Abstract
Objective
The pathogenesis of orthostatic tremor (OT) is unknown. We investigated OT‐related white matter changes and their correlations with scores from a neuropsychological testing battery.
Methods
Diffusion tensor imaging measures were compared between 14 OT patients and 14 age‐ and education‐matched healthy controls, using whole‐brain tract‐based spatial statistics analysis. Correlations between altered diffusion metrics and cognitive performance in OT group were assessed.
Results
In all cognitive domains (attention, executive function, visuospatial ability, verbal memory, visual memory, and language), OT patients’ cognitive performance was significantly worse than that of healthy controls. OT patients demonstrated altered diffusivity metrics not only in the posterior lobe of the cerebellum (left cerebellar lobule VI) and in its efferent cerebellar fibers (left superior cerebellar peduncle), but also in medial lemniscus bilaterally (pontine tegmentum), anterior limb of the internal capsule bilaterally, right posterior limb of the internal capsule, left anterior corona radiata, right insula, and the splenium of corpus callosum. No relationship was found between diffusion measures and disease duration in OT patients. Diffusion white matter changes, mainly those located in right anterior limb of the internal capsule, were correlated with poor performance on tests of executive function, visuospatial ability, verbal memory, and visual memory in OT patients.
Interpretation
White matter changes were preferentially located in the cerebellum, its efferent pathways, as well as in the pontine tegmentum and key components of the frontal–thalamic–cerebellar circuit. Further work needs to be done to understand the evolution of these white matter changes and their functional consequences.
Introduction
Orthostatic tremor (OT), also called shaky‐leg syndrome,1 is one of the most intriguing tremor disorders. OT might actually be a group of diseases, united by the presence of leg tremor, yet characterized by etiological and clinical heterogeneity.2 We are now witnessing an expansion of the construct of OT to include not only motor symptoms (e.g., leg tremor), but the presence of other clinical features (e.g., cognitive and personality changes and cerebellar signs), different patterns of clinical progression, and the association with different types of parkinsonism.2
The pathogenesis of OT is unknown, although clinical and neuroimaging studies point to a role of the brainstem and cerebellum.3, 4, 5, 6, 7, 8, 9, 10 As in other tremor disorders, such as essential tremor,11, 12 the pathogenesis of OT may be related to changes in the cerebello‐thalamo‐cortical network (the so called “tremor” network).13 However, in OT, changes might be more widespread, including the pontine tegmentum and mesiofrontal cortical areas, as evidenced in a recent 18F‐fluorodeoxyglucose‐positron emission tomography study.14 In line with this, OT patients might suffer deficits in cognitive and neuropsychiatric areas, especially those thought to involve the integrity of the prefrontal cortex, which, in turn, also suggests changes of limbic cortico‐striato‐thalamo‐cortical loops.15 On the other hand, in one resting state functional magnetic resonance imaging (MRI) study, changes in connectivity in particular resting‐state networks was detected.16 More specifically, OT patients showed increased connectivity in resting‐state networks involved in cognitive processes (e.g., default mode network and frontoparietal networks) and decreased connectivity in the cerebellum and sensorimotor networks.16 To date, there have been few studies. Hence, there is a need to investigate the white matter microstructure changes as well as their correlation with nonmotor signs in OT (i.e., cognitive dysfunction) and to identify their anatomical origin. Identifying the relevant white matter areas associated with OT would help to arrive at a better understanding of the pathogenesis of this enigmatic disease.
Diffusion tensor imaging (DTI) is an analysis technique of diffusion‐weighted images (DWI) that permits one to quantify microstructural white matter changes.17 Among several DTI parameters used to assess diffusion and, indirectly, fiber tract microstructure, are fractional anisotropy (FA), which measures the total magnitude of water directional movement along the axonal fibers, and mean diffusivity (MD), which measures the mean diffusion of each direction.17 Other diffusivity indices are radial diffusivity (RD), which measures the diffusion perpendicular to axons, and it is related with myelin degradation, and axial diffusivity (AD), which describes the diffusion parallel to the axon and is related to axonal injury.17
There are two main methods for analyzing DTI images that allow full brain coverage: voxel‐based analysis and whole‐brain tract‐based spatial statistics (TBSS) analysis. This latter has become an extremely popular software because it attempts to alleviate several of the drawbacks of conventional voxel‐based analysis, such as the alignment and smoothing problems.18 The main advantage of whole‐brain TBSS analysis is the substitution of the image alignment procedure by projecting all FA images onto a white matter skeleton.18 Statistical tests are performed on the skeleton, so this approach does not require data smoothing and it also gains statistical power by reducing dimensionality.18
To our knowledge, there are no published data on white matter microstructural changes data in OT patients. Our primary aim was to use whole‐brain TBSS analysis to assess white matter microstructural changes in OT patients compared with healthy controls (HCs). We hypothesized that there would be white matter changes in the pons and cerebellum in OT, and, possibly in key components of the frontal–thalamic–cerebellar circuit. As a secondary aim, in OT patients, we searched for correlations of white matter changes with scores from a neuropsychological testing battery.
Methods
All the participants included in the study gave their written informed consent after full explanation of the procedure. The study, which was conducted in accordance with the principles of the Helsinki Declaration, as revised in 2013, was approved by the ethical standards committee on human experimentation at the University Hospital “12 de Octubre” (Madrid).
Participants
Patients with OT were consecutively recruited from December 2011 to May 2013 from the outpatient neurology clinics of the University Hospital “12 de Octubre” in Madrid (Spain), a public hospital, which covers an area of more than 400,000 inhabitants. The patients, who were referred to the outpatient neurology clinics with a subjective feeling of unsteadiness or tremor when standing, which was absent while walking, seated, or supine, were examined by three neurologists with expertise in movement disorders (J.B.‐L., A.S.‐F., and M.M.). They were classified as having OT using the Consensus Statement on Tremor by the Movement Disorder Society.19 The diagnosis of OT was electromyographically confirmed before inclusion in the study. The neurological examination comprised a general neurological examination and the motor portion of the Unified Parkinson’s Disease Rating Scale (m‐UPDRS).20 Mild Parkinsonian signs (MPS) were defined as present when any one of the following conditions was met: (1) two or more m‐UPDRS20 ratings = 1; or (2) one m‐UPDRS20 rating = 2; or (3) the m‐UPDRS rest tremor rating = 1.21
OT patients were 1:1 frequency‐matched with HCs. Frequency‐matching was based on age (5‐year age categories), and years of education.
