Abstract
Background:
Blood transfusion therapy is lifesaving for beta-thalassemia major patients, yet it indirectly causes complications such as oxidative stress and liver dysfunction. In the present study, we investigated the effect of quercetin supplementation on oxidative stress and liver function in beta-thalassemia major patients.
Materials and Methods:
In this double-blind clinical trial, 84 beta-thalassemia patients who received desferrioxamine (DFO) were randomly assigned to two groups; the treatment group received 500 mg quercetin tablet daily for 12 weeks, and the control group received placebo. In addition to demographic and anthropometric assessment, malondialdehyde (MDA), total antioxidant capacity (TAC), superoxide dismutase (SOD), glutathione peroxidase (GPx), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) were biochemically assessed to detect the effect of quercetin on oxidative stress and liver function, respectively. The data were analyzed using SPSS 21. P < 0.05 was considered statistically significant.
Results:
Before adjusting for confounding variables, within-group comparison showed that quercetin supplementation reduced ALT (P < 0.001) and TAC (P < 0.001) significantly. Between-group comparison using analysis of covariance analysis though showed that quercetin could significantly reduce ALT (P = 0.002), but there was an insignificant increase in SOD and TAC, and insignificant decrease in GPx, MDA, AST, and ALP (P > 0.05).
Conclusion:
According to our results, consumption of 500 mg quercetin supplement daily for 3 months along with DFO treatment might be able to alter liver function, but not the oxidative stress in beta-thalassemia major patients.
Keywords: Beta-thalassemia, liver, oxidative stress, quercetin
INTRODUCTION
Beta-thalassemia major is a severe, progressive anemia occurs due to defects in the rate of synthesis of beta-globin chains of hemoglobin. Regular blood transfusion is a vital treatment in these patients to prevent the consequences of anemia. Excessive load of iron is an inevitable complication caused by blood transfusion, and this load is way more than the body's capability to remove iron. The first mechanism to reduce free iron is for it to be trapped and saved in the form of ferritin and transferrin. When the iron-binding capacity of these two exceeds, iron deposits in organs such as liver and also generates harmful free radicals which causes tissue and multiorgan damage.[1,2] To avoid the iron overload, chelation therapy with desferrioxamine (DFO), the most widely used iron chelator, is an effective treatment associated with a significant decrease in ferritin level, and the rate of iron-induced complications and can dramatically increase the survival of transfusion-dependent thalassemia patients.[2] However, one-third of the patients are reported to develop an excessive body iron load due to the difficulties in complying with the self-administered subcutaneous infusions 5–6 days a week and lack of access to the iron-chelating drugs.[2,3]
Quercetin, a flavonoid with antioxidative properties, is found in foods such as apples and onions and drinks such as tea and red wine[4] and is consumed 10–100 mg daily through these foods.[5] A large body of evidence has shown its beneficial effects on various conditions and diseases such as allergy and diabetes.[6,7] Previous studies on animal models and in vitro studies have illustrated that administration of this flavonoid ameliorates iron overload due to its iron-chelating ability.[8,9] Furthermore, as an antioxidant, quercetin protects the liver against protein oxidation and lipid peroxidation and decreases oxidative stress.[10] Since various factors affect the bioavailability and metabolism of quercetin and because of disunity about the safety and efficacy of this supplement, the dose and duration of intervention with quercetin must be cautiously chosen in clinical trials.[7] Oral dosage of 100–250 mg quercetin 3 times a day has shown to possess therapeutic benefits, with high tolerability and the least adverse effects.[11]
To the best of our knowledge, no study has examined the effect of quercetin supplementation on the oxidative stress and hepatic status of beta-thalassemia major patients. We therefore investigated the efficacy and safety of quercetin supplementation in transfusion-dependent beta-thalassemia major patients who only use DFO as an iron chelator.
