ABSTRACT
Background
Individuals with Parkinson's disease often experience periods of off time when their motor symptoms are poorly controlled, significantly impacting their lives.
Objectives
To identify the consequences of motor fluctuations on day‐to‐day activities and areas of unmet treatment priority among individuals with moderate to advanced Parkinson's disease, to assess whether existing patient‐reported outcome instruments adequately capture these consequences and priorities, and based on these evaluations, to adapt an existing or develop a new instrument.
Methods
The research was conducted in 2 stages: concept exploration and content confirmation. Concept exploration included direct input from individuals with Parkinson's disease representing the intended context of use via concept elicitation interviews. Content confirmation and item refinement was achieved through 5 rounds of cognitive debriefing. Final rounds of cognitive debriefing also included usability testing of the draft instrument for electronic data capture.
Results
Concept elicitation interviews were conducted among 29 individuals with Parkinson's disease (55% male; mean age 60.8 years). Concept saturation was achieved quickly with more than 90% of concepts identified by the end of the 16th interview. None of the existing outcome instruments were found to be fit for purpose in the intended context of use; therefore, a new instrument was developed. After 5 rounds, cognitive debriefing participants indicated clear and consistent interpretation of the items.
Conclusions
Evidence from this study supports the content validity of the Parkinson's Disease Activities of Daily Living, Interference and Dependence Instrument as the basis of a clinical trial endpoint for capturing priority treatment benefit outcomes to individuals with moderate to advanced Parkinson's disease experiencing motor fluctuations.
Keywords: Parkinson's disease, motor fluctuations, patient‐reported outcomes, activities of daily living, independence
Parkinson's disease (PD) is a progressive neurodegenerative disorder that affects more than 10 million people worldwide.1 There is no proven neuroprotective or disease‐modifying therapy. Symptoms vary by individual and state of disease progression; both motor and nonmotor functioning are impacted. The most common motor symptoms include tremor, bradykinesia, rigidity or muscle stiffness that can be painful and limit range of motion, and balance problems.2 PD can significantly affect a patient's health‐related quality of life. In particular, individuals with PD frequently express frustration with the day‐to‐day unpredictability in symptoms.3
The gold standard for symptomatic treatment of moderate to advanced PD is dopamine replacement using levodopa coupled with carbidopa. Therapy is taken multiple times per day and associated with a range of adverse effects with long‐term use.4 These include daily fluctuations in efficacy, resulting in periods of off time during which symptoms are poorly controlled4 and dyskinesia, or uncontrollable movement affecting one or more body parts.5 Over time, the inadequate control of motor symptoms leads to significant impacts on a patient's ability to perform the basic activities of daily living (ADLs).6
The objectives of this study were to identify important consequences to day‐to‐day functioning resulting from PD and areas of unmet priority related to treatment for individuals with moderate to advanced PD experiencing motor fluctuations (the target population), assess whether existing patient‐reported outcome (PRO) instruments adequately capture these consequences and unmet priorities and whether they meet the evidentiary requirements of the U.S. Food and Drug Administration (FDA) Guidance for Industry Patient‐Reported Outcome Measures,7 and based on these evaluations, adapt or develop de novo a PRO instrument for electronic implementation. The intended context of use (COU) for the PRO is as a clinical trial treatment benefit outcome associated with improved consistency of treatment effect for individuals with moderate to advanced PD experiencing motor fluctuations.
Methods
The research was conducted in 2 stages: concept exploration and content confirmation (Fig. 1). Following the FDA PRO guidance, concept exploration included direct input from individuals representing the intended COU via concept elicitation (CE) interviews. Content confirmation and item refinement was achieved through cognitive debriefing (CD).8 The final rounds of CD also included usability testing (UT) of the draft instrument as a mobile application for data capture. CE, CD, and CD/UT interviews were conducted as cross‐sectional, noninterventional, qualitative one‐on‐one interviews with individuals representing the target population.
Figure 1.