HCs were recruited either from relatives or friends of the health professionals working at the University Hospital “12 de Octubre” of Madrid (Spain) or among the relatives of patients who came to the neurological clinics for reasons other than OT (e.g., headache, dizziness). None of the HCs reported having a first‐degree or second‐degree relative with OT or essential tremor. Each HC underwent a standardized neurological examination performed by one of two neurologists (J.P.R. and A.S.‐F.) to further rule out any neurological conditions. None of the HCs were excluded due to refusal to perform MRI.
A senior neuropsychologist (V.P.‐M., see acknowledgments), specializing in cognitive problems associated with movement disorders, performed a mental status examination on each patient and control, applying DSM‐IV criteria and excluding those persons who had dementia.
None of the participants were excluded because of neurological comorbidities, dementia, or structural abnormalities on conventional MRI images. According to Fazekas visual rating scale, all participants had Fazekas score ≤ 1 (i.e., normal in the elderly).22
Procedure
Clinical characteristics were obtained from review of records from their outpatient neurological care. Participants were asked to bring all medications taken in the past 1 week to the clinic where the study researchers viewed and recorded the name and the dose of each one, with special emphasis on medications that potentially affect cognitive function (e.g., anxiolytics, stimulants, antipsychotics, antidepressants, antihistamines, or antiepileptics drugs). All participants underwent a neuropsychological assessment of cognitive functioning, including attention, executive function, visuospatial ability, verbal memory, visual memory, and language (Table 1).10, 16, 23 Depressive symptoms severity was assessed using the original 17‐item version of the Hamilton Depression Rating Scale.24
Table 1.
Comparison of demographic, clinical, and neuropsychiatric domains of orthostatic tremor patients versus HCs.
| Orthostatic tremor patients (N = 14) | HCs (N = 14) | P value | |
|---|---|---|---|
| Age in years | 65.0 (66.9) ± 13.9 | 63.3 (65.0) ± 12.9 | 0.740a |
| Sex (female) | 12 (85.7%) | 9 (64.3%) | 0.190 |
| Education in years | 8.0 (8.0) ± 4.9 | 10.0 (10.0) ± 3.3 | 0.205a |
| Depressive symptoms | |||
| 17‐item Hamilton Depression Rating Scale total score | 7.1 (6.0) ± 6.7 | 7.6 (7.0) ± 6.4 | 0.843a |
| On medications with central nervous system effects | 1 (7.1%) | 4 (28.6%) | 0.326 |
| Age at onset, years | 55.6 (60.0) ± 14.7 | – | |
| Disease duration, years | 9.5 (7.2) ± 6.9 | – | |
| EuroQol‐5D index score | 0.8 (0.7) ± 0.2 | – | |
| EuroQol visual analogue scale | 68.2 (77.5) ± 24.5 | ||
| Cognitive domains | |||
| Attention | |||
| Direct Digit Span subtest from the WAIS‐III | 5.2 (5.0) ± 1.5 | 7.2 (6.5) ± 2.6 | 0.035b |
| Coding‐Digit Symbol subtest from the WAIS‐III | 39.0 (28.5) ± 31.4 | 167.1 (73.0) ± 240.5 | 0.013b |
| Executive function | |||
| Stroop Color–Word Trial | 24.6 (22.0) ± 13.9 | 30.2 (36.5) ± 15.7 | 0.346a |
| Wisconsin Card Sorting Test | |||
| Perseverations | 34.3 (26.0) ± 29.3 | 33.8 (28.5) ± 30.5 | 0.964a |
| Nonperseverative errors | 66.6 (69.0) ± 26.4 | 36.3 (24.5) ± 27.2 | 0.007a |
| Similarities subtest from the WAIS‐III | 11.4 (9.5) ± 4.9 | 23.2 (19.5) ± 10.0 | <0.001b |
| Indirect Digit Span subtest from the WAIS‐III | 3.1 (3.0) ± 1.3 | 4.8 (5.0) ± 1.2 | 0.002b |
| Controlled Oral Word Association Test | 26.1 (26.5) ± 21.8 | 38.1 (41.0) ± 15.0 | 0.102a |
| Tower of London (time of execution in seconds) | 551.0 (492.5) ± 307.9 | 258.4 (229.5) ± 214.6 | 0.011b |
| Frontal Battery Assessment | 14.3 (15.0) ± 3.2 | 18.4 (17.0) ± 5.2 | 0.022b |
| Visuospatial ability | |||
| Benton Judgment of Line Orientation Test | 8.3 (8.5) ± 3.2 | 14.0 (10.5) ± 10.1 | 0.085b |
| Hooper Visual Organization Test | 28.6 (29.0) ± 14.3 | 44.3 (46.5) ± 9.6 | 0.002a |
| Verbal memory | |||
| WMS‐III Word List | |||
| Learning trials total | 26.3 (24.0) ± 7.2 | 24.4 (28.0) ± 13.4 | 0.653a |
| Immediate recall | 5.0 (4.5) ± 2.4 | 11.3 (8.5) ± 7.9 | 0.002b |
| Delayed recall | 4.6 (4.0) ± 2.8 | 6.9 (7.0) ± 2.5 | 0.032a |
| Recognition | 19.8 (20.5) ± 4.0 | 19.1 (22.0) ± 7.7 | 0.401b |
| Visual memory | |||
| Brief Visuospatial Memory Test‐Revised | |||
| Learning trials | 15.1 (10.5) ± 12.3 | 24.7 (31.0) ± 11.9 | 0.031b |
| Delayed recall trial | 5.5 (4.5) ± 4.8 | 12.6 (12.0) ± 5.2 | 0.001b |
| Recognition trial | 11.7 (12.0) ± 0.5 | 11.1 (12.0) ± 1.9 | 0.804b |
| Language | |||
| Boston Naming Test | 41.0 (37.0) ± 11.1 | 51.9 (53.5) ± 11.3 | 0.014b |
| Total number of animals as possible in 1 minute | 15.0 (13.0) ± 7.6 | 26.5 (27.0) ± 9.6 | 0.001b |
Mean (median) ± SD and frequency (%) are reported. aStudent’s t tests or bMann–Whitney U test were used for comparisons of continuous data; chi‐square test for sex and Fisher exact test for the intake of medications with central nervous system effects. WAIS‐III = Wechsler Adult Intelligence Scale‐Third Edition. WMS‐III = Wechsler Memory Scale‐Third Edition. Significantly different values are in bold font.
