Abstract
DOCK family proteins are evolutionarily conserved guanine nucleotide exchange factors for Rho GTPase with different cellular functions. It has been demonstrated that DOCK1 had adverse prognostic effect in acute myeloid leukemia (AML). We first analyzed data of 85 AML patients who were treated with chemotherapy and had available DOCK1 to DOCK11 expression information and found that DOCK1 and DOCK2 had prognostic significance in AML. In view of the known prognosis of DOCK1 in AML, we then explored the prognostic role of DOCK2. One hundred fifty-six AML patients with DOCK2 expression data were extracted from The Cancer Genome Atlas (TCGA) database and enrolled in this study. Patients were divided based on treatment modality into the chemotherapy group and the allogeneic hematopoietic stem cell transplant (allo-HSCT) group. Each group was divided into two groups by the median expression levels of DOCK2. In the chemotherapy group, high DOCK2 expression was associated with longer event-free survival (EFS, P=0.001) and overall survival (OS, P=0.007). In the allo-HSCT group, EFS and OS were not significantly different between high and low DOCK2 expression groups. Multivariate analysis showed that high DOCK2 expression was an independent favorable prognostic factor for both EFS and OS in all patients (all P<0.05). In conclusion, our results indicated that high DOCK2 expression, in contrast to DOCK1, conferred good prognosis in AML.
Keywords: acute myeloid leukemia, DOCK2, allogeneic hematopoietic stem cell transplantation, chemotherapy, prognosis
Introduction
Genetic abnormality is not only the pathogenic basis of acute myeloid leukemia (AML) 1 but also has important prognostic implications. For example, NPM1 mutations and double CEBPA mutations are associated with favorable prognosis in cytogenetically normal AML (CN-AML) 2, 3, while DNMT3A and WT1 mutations are adverse prognostic factors 4, 5.
The dedicators of cytokinesis (DOCK) family, including DOCK1 to DOCK11 proteins, are evolutionarily conserved guanine nucleotide exchange factors (GEF) for the Rho GTPases Rac. It is involved in various pathologies including cancers, immune disorders, and central nervous system diseases 6. For instance, high DOCK1 expression is an unfavorable prognostic marker in breast cancer and ovarian cancer 7,8, and it induces migration and invasion of malignant cells in lung and brain cancer 9, 10. Fukui Y et al found that DOCK2 is only expressed in hematopoietic tissues 11. In addition, DOCK2 is also associated with the development of various cancers 12.
Previous study has shown that high DOCK1 expression conferred poor prognosis in AML 13, but the prognosis value of the other DOCK family members in AML is unclear. We screened all the DOCK family members and found that DOCK2 also had independent prognostic significance in AML.
Materials and Methods
Patients
From The Cancer Genome Atlas (TCGA) database (https://cancergenome.nih.gov/), 156 AML patients with DOCK2 expression data were enrolled in this study 14. All patients were between ages 18 and 88, registered between November 2001 and March 2010, were selected from a set of more than 400 samples to reflect a realworld distribution of subtypes. Eighty-five patients were treated with chemotherapy alone, and other 71 received allo-HSCT. Patients treated with chemotherapy alone were defined as the chemotherapy group; patients who underwent allo-HSCT were defined as the allo-HSCT group. Then, each group was divided into two subgroups by the respective median DOCK2 expression levels. All clinical and molecular information including DOCK2 expression levels were publicly accessible from the TCGA website. All patients provided written informed consent and the research was approved by the Human Research Ethics Committee of Washington University. Primary endpoints were event-free survival (EFS) and overall survival (OS). EFS was defined as the time from diagnosis to removal from the study due to the absence of complete remission, relapse or death or was censored at the last follow-up. OS was defined as the time from diagnosis to death or was censored at the last follow-up.
Statistical Analysis
The clinical and molecular characteristics of patients were summarized using descriptive statistics. The Mann-Whitney U test and the chi-square test were used to compare continuous and categorical data, respectively. EFS and OS were estimated with the Kaplan-Meier method and compared using the log-rank test. Cox proportional hazard model was constructed for EFS and OS to identify possible prognostic factors among the clinical and molecular variables. All statistical analyses were performed by SPSS software 20.0 and GraphPad Prism software 5.0. For all statistical analyses, P-values were two-sided and P<0.05 was considered significant.
Results
Comparison of EFS and OS between different expression levels of DOCK1-11
To assess the prognostic significance of DOCK family in AML, EFS and OS patients with high and low expression groups of each DOCK family proteins were compared (Table 1). The results showed that DOCK1 was an adverse prognostic factor and DOCK2 was a favorable prognostic factor in AML. However, other DOCK members had no effect on EFS and OS.
Table 1.
