevoflurane in endotoxin-injured alveolar epithelial cells. Anesth Analg 2007; 104: 638–45 [DOI] [PubMed] [Google Scholar]
- 5.Yue T, Roth Z’graggen B, Blumenthal S, Neff SB, Reyes L, Booy C, Steurer M, Spahn DR, Neff TA, Schmid ER, Beck-Schimmer B: Postconditioning with a volatile anaesthetic in alveolar epithelial cells in vitro. Eur Respir J 2008; 31: 118–25 [DOI] [PubMed] [Google Scholar]
- 6.Boost KA, Leipold T, Scheiermann P, Hoegl S, Sadik CD, Hofstetter C, Zwissler B: Sevoflurane and isoflurane decrease TNF-alpha-induced gene expression in human monocytic THP-1 cells: potential role of intracellular IkappaBalpha regulation. Int J Mol Med 2009; 23: 665–71 [DOI] [PubMed] [Google Scholar]
- 7.Lee HT, Chen SW, Doetschman TC, Deng C, D’Agati VD, Kim M: Sevoflurane protects against renal ischemia and reperfusion injury in mice via the transforming growth factor-beta1 pathway. Am J Physiol Renal Physiol 2008; 295: F128–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HT, Kim M, Jan M, Emala CW: Anti-inflammatory and antinecrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells. Am J Physiol Renal Physiol 2006; 291: F67–78 [DOI] [PubMed] [Google Scholar]
- 9.Lee HT, Kim M, Song JH, Chen SW, Gubitosa G, Emala CW: Sevoflurane-mediated TGF-beta1 signaling in renal proximal tubule cells. Am J Physiol Renal Physiol 2008; 294: F371–8 [DOI] [PubMed] [Google Scholar]
- 10.Sun XJ, Li XQ, Wang XL, Tan WF, Wang JK: Sevoflurane inhibits nuclear factor-kappaB activation in lipopolysaccharide-induced acute inflammatory lung injury via toll-like receptor 4 signaling. PLoS One 2015; 10: e0122752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe K, Iwahara C, Nakayama H, Iwabuchi K, Matsukawa T, Yokoyama K, Yamaguchi K, Kamiyama Y, Inada E: Sevoflurane suppresses tumour necrosis factor-alpha-induced inflammatory responses in small airway epithelial cells after anoxia/reoxygenation. Br J Anaesth 2013; 110: 637–45 [DOI] [PubMed] [Google Scholar]
- 12.Zhong C, Zhou Y, Liu H: Nuclear factor kappaB and anesthetic preconditioning during myocardial ischemia-reperfusion. Anesthesiology 2004; 100: 540–6 [DOI] [PubMed] [Google Scholar]
- 13.Schlapfer M, Leutert AC, Voigtsberger S, Lachmann RA, Booy C, Beck-Schimmer B: Sevoflurane reduces severity of acute lung injury possibly by impairing formation of alveolar oedema. Clin Exp Immunol 2012; 168: 125–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kellner P, Muller M, Piegeler T, Eugster P, Booy C, Schlapfer M, Beck-Schimmer B: Sevoflurane Abolishes Oxygenation Impairment in a Long-Term Rat Model of Acute Lung Injury. Anesth Analg 2017; 124: 194–203 [DOI] [PubMed] [Google Scholar]
- 15.Herrmann IK, Castellon M, Schwartz DE, Hasler M, Urner M, Hu G, Minshall RD, Beck-Schimmer B: Volatile anesthetics improve survival after cecal ligation and puncture. Anesthesiology 2013; 119: 901–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlapfer M, Piegeler T, Dull RO, Schwartz DE, Mao M, Bonini MG, Z’Graggen BR, Beck-Schimmer B, Minshall RD: Propofol increases morbidity and mortality in a rat model of sepsis. Crit Care 2015; 19: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visvabharathy L, Xayarath B, Weinberg G, Shilling RA, Freitag NE: Propofol Increases Host Susceptibility to Microbial Infection by Reducing Subpopulations of Mature Immune Effector Cells at Sites of Infection. PLoS One 2015; 10: e0138043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kowalski C, Zahler S, Becker BF, Flaucher A, Conzen PF, Gerlach E, Peter K: Halothane, isoflurane, and sevoflurane reduce postischemic adhesion of neutrophils in the coronary system. Anesthesiology 1997; 86: 188–95 [DOI] [PubMed] [Google Scholar]
- 19.Mobert J, Zahler S, Becker BF, Conzen PF: Inhibition of neutrophil activation by volatile anesthetics decreases adhesion to cultured human endothelial cells. Anesthesiology 1999; 90: 1372–81 [DOI] [PubMed] [Google Scholar]
- 20.Muller-Edenborn B, Frick R, Piegeler T, Schlapfer M, Roth-Z’graggen B, Schlicker A, Beck-Schimmer B: Volatile anaesthetics reduce neutrophil inflammatory response by interfering with CXC receptor-2 signalling. Br J Anaesth 2015; 114: 143–9 [DOI] [PubMed] [Google Scholar]