As there are no validated instruments specifically designed to quantify OT severity among patients, we assessed its impact on their health‐related quality of life using the Spanish version of EuroQol‐5 dimension.25 This instrument was chosen given that it has been used in other related tremor disorders such as Parkinson’s Disease26 and essential tremor.27
MRI data and acquisition and analysis
HCs and patients were placed in the scanner, and to prevent motion artifacts, they were immobilized with a custom‐fit blue bag vacuum mold (Medical Intelligence, Inc.). They were told to relax with their eyes closed, and to attenuate scanner noise we used noise‐reduction headphones and earplugs.
All subjects underwent MRI examination in a 3T Signa HDx MR scanner (General Electric Healthcare, Waukesha, WI) using an eight‐channel phased array coil. The protocol for the DWI acquisition consisted of three images without diffusion gradients (b = 0 s/mm2) and 45 images measured with 45 directions (b = 1000 s/mm2) isotropically distributed in space (axial acquisition, echo time = 89 msec, repetion time = 10100 msec, slice thickness = 2.6 mm with no gap, resolution = 2.6 x 2.6 x 2.6 mm, flip‐angle = 90º, and field of view = 250 mm).
All DWI data were preprocessed with FMRIB’s Diffusion Toolbox (FDT, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FDT/), part of FMRIB Software Library (FSL version 5.0.10).28, 29 Image artifacts, due to eddy current distortions, head motion, and field map were corrected using the EDDYCORRECT, FUGUE, and PRELUDE functions respectively. Then, the average b0 images were brain‐extracted to remove all nonbrain tissue using the FMRIB’s Brain Extraction Toolbox. After that, the brain mask was applied to the remainder of the DWI. Next, DTIFIT function was used for fitting the diffusion tensor model for each voxel via linear regression to create FA, MD, AD, and RD maps.
Voxel‐wise analyses of whole‐brain white matter measures were performed with the TBSS package (http://www.fmrib.ox.ac.uk/fsl/tbss/index.html).30 In summary, all the individual FA images were aligned into a common space by nonlinear registration using the FNIRT tool and the FMRIB58_FA standard‐space template. After that, images were averaged to create a mean FA image, which was thinned to create a white matter tract “skeleton” using an FA threshold of 0.2 to exclude non‐white matter voxels. Aligned FA data for each subject were then projected onto this skeleton, resulting in an alignment‐invariant representation of the central trajectory of white matter pathways for all subjects. The individual registration and skeleton projections obtained in the previous FA analysis was then applied to the other diffusion‐derived MD, AD, and RD images.
The voxel‐wise analysis in FA, MD, AD, and RD images comparing controls and OT patients was done using permutation‐based inference for nonparametric statistical thresholding (FSL’s “randomize” function)31 and a two‐sample t‐test using the total intracranial volume, sex, and age as confounding regressors. To allow robust statistical inference, the number of permutations was set to 5000. A significance level of P < 0.001 was selected for between‐group comparisons using the randomized tool’s threshold‐free cluster enhancement (TFCE) option.32 To identify white matter tracts, we used the ICBM‐DTI‐81 white matter atlas provided by FSL.33, 34 Finally, we identified significant white matter clusters by their cluster size and by their coordinates (using the Montreal Neurological Institute coordinate system).
Statistical analyses of clinical and neuropsychological data
Statistical analyses for the clinical and neuropsychological scores were performed using Statistical Package for the Social Sciences (SPSS) Version 22.0 (SPSS, IBM Corporation). Age, years of education, and neuropsychological variables were compared using two independent sample t‐tests for continuous and normally distributed data, and Mann–Whitney U test for nonnormally distributed data, where appropriate. The chi‐square test and Fisher’s exact test were used to analyze differences in categorical variables.
Linear regression analyses were performed to assess differences between OT patients and HCs in neuropsychological scores. Those scores were adjusted for age, sex, years of education, and depressive symptoms. Using a one‐sample Kolmogorov–Smirnov test, we tested whether neuropsychological and personality scores were normally distributed, and, for several scores, a logarithmic transformation was performed prior to linear regression analyses.
Correlations between disease duration, EuroQol‐5 score, 17‐item Hamilton Depression Rating Scale total score, neuropsychological scores, and altered diffusion metrics in OT patients were determined using Pearson’s or Spearman’s correlation coefficients when appropriate. For the diffusion metrics, we used the average value for each cluster.
Results
Clinical details of the OT patients have been published elsewhere.10 All 14 OT patients were right‐handed. There was a female preponderance (85.7%) with a mean age of onset of 55.6 years (Table 1). Nine (64.3%) patients presented with primary OT and five (35.7%) had MPS. The nine patients with primary OT patients were younger to the five OT patients who had MPS (mean age = 59.1 ± 13.8 years vs. 75.7 ± 4.6 years, Student's t test = −2.583, P = 0.024), but had a similar perception of their health‐related quality of life (mean EuroQol‐5D index score = 0.8 ± 0.1 vs. 0.7 ± 0.1, Student's t test = 1.568, P = 0.143). In addition, there was no sex difference (seven [77.8%] vs. five [100.0%] women, chi‐square = 1.296, P = 0.255).
None had cerebellar atrophy on structural brain MRI. Before MRI procedure, all 14 OT patients underwent electromyographic analysis, which revealed a synchronous 10‐ to 18‐Hz leg tremor present only on standing. No patients were being treated with medication for OT.
