Abstract
Background:
Hepatocellular carcinoma (HCC) is one of the most common primary hepatic malignancies and growing challenges of global health. In this study, for the first time in Iran, we investigated the 5-year survival rate and prognostic factors in patients with HCC.
Materials and Methods:
In this historical cohort study, we examined the medical records of 227 HCC patients who were registered in the central tumor registry of our institution from September 2007 to September 2017. Demographic data, clinical parameters, received treatments, and survival curves from time of diagnosis were evaluated. Kaplan–Meier was used for univariate analysis, and multivariable analysis was performed by Cox regression.
Results:
A total of 208 (91.63%) patients were dead. The 5-year survival rate was estimated 19 (8.37%). The average follow-up in this study was 14.3 months. Overall median survival rate was 12.1 months. Univariate analysis showed that tumor size, metastasis, number of involved lymph node, hepatitis type, and treatment were significantly related to the survival rate, and Cox regression analysis revealed that the tumor size >3 cm (hazard ratio [HR] = 3.06, 95% confidence interval [CI] = 1.68–4.97; P = 0.027), involved lymph nodes >2 (HR = 4.12, 95% CI = 2.66–6.38; P = 0.001), metastasis (HR = 3.87, 95% CI = 3.13–6.54; P = 0.011), combination therapy with surgery and chemotherapy (HR = 0.4, 95% CI = 0.15–0.79; P = 0.023), and coinfection with hepatitis B virus and hepatitis C virus (HR = 2.11, 95% CI = 1.81–4.6; P = 0.036) are the most relevant prognostic factors with 5-year survival rate in patients with HCC.
Conclusion:
Results of this study will help estimate survival rates for patients with HCC according to their clinical status.
Keywords: Five-year survival rate, hepatocellular carcinomas, prognostic factors
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary hepatic malignancy in adults that originates from hepatocytes. Some studies have reported that it is the sixth[1] and some fifth common cancer in the world.[2,3,4] HCC is one of the important and growing challenges of global health, especially in developing countries, and the fourth cause of death due to cancer.[5]
The assessment of the incidence and severity of this cancer indicate a high mortality (93%).[2,6] The highest prevalence of HCC is in Africa and Eastern Asia, and the lowest prevalence is in South America and Europe.[7] The incidence and prevalence of this cancer in men are higher than that in women.[8] The main causes of HCC are hepatitis B virus (HBV) and hepatitis C virus (HCV), cirrhosis, alcohol-related liver disease,[1] and metabolic diseases such as hemochromatosis and alpha-1-antitrypsin deficiency. The first risk factor of HCC is cirrhosis.[9,10] Despite significant advances in the etiology of HCC, the 5-year survival rate has been estimated very low (5%–14%).[11,12] In cases that HCC leads to death, the survival rate of patients depends on many factors, such as portal vein thrombosis, tumor size, alpha-fetoprotein (AFP), and tumor stage.[13] Therefore, determining the survival rate of HCC patients after diagnosis and studying the relationship between different factors with this rate can be a subject of numerous studies in the field of epidemiology. Most of the retrospective studies on HCC have focused on epidemiology and risk factors for HCC.[14,15,16,17] In Iran, only one study has studied the incidence and risk of HCC factors.[18] In this study, we investigated the 5-year survival rate of HCC patients and prognostic factors assessed in HCC patients referred to Rasoul-e-Akram Hospital, Tehran, Iran, from September 2007 to September 2017 for the first time in Iran, we investigated the 5-year survival rate of HCC patients and prognostic factors assessed in HCC patients referred to Rasoul-e-Akram Hospital, Tehran, Iran, from September 2007 to September 2017.
METHODS
Study design and setting
In this historical cohort study, we examined the medical records of 227 HCC patients who referred to Rasoul-e-Akram Hospital, Iran University of Medical Sciences, Tehran, Iran, from September 2007 to September 2017 for the treatment or liver surgery (tumor removal or chemotherapy). HCC was diagnosed based on the European Association for the Study of the Liver guidelines[6] by an expert oncologist. This study was approved by the Ethics Committee of Iran University of Medical Sciences. All researches during this study adhered to the principles of Helsinki Declaration.