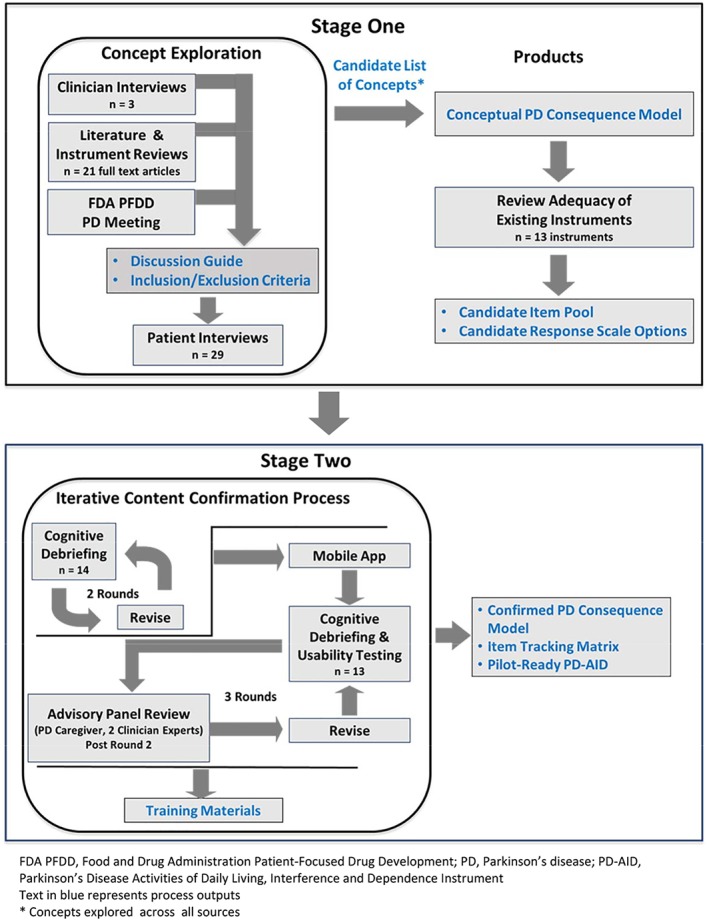
Schematic showing the details of the 2‐stage research process. Text in blue represents process outputs. FDA, Food and Drug Administration; PD, Parkinson's disease; PD‐AID, Parkinson's Disease Activities of Daily Living, Interference and Dependence Instrument; PFDD, patient‐focused drug development. *Concepts explored across all sources.
Concept Exploration
Activities in this stage were designed to explore the consequences of PD and PD treatment. Particular areas of interest were to identify unmet needs and areas of priority for individuals with PD and evaluate the suitability of existing instruments for the intended COU. To be certain that conceptual breadth was adequately explored and the relative importance of concepts across sources confirmed, these methods incorporated triangulation across sources (ie, literature, clinical experts, patients).
Clinical Expert Interviews
Three clinical experts in the field of neurology, whose identity was blinded to the study team, were interviewed to gain a clinical perspective on and an improved understanding of the consequences of PD. Motor symptoms were of particular interest in terms of impact on day‐to‐day functioning. The impact of medications was also discussed. The interviews lasted up to an hour and were conducted by telephone using a semistructured interview guide.
Literature Review
A targeted review was conducted to identify concepts reported in the literature on the consequences and functional impacts related to the symptoms and management of PD as reported by patients. The search was conducted in 2016 using MEDLINE and Embase databases and was limited to English‐language papers and human adult studies published since the year 2000 or seminal articles published before 2000.
Conceptual PD Consequence Model
The findings from the expert interviews, literature review, and the September 22, 2015, FDA patient‐focused drug development meeting on PD3 informed a draft conceptual PD consequence model (Fig. 2). CE interviews served to refine and confirm the model.
Figure 2.
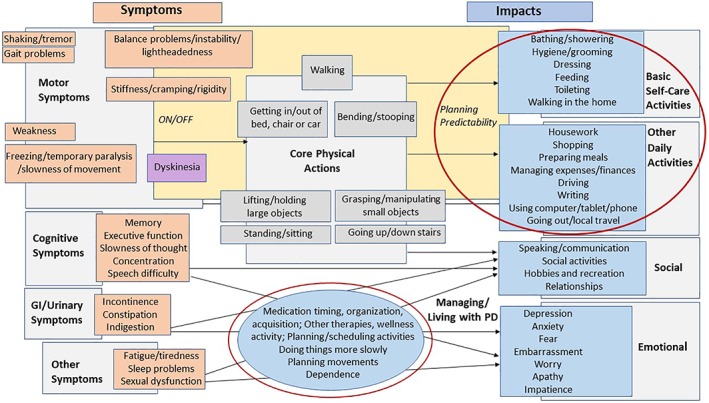
Conceptual PD consequence model illustrating the 5 domains (ie, core physical actions, basic self‐care activities, other daily activities, social impacts, and emotional impacts) and activities most impacted by PD as expressed during the concept elicitation interviews. PD, Parkinson's disease.
