Abstract
Objective
Cognitive variability is a potentially important source of heterogeneity in longitudinal cognitive profiles. We examined the extent to which common age‐related neuropathologies including Lewy bodies and Alzheimer’s disease (AD) contribute to yearly variability in late life cognition.
Methods
Data came from 1321 community‐dwelling older adults who were followed annually for up to 23 years, died and underwent brain autopsy. Cognition was assessed via a comprehensive testing battery. Uniform neuropathologic evaluations assessed burdens of Lewy bodies, AD, infarcts, TDP, hippocampal sclerosis, amyloid angiopathy, atherosclerosis, and arteriolosclerosis. Using mixed effects models, yearly variability in cognition, characterized as within‐person variability of annual cognitive scores, was regressed on the nine neuropathologic indices.
Results
The average age of decedents was 90 years and 69% were female. At autopsy, about two thirds met the pathologic criteria for AD. Neocortical Lewy bodies were present in 13% of the individuals. Other neuropathologic conditions also were common. All neuropathologic indices except for microinfarcts were associated with cognitive decline. Individuals with neocortical Lewy bodies had almost twice the yearly variability in cognition compared to those without. Individuals with AD had about 70% more variability compared to those without. Yearly variability was present among persons with vascular diseases but to a lesser degree than neocortical Lewy bodies and AD.
Interpretation
Lewy body pathology is associated with pronounced variability in annual cognitive assessments but this finding is not unique to this pathology. Comparable variability is present among persons with AD pathology and to a lesser extent among persons with cerebrovascular pathologies.
Introduction
Late life cognitive trajectories are heterogeneous, with some persons declining relatively fast, others slower, and still others not declining at all. Characterizing distinct longitudinal cognitive profiles and identifying their determinants are essential to prevent cognitive impairment and dementia in old age. It is well‐documented that multiple neuropathologies contribute to a substantial proportion of between‐person variation in the rate of cognitive decline. These pathologies increase the risk of decline as well as accelerate such decline. Notably, however, the rate of decline only captures one dimension of late life cognitive trajectories. Another potentially important dimension is the variability between cognitive assessments within same individuals. However, this within‐person variability in cognition has received relatively little attention in cognitive aging research and is largely dismissed as random noise. Studies that link age‐related neuropathologies with within‐person cognitive variability are extremely limited. One report from our group shows that the presence of neocortical Lewy bodies is associated with significant variation across annual cognitive assessments.1 To date, we are not aware of any study that has systematically investigated the associations of multiple common age‐related neuropathologies with cognitive variability; thus, the neuropathologic basis of aging‐related cognitive variability remains largely unknown.
To fill this knowledge gap, we leveraged annual cognitive and postmortem neuropathologic data from over 1300 community‐based older adults who participated in two cohort studies of aging and dementia, died and underwent brain autopsy, to examine the extent to which multiple age‐related neuropathologies contribute to cognitive variability. Specifically, we characterized cognitive variability as within‐person variability of annual cognitive scores relative to person‐specific trajectories of decline (i.e., residual variance). We regressed residual variance on nine neuropathologic indices including neocortical Lewy bodies, AD, macroscopic infarcts, microinfarcts, hyper‐phosphorylated transactive response DNA‐binding protein 43 (TDP), hippocampal sclerosis, amyloid angiopathy, atherosclerosis, and arteriolosclerosis. Based on our previous finding, we hypothesized that individuals with neocortical Lewy bodies would exhibit the greatest cognitive variability compared to those with AD and other neuropathologies.
Methods
Study participants
The Religious Orders Study (ROS) and the Rush Memory and Aging Project (MAP) are two ongoing clinicopathologic cohort studies.2 ROS started in 1994, and participants are older Catholic nuns, priests and brothers from more than 40 groups across the United States. MAP started in 1997, and participants are older lay persons from continuous care retirement communities, subsidized housing facilities and local churches throughout northeastern Illinois, the United States. To facilitate combined analyses, both studies share a common core of study protocol and testing batteries.
ROSMAP participants were enrolled without known dementia, all agreed to annual evaluations and brain donation after death. A written informed consent and Anatomical Gift Act were obtained from each participant. Both studies were approved by an Institutional Review Board of Rush University Medical Center. The follow‐up rate was about 95% among the survivors and the autopsy rate approached 87% among the deceased. At the time of analysis on February 28, 2019, a total of 3567 ROSMAP participants completed baseline evaluation and 1843 had died. Of the deceased, 1600 had undergone brain autopsies and 1572 had neuropathologic evaluations signed‐off by a board certified neuropathologist. We excluded 106 individuals with only one cognitive assessment before death and 145 individuals with incomplete neuropathology data. The analysis was conducted on the remaining 1321 individuals. The mean age at death was 89.9 years (Standard deviation [SD] = 6.4) and mean education was 16.2 years (SD = 3.6), and 68.9% (N = 910) were female.
