Abstract
Plasmids are key vehicles of horizontal gene transfer and contribute greatly to bacterial genome plasticity. In this work, we studied a group of plasmids from enterobacteria that encode phylogenetically related mobilization functions that populate the previously non-described MOBQ4 relaxase family. These plasmids encode two transfer genes: mobA coding for the MOBQ4 relaxase; and mobC, which is non-essential but enhances the plasmid mobilization frequency. The origin of transfer is located between these two divergently transcribed mob genes. We found that MPFI conjugative plasmids were the most efficient helpers for MOBQ4 conjugative dissemination among clinically relevant enterobacteria. While highly similar in their mobilization module, two sub-groups with unrelated replicons (Rep_3 and ColE2) can be distinguished in this plasmid family. These subgroups can stably coexist (are compatible) and transfer independently, despite origin-of-transfer cross-recognition by their relaxases. Specific discrimination among their highly similar oriT sequences is guaranteed by the preferential cis activity of the MOBQ4 relaxases. Such a strategy would be biologically relevant in a scenario of co-residence of non-divergent elements to favor self-dissemination.
Keywords: mobilizable plasmids, horizontal gene transfer, MOBQ relaxase, cis-acting relaxase, plasmid coexistence, bacterial conjugation
Introduction
Mobilizable plasmids are small genetic elements transmissible by conjugation with the assistance of a helper conjugative plasmid. They encode a relaxase, and usually a relaxase accessory protein (RAP), which are in charge of the conjugative DNA processing at a specific site of the origin of transfer (oriT) called nic. Mobilizable plasmids lack the transfer genes required for establishing a conjugative bridge (mating pair formation system, MPF) to the recipient cell, as well as the type IV coupling protein (T4CP) that puts in contact relaxosome and MPF and thus depend on conjugative plasmids to be transferred (Garcillán-Barcia and de la Cruz, 2013).
According to their relaxase, transmissible plasmids were phylogenetically classified into MOB families (Francia et al., 2004; Garcillán-Barcia et al., 2009). Currently, nine relaxase MOB classes are defined, and five of them (MOBP, MOBF, MOBQ, MOBH, and MOBC) are prevalent in transmissible plasmids hosted in γ-Proteobacteria. Plasmids gathered in a relaxase MOB family share similar genomic traits. Relaxase MOB classification has thus shown to be a good predictor of the plasmid backbone (Garcillán-Barcia and de la Cruz, 2013; Fernandez-Lopez et al., 2017). Mobilizable plasmids resident in γ-Proteobacteria form phylogenetically related clusters mainly within two relaxase MOB classes: MOBP and MOBQ (Garcillán-Barcia et al., 2009). Relevant examples are ColE1-like plasmids, grouped in family MOBP5; IncQ1 plasmids, such as RSF1010/R1162, gathered in MOBQ11; and IncQ2 plasmids, such as pTC-F14, in family MOBP14 (Garcillán-Barcia et al., 2009; Garcillán-Barcia and de la Cruz, 2013). An additional clade of small plasmids encoding MOBQ relaxases, previously classified as MOBQu, and here redefined as MOBQ4, was observed in a phylogenetic reconstruction of this relaxase family (Garcillán-Barcia et al., 2009).
A pair of degenerate primers specific for MOBQ4 plasmids was implemented in the Degenerate PCR MOB Typing (DPMT) approach developed by Alvarado et al. (2012) to detect and classify transmissible plasmids. This method revealed the abundance of MOBQ4 plasmids in clinical isolates of enterobacteria (Alvarado et al., 2012; Garcillán-Barcia et al., 2015), previously unnoticed by other plasmid typing methods. Whole-genome sequencing of clinical E. coli isolates also uncovered the presence of this kind of plasmids (Brolund et al., 2013; de Toro et al., 2014; Lanza et al., 2014). Prototype plasmids pIGWZ12 and ColE9-J (ColE2-like) cluster within the MOBQ4 clade. They are stable, theta-replicating, high copy-number, narrow host-range plasmids, whose replication systems have been extensively studied (Yasueda et al., 1989, 1994; Yagura et al., 2006; Zaleski et al., 2006, 2015). Here, we uncovered the diversity of MOBQ4 plasmids, determined the helper conjugative plasmids responsible for their dissemination, and established their behavior in terms of stability and transfer.
Materials and Methods
Plasmid Construction
MOBQ4 plasmid derivatives were constructed by isothermal assembly of linear DNA fragments from PCR reactions, following the Gibson method (Gibson et al., 2009, 2015). The MOBQ41 backbone (replication and mobilization regions), based on the complete sequence of the pE2022_4 plasmid [GenBank Acc. No. KT693143 (Lanza et al., 2014)], was linked to a kanamycin-resistance gene [coordinates 272 to 1216 of pSEVA211, GenBank Acc. No. JX560326 (Silva-Rocha et al., 2013)] and a cerulean fluorescent protein gene [coordinates 41 to 1091 of pNS2-φVL (Dunlop et al., 2008)], generating plasmid pRC1. The MOBQ42 backbone (replication and mobilization regions) was obtained by PCR amplification from the E. coli isolate HUMV 04/979 (Garcillán-Barcia et al., 2015), which contains a ColE9-J-like plasmid (coordinates 5102 to 7577, GenBank Acc. No. NC_011977.1). It was joined to a chloramphenicol resistance gene (coordinates 272–1072 of pSEVA311, GenBank Acc. No. JX560331 (Silva-Rocha et al., 2013)] and mCherry fluorescent protein gene (cfp, coordinates 1092–2117 of pNS2-φVL (Dunlop et al., 2008)], generating plasmid pRC2. MOBQ4 plasmids lacking the mobC ORF (from start to stop codon) were constructed by self-ligation of a single PCR fragment from either pRC1 or pRC2, producing plasmids pRC3 and pRC4, respectively.
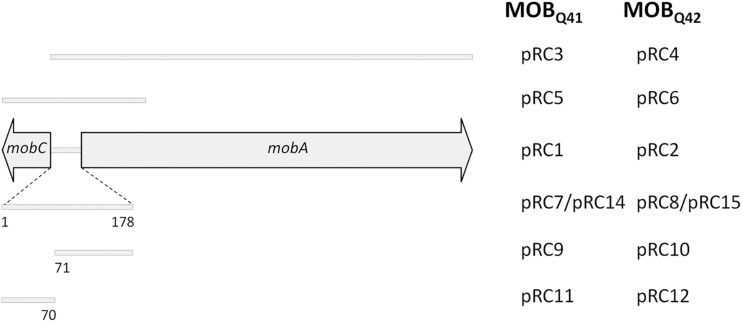
Additional plasmids were constructed to delimit the oriT region. A schematic representation of the fragments included in each construction is depicted in Figure 1. Such fragments were individually assembled to coordinates 1–1030 and 1360–3001 of vector pSEVA631 (GenBank Acc. No. JX560348). Plasmids pRC5 and pRC6 contained a fragment including the mobC gene, the 178bp intergenic region between mobC and mobA and the first 400 nucleotides of the mobA gene from pRC1 and pRC2, respectively. Plasmids pRC7 and pRC8 included only the 178bp intergenic fragment (Supplementary Figure S1), located between genes mobA and mobC of pRC1 and pRC2, respectively. Plasmids pRC14 and pRC15 contain the oriT regions of pRC7 and pRC8 but cloned in the inverse orientation. Plasmids pRC11 and pRC9, respectively included portions 1–70 and 71–178 of the intergenic fragment between genes mobA and mobC of pRC1, while the same portions from pRC2 were included in pRC12 and pRC10, respectively. A pSEVA631 fragment containing coordinates 1–1030 and 1360–3001 was self-ligated, generating the non-mobilizable vector pRC13, which was used as a control in the mating experiments.
FIGURE 1.
Schematic representation of the MOBQ4 DNA segments included in a series of recombinant plasmids. The mobilization region of MOBQ4 plasmids includes mobC and mobA genes, represented by large, horizontal gray arrows. The extent of the mobilization region included in each construction is represented by a gray bar. The plasmid names for the MOBQ41–based constructions are listed in the left column, while those for MOBQ42-based constructions are in the right column. Plasmids pRC1, pRC2, pRC3, and pRC4 also include the replication module of MOBQ41 or MOBQ42 plasmids.