Comparison of EFS and OS between different expression levels of Dock1-11 based on chemotherapy.
| Variables | EFS | OS | |||
|---|---|---|---|---|---|
| χ2 | P-value | χ2 | P-value | ||
| Dock1 (high vs. low) | 14.908 | 0.000 | 14.343 | 0.000 | |
| Dock2(high vs. low) | 13.331 | 0.000 | 11.748 | 0.001 | |
| Dock3 (high vs. low) | 0.030 | 0.863 | 0.000 | 0.999 | |
| Dock4(high vs. low) | 1.598 | 0.206 | 1.658 | 0.198 | |
| Dock5(high vs. low) | 0.153 | 0.695 | 0.021 | 0.884 | |
| Dock6(high vs. low) | 0.930 | 0.335 | 0.312 | 0.576 | |
| Dock7 (high vs. low) | 0.552 | 0.457 | 1.261 | 0.262 | |
| Dock8(high vs. low) | 0.288 | 0.591 | 0.419 | 0.518 | |
| Dock9(high vs. low) | 0.170 | 0.680 | 0.497 | 0.481 | |
| Dock10(high vs. low) | 0.011 | 0.916 | 0.009 | 0.923 | |
| Dock11(high vs. low) | 0.054 | 0.817 | 0.002 | 0.968 | |
Abbreviations: EFS, event-free survival; OS, overall survival.
Association between DOCK2 expression and patient's characteristics
Comparison of clinical and molecular characteristics between different expression subgroups within chemotherapy and allo-HSCT groups were summarized in Table 2. In the chemotherapy group, high DOCK2 expression group had more good-risk patients (P=0.014), fewer poor-risk patients (P=0.002) and less TP53mutations (P=0.049) than low expression group. Six patients among the low expression group harbored CBFβ-MYH11, which was not found in the high expression group (P=0.012). No significant difference was found in age, sex distribution, peripheral white blood cell (WBC) count and bone marrow blast (BM) percentage at diagnosis, French-American-British (FAB) classification, frequency of other recurrent genetic mutations (FLT3-ITD, NPM1, CEBPA, IDH1/IDH2, RUNX1, MLL-PTD, NRAS/KRAS, TET2, WT1 and TP53), or relapse rate between the high and low expression subgroups.
Table 2.
Clinical and molecular characteristics of patients according to DOCK2 levels
| Characteristics | Chemotherapy group | Allo-HSCT group | |||||
|---|---|---|---|---|---|---|---|
| High DOCK2 (n = 42) |
Low DOCK2 (n = 43) |
P | High DOCK2 (n = 35) |
Low DOCK2 (n = 36) |
P | ||
| Age/years, median (range) | 66.5 (22-77) | 66 (33-88) | 0.324* | 51 (22-69) | 52.5 (18-72) | 0.890* | |
| Age group/n (%) | 0.311§ | 0.205§ | |||||
| < 60 years | 15 (35.7) | 11 (25.6) | 28 (80.0) | 24 (66.7) | |||
| ≥ 60 years | 27 (64.3) | 32 (74.4) | 7 (20.0) | 12 (33.3) | |||
| Gender/n (%) | 0.591§ | 0.288§ | |||||
| Male | 21 (50.0) | 24 (55.8) | 18 (51.4) | 23 (63.9) | |||
| Female | 21 (50.0) | 19 (44.2) | 17 (48.6) | 13 (36.1) | |||
| WBC/×109/L, median (range) | 15.2(1.0-171.9) | 12.3(0.7-297.4) | 0.329* | 30.9(1.2-223.8) | 27.7(0.6-90.4) | 0.200* | |
| BM blast/%, median (range) | 71 (30-97) | 74 (32-99) | 0.379* | 71 (34-100) | 70 (30-97) | 0.809* | |
| PB blast/%, median (range) | 23 (0-91) | 25 (0-98) | 0.972* | 48 (0-96) | 53 (0-90) | 0.801* | |
| FAB subtypes/n(%) | |||||||
| M0 | 4 (9.5) | 3 (7.0) | 0.713§ | 3 (8.6) | 6 (16.7) | 0.478§ | |
| M1 | 7 (16.7) | 13 (30.2) | 0.140§ | 14 (40.0) | 9 (25.0) | 0.177§ | |
| M2 | 12 (28.6) | 9 (20.9) | 0.414§ | 8 (22.9) | 10 (27.8) | 0.634§ | |
| M3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.8) | 1.000§ | ||
| M4 | 11 (26.2) | 9 (20.9) | 0.568§ | 8 (22.9) | 5 (13.9) | 0.329§ | |