MATERIALS AND METHODS
Patient recruitment
This study was a randomized, placebo-controlled double-blind clinical trial. The study protocol was approved by the Ethical Committee of Iran University of Medical Sciences (95-04-27-30063). This study was also registered in the Iranian Registry of Clinical Trials (IRCT201701172709N43). The sample size was calculated based on comparing two independent means using G-power software (Heinrich-Heine-Universität, Düsseldorf, Germany). Eighty-four thalassemia major patients were voluntarily recruited at Zafar Thalassemia Clinic, Tehran, Iran, from April 2017 to March 2018. The permuted block randomization method was used with quadruple blocks. According to the 84 participants sampled, 21 blocks were produced, and in order to apply the concealment in the randomization process, unique codes were written on the pharmaceutical boxes, which were generated by the software. A university staff who was not aware of the aims of the study and was provided with the codes and tablets, put the tablets in the boxes and gave each a code. By recruiting each individual into this study, the supplement boxes were assigned to the individuals, and neither the researcher nor the patient was aware of the type of treatment. The statistics expert who analyzed the data was also blinded to group assignments. Participants were randomly assigned into two groups, receiving either quercetin (n = 42, 500 mg daily) or starch-containing placebo (n = 42, 500 mg daily) supplement for 12 weeks after lunch. The intervention dosage (500 mg) was determined according to the previous clinical trials using quercetin which was 100–250 mg 3 times a day.[6] Solaray provided us with the quercetin powder (Solaray, USA, CAS Number: 117-39-5) and Pharmaceutical Research Center of Tehran University of Medical Sciences converted the quercetin powder into tablets and made the placebo tablets in the same color, shape, and smell. It has been demonstrated that oral administration of up to 4 g/day quercetin has no side effects in human.[12] We monitored the compliance of the volunteers by counting the returned capsules at the end of the trial. Participants were asked to maintain their usual diet and physical activity during the study.
The inclusion criteria were blood transfusion at least once a month, 18–40 years of age, no intake of multivitamin-mineral supplement during the latter 6 months (except Vitamin C), having regular menstruation in women, and being under exclusive chelation therapy with DFO. Filling the questionnaires incompletely and having <80% compliance of taking the quercetin tablets were the exclusion criteria. Patients with metabolic or infectious diseases, pregnancy and lactation, smoking or alcohol consumption were not recruited to this study. Furthermore, patients who were prescribed with interfering medication (e.g., contraceptives, antihypertensive, anti-glycemic, or anti-inflammatory drug) were excluded from the study. Change in the dose or kind of iron-chelating drug or Vitamin C also led to the exclusion of the patients. All the participants were informed about the purpose of the study and signed a written informed consent. Patients were free to discontinue the trial at any time during the study.
Anthropometric and biochemical assessment
Bodyweight was measured using a scale (Seca, Germany) without shoes and with light clothing. Height was measured by a metric mounted tape with patients wearing no shoes. Body mass index (BMI) was calculated as weight over squared height (kg/m2). Dietary data were collected using three 24-h diet recalls (2 typical days and 1 holiday), and physical activity was assessed using the International Physical Activity Questionnaire Baecke. Other demographic data were extracted from the patient's medical record or obtained from the patients themselves during the interview. All demographic data were measured once before and once after the intervention. Blood samples (12 ml) were collected twice before and after the 12-week supplementation period after 12 h of fasting and at least 10 days after the latest blood transfusion. Samples were then centrifuged at 4000 rpm for 5 min, and sera were stored at −80°C until further analysis. Biochemical parameters including malondialdehyde (MDA), total antioxidant capacity (TAC), superoxide dismutase (SOD), glutathione peroxidase (GPx), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) were investigated. TAC and MDA were measured with ZellBio kits (Germany), SOD and GPx with Bioassay Technology Laboratory kits (China) and hepatic indices were measured by Pars Azmoon enzymatic methods (Iran).
Statistical analysis
After determining the normality of the variables using numerical statistics (such as graphs) and normality tests (such as Shapiro–Wilk test), between-group analyses was performed using independent t-test or its nonparametric equivalent, Mann–Whitney test. Within-group analysis was performed using paired t-test or its nonparametric equivalent Wilcoxon. Analysis of covariance (ANCOVA) was used to compare the two groups after the intervention with adjustment for variables that were statistically different in the baseline. Results were expressed as mean ± standard deviation or the median (interquartile range), and for categorical variables which were tested with Chi-square or Fisher's exact test, frequency (percentage) are reported. Per-protocol analysis was used in this clinical trial. P < 0.05 was considered statistically significant. All analyses were performed using Statistical Package for Social Sciences (IBM Corp. Released 2012.IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY, USA: IBM Corp.,).