The 14 right‐handed OT patients (12 women and 2 men) were compared with 14 right‐handed HCs (9 women and 5 men). The OT patients did not differ from the HCs in terms of age, sex, years of education, and depressive symptoms (Table 1). The results of neuropsychological testing, which were performed between 1 and 3 weeks before MRI, are shown in Table 1. In all domains, OT patients’ cognitive performance was significantly worse than that of the HCs.
OT patients and HCs did not differ to a significant degree in terms of demographic features; however, if the sample size had been larger, several of these features could have differed significantly. Therefore, we performed adjusted analyses to take any potential confounding into account. In linear regression analyses that adjusted for age in years, sex, years of education, and depressive symptoms, we found that diagnosis (OT vs. HC) was associated with poor performance on most neuropsychological test scores, particularly on tests of attention, executive function, visuospatial ability, verbal memory, visual memory, and language (Table 2).
Table 2.
Linear regression analyses using each neuropsychological test score as the outcome variable in separate adjusted models.
| Outcome variable1 | Orthostatic tremor (N = 14) | ||
|---|---|---|---|
| Beta | P value | ||
| Attention | Direct Digit Span test from the WAIS‐III | −0.315 | 0.081 |
| Coding‐Digit Symbol subtest from the WAIS‐III | −0.488 | 0.011 | |
| Executive Function | Stroop Color–Word Trial | −0.176 | 0.245 |
| Wisconsin Card Sorting Test | |||
| Perseverations | −0.021 | 0.919 | |
| Nonperseverative errors | 0.436 | 0.019 | |
| Similarities subtest from the WAIS‐III | −0.525 | <0.001 | |
| Indirect Digit Span subtest from the WAIS‐III | −0.406 | 0.021 | |
| Controlled Oral Word Association Test | −0.179 | 0.263 | |
| Tower of London (time of execution in seconds) | 0.441 | 0.015 | |
| Frontal Assessment Battery | −0.381 | 0.052 | |
| Visuospatial ability | Benton Judgment of Line Orientation Test | −0.198 | 0.241 |
| Hooper Visual Organization Test | −0.444 | 0.001 | |
| Verbal memory | WMS‐III Word List | ||
| Learning list | 0.121 | 0.369 | |
| Immediate recall | −0.437 | 0.018 | |
| Delayed recall | −0.333 | 0.034 | |
| Recognition | 0.303 | 0.196 | |
| Visual memory | Brief Visuospatial Memory Test ‐Revised | ||
| Learning trials total | −0.336 | 0.057 | |
| Delayed free recall trial | −0.451 | 0.013 | |
| Recognition trial | 0.294 | 0.149 | |
| Language | Boston Naming Test | −0.419 | 0.007 |
| Total number of animals as possible in 1 minute | −0.419 | 0.007 | |
Adjusted for age, sex, years of education, and depressive symptoms. Significant values are in bold font.
Primary Aim: Comparison of DTI metrics between OT patients and HCs
OT patients demonstrated decreased FA values in the anterior limb of internal capsule bilaterally, right insula, and splenium of corpus callosum compared with HCs (See Figure 1 and Table 3) for more details (all P TFCE‐corrected < 0.001). In addition, MD values in the left superior cerebellar peduncle, RD values in left cerebellar lobule VI and medial lemniscus bilaterally, and AD values in right posterior limb of the internal capsule and the left anterior corona radiata, were significantly increased in OT patients compared with HCs (all P TFCE‐corrected < 0.001).
Figure 1.
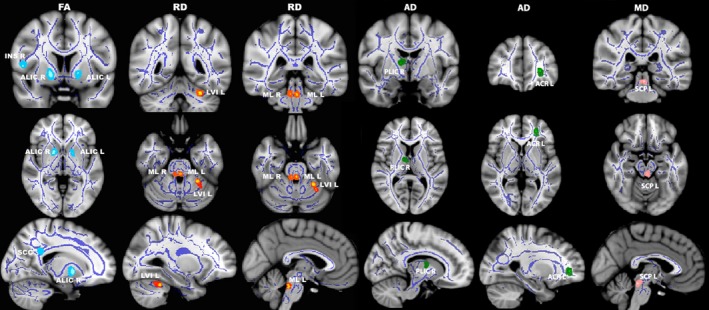
Differences in fractional anisotropy (first column), radial diffusivity (second and third columns), axial diffusivity (fourth and fifth columns), and mean diffusivity (sixth column) values in orthostatic tremor patients versus healthy controls. Results from between‐group comparison showing clusters with significantly decreased fractional anisotropy (light blue colormap) and increased radial (red–yellow colormap), axial (green colormap) and mean (pink colormap) diffusivity values in orthostatic tremor patients compared with healthy controls (P TFCE‐corrected < 0.001). The group‐averaged white matter skeleton (fractional anisotropy threshold> 0.2) is shown in dark blue. Group differences are mapped onto a standard T1 Montreal Neurological Institute template. Images are in radiological convention (i.e., findings in the left hemisphere are displayed on the right and vice versa). The color bar represents statistical significance (1 minus P value). FA, fractional anisotropy; RD, radial diffusivity; AD, axial diffusivity; MD, mean diffusivity; P TFCE‐corrected, threshold‐free cluster enhancement corrected P; ALIC L/R left and right anterior limb of internal capsule; INS R; right insula; SCC, splenium of corpus callosum; LVI L, left cerebellar lobule VI; ML L/R, left and right medial lemniscus; PLIC R, right posterior limb of the internal capsule; ACR L, left anterior corona radiata; SCP L, left superior cerebellar peduncle.
Table 3.