Longitudinal cognitive measures and clinical diagnosis
A uniform cognitive assessment was administered at baseline and during annual follow‐up visits (mean follow‐up years: 8.4, SD = 5.0, range = 1–23). A battery of 17 cognitive performance tests was used to construct a summary measure of global cognition.3 The tests for episodic memory include word list memory, word list recall, word list recognition, immediate and delayed recall of logical memory story A and the East Boston Story. The tests for semantic memory include Boston naming, category fluency, and a word reading test. The tests for working memory include digit span forward, digit span backward, and digit ordering. The tests for perceptual speed include symbol digit modalities and number comparison. The tests for visuospatial ability include judgment of line orientation and progressive matrices. Raw scores of each test were first standardized into z‐scores using the baseline mean and standard deviation of the ROSMAP cohort, and global cognitive score was then calculated by averaging z‐scores of the 17 tests, with higher scores representing better cognition. The composite measure has important advantages in that it reduces floor and ceiling effects and is suitable for modeling change over time.
Annual evaluations also include a medical history interview and a neurological exam. Based on these results and those from the cognitive assessment, dementia diagnosis and its most likely etiology were provided by a clinician.4 After a participant died, a neurologist with expertise in dementia reviewed all available clinical data and rendered a final summary opinion regarding the most plausible diagnosis at the time of death. Clinical diagnoses were blinded to all postmortem data.
Neuropathologic evaluation
After death, brain was removed and hemisected following standard procedure, as previously described.5 The hemispheres were cut into 1 cm coronal slabs. Both hemispheres were examined for gross pathology including chronic macroscopic infarcts. The hemisphere with visible pathology was chosen and prepared for histological evaluation. Briefly, the fresh slabs were fixed in 4% paraformaldehyde. Tissue blocks from predetermined regions were dissected, embedded in paraffin, and cut into 6‐micron sections for diagnostic purpose.
Neuropathologies of Lewy bodies, AD, and other degenerative as well as vascular conditions were systematically assessed among all autopsied persons by neuropathologists blinded to all clinical and cognitive data.1 Nigra‐predominant, limbic‐ and neocortical‐type Lewy bodies were identified using α‐synuclein immunostaining (LB509; 1:150 or 1:100, Zymed Labs, Invitrogen, Carlsbad, CA; and pSyn#64; 1:20,000; Wako Chemicals, Richmond, VA). It was shown previously that odds of dementia and cognitive decline are driven by Lewy bodies of neocortical type, therefore our analyses focused on the presence of neocortical Lewy bodies (i.e. mid frontal, mid temporal, and inferior parietal). Sections from five cortical regions (i.e. mid frontal, mid temporal, inferior parietal, entorhinal, and hippocampus) were stained using modified Bielschowsky silver stains, and neuritic plaques, diffuse plaques, and neurofibrillary tangles were counted to determine the burden of AD pathology. Individuals with intermediate or high likelihood of AD according to modified NIA‐Reagan criteria were classified as having a pathologic diagnosis of AD. TDP staging (1:localized to amygdala only, 2: extended to other limbic regions, and 3: extended to neocortical regions) was determined using a phosphorylated monoclonal TAR5P‐1D3 antibody (pS409/410; 1:100, Ascenion, Munich, Germany).6 The presence of hippocampal sclerosis was identified by severe neuronal loss and gliosis on H&E‐stained sections in CA1 or subiculum.7 Chronic macroscopic infarcts identified during gross examination were confirmed histologically. The presence of microinfarcts was determined on sections in a minimum of nine regions stained with hematoxylin and eosin (H&E).8 Sections in 4 neocortical regions (i.e. mid frontal, mid temporal, angular and calcarine) were immunostained for β‐amyloid (4G8; 1:9000, Covance Labs, Madison, WI; 6F/3DDako; 1:50, North America Inc., Carpinteria, CA; and 10D5; 1:600, Elan Pharmaceuticals, San Francisco, CA). Meningeal and parenchymal vessels were assessed for amyloid deposition. Individuals with moderate or severe amyloid angiopathy were identified.9 Moderate or severe atherosclerosis was determined by visually examining the cerebral arteries and visible branches of the circle of Willis. Small vessels of the anterior basal ganglia were assessed for moderate or severe arteriolosclerosis.10
In our current protocol, some neuropathologies are measured with greater precision than others (e.g., some are only classified as present vs absent). To facilitate comparison between neuropathologies, ordinal (i.e. TDP) or semiquantitative measures (i.e. amyloid angiopathy, atherosclerosis and arteriolosclerosis) were dichotomized for all the analyses.