Patient CE Interviews
To be eligible to participate in the CE interviews, individuals were required to be male or female and aged 45 to 85 years with a physician‐confirmed diagnosis of PD and a self‐reported Hoehn and Yahr9 stage ≤3. The participants were required to be currently using levodopa ≥4 times daily and responding, be able to recognize levodopa “wearing off,” and have an average self‐reported off time ≥2.5 hours daily or ≥2 hours off time based on clinician review of patient profile and number of daily medications for PD. The ability to read and understand English was necessary, as was a willingness and ability to participate in a 60‐ to 90‐minute audio‐recorded and transcribed interview about their current and past PD symptoms. Individuals with a history of surgical intervention for PD were excluded.
Candidate participants were identified by a recruitment agency via their internal database, patient panels, and physician referral. Effort was made to recruit geographically diverse participants who were clinically and demographically representative of the intended COU. All interviews were conducted by researchers trained in qualitative interview methods and familiar with the study objectives. Participants were recruited from the following: Los Angeles and San Francisco, CA; Miami, FL; Chicago, IL; Gaithersburg, MD; Boston, MA; New York City, NY; and, Dallas, TX.
Findings from the expert interviews, literature review, and the FDA patient‐focused drug development meeting informed the development of a CE protocol and patient interview guide. Interviews were then conducted with patient participants to gather information about the consequential impacts of PD on their lives and explore and prioritize areas of unmet need. During the CE interviews, the participants were asked open‐ended questions about their PD symptoms and the effect on their lives and day‐to‐day functioning. They were also asked to rank their symptoms and impacts based on the level of interference in day‐to‐day life. Follow‐up probing was based on impacts gleaned from the literature review, expert interviews, and a detailed report summarizing the transcripts and recordings from the FDA patient‐focused drug development meeting on PD. The interview format was semistructured and flexible so that topics could be covered as they emerged organically as well as generate new topics not covered in the interview guide. Participants were compensated following the interviews.
Instrument Review
The conceptual PD consequence model was used to identify target concepts representing unmet treatment priorities for the intended COU. These concepts were then compared with measures identified in an instrument review to determine whether to revise an existing instrument or develop one de novo. The existing instruments were also reviewed against the evidence requirements of the FDA PRO guidance for industry to support their content validity (ie, evidence of PD patient involvement in their development) and fit‐for‐purpose evidence for the intended COU.
Content Confirmation
The results from the concept exploration stage provided the basis for a decision to develop a de novo instrument. The principles for developing the de novo instrument item content appear in the results.
CD Interviews and UT
A total of 5 iterative rounds of CD interviews8 were conducted to confirm item content of the draft instrument, thus allowing for refinements to be made between rounds with retesting of changes in subsequent rounds. The first 2 rounds involved CD only; the last 3 rounds combined CD and UT of a mobile electronic version of the draft instrument. A caregiver of a PD participant and 2 clinicians comprised an advisory panel that reviewed the draft instrument and provided feedback.
For each round, a cohort of participants with no prior exposure to the draft instrument was asked to complete it. Following completion of the instrument, the CD and CD/UT interviews were conducted using a semistructured interview guide where participants were asked to think aloud and articulate their understanding of instructions for completion, express in their own words the intent of individual items, and share their thought process behind retrieving information to provide a response to the items. Finally, once the information for responding was in mind, interviewers observed any ease or difficulty participants had with selecting a response on the candidate scales. For CD/UT, the participants were also asked a series of questions related to the usability of the mobile application.
Data Analysis
Descriptive statistics were used to summarize the sociodemographic and clinical characteristics of the PD participants. Deidentified transcripts from all of the interviews were produced for analysis.
For the CE interview data, a content and thematic analysis was conducted, according to the methodology described by Joffe and Yardley.10 A codebook was developed in which unique codes were assigned to emerging concepts based on the interview guide. Two coders independently assigned codes to the transcripts from early interviews. Discrepancies that required adjudication were addressed by a coding lead, who conducted many of the interviews and was familiar with the data. During this process, the codebook was refined to account for emerging themes and concepts not captured initially. MAXQDA (version 11.0.4; VERBI GmbH, Berlin, Germany) was used to facilitate the qualitative data analysis.
CD and CD/UT interview data were analyzed iteratively using an approach designed to identify issues of ambiguity in interpretation at the item level, difficulty selecting a response, and issues related to administration, including instructions, branching patterns, and usability of the mobile app. As with the CE interviews, the analysis was facilitated by a codebook as well as independent verification of code application across analysts. The evolution of the instrument based on the CD and CD/UT analyses was documented using an item tracking matrix that recorded modifications to the instrument, when they were made, and the rationale for the modifications.