Eligibility criteria and follow-up
Inclusion criteria in the study were diagnosis of HCC, file and data availability, and at least 6-month follow-up. Exclusion criteria in the study were file and data unavailability, comorbidity with other cancers, and fewer than 6-month follow-up. The follow-up of patients was continued until the end of the study period or until the patient died. Base on the reviewers' comments: Cirrhosis was evaluated for its positivity or negativity, and if cirrhosis was positive, the extent was classified according to the Child–Pugh classification (A-C).[8]
The main variables in 5-year survival assay consisting of tumor size (<1, 1–3, and > 3 cm), number of involved lymph node (>2 or < 2), and presence of metastasis (positive or negative) were used to categorize the severity and survival rate estimation of HCC. Hepatitis types (HBV, HCV, HBV+HCV, and none), Child–Pugh class, treatment procedure (surgery, chemotherapy), involvement of right or left lobe, cirrhosis, and AFP level were the prognostic factors in 5-year survival assay.
Outcome
The main outcome of this study was 5-year survival rate in patients with HCC based on clinical data. Survival rate was defined as the interval between HCC diagnosis and death. Follow-up courses for patients were every 3 months. Patients who had an undetermined situation in follow-up were excluded from the study.
Data gathering
Demographic (age, sex, education, familial history, and alcohol consumption) and clinical data (hepatitis type, alkaline phosphatase levels >400 ng/ml, tumor size, number of involved lymph nodes, metastases, cirrhosis, type of treatment, diagnosis time, and dead or survival of patients) were recorded by referring to the cancer registry system of patients in the hospital archives department.
Data analysis
Data analysis was performed using SPSS version 16 Software (IBM Inc., Chicago, IL, USA). Kaplan–Meier analysis with a log-rank test was performed to compare the survival distributions of baseline variables including demographic data (sex, age, and family history), clinical parameters (Child-Pugh classification, tumor size, number of involved lymph node, presence of metastasis, hepatitis type, involvement of right or left lobe, cirrhosis, and AFP level), treatments received, and survival curves from the time of diagnosis. Univariate Cox regression analysis with P < 0.15 was used to determine the affecting factors. Variables with 0.15 in the log-rank test were entered into a Cox multivariable analysis with the backward selection method. The Cox-proportional hazards model was used for the multivariable analysis. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Statistical significance was defined as P < 0.05.
RESULTS
Demographic and clinical data
One hundred and seventy-eight (78.4%) cases of HCC were male, and 49 (21.6%) cases were female. The male-to-female ratio was 3.6. The mean age of the patients was 28 ± 62 years (35–84 years). The history of hepatitis HBV, HCV, HBV + HCV, and none were reported in 109 (48%), 74 (32.6%), 18 (8%), and 26 (11.4%) patients, respectively. Family history of HCC (at least one of the first-degree relatives) was reported in 16 (7%) patients. Surgery and chemotherapy cotreatment were reported as the most common treatment (89, 39.2% patients). The level of AFP was >400 ng/ml in 96 (42.3%), 10–400 ng/ml in 64 (28.1%), and ≤10 ng/ml in 67 (29.6%) patients [Table 1].
Table 1.
Distribution of demographic characteristics and details of tumor features
| Variables | Frequency (%) |
|---|---|
| Age (mean±SD) | 62±28 |
| Mean follow-up (months) | 14.2±7 |
| Median survival (months) | 12.7±7.6 |
| Sex | |
| Male | 178 (75) |
| Female | 49 (25) |
| Family history | |
| Positive | 16 (7) |
| Negative | 211 (93) |
| Hepatitis type | |
| HBV | 109 (48) |
| HCV | 74 (32.6) |
| HBV + HCV | 18 (8) |
| None | 26 (11.4) |
| Alcohol consumption (positive) | 85 (37.5) |
| Treatment type | |
| Surgery | 31 (13.6) |
| Chemotherapy | 58 (25.5) |
| Surgery and chemotherapy | 89 (39.2) |
| Other | 49 (21.7) |
| Hemoglobin (g/dl) | 14.12±3.24 |
| Platelet count×103 (g/dl) | 211.4±28.2 |
| Serum AFP level (ng/mL) | |
| <10 | 67 (29.6) |
| 10-400 | 64 (28.1) |
| >400 | 96 (42.3) |
| Tumor size (cm) | |
| <1 | 28 (12.4) |
| 1-3 | 33 (14.5) |
| >3 | 166 (73.1) |
| Metastasis | |
| Positive | 69 (30.2) |
| Negative | 114 (50) |
| A known | 44 (19.8) |
| Number of involved lymph node | |
| ≤2 | 74 (32.6) |
| >2 | 153 (77.4) |
| Cirrhosis | |
| Positive | 169 (74.5) |
| Negative | 58 (25.5) |
| Child-Pugh class | |
| A | 145 (85.8) |
| B | 20 (11.8) |
| C | 4 (2.4) |
| Involved liver lobe | |
| Right | 79 (34.9) |
| Left | 41 (17) |
| Both | 107 (47.1) |
SD=Standard deviation; HBV=Hepatitis B virus; HCV=Hepatitis C virus; AFP=Alpha-fetoprotein
One hundred and sixty-nine (74.5%) patients had liver cirrhosis. The Child–Pugh class was the A in 134 (79.3%), B in 20 (11.8%), and C in 15 (8.9%) patients. Encephalopathy, sepsis, and edema were estimated at 82 (36%), 87 (38%), and 87 (36%) patients, respectively. Seventy-nine (34.9%) cases of HCC in the right lobe of the liver, 41 (17%) cases in the left lobe, and 107 (47.1%) cases concurrently in both lobes were reported. The tumor size was >3 cm in 166 (73.1%), 1–3 cm in 33 (14.5%), and <3 cm in 28 (12.4%) patients. The metastasis to other organs was reported in 69 (30.3%) patients. In 153 (78%) of patients, more than two lymph nodes were involved. In 107 (47.1%) patients, both lobes of the liver (left and right) were involved. The rate of involvement for the right and left lobe was 79 (34.9%) and 41 (17%), respectively [Table 2].
