Abstract
Parkinson’s disease (PD) is a neurodegenerative disease characterized by motor impairment and the accumulation of alpha-synucleinopathy (α-syn), which can affect different levels of the brain-gut axis. There is a two-way communication between the gastrointestinal tract, and brain that includes the gut microbiota. This bidirectional communication between the gut microbiota and the brain includes many pathways, such as immune mechanisms, the vagus nerve, and microbial neurometabolite production. The common cause of constipation in PD is thought to be the accumulation of α-syn proteins in the enteric nervous system. Recent studies have focused on changes in microbial metabolites and gut microbiota dysbiosis. Microbiota dysbiosis is associated with increased intestinal permeability, intestinal inflammation, and neuroinflammation. Many factors, such as unbalanced nutrition, antibiotic use, age, and infection, result in alteration of microbial metabolites, triggering α-syn accumulation in the intestinal mucosa cells. Increased evidence indicates that the amount, type, and balance of dietary macronutrients (carbohydrates, proteins, and fats); high consumption of vegetables, fruits, and omega-3 fatty acids; and healthy diet patterns such as the Mediterranean diet may have a great protective impact on PD. This review focuses on the potential benefits of prebiotics, probiotics, and synbiotics to regulate microbiota dysbiosis along with the effect of diet on the gut microbiota in PD.
Keywords: Parkinson’s disease, alpha-synuclein, microbiota, nutrition, probiotics, gut-brain axis
INTRODUCTION
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, and it affects 0.5–1% of the population aged 65–69 years and 1–3% of the population above 80 years of age [1]. It is characterized by the aggregation and accumulation of alpha-synucleinopathy (α-syn) proteins in the central nervous system (CNS) and other neural structures. The classical motor symptoms like bradykinesia, resting tremor, rigidity, and late postural instability result from the death of dopamine-producing cells in the substantia nigra. In addition, PD patients frequently exhibit non-motor symptoms including symptoms affecting the olfactory (relating to loss of smell), gastrointestinal, cardiovascular, and urogenital systems [2]. The most common gastrointestinal system (GIS) symptoms are constipation, loss of appetite, dysphagia, drooling, and gastroesophageal reflux. Moreover, gastrointestinal function is further exacerbated following progression of the disease [3, 4]. Constipation is the most common autonomic symptom in PD [2, 5] and is reported to be seen in approximately 80% of PD patients [5]. The accumulation of α-syn protein in the enteric nervous system leads to increased intestinal permeability, oxidative stress, and local inflammation. This causes neurodegenerative changes in the enteric nervous system (ENS) and may result in prolonged intestinal permeability [6], and constipation [7, 8]. Lewy bodies, formed as a result of misfolding α-syn proteins, are found in CNS structures, peripheral autonomous systems, and the ENS [9, 10]. The ENS is a network of neurons in the GIS wall. It plays a major role in bidirectional communication between GIS and CNS [11, 12]. Lewy body pathology in ENS may represent the pathohistological correlate of gastrointestinal symptoms in PD. Current hypotheses suggest that ENS might be one of the first sites where Lewy body pathology appears in PD [9, 10]. Lewy bodies and α-syn proteins may appear in the gut before they appear in the brain, and these observations reveal the hypothesis that PD starts in the gut and spreads to the brain. Increased intestinal permeability in conjunction with the presence of α-syn in the gut at early stages of the disease may cause the spreading of the disease [13].
The role of nutrition in the development and prevention of diseases has always been remarkable. New evidence suggests that the effect of diet on brain health is not because of a diet-induced inflammatory response but is because of the effect of the composition of the diet on the gut microbiome [14]. The gut microbiota is essential for human health and the immune system. It also plays a major role in the bidirectional communication between the gut and the brain [15,16,17]. Recent research has shown that changes in gut microbiota can influence the physiological, behavioral, and cognitive functions of the brain [12, 18]. Gut microbiota affects brain activity via the microbiota-gut-brain axis under both physiological and pathological disease conditions [6]. Combinations of specific nutrients, which include neuronal precursors and cofactors, can prevent synaptic loss and may reduce membrane-related pathology in the CNS and ENS [19]. In recent studies, fecal short chain fatty acid (SCFA) concentrations were significantly reduced in PD patients [7, 9, 20] and Prevotellaceae were reduced in the feces of PD patients was reduced as compared with that of control [7]. The formation of the gut microbiota is influenced by many dietary factors. Dietary components plays an important role in the control of gut microbial populations and, thus, in the prevention, management, and treatment of certain diseases. Some dietary factors considered to be effective in patients with PD include vegetables, fruits, fish, protein, fat [21], carbohydrates, fiber [9, 20], polyphenols [22], the Mediterranean-type diet [23, 24], and the Western-type diet [25]. Therefore, this review focuses on the effects of dietary factors on the microbiota composition of PD patients and therapeutic treatment approaches.
GUT-BRAIN AXIS IN PARKINSON’S DISEASE
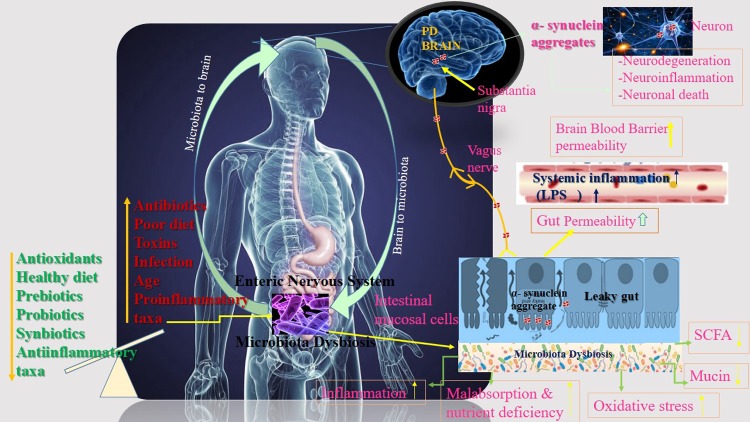
Recent research has shown that changes in intestinal microbiota can affect the physiological, behavioral, and cognitive functions of the brain [12, 18]. The gut-brain axis (GBA) is a complex bidirectional communication system between the gut and the brain mediated by hormonal, immunological, and neural signals [15,16,17]. GBA includes immune mediators such as cytokines, production of bacterial metabolites [26], and signal stimulation to the brain through direct action on the vagus nerve [26, 27]. This axis is also responsible for the modulation of digestive processes under physiological conditions. Dysregulation of the axis, gut dysbiosis, and inflammation are associated with various diseases including mental disorders (anxiety and depression), Alzheimer’s, inflammatory bowel disease, and obesity [15, 28,29,30]. In a study conducted on germ-free (GF) mice, to investigate the role of intestinal microbiota and bacterial metabolites in the pathogenesis of PD, a relationship between intestinal microbiota and behavior-related brain function was found. The microbial colonization process initiates signaling mechanisms that affect neuronal circuits involved in motor control and anxiety behavior. At the same time, GF mice compared with specific pathogen-free (SPF) mice show elevated noradrenaline (NA), dopamine (DA), and serotonin (5-HT) turnover rates in the striatum [31]. Serotonin is a neurotransmitter that plays an important role in the GBA, affecting both ENS and CNS levels. The gut microbiota stimulates the synthesis of various neuroactive molecules such as serotonin, acetylcholine, melatonine, gamma aminobutyric acid (GABA), catecholamine, and histamine [16, 32]. These neurotransmitters affect the regulation and control of blood flow, as well as intestinal motility, absorption of nutrients, the gastrointestinal immune system, and microbiota. Especially in pathological conditions like inflammatory bowel diseases and PD, these neurotransmitters can cause various gastrointestinal symptoms [33]. Although dysbiosis has been reported in PD, it is not yet clear whether changes in the microbiota are a trigger for PD pathology. Fecal microbiota from PD patients or controls were transplanted into individual groups of GF recipient animals. The microbiota derived from individuals with PD promoted increased α-syn-mediated motor dysfunction [34]. Evidence suggests that changes in the microbiota and its metabolites may be an important risk factor for PD through the gut-brain axis. Many factors such as unbalanced nutrition, antibiotics, age, and infection lead to changes in the gut mucosal cells which cause α-syn accumulation. The vagal nerve might provide a path for the spreading of α-syn pathology from the ENS to the brain. The aggregated α-syn spreads towards CNS via the the vagus nerve. Eventually, the aggregated α-syn arrives at the substantia nigra. The accumulation of α-syn in the brain has been linked to neurodegeneration, neuroinflammation, and neuronal death [35, 36]. The microbiota-gut brain axis communication pathway linking gut microbial dysbiosis with brain function in PD is shown in Fig. 1.
Fig. 1.
Microbiota-gut brain axis communication in the pathogenesis of Parkinson’s disease. Multiple factors affecting the composition of gut microbiota.
INTESTINAL BACTERIAL COMPOSITION AND PARKINSON DISEASE
Alterations in the number as well as composition of gut microbiota and microbial metabolites are found in PD patients. Intestinal dysbiosis in PD has been reported in 12 articles from six countries: one from Finland, Japan and Russia, two from the USA, three from China, and four from Germany.