White matter clusters showing decreased fractional anisotropy and increased mean diffusivity, radial diffusivity, and axial diffusivity in orthostatic tremor patients versus healthy controls (P TFCE‐corrected < 0.001).
| White matter areas | Difference in mean | t‐statistic | Montreal Neurological Institute coordinates | Cluster size (number of voxels) | P value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Right anterior limb of internal capsule1 | 0.016 | 2.4 | 16 | 6 | −5 | 20 | 0.002 |
| Left anterior limb of internal capsule1 | 0.024 | 2.3 | −17 | 4 | −1 | 10 | 0.005 |
| Right insula1 | 0.029 | 3.1 | 51 | 5 | 8 | 5 | 0.006 |
| Splenium of corpus callosum1 | 0.030 | 2.8 | 15 | −47 | 30 | 5 | 0.001 |
| Left superior cerebellar peduncle2 | −2.78 × 10−5 | 3.4 | 0 | −33 | −16 | 5 | 0.008 |
| Left cerebellar lobule VI3 | −1.38 × 10−5 | 2.7 | −28 | −47 | −28 | 10 | 0.005 |
| Left medial lemniscusc | −1.61 × 10−5 | 1.9 | 0 | −35 | −26 | 5 | 0.006 |
| Right medial lemniscus3 | −1.95 × 10−5 | 2.1 | 7 | −35 | −26 | 5 | 0.009 |
| Right posterior limb of the internal capsule4 | −9.47 × 10−5 | 2.7 | 12 | −5 | 8 | 5 | 0.007 |
| Left anterior corona radiata4 | −1.12 × 10−4 | 2.5 | −25 | 43 | 2 | 5 | 0.003 |
White matter clusters with 1decreased fractional anisotropy, 2increased mean diffusivity, 3increased radial diffusivity, and 4increased axial diffusivity.
P TFCE‐corrected, threshold‐free cluster enhancement corrected. Differences in mean values between orthostatic tremor patients and healthy controls.
In the secondary analyses, we excluded the five OT patients with MPS, and the results were similar (data not shown).
Secondary aim: Correlation Analyses
The correlations between diffusion measures, which were statistically different at a P TFCE‐corrected < 0.001 level between both groups, and the clinical and neuropsychological tests in the OT group are shown in Table 4. Diffusion white matter changes, mainly those located in right anterior limb of the internal capsule, were correlated with worse performance on executive function, visuospatial ability, verbal memory, and visual memory. Furthermore, there was a significant positive correlation between FA in the right anterior limb of internal capsule and the EuroQol‐5D index score (i.e., the lower the FA, the worse health‐related quality of life). No significant relationship was found between diffusion tensor imaging measures and disease duration in OT patients.
Table 4.
Matrix of significant correlations among diffusion measures statistically different at a P TFCE‐corrected < 0.001 and the clinical and neuropsychological tests in orthostatic tremor patients.
| Right posterior limb of the internal capsule (axial diffusivity) | Left anterior limb of the internal capsule (fractional anisotropy) | Right anterior limb of the internal capsule (fractional anisotropy) | Right insula (fractional anisotropy) | |
|---|---|---|---|---|
| EuroQol‐5D index score | 0.649 (P = 0.012)1 | |||
| Cognitive domains | ||||
| Executive function | ||||
| Stroop Color–Word Trial | 0.667 (P = 0.018)1 | |||
| Wisconsin Card Sorting Test | ||||
| Perseverations | ||||
| Nonperseverative errors | ||||
| Similarities subtest from the WAIS‐III | 0.544 (P = 0.044)2 | |||
| Tower of London (time of execution in seconds) | 0.635 (P = 0.015)2 | |||
| Visuospatial ability | ||||
| Hooper Visual Organization Test | 0.606 (P = 0.028)1 | |||
| Verbal memory | ||||
| WMS‐III Word List | ||||
| Learning trials total | 0.777 (P = 0.001)1 | 0.668 (P = 0.009)1 | ||
| Immediate recall | 0.771 (P = 0.001)2 | |||
| Delayed recall | 0.561 (P = 0.037)1 | 0.676 (P = 0.008)1 | ||
| Recognition | 0.603 (P = 0.022)2 | 0.610 (P = 0.021)2 | ||
| Visual memory | ||||
| Brief Visuospatial Memory Test‐Revised | ||||
| Learning trials | 0.564 (P = 0.036)2 | |||
| Delayed recall trial | 0.632 (P = 0.015)2 | |||
Pearson product–moment correlation coefficient.
Spearman's rank correlation coefficient.
Discussion
In the current study, we used whole‐brain TBSS analysis to assess white matter microstructure in OT, finding not only altered diffusivity metrics in the posterior lobe of the cerebellum (left cerebellar lobule VI) and in its efferent cerebellar fibers (left superior cerebellar peduncle), but also in the medial lemniscus bilaterally (pontine tegmentum), anterior limb of the internal capsule bilaterally, left anterior corona radiata, and right insula), the splenium of corpus callosum, and the right anterior limb of internal capsule.
Furthermore, decreased FA, mainly located in right anterior limb of the internal capsule, but also in left anterior limb of the internal capsule and in the right insula was correlated with worse performance on executive function, visuospatial ability, verbal memory, and visual memory. Increased AD in the right posterior limb of the internal capsule was also correlated with the performance of the Tower of London (executive function). The ultimate mechanism linking white matter changes with specific cognitive domains in OT is unknown. It is obvious that correlation and causality are not the same. Demonstrating that the white matter changes preceded the cognitive deficits would be a step forward in establishing causality; notwithstanding, in the lack of a prospective, longitudinal study, this is not possible. However, it is more biologically plausible that the white matter changes resulted in the cognitive deficits than vice versa.
Among the brain functional loops that are related to tremor generation, one of the most important is the Guillain–Mollaret triangle, which links the dentate nucleus of the cerebellum, via the superior cerebellar peduncles, with the contralateral red nucleus and the inferior olive (the so called dentate‐rubro‐olivary tract).35 Damage in the dentate or their outflow tract to the contralateral ventrolateral thalamus leads to cerebellar intention tremor.36 In this study, increased MD values of the left superior cerebellar peduncle suggest altered output from the cerebellum in OT.