Results
Concept Exploration
Clinical Expert Interviews
All 3 clinical experts reported that PD affects nearly all motor activity, with hallmark signs and symptoms such as bradykinesia, tremor, and unpredictable stiffness. Furthermore, they reported that motor symptom fluctuation is a pattern of PD itself that interacts with the pattern of levodopa's efficacy to produce on and off periods in terms of symptom alleviation and that could become less predictable with disease progression and further complicated by dyskinesia. There was consensus among the clinicians that increased on time as well as predictability of symptom control coupled with a reduction in dyskinetic side effects represented the greatest unmet needs regarding current therapies used to treat PD motor symptoms outside of disease modification and slowing of progression.
Literature Review
After removing duplicates and targeting the most relevant citations, 49 abstracts were reviewed; 21 full‐text articles were obtained for data extraction. The literature showed that PD impacts almost every facet of patients’ day‐to‐day lives and ADLs. Furthermore, the findings suggested that motor symptom impacts were the result not only of the symptoms themselves but also the unpredictable daily variations in symptoms, off periods, and the duration of medication effects.
Protocol and Other Changes
To meet sample size requirements, the following revisions were made to the original screening criteria: reported off time was reduced from 2.5 to 2 hours and the frequency of levodopa medication was reduced from ≥4 to ≥3 daily. In addition, clinician review of additional clinical characteristics (eg, time since diagnosis and levodopa dose) was considered along with the revised criteria to confirm eligibility. During early CE interviews, 9 participants were included based on self‐reported eligibility information, but during the interview, information emerged suggesting discrepancies, for example, that these participants were not eligible either because they had <2 hours of off time, took levodopa <4 times per day, had a history of deep brain stimulation surgery, or were not well enough to participate. Analyses were conducted both with and without the data for these participants to explore any impact on results.
Demographics and Clinical Characteristics
In all, 29 individuals from the target PD population participated in the CE interviews (55% male; mean age 68.0 years; 90% white); 3 also participated in CD interviews. Participants reported an average of 4.8 years on a levodopa therapy with a current prescription load of 2 PD‐related medications. The demographic and clinical characteristics were similar regardless of the screening criteria used (original or revised) as well as for participants whose responses were discrepant (Table 1).
Table 1.
Participant demographics and clinical characteristics
| CHARACTERISTIC | CE SAMPLE, N = 29* | CD (N = 14) AND CD/UT (N = 13) SAMPLEs, N = 27 |
|---|---|---|
| Sex, n (%) | ||
| Male | 16 (55) | 11 (41) |
| Female | 13 (45) | 16 (59) |
| Age, y, mean (range) | 68.0 (49–81) | 65.4 (46–80) |
| Race, n (%) | ||
| White | 26 (90) | 27 (100) |
| Black | 2 (7) | 0 |
| Asian | 1 (3) | 0 |
| Ethnicity, n (%)a | ||
| Hispanic | 1 (3) | 0 |
| Non‐Hispanic | 27 (93) | 27 (100) |
| Employment, n (%) | ||
| Full‐time | 2 (7) | 7 (26) |
| Part‐time | 5 (17) | 6 (22) |
| Retired | 20 (69) | 13 (48) |
| Homemaker | 1 (3) | 1 (4) |
| Disabled | 1 (3) | 0 |
| Years since diagnosis, mean (range) | 6.5 (1–15) | 8.2 (1–20) |
| Years on levodopa, mean (range) | 4.8 (1–11) | 7.7 (1–15) |
| No. of current PD medications, mean (range) | 2.1 (1–6) | 2.4 (1–4) |
| Dyskinesia, n (%) | ||
| YES | 4 (14) | 17 (63) |
| No | 25 (86) | 10 (37) |
CE total includes 3 individuals who participated in combined CE/CD interviews.
Missing values.
CE, concept elicitation; CD, cognitive debriefing; UT, usability testing; PD, Parkinson's disease.
CE Interviews
Overall, the 29 participants spontaneously reported 97 key consequence concepts. Saturation, the point in the interview process when participant interviews are no longer yielding new information,11 was achieved quickly with more than 90% of concepts (88 of 97) identified by the end of the 16th interview; 95% of concepts (93/97) were identified by the end of the 17th interview, and 100% of concepts were identified by the end of the 25th interview. Despite eligibility discrepancies for 9 of the CE participants, the concepts that emerged from these interviews were comparable with those identified by the 20 CE participants who met the eligibility requirements. When only participants meeting the original or revised eligibility criteria were considered, complete saturation was achieved after the 18th interview. The participants in the discrepant group did not identify any novel concepts.