Statistical analysis
We fit mixed effects models to the annual cognitive scores as a longitudinal continuous outcome and simultaneously examined the associations of neuropathologic indices with cognitive trajectory as well as yearly cognitive variability. We first examined linear decline.11 Here, the mean level of cognition and annual rate of cognitive decline (i.e. the intercept α and slope β) were modeled as linear functions of the demographics and nine neuropathologic measures. In addition, person‐specific variation from the mean was captured by random intercept γi 0 and slope γi 1. The basic model structure for cognitive score of individual i at visit j, Cogij, can be specified as below,
Yearly cognitive variability was characterized using the variance of residuals eij, i.e. the within‐person variability of deviation of each observed cognitive score from person‐specific linear trajectory of decline (Fig. 1A). The residual variance was modeled simultaneously as a log‐linear function of the nine neuropathologic measures. The exponent of individual coefficient for each neuropathologic index is an estimate of the ratio of cognitive variability among persons with and without the corresponding neuropathology.
Figure 1.
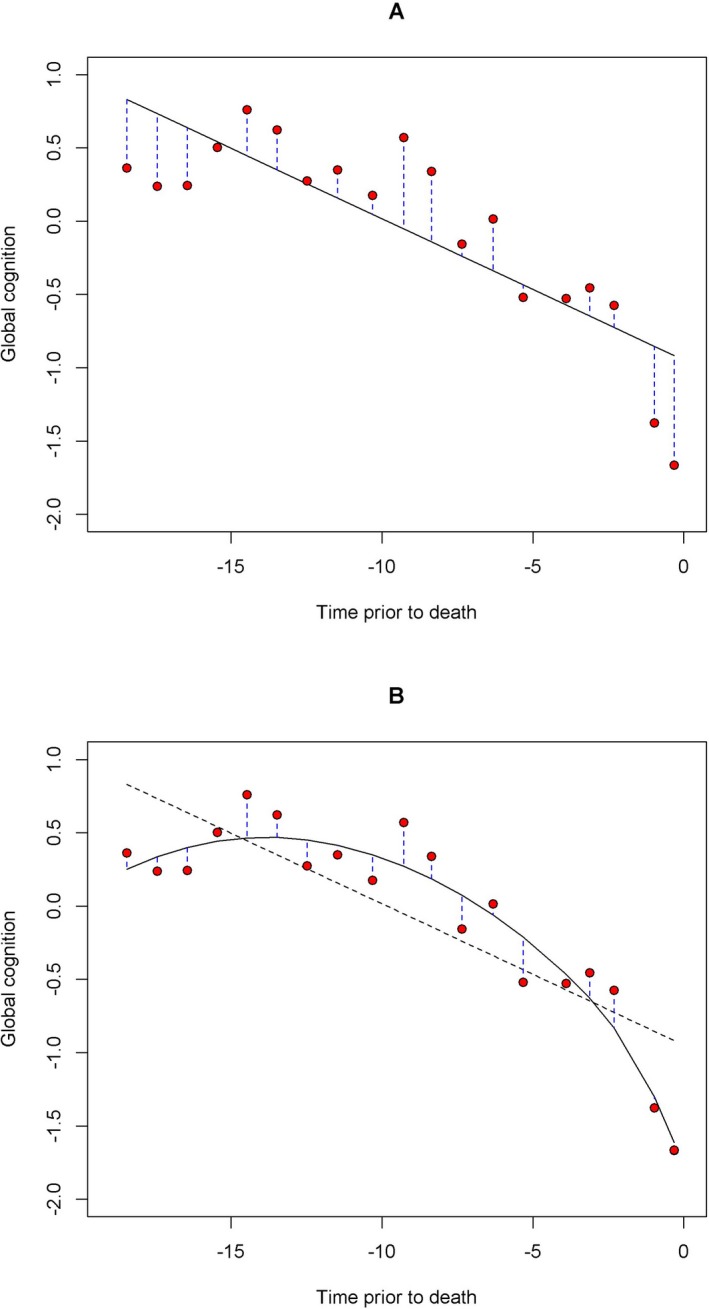
Within‐person variability in cognition. The figure illustrates how the variance of the residuals of cognitive scores was used to characterize cognitive variability. The red circles are observed cognitive scores over time for a representative participant. Both linear (Panel A) and nonlinear person‐specific cognitive decline (Panel B) were fit to the data using linear and functional mixed effects models, and residuals were defined as the deviations of the observed cognitive score from the fitted values (blue vertical dashed lines). The black dashed line on Panel B is the fitted line as in Panel A (solid back line).