Of special interest is the increased MD values of the left cerebellar lobule VI, which is part of the sensorimotor cerebello‐cerebral network. The involvement of this structure is in line with the results of a recent multimodal approach addressing the morphological and functional alterations of OT.9 Specifically, the bilateral decrease in gray matter volume in cerebellar lobule VI correlated negatively with disease duration and positively with clinical severity.9 In addition, the cerebellar lobule VI showed increased functional connectivity with the bilateral supplementary motor area, which was associated with more severe tremor.9 Furthermore, the cerebellar lobule VI showed increased functional connectivity with the primary motor cortex leg and trunk area.9 Lastly, after repeated cerebellar stimulation, the bilateral cerebellar lobule VI showed a decreased functional connectivity with both the supplementary motor area and the primary motor cortex leg and trunk area compared to baseline.9
The increased RD values in the medial lemniscus bilaterally are also of interest. The medial lemniscus, which comprises the most important afferent fiber tracts of the exteroceptive sensitivity from the caudal medulla to the ventral posterolateral nucleus of the thalamus, and subsequently the primary somatosensory cortex, are located in the pontine tegmentum.37 These structures could be involved in the pathophysiology of OT. In a recent 18F‐fluorodeoxyglucose‐positron emission tomography study, increased regional cerebral glucose metabolism in the pontine tegmentum, the posterior cerebellum, the ventral intermediate and ventral posterolateral nucleus of the thalamus, and the primary motor cortex bilaterally were found compared to controls. However, glucose metabolism was decreased in mesiofrontal cortical areas and the bilateral anterior insula while lying and standing.14 The involvement of the pontine tegmentum seems to be exclusive in OT, unlike other tremor disorders.14 Taken together, one could hypothesize that, in OT, upright stance would cause abnormal hyperexcitability in an already damaged medial lemniscus sensory pathway that would trigger the cerebello‐thalamo‐cortical network.
Of additional interest, we found that some of the affected areas (left superior cerebellar peduncle, anterior limb of internal capsule bilaterally, left anterior corona radiate, right insula, right posterior limb of internal capsule and splenium of corpus callosum) are associated with cognition in some way. First, the superior cerebellar peduncle and the anterior limb of the internal capsule are key components of the frontal–thalamic–cerebellar circuit.38 The anterior limb of the internal capsule connects prefrontal cortex with the thalamus and brainstem and is associated with different aspects of emotion, motivation, cognition processing, and decision‐making.39 Second, the corticospinal tract forms part of the descending spinal tract system that connects motor cortex to the brain stem and spinal cord.40 These tracts converge into the corona radiata and continue through the posterior limb of the internal capsule to the cerebral peduncle on their way to the lateral funiculus.40 Corticospinal tract originates mainly from the primary motor cortex; however, projections from other areas including the somatosensory, cingulate, and insular cortices are also found.41 Hence, the corticospinal tract and, obviously, the posterior limb of internal capsule, might be involved in a variety of functions, including cognition. Third, although the function of insular cortex is unknown, it has been associated with cognitive and emotional processes.42 Finally, the involvement of the splenium of the corpus callosum is related to a key default mode cortical network hub, the posterior cingulate cortex.43, 44 The default mode network is well known to be involved in cognitive processes,43 and previous research by our group has reported abnormal functioning of default mode network regions in OT.16 Overall, damage of all those structures might alter the connectivity between brain regions involved in mood and cognition,45 and therefore be associated with nonmotor manifestations that have been related to OT, such as cognitive deficits or personality changes.15
The study had a primary and a secondary aim. For the primary aim, the p values we report are very low (P = 0.001–0.009, Table 2) and not likely due to Type I error. The P values for our secondary aim were less robustly significant, so one must consider Type I error. Arguing against Type I error, however, is that the r values themselves were large (r = 0.56–0.77), which suggests that the associations are genuine rather than spurious. The p values associated with those r values were modestly significant, that is probably a reflection of a modest statistical power in the setting of a small sample.
Our study has limitations. First, the sample size was relatively small. However, in general, neuroimaging studies of OT only include small sample sizes.9, 14 OT is a very rare disease and hence it is rather difficult to recruit patients for case–control studies. Although there are no epidemiological data, in the follow‐up evaluation of the Neurological Disorders of Central Spain study,46 neurologists detected only one patient with OT in a cohort of approximately 4000 elderly subjects (data not published). Notwithstanding, our sample was adequate to detect a number of white matter differences in several areas between the two study groups, which are in line with previous research. Second, the small sample did not allow us to separate subjects based on the type of OT (primary vs. those cases with MPS). However, our aim was to examine whether OT patients in general had white matter differences when compared with matched HCs. All the same, after excluding those OT patients with MPS, the results were similar. Finally, the results of our correlation analyses should be interpreted carefully because they were not controlled for multiple comparisons. However, as this was a study of a rare condition, the sample size was small. Small sample size lessens the likelihood of detecting a statistically significant difference during statistical testing even when one truly exists (i.e., Type II statistical error). In this situation, investigators generally concentrate less on the p value and the adjustment for multiple comparisons and they concentrate more on the magnitude of the correlation coefficients (e.g., whether r = 0.3, r = 0.4, r = 0.5, r = 0.6). That was our approach and that was the value of our data. This study also had several strengths. First, this is the first study that has been designed to detect microstructural white matter changes in OT, and to search for correlations of these changes with scores from a neuropsychological testing battery to understand their pathophysiological implications. Second, assessments were conducted prospectively in a standardized manner. Finally, the tests included are among the most sensitive neuropsychological measures to detect cognitive impairment in tremor disorders.47, 48
In closing, diffusion white matter changes were preferentially located in the cerebellum, its efferent pathways, as well as in the pontine tegmentum and key components of the frontal–thalamic–cerebellar circuit. Abnormalities in this latter circuit could be related to the pathogenesis of cognitive deficits in OT. Further work needs to be done to understand the evolution of these white matter changes and their functional consequences.