- 21.Tyther R, O’Brien J, Wang J, Redmond HP, Shorten G: Effect of sevoflurane on human neutrophil apoptosis. Eur J Anaesthesiol 2003; 20: 111–5 [DOI] [PubMed] [Google Scholar]
- 22.Wynn TA, Chawla A, Pollard JW: Macrophage biology in development, homeostasis and disease. Nature 2013; 496: 445–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray PJ, Wynn TA: Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011; 11: 723–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janeway CA Jr, Travers P, Walport M, Shlomchik MJ: Immunobiology: The Immune System in Health and Disease. 5th edition., 5th edition. New York, Garland Science, 2001 [Google Scholar]
- 25.Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, van Rooijen N, Mempel TR, Whelan SP, von Andrian UH: Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 2007; 450: 110–4 [DOI] [PubMed] [Google Scholar]
- 26.Baig MS, Zaichick SV, Mao M, de Abreu AL, Bakhshi FR, Hart PC, Saqib U, Deng J, Chatterjee S, Block ML, Vogel SM, Malik AB, Consolaro ME, Christman JW, Minshall RD, Gantner BN, Bonini MG: NOS1-derived nitric oxide promotes NF-kappaB transcriptional activity through inhibition of suppressor of cytokine signaling-1. J Exp Med 2015; 212: 1725–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelm M, Feelisch M, Deussen A, Schrader J, Strauer BE: The Role of Nitric-Oxide in the Control of Coronary Vascular Tone in Relation to Partial Oxygen-Pressure, Perfusion-Pressure, and Flow. Journal of Cardiovascular Pharmacology 1991; 17: S95–S991723129 [Google Scholar]
- 28.Ninkovic J, Roy S: High throughput fluorometric technique for assessment of macrophage phagocytosis and actin polymerization. J Vis Exp 2014: e52195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hed J: Methods for distinguishing ingested from adhering particles. Methods Enzymol 1986; 132: 198–204 [DOI] [PubMed] [Google Scholar]
- 30.Scott AJ, Woods JP: Monitoring internalization of Histoplasma capsulatum by mammalian cell lines using a fluorometric microplate assay. Med Mycol 2000; 38: 15–22 [DOI] [PubMed] [Google Scholar]
- 31.Ghosn EE, Cassado AA, Govoni GR, Fukuhara T, Yang Y, Monack DM, Bortoluci KR, Almeida SR, Herzenberg LA: Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci U S A 2010; 107: 2568–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redel A, Stumpner J, Tischer-Zeitz T, Lange M, Smul TM, Lotz C, Roewer N, Kehl F: Comparison of isoflurane-, sevoflurane-, and desflurane-induced pre- and postconditioning against myocardial infarction in mice in vivo. Exp Biol Med (Maywood) 2009; 234: 1186–91 [DOI] [PubMed] [Google Scholar]
- 33.Ray A, Dittel BN: Isolation of mouse peritoneal cavity cells. J Vis Exp 2010; 35: 1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cruz MT, Duarte CB, Goncalo M, Carvalho AP, Lopes MC: LPS induction of I kappa B-alpha degradation and iNOS expression in a skin dendritic cell line is prevented by the janus kinase 2 inhibitor, Tyrphostin b42. Nitric Oxide 2001; 5: 53–61 [DOI] [PubMed] [Google Scholar]
- 35.Johnson TE, Michel BA, Meyerett C, Duffy A, Avery A, Dow S, Zabel MD: Monitoring immune cells trafficking fluorescent prion rods hours after intraperitoneal infection. J Vis Exp 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Gonzalez R, Baluja A, Veiras Del Rio S, Rodriguez A, Rodriguez J, Taboada M, Brea D, Alvarez J: Effects of sevoflurane postconditioning on cell death, inflammation and TLR expression in human endothelial cells exposed to LPS. J Transl Med 2013; 11: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steurer M, Schlapfer M, Steurer M, Z’Graggen B R, Booy C, Reyes L, Spahn DR, Beck-Schimmer B: The volatile anaesthetic sevoflurane attenuates lipopolysaccharide-induced injury in alveolar macrophages. Clin Exp Immunol 2009; 155: 224–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim SH, Li M, Pyeon TH, So KY, Kwak SH: The volatile anesthetic sevoflurane attenuates ventilator-induced lung injury through inhibition of ERK½ and Akt signal transduction. Korean J Anesthesiol 2015; 68: 62–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiklund A, Gustavsson D, Ebberyd A, Sundman E, Schulte G, Jonsson Fagerlund M, Eriksson LI: Prolonged attenuation of acetylcholine-induced phosphorylation of extracellular signal-regulated kinase ½ following sevoflurane exposure. Acta Anaesthesiol Scand 2012; 56: 608–15 [DOI] [PubMed] [Google Scholar]