Cognitive trajectories of decline in old age can be nonlinear. To ensure our results were robust, we repeated the analyses using a functional mixed effects model that allowed for nonlinear decline in cognition.12, 13 That is, in the linear mixed effects model, the cognitive trajectory is determined by intercept and slope and the associations of neuropathologies with decline are proportional over time. By contrast, the functional mixed effects model fits a nonlinear trajectory without proportional assumption over time for the associations. All the functional coefficients θ(tij) are modeled using B‐spline expansions with two equal‐quantile interior knots.
Similar to the linear model, the cognitive variability was quantified using the residual variance (Fig. 1B) and modeled as a log‐linear function of the neuropathologic measures.
The linear mixed effects models were fit using the NLMIXED procedure in SAS/STAT version 9.4 (SAS Institute, Cary, NC) and the functional mixed effects models were fit using the nlme package in R programs version 3.4.2 (The R Foundation for Statistical Computing). Statistical significance was determined at α level of 0.05. Throughout the analyses, all the nine neuropathologic measures were simultaneously included in the model such that the associations of each neuropathology with cognitive decline and cognitive variability were estimated after controlling for other pathologies.
Results
Clinical and neuropathologic characteristics
A total of 1321 deceased participants were included in the analyses. Of those, 410 (31.0%) had no cognitive impairment at death, 307 (23.2%) had mild cognitive impairment, and 604 (45.7%) were diagnosed with dementia. Five hundred and thirteen (84.9%) of the 604 dementia cases were Alzheimer’s dementia with no other condition. Seventy (11.6%) were Alzheimer’s dementia with another condition (47 vascular, 11 Lewy body, and 12 miscellaneous conditions such as depression and delirium). Only a very small fraction (N = 21) were diagnosed with non‐Alzheimer’s dementia, including nine with vascular dementia and seven with dementia with Lewy bodies.
Neuropathologic examination revealed that neocortical Lewy bodies were present in 13.3% of the sample (N = 175), nearly two thirds met pathologic criteria for AD (N = 874), approximately a third (N = 433) had TDP pathology extending beyond amygdala, and 10% (N = 132) had hippocampal sclerosis. Cerebrovascular conditions also were common. Chronic macroscopic infarcts and microinfarcts were reported in 35.8% and 29.7% of the sample respectively. Moderate or severe amyloid angiopathy, atherosclerosis, and arteriolosclerosis were each present in over 30% (Table 1).
Table 1.
Characteristics of study participants.
| Variable | Mean (SD) or N (percent) |
|---|---|
| Age at death | 89.9 (6.4) |
| Education | 16.2 (3.6) |
| Female | 910 (68.9%) |
| Follow‐up years | 8.4 (5.0) |
| Baseline global cognition | −0.10 (0.61) |
| Global cognition proximate to death | −1.00 (1.19) |
| Final clinical diagnosis | |
| No cognitive impairment | 410 (31.0%) |
| Mild cognitive impairment | 307 (23.2%) |
| Alzheimer’s dementia | 583 (44.1%) |
| Other dementia | 21 (1.6%) |
| Pathologic AD (Reagan criteria) | 874 (66.2%) |
| Macroscopic infarctsa | 473 (35.8%) |
| Microscopic infarctsa | 392 (29.7%) |
| Cortical Lewy bodiesa | 175 (13.3%) |
| TDPb | 433 (32.8%) |
| Hippocampal sclerosisa | 132 (10.0%) |
| Cerebral amyloid angiopathyc | 491 (37.2%) |
| Atherosclerosisc | 429 (32.5%) |
| Arteriolosclerosisc | 413 (31.3%) |
Present.
TDP inclusions in limbic or neocortical regions.
Moderate or severe.
Mixed pathologies were common. Of 175 individuals with neocortical Lewy bodies, only six had no other comorbid pathologic conditions; almost 75% also met criteria for pathologic AD. Similarly, of the 874 with pathologic AD, <10% (N = 84) had AD only; nearly 85% also had infarcts or other vascular conditions.
Associations of neuropathologies with linear decline and variability
We examined the associations of nine neuropathologic indices with annual rate of cognitive decline using a single linear mixed effects model. Except for microscopic infarcts, each of the other eight neuropathologies was associated with cognitive decline. After controlling for neuropathologies, a representative female with 85 years of age and 16 years of education had a mean rate of decline of −0.030 standard units per year (standard error [SE] = 0.006, P < 0.001). Neocortical Lewy bodies and AD were associated with the fastest rates of decline, followed by hippocampal sclerosis and TDP. These results are consistent with our prior report that AD has the most potent effect on cognitive decline but other less frequent pathologies including neocortical Lewy bodies and hippocampal sclerosis also show strong effects.14 The impact of vascular diseases is less potent compared to degenerative conditions (Table 2).
Table 2.