Scheperjans et al. [7] reported that alteration in the composition of gut microbiota in PD has been reported. They compared the fecal microbiomes of 72 PD patients and 72 control subjects by the pyrosequencing of the bacterial 16S ribosomal RNA gene. The abundance of Prevotellaceae in feces of PD patients with postural instability and gait difficulties was reduced by 77.6% as compared with controls, and there was a higher abundance of Enterobacteriaceae was found among those patients with postural instability along with gait difficulty phenotype compared with those with tremor-dominant PD. Keshavarzian et al. [20] found that members of the genus Faecalibacterium were significantly more abundant in the mucosa of controls than in PD patients. Putative “proinflammatory” Proteobacteria of the genus Ralstonia were significantly more abundant, and anti-inflammatory SCFA producers genera Blautia, Coprococcus, and Roseburia were significantly decreased in PD patients. One clinical study reported increased levels of Akkermansia, Lactobacillus, Bifidobacterium, and decreased levels of Lachnospiraceae in PD patients compared with controls [13]. Another study found that the abundance of Lactobacillus was higher despite the fact that Clostridium coccoides, Bacteroides fragilis, and Clostridium leptum were lower in the fecal samples of PD patients [37]. In a recent study, changes in the content of 9 genera and 15 species of microorganisms were revealed in PD patients decreased contents of Dorea, Bacteroides, Prevotella, Faecalibacterium, Bacteroides massiliensis, Stoquefichus massiliensis, Bacteroides coprocola, Blautia glucerasea, Dorea longicatena, Bacteroides dorei, Bacteroides plebeius, Prevotella copri, Coprococcus eutactus, and Ruminococcus callidus, and increased contents of Christensenella, Catabacter, Lactobacillus, Oscillospira, Bifidobacterium, Christensenella minuta, Catabacter hongkongensis, Lactobacillus mucosae, Ruminococcus bromii, and Papillibacter cinnamivorans [38]. In a study conducted in southern China, the abundance of Lachnospiraceae was decreased by 42.9% in patients with PD, whereas Bifidobacteriaceae were increased enriched in patients with PD compared with age-matched controls [39]. Another study conducted in China showed that genera Clostridium IV, Aquabacterium, Holdemania, Sphingomonas, Clostridium XVIII, Butyricicoccus, and Anaerotruncus are enriched in the feces of PD patients after adjustment according to age, gender, body mass index (BMI), and constipation. Furthermore, genera Escherichia and Shigella are negatively associated with disease duration [40]. A study reported that the putative cellulose-degrading bacteria from the genera Blautia, Faecalibacterium, and Ruminococcus are significantly decreased in PD patients compared with healthy controls. The putative pathobionts from the genera Escherichia-Shigella, Streptococcus, Proteus, and Enterococcus were significantly increased in PD subjects [41].
In a northern German cohort study, it was shown that elevated levels of Lactobacillaceae, Barnesiellaceae, and Enterococcacea occur in fecal samples of PD patients [42]. Another study showed that genera Akkermansia and Prevotella are significantly more abundant in PD patients compared with healthy controls [43]. Another study found that the bacterial phylum Bacteroidetes and the bacterial family Prevotellaceae were decreased and that Enterobacteriaceae were more abundant in fecal samples from PD patients compared with matched controls [9]. Bedarf et al. [44] compared the fecal microbiomes of 31 early-stage, L-DOPA-naïve PD patients with those of 28 age-matched controls. They found increased numbers of Verrucomicrobiaceae (Akkermansia muciniphila) and unclassified Firmicutes, whereas Prevotellaceae (Prevotella copri) and Erysipelotrichaceae (Eubacterium biforme) were markedly decreased in PD patients. A summary of the changes observed in the gut microbiota in patients with PD is given in Table 1. This table is revised from Sun and Shen [36].
Table 1. A summary of the changes observed in the gut microbiota in patients with Parkinson’s disease (Revised from Sun and Shen [36]).
| İncreased | Decreased | Country | Reference |
|---|---|---|---|
| Enterobacteriaceae | Prevotellaceae | Finland | [7] |
| Akkermansia, | Lachnospiraceae | USA | [13] |
| Lactobacillus | |||
| Bifidobacterium | |||
| Ruminococcaceae | |||
| Proteobacteria (Genus Ralstonia ) | Faecalibacterium | USA | [20] |
| Blautia, | |||
| Coprococcus | |||
| Roseburia | |||
| Lactobacillus | Clostridium coccoides | Japan | [37] |
| Bacteroides fragilis | |||
| Clostridium leptum | |||
| Christensenella | Dorea | Russia | [38] |
| Catabacter | Bacteroides | ||
| Lactobacillus | Prevotella | ||
| Oscillospira | Faecalibacterium | ||
| Bifidobacterium | Bacteroides massiliensis | ||
| Christensenella minuta | Stoquefichus massiliensis | ||
| Catabacter hongkongensis | Bacteroides coprocola | ||
| Lactobacillus mucosae, | Blautia glucerasea | ||
| Ruminococcus bromii, | Dorea longicatena | ||
| Papillibacter cinnamivorans | Bacteroides dorei | ||
| Bacteroides plebeus | |||
| Prevotella copri | |||
| Coprococcus eutactus | |||
| Ruminococcus callidus | |||
| Bifidobacteriaceae | Lachnospiraceae | China | [39] |
| Cluster IV | Lactobacillus | China | [40] |
| Aquabacterium | Sediminibacterium | ||
| Holdemania | |||
| Sphingomonas | |||
| Cluster XVIII | |||
| Butyricicoccus | |||
| Anaerotruncus | |||
| Escherichia-Shigella | Blautia | China | [41] |
| Streptococcus | Faecalibacterium | ||
| Proteus | Ruminococcus | ||
| Enterococcus | |||
| Enterobacteriaceae | Bacteroidetes | Germany | [9] |
| Bifidobacterium | Prevotellaceae | ||
| Faecalibacterium prausnitzii | |||
| Lactobacillaceae | |||
| Enterococcaceae | |||
| Lactobacillaceae | - | Germany | [42] |
| Barnesiellaceae | |||
| Enterococcacea | |||
| Akkermansia | - | Germany | [43] |
| (Akkermansia parent taxa Verrucomicrobiaceae, Verrucomicrobiales, Verrucomicrobia) | |||
| Prevotella | |||
| Verrucomicrobiaceae (Akkermansia muciniphila) | Prevotellaceae (Prevotella copri) | Germany | [44] |
| Unclassified Firmicutes | Erysipelotrichaceae (Eubacterium biforme) | ||
Recent studies have suggested that Helicobacter pylori infections are associated with negative interaction with the gut microbiota. H. pylori infections produce dramatic changes in the gastric microenvironment, simultaneously influence the gastric microbiota, and also impacts the intestinal commensal communities [45, 46]. H. pylori infection was reported years ago in PD patients [40], and there are also several studies suggesting that the potential relationship between H. pylori and Parkinson’s disease is controversial [9, 40,41,42,43]. H. pylori infection was associated with an increased risk of Parkinson’s disease. A recent retrospective study involving 2,105 H. pylori infected subjects and 9,105 matched uninfected controls found that those who were infected were 2- to 3-fold more likely to be PD patients than those who were uninfected [9]. In another study, H. pylori was reported in 32% of PD patients [43]. Therefore, the presence of H. pylori infections should not be ignored when evaluating the gut microbiota in PD patients.
A pro-inflammatory microbiota profile in the PD patient’s intestinal tract might increase gut permeability, allowing leakage of bacterial products and inflammatory mediators from the intestines [11]. A dysregulated microbiota-gut-brain axis in PD might underlie gastrointestinal dysfunctions which predominantly emerge many years prior to the PD diagnosis, corroborating the theory that the pathological process spreads from the gut to the brain [2, 4]. Changes in the gut microbiota also affect GIS epithelial cells, the immune system, and the ENS (both neurons and glial cells) [47]. Taken together, these results may suggest that changes in the intestinal microbiota may have a direct effect on the CNS through the GBA via chronic mild systemic inflammation [48]. For this reason, an adequete and balanced antioxidant-rich diet may play a potential role in preventing proinflammatory conditions.
Nowadays, based on the current understanding of gut microbial dysbiosis, fecal microbiota transplantation is used to regulate immunological mechanisms though the microbiota-gut-brain axis for the treatment of autism, multiple sclerosis, and other CNS diseases [49]. A previous study reported that the fecal microbiota transplantation in mice with PD reduced the activation of microglia in addition to astrocytes in the substantia nigra and reduced expression of TLR4/TNF-α signaling pathway components in the gut and the brain [50]. However, there are still concerns regarding safety that remain to be addressed. As far as we know, there have been no randomised controlled studies. So, further studies on a new therapeutic approach are needed in relation to the gut microbiota and fecal microbiota transplantation.
EFFECT OF DIETARY THERAPY APPROACHES ON MICROBIOTA IN PD
Dietary fiber, antioxidants, healthy diet patterns, prebiotics, probiotics, synbiotics, etc., may impact the gut microbiota composition, enhance intestinal epithelial integrity, and reduce the proinflammatory response, impacting the initiation of PD. For example, antibiotics, poor diet, toxins, etc., may lead to a pathological process in the enteric cell plexus causing mucosal inflammation, oxidative stress, and decreased mucin and SCFA, thereby initiating α-synuclein accumulation.