Stability Assays
Plasmids pRC1 and pRC2 were introduced in the recA+ and recA– isogenic strains UB1636 (F– lys his trp rpsL) (Achtman et al., 1971) and UB1637 (F– lys his trp rpsL recA56) (de la Cruz and Grinsted, 1982), either independently to check for their stability or both together to check for their compatibility. Single colonies were inoculated in Lysogeny-Broth (LB) supplemented with kanamycin at 50 μg/ml (for pRC1-containing strains) or chloramphenicol at 25 μg/ml (for pRC2-containing strains) and grown to saturation at 37°C with agitation (150 rpm). A volume of 9.7 μl was transferred from saturated cultures to 10 mL of fresh LB media without antibiotics and grown to saturation in the same conditions. Rounds of transfer and growth were repeated up to 80 generations. The proportion of plasmid-bearing cells in the population was monitored by replica-plating 100 colonies in LB-agar supplemented with the appropriate antibiotics every 10 generations. A larger number of cells was inspected by fluorescence microscopy and, in the case of pRC1-containing cells, also by flow cytometry. Live cells were visualized using a Leica AF6500 microscope at 63x magnification. CFP and mCherry signals were monitored using BP filters (Excitation 434/17 – Emission 479/40 for CFP, Excitation 562/40 – Emission 641/75 for mCherry). Images were obtained using an iXon885 EM CCD Camera (Andor) and up to 1000 cells were analyzed in each case. Fluorescence emission was measured by flow cytometry using a FACS Canto II flow cytometer (Becton Dickinson) equipped with a 488 nm solid state laser for excitation. The cyan fluorescence of 20,000 events was detected using a 525/20 filter.
Mating Assays
Conjugative plasmids used in this work are listed in Supplementary Table S1. They were tested as helpers of the MOBQ4 plasmids in surface mating experiments, following the procedure described by del Campo et al. (2012). E. coli strain DH5α (F– endA1 glnV44 thi-1 recA1 relA1 gyrA96 deoR nupG purB20 φ80dlacZΔM15 Δ(lacZYA-argF)U169, hsdR17(rK–mK+(), λ–) (Grant et al., 1990) containing different plasmid combinations was used as donor and BW25113 (lacIq rrnBT14 ΔlacZWJ16 hsdR514 ΔaraBADAH33 ΔrhaBADLD78), BW25993 (lacIq hsdR514 ΔaraBADAH33 ΔrhaBADLD78) (Datsenko and Wanner, 2000) as recipient. Donor and recipient strains were mixed in a 1:1 ratio, deposited onto an LB-agar surface and incubated for 1 h at 37°C (except when drR27 was used as a helper, in which case matings were carried out at 25°C). Then, the mixture was resuspended in LB and plated in the presence of appropriate antibiotics. Conjugation frequencies were expressed as the number of transconjugants per donor cell.
Phylogenetic Analysis
The 300 N-terminal residues of the MobA relaxase of plasmid ColE9-J were used as a query in a BLASTP search (Altschul et al., 1997) (e-value: 1xE-3). The homologous sequences were aligned using MUSCLE (Edgar, 2004). TrimAl v1.4 was used to calculate the average identity between sequences in the alignment (Capella-Gutiérrez et al., 2009). ProtTest 3 was used to estimate the best model of protein evolution for our set (Guindon and Gascuel, 2003; Darriba et al., 2011). RAxML version 7.2.7 (Stamatakis, 2006) was used for phylogenetic reconstruction. Using the JTTGAMMA model 10 maximum likelihood (ML) searches trees were inferred and support values were assigned to each node of the best tree from 1000 bootstrap searches. Relaxase of the pXF5847 plasmid (GenBank Acc. no. YP_009076807.1) was used as outgroup.
3D Structure Prediction
Phyre2 was used to predict the 3D structure of the MobA relaxase domains of plasmids pE2022_4 and ColE9-J (Kelley et al., 2015), which were visualized using PyMOL (Schrödinger, 2015).
Results and Discussion
Analysis of MOBQ4 Plasmids
MOBQ is a broad relaxase class that encompasses several families, each of which includes related plasmid backbones: MOBQ1 comprises relaxases of mobilizable broad host-range IncQ1-like plasmids; MOBQ2, conjugative relaxases of pTi and many rhizobial plasmids; MOBQ3, conjugative broad host-range plasmids resident in gram-positive, such as pIP501 (Garcillán-Barcia et al., 2009). In this previous study, many MOBQ plasmids were not ascribed to a specific subclassification due to either low resolution of the clades or lack of information on the plasmid members. Here, we focused on one of these poorly defined clades, now named MOBQ4, prompted by the fact that these relaxases have been recurrently detected in enterobacterial clinical isolates (Alvarado et al., 2012; Brolund et al., 2013; de Toro et al., 2014; Lanza et al., 2014; Garcillán-Barcia et al., 2015).
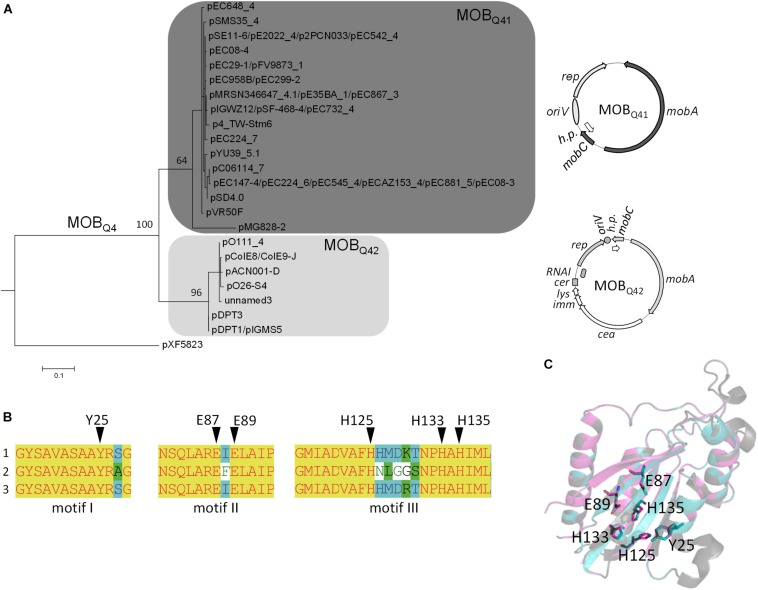
The phylogenetic reconstruction, based on the first N-terminal 300 residues of MOBQ4 relaxases produced two clusters, MOBQ41 and MOBQ42 (Figure 2A and Supplementary Table S2). This relaxase domain contains the three relaxase motifs (Figure 2B) and share 84% average amino acid identity (97 and 90% for individual MOBQ41 and MOBQ42 groups, respectively). The 3D structure prediction of the relaxase domain of MOBQ41 and MOBQ42 plasmids rendered MOBQ relaxases NES [plasmid pLW1043, PDB Acc. No. 4HT4 (Edwards et al., 2013)] and MobA [plasmid R1162/RSF1010, PDB Acc. No. 2NS6, (Monzingo et al., 2007)] as best hits (100% confidence). The superimposed structures pointed to MOBQ4 amino acids Y25 (motif I), E87 and E89 (motif II), and H125, H133 and H135 (motif III) as homologs of the MobA_R1162 catalytic residues Y25, E74 and E76, and H112, H120 and H122, respectively (Figure 2C). Contrary to the high conservation of the N-terminal domain among members of both MOBQ4 subgroups, the amino acid identity of the C-terminal part of the MOBQ4 relaxases dropped to 35%. This C-terminal domain exhibited low homology to SogL primases of IncI1 plasmids.
FIGURE 2.