Table 2.
Predictors of 5-year survival rate using the method Kaplan-Meier and log-rank test
| Variables | Frequency, n (%) | 5-survival rate (%) | Median survival (month) | Log-rank test | P | |
|---|---|---|---|---|---|---|
| Alive patients (n=208) | Died patients (n=19) | |||||
| Age (year) | ||||||
| ≤60 | 62 (29.9) | 6 (31.5) | 8.80 | 13.2±3.1 | 1.68 | 0.23 |
| >60 | 146 (70.1) | 13 (68.5) | 8.10 | 12.5±3.4 | ||
| Sex | ||||||
| Female | 45 (21.7) | 4 (21.1) | 8.20 | 14.0±2.8 | 3.03 | 0.21 |
| Male | 163 (78.3) | 15 (78.9) | 8.12 | 12.4±3.01 | ||
| Family history | ||||||
| Negative | 193 (93) | 18 (94.7) | 8.94 | 12.77±4.8 | 3.24 | 0.19 |
| Positive | 15 (7) | 1 (5.3) | 9.10 | 11.5±2.8 | ||
| Tumor size (cm) | ||||||
| <1 | 54 (26) | 7 (36.8) | 11.50 | 18.7±6.1 | 6.24 | 0.001 |
| 1-3 | 87 (41.8) | 9 (47.5) | 9.30 | 12.22±4.2 | ||
| >3 | 67 (32.2) | 3 (15.7) | 4.20 | 8.1±3.4 | ||
| Metastasis | ||||||
| Absence | 102 (49) | 12 (63.1) | 10.50 | 16.1±5.8 | 5.31 | 0.021 |
| Presence | 66 (31.8) | 3 (25) | 4.20 | 9.2±4.11 | ||
| A known | 40 (19.2) | 4 (33.3) | 9 | 12.2±5.2 | ||
| n | ||||||
| ≤2 | 61 (29.3) | 13 (68.4) | 17.60 | 18.88±5.6 | 9.75 | 0.001 |
| >2 | 147 (70.7) | 6 (31.6) | 3.90 | 9.7±5.8 | ||
| Cirrhosis | ||||||
| Positive | 54 (26) | 4 (21) | 7.50 | 11.9±4.1 | 3.64 | 0.33 |
| Negative | 154 (74) | 15 (79) | 8.90 | 13.01±3.6 | ||
| Liver lobe | ||||||
| Right | 73 (35) | 6 (31.6) | 7.50 | 13.1±4.5 | 2.024 | 0.45 |
| Left | 38 (18.2) | 4 (21) | 9.70 | 13.5±4.3 | ||
| Both | 98 (46.8) | 9 (47.4) | 8.40 | 12.17±4.9 | ||
| serum AFP level (ng/mL) | ||||||
| <400 | 102 (49) | 9 (47.3) | 8.80 | 12.09±4.7 | 1.47 | 0.66 |
| ≥400 | 106 (51) | 10 (52.7) | 9.40 | 13.31±3.9 | ||
| Child-Pugh class | ||||||
| None | 54 (26) | 4 (21) | 6.90 | 13.5±4.12 | 0.684 | 0.51 |
| A | 121 (58.2) | 13 (68.4) | 9.70 | 12.3±4.13 | ||
| B | 19 (9.1) | 1 (5.3) | 5 | 11.8±3.2 | ||
| C | 14 (6.7) | 1 (5.3) | 6.60 | 10.4±3.16 | ||
| Treatment type | ||||||
| Surgery | 29 (13.9) | 2 (10.5) | 6.50 | 9.1±3.24 | 4.27 | 0.027 |
| Chemotherapy | 54 (26) | 4 (21.1) | 6.90 | 9.21±3.4 | ||
| Surgery + Chemotherapy | 78 (37.5) | 11 (57.9) | 12.40 | 18.05±4.6 | ||
| Other | 47 (22.6) | 2 (10.5) | 4.10 | 8.14±4.7 | ||
| Hepatitis type | ||||||
| None | 22 (10.6) | 4 (21) | 15.40 | 19.1±5.1 | 4.11 | 0.037 |