Neuropathologies and rate of cognitive decline.
| Estimate | SE | P | |
|---|---|---|---|
| Mean rate of decline | −0.030 | 0.006 | <0.0001 |
| Presence of AD | −0.056 | 0.006 | <0.0001 |
| Presence of cortical Lewy bodies | −0.050 | 0.008 | <0.0001 |
| Presence of macroscopic infarcts | −0.017 | 0.006 | 0.002 |
| Presence of microinfarcts | 0.004 | 0.006 | 0.456 |
| TDP inclusions, limbic or neocortical | −0.033 | 0.006 | <0.0001 |
| Presence of hippocampal sclerosis | −0.039 | 0.009 | <0.0001 |
| Moderate or severe amyloid angiopathy | −0.016 | 0.005 | 0.004 |
| Moderate or severe atherosclerosis | −0.020 | 0.006 | 0.001 |
| Moderate or severe arteriolosclerosis | −0.016 | 0.006 | 0.005 |
SE, standard error.
The model was adjusted for age, sex and education.
In the same linear mixed effects model used to examine the relation of neuropathologies with cognitive decline, we simultaneously examined the residual variance as a log‐linear function of neuropathologies (Table 3). Our results revealed that neocortical Lewy bodies were strongly associated with yearly cognitive variability: individuals with neocortical Lewy bodies had almost twice the variability in cognition compared to those without (Estimate = 1.875, 95% confidence intervals [CI] = 1.712–2.038, P < 0.001). However, AD also was strongly associated with yearly cognitive variability, with individuals with AD exhibiting about 70% more variability (Estimate = 1.700, 95% CI = 1.592–1.809, P < 0.001) in cognition compared to those without. Notably, the variability due to neocortical Lewy bodies did not differ significantly from that due to AD (P = 0.079), suggesting that the two pathologies contribute similarly to yearly cognitive variability. To examine potential synergistic effects of AD and neocortical Lewy bodies on cognitive trajectories, we augmented the model by including interaction terms between the two. We did not observe a significant interaction for either cognitive decline or cognitive variability (both Ps> 0.05), suggesting that the effects of the two pathologies are additive.
Table 3.
Neuropathologies and variability in cognition (Linear model).
| Estimatea | 95% CI | P | ||
|---|---|---|---|---|
| Presence of AD | 0.531 | 0.467 | 0.594 | <0.0001 |
| Presence of cortical Lewy bodies | 0.629 | 0.542 | 0.716 | <0.0001 |
| Presence of macroscopic infarcts | 0.224 | 0.161 | 0.287 | <0.0001 |
| Presence of microinfarcts | 0.048 | −0.016 | 0.113 | 0.140 |
| TDP inclusions, limbic or neocortical | 0.051 | −0.015 | 0.118 | 0.128 |
| Presence of hippocampal sclerosis | 0.136 | 0.034 | 0.237 | 0.009 |
| Moderate or severe amyloid angiopathy | 0.055 | −0.007 | 0.116 | 0.085 |
| Moderate or severe atherosclerosis | 0.193 | 0.128 | 0.258 | <0.0001 |
| Moderate or severe arteriolosclerosis | 0.251 | 0.185 | 0.317 | <0.0001 |
SE, standard error; CI, Confidence interval.
Estimates for the association of pathologies with variability are in log scale.
Vascular pathologies were associated with increased variability, but the increases in variability were considerably less than those associated with neocortical Lewy bodies and AD. Specifically, the presence of macroscopic infarcts was associated with 25% (Estimate = 1.251, 95% CI = 1.592–1.809) more variability. Moderate or severe atherosclerosis and arteriolosclerosis were associated with 21% (Estimate = 1.213, 95% CI = 1.134–1.292) and 29% (Estimate = 1.285, 95% CI = 1.200–1.369) more variability respectively.
In contrast with its strong association with cognitive decline, hippocampal sclerosis only minimally affected variability, with those with HS showing 15% more variability compared to those without (Estimate = 1.145, 95% CI = 1.029–1.261). We did not observe associations of microinfarcts, TDP, or amyloid angiopathy with cognitive variability.
Associations of neuropathologies with nonlinear decline and variability
Cognitive decline in old age is often nonlinear, and this nonlinearity also contributes to an increase in within‐person variability from a linear fit (Fig. 1). Thus, using a functional mixed effects model that allows for nonlinear trajectories, we re‐examined the associations of neuropathologies with cognitive decline and variability. The associations with cognitive decline across different pathologies were consistent with those from the linear model (Fig. 2). Notably, the functional model revealed strong nonlinear associations of AD, neocortical Lewy bodies and TDP with cognitive decline, such that the difference in rates of cognitive decline between individuals with and without AD became evident decade before death, whereas the associations of neocortical Lewy bodies and TDP with cognitive decline occurred much closer to death. As expected, the overall variability due to individual pathology was lower after accounting for the nonlinear associations with cognitive decline. Importantly, however, the main results persisted. Specifically, neocortical Lewy bodies and AD were strongly associated with cognitive variability (Table 4). Vascular conditions were implicated to a lesser extent.