RESULTS
From 84 patients, 71 (84.52%) remained for the final analysis (40 in quercetin and 31 in placebo group). In the placebo group, 6 participants (7.14%) left the study due to gastrointestinal complications, one patient (1.19%) reported hair loss and two patients (2.38%) had <80% compliance. Four patients (4.76%) from both placebo and quercetin group withdrew the trial for personal reasons [Figure 1]. The main reason for the loss of sample size was gastrointestinal complications. This adverse effect was observed for 7 patients (8.33%), 6 patients in the placebo group (7.14%), and only 1 patient in the quercetin group (1.19%). Although we lost 13 patients (15.47%), our results are still reliable as the post hoc power computed after the trial for MDA was 0.83 and for GPx was 0.82.
Figure 1.
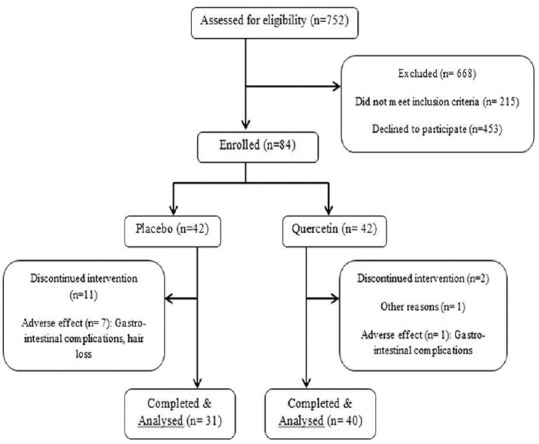
Study design (n = number of patients)
All anthropometric and demographic characteristics plus macro and micronutrient intake of the participants were alike in the two studied groups, except for physical activity (P = 0.02), and prime-age of DFO treatment (P = 0.03) which was significantly higher in the quercetin group and Fe (P = 0.006), folic acid (P = 0.03), and Cu (P = 0.04) dietary intakes which were significantly higher in the placebo group [Table 1]. Patients kept having the same BMI, physical activity, and dietary intake during the trial.
Table 1.
Baseline characteristics of patients according to treatment allocation: mean±standard deviation unless otherwise stated
| Quercetin (n=42) | Placebo (n=42) | P | |
|---|---|---|---|
| Age (years) | 27.88±4.73 | 27.71±5.49 | 0.882 |
| Height (cm) | 161.59±7.92 | 164.91±7.88 | 0.059 |
| Weight (kg) | 57.35±5.60 | 58.28±7.27 | 0.513 |
| BMI | 22.00±1.91 | 21.44±1.94 | 0.190 |
| Hb (g/dl) | 8.53±0.36 | 8.55±0.45 | 0.605 |
| Physical activity (MET-minute/week) | 528.00±609.18 | 198.00±222.75 | 0.026 |
| DFO dosage (ampoules per week)* | 24 (4) | 28 (10) | 0.063 |
| Prime age of DFO treatment (months old)* | 24 (36) | 24 (12) | 0.031 |
| Energy (Kcal)* | 1488.00 (975.06) | 1646.26 (945.30) | 0.170 |
| Protein (g)* | 58.93 (28.58) | 60.35 (39.57) | 0.566 |
| Carbohydrate (g) | 262.26 (95.07) | 272.42 (104.79) | 0.643 |
| Fat (g)* | 55.05 (72.27) | 47.59 (56.60) | 0.423 |
| Vitamin C (mg) | 89.34±47.12 | 92.95±45.16 | 0.722 |
| Ca (mg) | 639.83±353.23 | 644.75±302.63 | 0.946 |
| Vitamin D (IU)* | 1.57 (2.58) | 2.37 (2.72) | 0.135 |
| Zinc (mg) | 7.54±4.10 | 8.73±3.93 | 0.177 |
| Fe (mg)* | 10.62 (7.14) | 12.02 (5.94) | 0.006 |
| Folic acid (mcg)* | 168.09 (180.90) | 173.17 (207.54) | 0.036 |
| Cu (mg)* | 0.84 (0.90) | 1.20 (1.32) | 0.041 |
| Sex₤ | |||
| Female | 30 (71.4) | 23 (54.8) | 0.113 |
| Male | 12 (28.6) | 19 (45.2) | |
| Splenectomy₤ | |||
| Yes | 20 (47.6) | 16 (38.1) | 0.509 |