Fiber, carbohydrates and microbiota
Dietary fiber means carbohydrate polymers with ten or more monomeric units according to the CODEX Alimentarius Commission [51]. It is categorized into two groups: as soluble and insoluble. There are two basic types of food fiber—insoluble fiber, which does not dissolve in water and is not fermented by the gut’s bacteria, in addition to soluble fiber, which does dissolve in water and is fermented by the colon’s microorganisms or bacteria. The polysaccharides pectin, and mucilage are examples of soluble fiber, whereas cellulose, hemicellulose, and lignin are all insoluble forms [52, 53]. SCFAs (non-digestible carbohydrates) are produced by fermentation of intestinal microbiota bacteria in the large intestine and provide up to 10% of the daily caloric requirements in humans [54, 55].
SCFAs may have a beneficial effect on PD as they increase the motility of the gastrointestinal tract by modulating ENS activity [9]. In recent studies, it has been pointed out that there are low levels of SCFAs in the stool samples of PD patients and that the chance in SCFAs indicate a change in the intestinal microbiota composition [9, 20]. Unger et al. [9], analyzed SCFA concentrations (using gas chromatography) and microbiota composition (using quantitative PCR) in the fecal samples of 34 PD patients in addition to 34 age-matched controls who were analyzed. Fecal SCFA concentrations were significantly reduced in PD patients, their levels of the the bacterial phylum Bacteroidetes and the bacterial family Prevotellaceae were reduced, and Enterobacteriaceae were more abundant in fecal samples from PD patients compared with those of matched controls [9]. It should be noted that especially, Prevotellaceae has various functional role which promotes a healthy intestinal environment. This enterotype is related to higher levels of health-promoting neuroactive short chain fatty acids and a high capacity for biosynthesis of thiamine as well as folate [7]. On the other hand, the decreased abundance of the Prevotellaceae bacteria family might be related to reduced mucin synthesis and increased intestinal permeability [56, 57]. Increased intestinal permeability may provoke local and systemic exposure to bacterial endotoxins, which may lead to increased α-syn expression in the large intestine [57, 58].
Since the reduction of beneficial bacteria leads to a decrease in the production of SCFAs [56], supporting adequate fiber intake in PD patients could slow down the progression of the disease by affecting the bacterial composition positively, and possibly solve many problems including gastrointestinal dysmotility.
Dietary fatty acids (Omega-3) and microbiota
Fatty acids (FAs) are major components in neuronal cell membranes and synapses and are essential for maintaining their structure and function. FAs have also been found to have anti-inflammatory, antioxidative, and neuroprotective properties. The FA composition of the cell membranes is affected by diet [59]. There remains much to be learned about the effects of dietary factors on the development of PD [60]. Evidence from animal models suggests that dietary fatty acids, especially docosahexaenoic acids (DHA), regulate oxidative stress in the brain [61, 62]. A case-control study has investigated the association between dietary fatty acid intake of individuals and the risk of PD in Japan. Cholesterol and arachidonic acid intake were significantly positively associated with the risk of PD. However, intake of total fat, saturated fatty acids, monounsaturated fatty acids, n-3 polyunsaturated fatty acids, α-linolenic acid, eicosapentaenoic acid, docosahexaenoic acid, n-6 polyunsaturated fatty acids, linoleic acid, and the ratio of n-3 to n-6 polyunsaturated fatty acid intake were not associated with PD [60].
In recent studies, identification of the relationship between omega-3 fatty acids and the human gut microbiota has provided a new insight into the composition of the microbiota [63,64,65]. Omega-3 (PUFAs), like DHA, have anti-inflammatory properties which might reduce oxidative stress and therefore reduce alpha-synuclein accumulation [19, 66]. Holmqvist et al. [67], demonstrated in an in vivo animal model from PD patient brain lysate is taken up and transported retrogradely over a long distance via the vagal nerves from the gut to the brain after injection into the intestinal wall in an in vivo animal model [34]. In another study, colonization of α-syn-overexpressing mice with microbiota from PD patients enhanced physical impairments compared with microbiota transplants from healthy human donors. These findings indicated that gut bacteria regulate movement disorders in mice and suggest that alterations in the human microbiome represent a risk factor for PD [34]. There has only been one study evaluating the effect of fish oil on the brain-gut axis in the mice with PD. When oral or intrastriatal rotenone (neurotoxin) was administered to mice, both oral and intrastriatal rotenone induced similar PD-like motor deficits, dopaminergic cell loss, delayed intestinal transit, inflammation, and α-syn accumulation in the colon. In addition, it was found that GIS and rotetone-induced motor dysfunctions were prevented in these mice following a uridine and DHA-containing dietary intervention. So, a the dietary intervention may provide benefits in the prevention of motor and non-motor symptoms in PD [19]. Another recent study revealed that found that dietary fat intake may modify PD risk directly or by altering the response to environmental neurotoxicants including pesticides. PUFA intake was consistently associated with lower (PD) risk, and dietary fats modified the association of PD risk with pesticide exposure. It was concluded that a diet high in PUFAs and low in saturated fat might reduce the risk of PD [68]. All these results may be related to bidirectional communication between the gut and the brain for the formation of the Parkinson’s-like phenotype and pathology. Although the relationship between dietary fatty acids and PD has not been fully clarified in the literature [59, 60], it can be said that dietary interventions may help prevent both motor and non-motor symptoms in PD [19].
Polyphenols and microbiota
Dietary phenolics and polyphenols are natural compounds found in foods such as vegetables, fruit, cereals, tea, coffee, and wine [69]. Phenolics are characterized by having at least one aromatic ring with one or more hydroxyl groups attached. Phenolics occurring naturally can be classified into two large groups: flavonoids and nonflavonoids [70,71,72]. The rate of total polphenol absorption in the small intestine after deconjugation reactions such as deglycosylation is relatively low (5–10%) in comparison with other macro- or micronutrients [73, 74]. The remaining 90–95% of polyphenols, which are not absorbed, produce various metabolites of physiological importance in the colon [74]. The interaction between the phenolic compounds as dietary components and gut microbiota has gained a lot of attention in the last few years [75, 76]. Some polyphenols, such as flavan-3-ols, proanthocyanidins, and hydrolyzable tannins (ellagitannins) have been demonstrated to exert both selective prebiotic effects and selective antimicrobial effects against pathogenic gut bacteria [77]. First, polyphenols are biotransformed into their metabolites by gut microbiota, which results in the increased bioavailability of polyphenols. Second, they modulate the composition of the gut microbial population, mostly through the inhibition of pathogenic bacteria and the stimulation of beneficial bacteria. In the latter, they may act as prebiotic metabolites and enrich the beneficial bacteria [9]. Therefore, the interaction between dietary polyphenols and gut microbiota may result in an impact on the health of the human host [78].
Polyphenols are deconjugated by bacterial glycosidases, glucuronidases, in addition to sulfatases and further fermented to a wide range of low-molecular-weight phenolic acids. Therefore, the gut microbiota has a critical role in the bioavailability of polyphenols and has been shown to regulate the health-promoting activity through conversion into more active derivatives [79]. In addition to anti-inflammatory, antioxidant, antiproliferative, antidiabetic and antimicrobial effects [80], polyphenols have been reported to exert their neuroprotective actions via the potential to protect neurons against injury induced by neurotoxins, via an ability to suppress neuroinflammation and the potential to promote memory, learning, and cognitive function [81]. Along with all these effects, there are also important roles in the microbiota. The bioactive components of tea rich in polyphenols have been found to inhibit the growth of pathogenic bacteria such as H. pylori, [22] Staphylococcus aureus, and E. coli [82]. It has been found that citrus polyphenols such as hesperidin, naringenin, poncirin, and diosmetin have an inhibitory effect on the growth of H. pylori [83]. Queipo-Ortuño et al. [84] evaluated the effect of a moderate intake of red wine polyphenols on select gut microbial groups implicated in host health benefits. They found that compared with baseline, daily consumption of red wine polyphenols for 4 weeks significantly increased the numbers of Enterococcus, Prevotella, Bacteroides, Bifidobacterium, Bacteroides uniformis, Eggerthella lenta, and Blautia coccoides-Eubacterium rectale groups. To the best of our knowledge, the effect of polyphenol intake on the gut microbiota of PD patients has not been examined. However, a study has reported that the influence of the bioavailability of flavonoid metabolites may interfere with the misfolding of alpha (α)-synuclein, a process that plays a central role in Parkinson’s disease and other α-synucleinopathies. The study worked on two experimental groups of humanized gnotobiotic mice with compositionally diverse gut bacteria were orally treated with a flavanol-rich preparation (FRP). The study demonstrated that gnotobiotic mice with compositionally diverse human microbiotas generate unique phenolic acid profiles in the cecum after orally consuming FRP flavanols [85]. So, polyphenols might influence the microbiota through their effects on the high levels of H. pylori [86] and low levels of Prevotella [9] in PD. In addition, polyphenols could be used in therapies for PD with respect to the impact of inflammation and α-syn misfolding. Epidemiologic studies have found an inverse relationship between the consumption of high amounts of vegetables and fruits in PD [87, 88]. For this reason, increasing the consumption of vegetables and fruits in the prevention of PD could have positive effects on the intestinal microbiota composition by increasing the intake of antioxidant vitamins and polyphenols.