Figure 2.
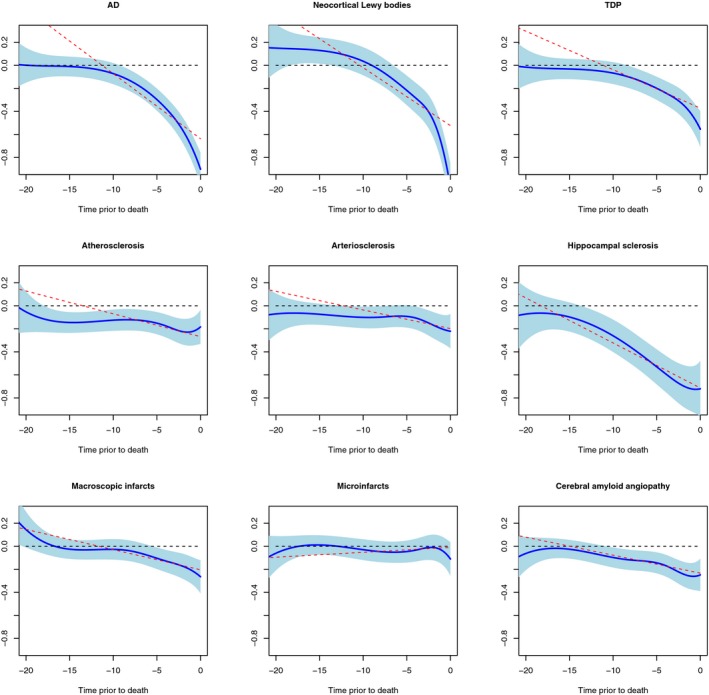
Associations of neuropathologies with trajectories of cognitive decline. Each panel shows both linear and nonlinear associations of corresponding neuropathology with trajectories of decline. Solid blue line and light blue shaded area are the pointwise estimates and 95% confidence band from the nonlinear fit. The red dashed line is the pointwise estimates from the linear fit. The black dashed line is the horizontal reference line at zero.
Table 4.
Neuropathologies and variability in cognition (Functional model).
| Estimatea | 95% CI | P | ||
|---|---|---|---|---|
| Presence of AD | 0.322 | 0.251 | 0.393 | <0.0001 |
| Presence of cortical Lewy bodies | 0.380 | 0.285 | 0.475 | <0.0001 |
| Presence of macroscopic infarcts | 0.150 | 0.085 | 0.216 | <0.0001 |
| Presence of microinfarcts | 0.052 | −0.034 | 0.137 | 0.237 |
| TDP inclusions, limbic or neocortical | −0.069 | −0.207 | 0.069 | 0.326 |
| Presence of hippocampal sclerosis | 0.041 | −0.171 | 0.252 | 0.706 |
| Moderate or severe amyloid angiopathy | −0.016 | −0.086 | 0.055 | 0.664 |
| Moderate or severe atherosclerosis | 0.241 | 0.170 | 0.313 | <0.0001 |
| Moderate or severe arteriolosclerosis | 0.259 | 0.186 | 0.331 | <0.0001 |
SE, standard error; CI, Confidence interval.
Estimates for the association of pathologies with variability are in log scale.
Discussion
Prior studies of longitudinal cognitive data largely focus on the rate of cognitive decline assuming that within‐person variability across cognitive assessments is random noise. Using clinicopathologic data from over 1300 community‐based older adults, we investigated the associations of multiple neuropathologies with both the rate of cognitive decline and yearly cognitive variability. This study provides empirical evidence that within‐person variability is not simply random noise but rather reflects a systematic source of individual differences that can be attributed to multiple neuropathologies. Findings from this study provide novel insight into the relationship between diverse neuropathologies and multiple dimensions of late life cognitive decline. Our results suggest that neocortical Lewy bodies and AD are the main drivers of late life cognitive decline. Furthermore, yearly variability in cognition, characterized as within‐person variability of annual cognitive scores relative to person‐specific trajectories of decline, is not specific to Lewy bodies. Both neocortical Lewy bodies and AD are important determinants of yearly cognitive variability, and their effects on variability are of similar magnitudes. TDP and HS are also important drivers of cognitive decline, but they are not strongly associated with yearly cognitive variability. Separately, vascular conditions are relatively weakly associated with both decline and variability.