The MOBQ4 relaxase family. (A) Maximum-likelihood phylogenetic reconstruction of the N-terminal domain of MOBQ4 relaxases is shown. MobA relaxase of plasmid pXF5843 was used as outgroup. Bootstrap values of relevant nodes are indicated. Families Q41 and Q42 are shadowed in dark and light gray, respectively. A prototype backbone of each MOBQ4 group is represented to the right of the corresponding clade. Genes of the mobilization module are represented in the same gray color pattern. The elements of the replication module are dotted (for MOBQ41) or striped (for MOBQ42). Genes of the colicin operon and the hypothetical proteins are depicted in a white background. (B) Multiple alignment of the three conserved MOBQ relaxase motifs (Garcillán-Barcia et al., 2009). (1) MOBQ41 relaxases, with the exception of pMG828-2; (2) pMG828-2; (3) MOBQ42 relaxases. Putative catalytic residues are indicated by black triangles over the amino acid sequences. (C) Protein 3D-structure superposition of MOBQ41, MOBQ42 and MOBQ11 relaxases. In gray, the minimal relaxase domain of MobA of plasmid R1162/RSF1010 used as a model (PDB 2NS6); in blue and magenta the predicted structures of the homologous domains of the MOBQ41 (MobA of pE2022_4) and MOBQ42 (MobA of ColE9-J) relaxases, respectively. Key residues of MOBQ4 relaxases are highlighted as sticks.
Each MOBQ4 subclade groups highly related backbones (Figure 2A). MOBQ41 are cryptic, small-size plasmids (Supplementary Table S2). Their backbone contains only four genes encoding a replication initiation protein (Rep), a relaxase (MobA), a putative relaxase accessory protein (MobC) and a hypothetical protein. The genes for the last two are generally not annotated. Besides the above-mentioned replication and mobilization genes, MOBQ42 plasmids also contain a colicin operon, including colicin, immunity and lysis genes, following the synteny of Group A nuclease colicins (Cascales et al., 2007). Plasmids ColE9-J and pO111_4 contain a second, partial colicin operon.
The MOBQ4 subdivision in two relaxase groups matches with the presence of two different replicons (Supplementary Table S2) and this family thus encompasses at least two plasmid species as defined by Fernandez-Lopez et al. (2017). MOBQ41 plasmids encode a replication initiation protein that belongs to the Rep_3 superfamily [PF01051 in the Pfam classification (Finn et al., 2016)], with no defined group in the PlasmidFinder classification (Carattoli et al., 2014). MOBQ42 plasmids encode ColE2-like initiators (Pfam PF03090 + PF08708), classified as Col156 by PlasmidFinder. Plasmids pIGWZ12 and ColE9-J exemplify each cluster. They are stable, theta-replicating, high copy number plasmids (15 and 10 copies per chromosome molecule, respectively (Takechi et al., 1994; Zaleski et al., 2012). The origin of replication of plasmid pIGWZ12 was located upstream the rep gene. It contains iterons, an A+T rich region and four DnaA boxes (Zaleski et al., 2006, 2015). The iterons were found to be the incompatibility determinants (Zaleski et al., 2015). ColE2-like plasmids, such as ColE9-J, form a group of closely related elements that share an identical priming mechanism, mediated by the plasmid-encoded Rep protein (Horii and Itoh, 1988; Itoh and Horii, 1989; Yasueda et al., 1989; Hiraga et al., 1994). The origin of replication consists of 32 bp located downstream of the rep gene, containing two directly repeated sequences (Kido et al., 1991; Nomura et al., 1991; Yagura and Itoh, 2006; Yagura et al., 2006). In ColE2-like plasmids, the rep gene expression is post-transcriptionally controlled by a plasmid-encoded RNA (RNAI), which binds the untranslated 5′ region of the rep mRNA, preventing its translation (Sugiyama and Itoh, 1993; Takechi et al., 1994; Yasueda et al., 1994). MOBQ42 plasmids contain a cer-like site (Hiraga et al., 1994), an indication that they use a host site-specific recombination system for resolving multimers to monomers as ColE1-like plasmids do (Summers and Sherratt, 1984, 1988; Summers, 1998).
All completely sequenced MOBQ4 plasmids come from hosts of the Enterobacteriaceae family (Supplementary Table S2). They were isolated from different backgrounds: Salmonella enterica isolated from pork meat (pSD4.0) (Bleicher et al., 2013), pork feces (p4_TW-Stm6) (Dyall-Smith et al., 2017) and human systemic infection (pYU39_5.1) (Calva et al., 2015), multidrug-resistant environmental E. coli (pSMS35_4) (Fricke et al., 2008), commensal E. coli (pSE11-6) (Oshima et al., 2008), enterohemorrhagic E. coli strains of the O26 and O111 serogroups (pO26-S4 and pO111_4) (Ogura et al., 2009; Fratamico et al., 2011), extended-spectrum beta-lactamase producing E. coli clinical isolates (pE2022_4, pFV9873_1, pEC147-3 and pEC08-6) (Brolund et al., 2013; Lanza et al., 2014), E. coli isolated from human urinary tract (pVR50F) (Beatson et al., 2015) and bloodstream infections (pSF-468-4) (Stephens et al., 2015), as well as porcine extraintestinal pathogenic E. coli strain (p2PCN033) (Liu et al., 2015), among others (Supplementary Table S2). None of these plasmids contain antibiotic-resistance genes. There is still no clue on the selective advantage provided by the cryptic MOBQ41 plasmids. In the case of MOBQ42 plasmids, the fact that all carry colicin operons, a priori an advantageous trait for the bacterial host, could explain the abundance of this type of plasmids. For example, the MOBQ42 plasmid pDPT1 was stably acquired by a Vietnamese Shigella sonnei strain in the mid-1990s, and became fixed in the evolving bacterial population (Holt et al., 2013). The colicin E5 produced by pDPT1 was highly bactericidal against non-immune Shigella and E. coli strains. The acquisition of the pDPT1 colicin plasmid, coinciding with the high increase of dysentery produced by this strain, suggests that pDPT1 conferred a beneficial function to its host (Holt et al., 2013).
Stability and Co-residence of MOBQ4 Plasmids
To study the MOBQ4 plasmids, two derivatives were constructed, pRC1 and pRC2. They included the replication and mobilization modules of the MOBQ41 and MOBQ42 backbones, respectively. Antibiotic-resistance and fluorescent protein genes were also included as reporters. Plasmid stability and compatibility were assayed in recA+ and recA– E. coli strains by propagating the plasmids either alone or in combination during 80 generations. Despite the cargoes loaded in plasmids pRC1 and pRC2, the percentage of plasmid retention in the bacterial population was 100%, suggesting that the MOBQ4 backbone confers a minimized fitness cost to its enterobacterial host (San Millan and MacLean, 2017). Besides stability in E. coli, both MOBQ4 plasmid species also exhibited full compatibility (100% retention of both after 100 generations), as could be expected due to their different replicons (Novick, 1987), and ruling out other plasmid-encoded traits out of the replication module that could interfere with the stable vertical inheritance of each other.
Mobilization of MOBQ4 Plasmids by Different MPF Systems
Since mobilizable plasmids do not encode the mating pair formation system neither the T4CP, their transfer relies on auto-transmissible plasmids. We wondered which conjugative plasmids could be responsible for the dissemination of the MOBQ4 plasmids. Not all conjugative plasmids are equally efficient at supplying these functions to a specific mobilizable plasmid (Cabezón et al., 1994, 1997). The contacts established between the relaxosome of the mobilizable plasmid and the T4CP-MPF of the helper plasmid are crucial in the transfer process. ColE1-like MOBP5 plasmids are efficiently mobilized by IncF-MOBF12 (e.g., F) and IncI1-MOBP12 (e.g., R64drd11) plasmids (Cabezón et al., 1997). IncQ1-MOBQ1 plasmids, such as RSF1010, are transferred by IncP1-MOBP11 helper plasmids (e.g., RP4) (Cabezón et al., 1997; Meyer, 2009). pMV158-like plasmids (MOBV1) are mobilized by IncP1-MOBP11 and Inc18-MOBQ3 (e.g., pIP501) plasmids (Lorenzo-Díaz et al., 2014).