| M5 | 7 (16.7) | 6 (14.0) | 0.728§ | 1 (2.9) | 3 (8.3) | 0.614§ | |
| M6 | 1 (2.4) | 0 (0.0) | 0.494§ | 0 (0.0) | 1 (2.8) | 1.000§ | |
| M7 | 0 (0.0) | 2 (4.7) | 0.494§ | 0 (0.0) | 1 (1.4) | 1.000§ | |
| Karyotype/n(%) | |||||||
| Normal | 21 (50.0) | 19 (44.2) | 0.591§ | 11 (29.7) | 23 (62.2) | 0.005§ | |
| Complex | 3 (7.1) | 9 (20.9) | 0.117§ | 7 (18.9) | 5 (13.5) | 0.528§ | |
| inv(16)/CBFβ-MYH11 | 6 (14.3) | 0 (0.0) | 0.012§ | 5 (13.5) | 0 (0.0) | 0.054§ | |
| 11q23/MLL | 0 (0.0) | 3 (7.0) | 0.241§ | 2 (5.4) | 1 (2.7) | 1.000§ | |
| t(15;17)/PML-RARA | 0 (0.0) | 0 (0.0) | 1 (2.7) | 1 (2.7) | 1.000§ | ||
| t(9;22)/BCR-ABL1 | 0 (0.0) | 1 (2.3) | 1.000§ | 2 (5.4) | 0 (0.0) | 0.493§ | |
| t(8;21)/RUNX1-RUNX1T1 | 4 (9.5) | 2 (4.7) | 0.433§ | 0 (0.0) | 1 (2.7) | 1.000§ | |
| Others | 8 (19.0) | 9 (20.9) | 0.828§ | 4 (10.8) | 2 (5.4) | 0.674§ | |
| Risk/n(%) | |||||||
| Good | 10 (23.8) | 2 (4.7) | 0.014§ | 5 (14.3) | 2 (5.6) | 0.260§ | |
| Intermediate | 26 (61.9) | 20 (46.5) | 0.154§ | 23 (65.7) | 17 (47.2) | 0.116§ | |
| Poor | 6 (14.3) | 19 (44.2) | 0.002§ | 6 (17.1) | 17 (47.2) | 0.007§ | |
| FLT3-ITD/n(%) | 0.366§ | 0.730§ | |||||
| Presence | 9 (21.4) | 6 (14.0) | 9 (25.7) | 8 (22.2) | |||
| Absence | 33 (78.6) | 37 (86.0) | 29 (78.4) | 28 (75.7) | |||
| NPM1/n(%) | 0.440§ | 0.246§ | |||||
| Mutation | 15 (35.7) | 12 (27.9) | 11 (31.4) | 7 (19.4) | |||
| Wildtype | 27 (64.3) | 31 (72.1) | 24 (68.6) | 29 (80.6) | |||
| CEBPA/n(%) | 0.557§ | 0.033§ | |||||
| Single mutation | 1 (2.4) | 2 (4.7) | 4 (11.4) | 1 (2.8) | |||
| Double mutation | 0 (0.0) | 0 (0.0) | 3 (8.6) | 0 (0.0) | |||
| Wild type | 41 (97.6) | 41 (95.3) | 28 (80.0) | 35 (97.2) | |||
| DNMT3A/n(%) | 0.859§ | 0.044§ | |||||
| Mutation | 11 (26.2) | 12 (27.9) | 12 (34.3) | 5 (13.9) | |||
| Wildtype | 31 (73.8) | 31 (72.1) | 23 (65.7) | 31 (86.1) | |||
| IDH1/IDH2/n(%) | 0.366§ | 0.730§ | |||||
| Mutation | 9 (21.4) | 6 (14.0) | 9 (25.7) | 8 (22.2) | |||
| Wildtype | 33 (78.6) | 37 (86.0) | 26 (74.3) | 28 (77.8) | |||
| RUNX1/n(%) | 0.156§ | 0.260§ | |||||
| Mutation | 6 (14.3) | 2 (4.7) | 2 (5.7) | 6 (16.7) | |||
| Wildtype | 36 (85.7) | 41 (95.3) | 33 (94.3) | 30 (83.3) | |||
| WT1/n(%) | 1.000§ | 0.478§ | |||||
| Mutation | 1 (2.4) | 1 (2.3) | 5 (14.3) | 3 (8.3) | |||
| Wildtype | 41 (97.6) | 42 (97.7) | 30 (85.7) | 33 (91.7) | |||
| MLL-PTD/n(%) | 0.360§ | 0.614§ | |||||
| Presence | 1 (2.4) | 4 (9.3) | 1 (2.9) | 3 (8.3) | |||
| Absence | 41 (97.6) | 39 (90.7) | 34 (97.1) | 33 (91.7) | |||
| NRAS/KRAS/n(%) | 0.505§ | 0.710§ | |||||
| Mutation | 7 (16.7) | 5 (11.6) | 4 (11.4) | 3 (8.3) | |||
| Wildtype | 35 (83.3) | 38 (88.4) | 31 (88.6) | 33 (91.7) | |||
| TET2/n(%) | 0.778§ | 1.000§ | |||||
| Mutation | 5 (11.9) | 6 (14.0) | 2 (5.7) | 2 (5.6) | |||
| Wildtype | 37 (88.1) | 37 (86.0) | 33 (94.3) | 34 (94.4) | |||
| TP53/n(%) | 0.049§ | 0.115§ | |||||
| Mutation | 2 (4.8) | 9 (20.9) | 0 (0.0) | 4 (11.1) | |||
| Wildtype | 40 (95.2) | 34 (79.1) | 35 (100.0) | 32 (88.9) | |||
| Relapse/n(%) | 0.227§ | 0.614§ | |||||
| Yes | 18 (42.9) | 13 (30.2) | 25 (71.4) | 23 (63.9) | |||
| No | 24 (57.1) | 30 (69.8) | 10 (28.6) | 13 (36.1) | |||
Abbreviations: WBC: white blood cell; BM: bone marrow; PB: peripheral blood; FAB: French American British.