Of note, although we observed considerable variability associated with Lewy body pathology and this may be related to the fluctuations reported in dementia with Lewy bodies (DLB), the yearly cognitive variability measured in this study is not the same as the cognitive fluctuation typically described in DLB. The latter often involves diurnal variations in attention and alertness. We speculate that it is possible that intraday cognitive fluctuation in the long term can result in pronounced variability in cognitive performance between annual assessments. Prior studies suggest that cognitive fluctuation is more frequent in patients with DLB compared to other types of dementia,15, 16, 17 such that fluctuation was reported in 89% of patients with DLB, 64% with Alzheimer’s dementia, and 23% with vascular dementia. Furthermore, the magnitude of fluctuation is highest among patients with DLB compared with those with Alzheimer’s, vascular or Parkinson’s dementia. These clinical findings suggest that prominent fluctuation in cognition may reflect the presence of Lewy body pathology. However, studies linking age‐related neuropathologies with cognitive fluctuation suffer from small sample size. One study reported that cognitive fluctuation was present in 10 of 27 patients (37%) who had both autopsy confirmed Lewy bodies and AD but no cognitive fluctuation was observed in the five patients with Lewy bodies without AD.18 Another study reported no difference in cognitive fluctuation among patients with AD (5/19), Lewy bodies (9/18), or AD with Lewy bodies (8/22).19 In this study, we used a data‐driven approach to characterize yearly variability in cognition by applying a novel statistical approach to longitudinal cognitive data derived from annual performance‐based assessments. Importantly, this approach captures cognitive variability while accounting for the trajectory of cognitive decline many years before death. Our findings are overall supportive of the hypothesis that Lewy bodies are indeed a major contributor to yearly variations in cognition as determined from detailed annual evaluations.
Using data from the same cohorts, we previously reported pronounced yearly variability in cognition in individuals with neocortical Lewy bodies.1 In this study, by simultaneously examining cognitive decline and cognitive variability within the same modeling framework and with a larger sample and longer follow‐up, we further extend our previous findings in several important ways. First, we confirmed that individuals with neocortical Lewy bodies have more pronounced yearly cognitive variability. In addition, we quantified the magnitude of variability associated with this pathology. In terms of linear cognitive decline, individuals with neocortical Lewy bodies had almost twice as much yearly variability compared to individuals without neocortical Lewy bodies. In a more flexible functional model that captures nonlinear decline, we observed nearly 50% more yearly cognitive variability in individuals with neocortical Lewy body pathology.
Second, we quantified the increases in variability associated with multiple other neuropathologies and compared these increases with that of neocortical Lewy bodies. We found that pronounced yearly variability in cognition is not unique to Lewy body pathology. Individuals with AD exhibited similar cognitive variability, and the magnitude of variability due to AD was not different statistically from that due to neocortical Lewy bodies. Separately, macroscopic infarcts, large and small vessel diseases also exhibited yearly cognitive variability, but with a smaller magnitude compared with neocortical Lewy bodies and AD. It is worth noting that while both neocortical Lewy bodies and AD show comparable magnitudes of yearly cognitive variability, whether these two pathologies have distinct patterns of variability is unknown and further investigation is warranted.
Finally, our findings suggest that, while neocortical Lewy bodies, AD and select vascular diseases had significant impact on both cognitive decline and variability, not all neuropathologies follow the same pattern. Notably, TDP and hippocampal sclerosis differentially affect cognitive decline and cognitive variability. The presence of hippocampal sclerosis had a profound impact on the rate of cognitive decline. By contrast, individuals with hippocampal sclerosis had only 15% more variability in linear decline, and the association with variability was not observed when nonlinear decline was considered. Similarly, we confirmed that TDP pathology is associated with progressive cognitive decline, but it does not appear to contribute to yearly variability in cognition. Future studies are warranted to replicate such dissociations and to explore potential underlying neurobiological mechanisms.
To our knowledge, this is the largest study investigating multiple age‐related neuropathologies in relation to variability in cognition. All participants in this study were without known dementia at baseline and followed prospectively until death. During the course of follow‐up, some participants developed cognitive impairment or dementia while others remained cognitively intact. Furthermore, systematic postmortem evaluations assessed several common age‐related neuropathologies, which allowed us to comprehensively compare neuropathologic contributions to both cognitive decline and variability. The limitations of ROSMAP involve the generalizability of the reported findings. In particular, all participants in ROSMAP have agreed to brain donation at death, and a majority of the participants are non‐Latino whites. Neuropathologic data collections from African American and Latino participants are currently underway but the data are not mature enough for formal analysis. Most participants are older with higher education than the general population. Furthermore, although it will be important to consider the degree to which the burden of neuropathologies is associated with late life cognitive decline and variability, we were not able to consider this in these analyses. In addition, the majority of dementia cases in this study were Alzheimer’s dementia, which needs to be taken into consideration while interpreting these findings. Future studies should attempt to address these limitations so as to further clarify the extent to which different age‐related diseases contribute to various components of late life cognitive trajectories.