| HBV | 100 (48) | 9 (47.4) | 8.30 | 12.1±4.8 | ||
| HCV | 68 (32.7) | 6 (31.6) | 8.10 | 11.88±4.6 | ||
| HBV + HCV | 18 (3.7) | 0 (0) | 0 | 7.26±2.1 | ||
HBV=Hepatitis B virus; HCV=Hepatitis C virus; AFP=Alpha-fetoprotein
Survival rate
The average follow-up in this study was 14.3 months. The overall median survival rate of 389 patients was 12.7 months. Two hundred and eight (91.63%) patients were dead. Based on Kaplan–Meier analysis, the 5-year survival rate was estimated 8.37%, while 1-year and 3-year survival rates were 60.5% and 27.6%, respectively [Figure 1], and the follow-up durations were 14.3 ± 7 and 21.5 ± 8.6 months, respectively [Table 1].
Figure 1.
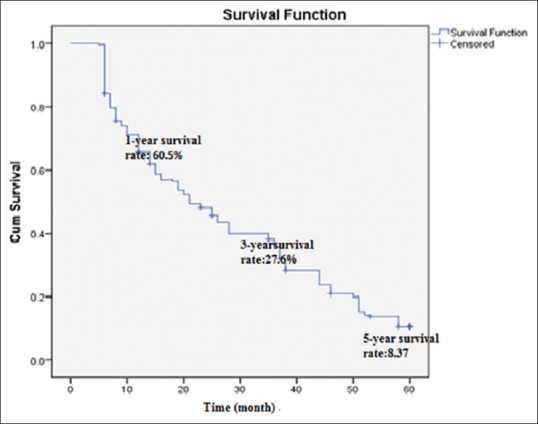
Kaplan–Meier chart, 5-year survival rate of 227 hepatocellular carcinoma patients
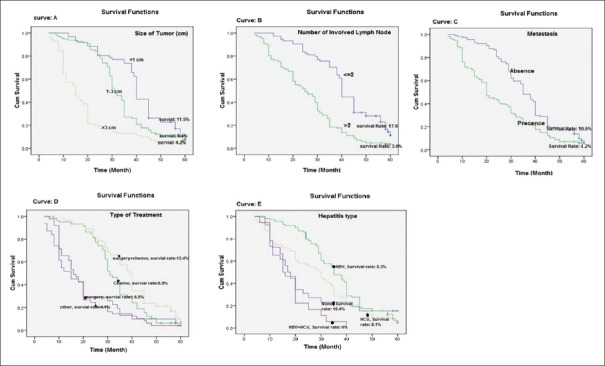
The median survival rate in patients with tumor size >3 cm, 1–3 cm, and <1 cm was 8.1, 18.7, and 12.22 months, respectively. The median survival rate was significantly lower in patients with tumor size of >3 cm (log-rank = 6.24, P 0.001). The median survival rate was significantly lower in patients with metastasis to other organs versus patients who did not have metastasis (9.4 vs. 16.1 months) (log-rank = 5.31; P 0.021). The median survival rate in patients with the number of involved lymph node >2 (9.7%) was significantly lower than survival rate in patients with the number of involved lymph node ≤2 (18.88%) (log-rank = 9.75; P = 0.001). Patients with HCV had a median survival rate of 11.88 months, and patients with HBV had the poorest median survival of 12.1 months. The median survival rate was estimated 0% in patients who simultaneously had HBV and HCV infections (log-rank = 4.11; P = 0.037). Finally, patient survival was analyzed on the basis of the treatments that the patients received. As shown in Figure 2, there was significant segregation in survival curves. As expected, patients who underwent surgery and chemotherapy cotreatment therapy had the best median survival rate of 18.05 months, followed by patients receiving surgery alone (9.1 months) and chemotherapy alone (9.21 months). There was no significant difference between sex, age, family history, AFP levels, type of liver lobe involvement, and Child–Pugh class with 5-year survival rate in univariate analysis (P > 0.05) [Table 2].
Figure 2.
