Abstract
Propolis possesses several immunological functions. We recently generated a conditional Ca2+ biosensor yellow cameleon (YC3.60) transgenic mouse line and established a five-dimensional (5D) (x, y, z, time, and Ca2+ signaling) system for intravital imaging of lymphoid tissues, including Peyer’s patches (PPs). To assess the effects of propolis on immune cells, we analyzed Ca2+ signaling in vitro and in vivo using CD11c-Cre/YC3.60flox transgenic mice, in which CD11c+ dendritic cells (DCs) specifically express YC3.60. We found that propolis induced Ca2+ signaling in DCs in the PPs. Intravital imaging of PPs also showed that an intraperitoneal injection of propolis augmented Ca2+ signaling in CD11c+ cells, suggesting that propolis possesses immune-stimulating activity. Furthermore, CD11c+ cells in PPs in mice administrated propolis indicated an increase in Ca2+ signaling. Our results indicate that propolis induces immunogenicity under physiological conditions.
Keywords: calcium signaling, gut, imaging, propolis, B cell, dendritic cell
INTRODUCTION
Propolis is produced by honeybees and is composed of resin, pollen, and other constituents from plants around beehives together with wax secreted by the bees. Therefore, the constituents of propolis collected worldwide are wildly diverse depending on the regions. Since ancient times, it has been used as an internal and external medicine worldwide. Propolis exhibits multiple biological functions, such as immunomodulatory, antioxidant, anti-inflammatory, anticancer, antibacterial, antifungal, antiviral, and antiparasitic functions, and is consumed as a health food [1,2,3,4,5]. Among the various types of propolis, Brazilian green propolis is well studied because plants for its constituents have been clarified [6]. Brazilian green propolis has been reported to prevent and alleviate pollen allergy in human studies [7, 8]. Furthermore, it functions to alleviate colds and recovery from colds [9]. It is also effective for improving immune system, especially in aged mice [10]. It has an adjuvant activity in vaccination of herpes simplex virus in cows [11] and mice [12, 13]. Moreover, propolis prevents the production of inflammatory cytokines and elevates anti-inflammatory cytokines in mice [14]. To clarify the biological properties of propolis, further physiological analyses are required.
Calcium ions (Ca2+) are universal second messengers that have multiple functions in most cells. In the immune system, the stimulation of immune receptors, including the B-cell antigen receptor, induces intracellular Ca2+ mobilization concomitant with other signaling events such as the phosphorylation of cellular substrates [15,16,17,18]. Ca2+ signaling regulates the mitogen-activated protein kinase, nuclear factor of activated T cells, and nuclear factor-κB pathways in B cells and plays a crucial role in B-cell development and function during humoral immune responses [15, 17].
We generated a conditional mouse model that expresses the Ca2+ indicator yellow cameleon 3.60 (YC3.60). YC3.60 is a double-chromophore indicator that employs Förster/fluorescence resonance energy transfer between the cyan fluorescent protein (CFP) and a circularly permuted variant of the yellow fluorescent protein (YFP) Venus [19]. Ca2+ signaling is monitored by measuring the ratio of YFP to CFP (YFP/CFP). Therefore, YC3.60 is suitable for in vivo whole-body imaging in mice, particularly for identifying migrating immune cells.
Although propolis has health-promoting benefits, its mechanism of action is not completely understood. Therefore, to evaluate the effect of propolis on the immune system, in vitro and in vivo Ca2+ signaling in immune cells was evaluated using calcium biosensor transgenic mice. This transgenic mouse line conditionally expresses YC3.60 to visualize the temporal and spatial dynamics of Ca2+ signaling in immune cells, enabling the analysis of specific-cell functions under both normal physiological and pathological conditions. Here we analyzed the physiological effects of propolis on the gut immune cells using intravital imaging in Ca2+ biosensor mice.
MATERIALS AND METHODS
Propolis
A Brazilian green propolis ethanol extract, including 55% propolis extract as a solid content, was obtained from Yamada Bee Company, Inc. (Okayama, Japan). The extract was standardized to contain a minimum of 8.0% artepillin C and a minimum of 0.14% culifolin.
YC3.60 reporter mice
The floxed YC3.60 reporter (YC3.60flox) mouse line [20] was crossed with a CD11c-Cre mouse line [21], resulting in CD11c+ cell-specific YC3.60 expression in YC3.60flox/CD11c-Cre mice because of the loss of the loxP-flanked neomycin cassette. The YC3.60flox mouse line was also crossed with an IgG1-Cre mouse line [22], resulting in IgG1+ cell-specific YC3.60 expression. CD19-Cre/YC3.60flox mice were described previously [20]. All mice were maintained in our animal facility under specific pathogen-free conditions according to the guidelines of the Tokyo Medical and Dental University for animal care.