Authors Contribution
Dr. Benito‐León (jbenitol67@gmail.com) collaborated in: 1) the conception, organization and execution of the research project; 2) the statistical analysis design, and; 3) the writing of the manuscript first draft and the review and critique of the manuscript. Dr. Romero (p.romero.prof@ufv.es) collaborated in: 1) the conception, organization of the research project, and; 2) the review and critique of the manuscript. Dr. Louis (elan.louis@yale.edu) collaborated in: 1) the conception, organization of the research project, and; 2) the review and critique of the manuscript. Dr. Sánchez‐Ferro (alvarosferro@hotmail.com) collaborated in: 1) the organization of the research project, and; 2) the review and critique of the manuscript. Dr. Matarazzo (michele.matarazzo@gmail.com) collaborated in: 1) the organization of the research project, and; 2) the review and critique of the manuscript. Dr. Molina‐Arjona (jmolinaa@salud.madrid.org) collaborated in: 1) the conception, organization of the research project, and; 2) the review and critique of the manuscript. Dr. Mato‐Abad (virginia.mato@urjc.es) collaborated in: 1) the conception, organization of the research project; 2) the statistical analysis design, and; 3) the review and critique of the manuscript.
Conflict of Interest
The authors declare no competing financial interests.
Acknowledgments
We acknowledge the neuropsychologist Verónica Puertas‐Martín, for her assistance with the project.
Funding Information
This research was supported by FEDER funds. Dr. Benito‐León is supported by the National Institutes of Health, Bethesda, MD, USA (NINDS #R01 NS39422), European Commission (grant ICT‐2011‐287739, NeuroTREMOR), the Ministry of Economy and Competitiveness (grant RTC‐2015‐3967‐1, NetMD—platform for the tracking of movement disorder), and the Spanish Health Research Agency (grant FIS PI12/01602 and grant FIS PI16/00451). Dr. Romero is supported by the European Commission (grant ICT‐2011‐287739, NeuroTREMOR) and the Spanish Ministry of Economy and Competitiveness (grant DPI‐2015‐68664‐C4‐1‐R, NeuroMOD). Dr. Louis has received research support from the National Institutes of Health: NINDS #R01 NS094607 (principal investigator), NINDS #R01 NS085136 (principal investigator), NINDS #R01 NS073872 (principal investigator), NINDS #R01 NS085136 (principal investigator) and NINDS #R01 NS088257 (principal investigator). He has also received support from the Claire O'Neil Essential Tremor Research Fund (Yale University). Dr. Sánchez‐Ferro is supported by the Consejería de Educación, Juventud y Deporte de la Comunidad de Madrid and the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme (FP7/2007‐2013).
Funding Statement
This work was funded by FEDER funds grant ; National Institutes of Health grants #R01 NS39422, #R01 NS094607, #R01 NS085136, #R01 NS073872, and #R01 NS088257; European Commission grant ICT‐2011‐287739; Ministry of Economy and Competitiveness grant RTC‐2015‐3967‐1; Spanish Health Research Agency grants FIS PI12/01602 and FIS PI16/00451; Spanish Ministry of Economy and Competitiveness grant DPI‐2015‐68664‐C4‐1‐R; Claire O'Neil Essential Tremor Research Fund grant ; Consejería de Educación, Juventud y Deporte de la Comunidad de Madrid and the People Programme grant ; European Union’s Seventh Framework Programme grant FP7/2007‐2013.
Data availability statement
Anonymized data will be shared by request from any qualified investigator, only for purposes of replicating procedures and results.
References
- 1. Benito‐León J, Porta‐Etessam J. Shaky‐leg syndrome and vitamin B12 deficiency. N Engl J Med. 2000;342:981. [DOI] [PubMed] [Google Scholar]
- 2. Benito‐León J, Domingo‐Santos A. Orthostatic tremor: an update on a rare entity. Tremor Other Hyperkinet Mov (N Y). 2016;6:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benito‐León J, Rodríguez J. Orthostatic tremor with cerebellar ataxia. J Neurol 1998;245:815. [DOI] [PubMed] [Google Scholar]
- 4. Setta F, Manto MU. Orthostatic tremor associated with a pontine lesion or cerebellar disease. Neurology. 1998;51:923. [DOI] [PubMed] [Google Scholar]
- 5. Manto MU, Setta F, Legros B, et al. Resetting of orthostatic tremor associated with cerebellar cortical atrophy by transcranial magnetic stimulation. Arch Neurol 1999;56:1497–500. [DOI] [PubMed] [Google Scholar]
- 6. Feil K, Bottcher N, Guri F, et al. Long‐term course of orthostatic tremor in serial posturographic measurement. Parkinsonism Relat Disord 2015;29:905–910. [DOI] [PubMed] [Google Scholar]
- 7. Wills AJ, Thompson PD, Findley LJ, Brooks DJ. A positron emission tomography study of primary orthostatic tremor. Neurology 1996;46:747–752. [DOI] [PubMed] [Google Scholar]
- 8. Benito‐León J, Rodríguez J, Ortí‐Pareja M, et al. Symptomatic orthostatic tremor in pontine lesions. Neurology 1997;49:1439–1441. [DOI] [PubMed] [Google Scholar]
- 9. Gallea C, Popa T, Garcia‐Lorenzo D, et al. Orthostatic tremor: a cerebellar pathology? Brain 2016;139(Pt 8):2182–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benito‐León J, Louis ED, Mato‐Abad V, et al. In vivo neurometabolic profiling in orthostatic tremor. Medicine (Baltimore). 2016;95:e4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benito‐León J, Louis ED. Update on essential tremor. Minerva Med 2011;102:417–439. [PubMed] [Google Scholar]
- 12. Benito‐León J, Louis ED. Essential tremor: emerging views of a common disorder. Nat Clin Pract Neurol 2006;2:666–678; quiz 2p following 91. [DOI] [PubMed] [Google Scholar]
- 13. Lenka A, Pal PK, Bhatti DE, Louis ED. Pathogenesis of primary orthostatic tremor: current concepts and controversies. Tremor Other Hyperkinet Mov (N Y) 2017;7:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schoberl F, Feil K, Xiong G, et al. Pathological ponto‐cerebello‐thalamo‐cortical activations in primary orthostatic tremor during lying and stance. Brain 2017;140:83–97. [DOI] [PubMed] [Google Scholar]