Five-year survival rate (Kaplan–Meyer) of patients with hepatocellular carcinoma based on the clinical and pathologic characteristics of patients
Univariate and multivariate analyses of potential factors affecting patient survival were performed to identify those risk factors, which predict the survival of patients. Results of univariate analysis for factors associated with overall survival are shown in Table 3. Tumor size >3 cm, metastasis, number of involved lymph node >2, coinfection with HBV and HCV, and combination treatment with surgery plus chemotherapy were associated with diminished overall survival.
Table 3.
Univariate analysis to identify variables associated with overall survival
| Variables | HR | 95% CI | P |
|---|---|---|---|
| Age, ≥60 versus <60 (years) | 1.25 | 1.16-2.4 | 0.37 |
| Sex, male versus female | 2.54 | 1.12-3.77 | 0.23 |
| Family history, positive versus negative | 2.31 | 0.71-4.91 | 0.45 |
| Tumor size (cm) | |||
| <1 | Reference | ||
| 1-3 | 1.72 | 1.25-10.54 | 0.45 |
| >3 | 3.24 | 1.9-5.31 | 0.021 |
| Metastasis | |||
| Absence | Reference | ||
| Presence | 4.31 | 2.14-6.42 | 0.001 |
| A known | 1.7 | 0.68-3.87 | 0.24 |
| Involved lymph nodes >2 versus ≤2 | 3.89 | 1.67-5.63 | 0.001 |
| Cirrhosis, positive versus negative | 1.94 | 0.77-4.150 | 0.24 |
| Child-Pugh class | |||
| None | Reference | ||
| A | 2.31 | 0.91-4.87 | 0.27 |
| B | 1.61 | 1.01-2.67 | 0.38 |
| C | 1.04 | 0.8-2.5 | 0.57 |
| Liver lobe of involved | |||
| Right | Reference | ||
| Left | 2.45 | 0.5-3.15 | 0.44 |
| Both | 3.6 | 0.9-4.6 | 0.3 |
| Serum AFP level (ng/mL) | |||
| <10 | Reference | ||
| 10-400 | 0.88 | 0.52-3.54 | 0.26 |
| >400 | 0.97 | 0.61-2.11 | 0.61 |
| Treatment type | |||
| Surgery | Reference | ||
| Chemotherapy | 0.91 | 0.51-3.29 | 0.28 |
| Surgery + chemotherapy | 0.38 | 0.11-0.82 | 0.001 |
| Other | 2.37 | 1.5-4.2 | 0.046 |
| Hepatitis type | |||
| None | Reference | ||
| HBV | 2.1 | 1.24- 3.58 | 0.088 |
| HCV | 2.6 | 1.51-4.3 | 0.095 |
| HBV + HCV | 3.4 | 1.77-5.19 | 0.001 |
HR=Hazards ratio, CI=Confidence interval; HBV=Hepatitis B virus; HCV=Hepatitis C virus; AFP=Alpha-fetoprotein
Multivariable analysis results showed that tumor size higher than 3 cm (HR = 3.06, 95% CI = 1.68–4.97, P = 0.027), involved lymph nodes >2 (HR = 4.12, 95% CI = 2.66–6.38, P = 0.001), metastasis to other organs (HR = 3.87, 95% CI = 3.13–6.54, P = 0.011), combination treatment with surgery plus chemotherapy (HR = 0.4, 95% CI = 0.15–0.79, P = 0.023), and coinfection with HBV and HCV (HR = 2.11, 95% CI = 1.81–9.65, P = 0.036) were identified to be independent risk factors affecting patient survival as shown in Table 4 by multivariate analysis.
Table 4.