Flow cytometry
Flow cytometry analysis was performed using a MACSQuant Analyzer (Miltenyi Biotec). VioletFluor™ 450-labeled anti-B220, FTIC-labeled anti-CD4, and APC-labeled anti-CD86 antibodies were purchased from TONBO Biosciences, and phycoerythrin (PE)-labeled anti-CD69 antibodies were purchased from BioLegend. Dead cells were excluded by propidium iodide staining. Data analysis was conducted with FlowJo (FlowJo, LLC).
In vitro culture assay
Spleen cells from C57BL/6 mice (3 × 106 cells) were suspended in RPMI-1640 supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, 50 μM 2-mercaptoethanol, 0.3 g/l L-glutamine, and 10% fetal bovine serum and seeded at 1 mL/well into 24-well culture plates. Cells were incubated in 5% CO2 at 37°C for 48 hr.
Fluorescent microscopy
Organs and tissues were observed under an M165 FC fluorescent stereoscope with an FL600 (Leica).
Intravital and in vitro microscope
PPs and IECs from anesthetized mice were imaged. PPs were surgically exposed, immobilized on a microscope stage, and maintained at 37°C [20]. For image acquisition, a Nikon A1 laser scanning confocal microscope with a 20 × objective and NIS-Elements AR software was used, as previously described [20]. We also used dichroic mirrors (DM457/514) and two band-pass emission filters (482/35 for CFP; 540/30 for YFP). The YFP/CFP ratio was obtained by excitation at 458 nm. PE and Alexa-647 were excited at 488 nm and 633 nm, respectively, and DM405/488/561/640 and band-pass emission filters (525/50, 595/50, and 700/75) were used for image acquisition. Images of purified spleen cells in PBS were also obtained as described above. Acquired images were analyzed using the Nis-Elements software (Nikon).
In vivo stimulatory assay
We injected 50 µg of propolis in PBS into the peritoneal cavity of mice. After 2 hr, mice were subjected to intravital imaging analysis. PBS was intraperitoneally injected into the mice as a control, as previously described [20].
Statistical analysis
Statistical analysis was performed using the unpaired Student’s t-test. A p value of <0.05 was considered statistically significant.
RESULTS
Effects of propolis on splenocytes
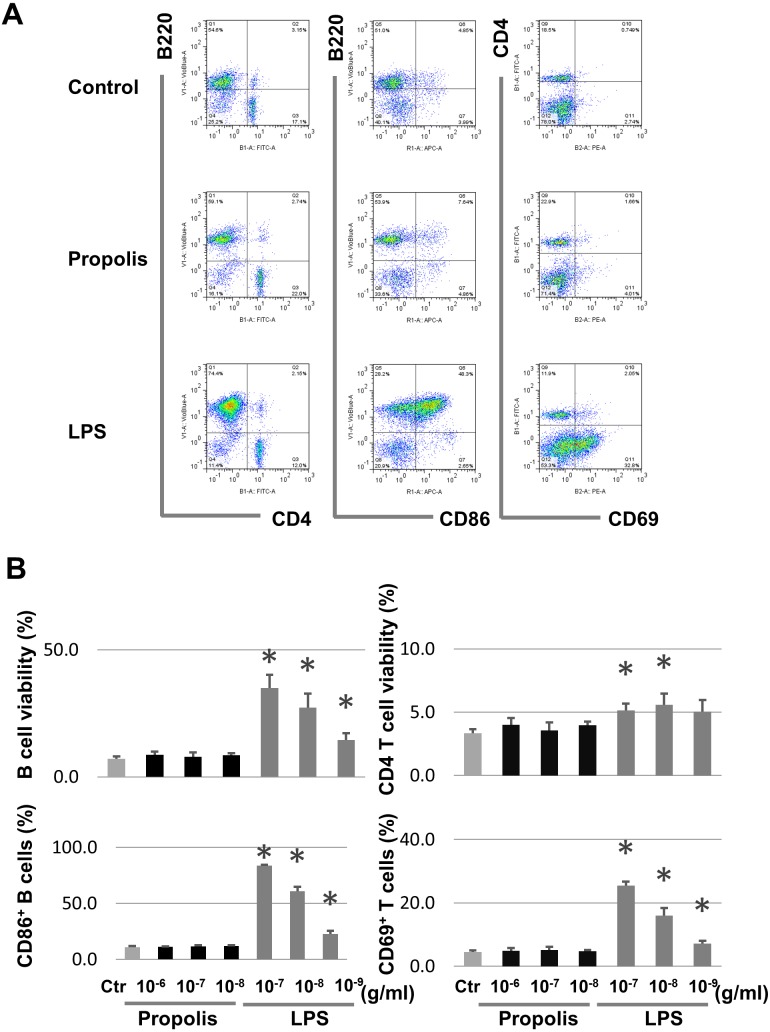
There are various types of propolis worldwide. Here, we used an ethanol extract of Brazilian propolis provided by Yamada Bee Company, Inc. Although the effect of propolis on splenocytes has been previously reported [23], the results of the study were controversial possibly because of the use of different constituents of propolis. Therefore, in this study, we first tested the effect of the propolis that we used on splenocytes. Splenocytes from C57BL/6 mice were cultured, and various doses of propolis were applied. The viability and expression of lymphocyte activation markers were then measured. Propolis did not have a remarkable effect on the viability of the splenocytes or on the expression of CD86 on B cells and CD69 on T cells. LPS, used as a positive control, induced CD86 and CD69 expression and increased the viability of the splenocytes (Fig. 1). These data suggest that Brazilian propolis does not possess lymphocyte proliferation activity, at least in vitro.