*denotes Mann-Whitney U test; §denotes chi-square test.
In the allo-HSCT group, high DOCK2 expression group had fewer poor-risk patients (P=0.007), fewer normal karyotype patients (P=0.005), more CEBPA (P=0.033) and DNMT3A mutations (P=0.044) than the low expression group. No significant difference was found in age, sex distribution, BM blasts, FAB classification, frequent AML mutations (FLT3-ITD, NPM1, IDH1/IDH2, RUNX1, MLL-PTD, NRAS/KRAS, TET2, WT1 and TP53), or relapse rate between two subgroups.
Prognostic value of DOCK2 in AML
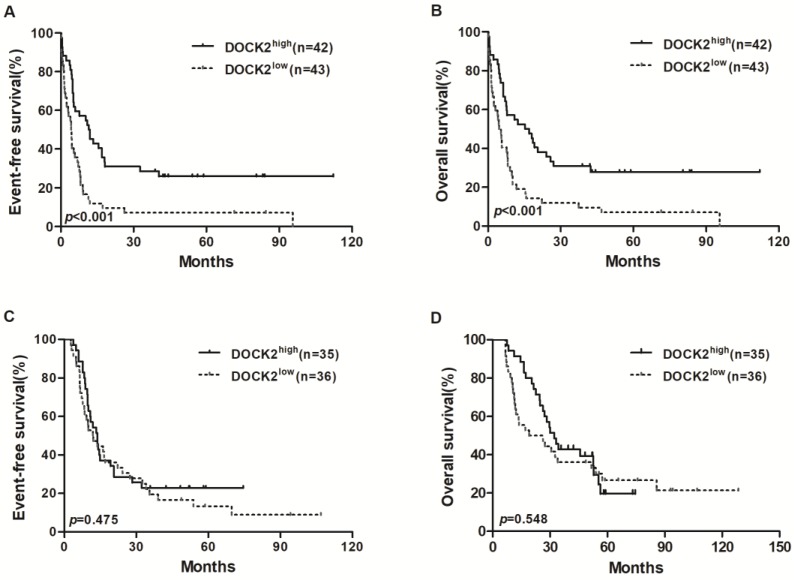
In the chemotherapy group, high DOCK2 expressers had longer EFS and OS (all P<0.001; Figure 1A and 1B) than low expressers, but survival was not significantly different between DOCK2 high and low expression subgroups in the allo-HSCT group (Figure 1C and 1D).
Figure 1.
Kaplan-Meier curves of EFS and OS in the chemotherapy and allo-HSCT groups. (A, B) In the chemotherapy group, high DOCK2 expressers had longer EFS and OS than low expressers. (C, D) EFS and OS were not significantly different between high and low DOCK2 expression subgroups in the allo-HSCT group.
We chose DOCK2 expression levels (low vs. high), therapy method (chemotherapy vs. allo-HSCT), age (<60 vs. ≥60 years), WBC counts (<20×109/L vs. ≥20×109/L), FLT3-ITD (positive vs. negative) and common AML mutations (NPM1, DNMT3A, IDH1/IDH2, RUNX1, WT1, CEBPA and TP53, mutated vs.wild) to construct multivariate analysis of EFS and OS.