The Mediterranean diet and western diets
The Mediterranean diet (MD) is regarded as one of the healthiest eating patterns [25]. It is characterized by high amounts of vegetables, fruits, grains, fish, and polyunsaturated fatty acids, and is especially based on olive oil and moderate wine consumption [25, 89]. Many components of MD have positive effects on health and can lead to a reduction in the incidence of many diseases, such as cancer, obesity, metabolic syndrome, cardiovascular diseases, neurodegenerative diseases, diabetes, and inflammatory bowel disease [90]. The possible biological mechanisms of MD that prevent chronic diseases are attributed to the consumption of components such as antioxidants, polyphenols, and monounsaturated-polyunsaturated fatty acids [91, 92]. MD are the main determinants of intestinal microbial diversity, and dietary components affect both the microbial population and their metabolic activities in the early stages of life [25]. There are some contradictory results regarding compliance with MD in PD. In a case-control study, there was no difference in adherence to the Mediterranean diet in patients with PD [93], whereas in another study, adherence to MD in patients with PD was found to be lower than that in the control group [23]. In addition, little is known about the effects of diet patterns, dietary components, and nutrients on the gut microbiota. A higher ratio of Firmicutes–Bacteroidetes was related to lower adherence to MD, and greater presence of Bacteroidetes was associated with lower animal protein intake [24]. High-level consumption of plant-derived foods consistent with (MD) is associated with beneficial microbiome-related metabolomic profiles [94]. Prevotella concentrations were significantly reduced in PD patients [7, 9]. Prevotella is associated with plant-rich diets (high levels of complex carbohydrates and fruit/vegetable intake) [24, 95] along with fiber intake [96, 97]. For this reason, the impact of a Mediterranean diet pattern on microbiota may be very important in patients with PD.
The Western diet is characterized by high intake of protein (animal and processed meat products), saturated fat, refined grain, sugar, alcohol, salt, and high fructose corn syrup and low intake of fruits and vegetables [98, 99]. It promotes inflammation that arises from both structural and behavioral changes in the resident microbiome [100]. The Western diet can lead to increased levels of endotoxin-producing bacteria in the intestinal tracts of both humans and mice, resulting in metabolic endotoxemia [101, 102]. Lipopolysaccharides (LPS), commonly referred to as endotoxins, are components of the cell wall of gram-negative bacteria found in the gut microbiota [103]. It has been found that the Western diet induces changes in the barrier function mechanism associated with metabolic endotoxemia in rats [104]. Pro-inflammatory stimulants of Toll-like receptor-2 and Toll-like receptor-4 (pathogen-associated molecular patterns, PAMPs), are abundant in some processed foods [105]. Diet-induced inflammation could be mediated partly by the PAMPs produced by microbes in processed foods. PAMPs (such as LPS and other Toll like receptor stimulants) arise from bacterial growth during the process between food preparation and heat treatment, which are likely to be extended in industrial processing compared with home cooking [100].
There are some contradictory results regarding compliance with the Western diet in PD. In an epidemiological study utilizing the Health Professionals Follow-Up Study (1986–2002) and Nurses’ Health Study (1984–2000), 49,692 men and 81,676 women free of (PD) at baseline were included, and 508 of them were diagnosed with PD after 16 years of follow up. In the study, the prudent dietary pattern, characterized by high intake of fruit, vegetables, and fish, was inversely associated with PD risk, but the Western pattern was not [88]. Although there have not been any studies in the literature that have assessed the relationship between intestinal microbiota and the Western diet in PD, the effect of the Western diet on microbiota, has been evaluated in various studies [106, 107]. The gut microbiota diversity and composition were remarkably changed in apolipoprotein E (apoE) knockout (KO) mice compared with wild-type (WT) mice, especially on a Western diet. Firmicutes and Clostridia (from class to family) were found to be enriched in apoE KO mice on a Western diet [106]. The relative abundance of Firmicutes could result in an increased amount of metabolic endotoxins such as lipopolysaccharides [108]. A Western diet could aggravate the inflammatory process and endotoxin-production, so these mechanisms may explain the impact on disease onset or prognosis of PD.
COMPLEMENTARY THERAPEUTIC APPROACHES
Probiotics
Probiotics that have essential beneficial effects on human health are defined as live microorganisms. In the early 20th century, Metchnikoff discovered “healthy bacteria”, and the interest in probiotics in food markets has increased [109]. The requirements that a probiotic organism should meet are as follows: resistance to gastric acidity, resistance to bile and pancreatic enzymes, adherence to intestinal mucosa cells, colonization capacity, remain alive for long periods during transportation and storage so they can effectively colonize a host, production of antimicrobial substances against pathogenic bacteria, and absence of translocation [110]. Probiotics are one of the treatment modalities that can be involved in the modulation of intestinal microbiota [111]. The most commonly used probiotic microorganisms are lactobacilli, enterococci, bifidobacteria, and a mixture of different beneficial bacteria [112]. The findings of gut microbiota in constipation are inconsistent [113, 114] and currently no consensus exists. In one study, Bifidobacterium and Lactobacillus were shown to be significantly less abundant in adult patients with constipation [113]. However, in a cross-sectional pilot study, the conventional probiotic genera Lactobacillus and bifidobacteria were not decreased in the microbiomes of constipated patients, and a significantly decreased abundance of Prevotella and increased presence of several genera of Firmicutes were observed in constipated patients compared with controls [114]. In a systematic review and meta-analysis including 14 randomized controlled trials, various Lactobacillus and Bifidobacterium strains were found to have the potential to have a positive effect on functional constipation by improving gut transit time, stool frequency, and stool consistency [115]. Regarding the use of probiotics for the treatment of patients with PD, the studies to date are inadequate. Cassani et al. [116] assessed the effects of milk fermented with the probiotic strain Lactobacillus casei Shirota in PD patients with constipation according to the Rome III criteria. After probiotic intake, there was a statistically significant increase in the frequency of stool and normal consistency and significant reduction the number of patients who felt bloating, abdominal pain, and experienced a sensations of incomplete emptying. On the other hand, probiotics play an important role in providing normal microbial balance. It has been reported that they have a potential role in the treatment along with the prevention of anxiety and depression via the brain-gut axis [28]. In addition, gut microbiota can affect various neurological outcomes, such as cognition, learning, and memory [117]. In a double-blind, placebo-controlled clinical trial that included 40 patients with a diagnosis of major depressive disorder (MDD) based on the (DSM-IV) and whose ages ranged between 20 and 55 year, the suplementation group received Lactobacillus acidophilus (2 × 109 CFU/g), Lactobacillus casei (2 × 109 CFU/g), and Bifidobacterium bifidum (2 × 109 CFU/g) in a capsule for 8 weeks. Probiotic administration in patients with MDD for 8 weeks had beneficial effects on the Beck’s Depression Inventory and some metabolic markers [118]. Bifidobacteria treatment also resulted in a reduced 5-hyroxyindoleacetic acid concentration in the frontal cortex and a decrease in dihydroxyphenylacetic acid in the amygdaloid cortex. The attenuation of pro-inflammatory immune responses and the elevation of the serotonergic precursor tryptophan by bifidobacteria treatment provides encouraging evidence in support of the proposition that this probiotic might have acquire antidepressant properties [119]. The effects of probiotics on severe cognitive disorders and metabolic disorders in Alzheimer’s patients have also been investigated. In a randomized, double-blinded, and controlled clinical trial, patients were administered either milk (control group) or a mixture of probiotics (probiotic group). The probiotic supplemented group took 200 mL/day probiotic milk containing Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum (2 × 109 CFU/g for each) for 12 weeks. The Mini-Mental State Examination (MMSE) score was recorded for all subjects before and after the treatment. After 12 a week of the intervention, the probiotic-treated patients showed a significant improvement score compared with the control group in the MMSE score [120]. In a randomized, double-blind, placebo-controlled clinical trial was conducted on 50 patients with PD, participants were randomly allocated into two groups to take either 8 × 109 CFU/day of a probiotics containing, Lactobacillus acidophilus, Bifidobacterium bifidum, L. reuteri, and Lactobacillus fermentum or a placebo (n=25 each group, one capsule daily) for 12 weeks. Probiotic supplementation for 12 weeks in PD patients significantly downregulated gene expression of proinflammatory IL-1, IL-8, TNF-α; and upregulated TGF-β along with peroxisome proliferator-activated receptor gamma (PPAR-γ). However, probiotic supplementation did not affect the gene expression of Vascular endothelial growth factor (VEGF) and low-density lipoprotein receptor (LDLR) [121]. Another randomized, double-blind, placebo-controlled clinical trial, the impact of probiotic supplementation (8 × 109 CFU/day probiotic or placebo) for 12 weeks on the movement and the metabolic parameters were evaluated in 60 people with PD. Compared with the placebo, consuming the probiotics decreased the Movement Disorders Society-Unified the Parkinson’s Disease Rating Scale (MDS-UPDRS) scores; reduced high-sensitivity C-reactive protein, malondialdehyde, and insulin levels, insulin resistance, enhanced glutathione levels, and insulin sensitivity [122]. Therefore, it is not possible to give a clear recommendation for probiotic supplementation in patients with PD, due to insufficiency of the randomized controlled trials to date, lack of knowledge about the effect on levodopa, and insufficient knowledge of the recommended dose amount, optimal duration of treatment, and reliability.