Fig. 1.
Effect of propolis on survival and activation of spleen cells.
(A) Effect of propolis on activation of spleen cells. Spleen cells were cultured without or with propolis for 2 days, and CD86 and CD69 expressions were analyzed. Cells were cultured with LPS as a positive control for B cells. (B) Dose dependency of propolis on survival and activation of spleen cells. *p<0.05 (t-test).
Propolis induces Ca2+ signaling in B cells and dendritic cells in vitro
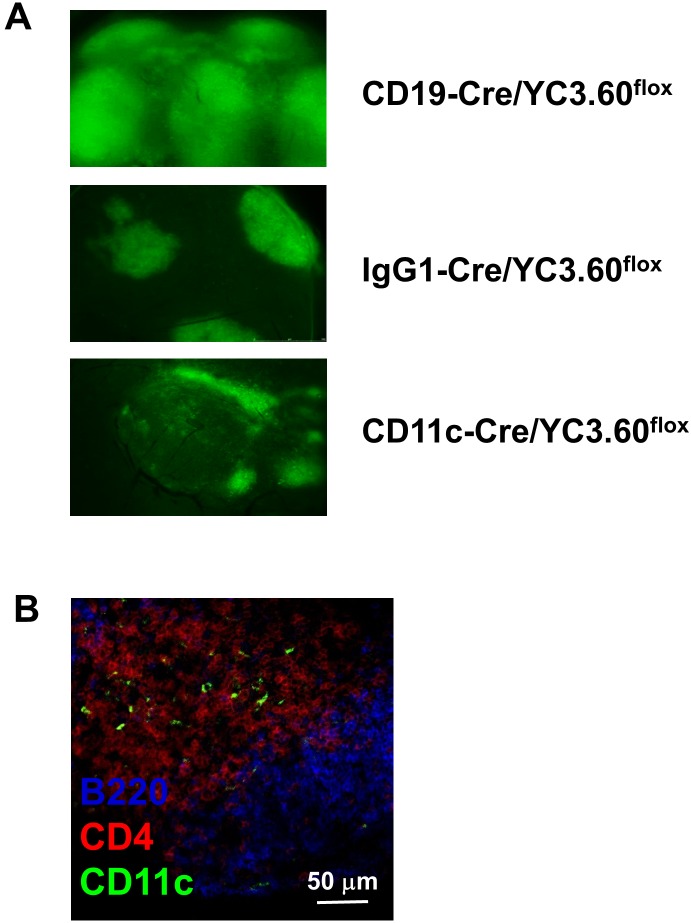
We evaluated the effect of propolis on Ca2+ signaling in various immune cells. Monocyte-derived cells such as dendritic cells (DCs) express various receptors such as Toll-like receptors, NOD family receptors, RIG family receptors, and lectin receptors to regulate pathogens [24]. CD11c is a well-known surface marker of DCs; other subpopulations of cell types such as B cells and macrophages also express CD11c on their cell surface. To examine whether propolis induces Ca2+ signaling in DCs, we crossed YC3.60flox mice [20] with CD11c-Cre mice [21]. We then measured YC3.60 expression. In Peyer’s patches (PPs), CD11c-Cre/YC3.60flox mice showed extrafollicular localization of YC3.60-expressing cells, whereas CD19-Cre/YC3.60flox and IgG1-Cre/YC3.60flox mice showed YC3.60-expressing cells in the follicular and second follicular regions, respectively (Fig. 2A). In the spleen, YC3.60-expressing cells were sparsely located in the T-cell regions (Fig. 2B). These results indicate that DCs express YC3.60 in the CD11c-Cre/YC3.60flox mice.
Fig. 2.
Property of CD11c-Cre/YC3.60flox mice.
(A) Representative images of Peyer’s patches of cell-specific YC3.60 mice. Peyer’s patches were analyzed by fluorescent microscopy (n>3 mice). YC3.60-expressing cells are shown in green. (B) YC3.60-expressing cells in the spleen of CD11c-Cre/YC3.60flox mice. The spleen was fixed with 4% PFA and then sliced and stained with anti-B220 mAb (blue) and anti-CD4 mAb (red). YC3.60-expressing cells are shown in green.