The following 5 major domains emerged from the CE interviews: core physical actions, basic self‐care activities, other daily activities, social impacts, and emotional impacts (Fig. 2). The core physical actions most affected included walking (76% of participants), standing (69%), lifting objects (66%), and getting into and out of bed (66%), some of which required assistance from another person or the use of an assistive device.
The most commonly reported basic self‐care activities affected by PD symptoms were bathing/showering (69% of participants), dressing (66%), and grooming/personal hygiene (59%). Other activities reported as being impacted by PD symptoms included driving (86% of participants), preparing food (62%), shopping (59%), and writing (59%). The participants reported that they often needed additional time to complete their daily activities because of slowness in mental processing and/or movement. The most frequently reported emotional impacts were anxiety/worry/fear (93% of participants), frustration (69%), and lack of motivation/apathy (48%).
More than half of the participants interviewed reported tiring easily (62%), loss of independence (59%), and slowing down (55%) as key aspects of living with and managing their disease. Most participants reported a constant awareness of their PD, having to think about everything they do, and a need to manage their disease during the entire course of the day. All of the participants viewed less off/more on time, even minimal amounts, as meaningful and beneficial.
There was a general consistency across all sources that PD leads to limitations in core physical actions stemming from motor symptoms (Table 2). These are wide‐ranging and varied, affecting nearly all aspects of the participants’ daily lives from basic self‐care to social activity. Disease management was shown to be correspondingly burdensome, requiring adjustment and planning around medication and symptoms alike to accomplish day‐to‐day activities and maintain independence. The burden of PD management was related to managing both symptoms and medication, considered significant and variably experienced throughout the day with impacts reflecting participants’ individual schedules and responsibilities.
Table 2.
Concepts elicited across all sources
| Concept | Participants, N = 29 | Clinician Experts, N = 3 | Qualitative Literature, N = 10* | Existing Instruments, N = 13** |
|---|---|---|---|---|
| Core self‐care activities | ||||
| Bathing/showering | 81% | 2 | 2 | 4 |
| Getting dressed/undressed | 73% | 1 | 2 | 7 |
| Hygiene/grooming | 65% | 3 | 1 | 6 |
| Walking in the home | 81% | 3 | – | 3 |
| Getting in/out of bed | 73% | 3 | – | 3 |
| Feeding yourself | 46% | 3 | 2 | 6 |
| Getting on/off the toilet | 42% | 2 | 1 | 3 |
| Other ADLs | ||||
| Driving | 92% | 3 | 1 | 2 |
| Shopping | 65% | 2 | 1 | 2 |
| Preparing food | 69% | 3 | 1 | 3 |
| Household activities | 62% | 1 | 2 | 6 |
| Working | 50% | 2 | – | – |
| Getting in/out of vehicle | 46% | – | – | 2 |
| Using a computer/tablet/phone | 54% | 3 | 1 | 2 |
| General activity | 38% | – | 6 | – |
| Interpersonal/social | ||||
| Relationship with others (not spouse/partner) | 81% | 2 | 1 | 2 |
| Relationship with spouse/partner | 62% | 2 | 1 | 2 |
| Social activities | 46% | 3 | 4 | 5 |
| Writing | 62% | – | 1 | 6 |
| Talking/communicating with others | 35% | 3 | 2 | 6 |
| Emotion | ||||
| Anxiety | 92% | 3 | 2 | 4 |
| Frustration | 73% | 2 | – | – |
| Depression | 46% | 2 | 4 | 2 |
| Isolation | 42% | 2 | – | 1 |
| Embarrassing | 38% | 2 | – | 1 |
| Fear of falling | 24% | – | – | 1 |
| Managing/living with PD | ||||
| Dependence | 65% | 2 | 3 | 5 |
| Slowness/taking longer | 62% | 2 | 3 | 6 |
| Unpredictability/planning | 27% | 3 | 1 | – |
These 10 articles plus another 11 on PRO instruments make up the 21 full‐text articles reviewed during the concept exploration stage.
8 of the 13 PROs reviewed during the concept exploration stage were further evaluated for fit‐for‐purpose adequacy for stated context of use; only 5 were found to be both motor and PD specific.
ADLs, activities of daily living; PD, Parkinson's disease; PROs, patient‐reported outcomes.
Conceptual PD Consequence Model
The resulting conceptual PD consequence model (Fig. 2) positions motor symptoms among other PD symptoms and shows key impacts resulting from impairment to specific categories of physical action. The model served as the basis for identifying the concepts that represent prioritized areas of unmet need (red circles) by patients. Specifically, we identified impacts that represented consequences of inconsistent motor symptom control as this was our intended COU.