We looked for reports providing indirect evidence on MOBQ4 plasmid mobilization through conjugation. In a survey for the presence of transmissible plasmids in a multidrug E. coli collection, MOBQ4 transconjugants were obtained from seven out of the eight MOBQ4 containing clinical isolates (Garcillán-Barcia et al., 2015). In all cases, a MOBP12-MPFI plasmid, presumptively the helper, was also present in both, donor and transconjugant cells. Similarly, the MOBQ41 plasmid pSD4.0 and the IncI1 plasmid pSD107 were found in E. coli transconjugants arisen from a mating with Salmonella enterica (Bleicher et al., 2013).
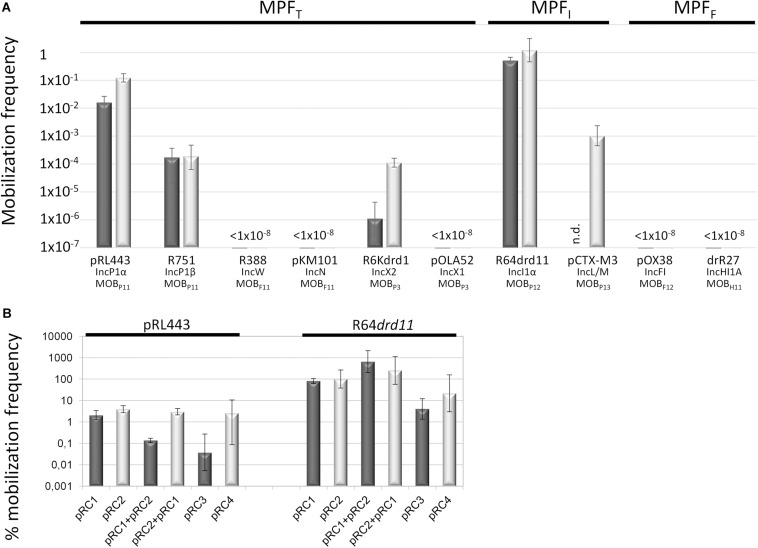
Three conjugative MPF types (MPFT, MPFF, and MPFI) are prevalent in Enterobacteriaceae (Smillie et al., 2010; Guglielmini et al., 2014), the taxonomic family where MOBQ4 plasmids have been found. In this study, a set of conjugative plasmids representative of these MPF families were tested as helpers for the mobilization of MOBQ4 plasmids (Supplementary Table S1). Not all of them were equally efficient (Figures 3A,B and Supplementary Table S3). R64drd11, the prototype of IncI1α-MOBP12 plasmids, which encodes a MPFI conjugative apparatus, was the most efficient helper. Another MPFI plasmid, pCTX-M3 (IncL/M-MOBP13), was also an efficient helper. Co-residence with MPFI plasmids has been reported for the MOBQ4 plasmids pSE11-6 (Oshima et al., 2008), pSD4.0 (Bleicher et al., 2013), pEC147-4 (Brolund et al., 2013), pO26-S4 (Fratamico et al., 2011), pDPT1 (Holt et al., 2013), and pE2022_4 (Lanza et al., 2014).
FIGURE 3.
Mobilization frequencies of MOBQ4 plasmids by a series of helper plasmids. The mobilization frequency was calculated as the number of transconjugants containing the MOBQ4 plasmid per donor cell. Figures are the average of at least six independent experiments. (A) Dark- and light-gray bars indicate the mobilization frequencies of pRC1 (MOBQ41) and pRC2 (MOBQ42) plasmids, respectively. Below the bars the helper plasmid used in each case, as well as its corresponding Inc and MOB groups, are indicated. The MPF types of the helper plasmids are indicated in the upper part of the figure. (B) The mobilization efficiencies of the MOBQ4 plasmids are relativized to the helper plasmid transfer rates (100%). The mobilization efficiencies of MOBQ41 and MOBQ42-based constructions are represented by dark- and light-gray bars, respectively. pRC1 + pRC2 indicates the mobilization frequency of pRC1 when coresident with pRC2. pRC2 + pRC1 indicates the mobilization frequency of pRC2 when coresident with pRC1.
On the other hand, MPFF-type plasmids [e.g., IncF-MOBF12 (F) or IncHI1-MOBH11 (R27) plasmids], which show high prevalence in enterobacteria, were not appropriate for MOBQ4 mobilization. MPFT plasmids behaved unevenly as MOBQ4 mobilizers. IncP1-MOBP11 (RP4 and R751) and IncX2-MOBP3 (R6Kdrd1) plasmids rendered MOBQ4 transconjugants, while IncW-MOBF11 (R388), IncN-MOBF11 (pKM101) or IncX1-MOBP3 (pOLA52) did not. Contrary to IncP, IncW and IncN plasmids, most IncF, IncI1, IncH, and IncX plasmids are naturally repressed for conjugation. In this study, we used derepressed variants of IncF (pOX38 and R100-1), IncI1α (R64drd11), IncHI1 (drR27), and IncX2 (R6Kdrd1) plasmids, but not a derepressed IncX1. IncX1 and IncX2 plasmids are highly similar in their conjugation genes. Taking into account that the IncX2 derepressed plasmid R6Kdrd1 was not efficient at mobilizing MOBQ4 plasmids (Figure 3A and Supplementary Table S3), and that the IncX1 plasmid pOLA52 self-transfers at low frequency (around 10–4 per donor) (Sørensen et al., 2003), the lack of mobilization of the MOBQ4 plasmids pRC1 and pRC2 by pOLA52 is not surprising. The widely different mobilization efficiencies displayed by the two IncP1-MOBP11 helpers used is more curious. RP4 and R751 are prototypes of the α and β divisions of the IncP1 backbones, respectively. Despite the high conservation of their transfer genes, the kanamycin-sensitive RP4 derivative, pRL443, was 100–1000 times more efficient than R751 as a MOBQ4 helper. Noticeable differences were also observed for these two conjugative plasmids at transferring IncQ2-MOBP14 mobilizable plasmids pTC-F14 and pTF-FC2 (van Zyl et al., 2003). The common characteristic of the MOBQ4 mobilizers was their belonging to the MOBP relaxase class. This could indicate that the MOBQ4 relaxosomes interact more efficiently with the T4 encoded by these MOBP plasmids.
Effect of Co-residence in the MOBQ4 Plasmid Mobilization
Bacterial co-infection with multiple plasmids is common in nature (San Millan et al., 2014). Co-residence of compatible plasmids may lead to intracellular interactions that negatively or positively affect plasmid transfer rates (Gama et al., 2017a,b,c; Getino et al., 2017). Among them, plasmid-encoded fertility inhibition systems that block transmission of unrelated plasmids from the same donor cell have been intensively studied (Maindola et al., 2014; Gama et al., 2018; Getino and de la Cruz, 2018). Besides, competition of two relaxosomes for the same T4CP-MPF can result in the preponderance of one them (Cascales et al., 2005), a fact relevant for any mobilizable plasmid. Cohabitation of two or more mobilizable plasmids that use the same mating apparatus could affect each other’s transfer. To test whether the mobilization of the MOBQ41 plasmid was affected by co-residence with a MOBQ42 plasmid and vice versa, pRC1 and pRC2 were introduced conjointly with the helper plasmid (either pRL443 or R64drd11) in the same cell (Figure 3B). Curiously, presence of pRC1 did not produce a significant variation in pRC2 transfer. In turn, pRC2 produced one-log decrease in pRC1 transfer by pRL443. However, this moderate negative effect was not exhibited when using R64drd11 as a helper: on the contrary, pRC2 presence resulted in one-log increase in pRC1 transfer. Testing different combinations of MOBQ41, MOBQ42 and helpers would be necessary to deeper assess the impact of residing together in MOBQ4 horizontal propagation.
mobC Deletion Effect in the Mobilization Efficiency
Many conjugative and mobilizable plasmids encode RAPs that recognize and bind their cognate oriT sequence probably favoring a single-stranded state around the nic site (de la Cruz et al., 2010). Deletion of RAP genes trwA of R388 (Moncalián et al., 1997), nikA of R64 (Furuya et al., 1991), mobB and mobC of plasmids pTC-F14 and pTF-FC2 (van Zyl et al., 2003), traJ and traK of RP4 (Guiney et al., 1989), mobC of R1162/RSF1010 (Brasch and Meyer, 1986), and mbeC of ColE1 (Varsaki et al., 2009) resulted in drastic decrease of plasmid transfer. All MOBQ4 plasmids encode a gene, called mobC, which is located adjacent to oriT and transcribed opposite to the mobA relaxase gene (Figure 1). Most of the mobC genes are not annotated, so we updated their annotation, as listed in Supplementary Table S2. The MobC proteins of MOBQ4 plasmids are small (less than 100 amino acids) and showed no homology to other RAPs (by using PSI-Blast). To check whether MobC plays a role in the MOBQ4 plasmid mobilization, mobC deletion mutants were constructed from pRC1 and pRC2, respectively producing pRC3 and pRC4 (Figure 1). A moderate decrease in mobilization was observed in the mobC– variants: 1.5-log reduction for pRC3 and 0.6-log for pRC4, when using R64drd11 as a helper (Figure 3B). MobC is thus not absolutely essential for MOBQ4 plasmid mobilization. This is an interesting difference to other plasmid groups, which should be further investigated. It is conceivable that some MOBQ4 plasmids can be found, the mobilization of which is independent of RAPs.