Prebiotics
Prebiotics are dietary substances (mostly consisting of nonstarch polysaccharides and oligosaccharides poorly digested by human enzymes) which nurture a selected group of microorganisms living in the gut. They favor the growth of beneficial bacteria over that of harmful ones [123]. An ideal prebiotic should 1) be resistant to the action of acids in the stomach, bile salts, and other hydrolyzing enzymes in the intestines, 2) not be absorbed in the upper gastrointestinal tract, 3) be easily fermentable by the beneficial intestinal microflora [124, 125]. Prebiotics are nondigestible nutrients. Short-chain nondigestible carbohydrates, inulin-type fructans, fructooligosaccharides (FOS), and galactooligosaccharides (GOS) are the most important prebiotics. They target bacterial groups which are generally Bifidobacterium and Lactobacillus. Fructans such as inulin and FOS are naturally present in different foods [126, 127]. Prebiotic forms a group of diverse carbohydrate ingredients. These include vegetables, fruits, cereals, and other edibles of plant origins. Some of the sources of prebiotics include tomatoes, artichokes, bananas, asparagus, berries, garlic, onions, chicory, green vegetables, and cereals such as oats, flax seeds, and barley [128].
Prebiotics have beneficial effects on cardiovascular diseases, type 2 diabetes/glycemic control, appetite control, obesity, cancer, immune function, and inflammation [129]. Inulin-like fructan consumption can also be beneficial because it stimulate intestinal movements by affecting microflora [130] in PD patients with constipation. Very recently, it has been demonstrated that treatment with microbial-produced SCFAs could rescue impaired microglial function impaired in GF animals [131]. It has been suggested that SCFAs resulting from fermentation of dietary fiber could have epigenetic and neuromodulatory effects through histone acetylation and improve cognitive functions for neurodevelopmental and neurodegenerative diseases [132]. In a study by Savignac and colleagues [133], healthy rats were gavaged with FOS, GOS, or water for five weeks. After prebiotic intake, it was found that the increase of hippocampal brain-derived neurotrophic factor (BDNF) and increased N-methyl-d-aspartate receptor (NMDAR) subunits translate to improved cognitive performance. This study demonstrates that prebiotics may play a role in the neurological preservation of the CNS. There are no studies that have evaluated the effects of prebiotics in PD alone. However, prebiotics could be considered an effective treatment for both constipation and gastrointestinal disturbance.
Synbiotics
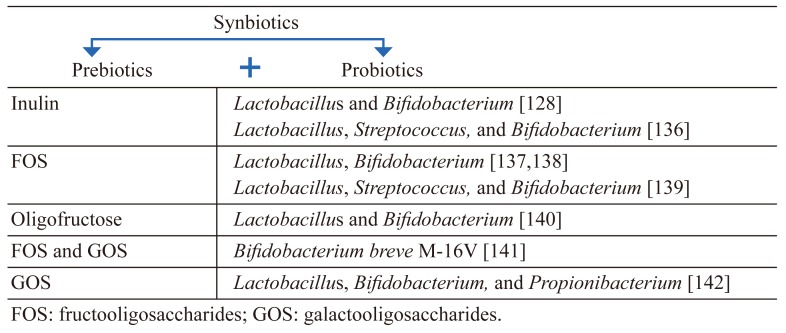
The word ‘synbiotics’ alludes to synergism, and this term should be reserved for products in which prebiotic compound(s) selectively favors the probiotic organism(s) [134]. Synbiotics have both probiotic and prebiotic properties. They were also created to overcome some possible difficulties in the survival of probiotics in the gastrointestinal tract. The principal purpose of this type of combination is the improvement of the survival of probiotic microorganisms in the gastrointestinal tract [127]. Polysaccharides such as inulin, reflux starch, cellulose, hemicellulose, and pectin may potentially be prebiotics. Examples of prebiotics and probiotics most commonly used together as synbiotics are are indicated in Table 2 (Adapted [128, 135, 136]). Synbiotic intake may efficiently restore the balance of gut microbiota and improve gastrointestinal functions. A randomized, placebo-controlled trial suggested that dietary supplementation with a synbiotic (the synbiotic [BIFICOPEC]containing 0.63 g of bifid triple viable capsules [BIFICO] and 8 g of soluble dietary fiber) improved evacuation-parameters-associated symptoms and colonic motility in patients with slow transit constipation [137]. GIS-related problems in particular are of great importance for patients with PD. For example, in a randomized, double-blind, placebo-controlled trial, on PD patients with Rome III-confirmed constipation based on 2-week stool diary data at baseline, patients were randomly assigned (80 in the experimental group, 40 placebo group) to either fermented milk containing multiple probiotic strains and prebiotic fiber, or placebo once daily for 4 weeks. Consumption of the fermented milk containing probiotics and prebiotics resulted in a higher increase in the number of bowel movements and relieved constipation [138]. The coexistence of prebiotics and probiotic microorganisms may be a therapeutic approach to the prevention and treatment of many diseases, including PD, in terms of the ability to regulate the microbiota composition.
Table 2. Examples of synbiotics used in human nutrition (Adapted [128, 135, 136]).
CONCLUSION
Little is known about the role of the molecular causes of altered homeostasis in PD. However, in recent evidence, some motor and non-motor symptoms observed in PD may be attributed to intestinal microbiota dysbiosis. PD patients are characterized by an altered gut microbiota composition and impairment of the intestinal barrier and enteric neuroimmune system that result in enteric inflammation which contributes to neuroinflammation and neurodegeneration in CNS.
Recent advances have highlighted in the understanding of probiotic modulation of neurological and neuropsychiatric disorders via the gut-brain axis. The intestinal microbiota can directly or indirectly alter the neurochemistry of the brain by influencing different physiological and behavioral outcomes through modulation of neuroendocrine pathways. The diversity and amount of microorganisms in the gut may affect both the ENS and CNS. Therefore, a better understanding of the gut-brain axis may prevent the pathogenesis of the disease. In this context, dietary approaches, probiotics, prebiotics, and synbiotics will play an important role. However, the studies conducted on the microbiota-gut-brain axis in patients with PD to date are inadequate, and randomized controlled trials are needed to evaluate nutritional or probiotic supplementation for gut microbiota dysbiosis.
REFERENCES
- 1.Toulouse A, Sullivan AM. 2008. Progress in Parkinson’s disease-where do we stand? Prog Neurobiol 85: 376–392. [DOI] [PubMed] [Google Scholar]
- 2.Mulak A, Bonaz B. 2015. Brain-gut-microbiota axis in Parkinson’s disease. World J Gastroenterol 21: 10609–10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park H, Lee JY, Shin CM, Kim JM, Kim TJ, Kim JW. 2015. Characterization of gastrointestinal disorders in patients with parkinsonian syndromes. Parkinsonism Relat Disord 21: 455–460. [DOI] [PubMed] [Google Scholar]
- 4.Sherwin E, Dinan TG, Cryan JF. 2018. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann N Y Acad Sci 1420: 5–25. [DOI] [PubMed] [Google Scholar]
- 5.Klingelhoefer L, Reichmann H. 2015. Pathogenesis of Parkinson disease—the gut-brain axis and environmental factors. Nat Rev Neurol 11: 625–636. [DOI] [PubMed] [Google Scholar]
- 6.Nair AT, Ramachandran V, Joghee NM, Antony S, Ramalingam G. 2018. Gut microbiota dysfunction as reliable non-invasive early diagnostic biomarkers in the pathophysiology of Parkinson’s disease: a critical review. J Neurogastroenterol Motil 24: 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P. 2015. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30: 350–358. [DOI] [PubMed] [Google Scholar]
- 8.Orimo S, Ghebremedhin E, Gelpi E. 2018. Peripheral and central autonomic nervous system: does the sympathetic or parasympathetic nervous system bear the brunt of the pathology during the course of sporadic PD? Cell Tissue Res 373: 267–286. [DOI] [PubMed] [Google Scholar]
- 9.Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Bürmann J, Faßbender K, Schwiertz A, Schäfer KH. 2016. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 32: 66–72. [DOI] [PubMed] [Google Scholar]
- 10.Clairembault T, Leclair-Visonneau L, Neunlist M, Derkinderen P. 2015. Enteric glial cells: new players in Parkinson’s disease? Mov Disord 30: 494–498. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Pardo P, Hartog M, Garssen J, Kraneveld AD. 2017. Microbes tickling your tummy: the importance of the gut-brain axis in Parkinson’s disease. Curr Behav Neurosci Rep 4: 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. 2014. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci 34: 15490–15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, Peddada SD, Factor SA, Molho E, Zabetian CP, Knight R, Payami H. 2017. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord 32: 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu X, Wang T, Jin F. 2016. Alzheimer’s disease and gut microbiota. Sci China Life Sci 59: 1006–1023. [DOI] [PubMed] [Google Scholar]
- 15.Jiang C, Li G, Huang P, Liu Z, Zhao B. 2017. The gut microbiota and Alzheimer’s disease. J Alzheimers Dis 58: 1–15. [DOI] [PubMed] [Google Scholar]
- 16.Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF. 2017. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res 179: 223–244. [DOI] [PubMed] [Google Scholar]
- 17.Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. 2015. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 9: 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins TA, Nguyen JC, Polglaze KE, Bertrand PP. 2016. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients 8: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Pardo P, Dodiya HB, Broersen LM, Douna H, van Wijk N, Lopes da Silva S, Garssen J, Keshavarzian A, Kraneveld AD. 2018. Gut-brain and brain-gut axis in Parkinson’s disease models: Effects of a uridine and fish oil diet. Nutr Neurosci 21: 391–402. [DOI] [PubMed] [Google Scholar]
- 20.Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, Shannon KM, Shannon KM. 2015. Colonic bacterial composition in Parkinson’s disease. Mov Disord 30: 1351–1360. [DOI] [PubMed] [Google Scholar]
- 21.Rajoka MSR, Shi J, Mehwish HM, Zhu J, Li Q, Shao D, Huang Q, Yang H. 2017. Interaction between diet composition and gut microbiota and its impact on gastrointestinal tract health. Food Science and Human Wellness 6: 121–130. [Google Scholar]
- 22.Ankolekar C, Johnson D, Pinto MS, Johnson K, Labbe R, Shetty K. 2011. Inhibitory potential of tea polyphenolics and influence of extraction time against Helicobacter pylori and lack of inhibition of beneficial lactic acid bacteria. J Med Food 14: 1321–1329. [DOI] [PubMed] [Google Scholar]