| No | 22 (52.4) | 26 (61.9) |
*Median (interquartile range); ₤Frequency (%). BMI=Body mass index; Hb=Hemoglobin; DFO=Desferrioxamine; Ca=Calcium; Fe=Iron; Cu=Copper; MET=Metabolic equivalent of task
As shown in Table 2, ANCOVA analysis showed that 12-week supplementation with quercetin could significantly decrease ALT compared to the placebo group, while AST remained unchanged during the trial. Before adjustment for baseline values, although not significant, ALP showed a reduction in the quercetin group (from 179 to 172 IU/L), while its amount increased in the placebo group (from 219 to 227 IU/L) (P > 0.05). In terms of indicators of oxidative status, TAC increased significantly in the quercetin group (P = 0.01), yet this significance disappeared after adjustment for the parameters that were different at the baseline (P = 0.14). MDA, GPx, and SOD showed no significant changes during the trial.
Table 2.
Results of within- and between-group comparison: median (interquartile range) unless otherwise stated
| Parameter | Quercetin (n=40) | Placebo (n=31) | Pb | Pc | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | P1 | Before | After | Pa | |||
| AST (IU/L) | 31.00 (42.00) | 24.00 (31.75) | <0.001 | 47.00 (36.25) | 39.00 (23.00) | <0.001 | 0.293 | 0.163 |
| ALT (IU/L) | 25.00 (57.25) | 17.00 (28.50) | <0.001 | 38.00 (28.50) | 31.00 (33.50) | 0.178 | 0.195 | 0.002 |
| ALP (IU/L) | 179.00 (83.25) | 172.00 (82.00) | 0.197 | 219.50 (107.75) | 227.50 (133.75) | 0.885 | 0.043 | 0.107 |
| TAC (mmol/L) | 2.89 (0.74) | 3.44 (0.97) | <0.001 | 2.81 (0.79) | 2.86 (0.92) | 0.593 | 0.010 | 0.142 |
| GPx (U/L) | 660.00 (763.95) | 487.90 (732.95) | 0.971 | 564.00 (882.73) | 432.90 (738.58) | 0.277 | 0.169 | 0.093 |
| SOD (U/L) | 464.25 (596.75) | 631.95 (348.38) | 0.438 | 441.90 (563.28) | 466.90 (485.03) | 0.739 | 0.158 | 0.111 |
| MDA (µmol/L)* | 18.59±6.21 | 17.31±7.19 | 0.401 | 16.87±5.23 | 16.37±5.33 | 0.549 | 0.497 | 0.995 |
*Mean±SD, aWithin-group comparison; bBetween-group comparison after the trial (nonadjusted); cBetween-group comparison (adjusted). MDA=Malondialdehyde; TAC=Total antioxidant capacity; SOD=Superoxide dismutase; GPx=Glutathione peroxidase; AST=Aspartate aminotransferase; ALT=Alanine aminotransferase; ALP=Alkaline phosphatase; SD=Standard deviation
DISCUSSION
In the present study, the effect of quercetin, a compound of alternative medicine, on oxidative stress and hepatic function of beta-thalassemia major patients have been investigated for the first time. According to the findings of this clinical trial, 12-week treatment with 500 mg quercetin in comparison to placebo could not reduce the oxidative stress, but it significantly affected the hepatic enzymes without affecting the BMI. Quercetin was well tolerated, and our participants experienced no serious complications.