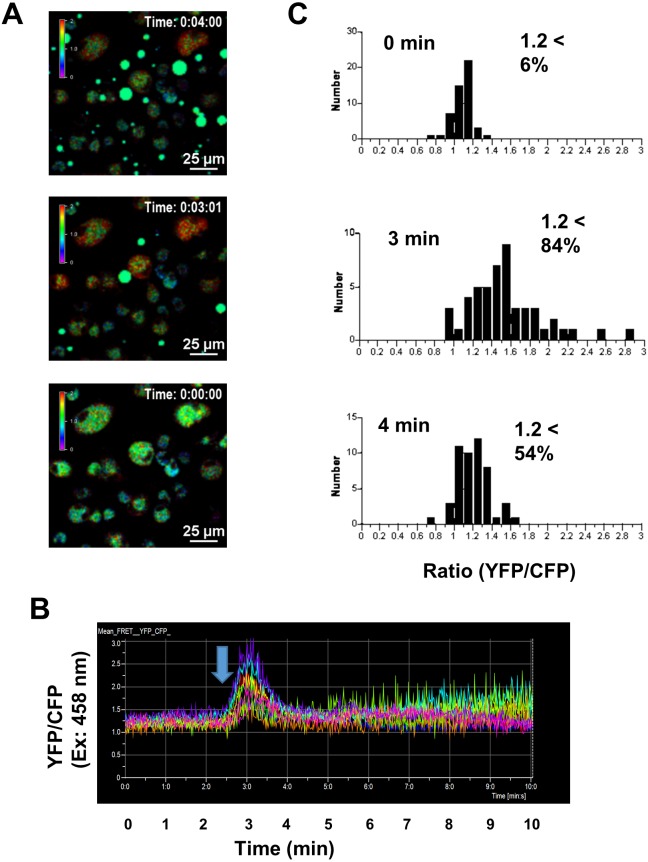
Next, we examined whether propolis induces Ca2+ signaling in DCs. As shown in Fig. 3A, propolis induced Ca2+ signaling in bone marrow-derived DCs from the CD11c-Cre/YC3.60flox mice. Intracellular Ca2+ concentrations of DCs were transiently elevated just after adding propolis, and the Ca2+ concentrations immediately decreased (Fig. 3B). We also examined whether propolis stimulates B cells, as another type of immune cells. Propolis also induced transient Ca2+ signaling in B cells from CD19-Cre/YC3.60flox mice (Fig. 4). These results strongly suggest that propolis directly stimulates B cells.
Fig. 3.
Ca2+ signaling images mediated by propolis in DCs in vitro.
(A) Representative Ca2+ signaling images in DCs from CD11-Cre/YC3.60 mice. Radiometric images (YFP/CFP at excitation of 458 nm) are shown. Propolis in PBS (final concentration: 10 μM) was added to the cell culture at the time point of 2 min 40 sec. A rainbow parameter indicates relative Ca2+ concentrations. (B) Time course for fluorescence intensities of the YFP/CFP ratio on excitation at 458 nm in the cells (n=10; frame=855, 1-sec interval). (C) Distribution of intracellular Ca2+ concentrations of randomly selected cells at the indicated time points (n=100). The results are representative of three experiments.
Fig. 4.
Ca2+ signaling images mediated by propolis in B cells in vitro.
(A) Representative Ca2+ signaling images in spleen B cells from CD19-Cre/YC3.60 mice. Ratiometric images (YFP/CFP excitation at 458 nm) are shown. Propolis in PBS (final concentration: 10 μM) was added to the cell culture at the indicated time point. A rainbow parameter indicates relative Ca2+ concentrations. (B) Time course for fluorescence intensities of the YFP/CFP ratio on excitation at 458 nm in the cells (n=10). (C) Distribution of time-integrated intracellular Ca2+ concentrations of randomly selected cells (n=10; scale bar, 25 μm; frame=19, 10-sec interval). These are representative results of three experiments.