- 23.Alcalay RN, Gu Y, Mejia-Santana H, Cote L, Marder KS, Scarmeas N. 2012. The association between Mediterranean diet adherence and Parkinson’s disease. Mov Disord 27: 771–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Mantrana I, Selma-Royo M, Alcantara C, Collado MC. 2018. Shifts on gut microbiota associated to Mediterranean diet adherence and specific dietary intakes on general adult population. Front Microbiol 9: 890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Chierico F, Vernocchi P, Dallapiccola B, Putignani L. 2014. Mediterranean diet and health: food effects on gut microbiota and disease control. Int J Mol Sci 15: 11678–11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherwin E, Sandhu KV, Dinan TG, Cryan JF. 2016. May the force be with you: the light and dark sides of the microbiota–gut–brain axis in neuropsychiatry. CNS Drugs 30: 1019–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marizzoni M, Provasi S, Cattaneo A, Frisoni GB. 2017. Microbiota and neurodegenerative diseases. Curr Opin Neurol 30: 630–638. [DOI] [PubMed] [Google Scholar]
- 28.Clapp M, Aurora N, Herrera L, Bhatia M, Wilen E, Wakefield S. 2017. Gut microbiota’s effect on mental health: the gut-brain axis. Clin Pract 7: 987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonaz BL, Bernstein CN. 2013. Brain-gut interactions in inflammatory bowel disease. Gastroenterology 144: 36–49. [DOI] [PubMed] [Google Scholar]
- 30.Agustí A, García-Pardo MP, López-Almela I, Campillo I, Maes M, Romaní-Pérez M, Sanz Y. 2018. Interplay between the gut-brain axis, obesity and cognitive function. Front Neurosci 12: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S, Hibberd ML, Forssberg H, Pettersson S. 2011. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA 108: 3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. 2014. Minireview: Gut microbiota: the neglected endocrine organ. Mol Endocrinol 28: 1221–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittal R, Debs LH, Patel AP, Nguyen D, Patel K, O’Connor G, Grati M, Mittal J, Yan D, Eshraghi AA, Deo SK, Daunert S, Liu XZ. 2017. Neurotransmitters: the critical modulators regulating gut-brain axis. J Cell Physiol 232: 2359–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. 2016. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167: 1469–1480.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spielman LJ, Gibson DL, Klegeris A. 2018. Unhealthy gut, unhealthy brain: the role of the intestinal microbiota in neurodegenerative diseases. Neurochem Int 120: 149–163. [DOI] [PubMed] [Google Scholar]
- 36.Sun MF, Shen YQ. 2018. Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s disease. Ageing Res Rev 45: 53–61. [DOI] [PubMed] [Google Scholar]
- 37.Hasegawa S, Goto S, Tsuji H, Okuno T, Asahara T, Nomoto K, Shibata A, Fujisawa Y, Minato T, Okamoto A, Ohno K, Hirayama M. 2015. Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson’s disease. PLoS One 10: e0142164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrov VA, Saltykova IV, Zhukova IA, Alifirova VM, Zhukova NG, Dorofeeva YB, Tyakht AV, Kovarsky BA, Alekseev DG, Kostryukova ES, Mironova YS, Izhboldina OP, Nikitina MA, Perevozchikova TV, Fait EA, Babenko VV, Vakhitova MT, Govorun VM, Sazonov AE. 2017. Analysis of gut microbiota in patients with Parkinson’s disease. Bull Exp Biol Med 162: 734–737. [DOI] [PubMed] [Google Scholar]
- 39.Lin A, Zheng W, He Y, Tang W, Wei X, He R, Huang W, Su Y, Huang Y, Zhou H, Xie H. 2018. Gut microbiota in patients with Parkinson’s disease in southern China. Parkinsonism Relat Disord 53: 82–88. [DOI] [PubMed] [Google Scholar]
- 40.Qian Y, Yang X, Xu S, Wu C, Song Y, Qin N, Chen SD, Xiao Q. 2018. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav Immun 70: 194–202. [DOI] [PubMed] [Google Scholar]
- 41.Li W, Wu X, Hu X, Wang T, Liang S, Duan Y, Jin F, Qin B. 2017. Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci China Life Sci 60: 1223–1233. [DOI] [PubMed] [Google Scholar]
- 42.Hopfner F, Künstner A, Müller SH, Künzel S, Zeuner KE, Margraf NG, Deuschl G, Baines JF, Kuhlenbäumer G. 2017. Gut microbiota in Parkinson disease in a northern German cohort. Brain Res 1667: 41–45. [DOI] [PubMed] [Google Scholar]
- 43.Heintz-Buschart A, Pandey U, Wicke T, Sixel-Döring F, Janzen A, Sittig-Wiegand E, Trenkwalder C, Oertel WH, Mollenhauer B, Wilmes P. 2018. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord 33: 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bedarf JR, Hildebrand F, Coelho LP, Sunagawa S, Bahram M, Goeser F, Bork P, Wüllner U. 2017. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson’s disease patients. Genome Med 9: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopetuso LR, Napoli M, Rizzatti G, Scaldaferri F, Franceschi F, Gasbarrini A. 2018. Considering gut microbiota disturbance in the management of Helicobacter pylori infection. Expert Rev Gastroenterol Hepatol 12: 899–906. [DOI] [PubMed] [Google Scholar]
- 46.Schulz C, Schütte K, Koch N, Vilchez-Vargas R, Wos-Oxley ML, Oxley APA, Vital M, Malfertheiner P, Pieper DH. 2018. The active bacterial assemblages of the upper GI tract in individuals with and without Helicobacter infection. Gut 67: 216–225. [DOI] [PubMed] [Google Scholar]
- 47.Vizcarra JA, Wilson-Perez HE, Espay AJ. 2015. The power in numbers: gut microbiota in Parkinson’s disease. Mov Disord 30: 296–298. [DOI] [PubMed] [Google Scholar]
- 48.Ghaisas S, Maher J, Kanthasamy A. 2016. Gut microbiome in health and disease: linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol Ther 158: 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Evrensel A, Ceylan ME. 2016. Fecal microbiota transplantation and its usage in neuropsychiatric disorders. Clin Psychopharmacol Neurosci 14: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun MF, Zhu YL, Zhou ZL, Jia XB, Xu YD, Yang Q, Cui C, Shen YQ. 2018. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav Immun 70: 48–60. [DOI] [PubMed] [Google Scholar]
- 51.Jones JM. 2014. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap’. Nutr J 13: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lombardi VC, De Meirleir KL, Subramanian K, Nourani SM, Dagda RK, Delaney SL, Palotás A. 2018. Nutritional modulation of the intestinal microbiota; future opportunities for the prevention and treatment of neuroimmune and neuroinflammatory disease. J Nutr Biochem 61: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams BA, Grant LJ, Gidley MJ, Mikkelsen D. 2017. Gut fermentation of dietary fibres: physico-chemistry of plant cell walls and implications for health. Int J Mol Sci 18: 2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van de Wouw M, Schellekens H, Dinan TG, Cryan JF. 2017. Microbiota-gut-brain axis: modulator of host metabolism and appetite. J Nutr 147: 727–745. [DOI] [PubMed] [Google Scholar]
- 55.Verbeke K, Ferchaud-Roucher V, Preston T, Small AC, Henckaerts L, Krempf M, Wang H, Vonk RJ, Priebe MG. 2010. Influence of the type of indigestible carbohydrate on plasma and urine short-chain fatty acid profiles in healthy human volunteers. Eur J Clin Nutr 64: 678–684. [DOI] [PubMed] [Google Scholar]
- 56.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Doré J, Antolín M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Mérieux A, Melo Minardi R, M’rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P, MetaHIT Consortium.2011. Enterotypes of the human gut microbiome. Nature 473: 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB, Keshavarzian A. 2011. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One 6: e28032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelly LP, Carvey PM, Keshavarzian A, Shannon KM, Shaikh M, Bakay RA, Kordower JH. 2014. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov Disord 29: 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tan LC, Methawasin K, Tan EK, Tan JH, Au WL, Yuan JM, Koh WP. 2016. Dietary cholesterol, fats and risk of Parkinson’s disease in the Singapore Chinese Health Study. J Neurol Neurosurg Psychiatry 87: 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miyake Y, Sasaki S, Tanaka K, Fukushima W, Kiyohara C, Tsuboi Y, Yamada T, Oeda T, Miki T, Kawamura N, Sakae N, Fukuyama H, Hirota Y, Nagai M, Fukuoka Kinki Parkinson’s Disease Study Group.2010. Dietary fat intake and risk of Parkinson’s disease: a case-control study in Japan. J Neurol Sci 288: 117–122. [DOI] [PubMed] [Google Scholar]
- 61.Wu A, Ying Z, Gomez-Pinilla F. 2004. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma 21: 1457–1467. [DOI] [PubMed] [Google Scholar]
- 62.Hashimoto M, Tanabe Y, Fujii Y, Kikuta T, Shibata H, Shido O. 2005. Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognition learning ability in amyloid β-infused rats. J Nutr 135: 549–555. [DOI] [PubMed] [Google Scholar]
- 63.Pu S, Khazanehei H, Jones PJ, Khafipour E. 2016. Interactions between obesity status and dietary intake of monounsaturated and polyunsaturated oils on human gut microbiome profiles in the canola oil multicenter intervention trial (COMIT). Front Microbiol 7: 1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noriega BS, Sanchez-Gonzalez MA, Salyakina D, Coffman J. 2016. Understanding the impact of omega-3 rich diet on the gut microbiota. Case Rep Med 2016: 3089303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Byerley LO, Samuelson D, Blanchard E, 4th, Luo M, Lorenzen BN, Banks S, Ponder MA, Welsh DA, Taylor CM. 2017. Changes in the gut microbial communities following addition of walnuts to the diet. J Nutr Biochem 48: 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller RL, James-Kracke M, Sun GY, Sun AY. 2009. Oxidative and inflammatory pathways in Parkinson’s disease. Neurochem Res 34: 55–65. [DOI] [PubMed] [Google Scholar]
- 67.Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Björklund T, Wang ZY, Roybon L, Melki R, Li JY. 2014. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 128: 805–820. [DOI] [PubMed] [Google Scholar]
- 68.Kamel F, Goldman SM, Umbach DM, Chen H, Richardson G, Barber MR, Meng C, Marras C, Korell M, Kasten M, Hoppin JA, Comyns K, Chade A, Blair A, Bhudhikanok GS, Webster Ross G, William Langston J, Sandler DP, Tanner CM. 2014. Dietary fat intake, pesticide use, and Parkinson’s disease. Parkinsonism Relat Disord 20: 82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pandey KB, Rizvi SI. 2009. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2: 270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neveu V, Perez-Jiménez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, Scalbert A. 2010. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford) 2010: bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Andrés-Lacueva C, Medina-Remon A, Llorach R, Urpi-Sarda M, Khan N, Chiva-Blanch G, Zamora-Ros R, Rotches-Ribalta M, Lamuela-Raventos RM. 2010. Phenolic compounds: chemistry and occurrence in fruits and vegetables. Fruit and vegetable phytochemicals: Chemistry, nutritional value and stability. 53–80.