In trans Mobilization of oriT_MOBQ4-Containing Vectors
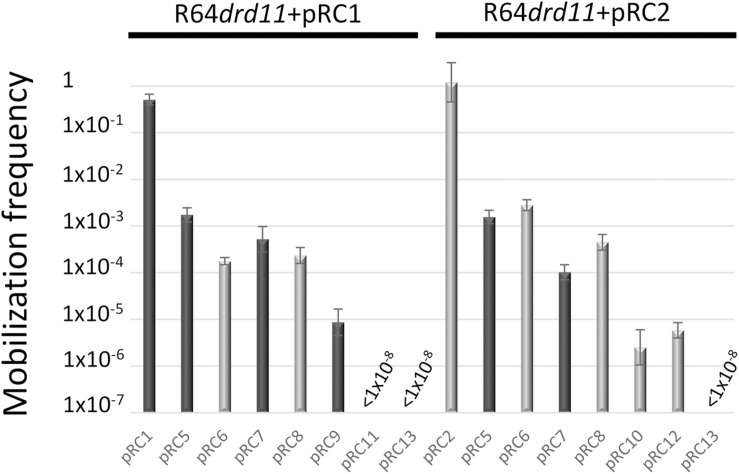
The 178 bp intergenic region comprised between the mobC and mobA genes of MOBQ4 plasmids was assembled with an oriT-lacking fragment of vector pSEVA631. The resulting constructions, pRC7 (for MOBQ41) and pRC8 (for MOBQ42) (Figure 1), were introduced in donor strains to check for their mobilization. The transfer proteins were supplied in trans: the corresponding mobilizable plasmid (pRC1 or pRC2) provided the relaxosomal proteins, while the conjugative plasmid (R64drd11) supplied the T4CP and MPF. Plasmids pRC7 and pRC8 were transferred to the recipient population, but 1000-fold less efficiently than their corresponding mobA+mobC+ partners (pRC1 and pRC2) (Figure 4). This result was confirmed by using plasmids pRC14 and pRC15, instead of pRC7 and pRC8, in the mobilization experiments. Plasmids pRC14 and pRC15 contained the same oriT region present in pRC7 and pRC8, but cloned in the inverse orientation. Besides, to avoid losing any oriT-related function, larger segments including also the mobC gene and the first 431 bp of the mobA gene [pRC5 and pRC6 (Figure 1)], were analyzed. Here again relaxase, T4CP and MPF components were provided in trans. Plasmids pRC5 and pRC6 behave similarly to pRC7 and pRC8, and were mobilized at least 500-fold less than pRC1 and pRC2 (Figure 4).
FIGURE 4.
In trans mobilization of oriT fragments. R64drd11-mediated mobilization frequencies of pRC1 and plasmids containing fragments of its oriT are represented by dark-gray bars, while those of pRC2 and its derivatives are in light-gray bars. The bars represent the average of at least six experiments.
MOBQ4 relaxases showed thus a cis-acting preference for their oriTs, performing at least 500-fold better on a cis than on a trans oriT substrate. The cis-acting preference is a characteristic exhibited by some DNA-binding proteins, such as the TnpA transposases of Tn10, Tn5 and Tn903 (Morisato et al., 1983; Derbyshire et al., 1990; DeLong and Syvanen, 1991). Relaxases generally lack a cis preference for their oriTs. There are only a few examples of relaxases that show preference for a cis-encoded substrate. The MOBP relaxase of transposon Tn1549 was found to be cis-acting (Tsvetkova et al., 2010). Notably, all plasmid-encoded cis-acting relaxases have been reported in members of the MOBQ class: TraA of plasmid pRetCFN42d (MOBQ2) (Pérez-Mendoza et al., 2006) and TraA of plasmid pIP501 (MOBQ3) (Arends et al., 2012). Nevertheless, other MOBQ relaxases, such as Nes_pSK41 (Pollet et al., 2016), as well as MobA of plasmids R1162/RSF1010 and pSC101 (Brasch and Meyer, 1986; Derbyshire and Willetts, 1987; Meyer, 2000) worked efficiently in trans.
The MOBQ4 relaxases were also tested for their specificity to act on a non-cognate MOBQ4 oriT. The oriTs of MOBQ41 and MOBQ42 plasmids differ in 10 nucleotides along their 178bp sequence (Supplementary Figure S1). Mobilization frequencies of oriT_MOBQ42 plasmids pRC6 or pRC8 by the MOBQ41 plasmid pRC1 + R64drd11, as well as oriT_MOBQ41 plasmids pRC5 or pRC7 by the MOBQ42 plasmid pRC2 + R64drd11, were similar to that obtained for the cognate systems, varying no more than one log (Figure 4).
To further delimit the oriT of MOBQ4 plasmids, the 178bp oriT fragments cloned in pRC7 and pRC8 (see Supplementary Figure S1) were subdivided in two portions, one containing oriT nucleotides 1–70 (pRC11 and pRC12) and the other containing oriT nucleotides 71–178 (pRC9 and pRC10) (Figure 1 and Supplementary Figure S1). Disruption of the 178bp oriT region resulted in a drastic loss of conjugation efficiency of the oriT-containing plasmid (Figure 4), as previously reported for pIGWZ12 (Zaleski et al., 2015).
The cis-acting preference of the MOBQ4 relaxases shown here is an example of biological orthogonality (de Lorenzo, 2011), that is, a mechanism to avoid interference. It implies that when two MOBQ4 plasmids are present in the same cell, the contribution of oriT cross-recognition by the heterologous MOBQ4 relaxase to plasmid transfer is not substantial. This feature could be essential to guarantee their efficient transfer, given the fact that both types of MOBQ4 plasmids use the same repertoire of conjugative helpers and share the same hosts.
Conclusion
MOBQ41 and MOBQ42 plasmids are able to coexist and spread in the E. coli population without affecting each other largely. They disseminate through bacterial conjugation, aided specially by MPFI conjugative plasmids, but neither of the MOBQ4 plasmids dominates the horizontal transfer process. Co-residence of MOBQ41 and MOBQ42 plasmids in the same host neither hindered nor boosted considerably their respective mobilization frequencies. Since both plasmids (MOBQ41 and MOBQ42) have a narrow host-range (they circulate among enterobacteria), their coexistence in natural environments is likely. In such ecological setting, specific discrimination among their highly similar oriT sequences would be guaranteed by the preferential cis activity of the MOBQ4 relaxase. Such strategy would be biologically relevant in a scenario of co-residence of non-divergent elements to favor self-dissemination.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
MG-B and FC conceived the study and designed the experiments. RC-L, AC, and MG-B performed the experiments. RC-L, AC, MG-B, and FC interpreted the data. MG-B and FC wrote the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors want to thank María Aramburu and Raúl Fernández-López for their technical assistance with the flow cytometer and the fluorescence microscopy, respectively. This manuscript has been released as a Pre-Print at bioRxiv (Garcillán-Barcia et al., 2019).