Propolis induces Ca2+ signaling in dendritic cells in vivo
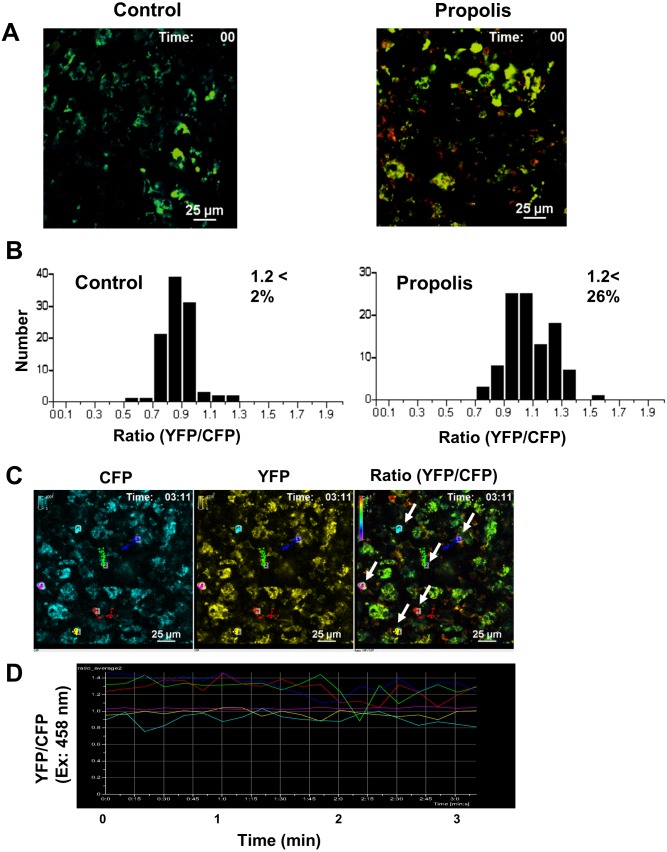
We demonstrated that propolis induces Ca2+ signaling in immune cells in vitro. Next, we examined the immunostimulatory action of propolis in vivo. We injected propolis into the peritoneal cavity of CD11c-Cre/YC3.60flox mice and then analyzed Ca2+ signaling in DCs using intravital imaging. DCs in PPs of propolis-injected mice exhibited elevated Ca2+ concentrations compared with those of control mice that exhibited low intracellular Ca2+ concentrations (Fig. 5A and B). Cells exhibiting high intracellular Ca2+ concentrations robustly migrated (Fig. 5C and D and Supplementary Movie 1). These results suggest that propolis activates DCs.
Fig. 5.
Representative Ca2+ signaling images in Peyer’s patches of the propolis-injected CD11c-Cre/YC3.60flox mice.
(A) Image of Peyer’s patches of a CD11c-Cre/YC3.60flox mouse in which propolis was injected intraperitoneally. Two hours after injection, the mouse was subjected to intravital imaging. Representative Ca2+ signaling image in the Peyer’s patches of a YC3.60flox/CD11c-Cre mouse injected with PBS as a control (left) and propolis/PBS (right). Only radiometric images (YFP/CFP excitation at 458 nm) are shown. Results are representative of at least three independent experiments (n=3 mice; scale bars, 25 μm; [left] frame=34, 2 sec interval; [right] frame=20, 10-sec interval). (B) Distribution of intracellular Ca2+ concentrations in randomly selected cells (n=100). (C) Tracking images of DCs in Peyer’s patches of a CD11c-Cre/YC3.60flox mouse intraperitoneally injected with propolis. Analyzed cells (right panel in A) are indicated by arrows. (D) Time course for fluorescence intensities of the YFP/CFP ratio on excitation at 458 nm in the cells. Results of the cells indicated in (C) are shown.
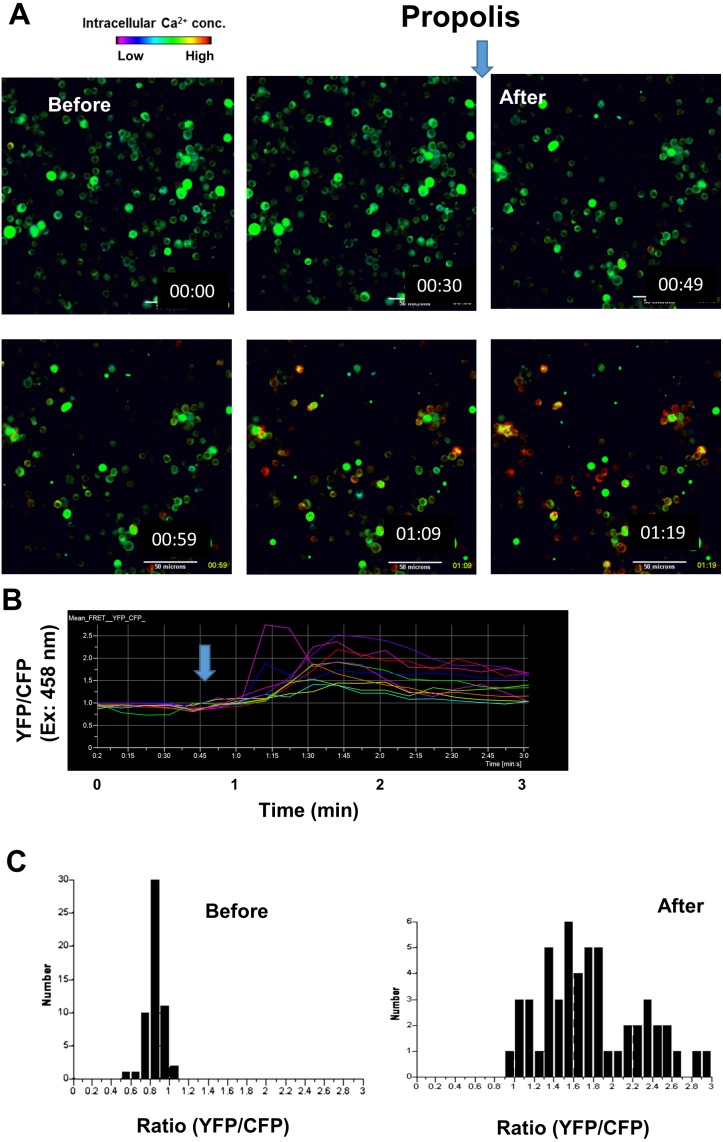
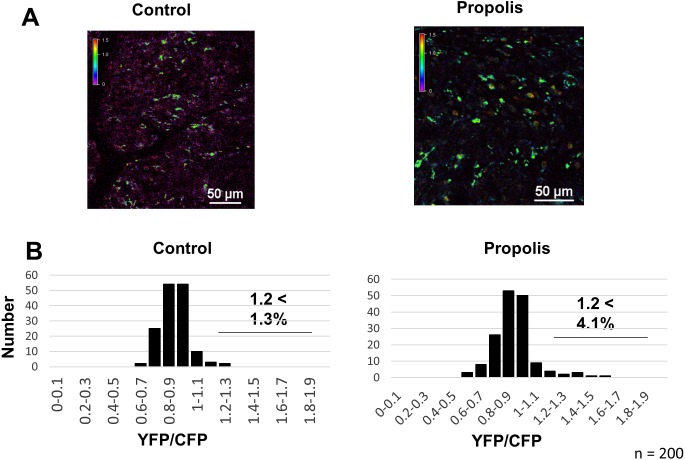
Oral administration of propolis elevates Ca2+ signaling in dendritic cells
Propolis stimulates B cells and DCs. To assess the effect of orally administered propolis on DCs in vivo, we measured Ca2+ signaling in DCs using intravital imaging. After free administration of 1% propolis for 1 week, we performed an intravital imaging analysis of PPs in CD11c-Cre/YC3.60flox mice. Compared with DCs in control mice, intracellular Ca2+ concentrations in DCs were increased by approximately three fold (Fig. 6). At the steady state concentration in pathogen-free mice, approximately 1% DCs exhibited high intracellular Ca2+ concentrations, suggesting that propolis regulates immune cells by modulating Ca2+ signaling.
Fig. 6.
Representative Ca2+ signaling images in Peyer’s patches of CD11c-Cre/YC3.60flox mice administered propolis.