- 40.Yufune S, Satoh Y, Akai R, Yoshinaga Y, Kobayashi Y, Endo S, Kazama T: Suppression of ERK phosphorylation through oxidative stress is involved in the mechanism underlying sevoflurane-induced toxicity in the developing brain. Sci Rep 2016; 6: 21859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhatt KH, Sodhi A, Chakraborty R: Role of mitogen-activated protein kinases in peptidoglycan-induced expression of inducible nitric oxide synthase and nitric oxide in mouse peritoneal macrophages: extracellular signal-related kinase, a negative regulator. Clin Vaccine Immunol 2011; 18: 994–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Boo S, Kopecka J, Brusa D, Gazzano E, Matera L, Ghigo D, Bosia A, Riganti C: iNOS activity is necessary for the cytotoxic and immunogenic effects of doxorubicin in human colon cancer cells. Mol Cancer 2009; 8: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai WC, Strieter RM, Zisman DA, Wilkowski JM, Bucknell KA, Chen GH, Standiford TJ: Nitric oxide is required for effective innate immunity against Klebsiella pneumoniae. Infect Immun 1997; 65: 1870–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mittal R, Gonzalez-Gomez I, Goth KA, Prasadarao NV: Inhibition of inducible nitric oxide controls pathogen load and brain damage by enhancing phagocytosis of Escherichia coli K1 in neonatal meningitis. Am J Pathol 2010; 176: 1292–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N, Chen H, Mudgett JS: Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 1995; 81: 641–50 [DOI] [PubMed] [Google Scholar]
- 46.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF: Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci U S A 1997; 94: 5243–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mastroeni P, Vazquez-Torres A, Fang FC, Xu Y, Khan S, Hormaeche CE, Dougan G: Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J Exp Med 2000; 192: 237–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miki S, Takeyama N, Tanaka T, Nakatani T: Immune dysfunction in endotoxicosis: role of nitric oxide produced by inducible nitric oxide synthase. Crit Care Med 2005; 33: 716–20 [DOI] [PubMed] [Google Scholar]
- 49.Fang FC: Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol 2004; 2: 820–32 [DOI] [PubMed] [Google Scholar]
- 50.Flannagan RS, Cosio G, Grinstein S: Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol 2009; 7: 355–66 [DOI] [PubMed] [Google Scholar]
- 51.Novalija E, Fujita S, Kampine JP, Stowe DF: Sevoflurane mimics ischemic preconditioning effects on coronary flow and nitric oxide release in isolated hearts. Anesthesiology 1999; 91: 701–12 [DOI] [PubMed] [Google Scholar]
- 52.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL: Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006; 311: 1770–3 [DOI] [PubMed] [Google Scholar]
- 53.Yamada Y, Laube I, Jang JH, Bonvini JM, Inci I, Weder W, Beck Schimmer B, Jungraithmayr W: Sevoflurane preconditioning protects from posttransplant injury in mouse lung transplantation. J Surg Res 2017; 214: 270–277 [DOI] [PubMed] [Google Scholar]
- 54.Zhao J, Wang F, Zhang Y, Jiao L, Lau WB, Wang L, Liu B, Gao E, Koch WJ, Ma XL, Wang Y: Sevoflurane preconditioning attenuates myocardial ischemia/reperfusion injury via caveolin-3-dependent cyclooxygenase-2 inhibition. Circulation 2013; 128: S121–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erol A, Reisli R, Reisli I, Kara R, Otelcioglu S: Effects of desflurane, sevoflurane and propofol on phagocytosis and respiratory burst activity of human polymorphonuclear leucocytes in bronchoalveolar lavage. Eur J Anaesthesiol 2009; 26: 150–4 [DOI] [PubMed] [Google Scholar]
- 56.Fahlenkamp AV, Coburn M, Rossaint R, Stoppe C, Haase H: Comparison of the effects of xenon and sevoflurane anaesthesia on leucocyte function in surgical patients: a randomized trial. Br J Anaesth 2014; 112: 272–80 [DOI] [PubMed] [Google Scholar]