In the chemotherapy group, multivariate analysis showed that age ≥60 years and TP53 mutations were independent risk factors for EFS and OS (all P<0.05), and high DOCK2 expression was an independent favorable factor for EFS and OS (all P<0.05, Table 3). In the allo-HSCT group, multivariate analysis showed that FLT3-ITD was an independent risk factor for EFS and OS (all P<0.05). WBC counts ≥20×109/L and WT1 mutations were independent risk factors for EFS. Mutations in RUNX1 and TP53 were independent risk factors for OS (all P<0.05, Table 4).
Table 3.
Multivariate analyses for EFS and OS based on chemotherapy
| Variables | EFS | OS | |||
|---|---|---|---|---|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value | ||
| DOCK2 (high vs. low) | 2.301 (1.381-3.835) | 0.001 | 1.974 (1.201-3.245) | 0.007 | |
| Age (< 60 v. ≥ 60 years) | 2.909 (1.550-5.460) | 0.001 | 2.582 (1.355-4.918) | 0.004 | |
| WBC (<20 vs. ≥20×109/L) | 1.382 (0.777-2.457) | 0.270 | 1.263 (0.719-2.220) | 0.416 | |
| NPM1,mutated v wild type | 0.653 (0.352-1.210) | 0.175 | 0.813 (0.439-1.504) | 0.509 | |
| DNMT3A, mutated v wild type | 0.674 (0.375-1.211) | 0.187 | 0.631 (0.357-1.117) | 0.114 | |
| FLT3-ITD, presence v absence | 0.801 (0.411-1.558) | 0.512 | 0.974 (0.495-1.916) | 0.939 | |
| IDH1/IDH2, mutated v wild typemutated v wild type | 1.077 (0.555-2.089) | 0.827 | 1.106 (0.560-2.185) | 0.772 | |
| RUNX1, mutated v wild type | 0.508 (0.218-1.185) | 0.117 | 0.500 (0.214-1.168) | 0.109 | |
| WT1, mutated v wild type | 0.638 (0.134-3.041) | 0.573 | 1.094 (0.134-8.929) | 0.933 | |
| CEBPA, mutated v wild type | 0.471 (0.139-1.596) | 0.226 | 0.461 (0.136-1.567) | 0.215 | |
| TP53, mutated v wild type | 0.351 (0.154-0.801) | 0.013 | 0.414 (0.184-0.933) | 0.033 | |
Abbreviations: EFS: Event-free survival; OS: Overall survival; WBC: white blood cell.
Table 4.
Multivariate analyses for EFS and OS based on allo-HSCT.
| Variables | EFS | OS | |||
|---|---|---|---|---|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value | ||
| DOCK2 (high vs. low) | 1.741 (0.921-3.294) | 0.088 | 1.386 (0.705-2.725) | 0.344 | |
| Age (< 60 v. ≥ 60 years) | 0.869 (0.453-1.670) | 0.674 | 1.174 (0.600-2.299) | 0.639 | |
| WBC (<20 vs. ≥20×109/L) | 2.151 (1.127-4.105) | 0.020 | 1.339 (0.687-2.612) | 0.391 | |
| NPM1,mutated v wild type | 1.878 (0.885-3.984) | 0.101 | 1.476 (0.621-3.507) | 0.378 | |
| DNMT3A, mutated v wild type | 0.711 (0.344-1.468) | 0.356 | 0.553 (0.258-1.183) | 0.127 | |
| FLT3-ITD, presencevabsence | 0.407 (0.201-0.826) | 0.013 | 0.451 (0.206-0.990) | 0.047 | |
| IDH1/IDH2, mutated v wild typemutated v wild type | 0.800 (0.354-1.806) | 0.591 | 1.058 (0.442-2.529) | 0.900 | |
| RUNX1, mutated v wild type | 0.822 (0.333-2.030) | 0.671 | 0.386 (0.155-0.958) | 0.040 | |
| WT1, mutated v wild type | 0.361 (0.137-0.949) | 0.039 | 0.607 (0.239-1.540) | 0.293 | |
| CEBPA, mutated v wild type | 1.358 (0.502-3.676) | 0.547 | 1.106 (0.402-3.042) | 0.846 | |
| TP53, mutated v wild type | 0.371 (0.112-1.222) | 0.103 | 0.155 (0.043-0.557) | 0.004 | |
Abbreviations: EFS: Event-free survival; OS: Overall survival; WBC: white blood cell.
In all patients, multivariate analysis showed that high DOCK2 expression and allo-HSCT were independent favorable factors for EFS and OS (all P<0.05). Age ≥60 years, WBC counts ≥20×109/L and mutations in DNMT3A, RUNX1 and TP53 were independent risk factors for EFS and OS (all P<0.05, Table 5).
Table 5.