(A) Image of PPs of a CD11c-Cre/YC3.60flox mouse after administration of propolis for one week. Representative Ca2+ signaling image in the PPs of a CD11c-Cre/ YC3.60flox mouse (right). Only radiometric images (YFP/CFP excitation at 458 nm) are shown (scale bars, 50 μm). (B) Distribution of intracellular Ca2+ concentrations of randomly selected regions (n=200). This is a representative image from >3 mice.
DISCUSSION
In this study, we evaluated the effect of propolis on immune cells by measuring intracellular Ca2+ signaling in DCs using conditional calcium biosensor YC3.60 transgenic mice. We found that propolis directly induced Ca2+ signaling in B cells and DCs. Furthermore, we demonstrated that propolis exhibited stimulatory activity in vivo. In addition, the oral administration of propolis stimulated DCs in PPs. Altogether, our data clearly indicated that propolis possesses immune-stimulating properties.
We also demonstrated, for the first time, that Brazilian propolis induced Ca2+ signaling in immune cells. A main constituent of Brazilian green propolis, Artepillin C, activates transient receptor potential ankyrin 1 (TRPA1) channels, which induce Ca2+ flux [25]. As lymphocytes express various TRP channels [26], propolis-induced Ca2+ signaling in B cells may be mediated by TRP channels such as TRPA1. However, the inhibitory effects of propolis on Ca2+ signaling have also been reported. Constituents of propolis, such as CAPE and pinocembrin, prevent TCR-mediated and angiotensin II-induced Ca2+ signaling in endothelial cells, respectively [27, 28], although these compounds were rare in Brazilian green propolis. Thus, propolis appears to contain various constituents that exhibit contradictory biological functions. Further investigations are required to clarify the distinctive roles of propolis in Ca2+ signaling.
Although propolis induced Ca2+ signaling in immune cells, it did not induce the expression of activation markers on lymphocytes and their proliferation. Propolis-induced Ca2+ signaling may exhibit synergistic effects and modulate or augment other signaling pathways [1,2,3,4,5]. Thus, the effects of propolis on multiple biological functions may be because of its multiple constituents.
We previously established a 5D (x, y, z, time, and Ca2+ signaling) intravital imaging system in calcium biosensor mice [20]. Using this mouse model, we revealed that propolis directly induced Ca2+ signaling in immune cells. As IECs secrete cytokines that regulate immune cells [29], propolis may indirectly regulate immune responses through IECs. Furthermore, we showed that orally administered propolis activated DCs, suggesting that these DCs activate T cells and/or B cells via their cognate interactions. Thus, propolis may affect immune responses both directly and indirectly. These observations may account for the previously reported multifunctional properties of propolis [23, 30].