According to the results of this study, quercetin supplementation could not reduce the oxidative stress in patients with beta-thalassemia major patients receiving DFO. This finding is consistent with that of Rashidi et al. who demonstrated that 12-week supplementation with Vitamin E and zinc did not affect the serum level of SOD and TAC in thalassemic patients.[13] Another interventional study showed that beta-carotene and Vitamin E supplementation could only reduce MDA, but failed to affect other oxidative markers.[14] However, some studies reported a significant effect of antioxidants on oxidative stress. For instance, 6-month supplementation with coenzyme Q10 could significantly plunged serum level of MDA and activity of anti-oxidative enzymes such as SOD and GPx. Animal studies have mostly reported a significant association between the quercetin intake and oxidative stress being reduced.[15,16,17]
A possible justification for our insignificant result could be the iron-binding property of the supplement given. Animal studies claim that quercetin can limit the rate of intestinal iron absorption.[18] Since our participants were told to take the supplement after lunch (to reduce the possibility of heartburn and stomachache), it is likely that quercetin has bound to iron and removed it through feces.[9] Therefore, serum quercetin level has not increased enough to be able to affect the oxidative stress. Another explanation could be the increased need of thalassemia patients to antioxidants. As our results indicate, our participants' dietary intake of vitamins and minerals are lower than the recommended amounts.[19] Previous investigations also reported the low serum antioxidant level in thalassemic patients.[20,21] To conclude, 500 mg quercetin intake during 12 weeks might have not been enough to meet the antioxidant needs of beta-thalassemic patients.
Regarding the three liver enzymes monitored, in spite of the significant changes observed for ALT in the adjusted and ALP in the nonadjusted model, the baseline serum levels of these enzymes were within the normal range (0–50 IU/L for ALT, 0–40 IU/L for AST, and 80–360 IU/L for ALP) and they remained within the normal range by the end of the trial. Animal studies claim that under the circumstances where liver enzymes are increased, quercetin can effectively reduce AST, ALT, and ALP.[22,23,24] Human interventional studies, on the other hand, reported various results. Moayedi et al. claimed that 9-month silymarin supplementation did not affect the level of serum hepatic enzymes.[25] Another study though claimed that 12-month supplementation with Vitamins A, C, and E could dramatically reduce AST and ALT serum level.[26]
Since the liver is the first organ to metabolize iron, it is the first organ that damages in the iron overload condition.[1] According to thalassemia monitoring guidelines, hepatic damage caused by iron overload usually demonstrates no clinical symptoms and the most sensitive and specific method of measuring liver status is through biopsy.[27] Hence, liver enzymes might not be proper indicators of the liver function. It is also worth mentioning that our participants did not have hepatitis which is the main reason for high liver enzyme levels. On the other hand, ALT, which reported to have significantly reduced by quercetin in the present study represents the hepatic status with the highest sensitivity.[28] It is suggested that thalassemic patients with high serum ALT level within the normal range might be at an early stage of liver fibrosis and need careful vigilance.[27] Thus, we can conclude that quercetin has been effective in improving liver function.
One of the limitations of this study is the information bias that might have occurred due to the self-reported dietary intake. Second, we lost a significant amount of sample size in the placebo group due to gastrointestinal complications. Although the placebo tablets were made of starch, cellulose, and other excipients, there is a possibility that our patients' digestive system had already been harmed by iron-chelating drugs, and the cellulose component of the placebo tablet just deteriorated the existing problem. Therefore, we seem to have to come up with another placebo composition with less harmful effects for thalassemia patients. The strengths of this study are the adjustment for various confounders, using validated and reliable measuring tools, and sampling from a referral clinic.
CONCLUSION
We conclude that quercetin might be effective in improving the hepatic status in beta-thalassemia major patients receiving DFO; however, the same effect was not observed for the oxidative stress. This could be due to low dosage of quercetin or short period of intervention. These results need to be further examined by studies with larger sample size, longer follow-up period, and different doses of quercetin. It is also suggested that the liver iron content and its health be investigated through a biopsy to clarify whether and how effectively quercetin can ameliorate the liver function.