Multivariate analyses for EFS and OS based on chemotherapy and allo-HSCT.
| Variables | EFS | OS | |||
|---|---|---|---|---|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value | ||
| DOCK2 (high vs. low) | 1.721 (1.175-2.518) | 0.005 | 1.489 (1.012-2.191) | 0.044 | |
| Chemotherapy v allo-HSCT | 1.599 (1.097-2.330) | 0.015 | 1.946 (1.301-2.910) | 0.001 | |
| Age (< 60 vs. ≥ 60 years) | 1.664 (1.107-2.500) | 0.014 | 1.957 (1.270-3.016) | 0.002 | |
| WBC (<20 vs. ≥20×109/L) | 1.649 (1.103-2.465) | 0.015 | 1.331 (0.883-2.005) | 0.172 | |
| NPM1, mutated v wild type | 0.936 (0.593-1.477) | 0.777 | 0.904 (0.559-1.461) | 0.680 | |
| DNMT3A, mutated v wild type | 0.578 (0.382-0.876) | 0.010 | 0.536 (0.352-0.816) | 0.004 | |
| FLT3-ITD, presence v absence | 0.781 (0.488-1.252) | 0.305 | 0.888 (0.533-1.478) | 0.646 | |
| IDH1/IDH2, mutated v wild typemutated v wild type | 1.135 (0.705-1.830) | 0.602 | 1.229 (0.746-2.026) | 0.419 | |
| RUNX1, mutated v wild type | 0.535 (0.294-0.974) | 0.041 | 0.413 (0.224-0.764) | 0.005 | |
| WT1, mutated v wild type | 0.612 (0.290-1.288) | 0.196 | 0.762 (0.352-1.650) | 0.491 | |
| CEBPA, mutated v wild type | 0.580 (0.273-1.232) | 0.156 | 0.671 (0.310-1.453) | 0.312 | |
| TP53, mutated v wild type | 0.310 (0.160-0.600) | 0.001 | 0.269 (0.137-0.531) | 0.000 | |
Abbreviations: EFS: Event-free survival; OS: Overall survival; WBC: white blood cell.
Correlation analysis of DOCK2 expression and genome-wide microRNA and gene expression profile
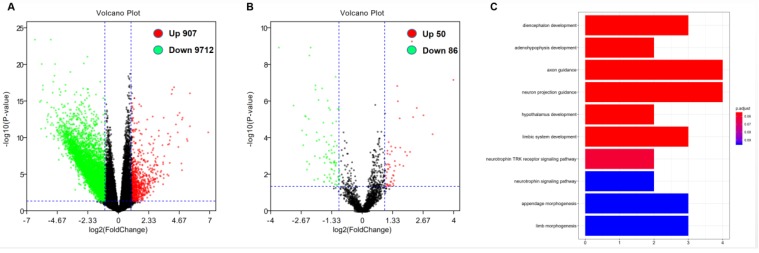
In order to further evaluate the role of DOCK2 in AML, we obtained DOCK2-associated gene expression profiles and mircroRNA from TCGA database through high-throughput sequencing. There were 907 genes were positively associated with DOCK2 expression, and 9712 genes were negatively associated with DOCK2 expression (P<0.05, fold change=1.5, Figure. 2A). Then, we identified 50 up-regulated and 86 down-regulated microRNAs that were significantly correlated with DOCK2 expression (P<0.05, fold change=1.5, Figure. 2B). Furthermore, gene ontology (GO) enrichment analysis suggested that the genes related to DOCK2 expression were mainly concentrated in "diencephalon development", "adenohypophysis development", "axon guidance", "neuron projection guidance", "hypothalamus development", "limbic system development", "neurotrophin TRK receptor signaling pathway", "neurotrophin signaling pathway", "appendage morphogenesis", and "limb morphogenesis" pathways (Figure. 2C).
Discussion
Our study showed that high DOCK2 expression was an independent favorable factor in AML patients who underwent chemotherapy alone, but not in patients who also underwent allo-HSCT. Consistent with previous studies, we also found that high DOCK1 expression was an adverse factor in AML 13.
Previous researches have demonstrated that TP53 mutation and older age were negative prognostic factors in AML 15,16, while CBFβ-MYH11 was associated with favorable prognosis in AML 17. Our study found that in high DOCK2 expression patients, there were more good-risk patients, more CBFβ-MYH11, and fewer TP53 mutations, suggesting that high expression of DOCK2 was more likely to co-exist with CBFβ-MYH11 rather than TP53 mutations. In the chemotherapy group, the survival analysis indicated that high DOCK2 expression was a favorable factor for EFS and OS, but it not in the allo-HSCT group, suggesting that the unfavorable effect of low DOCK2 expression might be overcome by allo-HSCT.