Our line of conditional YC3.60 mice enabled the analysis of propolis-induced Ca2+ signaling in specific cells under physiological conditions. For a comprehensive understanding of the biological effect of propolis, further studies are necessary.
AUTHOR CONTRIBUTIONS
T.A., S.Y., H.T., N. M. T., T. O. and H.K. designed the research, and T.A. wrote the manuscript; T.A., S.Y., H.T., N.M.T. and T.K. performed the experiments, analyzed the data and prepared the figures.
CONFLICT OF INTEREST
The authors declare no financial or commercial conflict of interest.
Supplementary Movie 1
Propolis was injected intraperitoneally in a CD11c-Cre/YC3.60flox mouse. After 2 hours of injection, the mouse was subjected to intravital imaging. Radiometric images (YFP/CFP excitation at 458 nm) are shown (scale bars, 25 μm; frame=34, 2-sec interval). The real acquisition time is indicated (top).
Acknowledgments
We are grateful to Dr. K. Rajewsky (MDC) for the CD19-Cre and IgG1-Cre mice, Dr. M. Okabe (Osaka University) for the CAG-Cre mice, Yamada bee company, Inc. for propolis and Drs. K. Hashimoto and H. Tani (Yamada Bee Company, Inc.) for critical reading of the manuscript. This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Yamada Foundation (to T.A.), the Joint Usage/Research Program of the Medical Research Institute, Tokyo Medical and Dental University (to S.Y., H.K. and N.M.T.), a Grant-in-Aid from the Cross-ministerial Strategic Innovation Promotion Program (SIP) (to N.M.T.), and the Canon Foundation (to T.A. and N.M.T.).
REFERENCES
- 1.Banskota AH, Tezuka Y, Kadota S. 2001. Recent progress in pharmacological research of propolis. Phytother Res 15: 561–571. [DOI] [PubMed] [Google Scholar]
- 2.Erdemli HK, Akyol S, Armutcu F, Akyol O. 2015. Antiviral properties of caffeic acid phenethyl ester and its potential application. J Intercult Ethnopharmacol 4: 344–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuo YY, Jim WT, Su LC, Chung CJ, Lin CY, Huo C, Tseng JC, Huang SH, Lai CJ, Chen BC, Wang BJ, Chan TM, Lin HP, Chang WS, Chang CR, Chuu CP. 2015. Caffeic acid phenethyl ester is a potential therapeutic agent for oral cancer. Int J Mol Sci 16: 10748–10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawicka D, Car H, Borawska MH, Nikliński J. 2012. The anticancer activity of propolis. Folia Histochem Cytobiol 50: 25–37. [DOI] [PubMed] [Google Scholar]
- 5.Sforcin JM, Bankova V. 2011. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol 133: 253–260. [DOI] [PubMed] [Google Scholar]
- 6.Bastos EM, Santana RA, Calaça-Costa AG, Thiago PS. 2011. Interaction between Apis mellifera L. and Baccharis dracunculifolia DC, that favours green propolis production in Minas Gerais. Braz J Biol 71: 727–734. [DOI] [PubMed] [Google Scholar]
- 7.Takeuchi H, Kitano H, Okihara K, Hashimoto K, Enomoto T. 2008. Efficacy and safety of propolis supplement on management of Japanese cedar pollinosis: a randomized double-blind, placebo-controlled trial in 2005. Pharmacometrics 75: 103–108. [Google Scholar]
- 8.Takeuchi H, Kitano H, Okihara K, Hashimoto K, Enomoto T. 2009. Oputimum dose of propolis supplements for the management of Japanese cedar pollinosis: a randomized double-blind, placebo-controlled trial in 2006. Pharmacometrics 76: 71–77. [Google Scholar]
- 9.Ohkuma A, Kanno T, Asama T, Doi-Takaki S, Kawauchi M, Tachifuji T, Hashimoto K. 2010. Effect of dietary supplement containing Brazilian propolis on the common cold. Pharmacometrics 79: 43–48. [Google Scholar]
- 10.Gao W, Wu J, Wei J, Pu L, Guo C, Yang J, Yang M, Luo H. 2014. Brazilian green propolis improves immune function in aged mice. J Clin Biochem Nutr 55: 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer G, Cleff MB, Dummer LA, Paulino N, Paulino AS, de Oliveira Vilela C, Campos FS, Storch T, D’Avila Vargas G, de Oliveira Hübner S, Vidor T. 2007. Adjuvant effect of green propolis on humoral immune response of bovines immunized with bovine herpesvirus type 5. Vet Immunol Immunopathol 116: 79–84. [DOI] [PubMed] [Google Scholar]
- 12.Fischer G, Conceição FR, Leite FP, Dummer LA, Vargas GD, Hübner SO, Dellagostin OA, Paulino N, Paulino AS, Vidor T. 2007. Immunomodulation produced by a green propolis extract on humoral and cellular responses of mice immunized with SuHV-1. Vaccine 25: 1250–1256. [DOI] [PubMed] [Google Scholar]