- 15. Benito‐León J, Louis ED, Puertas‐Martín V, et al. Cognitive and neuropsychiatric features of orthostatic tremor: a case‐control comparison. J Neurol Sci 2016;361:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benito‐León J, Louis ED, Manzanedo E, et al. Resting state functional MRI reveals abnormal network connectivity in orthostatic tremor. Medicine. 2016;95:e4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lerner A, Mogensen MA, Kim PE, et al. Clinical applications of diffusion tensor imaging. World Neurosurg 2014;82:96–109. [DOI] [PubMed] [Google Scholar]
- 18. Bach M, Laun FB, Leemans A, et al. Methodological considerations on tract‐based spatial statistics (TBSS). NeuroImage 2014;100:358–369. [DOI] [PubMed] [Google Scholar]
- 19. Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord 1998;13:2–23. [DOI] [PubMed] [Google Scholar]
- 20. Martinez‐Martín P, Gil‐Nagel A, Gracia LM, et al. Unified parkinson's disease rating scale characteristics and structure. The cooperative multicentric group. Mov Disord 1994;9:76–83. [DOI] [PubMed] [Google Scholar]
- 21. Louis ED, Schupf N, Manly J, et al. Association between mild parkinsonian signs and mild cognitive impairment in a community. Neurology 2005;64:1157–1161. [DOI] [PubMed] [Google Scholar]
- 22. Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987;149:351–356. [DOI] [PubMed] [Google Scholar]
- 23. Puertas‐Martín V, Villarejo‐Galende A, Fernández‐Guinea S, et al. A comparison study of cognitive and neuropsychiatric features of essential tremor and parkinson's disease. Tremor Other Hyperkinet Mov (N Y). 2016;6:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Macera CA, Sun RK, Yeager KK, Brandes DA. Sensitivity and specificity of death certificate diagnoses for dementing illnesses, 1988–1990. J Am Geriatr Soc 1992;40:479–481. [DOI] [PubMed] [Google Scholar]
- 26. Benito‐León J, Cubo E, Coronell C, Group AS . Impact of apathy on health‐related quality of life in recently diagnosed Parkinson's disease: the ANIMO study. Mov Disord 2012;27:211–218. [DOI] [PubMed] [Google Scholar]
- 27. Martínez‐Martín P, Jiménez‐Jiménez FJ, Carroza García E, et al. Most of the Quality of Life in Essential Tremor Questionnaire (QUEST) psychometric properties resulted in satisfactory values. J Clin Epidemiol 2010;63:767–773. [DOI] [PubMed] [Google Scholar]
- 28. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004;23(Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 29. Woolrich MW, Jbabdi S, Patenaude B, et al. Bayesian analysis of neuroimaging data in FSL. NeuroImage 2009;45(1 Suppl):S173–S186. [DOI] [PubMed] [Google Scholar]
- 30. Smith SM, Jenkinson M, Johansen‐Berg H, et al. Tract‐based spatial statistics: voxelwise analysis of multi‐subject diffusion data. NeuroImage 2006;31:1487–1505. [DOI] [PubMed] [Google Scholar]
- 31. Winkler AM, Ridgway GR, Webster MA, et al. Permutation inference for the general linear model. NeuroImage 2014;92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith SM, Nichols TE. Threshold‐free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 2009;44:83–98. [DOI] [PubMed] [Google Scholar]
- 33. Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage 2007;36:630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract‐specific quantification. NeuroImage 2008;39:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Manto M. Tremorgenesis: a new conceptual scheme using reciprocally innervated circuit of neurons. J Transl Med 2008;26:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Elble RJ. Origins of tremor. Lancet (London, England). 2000;355:1113–1114. [DOI] [PubMed] [Google Scholar]
- 37. Jang SH, Kwon HG. Anatomical location of the medial lemniscus and spinothalamic tract at the pons in the human brain: a diffusion tensor tractography study. Somatosens Mot Res 2013;30:206–209. [DOI] [PubMed] [Google Scholar]
- 38. Schmahmann JD, Pandya DN. Prefrontal cortex projections to the basilar pons in rhesus monkey: implications for the cerebellar contribution to higher function. Neurosci Lett 1995;199:175–178. [DOI] [PubMed] [Google Scholar]
- 39. Safadi Z, Grisot G, Jbabdi S, et al. Functional segmentation of the anterior limb of the internal capsule: linking white matter abnormalities to specific connections. J Neurosci 2018;38:2106–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jellison BJ, Field AS, Medow J, et al. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol 2004;25:356–369. [PMC free article] [PubMed] [Google Scholar]
- 41. Galea MP, Darian‐Smith I. Multiple corticospinal neuron populations in the macaque monkey are specified by their unique cortical origins, spinal terminations, and connections. Cereb Cortex 1994;4:166–194. [DOI] [PubMed] [Google Scholar]
- 42. Gasquoine PG. Contributions of the insula to cognition and emotion. Neuropsychol Rev 2014;24:77–87. [DOI] [PubMed] [Google Scholar]
- 43. Buckner RL, Andrews‐Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 44. Sharp DJ, Beckmann CF, Greenwood R, et al. Default mode network functional and structural connectivity after traumatic brain injury. Brain 2011;134(Pt 8):2233–2247. [DOI] [PubMed] [Google Scholar]
- 45. Alexander GE, Crutcher MD, DeLong MR. Basal ganglia‐thalamocortical circuits: parallel substrates for motor, oculomotor, "prefrontal" and "limbic" functions. Prog Brain Res 1990;85:119–146. [PubMed] [Google Scholar]
- 46. Benito‐León J, Bermejo‐Pareja F, Louis ED. Neurological Disorders in Central Spain Study G . Incidence of essential tremor in three elderly populations of central Spain. Neurology 2005;64:1721–1725. [DOI] [PubMed] [Google Scholar]
- 47. Benito‐León J, Louis ED, Posada IJ, et al. Population‐based case‐control study of cognitive function in early Parkinson's disease (NEDICES). J Neurol Sci 2011;310(1–2):176–182. [DOI] [PubMed] [Google Scholar]
- 48. Benito‐León J, Louis ED, Bermejo‐Pareja F, Neurological Disorders in Central Spain Study G . Population‐based case‐control study of cognitive function in essential tremor. Neurology 2006;66:69–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator, only for purposes of replicating procedures and results.