Independent variables predictive of 5-year survival by multivariate analysis
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Tumor size ≤3 versus 1-3 and <1 (cm) | 3.06 | 1.68-4.97 | 0.027 |
| Metastasis (absent versus present) | 3.88 | 3.13-6.54 | 0.011 |
| Involved lymph nodes >2 | 4.12 | 2.66-6.38 | 0.001 |
| Treatment with surgery + chemotherapy versus surgery and chemotherapy | 0.4 | 0.15-0.79 | 0.023 |
| Co infection with HBV + HCV versus HBV and HCV | 2.11 | 1.81-4.6 | 0.036 |
HR=Hazards ratio; CI=Confidence interval; HBV=Hepatitis B virus; HCV=Hepatitis C virus
DISCUSSION
Unlike most retrospective studies in the field of HCC that focus on the epidemiology and risk factors of HCC,[9,10,11,12] the present study evaluates the 5-year survival rate of patients with HCC based on their clinical conditions and treatment methods. In the present study, the results of 227 HCC patients who referred to the Rasoul-e-Akram Hospital in Tehran from September 2007 to September 2017 showed that 5-year survival rate is 8.37%. This is consistent with the reported survival rate in similar studies that reported 5-year survival rate is 5%–14% for patients with HCC.[13,14,15] In our study, the 1-year survival rate (60.5%) and 3-year survival rate (27.6%) were assessed in patients with HCC. This results consist with the findings of Otto et al., who reported that the 1-year survival rate is 62% in patients with HCC,[16] and Bordoni et al., who reported that the 3-year survival rate is 19%.[17] In our study, tumor size >3 cm, metastasis to other organ, involved lymph nodes >2, and simultaneous infection with both types of hepatitis (HBV, HCV) are the risk factors of lower survival rate in patients with HCC, and combined surgical treatment and chemotherapy are related to higher survival rate. Many studies have investigated various risk factors that reduce the survival rate of patients with HCC. For example, the results of Wang et al.'s study in 2019 showed that race, age, region, tumor stage, and tumor grade are risk factors associated with decreasing the survival rate of patients with HCC.[18] Fujii et al. in 2012 showed that the risk factors for reducing HCC survival include age and tumor stage.[19] However, there are few studies that have examined the risk factors mentioned in our study. Wu et al. in 2019 showed that primary liver tumor size, lymph node involvement, and metastasis are the most important risk factors associated with decreasing the survival rate of patients with HCC.[20] The results of our study are also consistent with the study by Wu et al. Our study results show that, in addition to the risk factors mentioned above, HCV and HBV coinfections are a risk factor for the survival rate of patients with HCC. One of the differences between the results of our study and Wang et al. 2019 is that in our study, age is not a risk factor for HCC survival rate. The reason for this can be related to the race of our population study (Asian race). In Asia, the death rate due to HCC is lower.[18,21]
Since determining the possible prognostic factors affecting the survival of patients can be useful in adopting the best treatment strategy for HCC, therefore, it is important to evaluate patients with HCC. In this study, prognostic factors were also studied. Finally, tumor size, metastasis, involved lymph nodes, hepatitis, and type of treatment were recognized as prognostic factors in the survival of patients with HCC. It should be noted that there has not been any study on HCC survival in Iran so far, and the present study is the only study in Iran that, first time, evaluates the survival rate of patients with HCC.
The results of the Cox regression analysis showed that the number of involved lymph nodes, metastasis, type of treatment, tumor size, and coinfection with both hepatitis B and C, respectively, were the most prognostic factors in the 5-year survival rate of patients with HCC. In our study, the mean age for HCC was 62 years, which confirms that HCC is more common in older patients.[22] In a systematic review and meta-analysis in 2009, Bruix and Llovet, reported the cirrhosis, type of treatment and tumor size are main prognostic factors for HCC.[23,24,25] Our study also confirmed the type of treatment and tumor size as prognostic factors for HCC. Zeeneldin et al. in 2015 and Selçuk in 2017 reported that metastasis is another prognostic factor in HCC.[26,27] The results of our study are consistent with these studies and suggest that metastasis is a factor reducing and affecting the survival rate of HCC patients. The findings of Kim et al. in 2006 showed that the number of affected lymph nodes is one of the prognostic factors related to a decreased survival rate of patients with HCC.[28] Our study also confirms that the number of involved lymph nodes is a prognostic factor for HCC survival. The results of our study showed that hepatitis C can be considered as one of the prognostic factors in the survival rate of HCC patients. Few studies have investigated hepatitis as a prognostic and effective factor in the survival rate of HCC patients. The results of our study are consistent with the results of the study by Abbas et al. in 2008. They reported that HCC prognosis in patients with hepatitis C is poorer than other HCC patients, and hepatitis C should be taken into consideration as a prognostic factor for HCC.[29] Some studies have suggested AFP as a prognostic factor in HCC, but our results do not confirm this. The reason for this difference can be related to the level of AFP, in studies where the level of AFP ≥1000 ng/ml is considered,[30] this factor is considered as a prognostic factor in HCC, but in studies with a level of AFP ≥400 ng/ml, this factor has not been confirmed as a prognostic factor for HCC.[27] In our study, probably due to the level of AFP ≥400 ng/ml, this factor has not been confirmed as a prognostic factor for HCC. Hence, if we were considering AFP ≥1000 ng/ml, our study also probably confirmed AFP as a prognostic factor in HCC. In general, knowing more about the importance of HCC leads to an early diagnosis of this disease at an early stage by diagnostic procedures. Early diagnosis of HCC in combination with awareness of prognostic factors leads to the choice of the best treatment option for patients with HCC. Like other retrospective studies in the field of HCC survival rate, in this study also, there is no precise investigation such as molecular biology studies of HCC. This Consequently was Base on the reviewers' comments: Consequently, may be lost some key data associated with HCC prognostic factors. Another limitation is the diagnosis of the disease and the entry of patients to the study based on clinical and general symptoms; if the diagnosis and entry into the study are based on microscopic studies, the outcomes associated with survival rates can be affected.