Instrument Review
A total of 13 PROs were identified as potential candidates for addressing the target concepts; however, only the following 5 were both specific to PD and designed to assess motor symptom‐based impacts on functioning:
Self‐Assessment Parkinson's Disease Disability Scale12
PD Quality of Life Scale13
PD Activities of Daily Living Scale14
Movement Disorder Society–Unified Parkinson's Disease Rating Scale15, 16
Parkinson's Disease Questionnaire17
When reviewed against the evidentiary recommendations of the FDA PRO guidance for industry, all 5 instruments were found to have gaps or limitations. Most lacked evidence of content validity (ie, direct input from individuals representative of the target population) as well as adequate fit‐for‐purpose evidence for the intended COU. Recall periods were either unspecified or were not appropriate for measuring daily activity. Although those instruments were specific to motor symptoms, none of them captured ADL impacts consequential to the physical actions that are affected. Importantly, none of the measures reviewed assessed disease management and/or planning regarding medication wearing off. Consequently, the existing measures were determined not to be fit for purpose to capture everyday life impact in the target population, and a decision was made to develop a de novo instrument.
De Novo Instrument Item Generation
The previous results provided the basis for employing the following principles in drafting the de novo instrument content. Items representing unmet treatment benefit, as prioritized by the PD participants, and that met all or most of the criteria listed below were considered candidates for item generation:
Day‐to‐day life activity that would give meaning to physical limitations (eg, focus on activities rather than physical actions, such as bending, gripping, standing)
Commonly reported and considered important by participants
Primarily motor symptom related
Could improve with treatment within the timeframe of a clinical trial
Not or inadequately covered by existing instruments
Items were developed using language employed by the CE participants and then grouped by domain in accordance with the conceptual PD consequence model (ie, basic self‐care activities, other daily activities, social impacts, emotional impacts, and disease management‐related impacts). Recall and response scale candidates were required to relate naturally to the manner in which CE participants experienced and described their PD. Activity‐related items were designed to assess the level of difficulty in performing the activity, thus the response scale was designed to capture levels reflecting no difficulty to extreme difficulty using a verbal rating scale. All items were drafted to capture difficulty/impact during the previous 24‐hour period. Following CE and item generation, a draft PRO instrument was created for CD interviews and later tested for usability with additional PD participants.
Content Confirmation
A total of 27 patients (59% female; mean age 65.4 years) participated in this stage of the research: 14 participated in the CD interviews and 13 in combined CD/UT interviews. The sample was entirely white and non‐Hispanic. The participants were diagnosed, on average, about 8 years prior to the study and took an average of 2.4 medications to treat their PD (Table 1).
The following 2 major structural changes were implemented as a result of CD: the instrument was split into a morning and evening diary for twice‐daily completion and branching logic was added for all activity items. The decision to split the instrument was primarily driven by the fact that a key area of unmet need is reducing off time, which CD participants described as most often experienced upon waking, thus affecting morning ADLs differently from the routine ADLs during the remainder of the day. The split was also implemented to minimize difficulties with recall because of diminished working memory associated with PD.
Branching logic was introduced for the activity items to encourage participants to focus their response on the initial gate questions, which were meant to ascertain whether an activity was performed with or without someone's assistance or not at all. Those reporting that no assistance was needed for a particular activity were asked about the level of difficulty in performing the activity independently as discrete levels. Those who did not perform the activity were asked whether this was a result of PD or other reasons. The phrase “on your own” was incorporated in the difficulty items’ question stem to further emphasize that the response was specific to performing the activity independently. By the final rounds, the participants reported few issues with item phrasing and the response scales, indicating clear and consistent interpretation across items and alignment with item development intentions. The participants generally found the electronic mobile application easy to use.
Feedback from the caregiver's review reinforced key findings from the CE stage of the study and the general approach taken regarding the development of an instrument measuring meaningful day‐to‐day PD consequences resulting from motor function impacts. Both clinician advisors noted that some of the content of the instrument was already captured in the existing measures, although they acknowledged that daily administration was unique to this new instrument. One agreed that the “management” items in the newly developed instrument were important and unique in capturing treatment benefit, beyond the concepts captured in other measures.
After completing both stages of qualitative development, the electronic Parkinson's Disease Activities of Daily Living, Interference and Dependence Instrument (PD‐AID) contained 11 items for daily completion in the morning and 18 for daily completion in the evening. The proposed COU for this novel PRO is as an instrument from which to define clinical trial endpoints for assessing treatment benefit to basic ADLs (which are a direct and proximal consequence of motor function impacts), interference as a result of the disease, and dependence on others for individuals with moderate to advanced PD experiencing motor fluctuations.