Financial support and sponsorship
This work was funded by the Vice Chancellor for research of Iran University of Medical Sciences, Tehran, Iran (grant number: 95-04-27-30063).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The study protocol was approved by the Ethical Committee of Iran University of Medical Sciences (code: 4949). This study was also registered in the Iranian Registry of Clinical Trials (IRCT201701172709N43). We would like to thank Professor Mohammadreza Vafa who provided us with precious tips and advice, and all the thalassemia major patients who cooperated and volunteered to participate in this study.
REFERENCES
- 1.Rund D, Rachmilewitz E. Beta-thalassemia. N Engl J Med. 2005;353:1135–46. doi: 10.1056/NEJMra050436. [DOI] [PubMed] [Google Scholar]
- 2.Jomova K, Valko M. Importance of iron chelation in free radical-induced oxidative stress and human disease. Curr Pharm Des. 2011;17:3460–73. doi: 10.2174/138161211798072463. [DOI] [PubMed] [Google Scholar]
- 3.Rachmilewitz EA, Giardina PJ. How I treat thalassemia. Blood. 2011;118:3479–88. doi: 10.1182/blood-2010-08-300335. [DOI] [PubMed] [Google Scholar]
- 4.Lee KW, Kim YJ, Kim DO, Lee HJ, Lee CY. Major phenolics in apple and their contribution to the total antioxidant capacity. J Agric Food Chem. 2003;51:6516–20. doi: 10.1021/jf034475w. [DOI] [PubMed] [Google Scholar]
- 5.Bischoff SC. Quercetin: Potentials in the prevention and therapy of disease. Curr Opin Clin Nutr Metab Care. 2008;11:733–40. doi: 10.1097/MCO.0b013e32831394b8. [DOI] [PubMed] [Google Scholar]
- 6.Gupta A, Birhman K, Raheja I, Sharma SK, Kar HK. Quercetin: A wonder bioflavonoid with therapeutic potential in disease management. Asian Pac J Trop Dis. 2016;6:248–52. [Google Scholar]
- 7.D'Andrea G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia. 2015;106:256–71. doi: 10.1016/j.fitote.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Leopoldini M, Russo N, Chiodo S, Toscano M. Iron chelation by the powerful antioxidant flavonoid quercetin. J Agric Food Chem. 2006;54:6343–51. doi: 10.1021/jf060986h. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Li H, Zhao Y, Gao Z. Dietary supplementation of baicalin and quercetin attenuates iron overload induced mouse liver injury. Eur J Pharmacol. 2006;535:263–9. doi: 10.1016/j.ejphar.2006.01.067. [DOI] [PubMed] [Google Scholar]
- 10.Lehmann C, Islam S, Jarosch S, Zhou J, Hoskin D, Greenshields A, et al. The utility of iron chelators in the management of inflammatory disorders. Mediators Inflamm. 2015;2015:516740. doi: 10.1155/2015/516740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubbard GP, Wolffram S, Lovegrove JA, Gibbins JM. Ingestion of quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in humans. J Thromb Haemost. 2004;2:2138–45. doi: 10.1111/j.1538-7836.2004.01067.x. [DOI] [PubMed] [Google Scholar]
- 12.Lamson DW, Brignall MS. Antioxidants and cancer, part 3: Quercetin. Altern Med Rev. 2000;5:196–208. [PubMed] [Google Scholar]
- 13.Rashidi M, Aboomardani M, Rafraf M, Arefhosseini SR, Keshtkar A, Joshaghani H. Effects of Vitamin E and zinc supplementation on antioxidants in beta thalassemia major patients. Iran J Pediatr. 2011;21:8–14. [PMC free article] [PubMed] [Google Scholar]
- 14.Soleiman M, Ahmad T, Maseoud Zanjanchi N, Ali Akbar M. The effects of beta-carotene and Vitamin E on erythrocytes lipid peroxidation in beta-thalassemia patients. J Res Med Sci. 2007;12:301–7. [Google Scholar]