CONCLUSION
The results of this study indicate that the 5-year survival rate of HCC is 8.37% based on clinical symptoms. Number of involved lymph nodes, metastasis, type of treatment, tumor size, and hepatitis, respectively, are the main prognostic factors in the survival rate of HCC patients. In fact, they are reducing the survival rate of HCC patients, while combination therapy (surgery + chemotherapy) improves the survival rate of HCC patients. These results are important in determining survival rates of HCC as well as the adoption of the best treatment plan. Finally, the results of this study can be considered in the design of clinical trials associated with different HCC treatments.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tunissiolli NM, Castanhole-Nunes MM, Pavarino ÉC, da Silva RF, da Silva RC, Maria Goloni-Bertollo E. Clinical, epidemiological and histopathological aspects in patients with hepatocellular carcinoma undergoing liver transplantation. Asian Pac J Cancer Prev. 2018;19:2795–802. doi: 10.22034/APJCP.2018.19.10.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: A review of available epidemiological data. J Hepatol. 2013;58:593–608. doi: 10.1016/j.jhep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Franceschi S, Raza SA. Epidemiology and prevention of hepatocellular carcinoma. Cancer Lett. 2009;286:5–8. doi: 10.1016/j.canlet.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 4.Kirchner G, Kirovski G, Hebestreit A, Schölmerich J, Schlitt HJ, Stoeltzing O, et al. Epidemiology and survival of patients with hepatocellular carcinoma in Southern Germany. Int J Clin Exp Med. 2010;3:169–79. [PMC free article] [PubMed] [Google Scholar]
- 5.Weinmann A, Koch S, Niederle IM, Schulze-Bergkamen H, König J, Hoppe-Lotichius M, et al. Trends in epidemiology, treatment, and survival of hepatocellular carcinoma patients between 1998 and 2009: An analysis of 1066 cases of a German HCC registry. J Clin Gastroenterol. 2014;48:279–89. doi: 10.1097/MCG.0b013e3182a8a793. [DOI] [PubMed] [Google Scholar]
- 6.Weishaupt N, Blesch A, Fouad K. BDNF: The career of a multifaceted neurotrophin in spinal cord injury. Exp Neurol. 2012;238:254–64. doi: 10.1016/j.expneurol.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Allgaier HP, Deibert P, Olschewski M, Spamer C, Blum U, Gerok W, et al. Survival benefit of patients with inoperable hepatocellular carcinoma treated by a combination of transarterial chemoembolization and percutaneous ethanol injection – A single-center analysis including 132 patients. Int J Cancer. 1998;79:601–5. doi: 10.1002/(sici)1097-0215(19981218)79:6<601::aid-ijc8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 8.Befeler AS, Palmer DE, Hoffman M, Longo W, Solomon H, Di Bisceglie AM. The safety of intra-abdominal surgery in patients with cirrhosis: Model for end-stage liver disease score is superior to child-turcotte-pugh classification in predicting outcome. Arch Surg. 2005;140:650–4. doi: 10.1001/archsurg.140.7.650. [DOI] [PubMed] [Google Scholar]
- 9.Kubicka S, Rudolph KL, Hanke M, Tietze MK, Tillmann HL, Trautwein C, et al. Hepatocellular carcinoma in Germany: A retrospective epidemiological study from a low-endemic area. Liver. 2000;20:312–8. doi: 10.1034/j.1600-0676.2000.020004312.x. [DOI] [PubMed] [Google Scholar]
- 10.Rabe C, Pilz T, Klostermann C, Berna M, Schild HH, Sauerbruch T, et al. Clinical characteristics and outcome of a cohort of 101 patients with hepatocellular carcinoma. World J Gastroenterol. 2001;7:208–15. doi: 10.3748/wjg.v7.i2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llovet JM, Beaugrand M. Hepatocellular carcinoma: Present status and future prospects. J Hepatol. 2003;38(Suppl 1):S136–49. doi: 10.1016/s0168-8278(02)00432-4. [DOI] [PubMed] [Google Scholar]
- 12.Caselitz M, Masche N, Flemming P, Stern C, Manns MP, Wagner S, et al. Prognosis of hepatocellular carcinoma according to new staging classifications. Dtsch Med Wochenschr. 2004;129:1725–30. doi: 10.1055/s-2004-829023. [DOI] [PubMed] [Google Scholar]