Author Contributions
Conception and design of the study: LY, TW, RSW, PAB. Acquisition and analysis of data: LY, TW, JAS, DAB. Drafting a significant portion of the manuscript or figures: LY, TW, RSW, SL, JAS, DAB, PAB.
Conflict of Interest
LY reports grants from National Institute on Aging. DAB reports grants from National Institutes of Health. JAS serves as a consultant for AVID radiopharmaceuticals, Grifols, National Hockey League and National Football League. PAB reports grants from National Institutes of Health/National Institute on Aging. Other authors have no relevant conflict of interest to disclose.
Acknowledgments
This study is funded by National institute on Aging grants (P30AG10161, R01AG15819, R01AG17917, R01AG34374, and R01AG042210). We are deeply indebted to all participants who contributed their data and biospecimens. We are thankful to the staff in the Rush Alzheimer’s Disease Center. Data used in this study are available through request via the RADC research resource sharing hub (https://www.radc.rush.edu/).
Funding Information
This study is funded by National institute on Aging grants (P30AG10161, R01AG15819, R01AG17917, R01AG34374, and R01AG042210).
Funding Statement
This work was funded by National Institute on Aging grants P30AG10161, R01AG042210, R01AG15819, R01AG17917, and R01AG34374.
References
- 1. Schneider JA, Arvanitakis Z, Yu L, et al. Cognitive impairment, decline and fluctuations in older community‐dwelling subjects with Lewy bodies. Brain 2012;135(Pt 10):3005–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bennett DA, Buchman AS, Boyle PA, et al. Religious orders study and rush memory and aging project. J Alzheimer's Dis 2018;64(s1):S161–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyle PA, Wilson RS, Yu L, et al. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol 2013;74:478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bennett DA, Schneider JA, Buchman AS, et al. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology 2005;25:163–175. [DOI] [PubMed] [Google Scholar]
- 5. Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 2009;66:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nag S, Yu L, Wilson RS, et al. TDP‐43 pathology and memory impairment in elders without pathologic diagnoses of AD or FTLD. Neurology 2017;88:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nag S, Yu L, Capuano AW, et al. Hippocampal sclerosis and TDP‐43 pathology in aging and Alzheimer disease. Ann Neurol 2015;77:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arvanitakis Z, Leurgans SE, Barnes LL, et al. Microinfarct pathology, dementia, and cognitive systems. Stroke 2011;42:722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu L, Boyle PA, Nag S, et al. APOE and cerebral amyloid angiopathy in community‐dwelling older persons. Neurobiol Aging 2015;36:2946–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arvanitakis Z, Capuano AW, Leurgans SE, et al. Relation of cerebral vessel disease to Alzheimer's disease dementia and cognitive function in elderly people: a cross‐sectional study. Lancet Neurol 2016;15:934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hedeker D, Mermelstein RJ, Demirtas H. An application of a mixed‐effects location scale model for analysis of Ecological Momentary Assessment (EMA) data. Biometrics 2008;64:627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rice JA, Wu CO. Nonparametric mixed effects models for unequally sampled noisy curves. Biometrics 2001;57:253–259. [DOI] [PubMed] [Google Scholar]
- 13. Guo W. Functional mixed effects models. Biometrics 2002;58:121–128. [DOI] [PubMed] [Google Scholar]
- 14. Boyle PA, Yu L, Wilson RS, et al. Person‐specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol 2018;83:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ballard C, O'Brien J, Gray A, et al. Attention and fluctuating attention in patients with dementia with Lewy bodies and Alzheimer disease. Arch Neurol 2001;58:977–982. [DOI] [PubMed] [Google Scholar]
- 16. Walker M, Ayre G, Cummings J, et al. Quantifying fluctuation in dementia with Lewy bodies, Alzheimer’s disease, and vascular dementia. Neurology 2000;54:1616–1625. [DOI] [PubMed] [Google Scholar]
- 17. Bliwise DL, Scullin MK, Trotti LM. Fluctuations in cognition and alertness vary independently in dementia with Lewy bodies. Mov Disord 2014;29:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hohl U, Tiraboschi P, Hansen LA, et al. Diagnostic accuracy of dementia with Lewy bodies. Arch Neurol 2000;57:347–351. [DOI] [PubMed] [Google Scholar]
- 19. Thomas AJ, Mahin‐Babaei F, Saidi M, et al. Improving the identification of dementia with Lewy bodies in the context of an Alzheimer's‐type dementia. Alzheimer's Res Ther 2018;10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]