- 15.Kanter M, Aktoz T, Aktas C, Ozen F, Yarali O, Kanter B. Role of quercetin in cadmium-induced oxidative stress, testicular damage, and apoptosis in rats. Anal Quant Cytopathol Histpathol. 2016;38:45–51. [PubMed] [Google Scholar]
- 16.Rocha de Oliveira C, Ceolin J, Rocha de Oliveira R, Gonçalves Schemitt E, Raskopf Colares J, De Freitas Bauermann L, et al. Effects of quercetin on polychlorinated biphenyls-induced liver injury in rats. Nutr Hosp. 2014;29:1141–8. doi: 10.3305/nh.2014.29.5.7362. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Deng Y, Tang Y, Yu H, Gao C, Liu L, et al. Quercetin protects rat hepatocytes from oxidative damage induced by ethanol and iron by maintaining intercellular liable iron pool. Hum Exp Toxicol. 2014;33:534–41. doi: 10.1177/0960327113499168. [DOI] [PubMed] [Google Scholar]
- 18.Lesjak M, Hoque R, Balesaria S, Skinner V, Debnam ES, Srai SK, et al. Quercetin inhibits intestinal iron absorption and ferroportin transporter expression in vivo and in vitro. PLoS One. 2014;9:e102900. doi: 10.1371/journal.pone.0102900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dietary Reference Intakes – National Agricultural Library. 2006. [Last accessed on 2018 Jun 02]. Available from: https://www.nal.usda.gov/sites/default/files/fnic_uploads/DRIEssentialGuideNutReq.pdf .
- 20.Fung EB. Nutritional deficiencies in patients with thalassemia. Ann N Y Acad Sci. 2010;1202:188–96. doi: 10.1111/j.1749-6632.2010.05578.x. [DOI] [PubMed] [Google Scholar]
- 21.Kooshki A, Towfighian T, Rahsepar FR, Akaberi A. The relationship between the antioxidants intake and blood indices of the children with thalassemia in Sabzevar and Mashhad. Pak J Nutr. 2010;9:716–9. [Google Scholar]
- 22.Bahar E, Lee GH, Bhattarai KR, Lee HY, Kim HK, Handigund M, et al. Protective role of quercetin against manganese-induced injury in the liver, kidney, and lung; and hematological parameters in acute and subchronic rat models. Drug Des Devel Ther. 2017;11:2605–19. doi: 10.2147/DDDT.S143875. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Bader MA, Sultana M, Raina R, Prawez S, Pankaj NK, Reshi RN, et al. Effect of quercetin against roundup® and/or fluoride induced biochemical alterations and lipid peroxidation in rats. Int J Pharm Sci Rev Res. 2015;34:168–75. [Google Scholar]
- 24.Uylaş MU, Şahin A, Şahintürk V, Alataş İÖ. Quercetin dose affects the fate of hepatic ischemia and reperfusion injury in rats: An experimental research. Int J Surg. 2018;53:117–21. doi: 10.1016/j.ijsu.2018.03.043. [DOI] [PubMed] [Google Scholar]
- 25.Moayedi B, Gharagozloo M, Esmaeil N, Maracy MR, Hoorfar H, Jalaeikar M. A randomized double-blind, placebo-controlled study of therapeutic effects of silymarin in β-thalassemia major patients receiving desferrioxamine. Eur J Haematol. 2013;90:202–9. doi: 10.1111/ejh.12061. [DOI] [PubMed] [Google Scholar]
- 26.Elalfy MS, Adly AA, Attia AA, Ibrahim FA, Mohammed AS, Sayed AM. Effect of antioxidant therapy on hepatic fibrosis and liver iron concentrations in β-thalassemia major patients. Hemoglobin. 2013;37:257–76. doi: 10.3109/03630269.2013.778866. [DOI] [PubMed] [Google Scholar]
- 27.Tubman VN, Fung EB, Vogiatzi M, Thompson AA, Rogers ZR, Neufeld EJ, et al. Guidelines for the standard monitoring of patients with thalassemia: Report of the thalassemia longitudinal cohort. J Pediatr Hematol Oncol. 2015;37:e162–9. doi: 10.1097/MPH.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray RK, Granner DK, Mayes P, Rodwell V. Harper's illustrated biochemistry. 28th ed. New York: McGraw-Hill; 2009. p. 588. [Google Scholar]