Footnotes
Funding. This work was supported by the Spanish Ministry of Economy and Competitiveness (BFU2017-86378-P, AEI/FEDER, UE, to FC) and Consejo Superior de Investigaciones Científicas (201820I143 to MG-B). We acknowledge support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.02557/full#supplementary-material
References
- Achtman M., Willetts N., Clark A. J. (1971). Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J. Bacteriol. 106 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., et al. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25 3389–3402. 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado A., Garcillán-Barcia M. P., de la Cruz F. (2012). A degenerate primer MOB typing (DPMT) method to classify gamma-proteobacterial plasmids in clinical and environmental settings. PLoS One 7:e40438. 10.1371/journal.pone.0040438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends K., Schiwon K., Sakinc T., Hübner J., Grohmann E. (2012). Green fluorescent protein-labeled monitoring tool to quantify conjugative plasmid transfer between gram-positive and gram-negative bacteria. Appl. Environ. Microbiol. 78 895–899. 10.1128/AEM.05578-5511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatson S. A., Ben Zakour N. L., Totsika M., Forde B. M., Watts R. E., Mabbett A. N., et al. (2015). Molecular analysis of asymptomatic bacteriuria Escherichia coli strain VR50 reveals adaptation to the urinary tract by gene acquisition. Infect. Immun. 83 1749–1764. 10.1128/IAI.02810-2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicher A., Schöfl G., Rodicio M. D. R., Saluz H. P. (2013). The plasmidome of a Salmonella enterica serovar derby isolated from pork meat. Plasmid 69 202–210. 10.1016/j.plasmid.2013.01.001 [DOI] [PubMed] [Google Scholar]
- Brasch M. A., Meyer R. J. (1986). Genetic organization of plasmid R1162 DNA involved in conjugative mobilization. J. Bacteriol. 167 703–710. 10.1128/jb.167.2.703-710.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brolund A., Franzén O., Melefors O., Tegmark-Wisell K., Sandegren L. (2013). Plasmidome-analysis of ESBL-producing escherichia coli using conventional typing and high-throughput sequencing. PLoS One 8:e65793. 10.1371/journal.pone.0065793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezón E., Lanka E., de la Cruz F. (1994). Requirements for mobilization of plasmids RSF1010 and ColE1 by the IncW plasmid R388: trwB and RP4 traG are interchangeable. J. Bacteriol. 176 4455–4458. 10.1128/jb.176.14.4455-4458.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezón E., Sastre J. I., de la Cruz F. (1997). Genetic evidence of a coupling role for the TraG protein family in bacterial conjugation. Mol. Gen. Genet. 254 400–406. 10.1007/s004380050432 [DOI] [PubMed] [Google Scholar]
- Calva E., Silva C., Zaidi M. B., Sanchez-Flores A., Estrada K., Silva G. G. Z., et al. (2015). Complete genome sequencing of a multidrug-resistant and human-invasive Salmonella enterica serovar typhimurium strain of the emerging sequence type 213 genotype. Genome Announc. 3:e663-15. 10.1128/genomeA.00663-615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capella-Gutiérrez S., Silla-Martínez J. M., Gabaldón T. (2009). trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25 1972–1973. 10.1093/bioinformatics/btp348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carattoli A., Zankari E., García-Fernández A., Voldby Larsen M., Lund O., Villa L., et al. (2014). In silico detection and typing of plasmids using plasmidfinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 58 3895–3903. 10.1128/AAC.02412-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascales E., Atmakuri K., Liu Z., Binns A. N., Christie P. J. (2005). Agrobacterium tumefaciens oncogenic suppressors inhibit T-DNA and VirE2 protein substrate binding to the VirD4 coupling protein. Mol. Microbiol. 58 565–579. 10.1111/j.1365-2958.2005.04852.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascales E., Buchanan S. K., Duché D., Kleanthous C., Lloubès R., Postle K., et al. (2007). Colicin biology. Microbiol. Mol. Biol. Rev. 71 158–229. 10.1128/MMBR.00036-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba D., Taboada G. L., Doallo R., Posada D. (2011). ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 27 1164–1165. 10.1093/bioinformatics/btr088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko K. A., Wanner B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97 6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz F., Frost L. S., Meyer R. J., Zechner E. L. (2010). Conjugative DNA metabolism in gram-negative bacteria. FEMS Microbiol. Rev. 34 18–40. 10.1111/j.1574-6976.2009.00195.x [DOI] [PubMed] [Google Scholar]
- de la Cruz F., Grinsted J. (1982). Genetic and molecular characterization of Tn21, a multiple resistance transposon from R100.1. J. Bacteriol. 151 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V. (2011). Beware of metaphors: chasses and orthogonality in synthetic biology. Bioeng. Bugs 2 3–7. 10.4161/bbug.2.1.13388 [DOI] [PubMed] [Google Scholar]
- de Toro M., Garcillán-Barcia M. P., De La Cruz F. (2014). Plasmid diversity and adaptation analyzed by massive sequencing of Escherichia coli plasmids. Microbiol. Spectr. 2 219–235. 10.1128/microbiolspec.PLAS-0031-2014 [DOI] [PubMed] [Google Scholar]
- del Campo I., Ruiz R., Cuevas A., Revilla C., Vielva L., de la Cruz F. (2012). Determination of conjugation rates on solid surfaces. Plasmid 67 174–182. 10.1016/j.plasmid.2012.01.008 [DOI] [PubMed] [Google Scholar]
- DeLong A., Syvanen M. (1991). Trans-acting transposase mutant from Tn5. Proc. Natl. Acad. Sci. U.S.A. 88 6072–6076. 10.1073/pnas.88.14.6072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire K. M., Kramer M., Grindley N. D. (1990). Role of instability in the cis action of the insertion sequence IS903 transposase. Proc. Natl. Acad. Sci. U.S.A. 87 4048–4052. 10.1073/pnas.87.11.4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire K. M., Willetts N. S. (1987). Mobilization of the non-conjugative plasmid RSF1010: a genetic analysis of its origin of transfer. Mol. Gen. Genet. 206 154–160. 10.1007/bf00326551 [DOI] [PubMed] [Google Scholar]
- Dunlop M. J., Cox R. S., Levine J. H., Murray R. M., Elowitz M. B. (2008). Regulatory activity revealed by dynamic correlations in gene expression noise. Nat. Genet. 40 1493–1498. 10.1038/ng.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Liu Y., Billman-Jacobe H. (2017). Genome sequence of an australian monophasic Salmonella enterica subsp. enterica typhimurium Isolate (TW-Stm6) carrying a large plasmid with multiple antimicrobial resistance genes. Genome Announc. 5 e793–17. 10.1128/genomeA.00793-717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32 1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. S., Betts L., Frazier M. L., Pollet R. M., Kwong S. M., Walton W. G., et al. (2013). Molecular basis of antibiotic multiresistance transfer in Staphylococcus aureus. Proc. Natl. Acad. Sci. U.S.A. 110 2804–2809. 10.1073/pnas.1219701110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lopez R., Redondo S., Garcillan-Barcia M. P., de la Cruz F. (2017). Towards a taxonomy of conjugative plasmids. Curr. Opin. Microbiol. 38 106–113. 10.1016/j.mib.2017.05.005 [DOI] [PubMed] [Google Scholar]
- Finn R. D., Coggill P., Eberhardt R. Y., Eddy S. R., Mistry J., Mitchell A. L., et al. (2016). The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 44 D279–D285. 10.1093/nar/gkv1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francia M. V., Varsaki A., Garcillán-Barcia M. P., Latorre A., Drainas C., de la Cruz F. (2004). A classification scheme for mobilization regions of bacterial plasmids. FEMS Microbiol. Rev. 28 79–100. 10.1016/j.femsre.2003.09.001 [DOI] [PubMed] [Google Scholar]