- 13.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 14.Capocaccia R, Sant M, Berrino F, Simonetti A, Santi V, Trevisani F. Hepatocellular carcinoma: Trends of incidence and survival in Europe and the United States at the end of the 20th century. Am J Gastroenterol. 2007;102:1661–70. doi: 10.1111/j.1572-0241.2007.01337.x. [DOI] [PubMed] [Google Scholar]
- 15.Montomoli J, Erichsen R, Nørgaard M, Høyer M, Hansen JB, Jacobsen JB. Survival of patients with primary liver cancer in central and Northern Denmark, 1998-2009. Clin Epidemiol. 2011;3(Suppl 1):3–10. doi: 10.2147/CLEP.S20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otto G, Heuschen U, Hofmann WJ, Krumm G, Hinz U, Herfarth C. Survival and recurrence after liver transplantation versus liver resection for hepatocellular carcinoma: A retrospective analysis. Ann Surg. 1998;227:424–32. doi: 10.1097/00000658-199803000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bordoni A, Cerny A, Bihl F, Alerci M, Mazzola P, Peverelli S, et al. Survival of hepatocellular carcinoma patients is significantly improving: A population-based study from Southern Switzerland. Cancer Epidemiol. 2014;38:679–85. doi: 10.1016/j.canep.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Gu X, Thrift AP. Factors associated with favorable survival outcomes for Asians with hepatocellular carcinoma: A sequential matching cohort study. PLoS One. 2019;14:e0214721. doi: 10.1371/journal.pone.0214721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujii H, Itoh Y, Ohnishi N, Sakamoto M, Ohkawara T, Sawa Y, et al. Factors associated with the overall survival of elderly patients with hepatocellular carcinoma. World J Gastroenterol. 2012;18:1926–32. doi: 10.3748/wjg.v18.i16.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C, Ren X, Zhang Q. Incidence, risk factors, and prognosis in patients with primary hepatocellular carcinoma and lung metastasis: A population-based study. Cancer Manag Res. 2019;11:2759–68. doi: 10.2147/CMAR.S192896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–91. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dohmen K, Shirahama M, Shigematsu H, Irie K, Ishibashi H. Optimal treatment strategy for elderly patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2004;19:859–65. doi: 10.1111/j.1440-1746.2003.03306.x. [DOI] [PubMed] [Google Scholar]
- 23.Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: A systematic review of 72 studies. Liver Int. 2009;29:502–10. doi: 10.1111/j.1478-3231.2008.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–24. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 25.Keramatinia A, Mohseny M, Akbari ME, Mosavi-Jarrahi A, Monfared ED, Amanpour F, et al. Determinants of survival of common childhood cancers in Iran. J Res Med Sci. 2018;23:101. doi: 10.4103/jrms.JRMS_835_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeeneldin AA, Salem SE, Darwish AD, El-Gammal MM, Hussein MM, Saadeldin M. Untreated hepatocellular carcinoma in Egypt: Outcome and prognostic factors. J Hepatocell Carcinoma. 2015;2:3–9. doi: 10.2147/JHC.S73828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selçuk H. Prognostic factors and staging systems in hepatocellular carcinoma. Exp Clin Transplant. 2017;15:45–9. doi: 10.6002/ect.TOND16.L11. [DOI] [PubMed] [Google Scholar]
- 28.Kim JH, Choi MS, Lee H, Kim DY, Lee JH, Koh KC, et al. Clinical features and prognosis of hepatocellular carcinoma in young patients from a hepatitis B-endemic area. J Gastroenterol Hepatol. 2006;21:588–94. doi: 10.1111/j.1440-1746.2005.04127.x. [DOI] [PubMed] [Google Scholar]
- 29.Abbas Z, Siddiqui AU, Luck NH, Hassan M, Mirza R, Naqvi A, et al. Prognostic factors of survival in patients with non-resectable hepatocellular carcinoma: Hepatitis C versus miscellaneous etiology. J Pak Med Assoc. 2008;58:602–7. [PubMed] [Google Scholar]
- 30.Hanazaki K, Kajikawa S, Koide N, Adachi W, Amano J. Prognostic factors after hepatic resection for hepatocellular carcinoma with hepatitis C viral infection: Univariate and multivariate analysis. Am J Gastroenterol. 2001;96:1243–50. doi: 10.1111/j.1572-0241.2001.03634.x. [DOI] [PubMed] [Google Scholar]