- 72.Serra D, Almeida LM, Dinis TC. 2018. Dietary polyphenols: a novel strategy to modulate microbiota-gut-brain axis. Trends Food Sci Technol 78: 224–233. [Google Scholar]
- 73.Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. 2005. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 81Suppl: 230S–242S. [DOI] [PubMed] [Google Scholar]
- 74.Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI. 2013. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem 24: 1415–1422. [DOI] [PubMed] [Google Scholar]
- 75.Mayta-Apaza AC, Pottgen E, De Bodt J, Papp N, Marasini D, Howard L, Abranko L, Van de Wiele T, Lee SO, Carbonero F. 2018. Impact of tart cherries polyphenols on the human gut microbiota and phenolic metabolites in vitro and in vivo. J Nutr Biochem 59: 160–172. [DOI] [PubMed] [Google Scholar]
- 76.Popa DE, Drăgoi CM, Arsene AL, Dumitrescu IB, Nicolae AC, Velescu BS, Burcea-Dragomiroiu GT. 2017. The relationship between phenolic compounds from diet and microbiota. In Phenolic Compounds-Biological Activity. [Google Scholar]
- 77.Espín JC, González-Sarrías A, Tomás-Barberán FA. 2017. The gut microbiota: a key factor in the therapeutic effects of (poly)phenols. Biochem Pharmacol 139: 82–93. [DOI] [PubMed] [Google Scholar]
- 78.Ozdal T, Sela DA, Xiao J, Boyacıoğlu D, Chen F, Capanoglu E. 2016. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 8: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dudek-Wicher RK, Junka A, Bartoszewicz M. 2018. The influence of antibiotics and dietary components on gut microbiota. Prz Gastroenterol 13: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mahendran G, Manoj M, Prasad KR, Bai VN. 2015. Antioxidants, anti-proliferative, anti-inflammatory, anti-diabetic and anti-microbial effects of isolated compounds from Swertia corymbosa (Grieb.) Wight ex CB Clark—an in vitro approach. Food Science and Human Wellness 4: 169–179. [Google Scholar]
- 81.Vauzour D. 2012. Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxid Med Cell Longev 2012: 914273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakayama M, Shigemune N, Tsugukuni T, Jun H, Matsushita T, Mekada Y, Kurahachi M, Miyamoto T. 2012. Mechanism of the combined anti-bacterial effect of green tea extract and NaCl against Staphylococcus aureus and Escherichia coli O157: H7. Food Control 25: 225–232. [Google Scholar]
- 83.Bae EA, Han MJ, Kim DH. 1999. In vitro anti-Helicobacter pylori activity of some flavonoids and their metabolites. Planta Med 65: 442–443. [DOI] [PubMed] [Google Scholar]
- 84.Queipo-Ortuño MI, Boto-Ordóñez M, Murri M, Gomez-Zumaquero JM, Clemente-Postigo M, Estruch R, Cardona Diaz F, Andrés-Lacueva C, Tinahones FJ. 2012. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am J Clin Nutr 95: 1323–1334. [DOI] [PubMed] [Google Scholar]
- 85.Ho L, Zhao D, Ono K, Ruan K, Mogno I, Tsuji M, Carry E, Brathwaite J, Sims S, Frolinger T, Westfall S, Mazzola P, Wu Q, Hao K, Lloyd TE, Simon JE, Faith J, Pasinetti GM. 2019. Heterogeneity in gut microbiota drive polyphenol metabolism that influences α-synuclein misfolding and toxicity. J Nutr Biochem 64: 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang HK, Wang JH, Lei WY, Chen CL, Chang CY, Liou LS. 2018. Helicobacter pylori infection is associated with an increased risk of Parkinson’s disease: a population-based retrospective cohort study. Parkinsonism Relat Disord 47: 26–31. [DOI] [PubMed] [Google Scholar]
- 87.Okubo H, Miyake Y, Sasaki S, Murakami K, Tanaka K, Fukushima W, Kiyohara C, Tsuboi Y, Yamada T, Oeda T, Shimada H, Kawamura N, Sakae N, Fukuyama H, Hirota Y, Nagai M, Fukuoka Kinki Parkinson’s Disease Study Group.2012. Dietary patterns and risk of Parkinson’s disease: a case-control study in Japan. Eur J Neurol 19: 681–688. [DOI] [PubMed] [Google Scholar]
- 88.Gao X, Chen H, Fung TT, Logroscino G, Schwarzschild MA, Hu FB, Ascherio A. 2007. Prospective study of dietary pattern and risk of Parkinson disease. Am J Clin Nutr 86: 1486–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Giacosa A, Barale R, Bavaresco L, Gatenby P, Gerbi V, Janssens J, Johnston B, Kas K, La Vecchia C, Mainguet P, Morazzoni P, Negri E, Pelucchi C, Pezzotti M, Rondanelli M. 2013. Cancer prevention in Europe: the Mediterranean diet as a protective choice. Eur J Cancer Prev 22: 90–95. [DOI] [PubMed] [Google Scholar]
- 90.Romagnolo DF, Selmin OI. 2017. Mediterranean diet and prevention of chronic diseases. Nutr Today 52: 208–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aguilera Y, Martin-Cabrejas MA, de Mejia EG. 2016. Phenolic compounds in fruits and beverages consumed as part of the mediterranean diet: their role in prevention of chronic diseases. Phytochem Rev 15: 405–423. [Google Scholar]
- 92.Farooqui AA, Farooqui T. 2018. Effects of Mediterranean diet components on neurodegenerative diseases. In Role of the Mediterranean diet in the brain and neurodegenerative diseases. 1–16. [Google Scholar]
- 93.Cassani E, Barichella M, Ferri V, Pinelli G, Iorio L, Bolliri C, Caronni S, Faierman SA, Mottolese A, Pusani C, Monajemi F, Pasqua M, Lubisco A, Cereda E, Frazzitta G, Petroni ML, Pezzoli G. 2017. Dietary habits in Parkinson’s disease: adherence to Mediterranean diet. Parkinsonism Relat Disord 42: 40–46. [DOI] [PubMed] [Google Scholar]
- 94.De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi C, Turroni S, Cocolin L, Brigidi P, Neviani E, Gobbetti M, O’Toole PW, Ercolini D. 2016. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 65: 1812–1821. [DOI] [PubMed] [Google Scholar]
- 95.Ley RE. 2016. Gut microbiota in 2015: Prevotella in the gut: choose carefully. Nat Rev Gastroenterol Hepatol 13: 69–70. [DOI] [PubMed] [Google Scholar]
- 96.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. 2010. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 107: 14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. 2011. Linking long-term dietary patterns with gut microbial enterotypes. Science 334: 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Statovci D, Aguilera M, MacSharry J, Melgar S. 2017. The impact of Western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front Immunol 8: 838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park Y, Subar AF, Hollenbeck A, Schatzkin A. 2011. Dietary fiber intake and mortality in the NIH-AARP diet and health study. Arch Intern Med 171: 1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zinöcker MK, Lindseth IA. 2018. The Western diet–microbiome-host interaction and its role in metabolic disease. Nutrients 10: 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pendyala S, Walker JM, Holt PR. 2012. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology 142: 1100–1101.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ghosh SS, Bie J, Wang J, Ghosh S. 2014. Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR-/- mice—role of intestinal permeability and macrophage activation. PLoS One 9: e108577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Caroff M, Karibian D. 2003. Structure of bacterial lipopolysaccharides. Carbohydr Res 338: 2431–2447. [DOI] [PubMed] [Google Scholar]
- 104.Guerville M, Leroy A, Sinquin A, Laugerette F, Michalski MC, Boudry G. 2017. Western-diet consumption induces alteration of barrier function mechanisms in the ileum that correlates with metabolic endotoxemia in rats. Am J Physiol Endocrinol Metab 313: E107–E120. [DOI] [PubMed] [Google Scholar]
- 105.Herieka M, Faraj TA, Erridge C. 2016. Reduced dietary intake of pro-inflammatory Toll-like receptor stimulants favourably modifies markers of cardiometabolic risk in healthy men. Nutr Metab Cardiovasc Dis 26: 194–200. [DOI] [PubMed] [Google Scholar]