Conclusion
The PD‐AID was developed for use in a clinical setting as a mobile application for twice daily completion to assess clinical benefit from the patient perspective (Supplementary Fig. S1). Specifically, the instrument targets concepts representing consequential everyday life impacts, that is, concepts that give meaning to PD motor symptoms in terms of their impact on everyday life activities.18 The basic ADLs addressed in the PD‐AID include transferring in and out of bed, on and off the toilet, in and out of a vehicle, moving about the home, bathing/showering, dressing, grooming, preparing meals, feeding one's self, and use of electronic touchscreens, a mouse, or keyboard. Because each activity is gated to ascertain whether the patient can perform it with or without assistance from another person, these gate questions will facilitate the creation of a dependence index. There are also morning and evening self‐assessment questions addressing overall dependence on others. Finally, interference with everyday life is assessed with 2 questions in the morning diary and 8 questions in the evening diary. The morning diary questions address delay to activities as a result of PD and how much PD interfered with getting ready for the day. The evening diary questions address PD interference with work (if employed) and leisure activities as well as the need to plan daily activities around expectations related to PD treatment wearing off, for example, prevent, delay, or cease activities. A review of the existing instruments revealed that no current instruments adequately captured these target concepts, and none were developed to FDA standards or to capture impact daily. This is important in the target population because symptoms and resulting impacts fluctuate day to day and within the course of the day and because the target PD population experiences deficits in working memory making long recall periods challenging. In sum, the culmination of evidence collected through best‐practice instrument development methods, as outlined in the FDA PRO guidance, supports the content validity of the PD‐AID for use in clinical trials for capturing patient priority treatment benefit among individuals with moderate to advanced PD experiencing motor fluctuations.
To assess whether the discrepancy between self‐reported eligibility information and information expressed during the CE interview may have created a potential limitation to this research, saturation was analyzed separately on the CE sample meeting original or revised recruitment criteria (n = 20) and the discrepant CE sample (n = 9). The discrepant sample did not identify any unique concepts indicating that the discrepancies did not lead to irrelevant content for the intended COU.
An important next step for establishing the PD‐AID as fit for purpose is to evaluate measurement properties such as internal consistency reliability, reproducibility, construct validity, and responsiveness. In addition, determining the structure, including any subscales, and a scoring algorithm along with guidance for interpreting and defining clinically meaningful changes in scores will be essential. Finally, we recommend that the PD‐AID items be evaluated for translatability and adaptation into additional languages and cultures prior to use outside the United States.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Qualitative Data Analysis: A. Execution, B. Review and Critique; (3) Instrument Design: A. Design and Structure, B. Content development, C. Review and Critique; (4) Manuscript Preparation: A. Writing of the first draft, B. Review and Critique
L.S.D.: 1A, 1B, 2A, 2B, 3A, 3B, 3C, 4A, 4B
E.F.: 1B, 1C, 2A, 2B, 3A, 3B, 4A, 4B
D.E.M.: 1A, 1B, 2A, 2B, 3B, 3C, 4A, 4B
J.D.: 1C, 2A, 3B, 4A, 4B
D.L.G.: 1A, 2B, 3B, 3C, 4A, 4B
Disclosures
Ethical Compliance Statement
This research was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki 2008.19 Protocols and materials developed for PD participant interviews in both stages of the project were approved by a central institutional review board (Salus Institutional Review Board, Austin, TX; no. 9002‐0435) in accordance with all applicable national guidelines/laws. All PD participants were briefed on the study objectives and provided written informed consent. The authors confirm that they have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
This study was funded by Pfizer. Ms. Deal and Ms. Myers are currently employees of Pfizer and were so employed at the time the study was conducted. Their compensation includes company stock. Dr. Gray was also employed by Pfizer at the time this study was conducted. He is now employed by Cerevel Therapeutics. Mr. Devine was an employee of ICON, plc at the time the study was conducted. His ICON compensation included company stock. Mr. Devine is now employed by Genentech. Ms. Flood was employed ICON, plc, at the time the study was conducted. Her ICON compensation included company stock. ICON was contracted by Pfizer in connection with the conduct of the study. Ms. Flood is now employed by AstraZeneca.
Financial Disclosures for the Previous 12 Months
The authors declare that there are no additional disclosures to report.
Supporting information
Figure S1. Parkinson's Disease Activities of Daily Living, Interference and Dependence Instrument mobile application sample morning and evening screens.