- 13.Fischer G, Paulino N, Marcucci MC, Siedler BS, Munhoz LS, Finger PF, Vargas GD, Hübner SO, Vidor T, Roehe PM. 2010. Green propolis phenolic compounds act as vaccine adjuvants, improving humoral and cellular responses in mice inoculated with inactivated vaccines. Mem Inst Oswaldo Cruz 105: 908–913. [DOI] [PubMed] [Google Scholar]
- 14.Machado JL, Assunção AK, da Silva MC, Dos Reis AS, Costa GC, Arruda DS, Rocha BA, Vaz MM, Paes AM, Guerra RN, Berretta AA, do Nascimento FR. 2012. Brazilian green propolis: anti-inflammatory property by an immunomodulatory activity. Evid Based Complement Alternat Med 2012: 157652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurosaki T, Shinohara H, Baba Y. 2010. B cell signaling and fate decision. Annu Rev Immunol 28: 21–55. [DOI] [PubMed] [Google Scholar]
- 16.Oh-hora M, Rao A. 2008. Calcium signaling in lymphocytes. Curr Opin Immunol 20: 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scharenberg AM, Humphries LA, Rawlings DJ. 2007. Calcium signalling and cell-fate choice in B cells. Nat Rev Immunol 7: 778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feske S. 2007. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol 7: 690–702. [DOI] [PubMed] [Google Scholar]
- 19.Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A. 2004. Expanded dynamic range of fluorescent indicators for Ca(2+) by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci USA 101: 10554–10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshikawa S, Usami T, Kikuta J, Ishii M, Sasano T, Sugiyama K, Furukawa T, Nakasho E, Takayanagi H, Tedder TF, Karasuyama H, Miyawaki A, Adachi T. 2016. Intravital imaging of Ca(2+) signals in lymphocytes of Ca(2+) biosensor transgenic mice: indication of autoimmune diseases before the pathological onset. Sci Rep 6: 18738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caton ML, Smith-Raska MR, Reizis B. 2007. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med 204: 1653–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casola S, Cattoretti G, Uyttersprot N, Koralov SB, Seagal J, Hao Z, Waisman A, Egert A, Ghitza D, Rajewsky K. 2006. Tracking germinal center B cells expressing germ-line immunoglobulin gamma1 transcripts by conditional gene targeting. Proc Natl Acad Sci USA 103: 7396–7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sforcin JM. 2007. Propolis and the immune system: a review. J Ethnopharmacol 113: 1–14. [DOI] [PubMed] [Google Scholar]
- 24.Lavelle EC, Murphy C, O’Neill LA, Creagh EM. 2010. The role of TLRs, NLRs, and RLRs in mucosal innate immunity and homeostasis. Mucosal Immunol 3: 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hata T, Tazawa S, Ohta S, Rhyu MR, Misaka T, Ichihara K. 2012. Artepillin C, a major ingredient of Brazilian propolis, induces a pungent taste by activating TRPA1 channels. PLoS One 7: e48072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feske S, Wulff H, Skolnik EY. 2015. Ion channels in innate and adaptive immunity. Annu Rev Immunol 33: 291–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, Pang XB, Chen BN, Gao L, Wang L, Wang SB, Wang SB, Liu DP, Du GH. 2013. Pinocembrin inhibits angiotensin II-induced vasoconstriction via suppression of the increase of [Ca2+]i and ERK1/2 activation through blocking AT1R in the rat aorta. Biochem Biophys Res Commun 435: 69–75. [DOI] [PubMed] [Google Scholar]
- 28.Nam JH, Shin DH, Zheng H, Kang JS, Kim WK, Kim SJ. 2009. Inhibition of store-operated Ca2+ entry channels and K+ channels by caffeic acid phenethylester in T lymphocytes. Eur J Pharmacol 612: 153–160. [DOI] [PubMed] [Google Scholar]
- 29.Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, Gallini CA, Redding K, Margolskee RF, Osborne LC, Artis D, Garrett WS. 2016. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351: 1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Figueiredo SM, Nogueira-Machado JA, Almeida BM, Abreu SR, de Abreu JA, Filho SA, Binda NS, Caligiorne RB. 2014. Immunomodulatory properties of green propolis. Recent Pat Endocr Metab Immune Drug Discov 8: 85–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Propolis was injected intraperitoneally in a CD11c-Cre/YC3.60flox mouse. After 2 hours of injection, the mouse was subjected to intravital imaging. Radiometric images (YFP/CFP excitation at 458 nm) are shown (scale bars, 25 μm; frame=34, 2-sec interval). The real acquisition time is indicated (top).