- 106.Liu B, Zhang Y, Wang R, An Y, Gao W, Bai L, Li Y, Zhao S, Fan J, Liu E. 2018. Western diet feeding influences gut microbiota profiles in apoE knockout mice. Lipids Health Dis 17: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Agus A, Denizot J, Thévenot J, Martinez-Medina M, Massier S, Sauvanet P, Bernalier-Donadille A, Denis S, Hofman P, Bonnet R, Billard E, Barnich N. 2016. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci Rep 6: 19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Poppleton DI, Duchateau M, Hourdel V, Matondo M, Flechsler J, Klingl A, Beloin C, Gribaldo S. 2017. Outer membrane proteome of Veillonella parvula: a diderm firmicute of the human microbiome. Front Microbiol 8: 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Metchnikoff II. 2004. The prolongation of life: optimistic studies. Springer Publishing Company, New York. [Google Scholar]
- 110.Boaventura C, Azevedo R, Uetanabaro A, Nicoli J, Braga LG. 2012. The benefits of probiotics in human and animal nutrition. In New Advances in the Basic and Clinical Gastroenterology. [Google Scholar]
- 111.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. 2014. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11: 506–514. [DOI] [PubMed] [Google Scholar]
- 112.Varankovich NV, Nickerson MT, Korber DR. 2015. Probiotic-based strategies for therapeutic and prophylactic use against multiple gastrointestinal diseases. Front Microbiol 6: 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Khalif IL, Quigley EMM, Konovitch EA, Maximova ID. 2005. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig Liver Dis 37: 838–849. [DOI] [PubMed] [Google Scholar]
- 114.Zhu L, Liu W, Alkhouri R, Baker RD, Bard JE, Quigley EM, Baker SS. 2014. Structural changes in the gut microbiome of constipated patients. Physiol Genomics 46: 679–686. [DOI] [PubMed] [Google Scholar]
- 115.Dimidi E, Christodoulides S, Fragkos KC, Scott SM, Whelan K. 2014. The effect of probiotics on functional constipation in adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 100: 1075–1084. [DOI] [PubMed] [Google Scholar]
- 116.Cassani E, Privitera G, Pezzoli G, Pusani C, Madio C, Iorio L, Barichella M. 2011. Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Gastroenterol Dietol 57: 117–121. [PubMed] [Google Scholar]
- 117.Parashar A, Udayabanu M. 2017. Gut microbiota: implications in Parkinson’s disease. Parkinsonism Relat Disord 38: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, Jafari P, Akbari H, Taghizadeh M, Memarzadeh MR, Asemi Z, Esmaillzadeh A. 2016. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition 32: 315–320. [DOI] [PubMed] [Google Scholar]
- 119.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. 2008. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res 43: 164–174. [DOI] [PubMed] [Google Scholar]
- 120.Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, Tamtaji OR, Hamidi GA, Salami M. 2016. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Front Aging Neurosci 8: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Borzabadi S, Oryan S, Eidi A, Aghadavod E, Daneshvar Kakhaki R, Tamtaji OR, Taghizadeh M, Asemi Z. 2018. The effects of probiotic supplementation on gene expression related to inflammation, insulin and lipid in patients with Parkinson’s disease: a randomized, double-blind, placebo controlled trial. Arch Iran Med 21: 289–295. [PubMed] [Google Scholar]
- 122.Tamtaji OR, Taghizadeh M, Daneshvar Kakhaki R, Kouchaki E, Bahmani F, Borzabadi S, Oryan S, Mafi A, Asemi Z. 2019. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr 38: 1031–1035. [DOI] [PubMed] [Google Scholar]
- 123.Guarner F, Khan AG, Garisch J, Eliakim R, Gangl A, Thomson A, Krabshuis J, Lemair T, Kaufmann P, de Paula JA, Fedorak R, Shanahan F, Sanders ME, Szajewska H, Ramakrishna BS, Karakan T, Kim N, World Gastroenterology Organization.2012. World gastroenterology organisation global guidelines: probiotics and prebiotics october 2011. J Clin Gastroenterol 46: 468–481. [DOI] [PubMed] [Google Scholar]
- 124.Kuo SM. 2013. The interplay between fiber and the intestinal microbiome in the inflammatory response. Adv Nutr 4: 16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pandey KR, Naik SR, Vakil BV. 2015. Probiotics, prebiotics and synbiotics—a review. J Food Sci Technol 52: 7577–7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kovács Z, Benjamins E, Grau K, Rehman AU, Ebrahimi M, Czermak P. 2013. Recent developments in manufacturing oligosaccharides with prebiotic functions. In Biotechnology of food and feed additives. Springer, Berlin, Heidelberg, pp. 257–295. [Google Scholar]
- 127.Conlon MA, Bird AR. 2014. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 7: 17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Markowiak P, Śliżewska K. 2017. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9: 1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Slavin J. 2013. Fiber and prebiotics: mechanisms and health benefits. Nutrients 5: 1417–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Quigley EMM. 2011. The enteric microbiota in the pathogenesis and management of constipation. Best Pract Res Clin Gastroenterol 25: 119–126. [DOI] [PubMed] [Google Scholar]
- 131.Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermöhlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M. 2015. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18: 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stilling RM, Dinan TG, Cryan JF. 2014. Microbial genes, brain & behaviour—epigenetic regulation of the gut-brain axis. Genes Brain Behav 13: 69–86. [DOI] [PubMed] [Google Scholar]
- 133.Savignac HM, Corona G, Mills H, Chen L, Spencer JP, Tzortzis G, Burnet PW. 2013. Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-D-aspartate receptor subunits and D-serine. Neurochem Int 63: 756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cencic A, Chingwaru W. 2010. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2: 611–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McFarlane C, Ramos CI, Johnson DW, Campbell KL. 2019. Prebiotic, probiotic, and synbiotic supplementation in chronic kidney disease: a systematic review and meta-analysis. J Ren Nutr 29: 209–220. [DOI] [PubMed] [Google Scholar]
- 136.Sáez-Lara MJ, Robles-Sanchez C, Ruiz-Ojeda FJ, Plaza-Diaz J, Gil A. 2016. Effects of probiotics and synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: a review of human clinical trials. Int J Mol Sci 17: 928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ding C, Ge X, Zhang X, Tian H, Wang H, Gu L, Gong J, Zhu W, Li N. 2016. Efficacy of synbiotics in patients with slow transit constipation: a prospective randomized trial. Nutrients 8: 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Barichella M, Pacchetti C, Bolliri C, Cassani E, Iorio L, Pusani C, Pinelli G, Privitera G, Cesari I, Faierman SA, Caccialanza R, Pezzoli G, Cereda E. 2016. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: an RCT. Neurology 87: 1274–1280. [DOI] [PubMed] [Google Scholar]
- 139.Asemi Z, Zare Z, Shakeri H, Sabihi SS, Esmaillzadeh A. 2013. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab 63: 1–9. [DOI] [PubMed] [Google Scholar]
- 140.Moroti C, Souza Magri LF, de Rezende Costa M, Cavallini DC, Sivieri K. 2012. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis 11: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.van der Aa LB, Heymans HS, van Aalderen WM, Sillevis Smitt JH, Knol J, Ben Amor K, Goossens DA, Sprikkelman AB, Synbad Study Group.2010. Effect of a new synbiotic mixture on atopic dermatitis in infants: a randomized-controlled trial. Clin Exp Allergy 40: 795–804. [DOI] [PubMed] [Google Scholar]
- 142.Kekkonen RA, Holma R, Hatakka K, Suomalainen T, Poussa T, Adlercreutz H, Korpela R. 2011. A probiotic mixture including galactooligosaccharides decreases fecal β-glucosidase activity but does not affect serum enterolactone concentration in men during a two-week intervention. J Nutr 141: 870–876. [DOI] [PubMed] [Google Scholar]