Acknowledgments
We acknowledge the patients who participated in this study along with their families and caregivers. We also extend our sincere appreciation to the members of the Clinical Advisory Panel. Triza Brion of ICON, plc, San Francisco, CA, assisted with data collection and analysis. Writing and editorial assistance was provided by Maria B. Vinall of Medical Communications Depot, Inc. Ms Vinall's services were paid for by Pfizer.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Parkinson's Foundation . Understanding Parkinsons/causes‐and‐statistics/statistics. http://parkinson.org/understanding-parkinsons/causes-and-statistics/statistics. Accessed July 22, 2018.
- 2. Mayo Clinic . Parkinson's disease—symptoms and causes. https://www.mayoclinic.org/diseases-conditions/parkinsons-disease/symptoms-causes/syc-20376055. Accessed June 30, 2018.
- 3. Center for Drug Evaluation and Research, U.S. Food and Drug Administration . The voice of the patient: a series of reports from the U.S. Food and Drug Administration's (FDA's) patient‐focused drug development initiative: Parkinson's disease. https://www.fda.gov/downloads/forindustry/userfees/prescriptiondruguserfee/ucm498266.pdf. Accessed August 25, 2019.
- 4. Mayo Clinic . Parkinson's disease—diagnosis & treatment. https://www.mayoclinic.org/diseases-conditions/parkinsons-disease/diagnosis-treatment/drc-20376062. Accessed June 30, 2018.
- 5. Vijayakumar D, Jankovic J. Drug‐induced dyskinesia, part 1: treatment of levodopa‐induced dyskinesia. Drugs 2016;76(7):759–777. [DOI] [PubMed] [Google Scholar]
- 6. Hechtner MC, Vogt T, Zollner Y, et al. Quality of life in Parkinson's disease patients with motor fluctuations and dyskinesias in five European countries. Parkinsonism Relat Disord 2014;20(9):969–974. [DOI] [PubMed] [Google Scholar]
- 7. U.S. Department of Health and Human Services , et al. Guidance for industry patient‐reported outcome measures: use in medical product development to support labeling claims. https://www.fda.gov/downloads/drugs/guidances/ucm193282.pdf. Accessed August 10, 2018. [DOI] [PMC free article] [PubMed]
- 8. Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity—establishing and reporting the evidence in newly developed patient‐reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 2—assessing respondent understanding. Value Health 2011;14(8):978–988. [DOI] [PubMed] [Google Scholar]
- 9. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17(5):427–442. [DOI] [PubMed] [Google Scholar]
- 10. Joffe H, Yardley L. Content and thematic analysis In: Marks DF, Yardley L, editors. Research Methods for Clinical and Health Psychology. 1st ed London: SAGE Publications Ltd; 2004:56–68. [Google Scholar]
- 11. Kerr C, Nixon A, Wild D. Assessing and demonstrating data saturation in qualitative inquiry supporting patient‐reported outcomes research. Expert Rev Pharmacoecon Outcomes Res 2010;10(3):269–281. [DOI] [PubMed] [Google Scholar]
- 12. Biemans MA, Dekker J, van der Woude LH. The internal consistency and validity of the Self‐Assessment Parkinson's Disease Disability Scale. Clin Rehabil 2001;15(2):221–228. [DOI] [PubMed] [Google Scholar]
- 13. Welsh M, McDermott MP, Holloway RG, Plumb S, Pfeiffer R, Hubble J. Development and testing of the Parkinson's disease quality of life scale. Mov Disord 2003;18(6):637–645. [DOI] [PubMed] [Google Scholar]
- 14. Hobson JP, Edwards NI, Meara RJ. The Parkinson's Disease Activities of Daily Living Scale: a new simple and brief subjective measure of disability in Parkinson's disease. Clin Rehabil 2001;15(3):241–246. [DOI] [PubMed] [Google Scholar]
- 15. Goetz CG, Fahn S, Martinez‐Martin P, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): process, format, and clinimetric testing plan. Mov Disord 2007;22(1):41–47. [DOI] [PubMed] [Google Scholar]
- 16. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23(15):2129–2170. [DOI] [PubMed] [Google Scholar]
- 17. Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson's disease. Qual Life Res 1995;4(3):241–248. [DOI] [PubMed] [Google Scholar]
- 18. Mlinac ME, Feng MC. Assessment of activities of daily living, self‐care, and independence. Arch Clin Neuropsychol 2016;31(6):506–516. [DOI] [PubMed] [Google Scholar]
- 19. World Medical Association . Declaration of Helsinki: ethical principles for medical research involving human subjects. https://www.wma.net/wp-content/uploads/2018/07/doh-oct2008.pdf. Accessed September 6, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Parkinson's Disease Activities of Daily Living, Interference and Dependence Instrument mobile application sample morning and evening screens.