DOCK2 has been shown to be a specific Rac activator in mature lymphocytes 18. It is involved in neutrophil chemotaxis 19 and NK cells differentiation 20. Previous study found that DOCK2 plays a key role in the regulation of cell proliferation in diffuse large B cell lymphoma and follicular lymphoma via the ERK signaling pathway 21. Nishihara H et al found that DOCK2 is associated with CrkL and regulates Rac1 in human leukemia cell lines 22. Another study revealed that DOCK2 regulates CXCR4 signaling in immature hematopoietic cells 23. In the present study, DOCK2 was associated with "neurotrophin TRK receptor signaling pathway", "neurotrophin signaling pathway", "appendage morphogenesis". We speculate that DOCK2 may play a prognostic role in leukemia by interacting with genes in these functional pathways.
A previous study suggested that knocking down DOCK2 could sensitize FLT3-ITD leukemic cells to cytarabine treatment through the inhibition of Rac1 pathway 24, whereas in this study, we observed a favorable prognostic impact of high DOCK2 expression in AML patients. This discrepancy might be related to the different research objects of the two studies, since we did not specifically study AML patients with FLT3-ITD.
DOCK2 may play different roles in the lymphoid and myeloid system 21. This is similar to LEF1. High LEF1 expression has been reported as a favorable prognostic factor in CN-AML 25, but it is also an adverse prognostic factor in adult B-precursor acute lymphoblastic leukemia 26. Low expression of DOCK2 is associated with poorer prognosis in colorectal cancer 27. However, the expression level of DOCK2 is positively correlated with the proliferation rate of CXCL13-induced prostate cancer cells 28. We theorized that DOCK2 had tissue-specific effects in different malignancies.
In summary, two of the 11 members of the DOCK family have prognostic significance in AML. DOCK1 has adverse prognostic effect and DOCK2 the opposite. This finding may further deepen the risk stratification system of AML.
Figure 2.
Genome-wide gene/microRNA expression profiles and cell signaling pathways associated with DOCK2 expression. (A) Volcano plot of differential gene expression. Up-regulated and down-regulated genes were labeled with red and green dots, respectively. (B) Volcano plot of differential microRNA expression. Up-regulated and down-regulated microRNAs were labeled with red and green dots, respectively. (C) Gene ontology (GO) enrichment analysis of genes related to DOCK2 expression.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81500118, 61501519), the China Postdoctoral Science Foundation funded project (project No.2016M600443) and PLAGH project of Medical Big Data (Project No.2016MBD-025.
References
- 1.Prada-Arismendy J, Arroyave JC, Röthlisberger S. Molecular Biomarkers in Acute Myeloid Leukemia. Blood Rev. 2017;31(1):63–76. doi: 10.1016/j.blre.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Döhner K, Schlenk RF, Habdank M. et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood. 2005;106(12):3740–3746. doi: 10.1182/blood-2005-05-2164. [DOI] [PubMed] [Google Scholar]
- 3.Dufour A, Schneider F, Metzeler K. et al. Acute myeloid leukemia with biallelic CEBPA gene mutations and normal karyotype represents a distinct genetic entity associated with a favorable clinical outcome. J Clin Oncol. 2010;28(4):570–577. doi: 10.1200/JCO.2008.21.6010. [DOI] [PubMed] [Google Scholar]
- 4.Lin N, Fu W, Zhao C. et al. Biologico-clinical significance of DNMT3A variants expression in acute myeloid leukemia. Biochem Biophys Res Commun. 2017;494:270–277. doi: 10.1016/j.bbrc.2017.10.041. [DOI] [PubMed] [Google Scholar]
- 5.Lyu X, Xin Y, Mi R. et al. Over expression of Wilms tumor 1 gene as a negative prognostic indicator in acute myeloid leukemia. PLoS ONE. 2014;9(3):e92470. doi: 10.1371/journal.pone.0092470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gadea G, Blangy A. Dock-family exchange factors in cell migration and disease. Eur J Cell Biol. 2014;93:466–477. doi: 10.1016/j.ejcb.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Laurin M, Huber J, Pelletier A. et al. Rac-specific guanine nucleotide exchange factor DOCK1 is a critical regulator of HER2-mediated breast cancer metastasis. Proc Natl Acad Sci U S A. 2013;110:7434–7439. doi: 10.1073/pnas.1213050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao F, Siu MK, Jiang L. et al. Overexpression of dedicator of cytokinesis I (Dock180) in ovarian cancer correlated with aggressive phenotype and poor patient survival. Histopathology. 