- Fratamico P. M., Yan X., Caprioli A., Esposito G., Needleman D. S., Pepe T., et al. (2011). The complete DNA sequence and analysis of the virulence plasmid and of five additional plasmids carried by shiga toxin-producing Escherichia coli O26:H11 strain H30. Int. J. Med. Microbiol. 301 192–203. 10.1016/j.ijmm.2010.09.002 [DOI] [PubMed] [Google Scholar]
- Fricke W. F., Wright M. S., Lindell A. H., Harkins D. M., Baker-Austin C., Ravel J., et al. (2008). Insights into the environmental resistance gene pool from the genome sequence of the multidrug-resistant environmental isolate Escherichia coli SMS-3-5. J. Bacteriol. 190 6779–6794. 10.1128/JB.00661-668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya N., Nisioka T., Komano T. (1991). Nucleotide sequence and functions of the oriT operon in IncI1 plasmid R64. J. Bacteriol. 173 2231–2237. 10.1128/jb.173.7.2231-2237.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gama J. A., Zilhão R., Dionisio F. (2017a). Co-resident plasmids travel together. Plasmid 93 24–29. 10.1016/j.plasmid.2017.08.004 [DOI] [PubMed] [Google Scholar]
- Gama J. A., Zilhão R., Dionisio F. (2017b). Conjugation efficiency depends on intra and intercellular interactions between distinct plasmids: plasmids promote the immigration of other plasmids but repress co-colonizing plasmids. Plasmid 93 6–16. 10.1016/j.plasmid.2017.08.003 [DOI] [PubMed] [Google Scholar]
- Gama J. A., Zilhão R., Dionisio F. (2017c). Multiple plasmid interference - pledging allegiance to my enemy’s enemy. Plasmid 93 17–23. 10.1016/j.plasmid.2017.08.002 [DOI] [PubMed] [Google Scholar]
- Gama J. A., Zilhão R., Dionisio F. (2018). Impact of plasmid interactions with the chromosome and other plasmids on the spread of antibiotic resistance. Plasmid 99 82–88. 10.1016/j.plasmid.2018.09.009 [DOI] [PubMed] [Google Scholar]
- Garcillán-Barcia M. P., Cuartas Lanza R., Cuevas A., de la Cruz F. (2019). Comparative analysis of MOBQ4 plasmids demonstrates that MOBQ is a cis-acting enriched relaxase protein family. bioRxiv org/ 10.1101/726927[Preprint]. [DOI] [Google Scholar]
- Garcillán-Barcia M. P., de la Cruz F. (2013). Ordering the bestiary of genetic elements transmissible by conjugation. Mob. Genet. Elements 3:e24263. 10.4161/mge.24263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcillán-Barcia M. P., Francia M. V., de la Cruz F. (2009). The diversity of conjugative relaxases and its application in plasmid classification. FEMS Microbiol. Rev. 33 657–687. 10.1111/j.1574-6976.2009.00168.x [DOI] [PubMed] [Google Scholar]
- Garcillán-Barcia M. P., Ruiz del Castillo B., Alvarado A., de la Cruz F., Martínez-Martínez L. (2015). Degenerate primer MOB typing of multiresistant clinical isolates of E. coli uncovers new plasmid backbones. Plasmid 77 17–27. 10.1016/j.plasmid.2014.11.003 [DOI] [PubMed] [Google Scholar]
- Getino M., de la Cruz F. (2018). Natural and artificial strategies to control the conjugative transmission of plasmids. Microbiol. Spectr 6 1–25. 10.1128/microbiolspec.MTBP-0015-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getino M., Palencia-Gándara C., Garcillán-Barcia M. P., de la Cruz F. (2017). PifC and osa, plasmid weapons against rival conjugative coupling proteins. Front. Microbiol. 8:2260. 10.3389/fmicb.2017.02260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. G., Young L., Chuang R.-Y., Venter J. C., Hutchison C. A., Smith H. O. (2009). Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6 343–345. 10.1038/nmeth.1318 [DOI] [PubMed] [Google Scholar]
- Gibson M. K., Forsberg K. J., Dantas G. (2015). Improved annotation of antibiotic resistance determinants reveals microbial resistomes cluster by ecology. ISME J. 9 207–216. 10.1038/ismej.2014.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S. G., Jessee J., Bloom F. R., Hanahan D. (1990). Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc. Natl. Acad. Sci. U.S.A. 87 4645–4649. 10.1073/pnas.87.12.4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmini J., Néron B., Abby S. S., Garcillán-Barcia M. P., de la Cruz F., Rocha E. P. C. (2014). Key components of the eight classes of type IV secretion systems involved in bacterial conjugation or protein secretion. Nucleic Acids Res. 42 5715–5727. 10.1093/nar/gku194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S., Gascuel O. (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52 696–704. 10.1080/10635150390235520 [DOI] [PubMed] [Google Scholar]
- Guiney D. G., Deiss C., Simnad V., Yee L., Pansegrau W., Lanka E. (1989). Mutagenesis of the Tra1 core region of RK2 by using Tn5: identification of plasmid-specific transfer genes. J. Bacteriol. 171 4100–4103. 10.1128/jb.171.7.4100-4103.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S., Sugiyama T., Itoh T. (1994). Comparative analysis of the replicon regions of eleven ColE2-related plasmids. J. Bacteriol. 176 7233–7243. 10.1128/jb.176.23.7233-7243.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt K. E., Thieu Nga T. V., Thanh D. P., Vinh H., Kim D. W., Vu Tra M. P., et al. (2013). Tracking the establishment of local endemic populations of an emergent enteric pathogen. Proc. Natl. Acad. Sci. U.S.A. 110 17522–17527. 10.1073/pnas.1308632110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii T., Itoh T. (1988). Replication of ColE2 and ColE3 plasmids: the regions sufficient for autonomous replication. Mol. Gen. Genet. 212 225–231. 10.1007/bf00334689 [DOI] [PubMed] [Google Scholar]
- Itoh T., Horii T. (1989). Replication of ColE2 and ColE3 plasmids: in vitro replication dependent on plasmid-coded proteins. Mol. Gen. Genet. 219 249–255. 10.1007/bf00261184 [DOI] [PubMed] [Google Scholar]
- Kelley L. A., Mezulis S., Yates C. M., Wass M. N., Sternberg M. J. E. (2015). The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10 845–858. 10.1038/nprot.2015.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido M., Yasueda H., Itoh T. (1991). Identification of a plasmid-coded protein required for initiation of ColE2 DNA replication. Nucleic Acids Res. 19 2875–2880. 10.1093/nar/19.11.2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza V. F., de Toro M., Garcillán-Barcia M. P., Mora A., Blanco J., Coque T. M., et al. (2014). Plasmid flux in Escherichia coli ST131 sublineages, analyzed by plasmid constellation network (PLACNET), a new method for plasmid reconstruction from whole genome sequences. PLoS Genet. 10:e1004766. 10.1371/journal.pgen.1004766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Zheng H., Yang M., Xu Z., Wang X., Wei L., et al. (2015). Genome analysis and in vivo virulence of porcine extraintestinal pathogenic Escherichia coli strain PCN033. BMC Genomics 16:717. 10.1186/s12864-015-1890-1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo-Díaz F., Fernández-López C., Garcillán-Barcia M. P., Espinosa M. (2014). Bringing them together: plasmid pMV158 rolling circle replication and conjugation under an evolutionary perspective. Plasmid 74 15–31. 10.1016/j.plasmid.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maindola P., Raina R., Goyal P., Atmakuri K., Ojha A., Gupta S., et al. (2014). Multiple enzymatic activities of ParB/Srx superfamily mediate sexual conflict among conjugative plasmids. Nat. Commun. 5:5322. 10.1038/ncomms6322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. (2000). Identification of the mob genes of plasmid pSC101 and characterization of a hybrid pSC101-R1162 system for conjugal mobilization. J. Bacteriol. 182 4875–4881. 10.1128/jb.182.17.4875-4881.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. (2009). Replication and conjugative mobilization of broad host-range IncQ plasmids. Plasmid 62 57–70. 10.1016/j.plasmid.2009.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncalián G., Grandoso G., Llosa M., de la Cruz F. (1997). oriT-processing and regulatory roles of TrwA protein in plasmid R388 conjugation. J. Mol. Biol. 270 188–200. 10.1006/jmbi.1997.1082 [DOI] [PubMed] [Google Scholar]
- Monzingo A. F., Ozburn A., Xia S., Meyer R. J., Robertus J. D. (2007). The structure of the minimal relaxase domain of MobA at 2.1 a resolution. J. Mol. Biol. 366 165–178. 10.1016/j.jmb.2006.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisato D., Way J. C., Kim H. J., Kleckner N. (1983). Tn10 transposase acts preferentially on nearby transposon ends in vivo. Cell 32 799–807. 10.1016/0092-8674(83)90066-90061 [DOI] [PubMed] [Google Scholar]
- Nomura N., Masai H., Inuzuka M., Miyazaki C., Ohtsubo E., Itoh T., et al. (1991). Identification of eleven single-strand initiation sequences (ssi) for priming of DNA replication in the F, R6K, R100 and ColE2 plasmids. Gene 108 15–22. 10.1016/0378-1119(91)90482-q [DOI] [PubMed] [Google Scholar]
- Novick R. P. (1987). Plasmid incompatibility. Microbiol. Rev. 51 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y., Ooka T., Iguchi A., Toh H., Asadulghani M., Oshima K., et al. (2009). Comparative genomics reveal the mechanism of the parallel evolution of O157 and non-O157 enterohemorrhagic Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 106 17939–17944. 10.1073/pnas.0903585106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K., Toh H., Ogura Y., Sasamoto H., Morita H., Park S.-H., et al. (2008). Complete genome sequence and comparative analysis of the wild-type commensal Escherichia coli strain SE11 isolated from a healthy adult. DNA Res. 15 375–386. 10.1093/dnares/dsn026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Mendoza D., Lucas M., Muñoz S., Herrera-Cervera J. A., Olivares J., de la Cruz F., et al. (2006). The relaxase of the rhizobium etli symbiotic plasmid shows nic site cis-acting preference. J. Bacteriol. 188 7488–7499. 10.1128/JB.00701-706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollet R. M., Ingle J. D., Hymes J. P., Eakes T. C., Eto K. Y., Kwong S. M., et al. (2016). Processing of nonconjugative resistance plasmids by conjugation nicking enzyme of staphylococci. J. Bacteriol. 198 888–897. 10.1128/JB.00832-815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Millan A., Heilbron K., MacLean R. C. (2014). Positive epistasis between co-infecting plasmids promotes plasmid survival in bacterial populations. ISME J. 8 601–612. 10.1038/ismej.2013.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Millan A., MacLean R. C. (2017). Fitness costs of plasmids: a limit to plasmid transmission. Microbiol. Spectr 5 601–612. 10.1128/microbiolspec.MTBP-0016-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrödinger L. (2015). The PyMOL Molecular Graphics System. Version 1. [Google Scholar]
- Silva-Rocha R., Martínez-García E., Calles B., Chavarría M., Arce-Rodríguez A., de Las Heras A., et al. (2013). The standard european vector architecture (SEVA): a coherent platform for the analysis and deployment of complex prokaryotic phenotypes. Nucleic Acids Res. 41 D666–D675. 10.1093/nar/gks1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie C., Garcillán-Barcia M. P., Francia M. V., Rocha E. P. C., de la Cruz F. (2010). Mobility of plasmids. Microbiol. Mol. Biol. Rev. 74 434–452. 10.1128/MMBR.00020-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen A. H., Hansen L. H., Johannesen E., Sørensen S. J. (2003). Conjugative plasmid conferring resistance to olaquindox. Antimicrob. Agents Chemother. 47 798–799. 10.1128/aac.47.2.798-799.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22 2688–2690. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- Stephens C. M., Skerker J. M., Sekhon M. S., Arkin A. P., Riley L. W. (2015). Complete genome sequences of four Escherichia coli ST95 isolates from bloodstream infections. Genome Announc 3:e1241-15. 10.1128/genomeA.01241-1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Itoh T. (1993). Control of ColE2 DNA replication: in vitro binding of the antisense RNA to the Rep mRNA. Nucleic Acids Res. 21 5972–5977. 10.1093/nar/21.25.5972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. (1998). Timing, self-control and a sense of direction are the secrets of multicopy plasmid stability. Mol. Microbiol. 29 1137–1145. 10.1046/j.1365-2958.1998.01012.x [DOI] [PubMed] [Google Scholar]
- Summers D. K., Sherratt D. J. (1984). Multimerization of high copy number plasmids causes instability: CoIE1 encodes a determinant essential for plasmid monomerization and stability. Cell 36 1097–1103. 10.1016/0092-8674(84)90060-90066 [DOI] [PubMed] [Google Scholar]
- Summers D. K., Sherratt D. J. (1988). Resolution of ColE1 dimers requires a DNA sequence implicated in the three-dimensional organization of the cer site. EMBO J. 7 851–858. 10.1002/j.1460-2075.1988.tb02884.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takechi S., Yasueda H., Itoh T. (1994). Control of ColE2 plasmid replication: regulation of rep expression by a plasmid-coded antisense RNA. Mol. Gen. Genet. 244 49–56. 10.1007/bf00280186 [DOI] [PubMed] [Google Scholar]
- Tsvetkova K., Marvaud J.-C., Lambert T. (2010). Analysis of the mobilization functions of the vancomycin resistance transposon Tn1549, a member of a new family of conjugative elements. J. Bacteriol. 192 702–713. 10.1128/JB.00680-689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zyl L. J., Deane S. M., Rawlings D. E. (2003). Analysis of the mobilization region of the broad-host-range IncQ-like plasmid pTC-F14 and its ability to interact with a related plasmid, pTF-FC2. J. Bacteriol. 185 6104–6111. 10.1128/jb.185.20.6104-6111.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varsaki A., Moncalián G., Garcillán-Barcia M., del P., Drainas C., de la Cruz F. (2009). Analysis of ColE1 MbeC unveils an extended ribbon-helix-helix family of nicking accessory proteins. J. Bacteriol. 191 1446–1455. 10.1128/JB.01342-1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagura M., Itoh T. (2006). The rep protein binding elements of the plasmid ColE2-P9 replication origin. Biochem. Biophys. Res. Commun. 345 872–877. 10.1016/j.bbrc.2006.04.168 [DOI] [PubMed] [Google Scholar]
- Yagura M., Nishio S.-Y., Kurozumi H., Wang C.-F., Itoh T. (2006). Anatomy of the replication origin of plasmid ColE2-P9. J. Bacteriol. 188 999–1010. 10.1128/JB.188.3.999-1010.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasueda H., Horii T., Itoh T. (1989). Structural and functional organization of ColE2 and ColE3 replicons. Mol. Gen. Genet. 215 209–216. 10.1007/bf00339719 [DOI] [PubMed] [Google Scholar]
- Yasueda H., Takechi S., Sugiyama T., Itoh T. (1994). Control of ColE2 plasmid replication: negative regulation of the expression of the plasmid-specified initiator protein, Rep, at a posttranscriptional step. Mol. Gen. Genet. 244 41–48. 10.1007/bf00280185 [DOI] [PubMed] [Google Scholar]
- Zaleski P., Wawrzyniak P., Sobolewska A., Łukasiewicz N., Baran P., Romańczuk K., et al. (2015). pIGWZ12–A cryptic plasmid with a modular structure. Plasmid 79 37–47. 10.1016/j.plasmid.2015.04.001 [DOI] [PubMed] [Google Scholar]
- Zaleski P., Wawrzyniak P., Sobolewska A., Mikiewicz D., Wojtowicz-Krawiec A., Chojnacka-Puchta L., et al. (2012). New cloning and expression vector derived from Escherichia coli plasmid pIGWZ12; a potential vector for a two-plasmid expression system. Plasmid 67 264–271. 10.1016/j.plasmid.2011.12.011 [DOI] [PubMed] [Google Scholar]
- Zaleski P., Wolinowska R., Strzezek K., Lakomy A., Plucienniczak A. (2006). The complete sequence and segregational stability analysis of a new cryptic plasmid pIGWZ12 from a clinical strain of Escherichia coli. Plasmid 56 228–232. 10.1016/j.plasmid.2006.05.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.