2011;59(6):1163–1172. doi: 10.1111/j.1365-2559.2011.04045.x. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Yang L, Fu H. et al. Association between Galphai2 and ELMO1/Dock180 connects chemokinesignalling with Rac activation and metastasis. Nat Commun. 2013;4:1706. doi: 10.1038/ncomms2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haizhong Feng, Bo Hu, Kun-Wei Liu. et al. Activation of Rac1 by Src-dependent phosphorylation of Dock180(Y1811) mediates PDGFRa-lphastimulated glioma tumorigenesis in mice and humans. J. Clin. Invest. 2011;121:4670–4684. doi: 10.1172/JCI58559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukui Y, Hashimoto O, Sanui T. et al. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature. 2001;412(6849):826–831. doi: 10.1038/35090591. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Meng F, Wang B. et al. Dock2 in the development of inflammation and cancer. Eur J Immunol. 2018;48(6):915–922. doi: 10.1002/eji.201747157. [DOI] [PubMed] [Google Scholar]
- 13.Sze-Hwei Lee, Yu-Chiao Chiu, Yi-Hung Li. et al. High expression of dedicator of cytokinesis 1 (DOCK1) confers poor prognosis in acute myeloid leukemia. Oncotarget. 2017;8(42):72250–72259. doi: 10.18632/oncotarget.19706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ley TJ, Miller C, Ding L. et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stengel A, Kern W, Haferlach T. et al. The impact of TP53 mutations and TP53 deletions on survival varies between AML, ALL, MDS and CLL: an analysis of 3307 cases. Leukemia. 2017;31(3):705–711. doi: 10.1038/leu.2016.263. [DOI] [PubMed] [Google Scholar]
- 16.Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. New Engl J Med. 2015;373:1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 17.Kurosawa S, Miyawaki S, Yamaguchi T. et al. Prognosis of patients with core binding factor acute myeloid leukemia after first relapse. Haematologica. 2013;98:1525–1531. doi: 10.3324/haematol.2012.078030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukui Y, Hashimoto O, Sanui T. et al. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature. 2001;412:826–831. doi: 10.1038/35090591. [DOI] [PubMed] [Google Scholar]
- 19.Nishikimi A, Fukuhara H, Su W. et al. Sequential regulation of DOCK2 dynamics by two phospholipids during neutrophil chemotaxis. Science. 2009;324:384–387. doi: 10.1126/science.1170179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakai Y, Tanaka Y, Yanagihara T. et al. The Rac activator DOCK2 regulates naturalkiller cell-mediated cytotoxicity in mice through the lytic synapse formation. Blood. 2013;122:386–393. doi: 10.1182/blood-2012-12-475897. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Nishihara H, Kimura T. et al. DOCK2 regulates cell proliferation through Rac and ERK activation in B cell lymphoma. Biochem Biophys Res Commun. 2010;395(1):111–115. doi: 10.1016/j.bbrc.2010.03.148. [DOI] [PubMed] [Google Scholar]
- 22.Nishihara H, Maeda M, Oda A. et al. DOCK2 associates with CrkL and regulates Rac1 in human leukemia cell lines. Blood. 2002;100(12):3968–3974. doi: 10.1182/blood-2001-11-0032. [DOI] [PubMed] [Google Scholar]
- 23.Kikuchi T, Kubonishi S, Shibakura M. et al. Dock2 participates in bone marrow lympho-hematopoiesis. Biochem Biophys Res Commum. 2008;367(1):90–96. doi: 10.1016/j.bbrc.2007.12.093. [DOI] [PubMed] [Google Scholar]
- 24.Wu M, Hamaker M, Li L. et al. DOCK2 Interacts with FLT3 and Modulates the Survival of FLT3-Expressing Leukemia Cells. Leukemia. 2017;31(3):688–696. doi: 10.1038/leu.2016.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhnl A, Gokbuget N, Kaiser M. et al. Overexpression of LEF1 predicts unfavorable outcome in adult patients with B-precursor acute lymphoblastic leukemia. Blood. 2011;118:6362–6367. doi: 10.1182/blood-2011-04-350850. [DOI] [PubMed] [Google Scholar]
- 26.Metzeler KH, Heilmeier B, Edmaier KE. et al. High expression of lymphoid enhancer-binding factor-1 (LEF1) is a novel favorable prognostic factor in cytogenetically normal acute myeloid leukemia. Blood. 2012;120:2118–2126. doi: 10.1182/blood-2012-02-411827. [DOI] [PubMed] [Google Scholar]
- 27.Yu J, Wu WK, Li X. et al. Novel recurrently mutated genes and a prognostic mutation signature in colorectal cancer. Gut. 2015;64:636–645. doi: 10.1136/gutjnl-2013-306620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Haibi CP, Singh R, Sharma PK. et al. CXCL13 mediates prostate cancer cell proliferation through JNK signalling and invasion through ERK activation. Cell Prolif. 2011;44:311–319. doi: 10.1111/j.1365-2184.2011.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]